Abstract
The discovery of a second estrogen receptor (ER), designated ERβ (NR3A2), has redefined our knowledge about the mechanisms underlying cellular signaling by estrogens and has broad implications for our understanding of regulation of estrogen-responsive tissues. Highly variable and even contrasting effects of estrogens in different tissues seem to be at least partially explained by different estrogen signaling pathways, involving ERα (NR3A1) and/or ERβ. To date, two key conclusions can be drawn from the significant body of work carried out on the specific roles of the two receptor subtypes in diverse estrogen target tissues. First, ERα and ERβ have different biological functions, as indicated by their specific expression patterns and the distinct phenotypes observed in ERα and ERβ knockout (αERKO and βERKO) mice. Second, ERα and ERβ appear to have overlapping but also unique sets of downstream target genes, as judged from a set of microarray experiments. Thus, ERα and ERβ have different transcriptional activities in certain ligand, cell-type, and promoter contexts, which may help to explain some of the major differences in their tissue-specific biological actions. The phenotypes observed for βERKO mice have suggested certain therapeutic areas to be further explored. The development of ERβ-selective ligands active in animal disease models indicates new avenues for clinical exploration. ERβ agonists are being explored and validated as drugs for a growing number of indications. Hopefully, some ERβ targeted drugs will prove to be efficient in enhancing human health.
Introduction
Estrogen is a key regulator of growth and differentiation in a broad range of target tissues, including the reproductive tract, mammary gland, and the central nervous and skeletal systems [Couse and Korach, 1999; Pettersson and Gustafsson, 2001]. Estrogen is also known to be involved in many pathological processes such as breast and endometrial cancer [Henderson et al., 1988] and osteoporosis [Horowitz, 1993]. The presence of an estrogen binding receptor protein was first reported in the early sixties by Elwood Jensen and colleagues [Jensen and Jacobson, 1962]. The cDNA encoding an estrogen receptor (ER) protein was cloned in the middle of the eighties [Green et al., 1986; Greene et al., 1986] and this receptor was long believed to be the only existing ER. However, in 1996, an additional ER was cloned from rat prostate [Kuiper et al., 1996]. This novel receptor was designated ERβ and consequently the originally cloned ER was renamed ERα. Orthologs of rat ERβ were later cloned from many species including human and mouse [Mosselman et al., 1996; Tremblay et al., 1997]. ERα and ERβ belong to the superfamily of nuclear receptors and specifically to the family of steroid receptors that act as ligand-regulated transcription factors [Beato, 1989; Evans, 1988]. Models of action involving cooperation, as well as competition, between the two ER proteins have been proposed [Matthews and Gustafsson, 2003].
Estrogenic therapy of today targets both ERα and ERβ. Specific targeting of ERα or ERβ would open up novel therapeutic opportunities, stratifying this hormonal treatment, thereby reducing undesired side effects. Examples of such unwanted effects include proliferation of the uterus and mammary gland, most likely mediated through ERα. The two receptor subtypes act in distinct ways in several estrogen target cells and tissues [Dahlman-Wright et al., 2006; Harris, 2007]. Two major conclusions may be drawn from this work. First, ERα and ERβ have different biological functions, as indicated by their distinct expression patterns and the different phenotypes reported for the two ER isoform knockout animals, respectively. Second, ERα and ERβ have overlapping yet unique roles in estrogen signaling, as judged from a number of gene expression profiling studies. This article will review the current state of knowledge of mechanisms of ERβ-mediated estrogen signaling, the role of ERβ in physiology and disease and potential diagnostic and pharmaceutical implications of ERβ.
The ERβ gene and protein structure
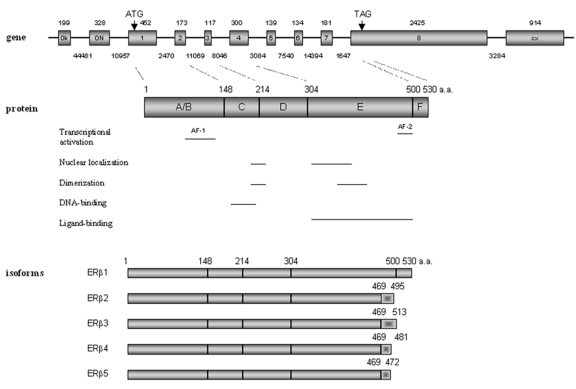
The human ERβ gene (ESR2) is located on chromosome 14 q23.2, and is ∼61.2 kb. The ERβ protein is produced from eight exons. Additionally, there are two untranslated exons, 0N and 0K, in the 5′ region and an exon at the 3′ end that can be spliced to exon 7 to produce the alternative ERβ isoform, ERβ2 [Kuiper et al., 1996; Kuiper and Gustafsson, 1997] (Figure 1). Human ERβ is a protein of 530 amino acids [Ogawa et al., 1998a]. Both the mouse and the rat ERβ genes contain open reading frames that encode proteins of 549 amino acids [Leygue et al., 1998]. A recent study of African, Caucasian and Asian populations failed to support the notion that a human ERβ548 exists [Xu et al., 2003].
Figure 1. Structure of the human ERβ gene, protein and functional domains, and mRNA isoforms.
Gene: exons are indicated with boxes and introns with lines. The numbers above each box indicate the size of the exons (bp); the numbers below each line designate the size of the respective introns (bp). Dotted lines between gene and protein point to protein domain junctions. Protein: numbers indicate the total size of the protein in amino acids. Isoforms: the shaded bar shows the divergent C-terminal regions among the isoforms.
ERβ is a member of the nuclear receptor superfamily and shares common structural characteristics with the other members of this family including five distinguishable domains [Gronemeyer and Laudet, 1995]. They are named the A/B, C, D, E and F domains, respectively (Figure 1). The N-terminal A/B domain is the most variable region and the human ERα and ERβ share less than 20% amino acid identity in this region, indicating that this domain may contribute to ER subtype-specific actions on target genes. This region harbors an activation function (AF-1) [Tora et al., 1989] that is ligand-independent and shows promoter- and cell-specific activity. The central C-domain is the DNA-binding domain (DBD), which is involved in specific DNA binding and receptor dimerization. This domain is highly conserved between ERα and ERβ and shares 95% amino acid identity. The D-domain works as a flexible hinge between the DBD and the ligand-binding domain (LBD), and is thus referred to as the hinge domain. This domain, which is not well conserved between ERα and ERβ (30%), appears to be important for nuclear translocation and has been reported to contain a nuclear localization signal [Picard et al., 1990]. The E-domain is referred to as the LBD and the ERα and ERβ share approximately 55% amino acid identity in this region. The LBD contains a hormone-dependent activation function (AF-2) [Tora et al., 1989] and is important for ligand binding and receptor dimerization. The LBDs of ERα and ERβ have very similar three-dimensional structures. However, the amino acids lining the ligand-binding cavities of ERα and ERβ differ in two positions [Brzozowski et al., 1997; Pike et al., 1999]. Furthermore, the ligand-binding cavity of ERβ is significantly smaller (∼20%) than that of ERα and this may have implications for the selective affinity and pharmacology of ligands [Brzozowski et al., 1997; Pike et al., 1999]. The F-domain has less than 20% amino acid identity between the two ER subtypes and the functions of this domain remain undefined.
