Summary
Ovarian hormones regulate prepulse inhibition (PPI) of the acoustic startle reflex. Results from studies in intact female rodents investigating sex, estrous cycle and ovarian hormone regulation of PPI are inconsistent. In experiment #1, we investigated whether PPI in female rats is influenced by the time of day of testing and the estrous cycle stage of the rat. PPI was examined across the day of proestrus (P) and diestrus 1 (D1) in female rats and compared to males. PPI in males and P females was significantly higher than in D1 females. PPI in males and D1 females was significantly affected by the time of day of testing with PPI being reduced in the afternoon and evening compared to morning. PPI in P females was not significantly affected by the time of day of testing. Previous studies have demonstrated estrous cycle regulation of central nervous system neurotensin (NT) neurons and peripherally administered NT receptor agonists regulate PPI in a manner similar to antipsychotic drugs. Experiment #2 of this study was designed to examine whether endogenous NT is involved in estrous cycle regulation of PPI. The NT receptor antagonist SR 142948A reduced the high levels of PPI during D1 and P. In contrast, when tested at a time of day in which PPI was low in D1 females, administration of both the typical antipsychotic drug haloperidol and the NT receptor antagonist significantly increased PPI. These data support an effect of time of day and estrous cycle stage on PPI in female rats. The estrous cycle variations in PPI are mediated in part by endogenous NT.
Keywords: sensorimotor gating, schizophrenia, ovarian hormones, proestrus, diestrus, antipsychotic drug
Introduction
Prepulse inhibition (PPI) of the acoustic startle reflex (ASR) is one measure commonly used to assess sensorimotor gating. The ASR is an animal’s defensive response to a sudden, intense acoustic stimulus, and PPI refers to the reduction in ASR amplitude when the acoustic stimulus (pulse) is preceded 30–500 msec by a weak non-startling prestimulus (prepulse). PPI is sexually dimorphic, with women consistently reported to be less inhibited by weak prepulses than men (Aasen et al., 2005; Della Casa et al., 1998; Kumari et al., 2004; Swerdlow et al., 1993; Swerdlow et al., 1995; Swerdlow et al., 1999; Swerdlow et al., 1997). In addition, PPI varies across the menstrual cycle (Jovanovic et al., 2004; Swerdlow et al., 1997).
Results from rodent studies investigating sex, estrous cycle and ovarian hormone regulation of PPI are less consistent (Bubeníková et al., 2005; Gogos and Van den Buuse, 2004; Gulinello et al., 2003; Koch, 1998; Plappert et al., 2005; Rupprecht et al., 1999; Vaillancourt et al., 2002; Van den Buuse and Eikelis, 2001). One explanation for the varied results of PPI studies in female rats is the significant intra-day variation in hormone levels across the female rats’ 4–5 day estrous cycle (Gorski et al., 1975) (figure 1A). In experiment #1, we investigated whether PPI in female rats is influenced by the time of day of testing and the estrous cycle stage of the rat. PPI was examined across the day of proestrus (P) and diestrus 1 (D1) in female rats and compared to males. We report that the time of day of PPI testing critically affects PPI in both male and female rats and that the effect of time of day on PPI in female rats is dependent on estrous cycle stage.
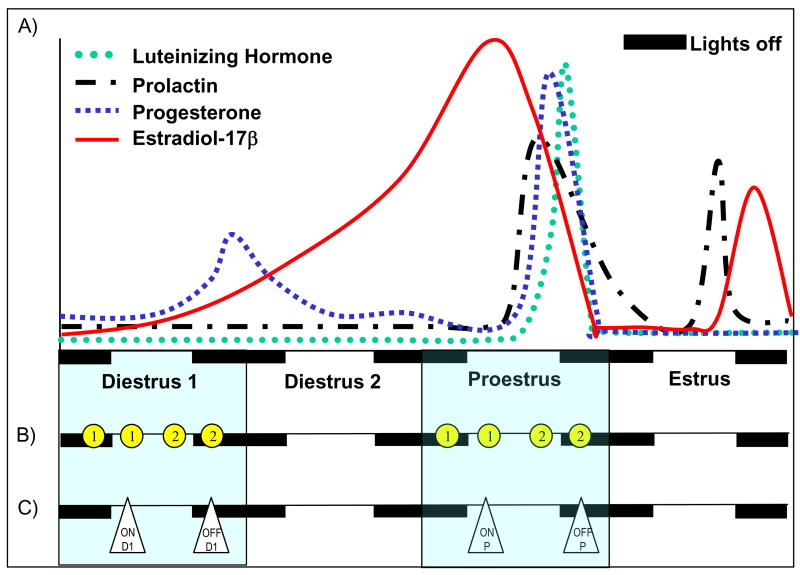
Figure 1.
