Abstract
Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes that degrade various components of the extracellular matrix (ECM). Members of the MMP family include collagenases, gelatinases, stromelysins, matrilysins and membrane-type MMPs. ProMMPs are cleaved into active forms that promote degradation of ECM proteins. Also, recent evidence suggests direct or indirect effects of MMPs on ion channels in the endothelium and vascular smooth muscle, and on other mechanisms of vascular relaxation/contraction. Endogenous tissue inhibitors of metalloproteinases (TIMPs) reduce excessive proteolytic ECM degradation by MMPs. The balance between MMPs and TIMPs plays a major role in vascular remodeling, angiogenesis, and the uterine and systemic vasodilation during normal pregnancy. An imbalance in the MMPs/TIMPs activity ratio may underlie the pathogenesis of vascular diseases such as abdominal aortic aneurysm, varicose veins, hypertension and preeclampsia. Downregulation of MMPs using genetic manipulations of endogenous TIMPs, or synthetic pharmacological inhibitors such as BB-94 (Batimastat) and doxycycline, and Ro-28-2653, a more specific inhibitor of gelatinases and membrane type 1-MMP, could be beneficial in reducing the MMP-mediated vascular dysfunction and the progressive vessel wall damage associated with vascular disease.
Keywords: MMP, TIMP, blood vessels, extracellular matrix, aneurysm, varicose veins
INTRODUCTION
Blood vessels are constantly adjusting to neuronal, hormonal and hemodynamic stimuli. Short-term alterations in vascular tone occur as a result of changes in endothelial cell function, and increased neurohormonal drive or altered sensitivity of vascular smooth muscle (VSM). On the other hand, chronic changes in vascular function often involve structural changes in the blood vessel architecture. Vascular remodeling involves lasting structural changes in the vessel wall in response to hemodynamic stimuli. The enduring changes in the composition/size of the blood vessel associated with vascular remodeling allow adaptation and repair.
Vascular remodeling may take one of several forms: 1) neointimal hyperplasia, 2) alteration of the arterial structural luminal diameter (inward or outward), and 3) alteration of tunica media mass (eutrophic, hypertrophic and hypotrophic). Eutrophic remodeling involves rearrangement of the same number and size of otherwise normal VSM cells around a smaller lumen, i.e. the same media cross-sectional area, leading to increased media:lumen ratio. In various forms of hypertension, including human essential hypertension, the resistance arteries undergo eutrophic inward remodeling, whereas large vessels demonstrate hypertrophy [1–4]. An example of hypotrophic remodeling is the formation of thin walled spiral arteries during pregnancy to allow adequate uteroplacental blood flow to the fetus.
While such classification may be useful, a chronic increase in blood flow in resistance-sized arteries may lead to increased arterial structural luminal diameter and wall mass. Also, arterial structural diameter refers to the maximally dilated diameter, and results from the elastic properties of the arterial wall. However, the diameter of an arterial segment in vivo depends not only on the structural diameter, but also on the degree of vasomotor tone and VSM contractile activity. Furthermore, the manner in which media, adventitia, and ultrastructural wall components, such as focal adhesion sites, and cytoskeleton extracellular matrix (ECM), may ultimately determine the diameter [4]. Specifically, degradation of ECM enables VSM cells to migrate and proliferate, and inflammatory cells to infiltrate the arterial wall during the remodeling process.
Matrix metalloproteinases (MMPs) are a family of structurally related, zinc-containing enzymes that degrade the ECM and connective tissue proteins [5,6]. The proteolytic effects of MMPs play an important role in vascular remodeling, cellular migration and the processing of ECM proteins and adhesion molecules [5–7]. Increasing evidence suggests additional effects of MMPs on other types of vascular cells such as the endothelium and smooth muscle, which may be important in the early stages of vascular remodeling in order to maintain blood flow to various organs. Under normal physiological conditions, the activities of MMPs are regulated at the level of transcription, activation of the precursor zymogens, and interaction with specific ECM components. Also, endogenous tissue inhibitors of MMPs (TIMPs) provide a balancing mechanism to prevent excessive degradation of ECM. An imbalance between MMPs and TIMPs could cause large increases in MMP activity and may lead to pathological changes in the vessel wall structure associated with vascular disease.
Several excellent reviews and research studies have provided detailed information regarding the biochemical structure of MMPs and the determinant of their effects on various components of the ECM [5–7]. It has also been suggested that flow-dependent vascular remodeling is involved in physiological processes, such as blood vessel growth and angiogenesis during development, wound healing, exercise training, and pregnancy as well as in pathological conditions including hypertension, ischemic diseases, and tumor growth [8]. The purpose of this review is to provide an insight into the biological activities of MMPs and their inhibitors in the vascular remodeling associated with angiogenesis and normal pregnancy, and their pathogenetic role in the development/progression of vascular diseases such as abdominal aortic aneurysm (AAA), varicose veins, hypertension and preeclampsia.
Biochemistry of MMPs
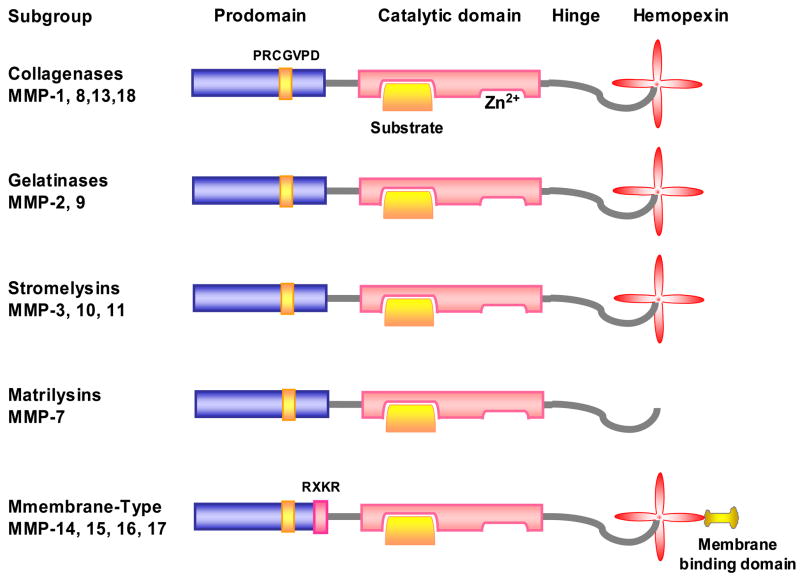
MMPs generally consist of a prodomain, catalytic domain, hinge region, and hemopexin domain (Fig. 1). Proteinases assigned to the MMP family have 3 molecular signatures:
Figure 1.
Structure of MMPs. MMP consists of a prodomain, catalytic domain, hinge region, and hemopexin domain. In the catalytic domain, MMP has a Zn2+ binding site, and a binding site for the specific substrate. MMP has cysteine switch motif PRCGXPD in the prodomain. Matrilysins lack a hemopexin domain. MT-MMP has an additional transmembrane binding domain.
Sequence homology with collagenase-1 (MMP-1).
Cysteine switch motif PRCGXPD in the prodomain that maintains MMPs in their proMMP zymogen form. The conserved cysteine chelates the active zinc site (Fig. 1). MMP-23 is an exception as it lacks the cysteine switch motif.
Zinc-binding motif bound by 3 histidine molecules with the conserved sequence HEXGHXXGXXH located in the catalytic domain.
Members of the MMP Family
Currently, 26 members of the MMP family have been identified in vertebrates; 23 of them have been found in humans [5–7,9] (Table 1). MMPs are divided into 6 groups:
Table 1.
Members of the MMP family in representative vascular and non-vascular tissues
| MMPs | Other Names | Mol. Wt. KDa | Tissue Distribution/Disease Condition | Collagen Substrate | Other Substrates | |
|---|---|---|---|---|---|---|
| Proform | Active | |||||
|
Vascular
Collagenases MMP-1 |
Collagenase-1 |
55 |
45 |
Fibroblast, Interstitial, Tissue collagenase |
I, II, III, VII, VIII, X |
Aggrecan, gelatin, MMP-2, MMP-9 |
| MMP-8 | Collagenase-2 | 75 | 58 | Neutrophil, or PMNL Collagenase | I, II, III, V, VII, VIII, X | Aggrecan, elastin, fibronectin, gelatin, laminin |
| MMP-13 | Collagenase-3 | 60 | 48 | SMC, varicose veins, pre-eclampsia, breast cancer | I, II, III, IV | Aggrecan, gelatin |
|
Gelatinases
MMP-2 |
Gelatinase-A |
72 |
66 |
Aortic aneurysm, varicose veins |
I, II, III, IV, V, VII, X, XI |
Aggrecan, elastin, fibronectin, gelatin, laminin, proteo-glycan, MMP-9, MMP-13 |
| MMP-9 | Gelatinase-B | 92 | 86 | Aortic aneurysm, varcose veins | IV, V, VII, X, XIV | Aggrecan, elastin, fibronectin, gelatin |
|
Stromelysins
MMP-3 |
Stromelysin-1 |
57 |
45 |
SMC, synovial fibroblasts, CAD, HTN, tumor invasion | II, III, IV, IX, X, XI | Aggrecan, elastin, fibronectin, gelatin, laminin, proteoglycan, MMP-7, MMP-8, MMP-13 |
| MMP-10 | Stromelysin-2 | 57 | 44 | Uterine. preeclampsia, arthritis, atherosclerosis, carcinoma cells | III, IV, V | Aggrecan, elastin, fibronectin, gelatin, laminin, MMP-1, -8 |
|
Matrilysins
MMP-7 |
Matrilysin-1 |
28 |
19 |
Uterine |
IV, X |
Aggrecan, elastin, fibronectin, gelatin, laminin, proteoglycan, MMP-1, MMP-2, MMP-9 |
|
Membrane-Type MMPs
MMP-14 |
MT1-MMP | 66 | 56 | Human fibroblasts, SMC, VSMC, uterine, angiogenesis | I, II, III | Aggrecan, elastin, fibronectin, gelatin, laminin, MMP-2, MMP-13 |
| MMP-15 | MT2-MMP | 72 | 50 | Fibroblasts, leukocytes, preeclampsia, cancer (breast, prostate, colon) | I | Fibronectin, gelatin, laminin, MMP-2 |
| MMP-16 | MT3-MMP | 64 | 52 | Leukocytes, angiogenesis human cancer | I | MMP-2 |
| MMP-24 | MT5-MMP | 57 | 53 | Leukocytes, brain tumor, astrocytoma/glioblastoma, | None identified | Fibrin, gelatin |
|
Other MMPs
MMP-11 |
Stromelysin-3 |
51 |
44 |
Uterine, Angiogenesis, hepatocellular carcinoma |
Does not cleave |
Aggrecan, fibronectin, laminin |
| MMP-12 | metalloelastase | 54 | 45 and 22 | Macrophage | IV | Elastin, fibronectin, gelatin, laminin |
| MMP-21 | XMMP | 62 | 49 | Human placenta | α1-anti-trypsin | |
|
Non-Vascular
MMP-18 |
Xenopus Collagenase-4 |
70 |
53 |
Xenopus (amphibian) |
I |
Gelatin |
| MMP-26 | matrilysin-2, endometase | 28 | 19 | Human endometrial tumor | IV | Gelatin, fibronectin, gelatin |
| MMP-17 | MT4-MMP | 57 | 53 | Brain specific cerebellum, breast cancer, | None identified | Fibrin, gelatin |
| MMP-25 | MT6-MMP, Leukolysin | 34 | 28 | Leukocytes, anaplastic astrocytomas, glioblastomas | IV | Gelatin, fibronectin, laminin, fibrin |
| MMP-19 | RASI-1 | 54 | 45 | Liver | IV | Fibronectin, aggrecan, COMP, laminin, gelatin |
| MMP-20 | Enamelysin | 54 | 22 | Tooth enamel | V | Aggrecan, amelogenin, COMP |
| MMP-22 | CMMP | 51 | Chicken fibroblasts | Unknown | Gelatin | |
| MMP-23 | cysteine array MMP | 28 | 19 | Reproductive tissues | Unknown | Unknown |
| MMP-28 | Epilysin | 56 | 45 | Skin keratinocytes | Unknown | Unknown |
CAD, coronary aretery disease; HTN hypertension; PMNL, polymorphonuclear leukocytes; SMC, smooth muscle cells; VSMC, vascular smooth muscle cells
Collagenases, including MMP-1, -8, -13, and -18 (Xenopus). Collagenases cleave interstitial collagens I, II, and III at a specific site three-fourths from the N-terminus, releasing ¼ and ¾ length fragments. They also cleave other ECM and non-ECM molecules.