ERβ promoters
The promoter organization of human ERβ has yet to be clarified. Hirata et al. [Hirata et al., 2001] have analysed the structure of the 5′-UTR of the ERβ mRNA in the normal uterine endometrium and liver using 5′-rapid amplification of the cDNA ends. This work has revealed two isoforms of ERβ mRNA containing different untranslated 5′-regions, which are generated by alternative splicing of two upstream exons, exon 0K and exon 0N (Figure 1), to exon 1. These results indicate that transcription of the human ERβ gene occurs from at least two different promoters, named promoter 0N and promoter 0K. Promoter 0N has been cloned and proven to have promoter activity [Li et al., 2000]. Our laboratory has cloned promoter 0K and our ongoing studies of successive 5′ promoter deletions suggest that sequences of promoter 0K between –534 and –1058 may contain binding sites for key transcription factors driving basal ERβ gene expression. Expression of the two transcripts originating from promoters 0N and 0K, respectively, has been detected in several human tissues, such as liver, ejaculated spermatozoa, uterine endometrium and myometrium [Hirata et al., 2001]. We have shown that transcripts from promoter 0N were more pronounced than those from promoter 0K in normal breast epithelial cells and a panel of breast cancer cell lines [Zhao et al., 2003]. It should be noted that full-length ERβ cDNA sequences containing neither exon 0N nor exon 0K [Ogawa et al., 1998a] have been reported, suggesting the presence of (an) additional promoter(s). Further studies aimed at characterization of promoters 0K and 0N and identification and characterization of putative additional promoters should aid in defining mechanisms for how expression of the human ERβ gene is regulated.
The expression level of ERβ is also regulated through chromatin condensation of promoter regions via hypermethylation of CpG islands in the ERβ promoter. Hypermethylation of the ERβ promoter is associated with a marked decrease of ERβ mRNA expression in breast cancer, prostate cancer as well as in cancer cell lines, and the inhibition of DNA-methyltransferases reactivates ERβ expression in these cell lines [Nojima et al., 2001; Rody et al., 2005; Zhao et al., 2003; Zhu et al., 2004]. It has been reported that hypermethylation of gene promoter CpG islands plays a significant role in the development and progression of various cancers [Dulaimi et al., 2004a; Dulaimi et al., 2004b; Umetani et al., 2005]. The identification of hypermethylated genes in tumors has become an accepted approach to assess tumor-related gene inactivation [Herman and Baylin, 2003]. The finding of ERβ gene silencing via promoter hypermethylation in tumors suggests an important role for the ERβ gene in cancer progression and may be used as a prognostic molecular biomarker. Interestingly, a significant increase of ERβ promoter methylation was recently reported as an epigenetic change in atherosclerosis and vascular aging [Kim et al., 2007]. Moreover, methylation of a CpG island at the ERβ promoter region was recently shown to be a primary mechanism responsible for differential expression of ERβ in endometriosis and endometrium [Xue et al., 2007].
ERβ mRNA isoforms
Multiple ERβ isoforms exist as a result of either alternative splicing of the last coding exons (exon 8 and exon 9, respectively), deletion of one or more coding exons, or alternative usage of untranslated exons in the 5′ region [Lewandowski et al., 2002]. Among them, five full-length transcripts, designated ERβ1-5, have been reported in human (Figure 1). The full-length human mRNA translated from 8 exons, encoding 530 amino acids, is named ERβ1. The full-length ERβ2-5 transcripts share identical sequences with ERβ1 from exon 1 to exon 7, but have unique sequences in place of exon 8 [Moore et al., 1998]. The predicted amino acid sequences of ERβ1-5 diverge at amino acid 469 within the LBD and extend to the C-terminus. ERβ4 and ERβ5 isoforms were originally identified as truncated transcripts containing only part of the common exon 7 and different exon 8 sequences [Moore et al., 1998]. A recent report confirmed their existence as full-length transcripts [Poola et al., 2005]. Their functional aspects were subsequently examined by Leung et al. [Leung et al., 2006]. In vitro studies show that ERβ4 and β5 can heterodimerize with ERβ1 and enhance its transactivation in a ligand-dependent manner. The expression of ERβ3 appears to be restricted to the testis [Moore et al., 1998] and functional studies on this isoform have not been performed.
To date, there are data supporting protein expression of several ERβ isoforms. The human variant ERβ2 (also called ERβcx) encodes a protein of 495 amino acid residues, with a molecular weight of 55.5 kDa. ERβ2 has a unique C-terminus, due to alternative splicing, where the amino acids corresponding to exon 8 are replaced with 26 unique amino acids [Ogawa et al., 1998b]. Thus, ERβ2 lacks the AF-2 core region and has undetectable affinity for estradiol and other tested ligands. Interestingly, ERβ2 was shown to inhibit ligand-induced ERα transcriptional activity on an ERE-reporter gene [Ogawa et al., 1998b]. Our laboratory has further investigated the possible molecular mechanism for the antagonistic effect of ERβ2 on ERα-mediated transactivation. Our results show that ERβ2 induces proteasome-dependent degradation of ERα, presumably through the formation of ERβ2/ERα heterodimers [Zhao et al., 2007]. We suggest that ERβ2-mediated degradation of ERα is at least one mechanism whereby expression of ERβ2 inhibits recruitment of ERα to the estrogen-responsive promoters, leading to suppression of ERα-regulated genes.
Molecular mechanisms of estrogen action
Estrogen action is exerted in target tissues via binding to one of the two ERs, ERα or ERβ. Like other steroid hormone receptors, ERs act as dimers to regulate transcriptional activation. Full transcriptional activation by ERs is mediated by synergism between two activation domains, AF-1 at the N terminus and AF-2 in the LBD. Both ERα and ERβ contain the potent AF-2 function, but unlike ERα, ERβ seems to have a weaker corresponding AF-1 function and thus depends more on the ligand-dependent AF-2 for its transcriptional activation function [Delaunay et al., 2000].