Experimental design. A) Stylized diagram of ovarian hormone levels across a 4 day estrous cycle in the rat (based on data from Gorski, 1975). B) Design of experiment #1: Baseline PPI in male and female rats. Two groups of animals were housed in adjacent rooms (5 males and 20 females per room). The rooms were maintained on 12h:12h light/dark cycles with the lights either on (Group 1 rats) or off (Group 2 rats) at 10:00 hrs. Each female rat was tested for PPI a total of 4 times: at 07:00 during D1 and P and at 13:00 hrs during D1 and P. ➀ Represents the time points of testing for rats housed on the light cycle with lights on at 10:00 hrs. ➁ Represents the time points of testing for rats housed on the light cycle with lights off at 10:00 hrs. There was at least a 3 day interval between test sessions. All male rats were tested for PPI a total of 6 times, 3 times at 07:00 hrs and 3 times at 13:00 hrs, with at least a 3 day interval between test sessions (not shown in figure). C) Design of experiment #2: Effect of the NTR antagonist and haloperidol on PPI in female rats. Animals were housed in adjacent rooms (20 females per room) maintained on 12h:12h light/dark cycles with the lights either on (ON) or off (OFF) at 10:00 hrs. All PPI testing was completed at 13:00 hrs. Females in each room were tested either during D1 or P. In the figure, ON-D1, OFF-D1, ON-P or OFF-P represent the time points of testing for each group of animals. Each animal within a group was tested for PPI a total of 4 times. The test sessions consisted of 1) baseline determination of PPI, 2) a single injection of haloperidol (0.1 mg/kg, i.p.) 30 minutes before PPI testing, 3) baseline PPI and 4) a single injection of the neurotensin receptor antagonist SR 142948A (0.1 mg/kg, i.p.) 1 hour before PPI testing. There was at least a 3 day interval between test sessions.
We then investigated a likely neurotransmitter substrate mediating estrous cycle regulation of PPI. The neuropeptide neurotensin (NT) is hypothesized to be an endogenous antipsychotic (Nemeroff, 1980). NT is regulated across the estrous cycle of rats in brain regions involved in PPI (Kinkead et al., 2000), and NT receptor (NTR) agonists regulate PPI in a manner similar to antipsychotic drugs (Feifel et al., 2003; 2004; Feifel et al., 1997; Feifel et al., 1999; Shilling et al., 2004; Shilling et al., 2003). It was therefore hypothesized that endogenous NT systems may mediate estrous cycle regulation of PPI. In experiment #2 of this study, we examined the effect of an NT receptor antagonist (SR 142948A) on PPI in D1 and P female rats. We report evidence supporting endogenous NT systems in regulation of estrous cycle regulation of PPI.
Antipsychotic drugs restore pharmacologic, environmental and genetic deficits in PPI (Geyer et al., 2001; Geyer et al., 2002). The effects of antipsychotic drugs across the estrous cycle of the female rat are unknown. Previously we have demonstrated that haloperidol increases NT tissue concentrations in the nucleus accumbens during D1, but not P (Kinkead et al., 2000). Based on these findings, we hypothesized that the effects of haloperidol on NT tissue concentrations in the nucleus accumbens would predict the effects of haloperidol on PPI in female rats such that, when haloperidol does not significantly increase NT tissue concentrations (P), haloperidol would not significantly increase PPI and that when haloperidol increased NT tissue concentrations (D1), haloperidol would also increase PPI. Experiment #2 of this study also examined the effect of haloperidol on PPI in D1 and P female rats. The results of this study demonstrated that the effect of haloperidol on PPI in female rats was dependent on the time of day and estrous cycle stage at the time of testing.