Gelatinases, including gelatinase-A (MMP-2) and gelatinase-B (MMP-9). They mainly digest denatured collagens (gelatins). They have 3 repeats of a type II fibronectin domain inserted in the catalytic domain, which bind to gelatin, collagens, and laminin [10].
Stromelysins, including stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10). MMP-3 has similar substrate specificity but higher proteolytic efficiency as compared to MMP-10.
Matrilysins, including matrilysin-1 (MMP-7) and matrilysin-2 (MMP-26, endometase). Matrilysins lack the hemopexin domain (Fig. 1).
Membrane-Type MMPs (MT-MMPs), including the type-I transmembrane proteins MT1-, MT2-, MT3-, and MT4-MMP (MMP-14, -15, -16, and -24), and the glycosylphosphatidylinositol (GPI)-anchored proteins MT5-, and MT6-MMP (MMP-17 and -25). MT1-MMP can digest type-I, -II, and III-collagen and other components of ECM, and can activate proMMP to MMP.
Other MMPs, including MMP-11, -12, -19, -20, -22, -23, and -28. MMP-11 (stromelysin-3) differs from MMP-3 (stromelysin-1) in its sequence and substrate specificity. MMP-12 (metalloelastase) digests elastin and other proteins. MMP-20 (enamelysin) digests amelogenin. MMP-22 was first cloned from chicken fibroblasts, and a human homologue was later identified; however, its function and substrate remain unclear. MMP-23 (cysteine array MMP) lacks the cysteine switch motif in the prodomain as well as the hemopexin domain which provides substrate specificity; instead, it has a cysteine-rich domain followed by an immunoglobulin-like domain. It is proposed to be a type II membrane protein harboring the transmembrane domain in the N-terminal part of the propeptide. Because it has a Furin recognition motif in the propeptide (convertase cleavage site which activates MMP), it is cleaved in the Golgi and released as an active enzyme into the extracellular space. MMP-28 (Epilysin ) is the latest addition to the MMP family [11].
Tissue Distribution
MMPs are produced by many tissues and cell types including the vascular cells (Table 1). MMPs are either secreted from the cell or anchored to the plasma membrane. MMPs are often bound with heparin sulfate glycosaminoglycans on the cell surface. Metalloelastase (MMP-12) is mainly expressed in macrophages and is essential for macrophage migration [12]. MMP-19 was identified by cDNA cloning from liver and as a T-cell–derived autoantigen from patients with rheumatoid arthritis (RASI) [13,14].
MMP Cleavage and Activation
Activation of MMPs pro-forms involves detaching of the hemopexin domain, and requires other MMPs or other classes of proteinases. MMP-3 activates a number of proMMPs, including the processing of proMMP-1 into fully active MMP-1 [15]. ProMMP-2 is not activated by general proteinases, and its activation takes place on the cell surface and by most MT-MMPs, but not MT4-MMP [1]. MT1-MMP-mediated activation of proMMP-2 requires TIMP-2 [16,17]. ProMMP-2 forms a complex with TIMP-2 through their C-terminal domains, thus permitting the N-terminal inhibitory domain of TIMP-2 to bind to MT1-MMP on the cell surface. The cell surface-bound proMMP-2 is then activated by an MT1-MMP that is free of TIMP-2. Alternatively, MT1-MMP inhibited by TIMP-2 can act as a “receptor” of proMMP-2. The MT1-MMP-TIMP-2-proMMP-2 complex is then presented to an adjacent free MT1-MMP for activation. Clustering of MT1-MMP on the cell surface through interactions of the hemopexin domain facilitates the activation process [18].
MMPs can be activated by heat treatment and low pH. MMPs are also activated by thiol-modifying agents such as 4-aminophenylmercuric acetate, mercury chloride, and N-ethylmaleimide, oxidized glutathione, sodium dodecyl sulfate, chaotropic agents, and reactive oxygen species. These agents mainly cause disturbance of the cysteine-zinc interaction at the cysteine switch [19]. Nitric oxide (NO) may activate proMMP-9 during cerebral ischemia by reacting with the thiol group of the cysteine switch and forming an S-nitrosylated derivative, suggesting that chemical activation of proMMP occurs in vivo [20].
MMP Substrates and Biological Activities
Substrate specificity for the MMPs is not fully characterized, and the hemopexin domain confers much of the substrate specificity to the MMPs [21]. Known substrates include most of the ECM components (fibronectin, vitronectin, laminin, entactin, tenascin, aggrecan, myelin basic protein, etc). Collagens type I, II, III, IV, V, VI, VII, VIII, IX, X, and XIV are known substrates of MMPs, with different efficacies (Table 1). MMPs may act cooperatively to bring about complete degradation of ECM proteins. Interstitial collagenase MMP-1 and MMP-8 are capable of degrading fibrillar helices. The resulting fragments unfold their triple helix conformation. So-formed single alpha-chain gelatins are further degraded into oligopeptides by less specific gelatinases (MMP-2 and MMP-9) [22]
The most common substrates used to study MMP activity are casein and gelatin. While gelatin (heat denatured collagen) is considered a valid substrate for the gelatinases MMP-2 and MMP-9, casein may not be a physiologically relevant substrate. Nevertheless, casein is used as a generic proteinase substrate because it is digested by a wide ra nge of proteinases.
Effects of MMPs on Endothelium and VSM
Despite the potent proteolytic activity of MMPs on the ECM proteins, little is known regarding the effects of MMPs on other vascular cell types, particularly the endothelium and vascular smooth muscle (VSM). We have recently shown that MMP-2 causes significant relaxation of the rat inferior vena cava (IVC). The MMP-2 induced venous relaxation does not appear to involve increased NO or prostacyclin (PGI2) production, as it is not blocked by the NOS inhibitor L-NAME or the cyclooxygenase inhibitor indomethacin. Interestingly, the MMP-2 induced venous relaxation is abolished by blockers of the large conductance Ca2+-activated K+ channels such as iberiotoxin, suggesting involvement of VSM hyperpolarization pathway [23].
We have also shown that phenylephrine (Phe)-induced contraction in rat aortic strips is inhibited by MMP-2 (~50%) and MMP-9 (~70%) [24]. The MMP-induced inhibition of aortic contraction is concentration- and time-dependent, and is reversible. Also, histological examination of MMP-treated tissues showed an intact tunica media and preserved elastin layer, thus confirming the vessel integrity and absence of degradation of the ECM. The reversibility of the effects of MMPs on Phe-induced contraction suggests that the actions of MMPs are not solely due to irreversible degradation of the ECM. Also, the inhibitory effects of MMPs on VSM contraction are not likely due to degradation of Phe or the α-adrenergic receptors because MMPs also inhibit prostaglandin F2α-induced contraction, suggesting that their effects are not specific to a particular agonist/receptor, but likely involve direct effects of MMPs on a common VSM contraction pathway downstream from receptor activation.
VSM contraction is triggered by increases in Ca2+ release from the intracellular stores and Ca2+ entry from the extracellular space. MMPs do not inhibit Phe-induced contraction in Ca2+-free solution suggesting that they do not inhibit the Ca2+ release mechanism from the intracellular stores. On the other hand, MMPs inhibit Phe-induced Ca2+ influx, suggesting that they may function by inhibiting Ca2+ entry from the extracellular space [24].
The specific mechanism by which MMPs inhibit Ca2+ entry is unclear, but could involve direct effects on the Ca2+ channels. Also, MMP-induced degradation of collagen produces Arg-Gly-Asp (RGD)-containing peptides, which bind to αvβ3 integrin receptors and inhibit Ca2+ entry into VSM [25]. MMPs may also stimulate protease-activated receptors (PARs) and activate signaling pathways that could lead to blockade of VSM Ca2+ channels [26]. This is supported by reports that proteases such as thrombin activate PARs and promote endothelium-dependent VSM relaxation by inhibiting Ca2+ influx [27]. Further studies are needed to define the role of integrins and PARs as possible molecular mechanisms via which MMPs could inhibit VSM contraction.
MMPs and Angiogenesis
Angiogenesis is the process of forming new blood vessels from existing ones and requires degradation of the vascular basement membrane and remodeling of the ECM in order to allow endothelial cells to migrate into the surrounding tissue. Angiogenic growth factors such as aFGF, bFGF, TGF-α, TGF-β, TNF-α, VEGF, and angiogenin are secreted by endothelial cells and supporting cells to accelerate the process of angiogenesis. These factors act as autocrine or paracrine growth factors to induce angiogenesis. Angiogenesis plays a role in tissue revascularization and in the pathogenesis and progression of endometriosis and cancer [28].