Evidence suggests that there are several distinct pathways by which estrogens, via ERs, can regulate biological processes [Hall et al., 2001]. In the classical model of ER action, ligand-activated ERs bind specifically to DNA at estrogen-responsive elements (EREs) through their DNA binding domains and bring coregulators to the transcription start site. The consensus ERE consists of two half-sites (aGGTCAnnnTGACCt) separated by a three-nucleotide spacer. However, many natural EREs deviate substantially from the consensus sequences [O'Lone et al., 2004]. Estrogen also modulates gene expression by a second mechanism in which ERs interact with other transcription factors, such as activating protein-1 (AP-1) and stimulating protein 1 (Sp1), through a process referred to as transcription factor cross talk [Bjornstrom and Sjoberg, 2005; Kushner et al., 2000; Saville et al., 2000]. An interesting difference between ERα and ERβ is observed on AP-1 sites. In the presence of estrogen, ERα induces AP-1-driven reporter activity, whereas ERβ has no effect [Paech et al., 1997]. Raloxifene binding to ERβ induces transcriptional activity through an AP-1 site, whereas binding to ERα results in minimal activation examined under the same conditions [Paech et al., 1997]. For ER:Sp1 complexes, that interact with the GC-rich Sp1 motif [Batistuzzo de Medeiros et al., 1997; Porter et al., 1997], alternative ligand responses have been reported. In a study by Zou et al., ERβ activated an RARα1 promoter-reporter construct presumably by the formation of an ER:Sp1 complex [Zou et al., 1999]. Antagonist binding to ERβ caused an increase in reporter gene expression. This effect was blocked by estrogen, which resembles the effect of ERβ on an AP-1 site. Moreover, ERα and ERβ also exhibit different transcriptional effects in regulation of the cyclin D1 promoter [Liu et al., 2002]. ERα mediates the stimulatory effect of estrogen on cyclin D1 expression, whereas ERβ has a repressive effect. However, both ERα and ERβ induce the expression of cyclin D1 in response to antiestrogens. These effects were traced to a cAMP response element (CRE) in the cyclin D1 promoter.
In addition, estrogen may elicit effects through non-genomic mechanisms where estrogen has been claimed to bind to ERs localized on the plasma membrane of target cells [Nelson et al., 1986; Razandi et al., 1999; Razandi et al., 2004]. It has been suggested that these effects may be the result of estrogen activation of MAPK and ERK signaling [Pedram et al., 2006] or release of intracellular calcium [Mermelstein et al., 1996]. However, such membrane-associated ER proteins have so far not been conclusively identified and the mechanistic details of activation through these non-genomic pathways remain to be characterized. Recently, Galluzzo et al. [Galluzzo et al., 2007] showed that ERβ palmitoylation is necessary for receptor localization at the plasma membrane and for the p38-dependent activation of downstream pro-apoptotic cascade.
In addition to these ligand-induced transcriptional activities of ERs, ligand-independent pathways to activate ERs have been described. Growth factor signaling or stimulation of other signaling pathways leads to activation of kinases that can phosphorylate and thereby activate ERs or associated coregulators in the absence of ligand [Kato et al., 1995]. For example it has been shown that the HER2 downstream signaling molecules ERK1 and ERK2 can phosphorylate ER, leading to enhanced sensitivity of the receptor to its cognate hormone and additionally to ligand-independent receptor activation [Martin et al., 2005]. Tremblay et al. [Tremblay et al., 1999] showed that phosphorylation of MAPK sites within ERβ AF-1 stimulates ligand- and AF-2-independent interaction with SRC-1 leading to transcriptional activation. Furthermore, one recent microarray study revealed that ERβ in the absence of ligand, stimulated and suppressed the activity of a number of genes that were normally only regulated by ERα in the presence of E2 [Chang et al., 2006].
In summary, ERα and ERβ exert differential transcriptional activities which, together with their distinct expression patterns, may serve as a basis for the major differences in their tissue-specific actions, the distinct phenotypes of αERKO and βERKO animals and the specific pharmacological effects exerted by ERα and ERβ selective compounds. This complexity is further enhanced by co-expression of splicing variants of ERs in the cells, and the ability of ERs to form homodimers and heterodimers.
Expression profiling reveals ERβ gene expression programs
There have been a number of studies in the past few years aimed at comprehensively unraveling the complete estrogen-regulated gene expression programs in cancer cells. These reports can be attributed to the introduction of microarrays for global gene expression profiling. To date, much of the work on identification of gene expression profiles has been focused on the role of ERα [Carroll and Brown, 2006; Lin et al., 2007a], but some microarray studies have examined the role of ERβ in regulating global gene expression programs. A summary of studies on expression profiling of ERβ-regulated genes is shown in Table 1.
Table 1. Expression profiling of E2-regulated genes through ERβ.
For each reference, the model cell lines used in the study, treatment conditions, and the profiling platforms are listed.
Several gene expression studies have been performed in breast cancer cell lines stably expressing ERβ [Chang et al., 2006; Lin et al., 2007b; Omoto et al., 2003; Secreto et al., 2007]. Chang et al. [Chang et al., 2006] conducted microarray analyses to investigate gene regulatory effects of ERβ in MCF7 cells. Of the genes modulated by ERβ, the greatest numbers were associated with transcriptional factors and signal transduction pathways. In particular, ERβ regulated multiple components of TGFβ signaling, consistent with the observations that TGFβ is normally associated with the suppression of breast cancer cell proliferation. Lin et al. [Lin et al., 2007b] identified a subset of 14 DNA replication and cell cycle-related genes that were specifically downregulated by ERβ in T47D cells. However, assessment of the 5′ regulatory regions of the four key downstream genes CDC2, CKS2, DNA2L and CDC6 did not identify the consensus ERE. This raises the possibility that either the expression of these genes involves trans-elements such as other transcription factors induced by ERβ or that ERβ is acting as a cofactor for other transcription factors such as AP-1. Recently, data obtained from microarray analyses of E2-stimulated Hs578T cells stably expressing either ERα or ERβ revealed that the patterns of E2-regulated gene expression were largely unique to either ER subtype [Secreto et al., 2007]. Gene expression profiles in aortas from αERKO and βERKO mice revealed also that ERα- and ERβ-dependent pathways regulate distinct sets of genes [O'Lone et al., 2007].
Estrogens exert profound effects on bone, a tissue that expresses ERα and ERβ. Thus, the second most frequently used model system has been a human osteoblast-like cell type, U2OS human osteosarcoma cells [Kian Tee et al., 2004; Monroe et al., 2003; Monroe et al., 2005; Stossi et al., 2004]. Because of the lack of endogenous expression of either ERα or ERβ, U2OS ERα or ERβ stably expressing cell lines provide a cell model, permitting investigation of ER-subtype specific actions on gene expression. These studies have compared the gene-regulatory activities of ERα and ERβ in U2OS cells and showed that ERα and ERβ share some common target genes, although each receptor also appears to have distinct sets of downstream target genes. For example, genes encoding cystatin D, autotaxin or stromal antigen 2 appear to be specifically regulated by E2 via ERβ in bone [Stossi et al., 2004]. An interesting finding was that E2 upregulated several genes associated with cell motility selectively via ERβ, fitting with a model of the selective E2 enhancement of the motility of ERβ-containing cells.