MMPs participate in the remodeling of basement membranes and degradation of components of the ECM. MMPs can also enhance angiogenesis by helping to detach pericytes from the vessels undergoing angiogenesis, by releasing ECM-bound angiogenic growth factors, by exposing cryptic proangiogenic integrin binding sites in the ECM, by generating promigratory ECM component fragments, and by cleaving endothelial cell-cell adhesions. MT1-MMP plays an important role in angiogenesis [29]. Augmentation in MMP activity is positively linked to an increase in the metastatic and angiogenic potential of tumors. Also, up-regulation of MMP-2, -7, -9, and stromelysin-3 mRNA has been reported in tumor invasion and metastasis [30].
MMPs may also contribute negatively to angiogenesis through the generation of endogenous angiogenesis inhibitors by proteolytic cleavage of certain collagen chains and plasminogen and by modulating cell receptor signaling by cleaving off their ligand-binding domains [31]. MMP-12, along with MMP-7 and MMP-9 may block angiogenesis by converting plasminogen to angiostatin, a potent angiogenesis antagonist. Also, dormant tumors may secrete inhibitory factors such as angiostatin, thrombospondins, and TIMPs that prevent the tumor from switching to the angiogenic phenotype and arrest the growth of tumors.
While angiogenic factors can induce MMP expression in endothelial and stromal cells, MMPs can enhance the availability/bioactivity of angiogenic factors. Degradation of ECM releases ECM/basement membrane-sequestered angiogenic factors, VEGF, bFGF, and TGF-β. MMP-1 and MMP-3 degrade perlecan in endothelial cell basement membranes to release bFGF. Connective tissue growth factor (CTGF) forms an inactive complex with VEGF165 and cleavage of CTGF by MMP-1, -3, -7, or -13 releases active VEGF165. MMP-2, -3, and -7 degrade the ECM proteoglycan decorin releasing latent TGF-1, while MMP-2 and -9 cleave the latency-associated peptide to activate TGF-β1. Up-regulation of MMP-9 expression in angiogenic islets results in release of VEGF from the ECM that is then responsible for induction of the angiogenic phenotype. Similarly, forced overexpression of MMP-9 in human breast cancer MCF-7 cells results in increased tumor angiogenesis, tumor growth, and VEGF/VEGFR-2 complex formation, suggesting that MMP-9 controls release of VEGF from the ECM [31].
MMPs and tissue remodeling during pregnancy
Embryo implantation and trophoblast invasion are tightly regulated processes, involving sophisticated communication between maternal decidual and fetal trophoblast cells. Decidualization is a prerequisite for successful implantation and is promoted by many factors including MMPs. Studies have shown endometrial production of proMMP-2, -3, -7, -9, and active MMP-2 [32]. Also, MMP-26 mRNA is expressed in the mouse uterus during the estrous cycle and early pregnancy and may play a role in the cycling changes in the uterus during the estrous cycle and in embryo implantation [33].
MMPs may also be involved in placental remodeling throughout pregnancy. In first trimester human placental tissue, MMP-2 expression/activity was observed in extravillous trophoblasts, and MMP-9 mainly in villous cytotrophoblasts. In full-term placental tissue, the MMP-2 expression was observed in the extravillous trophoblasts similar to that in first trimester, whereas the gelatinase activity was decreased or completely lost. The gelatinase activity was marked in early, but not term cytotrophoblasts. Invasive ability of early cytotrophoblasts was inhibited by TIMP-2 and anti-MMP-2 antibody. These data suggest that the invasive ability of trophoblasts may be regulated by gelatinases, especially MMP-2 [34]. This is supported by reports of polarized release of MMP-2 and -9 from cultured human placental syncytiotrophoblasts [35]. The studies also emphasize the importance of temporal regulation of MMPs to perform specific functions during the gestation period. Further studies are needed to identify the molecular signals involved in the regulation of MMPs expression and activity.
Increases in the plasma levels of MMPs have also been detected during normal pregnancy, suggesting a role for MMPs in the pregnancy-associated changes in vascular function [36]. In the pregnant bitches, at days 5–13 and 15–21 after mating, the activity of MMP-2 and -9 is significantly higher than in non-pregnant animals. Also, in the pregnant bitches, serum MMP activity is significantly correlated with the serum levels of estrogen [37].
MMPs could play a role in the uterine artery remodeling during pregnancy. In the uterine artery of late pregnant rats (day 21) the mRNA expression of MMP-2, -3, -7, -9, -12, -13, MT1-MMP, TIMP-1, and TIMP-2 is increased. MMP-2, MT1MMP, MMP-3 and TIMP-1 transcripts are also elevated at day 7. TIMP-1 and MMP-3 mRNA expression returned to virgin control values in the post-partum, whereas MMP-9 and -13 remained elevated or increased further. Gelatin zymography showed maximum elevation of MMP-2 at day 21. We should note that this study presented the MMP levels as geometric mean of gene expression in arbitrary units, and therefore the increases in MMPs during pregnancy were relative to the specific MMP baseline levels in the nonpregnant females, which varied dramatically for the different MMPs. Nevertheless, the data suggest that MMPs and TIMPs may play a role in remodeling of the uterine arteries in pregnant rats and may represent a mechanism to maintain uterine blood flow in late pregnancy. Continued elevated levels of some MMPs post-partum may contribute to vessel regression and return to a non-pregnant physiological state [38].
Increases in MMP-2 mRNA and protein expression of pro- and active forms of MMP-2 have been observed in small renal and mesenteric arteries from pregnant compared with virgin rats [39]. It has been suggested that vascular gelatinase may function upstream of the endothelial endothelin B (ETB) receptor and the NO signaling pathway in the renal vasodilatory response during pregnancy [40].
MMPs in Vascular Disease
In normal physiological vascular remodeling, the activities of MMPs are tightly regulated at the transcription level, activation of their pro-form or zymogens, the interaction with specific ECM components, and the inhibition by endogenous inhibitors. Factors that promote vessel remodeling upregulate MMP activities and include chronic changes in hemodynamics, vessel injury, inflammatory cytokines, and reactive oxygen species.
Increased MMP activity has been reported in various inflammatory, malignant and degenerative disorders. Also, loss of control of MMP activity could result in pathological vascular remodeling and vascular disease [1]. MMPs play a role in VSM cell migration and neointima formation after localized vascular injury. In atherosclerotic lesions, active MMPs may contribute to plaque destabilization by degrading ECM components. MMPs may also promote aneurysm formation by proteolytic degradation of the elastic lamina. Furthermore, MMPs may be involved in venous diseases such as varicose veins and in systemic vascular diseases such as hypertension and preeclampsia.
MMPs in Atherosclerosis and Coronary Restenosis
Inappropriate vascular remodeling, including its absence, underlies the pathogenesis of atherosclerosis and restenosis. Enhanced MMP expression has been detected in the atherosclerotic plaque, and activation of MMPs appears to facilitate atherosclerosis, plaque destabilization, and platelet aggregation. Specifically, a role of MMP-2 in atherosclerosis, plaque instability and rupture has been demonstrated during arterial lesion progression [41,42].
Dysregulated ECM metabolism may contribute to vascular remodeling during the development and complication of human atherosclerotic lesions. In a study examining the expression of MMPs in human atherosclerotic plaques and in uninvolved arterial specimens, the normal arteries stained uniformly for MMP-2 and TIMPs. In contrast, plaques’ shoulders and regions of foam cell accumulation displayed locally increased expression of MMP-1, stromelysin, and MMP-9. All plaque extracts contained activated forms of gelatinases determined zymographically and by degradation of 3H-collagen type IV. In situ zymography revealed gelatinolytic and caseinolytic activity in frozen sections of atherosclerotic, but not of uninvolved arterial tissues. It was suggested that focal overexpression of activated MMP may promote destabilization and complication of atherosclerotic plaques and provide novel targets for therapeutic intervention [43].
Areas of the atherosclerotic plaque rupture exhibit a paucity of VSM cells and an accumulation of macrophage-derived foam cells. On the basis of the collective ability of MMPs to degrade ECM proteins and the detection of increased MMP protein and activity in vulnerable plaques, it has been proposed that these enzymes reduce the strength of the fibrous cap and contribute to plaque rupture. Conversely, the ability of some MMPs to promote migration and proliferation of VSM cells suggests that they promote atherosclerotic plaque cap growth and stability. Investigating the effects of MMP inhibitors should help to resolve this controversy. Using the mouse brachiocephalic artery model of plaque instability, a recent study compared apolipoprotein E (apoE)/MMP-3, apoE/MMP-7, apoE/MMP-9, and apoE/MMP-12 double knockouts with their age-, strain-, and sex-matched apoE single knockout controls. Brachiocephalic artery plaques were larger in apoE/MMP-3 and apoE/MMP-9 double knockouts than in controls. The number of buried fibrous layers was also higher in the double knockouts, and both knockouts exhibited cellular compositional changes indicative of an unstable plaque phenotype. Conversely, lesion size and buried fibrous layers were reduced in apoE/MMP-12 double knockouts compared with controls, and double knockouts had increased VSM cell and reduced macrophage content in the plaque, indicative of a stable plaque phenotype. ApoE/MMP-7 double knockout plaques contained more VSM cells than controls, but neither lesion size nor features of stability were altered in these animals. Hence, MMP-3 and -9 appear to play protective roles, limiting plaque growth and promoting a stable plaque phenotype. MMP-12 supports lesion expansion and destabilization. MMP-7 has no effect on plaque growth or stability, although it is associated with reduced VSM cell content in plaques. These data demonstrate that MMPs are directly involved in atherosclerotic plaque destabilization and clearly show that members of the MMP family have widely differing effects on atherogenesis [44].
Some clinical studies support a role of MMPs in the development of acute coronary syndromes [45]. Circulating MMP levels are elevated in patients with acute myocardial infarction and unstable angina. Increased MMP expression is also observed after coronary angioplasty, suggesting that MMP expression may be involved in the formation of restenotic lesions. The MMP system may therefore represent a potential therapeutic target for the prevention of atherosclerotic lesion development, plaque rupture, and restenosis [46].
Abdominal Aortic Aneurysm (AAA)
AAA is a focal dilation of the aorta that is commonly observed in elderly individuals. AAA is a progressive degenerative disease that, if left uncorrected, could result in aortic rupture and death. Although large AAA can be corrected by surgical repair, no effective treatment is currently available to prevent the progressive aneurysm degeneration and increase in vessel diameter in patients with small AAA. Understanding the mechanisms of AAA formation would help identify new treatment strategies to retard the progressive growth of small AAA.