Clearly, future studies should aim at investigating changes in gene expression profiles in response to the modulation of the activity of endogenous ERβ and at identifying the first step in the signal cascade of ERβ, namely the global binding of ERβ to DNA in the context of intact chromatin.
Roles of ERβ in human cancers
Targeted disruption of ERβ in mice has suggested roles for ERβ in many tissues and organs, including the ovary, uterus, mammary gland, brain, immune system and ventral prostate [Harris, 2007].
Breast cancer
Estrogen is essential for growth and development of the mammary glands, and has been associated with promotion and growth of breast cancer. ERβ is found in both ductal and lobular epithelial and stromal cells of the rodent, whereas ERα is only found in the ductal and lobular epithelial cells and not in stroma [Gotteland et al., 1995]. ERβ expression in normal human breast and breast cancer specimens and the relationship between ERβ and other clinicopathological features and its role in response to endocrine treatment has been extensively investigated at both mRNA and protein levels. Consensus regarding a protective role of ERβ against breast cancer development seems to have been reached during the recent few years. ERβ is lost in a majority of breast tumors [Bardin et al., 2004a; Skliris et al., 2003], apparently by ERβ promoter methylation in breast cancer cells [Rody et al., 2005; Zhao et al., 2003]. Since promoter methylation is frequently observed in cancer [Garinis et al., 2002], these data suggest that ERβ is a possible tumor suppressor gene. In vitro studies indicated that ERβ is an important modulator of proliferation and invasion of breast cancer cells, thus supporting the hypothesis that the loss of ERβ expression could be one of the events leading to breast cancer development [Chang et al., 2006; Lazennec et al., 2001]. However, this hypothesis needs to be confirmed, because it has been shown that ERβ is expressed in the majority of breast tumors, with immunohistochemical staining in about 2/3 of breast tumors, similar to the percentage of tumors which express ERα. Currently, only the ERα form is measured for clinical decision-making and treatment of breast cancer patients.
Prostate cancer
Estrogens can have profound effects on prostate growth and differentiation as well as in the pathogenesis of prostate cancer [Ho et al., 2006]. Since ERβ was originally discovered in a rat prostate cDNA library, it was not surprising that ERβ is highly expressed in both rat and normal human prostate [Horvath et al., 2001; Kuiper et al., 1996]. In the adult rodent ventral prostate, ERβ is expressed in the epithelial cells, whereas ERα is expressed in the stroma [Adams et al., 2002]. The estrogenic effects in the prostate may therefore be exerted by both ERs, but in different cells. Our laboratory has shown that ERβ knockout (βERKO) mice display signs of prostatic hyperplasia with aging [Imamov et al., 2004; Weihua et al., 2000]. We also found that most epithelial cells express the proliferation antigen Ki-67 and the antiapoptotic factor BCLII in the prostates from βERKO mice. We thus hypothesize that ERβ has an antiproliferative and a prodifferentiative role in prostatic epithelium.
Colon cancer
Colon cancer incidence and mortality rates are lower in females compared with males, and numerous epidemiological studies suggest that estrogen replacement therapy (ERT) reduces the incidence of colorectal cancer in postmenopausal women. ERβ is the predominant ER in the colonic epithelium [Campbell-Thompson et al., 2001; Konstantinopoulos et al., 2003], suggesting that effects of estrogen in the colon are mediated by ERβ. In colons from βERKO mice, the number of proliferating cells was higher, and the migration of labelled cells from base to lumen of the crypts was faster when compared to wild-type mice [Wada-Hiraike et al., 2006]. Additionally, immunohistochemical staining revealed fewer apoptotic cells (cleaved caspase 3-positive), a significant decrease in expression of the epithelial differentiation marker, cytokeratin CK20, the adherens junction protein, α-catenin, and the hemidesmosomal protein, plectin, in βERKO mice [Wada-Hiraike et al., 2006]. These findings suggest a role for ERβ in the organization and architectural maintenance of the colon.
Ovarian cancer
Ovarian cancer is a disease with high mortality, mainly because of the lack of effective screening methods and late symptoms. At the time of diagnosis, patients are often at an advanced stage of the disease with occult metastases within the peritoneal cavity. The role of estrogens has been recently highlighted by the results of three large prospective studies showing increased ovarian cancer incidence and mortality in women who used long-term estrogen replacement therapy [Anderson et al., 2003; Lacey et al., 2002; Rodriguez et al., 2001]. A loss of ERβ expression or a decrease in ERβ/ERα ratio in epithelial ovarian cancer cells as compared with normal tissues has been reported by several groups [Bardin et al., 2004b; Brandenberger et al., 1998; Pujol et al., 1998; Rutherford et al., 2000]. Recently, one study showed that ERβ overexpression in ovarian cancer cells exerts antitumoral effects [Bardin et al., 2004b; Treeck et al., 2007]. Oral contraceptive (OC) use has been associated with a decreased risk of ovarian cancer. The study by Schildkraut et al. [Schildkraut et al., 2002] shows that the combination OC formulations with high-progestin potency appear to be associated with a greater reduction in ovarian cancer risk than those with low-progestin potency. Mechanisms underlying this reduction may include inhibition of ovulation and/or some direct biological effects of the progestin.
Validation of ERβ-selective agonists in animal disease models
As described above, studies in knockout animals have revealed a number of interesting phenotypes associated with the lack of ERβ signaling and thus generated hypotheses to be tested in animal disease models. Some examples where these hypotheses have been validated in animal models are outlined below.
Recently, ERβ-selective ligands have been characterized in several clinically relevant animal models. In a study by Walf and co-workers [Walf and Frye, 2005], vehicle, 17β-estradiol, or ER subtype selective agonists were administered acutely to female ovariectomized rats prior to behavioral testing. 17β-estradiol and the ERβ-selective agonist diaryl-propionitrile (DPN) [Meyers et al., 2001], but not the ERα-selective agonist propyl pyrazole triol (PPT) [Stauffer et al., 2000], showed antidepressant-like effects (reduced immobility) in the forced swim test, an animal model of depression. In an another study by Hegele-Hartung et al. [Hegele-Hartung et al., 2004], the effects of ER subtype selective agonists on ovarian biology were evaluated in hypophysectomized rats, gonadotropin-releasing hormone antagonist-treated mice, as well as intact rats. Their results showed that the ERβ agonist caused stimulation of early folliculogenesis, a decrease in follicular atresia, induction of ovarian gene expression, and stimulation of late follicular growth, accompanied by an increase in the number of ovulated oocytes, similar to 17β-estradiol. In contrast, the ERα agonist had little or no effect on these parameters, implying that direct estrogen effects on ovarian follicular development are mediated by ERβ. These results suggested that ERβ agonists might be useful as a novel therapeutic approach to improve ovarian function in sub- or infertile women. Moreover, an ERβ-selective agonist, ERB041, synthesized by Wyeth Research, has been shown to be effective against inflammatory pain [Leventhal et al., 2006]. This compound has previously been reported to be anti-inflammatory in animal models [Harris et al., 2003]. Furthermore, an ERβ agonist developed by Eli Lilly demonstrates involution of the ventral prostate in rodent prostate models [Norman et al., 2006]. More recently, studies performed with intact aromatase knockout mice demonstrated that the administration of an ERβ-specific agonist ablated preexisting prostatic epithelial hyperplasia, whereas an ERα-specific agonist did not [McPherson et al., 2007]. These findings suggested that ERβ-specific agonists might be valid candidates for new pharmacological approaches to manage dysregulated prostate growth.
Conclusions
Studies in βERKO mice together with high-throughput gene expression profiling approaches have furthered our understanding of the role of ERβ in physiology and provided important glimpses into the molecular basis of ERβ-mediated estrogen action in target cells. Future studies should include validation of ERβ as a target for candidate diseases and exploration of ERβ as a marker for clinical decision-making and treatment. Hopefully, the next decade will see a number of clinically useful ERβ-agonists.
Abbreviations
- 5′-UTR
5′-untranslated region
- AF
activation function
- DBD
DNA-binding domain
- ER
estrogen receptor
- ERE
estrogen-responsive elements
- ERT
estrogen replacement therapy
- LBD
ligand-binding domain
- SERM
selective estrogen receptor modulator
- αERKO
ERα knockout
- βERKO
ERβ knockout
References
- Adams J. Y., Leav I., Lau K. M., Ho S. M., Pflueger S. M. Expression of estrogen receptor β in the fetal, neonatal, and prepubertal human prostate. Prostate. 2002;52:69–81. doi: 10.1002/pros.10103. [DOI] [PubMed] [Google Scholar]
- Anderson G. L., Judd H. L., Kaunitz A. M., Barad D. H., Beresford S. A., Pettinger M., Liu J., McNeeley S. G., Lopez A. M. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial. Jama. 2003;290:1739–48. doi: 10.1001/jama.290.13.1739. [DOI] [PubMed] [Google Scholar]
- Bardin A., Hoffmann P., Boulle N., Katsaros D., Vignon F., Pujol P., Lazennec G. Involvement of estrogen receptor β in ovarian carcinogenesis. Cancer Res. 2004b;64:5861–9. doi: 10.1158/0008-5472.CAN-04-0552. [DOI] [PubMed] [Google Scholar]
- Bardin A., Boulle N., Lazennec G., Vignon F., Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004a;11:537–51. doi: 10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistuzzo de Medeiros S. R., Krey G., Hihi A. K., Wahli W. Functional interactions between the estrogen receptor and the transcription activator Sp1 regulate the estrogen-dependent transcriptional activity of the vitellogenin A1 io promoter. J Biol Chem. 1997;272:18250–60. doi: 10.1074/jbc.272.29.18250. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–44. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bjornstrom L., Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–42. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Brandenberger A. W., Tee M. K., Jaffe R. B. Estrogen receptor α (ER-α) and β (ER-β) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: down-regulation of ER-β in neoplastic tissues. J Clin Endocrinol Metab. 1998;83:1025–8. doi: 10.1210/jcem.83.3.4788. [DOI] [PubMed] [Google Scholar]
- Brzozowski A. M., Pike A. C., Dauter Z., Hubbard R. E., Bonn T., Engstrom O., Ohman L., Greene G. L., Gustafsson J. A., Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–8. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- Campbell-Thompson M., Lynch I. J., Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61:632–40. [PubMed] [Google Scholar]
- Carroll J. S., Brown M. Estrogen receptor target gene: an evolving concept. Mol Endocrinol. 2006;20:1707–14. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- Chang E. C., Frasor J., Komm B., Katzenellenbogen B. S. Impact of estrogen receptor β on gene networks regulated by estrogen receptor α in breast cancer cells. Endocrinology. 2006;147:4831–42. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- Couse J. F., Korach K. S. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Dahlman-Wright K., Cavailles V., Fuqua S. A., Jordan V. C., Katzenellenbogen J. A., Korach K. S., Maggi A., Muramatsu M., Parker M. G., Gustafsson J. A. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58:773–81. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- Delaunay F., Pettersson K., Tujague M., Gustafsson J. A. Functional differences between the amino-terminal domains of estrogen receptors α and β. Mol Pharmacol. 2000;58:584–90. doi: 10.1124/mol.58.3.584. [DOI] [PubMed] [Google Scholar]
- Dulaimi E., Ibanez de Caceres I., Uzzo R. G., Al-Saleem T., Greenberg R. E., Polascik T. J., Babb J. S., Grizzle W. E., Cairns P. Promoter hypermethylation profile of kidney cancer. Clin Cancer Res. 2004b;10:3972–9. doi: 10.1158/1078-0432.CCR-04-0175. [DOI] [PubMed] [Google Scholar]
- Dulaimi E., Hillinck J., Ibanez de Caceres I., Al-Saleem T., Cairns P. Tumor suppressor gene promoter hypermethylation in serum of breast cancer patients. Clin Cancer Res. 2004a;10:6189–93. doi: 10.1158/1078-0432.CCR-04-0597. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzo P., Caiazza F., Moreno S., Marino M. Role of ERbeta palmitoylation in the inhibition of human colon cancer cell proliferation. Endocr Relat Cancer. 2007;14:153–67. doi: 10.1677/ERC-06-0020. [DOI] [PubMed] [Google Scholar]