Increased MMP production/activity may play a role in the pathogenesis of AAA [47,48]. The plasma level and aortic wall expression of MMPs is increased in AAA, which especially in patients with imminent aneurysm rupture [49–53]. Specifically, MMP-2 and MMP-9 appear to play an important role in AAA formation [53–55]. In a study examining infrarenal aortic tissues obtained at surgical repair of AAA, aortic obstructive disease (AOD), or organ procurement for transplantation (control), intergroup statistical analysis of MMP-2 mRNA revealed that all 3 groups were statistically different, with the highest mRNA levels in AAA (control, 2.7±0.8; AOD, 4.0±0.4; and AAA, 27.3±6.3 pg MMP-2/μg RNA). In contrast to MMP-2, MMP-9 transcripts did not differ between AOD (6.6±4.6) and AAA (5.9±2.3 pg MMP-9/μg RNA), but both were higher than that in the control aortas (0.04±0.01) [56]. Also, studies have shown that the median concentration of MMP-2 in AAA wall is 0.04 μg/ml [57], and that of MMP-9 is 0.5 μg/g tissue weight [58]. Also, the levels of MMP-2 and MMP-9 isolated from fibroblasts and VSM cells cultured from AAA wall are between 0.01 to 1.2 μg/ml [54]. Furthermore, studies in patients with AAA have shown elevated plasma levels of MMP-2 and MMP-9 in the range of 0.06 to 0.6 μg/ml [50,51]. Targeted gene disruption of MMP-9 in mice suppresses the development of experimental AAA [59]. Also, both MMP-2 and -9 are necessary to induce experimental AAA formation in mice [60]. MMP-2 has the greatest elastolytic activity and is produced mainly by VSM and fibroblasts [61]. MMP-9 is the most abundantly expressed MMP in AAA tissue and is produced mainly by the aneurysm-infiltrating macrophages [53]. The mechanism of action of MMPs in aneurysm formation has largely been attributed to their proteolytic effects on the ECM proteins and subsequent weakening of the aortic wall [48]. However, our recent studies suggest additional effects of MMP-2 and -9 on the Ca2+-dependent mechanism of aortic VSM contraction that may have an important role in the early development of aneurysm [24]. The inhibitory effects of the MMPs on aortic VSM contraction were observed at concentrations similar to those observed in plasma and tissue samples of patients with AAA. Also, the observed MMP-induced inhibition of aortic VSM contraction occurred even in the absence of degradation of the connective tissue matrix of the vessel wall. Furthermore, MMP-9 is a more potent inhibitor of aortic contraction than MMP-2; consistent with the observations that MMP-9 is the dominant MMP in AAA wall [53].
The ECM proteins elastin and collagen provide significant structural support of the aorta. Although the contribution of VSM contraction to the overall tensile strength of the aortic wall is less clear, there is evidence to suggest that the aortic VSM contractile function may contribute to the structural integrity of the aortic wall. First, the tunica media comprises a major portion of the aortic wall and is formed mainly of circumferentially arranged alternating lamellae of VSM cells and elastin fibers. An active and tonic contraction of VSM is predicted to limit the tendency of the aorta to dilate in response to pulsatile forces generated with each cardiac cycle. Second, atrophy of the tunica media and depletion/apoptosis of VSM cells are consistent histological findings in AAA [62]. Third, disruption of the structural integrity of the tunica media e.g. in chronic aortic dissection, often leads to late aneurysm formation. Thus, the MMP-induced inhibition of VSM contraction may function synergistically with their degradation of the ECM, and thereby contribute to further weakening of the aortic wall and aneurysm formation.
MMPs in Varicose Veins
Varicose veins is a common vascular disorder of the lower extremity that affects 25% of the adult population. Although risk factors for varicose veins such as aging, female gender, pregnancy, obesity, and family history have been identified [63,64], the molecular mechanisms underlying the pathogenesis and progression of the disease remain unclear. Decreased elastin content has been implicated in the pathogenesis of varicose veins [65,66], but the role of collagen has not been clearly defined. Studies suggest increased [67], decreased [68], or unchanged [69] collagen content in the varicose vein wall. The net collagen content represents a balance between its biosynthesis and its degradation by specific MMPs such collagenases, gelatinases and stromelysins. The plasma and venous tissue levels of MMP-1, -2, -3, -9, and -13 are elevated in varicose veins, suggesting that MMPs may contribute to the pathogenesis of the disease [70–72]. MMPs have been detected in all histologic layers of the venous wall, and increased expression and activity have been demonstrated in thrombophlebitic varicose veins [71]. However, the presence of MMPs in varicose veins could be merely a component of the chronic inflammatory process. Also, increased MMP activity in the advanced thrombophlebitc stages does not address whether MMPs affect venous function at the early stages of varicose vein formation.
We have shown that MMP-2 at physiologically relevant 1 μg/ml concentration causes relaxation of Phe contraction in rat IVC segments by a mechanism involving hyperpolarization and activation of BKCa [23]. Our data raise the interesting possibility that a protracted MMP-2 induced venous relaxation could lead to progressive venous dilatation, chronic venous insufficiency and varicose vein formation. We should note that although large amounts of MMP-2 have been detected in the plasma and venous tissues of patients with varicose veins, other MMPs such as MMP-1, -3, -9, and -13 are also expressed in human varicose veins [70–72]. Expression patterns in intact and damaged skin suggest that MMP-28 might function in tissue hemostasis and wound repair [73]. Also, it is possible that some of the effects of MMP-2 could be due to activation of other endogenous MMPs [1]. Future studies should examine whether the effects of MMPs on the mechanisms of venous relaxation are unique to MMP-2, or are caused by other MMPs. Also, whether the observed effects of MMP on IVC could also be demonstrated in other venous tissues such as the human saphenous and femoral vein should be examined.
MMPs in Hypertension
Hypertension is a multifactorial disorder that involves pathological alterations in the renal, neuronal, hormonal, and vascular control mechanisms of the blood pressure. Hypertension is associated with vascular remodeling characterized by rearrangement of vascular wall components including ECM proteins. Recent studies have examined the role of MMPs and TIMPs in the vascular remodeling associated with hypertension. A clinical study in 44 hypertensive patients and 44 controls demonstrated that the plasma levels and activities of MMP-2, MMP-9, and TIMP-1 are increased in hypertensive patients, which may reflect abnormal ECM metabolism [74]. Other studies have shown different results and demonstrated that the plasma concentrations of active MMP-2 and MMP-9 are depressed in patients with essential hypertension. Also, a 6 months treatment with amlodipine normalized MMP-9 but not MMP-2 plasma concentrations [75]. These studies suggested a role of abnormal ECM metabolism in hypertension, and raised the interesting possibility that antihypertensive treatment may modulate collagen metabolism. In addition, it demonstrates that MMPs from the same family can have significant and different effects in vascular function and disease processes.
A recent study has examined the serum concentrations of carboxy-terminal telopeptide of collagen type I (CITP) as a marker of extracellular collagen type I degradation, total matrix MMP-1 (collagenase), total TIMP-1, and MMP-1/TIMP-1 complex in 37 patients with never-treated essential hypertension and in 23 normotensive control subjects. Serum concentrations of free MMP-1 and free TIMP-1 were calculated by subtracting the values of MMP-1/TIMP-1 complex from the values of total MMP-1 and total TIMP-1, respectively. Measurements were repeated in 26 hypertensive patients after 1 year of treatment with the ACE inhibitor lisinopril. Interestingly, baseline free MMP-1 was decreased and baseline free TIMP-1 was increased in hypertensives compared with normotensives. No significant differences were observed in the baseline values of CITP between the 2 groups. Hypertensive patients with baseline left ventricular hypertrophy exhibited lower free MMP-1 and CITP and higher free TIMP-1 than hypertensive patients without baseline left ventricular hypertrophy. Treated patients showed an increase in free MMP-1 and a decrease in free TIMP-1. In addition, serum CITP was increased in treated hypertensives compared with normotensive subjects. It was concluded that systemic extracellular degradation of collagen type I is depressed in patients with essential hypertension and can be normalized by treatment with lisinopril. A depressed degradation of collagen type I may facilitate organ fibrosis in hypertensive patients, namely, in those with left ventricular hypertrophy [76].
Studies have also examined the expression/activity of MMPs in internal mammary artery specimens obtained from normotensive and hypertensive patients undergoing coronary artery bypass surgery. Zymographic analysis indicated a decrease in total gelatinolytic activity of MMP-2 and -9 in hypertension. MMP-1 activity was also decreased by fourfold without a significant change in protein levels. Tissue levels of ECM inducer protein (EMMPRIN, a known stimulator of MMPs transcription), MMP activator protein (MT1-MMP), MMP-1, -2, and -9, as well as TIMP-1 and -2 were assessed by immunoblotting and revealed a significant decrease in MMP-9, EMMPRIN, and MT1-MMP levels in hypertension. In addition, measurement of plasma markers of collagen synthesis (procollagen type I amino-terminal propeptide [PINP]) and collagen degradation (carboxy-terminal telopeptide of collagen type I [ICTP]) indicated no difference in PINP levels but suppressed degradation of collagen in hypertension. These data demonstrate that not only MMP-1 and MMP-9, but MMP inducer and activator proteins are downregulated in the hypertensive state, which may result in enhanced collagen deposition in hypertension [77].
Experimental studies have also examined whether hypertension is associated with vascular remodeling and changes in the vascular tissue expression/activity of MMPs. It was found that the wall thickness was increased in the aorta of DOCA-salt vs. sham rats, as was medial area, but neither measure was altered in vena cava. In hypertension, MMP-2 expression and activity was increased in aorta but not vena cava, while MMP-9 was weakly expressed in both vessels. TIMP-2 expression was increased in aorta of DOCA rats compared to sham, but barely detectable in vena cava of sham or DOCA-salt hypertensive rats. These data suggest that vascular remodeling in the aorta of DOCA-salt hypertensive rats, observed as an increase in wall thickness and medial area, is linked to the action of specific MMPs such as MMP-2. The increase in TIMP-2 expression observed in the aorta from DOCA-salt rats is presumably an adaptive increase to the higher-than-normal levels of MMP-2 [78].
A recent study has further examined the effects of MMP-9 during the progression of hypertension. Wild-type and MMP-9(−/−) mice were treated with AngII, 1 μg/kg per min by minipump, plus a 5% NaCl diet for 10 days. It was found that the onset of AngII-induced hypertension was accompanied by increased MMP-9 activity in conductance vessels. The absence of MMP-9 activity results in vessel stiffness and increased pulse pressure. It was suggested that MMP-9 activation was associated with a beneficial role early on in hypertension by preserving vessel compliance and alleviating BP increase [79].
MMPs in Preeclampsia
Preeclampsia is a major complication of pregnancy with severe increases in BP, and MMP-mediated vascular remodeling may play a role in the pathogenesis of the disease [36]. MMP-2 is elevated in the plasma of women with preeclampsia [80] as well as in women who subsequently develop preeclampsia [81]. Changes in circulating MMP-9 and TIMP-1 and -2 levels have also been observed in gestational hypertension [82]. It has been speculated that increased net MMP-2 activity may contribute to the endothelial dysfunction that is central to the pathophysiology of preeclampsia [81]. However, recent studies tested the hypothesis that proteases intrinsic to syncytiotrophoblast microvillous membranes (STBM) are the cause of the in vitro endothelial changes. Purified STBM detected gelatinase activity and showed that it was due to MMP-9. Its presence was confirmed in this location by immunohistocytochemistry. Protease inhibitors caused a small reversal of the effects of STBM on morphology and no effect on inhibition of proliferation. It was concluded that the effect of STBM on endothelial cells is unlikely to be caused by intrinsic proteases [83].
The source of the increased plasma MMPs is unclear, but studies have suggested the placenta as a potential source. MMP-2 and -9 are increased in the placenta of diabetic rats at midgestation [84]. Also, low amount of MMP-1, -9 and -3 was detected in extracts from the wall of human umbilical cord artery. MMP-2 is the main collagenolytic enzyme in umbilical cord artery (UCA) wall (both latent and active form). Preeclampsia is associated with a distinct reduction in those MMPs content in comparison to control UCA. It was concluded that the decrease of MMP content and activity in the umbilical cord artery may be a factor that reduces the breakdown of collagen in the arterial wall. The accumulation of collagen with simultaneous reduction in elastin content in the UCA may be the factor that reduces the elasticity of arterial wall and decreases the blood flow in the fetus of women with preeclampsia [85].
Studies have examined the secretion of MMP by cultured human decidual endothelial cells from normal and preeclamptic pregnancies. MMP-9 and TIMP1 levels were similar between the two cell types; however, the basal and stimulated secretion of MMP-1 was markedly higher in normal compared with preclamptic endothelial cells. It was suggested that the lower MMP1 expression of decidual endothelial cells from preeclamptic women may inhibit endovascular invasion by cytotrophoblasts. These findings may, at least partly, explain the relative failure of trophoblasts to invade maternal decidual blood vessels in preeclamptic pregnancy [86].
MMP Inhibitors
Several endogenous and synthetic MMP inhibitors have been identified. TIMPs are specific endogenous inhibitors that bind MMPs in a 1:1 stoichiometry. Four TIMPs (TIMP-1, -2, -3, and -4) have been identified in vertebrates, and their expression is regulated during development and tissue remodeling [87]. TIMPs (21 to 29 kDa) have an N-terminal domain (125 aa) and C-terminal domain (65 aa); each containing 3 conserved disulfide bonds [88,89]. The N-terminal domain folds as a separate unit and is capable of inhibiting MMPs [88]. NMR and X-ray crystallography have resolved the structure of TIMP-1, TIMP-2, and the TIMP-1–MMP-3 and TIMP-2–MT1-MMP complex [90]. The TIMP molecule wedges into the active-site cleft of MMP in a manner similar to that of the substrate (Fig. 1). Cys1 is instrumental in chelating the active-site zinc with its N-terminal α-amino group and carbonyl group, thereby expelling the water molecule bound to the catalytic zinc. TIMPs inhibit all MMPs tested so far, except that TIMP-1 does not inhibit MT1-MMP [91]. The inhibitory properties of TIMP-3 are different from the rest, because it inhibits ADAMs (A Disintigrin And Metalloproteinase) [92]. TIMP-4 is localized mostly in vascular tissue.
Plasma α2-macroglobulins are general endopeptidase inhibitors that inhibit most proteinases by trapping them within the macroglobulin after proteolysis of the bait region of the inhibitor [93]. MMP-1 reacts with α2-macroglobulin more readily than with TIMP-1 in solution [94]. α2-macroglobulins have a Zn2+-binding site, and therefore could bind Zn2+ ion or other divalent cations such as Ca2+. Other Zn2+ chelators include tetracyclines such as doxicycline.
Tissue factor pathway inhibitor-2 is a serine protease inhibitor that inhibits MMPs [95]. A C-terminal fragment of the procollagen C-terminal proteinase enhancer protein and the secreted form, membrane-bound β-amyloid precursor protein inhibit MMP-2 [96,97]. RECK, a GPI-anchored glycoprotein that downregulates the levels of MMP-9 and active MMP-2 and suppresses angiogenic sprouting, leading to tumor cell-death, inhibits the proteolytic activity of MMP-2, MMP-9, and MT1-MMP [98]. Chlorotoxin, a scorpion toxin that has anti-invasive effects on glioma cells, inhibits MMP-2, but not MMP-1, MMP-3, or MMP-9 [99]. However, the mechanisms of MMP inhibition by these proteins are not known.
Synthetic broad-spectrum MMP inhibitors include hydroxamic acid derived inhibitors such as BB-94 (Batimastat), BB-1101, BB-2293, BB-2516 (marimastat), and CT1746 [100]. Batimastat and marimastat, are competitive MMP-inhibitors and Zn2+-chelating mimickers of collagen. More selective MMP inhibitors include the pyrimidine-2,4,6-trione derivative Ro-28-2653, which selectively inhibits MMP-2, MMP-9, and MT1-MMP, Ro-28-3555 (trocade), selective inhibitor of MMP-1 [101], and IW449, selective inhibitor of MMP-2 [100]. Other synthetic inhibitors include PD166793, a broad-spectrum MMP inhibitor, OPB-3206 (3S-[4-(N-hydroxyamino)-2R-isobutylsuccinyl] amino-1methoxy-3, 4-dihydrocarbostyril) [102], BAY12-9566, AG-3340, KBR-7785, KBR-8301, CDP-845 (CT-1746), metastat and AE-941 (Neovastat) [103].
Clinical Applications of MMP Inhibitors
A number of inhibitors of MMPs with potent anti-angiogenic activity are already in early stages of clinical trials, primarily to treat cancer and cancer-associated angiogenesis. However, because of the multiple effects of MMPs on angiogenesis, careful testing of these MMP inhibitors is necessary to show that they do not adversely enhance angiogenesis [104]. Initial clinical trials using MMP inhibitors as cancer treatments did not demonstrate efficacy in terms of reducing tumor progression partially due to the fact that most trials were done in patients with advanced stage disease, when the tumor vasculature is already well-established, and to the fact that MMPs play multiple roles in both angiogenesis and tumor progression. Also, broad spectrum MMP inhibitors can inhibit the ADAMTSs, which have anti-angiogenic activity. More specific MMP inhibitors are now being designed and tested. In addition, monoclonal antibodies to MT1-MMP, which inhibit its enzymatic activity and so activation of proMMP-2, have been shown to inhibit endothelial cell migration and invasion of collagen and fibrin gels. Another approach to inhibiting proMMP-2 activation is the use of recombinant hemopexin-like (PEX) domain. Lentiviral delivery of PEX to endothelial cells inhibited their invasion and tube formation in Matrigel in vitro and delivery of PEX to the chick chorioallantoic membrane inhibited bFGF-induced and tumor-induced angiogenesis in vivo [31].
Experimental studies have suggested significant effects of doxycycline in reducing the progression of AAA. There is also widespread interest in developing MMP inhibitors for the prevention of atherosclerotic plaque rupture. The MMP inhibitors, EDTA and 1,10-phenanthroline, as well as recombinant TIMP-1, reduced the activities of MMP-1, -2, -9 and -3 which colocalized with regions of increased immunoreactive MMP expression, i.e., the shoulders, core, and microvasculature of the plaques [43]. However, broad-spectrum MMP inhibition by RS-130830 may not have a beneficial effect on atherosclerosis in the apoE knockout mouse, indicating that more selective compounds would be preferable [105].
Because the results of clinical trials with small molecule inhibitors have been disappointing, there is a clear potential for the application of TIMPs as endogenous inhibitors [106]. Endogenous TIMPs have antiangiogenic activities. TIMP-2 inhibits bFGFinduced endothelial cell proliferation, TIMP-3 inhibits cell migration and proliferation of stimulated endothelial cells, and TIMP-4 inhibits endothelial cell tube formation in the basement membrane extract, Matrigel. Application of TIMPs as a therapeutic tool for vascular disease through gene therapy or direct protein application is in early phase of development [107]. Previous studies have demonstrated that gene transfer to overexpress TIMPs can reduce MMP activity and reduce intimal thickening in various models. For example, TIMP-1 gene transfer delivered by an adeno-associated virus (AAV) vector inhibits tumor growth and angiogenesis in a murine xenotransplant model [108]. Also, adenoviral overexpression of TIMP-1 in a mouse model of atherosclerosis showed a reduction in the lesion [109]. A recent study examined whether overexpression of TIMP-1 or -2 would attenuate atherosclerotic plaque development and instability in apoE(−/−) mice fed a high-fat diet for 7 weeks and injected intravenously with first-generation adenoviruses expressing the gene for human TIMP-1 (RAdTIMP-1) or TIMP-2 (RAdTIMP-2) or a control adenovirus (RAd66) and fed a high-fat diet for additional 4 weeks. Analysis of brachiocephalic artery plaques revealed that RAdTIMP-2, but not RAdTIMP-1 infection resulted in marked reduction in lesion area compared with that in control animals. Markers associated with plaque instability, assessed by VSM cell and macrophage content and the presence of buried fibrous caps, were significantly reduced by RAdTIMP-2. Effects on lesion size were not sustained with first-generation adenoviruses, but murine TIMP-2 overexpression mediated by Helper-dependent adenoviral vectors exerted significant effects on plaques assessed 11 weeks after infection. In an attempt to determine the mechanism of action, macrophages and macrophage-derived foam cells were treated with exogenous TIMP-2 in vitro. TIMP-2 significantly inhibited migration and apoptosis of macrophages and foam cells, whereas TIMP-1 failed to exert similar effects. It was concluded that overexpression of TIMP-2 but not TIMP-1 inhibits atherosclerotic plaque development and destabilisation, possibly through modulation of macrophage and foam cell behavior, and that Helper-dependent adenovirus technology is required for these effects to be maintained [110].