- Garinis G. A., Patrinos G. P., Spanakis N. E., Menounos P. G. DNA hypermethylation: when tumour suppressor genes go silent. Hum Genet. 2002;111:115–27. doi: 10.1007/s00439-002-0783-6. [DOI] [PubMed] [Google Scholar]
- Gotteland M., Desauty G., Delarue J. C., Liu L., May E. Human estrogen receptor messenger RNA variants in both normal and tumor breast tissues. Mol Cell Endocrinol. 1995;112:1–13. doi: 10.1016/0303-7207(95)03576-s. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Gilna P., Waterfield M., Baker A., Hort Y., Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–4. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–9. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Laudet V. Transcription factors 3: nuclear receptors. Protein Profile. 1995;2:1173–308. [PubMed] [Google Scholar]
- Hall J. M., Couse J. F., Korach K. S. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–72. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- Harris H. A. Estrogen receptor-β: recent lessons from in vivo studies. Mol Endocrinol. 2007;21:1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- Harris H. A., Albert L. M., Leathurby Y., Malamas M. S., Mewshaw R. E., Miller C. P., Kharode Y. P., Marzolf J., Komm B. S., Winneker R. C., Frail D. E., Henderson R. A., Zhu Y., Keith J. C., Jr. Evaluation of an estrogen receptor-β agonist in animal models of human disease. Endocrinology. 2003;144:4241–9. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- Hegele-Hartung C., Siebel P., Peters O., Kosemund D., Muller G., Hillisch A., Walter A., Kraetzschmar J., Fritzemeier K. H. Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc Natl Acad Sci U S A. 2004;101:5129–34. doi: 10.1073/pnas.0306720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B. E., Ross R., Bernstein L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1988;48:246–53. [PubMed] [Google Scholar]
- Herman J. G., Baylin S. B. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–54. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- Hirata S., Shoda T., Kato J., Hoshi K. The multiple untranslated first exons system of the human estrogen receptor β (ER β) gene. J Steroid Biochem Mol Biol. 2001;78:33–40. doi: 10.1016/s0960-0760(01)00071-1. [DOI] [PubMed] [Google Scholar]
- Ho S. M., Leung Y. K., Chung I. Estrogens and antiestrogens as etiological factors and therapeutics for prostate cancer. Ann N Y Acad Sci. 2006;1089:177–93. doi: 10.1196/annals.1386.005. [DOI] [PubMed] [Google Scholar]
- Horowitz M. C. Cytokines and estrogen in bone: anti-osteoporotic effects. Science. 1993;260:626–7. doi: 10.1126/science.8480174. [DOI] [PubMed] [Google Scholar]
- Horvath L. G., Henshall S. M., Lee C. S., Head D. R., Quinn D. I., Makela S., Delprado W., Golovsky D., Brenner P. C., O'Neill G., Kooner R., Stricker P. D., Grygiel J. J., Gustafsson J. A., Sutherland R. L. Frequent loss of estrogen receptor-β expression in prostate cancer. Cancer Res. 2001;61:5331–5. [PubMed] [Google Scholar]
- Imamov O., Morani A., Shim G. J., Omoto Y., Thulin-Andersson C., Warner M., Gustafsson J. A. Estrogen receptor β regulates epithelial cellular differentiation in the mouse ventral prostate. Proc Natl Acad Sci U S A. 2004;101:9375–80. doi: 10.1073/pnas.0403041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E. V., Jacobson H. I. Basic guides to the mechanism of estrogen action. Rec Prog Horm Res. 1962;18:387–414. [Google Scholar]
- Kato S., Endoh H., Masuhiro Y., Kitamoto T., Uchiyama S., Sasaki H., Masushige S., Gotoh Y., Nishida E., Kawashima H., Metzger D., Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Kian Tee M., Rogatsky I., Tzagarakis-Foster C., Cvoro A., An J., Christy R. J., Yamamoto K. R., Leitman D. C. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors α and β. Mol Biol Cell. 2004;15:1262–72. doi: 10.1091/mbc.E03-06-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kim J. Y., Song K. S., Lee Y. H., Seo J. S., Jelinek J., Goldschmidt-Clermont P. J., Issa J. P. Epigenetic changes in estrogen receptor β gene in atherosclerotic cardiovascular tissues and in-vitro vascular senescence. Biochim Biophys Acta. 2007;1772:72–80. doi: 10.1016/j.bbadis.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Konstantinopoulos P. A., Kominea A., Vandoros G., Sykiotis G. P., Andricopoulos P., Varakis I., Sotiropoulou-Bonikou G., Papavassiliou A. G. Oestrogen receptor β (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. Eur J Cancer. 2003;39:1251–8. doi: 10.1016/s0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- Kuiper G. G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J. A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper G. G., Gustafsson J. A. The novel estrogen receptor-β subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997;410:87–90. doi: 10.1016/s0014-5793(97)00413-4. [DOI] [PubMed] [Google Scholar]
- Kushner P. J., Agard D. A., Greene G. L., Scanlan T. S., Shiau A. K., Uht R. M., Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–7. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Lacey J. V., Jr., Mink P. J., Lubin J. H., Sherman M. E., Troisi R., Hartge P., Schatzkin A., Schairer C. Menopausal hormone replacement therapy and risk of ovarian cancer. Jama. 2002;288:334–41. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- Lazennec G., Bresson D., Lucas A., Chauveau C., Vignon F. ER β inhibits proliferation and invasion of breast cancer cells. Endocrinology. 2001;142:4120–30. doi: 10.1210/endo.142.9.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung Y. K., Mak P., Hassan S., Ho S. M. Estrogen receptor (ER)-β isoforms: a key to understanding ER-β signaling. Proc Natl Acad Sci U S A. 2006;103:13162–7. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal L., Brandt M. R., Cummons T. A., Piesla M. J., Rogers K. E., Harris H. A. An estrogen receptor-β agonist is active in models of inflammatory and chemical-induced pain. Eur J Pharmacol. 2006;553:146–8. doi: 10.1016/j.ejphar.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Lewandowski S., Kalita K., Kaczmarek L. Estrogen receptor β. Potential functional significance of a variety of mRNA isoforms. FEBS Lett. 2002;524:1–5. doi: 10.1016/s0014-5793(02)03015-6. [DOI] [PubMed] [Google Scholar]
- Leygue E., Dotzlaw H., Lu B., Glor C., Watson P. H., Murphy L. C. Estrogen receptor β: mine is longer than yours? J Clin Endocrinol Metab. 1998;83:3754–5. doi: 10.1210/jcem.83.10.5187-1. [DOI] [PubMed] [Google Scholar]