MMPs are implicated in neointima formation and hence vein graft failure, and gene transfer to elevate local levels of TIMPs is therefore a potential treatment. Gene therapy could improve human saphenous vein coronary vein-graft patency by reducing early thrombosis, neointimal hyperplasia and atherosclerosis. TIMPs reduce intimal migration of VSM cells, while TIMP-3 and the p53 tumor suppressor protein promote apoptosis [111]. Also, interventional studies have used lumenal application of a replication-defective recombinant adenovirus to overexpress TIMP-2 and observed the effects on neointimal thickening in a human saphenous vein organ culture model. Increased TIMP-2 expression was localiszd to lumenal surface cells, but increased total functional TIMP-2 secretion after 14 days culture. In situ zymography revealed a marked inhibition of gelatinolytic activity by TIMP-2 gene transfer throughout the vein segments. Neointima formation and neointimal cell numbers were reduced 79% and 71%, respectively. TIMP-2 overexpression had no effect on VSM cell proliferation, secretion of pro-MMP-2 or -9 and did not inhibit the processing of pro-MMP-2 to its active form. These data indicate that TIMP-2 overexpression reduces neointimal thickening, primarily by inhibiting MMP activity and hence VSM cell migration [112].
Local expression of TIMP-1 in a rat model of aneurysm prevented aneurysm progression and rupture [113]. However, expressing wild-type TIMPs could have drawbacks because multiple MMPs may be inhibited, and in the case of TIMP-3, ADAMs and ADAMTSs may be inhibited as well. Probably the best route to success will be the development of engineered TIMPs with altered specificity, to allow targeting of specific MMPs.
Perspective
MMPs play a significant role in vascular remodeling. Significant progress has been made in identifying the changes in the levels and activity of MMPs in several physiological and pathological conditions. However, more research is needed to define the effects of MMPs not only on ECM degradation, but also on other cell types that control vascular function, particularly the endothelium and VSM. Also, the effects of MMPs in vascular remodeling and vascular function in large as compared to small vessels need to be further examined. Additionally, more research is needed to identify the specific MMPs involved. However, the mere presence of MMP does not establish their catalytic capacity, as the zymogens lack activity, and TIMPs may block activated MMPs. Therefore, advanced techniques to measure the catalytic MMP activity, particularly in vivo are needed. The development of specific inhibitors of MMPs is an area of research that needs to be further investigated. Inhibiting the activity of specific MMPs or preventing their upregulation could ameliorate intimal thickening and reduce coronary restenosis and atherosclerosis. Targeting specific MMPs in aneurysm tissue may not reverse the aortic wall dilation, but could prevent progression of wall dilation, and in the instance of coronary artery disease plaque dislodgement and myocardial infarction. Future investigations should examine the role of MMPs and their inhibitors in the pathogenesis and treatment of systemic vascular diseases such hypertension and preeclamosia and localized venous diseases such as varicose veins. Investigations of the role of MMPs in vascular remodeling and vascular disease should also consider the effects of other proteinases and enzymes such as ADAMs and transglutaminase [114].
Acknowledgments
This work was supported by grants from the National Heart, Lung and Blood Institute (HL-65998, HL-70659).
List of Abbreviations
- AAA
abdominal aortic aneurysm
- ECM
extracellular matrix
- IVC
inferior vena cava
- MMP
matrix metalloproteinase
- NO
nitric oxide
- NOS
nitric oxide synthase
- PARs
protease-activated receptors
- PGI2
prostacyclin
- Phe
phenylephrine
- TIMP
tissue Inhibitor of metalloproteinase
- VSM
vascular smooth muscle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension. 1993;21:391–397. doi: 10.1161/01.hyp.21.4.391. [DOI] [PubMed] [Google Scholar]
- 2.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension. 1996;28:505–506. [PubMed] [Google Scholar]
- 3.Mulvany MJ. Small artery remodeling and significance in the development of hypertension. News Physiol Sci. 2002;17:105–109. doi: 10.1152/nips.01366.2001. [DOI] [PubMed] [Google Scholar]
- 4.De Mey JG, Schiffers PM, Hilgers RH, Sanders MM. Toward functional genomics of flow-induced outward remodeling of resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288(3):H1022–H1027. doi: 10.1152/ajpheart.00800.2004. [DOI] [PubMed] [Google Scholar]
- 5.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 6.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90(3):251–262. [PubMed] [Google Scholar]
- 7.Liu YE, Wang M, Greene J, Su J, Ullrich S, Li H, Sheng S, Alexander P, Sang QA, Shi YE. Preparation and characterization of recombinant tissue inhibitor of metalloproteinase 4 (TIMP-4) J Biol Chem. 1997;272(33):20479–20483. doi: 10.1074/jbc.272.33.20479. [DOI] [PubMed] [Google Scholar]
- 8.Dumont O, Loufrani L, Henrion D. Key role of the NO-pathway and matrix metalloprotease-9 in high blood flow-induced remodeling of rat resistance arteries. Arterioscler Thromb Vasc Biol. 2007;27(2):317–24. doi: 10.1161/01.ATV.0000254684.80662.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol. 2004;24(1):54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- 10.Allan JA, Docherty AJ, Barker PJ, Huskisson NS, Reynolds JJ, Murphy G. Binding of gelatinases A and B to type-I collagen and other matrix components. Biochem J. 1995;309:299–306. doi: 10.1042/bj3090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchenko GN, Strongin AY. MMP-28, a new human matrix metalloproteinase with an unusual cysteine-switch sequence is widely expressed in tumors. Gene. 2001;265:87–93. doi: 10.1016/s0378-1119(01)00360-2. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem. 1993;268:23824–23829. [PubMed] [Google Scholar]
- 13.Péndas AM, Knäuper V, Puente XS, Llano E, Mattei MG, Apte S, Murphy G, Lopéz-Otín C. Identification and characterization of a novel human matrix metalloproteinase with unique structural characteristics, chromosomal location, and tissue distribution. J Biol Chem. 1997;272:4281–4286. doi: 10.1074/jbc.272.7.4281. [DOI] [PubMed] [Google Scholar]
- 14.Kolb C, Mauch S, Peter HH, Krawinkel U, Sedlacek R. The matrix metalloproteinase RASI-1 is expressed in synovial blood vessels of a rheumatoid arthritis patient. Immunol Lett. 1997;57:83–88. doi: 10.1016/s0165-2478(97)00057-6. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki K, Enghild JJ, Morodomi T, Salvesen G, Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin) Biochemistry. 1990;29:10261–10270. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- 16.Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, van Westrum SS, Crabbe T, Clements J, d’Ortho MP, Murphy G. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A: a kinetic study. J Biol Chem. 1998;273:871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, Aoki T, Seiki M. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001;20:4782–4793. doi: 10.1093/emboj/20.17.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen LC, Noelken ME, Nagase H. Disruption of the cysteine-75 and zinc ion coordination is not sufficient to activate the precursor of human matrix metalloproteinase 3 (stromelysin 1) Biochemistry. 1993;32:10289–10295. doi: 10.1021/bi00090a003. [DOI] [PubMed] [Google Scholar]
- 20.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-Nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 21.Patterson ML, Atkinson SJ, Knäuper V, Murphy G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001;503:158–162. doi: 10.1016/s0014-5793(01)02723-5. [DOI] [PubMed] [Google Scholar]
- 22.Ammarguellat FZ, Gannon PO, Amiri F, Schiffrin EL. Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA-salt hypertensive rats: Role of ETA Receptors. Hypertension. 2002;39(part 2):679–684. doi: 10.1161/hy0202.103481. [DOI] [PubMed] [Google Scholar]
- 23.Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase-2 induced venous dilation via hyperpolarization and activation of K+ channels: Relevance to varicose vein formation. J Vasc Surg. 2007;45:373–80. doi: 10.1016/j.jvs.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chew DKW, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg. 2004;40:1001–10. doi: 10.1016/j.jvs.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Waitkus-Edwards KR, Martinez-Lemus LA, Wu X, Trzeciakowski JP, Davis MJ, Davis GE, Meininger GA. α4β1 Integrin activation of L-type calcium channels in vascular smooth muscle causes arteriole vasoconstriction. Circ Res. 2002;90(4):473–480. doi: 10.1161/hh0402.105899. [DOI] [PubMed] [Google Scholar]
- 26.Macfarlane S, Seatter M, Kanke T, Hunter G, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 27.Hamilton JR, Nguyen PB, Cocks TM. Atypical protease-activated receptor mediates endothelium-dependent relaxation of human coronary arteries. Circ Res. 1998;82:1306–11. doi: 10.1161/01.res.82.12.1306. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 29.Pepper MS. Extracellular proteolysis and angiogenesis. Thromb Haemost. 2001;86:346–355. [PubMed] [Google Scholar]
- 30.Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer. 2005;115(6):849–60. doi: 10.1002/ijc.20945. [DOI] [PubMed] [Google Scholar]
- 31.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–85. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones RL, Findlay JK, Farnworth PG, Robertson DM, Wallace E, Salamonsen LA. Activin A and inhibin A differentially regulate human uterine matrix metalloproteinases: potential interactions during decidualization and trophoblast invasion. Endocrinology. 2006;147(2):724–32. doi: 10.1210/en.2005-1183. [DOI] [PubMed] [Google Scholar]