- Li L. C., Yeh C. C., Nojima D., Dahiya R. Cloning and characterization of human estrogen receptor β promoter. Biochem Biophys Res Commun. 2000;275:682–9. doi: 10.1006/bbrc.2000.3363. [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Vega V. B., Thomsen J. S., Zhang T., Kong S. L., Xie M., Chiu K. P., Lipovich L., Barnett D. H., Stossi F., Yeo A., George J., Kuznetsov V. A., Lee Y. K., Charn T. H., Palanisamy N., Miller L. D., Cheung E., Katzenellenbogen B. S., Ruan Y., Bourque G., Wei C. L., Liu E. T. Whole-genome cartography of estrogen receptor α binding sites. PLoS Genet. 2007a;3:e87. doi: 10.1371/journal.pgen.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Strom A., Li Kong S., Kietz S., Thomsen J. S., Tee J. B., Vega V. B., Miller L. D., Smeds J., Bergh J., Gustafsson J. A., Liu E. T. Inhibitory effects of estrogen receptor β on specific hormone-responsive gene expression and association with disease outcome in primary breast cancer. Breast Cancer Res. 2007b;9:R25. doi: 10.1186/bcr1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. M., Albanese C., Anderson C. M., Hilty K., Webb P., Uht R. M., Price R. H., Jr., Pestell R. G., Kushner P. J. Opposing action of estrogen receptors α and β on cyclin D1 gene expression. J Biol Chem. 2002;277:24353–60. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- Martin L. A., Farmer I., Johnston S. R., Ali S., Dowsett M. Elevated ERK1/ERK2/estrogen receptor cross-talk enhances estrogen-mediated signaling during long-term estrogen deprivation. Endocr Relat Cancer. 2005;12 Suppl 1:S75–84. doi: 10.1677/erc.1.01023. [DOI] [PubMed] [Google Scholar]
- Matthews J., Gustafsson J. A. Estrogen signaling: a subtle balance between ER α and ER β. Mol Interv. 2003;3:281–92. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- McPherson S. J., Ellem S. J., Simpson E. R., Patchev V., Fritzemeier K. H., Risbridger G. P. Essential role for estrogen receptor β in stromal-epithelial regulation of prostatic hyperplasia. Endocrinology. 2007;148:566–74. doi: 10.1210/en.2006-0906. [DOI] [PubMed] [Google Scholar]
- Mermelstein P. G., Becker J. B., Surmeier D. J. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers M. J., Sun J., Carlson K. E., Marriner G. A., Katzenellenbogen B. S., Katzenellenbogen J. A. Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–51. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Monroe D. G., Secreto F. J., Subramaniam M., Getz B. J., Khosla S., Spelsberg T. C. Estrogen receptor α and β heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol. 2005;19:1555–68. doi: 10.1210/me.2004-0381. [DOI] [PubMed] [Google Scholar]
- Monroe D. G., Getz B. J., Johnsen S. A., Riggs B. L., Khosla S., Spelsberg T. C. Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERalpha or ERbeta. J Cell Biochem. 2003;90:315–26. doi: 10.1002/jcb.10633. [DOI] [PubMed] [Google Scholar]
- Moore J. T., McKee D. D., Slentz-Kesler K., Moore L. B., Jones S. A., Horne E. L., Su J. L., Kliewer S. A., Lehmann J. M., Willson T. M. Cloning and characterization of human estrogen receptor β isoforms. Biochem Biophys Res Commun. 1998;247:75–8. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- Mosselman S., Polman J., Dijkema R. ER β: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Nelson J., Clarke R., Murphy R. F. The unoccupied estrogen receptor: some comments on localization. Steroids. 1986;48:121–4. doi: 10.1016/0039-128x(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Nojima D., Li L. C., Dharia A., Perinchery G., Ribeiro-Filho L., Yen T. S., Dahiya R. CpG hypermethylation of the promoter region inactivates the estrogen receptor-β gene in patients with prostate carcinoma. Cancer. 2001;92:2076–83. doi: 10.1002/1097-0142(20011015)92:8<2076::aid-cncr1548>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Norman B. H., Dodge J. A., Richardson T. I., Borromeo P. S., Lugar C. W., Jones S. A., Chen K., Wang Y., Durst G. L., Barr R. J., Montrose-Rafizadeh C., Osborne H. E., Amos R. M., Guo S., Boodhoo A., Krishnan V. Benzopyrans are selective estrogen receptor β agonists with novel activity in models of benign prostatic hyperplasia. J Med Chem. 2006;49:6155–7. doi: 10.1021/jm060491j. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Inoue S., Watanabe T., Orimo A., Hosoi T., Ouchi Y., Muramatsu M. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor ofestrogen action in human. Nucleic Acids Res. 1998b;26:3505–12. doi: 10.1093/nar/26.15.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Inoue S., Watanabe T., Hiroi H., Orimo A., Hosoi T., Ouchi Y., Muramatsu M. The complete primary structure of human estrogen receptor β (hER β) and its heterodimerization with ER α in vivo and in vitro. Biochem Biophys Res Commun. 1998a;243:122–6. doi: 10.1006/bbrc.1997.7893. [DOI] [PubMed] [Google Scholar]
- O'Lone R., Knorr K., Jaffe I. Z., Schaffer M. E., Martini P. G., Karas R. H., Bienkowska J., Mendelsohn M. E., Hansen U. Estrogen receptors α and β mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21:1281–96. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- O'Lone R., Frith M. C., Karlsson E. K., Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–75. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- Omoto Y., Eguchi H., Yamamoto-Yamaguchi Y., Hayashi S. Estrogen receptor (ER) beta1 and ERbetacx/beta2 inhibit ERalpha function differently in breast cancer cell line MCF7. Oncogene. 2003;22:5011–20. doi: 10.1038/sj.onc.1206787. [DOI] [PubMed] [Google Scholar]
- Paech K., Webb P., Kuiper G. G., Nilsson S., Gustafsson J., Kushner P. J., Scanlan T. S. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–10. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Pedram A., Razandi M., Levin E. R. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pettersson K., Gustafsson J. A. Role of estrogen receptor β in estrogen action. Annu Rev Physiol. 2001;63:165–92. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- Picard D., Kumar V., Chambon P., Yamamoto K. R. Signal transduction by steroid hormones: nuclear localization is differentially regulated in estrogen and glucocorticoid receptors. Cell Regul. 1990;1:291–9. doi: 10.1091/mbc.1.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike A. C., Brzozowski A. M., Hubbard R. E., Bonn T., Thorsell A. G., Engstrom O., Ljunggren J., Gustafsson J. A., Carlquist M. Structure of the ligand-binding domain of oestrogen receptor β in the presence of a partial agonist and a full antagonist. Embo J. 1999;18:4608–18. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poola I., Abraham J., Baldwin K., Saunders A., Bhatnagar R. Estrogen receptors beta4 and beta5 are full length functionally distinct ERbeta isoforms: cloning from human ovary and functional characterization. Endocrine. 2005;27:227–38. doi: 10.1385/ENDO:27:3:227. [DOI] [PubMed] [Google Scholar]