- 33.Liu G, Zhang X, Lin H, Li Q, Wang H, Ni J, Amy Sang QX, Zhu C. Expression of matrix metalloproteinase-26 (MMP-26) mRNA in mouse uterus during the estrous cycle and early pregnancy. Life Sci. 2005;77(26):3355–65. doi: 10.1016/j.lfs.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 34.Isaka K, Usuda S, Ito H, Sagawa Y, Nakamura H, Nishi H, Suzuki Y, Li YF, Takayama M. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta. 2003;24(1):53–64. doi: 10.1053/plac.2002.0867. [DOI] [PubMed] [Google Scholar]
- 35.Sawicki G, Radomski MW, Winkler-Lowen B, Krzymien A, Guilbert LJ. Polarized release of matrix metalloproteinase-2 and -9 from cultured human placental syncytiotrophoblasts. Biol Reprod. 2000;63(5):1390–5. doi: 10.1095/biolreprod63.5.1390. [DOI] [PubMed] [Google Scholar]
- 36.Merchant SJ, Davidge ST. The role of matrix metalloproteinases in vascular function: implications for normal pregnancy and pre-eclampsia. BJOG. 2004;111(9):931–9. doi: 10.1111/j.1471-0528.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 37.Schafer-Somi S, Ali Aksoy O, Patzl M, Findik M, Erunal-Maral N, Beceriklisoy HB, Polat B, Aslan S. The activity of matrix metalloproteinase-2 and -9 in serum of pregnant and non-pregnant bitches. Reprod Domest Anim. 2005;40(1):46–50. doi: 10.1111/j.1439-0531.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 38.Kelly BA, Bond BC, Poston L. Gestational profile of matrix metalloproteinases in rat uterine artery. Mol Hum Reprod. 2003;9(6):351–8. doi: 10.1093/molehr/gag043. [DOI] [PubMed] [Google Scholar]
- 39.Jeyabalan A, Kerchner LJ, Fisher MC, McGuane JT, Doty KD, Conrad KP. Matrix metalloproteinase-2 activity, protein, mRNA, and tissue inhibitors in small arteries from pregnant and relaxin-treated nonpregnant rats. J Appl Physiol. 2006;100(6):1955–63. doi: 10.1152/japplphysiol.01330.2005. [DOI] [PubMed] [Google Scholar]
- 40.Jeyabalan A, Novak J, Danielson LA, Kerchner LJ, Opett SL, Conrad KP. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ Res. 2003;93(12):1249–57. doi: 10.1161/01.RES.0000104086.43830.6C. [DOI] [PubMed] [Google Scholar]
- 41.Beaudeux JL, Giral P, Brucker E, Foglietti MJ, Chapman MJ. Matrix metalloproteinases, inflammation and atherosclerosis: therapeutic perspectives. Clin Chem Lab Med. 2004;42:121–31. doi: 10.1515/CCLM.2004.024. [DOI] [PubMed] [Google Scholar]
- 42.Kadoglou NP, Daskalopoulou SS, Perrea D, Liapis CD. Matrix metalloproteinases and diabetic vascular complications. Angiology. 2005;56:173–89. doi: 10.1177/000331970505600208. [DOI] [PubMed] [Google Scholar]
- 43.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94(6):2493–503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A. 2005;102(43):15575–80. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003;59(4):812–23. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda U, Shimada K. Matrix metalloproteinases and coronary artery diseases. Clin Cardiol. 2003;26(2):55–9. doi: 10.1002/clc.4960260203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson RW, Parks WC. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:157–174. doi: 10.1111/j.1749-6632.1996.tb33307.x. [DOI] [PubMed] [Google Scholar]
- 48.Boyle JR, McDermott E, Crowther M, Wills AD, Bell PR, Thompson MM. Doxycycline inhibits elastin degradation and reduces metalloproteinase activity in a model of aneurysmal disease. J Vasc Surg. 1998;27(2):354–361. doi: 10.1016/s0741-5214(98)70367-2. [DOI] [PubMed] [Google Scholar]
- 49.Newman KM, Malon AM, Shin RD, Scholes JV, Ramey WG, Tilson MD. Matrix metalloproteinases in abdominal aortic aneurysm: characterization, purification, and their possible sources. Connect Tissue Res. 1994;30(4):265–276. doi: 10.3109/03008209409015042. [DOI] [PubMed] [Google Scholar]
- 50.Hovsepian DM, Ziporin SJ, Sakurai MK, Lee JK, Curci JA, Thompson RW. Elevated plasma levels of matrix metalloproteinase-9 in patients with abdominal aortic aneurysms: a circulating marker of degenerative aneurysm disease. J Vasc Interv Radiol. 2000;11(10):1345–1352. doi: 10.1016/s1051-0443(07)61315-3. [DOI] [PubMed] [Google Scholar]
- 51.Lindholt JS, Vammen S, Fasting H, Henneberg EW, Heickendorff L. The plasma level of matrix metalloproteinase 9 may predict the natural history of small abdominal aortic aneurysms. A preliminary study. Eur J Vasc Endovasc Surg. 2000;20(3):281–285. doi: 10.1053/ejvs.2000.1151. [DOI] [PubMed] [Google Scholar]
- 52.Sangiorgi G, D’Averio R, Mauriello A, Bondio M, Pontillo M, Castelvecchio S, Trimarchi S, Tolva V, Nano G, Rampoldi V, Spagnoli LG, Inglese L. Plasma levels of metalloproteinases-3 and -9 as markers of successful abdominal aortic aneurysm exclusion after endovascular graft treatment. Circulation. 2001;104:I288–I295. doi: 10.1161/hc37t1.094596. [DOI] [PubMed] [Google Scholar]
- 53.Sakalihasan N, Delvenne P, Nusgens BV, Limet R, Lapiere CM. Activated forms of MMP2 and MMP9 in abdominal aortic aneurysms. J Vasc Surg. 1996;24(1):127–133. doi: 10.1016/s0741-5214(96)70153-2. [DOI] [PubMed] [Google Scholar]
- 54.Crowther M, Goodall S, Jones JL, Bell PR, Thompson MM. Localization of matrix metalloproteinase 2 within the aneurysmal and normal aortic wall. Br J Surg. 2000;87(10):1391–1400. doi: 10.1046/j.1365-2168.2000.01554.x. [DOI] [PubMed] [Google Scholar]
- 55.Petersen E, Gineitis A, Wagberg F, Angquist KA. Activity of matrix metalloproteinase-2 and -9 in abdominal aortic aneurysms. Relation to size and rupture. Eur J Vasc Endovasc Surg. 2000;20(5):457–461. doi: 10.1053/ejvs.2000.1211. [DOI] [PubMed] [Google Scholar]
- 56.Davis V, Persidskaia R, Baca-Regen L, Itoh Y, Nagase H, Persidsky Y, Ghorpade A, Baxter BT. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 1998;18(10):1625–33. doi: 10.1161/01.atv.18.10.1625. [DOI] [PubMed] [Google Scholar]
- 57.Goodall S, Crowther M, Hemingway DM, Bell PR, Thompson MM. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation. 2001;104(3):304–309. doi: 10.1161/01.cir.104.3.304. [DOI] [PubMed] [Google Scholar]
- 58.Nagashima H, Aoka Y, Sakomura Y, Sakuta A, Aomi S, Ishizuka N, Hagiwara N, Kawana M, Kasanuki H. A 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, cerivastatin, suppresses production of matrix metalloproteinase-9 in human abdominal aortic aneurysm wall. J Vasc Surg. 2002;36(1):158–163. doi: 10.1067/mva.2002.123680. [DOI] [PubMed] [Google Scholar]
- 59.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105(11):1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110(5):625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wall SJ, Sampson MJ, Levell N, Murphy G. Elevated matrix metalloproteinase-2 and -3 production from human diabetic dermal fibroblasts. Br J Dermatol. 2003;149(1):13–16. doi: 10.1046/j.1365-2133.2003.05262.x. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150:993–1007. [PMC free article] [PubMed] [Google Scholar]
- 63.Carpentier PH, Maricq HR, Biro C, Pancot-Makinen CO, Franco A. Prevalence, risk factors, and clinical patterns of chronic venous disorders of lower limbs: a population-based study in France. J Vasc Surg. 2004;40:650–9. doi: 10.1016/j.jvs.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 64.Ruckley CV, Evans CJ, Allan PL, Lee AJ, Fowkes FG. Chronic venous insufficiency: clinical and duplex correlations. The Edinburgh Vein Study of venous disorders in the general population. J Vasc Surg. 2002;36:520–525. doi: 10.1067/mva.2002.126547. [DOI] [PubMed] [Google Scholar]
- 65.Venturi M, Bonavina L, Annoni F, Colombo L, Butera C, Peracchia A, Mussini E. Biochemical assay of collagen and elastin in the normal and varicose vein wall. J Surg Res. 1996;60(1):245–8. doi: 10.1006/jsre.1996.0038. [DOI] [PubMed] [Google Scholar]
- 66.Rose SS, Ahmed A. Some thoughts on the aetiology of varicose veins. J Cardiovasc Surg (Torino) 1986;27(5):534–43. [PubMed] [Google Scholar]
- 67.Gandhi RH, Irizarry E, Nackman GB, Halpern VJ, Mulcare RJ, Tilson MD. Analysis of the connective tissue matrix and proteolytic activity of primary varicose veins. J Vasc Surg. 1993;18(5):814–20. [PubMed] [Google Scholar]
- 68.Haviarova Z, Weismann P, Stvrtinova V, Benuska J. The determination of the collagen and elastin amount in the human varicose vein by the computer morphometric method. Gen Physiol Biophys. 1999;18(Suppl 1):30–3. [PubMed] [Google Scholar]
- 69.Kockx MM, Knaapen MW, Bortier HE, Cromheeke KM, Boutherin-Falson O, Finet M. Vascular remodeling in varicose veins. Angiology. 1998;49(11):871–7. doi: 10.1177/000331979804901101. [DOI] [PubMed] [Google Scholar]
- 70.Gillespie DL, Patel A, Fileta B, Chang A, Barnes S, Flagg A, Kidwell M, Villavicencio JL, Rich NM. Varicose veins possess greater quantities of MMP-1 than normal veins and demonstrate regional variation in MMP-1 and MMP-13. J Surg Res. 2002;106:233–8. doi: 10.1006/jsre.2002.6455. [DOI] [PubMed] [Google Scholar]
- 71.Kowalewski R, Sobolewski K, Wolanska M, Gacko M. Matrix metalloproteinases in the vein wall. Int Angiol. 2004;23:164–9. [PubMed] [Google Scholar]
- 72.Woosside KJ, Hu M, Burke A, Murakami M, Pounds LL, Killewich LA, Daller JA, Hunter GC. Morphologic characteristics of varicose veins: possible role of metalloproteinases. J Vasc Surg. 2003;38:162–169. doi: 10.1016/s0741-5214(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 73.Lohi J, Wilson CL, Roby JD, Parks WC. Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J Biol Chem. 2001;276:10134–10144. doi: 10.1074/jbc.M001599200. [DOI] [PubMed] [Google Scholar]
- 74.Derosa G, D’Angelo A, Ciccarelli L, Piccinni MN, Pricolo F, Salvadeo S, Montagna L, Gravina A, Ferrari I, Galli S, Paniga S, Tinelli C, Cicero AF. Matrix metalloproteinase-2, -9, and tissue inhibitor of metalloproteinase-1 in patients with hypertension. Endothelium. 2006;13(3):227–31. doi: 10.1080/10623320600780942. [DOI] [PubMed] [Google Scholar]