- Porter W., Saville B., Hoivik D., Safe S. Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol. 1997;11:1569–80. doi: 10.1210/mend.11.11.9916. [DOI] [PubMed] [Google Scholar]
- Pujol P., Rey J. M., Nirde P., Roger P., Gastaldi M., Laffargue F., Rochefort H., Maudelonde T. Differential expression of estrogen receptor-α and -β messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Res. 1998;58:5367–73. [PubMed] [Google Scholar]
- Razandi M., Pedram A., Greene G. L., Levin E. R. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–19. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Razandi M., Pedram A., Merchenthaler I., Greene G. L., Levin E. R. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18:2854–65. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- Rodriguez C., Patel A. V., Calle E. E., Jacob E. J., Thun M. J. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. Jama. 2001;285:1460–5. doi: 10.1001/jama.285.11.1460. [DOI] [PubMed] [Google Scholar]
- Rody A., Holtrich U., Solbach C., Kourtis K., von Minckwitz G., Engels K., Kissler S., Gatje R., Karn T., Kaufmann M. Methylation of estrogen receptor β promoter correlates with loss of ER-β expression in mammary carcinoma and is an early indication marker in premalignant lesions. Endocr Relat Cancer. 2005;12:903–16. doi: 10.1677/erc.1.01088. [DOI] [PubMed] [Google Scholar]
- Rutherford T., Brown W. D., Sapi E., Aschkenazi S., Munoz A., Mor G. Absence of estrogen receptor-β expression in metastatic ovarian cancer. Obstet Gynecol. 2000;96:417–21. doi: 10.1016/s0029-7844(00)00917-0. [DOI] [PubMed] [Google Scholar]
- Saville B., Wormke M., Wang F., Nguyen T., Enmark E., Kuiper G., Gustafsson J. A., Safe S. Ligand-, cell-, and estrogen receptor subtype (α/β)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem. 2000;275:5379–87. doi: 10.1074/jbc.275.8.5379. [DOI] [PubMed] [Google Scholar]
- Schildkraut J. M., Calingaert B., Marchbanks P. A., Moorman P. G., Rodriguez G. C. Impact of progestin and estrogen potency in oral contraceptives on ovarian cancer risk. J Natl Cancer Inst. 2002;94:32–8. doi: 10.1093/jnci/94.1.32. [DOI] [PubMed] [Google Scholar]
- Secreto F. J., Monroe D. G., Dutta S., Ingle J. N., Spelsberg T. C. Estrogen receptor α/β isoforms, but not betacx, modulate unique patterns of gene expression and cell proliferation in Hs578T cells. J Cell Biochem. 2007;101:1125–47. doi: 10.1002/jcb.21205. [DOI] [PubMed] [Google Scholar]
- Skliris G. P., Munot K., Bell S. M., Carder P. J., Lane S., Horgan K., Lansdown M. R., Parkes A. T., Hanby A. M., Markham A. F., Speirs V. Reduced expression of oestrogen receptor β in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J Pathol. 2003;201:213–20. doi: 10.1002/path.1436. [DOI] [PubMed] [Google Scholar]
- Stauffer S. R., Coletta C. J., Tedesco R., Nishiguchi G., Carlson K., Sun J., Katzenellenbogen B. S., Katzenellenbogen J. A. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem. 2000;43:4934–47. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Stossi F., Barnett D. H., Frasor J., Komm B., Lyttle C. R., Katzenellenbogen B. S. Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) α or ERbeta in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology. 2004;145:3473–86. doi: 10.1210/en.2003-1682. [DOI] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989;59:477–87. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Treeck O., Pfeiler G., Mitter D., Lattrich C., Piendl G., Ortmann O. Estrogen receptor {β}1 exerts antitumoral effects on SK-OV-3 ovarian cancer cells. J Endocrinol. 2007;193:421–33. doi: 10.1677/JOE-07-0087. [DOI] [PubMed] [Google Scholar]
- Tremblay G. B., Tremblay A., Copeland N. G., Gilbert D. J., Jenkins N. A., Labrie F., Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor β. Mol Endocrinol. 1997;11:353–65. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- Tremblay A., Tremblay G. B., Labrie F., Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor β through phosphorylation of activation function AF-1. Mol Cell. 1999;3:513–9. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- Umetani N., Mori T., Koyanagi K., Shinozaki M., Kim J., Giuliano A. E., Hoon D. S. Aberrant hypermethylation of ID4 gene promoter region increases risk of lymph node metastasis in T1 breast cancer. Oncogene. 2005;24:4721–7. doi: 10.1038/sj.onc.1208538. [DOI] [PubMed] [Google Scholar]
- Wada-Hiraike O., Imamov O., Hiraike H., Hultenby K., Schwend T., Omoto Y., Warner M., Gustafsson J. A. Role of estrogen receptor β in colonic epithelium. Proc Natl Acad Sci U S A. 2006;103:2959–64. doi: 10.1073/pnas.0511271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf A. A., Frye C. A. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Weihua Z., Saji S., Makinen S., Cheng G., Jensen E. V., Warner M., Gustafsson J. A. Estrogen receptor (ER) β, a modulator of ERalpha in the uterus. Proc Natl Acad Sci U S A. 2000;97:5936–41. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q., Lin Z., Cheng Y. H., Huang C. C., Marsh E., Yin P., Milad M. P., Confino E., Reierstad S., Innes J., Bulun S. E. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77:681–7. doi: 10.1095/biolreprod.107.061804. [DOI] [PubMed] [Google Scholar]
- Xu L., Pan-Hammarstrom Q., Forsti A., Hemminki K., Hammarstrom L., Labuda D., Gustafsson J. A., Dahlman-Wright K. Human estrogen receptor β 548 is not a common variant in three distinct populations. Endocrinology. 2003;144:3541–6. doi: 10.1210/en.2002-0118. [DOI] [PubMed] [Google Scholar]
- Zhao C., Matthews J., Tujague M., Wan J., Strom A., Toresson G., Lam E. W., Cheng G., Gustafsson J. A., Dahlman-Wright K. Estrogen receptor beta2 negatively regulates the transactivation of estrogen receptor α in human breast cancer cells. Cancer Res. 2007;67:3955–62. doi: 10.1158/0008-5472.CAN-06-3505. [DOI] [PubMed] [Google Scholar]
- Zhao C., Lam E. W., Sunters A., Enmark E., De Bella M. T., Coombes R. C., Gustafsson J. A., Dahlman-Wright K. Expression of estrogen receptor β isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene. 2003;22:7600–6. doi: 10.1038/sj.onc.1207100. [DOI] [PubMed] [Google Scholar]
- Zhu X., Leav I., Leung Y. K., Wu M., Liu Q., Gao Y., McNeal J. E., Ho S. M. Dynamic regulation of estrogen receptor-β expression by DNA methylation during prostate cancer development and metastasis. Am J Pathol. 2004;164:2003–12. doi: 10.1016/s0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou A., Marschke K. B., Arnold K. E., Berger E. M., Fitzgerald P., Mais D. E., Allegretto E. A. Estrogen receptor β activates the human retinoic acid receptor α-1 promoter in response to tamoxifen and other estrogen receptor antagonists, but not in response to estrogen. Mol Endocrinol. 1999;13:418–30. doi: 10.1210/mend.13.3.0253. [DOI] [PubMed] [Google Scholar]