- 75.Zervoudaki A, Economou E, Stefanadis C, Pitsavos C, Tsioufis K, Aggeli C, Vasiliadou K, Toutouza M, Toutouzas P. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J Hum Hypertens. 2003;17(2):119–24. doi: 10.1038/sj.jhh.1001518. [DOI] [PubMed] [Google Scholar]
- 76.Laviades C, Varo N, Fernandez J, Mayor G, Gil MJ, Monreal I, Diez J. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 1998;98(6):535–40. doi: 10.1161/01.cir.98.6.535. [DOI] [PubMed] [Google Scholar]
- 77.Ergul A, Portik-Dobos V, Hutchinson J, Franco J, Anstadt MP. Downregulation of vascular matrix metalloproteinase inducer and activator proteins in hypertensive patients. Am J Hypertens. 2004;17(9):775–82. doi: 10.1016/j.amjhyper.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 78.Watts SW, Rondelli C, Thakali K, Li X, Uhal B, Pervaiz MH, Watson RE, Fink GD. Morphological and biochemical characterization of remodeling in aorta and vena cava of DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H2438–48. doi: 10.1152/ajpheart.00900.2006. [DOI] [PubMed] [Google Scholar]
- 79.Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC, Lehoux S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50(1):212–8. doi: 10.1161/HYPERTENSIONAHA.107.089631. [DOI] [PubMed] [Google Scholar]
- 80.Narumiya H, Zhang Y, Fernandez-Patron C, Guilbert LJ, Davidge ST. Matrix metalloproteinase-2 is elevated in the plasma of women with preeclampsia. Hypertens Pregnancy. 2001;20(2):185–94. doi: 10.1081/PRG-100106968. [DOI] [PubMed] [Google Scholar]
- 81.Myers JE, Merchant SJ, Macleod M, Mires GJ, Baker PN, Davidge ST. MMP-2 levels are elevated in the plasma of women who subsequently develop preeclampsia. Hypertens Pregnancy. 2005;24(2):103–15. doi: 10.1081/PRG-200059836. [DOI] [PubMed] [Google Scholar]
- 82.Tayebjee MH, Karalis I, Nadar SK, Beevers DG, MacFadyen RJ, Lip GY. Circulating matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases-1 and -2 levels in gestational hypertension. Am J Hypertens. 2005;18(3):325–9. doi: 10.1016/j.amjhyper.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 83.de Jager CA, Linton EA, Spyropoulou I, Sargent IL, Redman CW. Matrix metalloprotease-9, placental syncytiotrophoblast and the endothelial dysfunction of pre-eclampsia. Placenta. 2003;24(1):84–91. doi: 10.1053/plac.2002.0871. [DOI] [PubMed] [Google Scholar]
- 84.Pustovrh MC, Jawerbaum A, Capobianco E, White V, Lopez-Costa JJ, Gonzalez E. Increased matrix metalloproteinases 2 and 9 in placenta of diabetic rats at midgestation. Placenta. 2005;26(4):339–48. doi: 10.1016/j.placenta.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 85.Galewska Z, Bankowski E, Romanowicz L, Jaworski S. Pre-eclampsia (EPH-gestosis)-induced decrease of MMP-s content in the umbilical cord artery. Clin Chim Acta. 2003;335(1–2):109–15. doi: 10.1016/s0009-8981(03)00296-1. [DOI] [PubMed] [Google Scholar]
- 86.Gallery ED, Campbell S, Arkell J, Nguyen M, Jackson CJ. Preeclamptic decidual microvascular endothelial cells express lower levels of matrix metalloproteinase-1 than normals. Microvasc Res. 1999;57(3):340–6. doi: 10.1006/mvre.1998.2142. [DOI] [PubMed] [Google Scholar]
- 87.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metallopro-teinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 88.Murphy G, Houbrechts A, Cockett MI, Williamson RA, O’Shea M, Docherty AJ. The N-terminal domain of tissue inhibitor of metallopro-teinases retains metalloproteinase inhibitory activity. Biochemistry. 1991;30:8097–8102. doi: 10.1021/bi00247a001. [DOI] [PubMed] [Google Scholar]
- 89.Williamson RA, Marston FA, Angal S, Koklitis P, Panico M, Morris HR, Carne AF, Smith BJ, Harris TJ, Freedman RB. Disulphide bond assignment in human tissue inhibitor of metalloproteinases (TIMP) Biochem J. 1990;268:267–274. doi: 10.1042/bj2680267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fernandez-Catalan C, Bode W, Huber R, Turk D, Calvete JJ, Lichte A, Tschesche H, Maskos K. Crystal structure of the complex formed by the membrane type 1-matrix metalloproteinase with the tissue inhibitor of metalloproteinases-2, the soluble progelatinase A receptor. EMBO J. 1998;17:5238–5248. doi: 10.1093/emboj/17.17.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Will H, Atkinson SJ, Butler GS, Smith B, Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic acti-vation: regulation by TIMP-2 and TIMP-3. J Biol Chem. 1996;271:17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- 92.Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5) J Biol Chem. 2001;276:12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- 93.Barrett AJ. 2-Macroglobulin. Methods Enzymol. 1981;80:737–754. doi: 10.1016/s0076-6879(81)80056-0. [DOI] [PubMed] [Google Scholar]
- 94.Cawston TE, Mercer E. Preferential binding of collagenase to _2-macroglobulin in the presence of the tissue inhibitor of metallopro-teinases. FEBS Lett. 1986;209:9–12. doi: 10.1016/0014-5793(86)81074-2. [DOI] [PubMed] [Google Scholar]
- 95.Herman MP, Sukhova GK, Kisiel W, Foster D, Kehry MR, Libby P, Schonbeck U. Tissue factor pathway inhibitor-2 is a novel inhibitor of matrix metalloproteinases with implications for atherosclerosis. J Clin Invest. 2001;107:1117–1126. doi: 10.1172/JCI10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mott JD, Thomas CL, Rosenbach MT, Takahara K, Greenspan DS, Banda MJ. Post-translational proteolytic processing of procollagen C-terminal proteinase enhancer releases a metalloproteinase inhibitor. J Biol Chem. 2000;275:1384–1390. doi: 10.1074/jbc.275.2.1384. [DOI] [PubMed] [Google Scholar]
- 97.Miyazaki K, Hasegawa M, Funahashi K, Umeda M. A metalloproteinase inhibitor domain in Alzheimer amyloid protein precursor. Nature. 1993;362:839–841. doi: 10.1038/362839a0. [DOI] [PubMed] [Google Scholar]
- 98.Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell. 2001;107:789–800. doi: 10.1016/s0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- 99.Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278:4135–4144. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- 100.Rosenberg GA, Estrada EY, Mobashery S. Effect of synthetic matrix metalloproteinase inhibitors on lipopolysaccharide-induced blood-brain barrier opening in rodents: Differences in response based on strains and solvents. Brain Res. 2007;1133:186–92. doi: 10.1016/j.brainres.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maquoi E, Sounni NE, Devy L, Olivier F, Frankenne F, Krell HW, Grams F, Foidart JM, Noel A. Anti-invasive, antitumoral, and antiangiogenic efficacy of a pyrimidine-2,4,6-trione derivative, an orally active and selective matrix metalloproteinases inhibitor. Clin Cancer Res. 2004;10(12 Pt 1):4038–47. doi: 10.1158/1078-0432.CCR-04-0125. [DOI] [PubMed] [Google Scholar]
- 102.Shono T, Motoyama M, Tatsumi K, Ulbrich N, Iwamoto Y, Kuwano M, Ono M. A new synthetic matrix metalloproteinase inhibitor modulates both angiogenesis and urokinase type plasminogen activator activity. Angiogenesis. 1998;2(4):319–29. doi: 10.1023/a:1009207820233. [DOI] [PubMed] [Google Scholar]
- 103.Wojtowicz-Praga S. Clinical potential of matrix metalloprotease inhibitors. Drugs R D. 1999;1(2):117–29. doi: 10.2165/00126839-199901020-00001. [DOI] [PubMed] [Google Scholar]
- 104.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9(2):267–85. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnson JL, Fritsche-Danielson R, Behrendt M, Westin-Eriksson A, Wennbo H, Herslof M, Elebring M, George SJ, McPheat WL, Jackson CL. Effect of broad-spectrum matrix metalloproteinase inhibition on atherosclerotic plaque stability. Cardiovasc Res. 2006;71(3):586–95. doi: 10.1016/j.cardiores.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 106.Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642–6650. doi: 10.1038/sj.onc.1204097. [DOI] [PubMed] [Google Scholar]
- 107.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: bio-logical actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 108.Zacchigna S, Zentilin L, Morini M, Dell’Eva R, Noonan DM, Albini A, Giacca M. AAV-mediated gene transfer of tissue inhibitor of metalloproteinases-1 inhibits vascular tumor growth and angiogenesis in vivo. Cancer Gene Ther. 2004;11(1):73–80. doi: 10.1038/sj.cgt.7700657. [DOI] [PubMed] [Google Scholar]
- 109.Rouis M, Adamy C, Duverger N, Lesnik P, Horellou P, Moreau M, Emmanuel F, Caillaud JM, Laplaud PM, Dachet C, Chapman MJ. Adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-1 reduces atherosclerotic lesions in apolipoprotein E-deficient mice. Circulation. 1999;100:533–540. doi: 10.1161/01.cir.100.5.533. [DOI] [PubMed] [Google Scholar]
- 110.Johnson JL, Baker AH, Oka K, Chan L, Newby AC, Jackson CL, George SJ. Suppression of atherosclerotic plaque progression and instability by tissue inhibitor of metalloproteinase-2: involvement of macrophage migration and apoptosis. Circulation. 2006;113(20):2435–44. doi: 10.1161/CIRCULATIONAHA.106.613281. [DOI] [PubMed] [Google Scholar]
- 111.White SJ, Newby AC. Gene therapy for all aspects of vein-graft disease. J Card Surg. 2002;17(6):549–55. doi: 10.1046/j.1540-8191.2002.01013.x. [DOI] [PubMed] [Google Scholar]
- 112.George SJ, Baker AH, Angelini GD, Newby AC. Gene transfer of tissue inhibitor of metalloproteinase-2 inhibits metalloproteinase activity and neointima formation in human saphenous veins. Gene Ther. 1998;5(11):1552–60. doi: 10.1038/sj.gt.3300764. [DOI] [PubMed] [Google Scholar]
- 113.Allaire E, Forough R, Clowes M, Starcher B, Clowes AW. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J Clin Invest. 1998;102:1413–1420. doi: 10.1172/JCI2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res. 2005;96(1):119–26. doi: 10.1161/01.RES.0000151333.56089.66. [DOI] [PubMed] [Google Scholar]