Methods
Animals
Male and Female Long-Evans rats (250–275g upon arrival, Charles River, Wilmington, MA) were housed in same sex groups of two or three with food and water provided ad libitum. All animals were handled individually within 3 days of arrival and daily thereafter. All animals were allowed to acclimate to the reverse light:dark cycle for 2 weeks before determination of estrous cycle stage. Vaginal smears were taken in order to determine the estrous cycle stage of the female rats. Based on the cell type and number in the smear, the rat was determined to be in diestrus 1 (D1), diestrus 2 (D2), proestrus (P) or estrus (E). Only those rats demonstrating two consecutive estrous cycles were included in the study. Animal protocols were approved by the Emory Institutional Animal Care and Use Committee and the “Principles of laboratory animal care” (http://www.nap.edu/readingroom/books/labrats/) were followed in compliance with NIH (http://grants.nih.gov/grants/olaw/olaw.htm) recommendations based on National Research Council guidelines (Research, 2003).
Drugs
The NT receptor antagonist SR 142948A (2-[[5-(2,6-dimethoxyphenyl)-1-(4-(N-(3-dimethylaminopropyl)-N-methylcarbamoyl)-2-iso propylphenyl)-1H-pyrazole3-carbonyl]amino] adamantane-2-carboxylic acid, hydrochloride) was a generous gift of Sanofi Recherche (Toulouse, France). SR 142948A was suspended in several drops of Tween 20 and brought to volume with 0.9% NaCl (drug vehicle = 0.9% NaCl plus several drops of Tween 20). Haloperidol (Sigma Chemical Co., St. Louis, MO) was dissolved in 0.3% tartaric acid. All drugs were administered in a fixed volume of 1.0 ml/kg body weight.
PPI Testing Procedure
PPI testing was performed in a ventilated startle chamber (San Diego Instruments, San Diego, CA). Startle amplitude was measured by converting the vibrations of a Plexiglas cylinder (resting platform) caused by the rat startle response into analogue signals by a piezoelectric unit. These signals were then digitized, represented as arbitrary startle units and stored in a personal computer. The testing session began with 5 min acclimatization to the startle chamber in the presence of 65 db background white noise. Testing consisted of eleven 120 db pulses alone and 18 pulses preceded (100 ms) by a prepulse 4, 8, or 12 db above background. Pulses were presented in a pseudorandom order with an average of 15 s between trials. At the end of the 5 minute acclimation period, there were 4 pulse alone trials followed by 7 additional pulse alone trials, pseudorandomly distributed throughout the prepulse-pulse trials. Habituation was analyzed by comparison of average startle amplitudes in pulse alone startle trials #1–4 compared to trials #5–11. Because a large degree of habituation to the pulse alone startle occurs in the initial pulse alone trials, it is common practice to disregard the initial pulse alone trials in computation of PPI (Bubeníková et al., 2005; Gogos and Van den Buuse, 2004; Koch, 1998; Plappert et al., 2005; Swerdlow et al., 2000; Vaillancourt et al., 2002). Percent PPI was calculated and analyzed using the average amplitude of pulse alone trials #5–11 using the formula: %PPI= 100− (prepulse/pulse startle amplitude × 100/pulse alone startle amplitude of trials #5–11). The onset latency (latency from stimulus to commencement of startle) was reported in msec. The peak latency (latency from stimulus to maximum startle amplitude) was defined as the point of maximal amplitude occurring within 150 msec of the pulse alone stimulus. Peak latency facilitation was calculated using the following formula: peak pulse alone latency-peak prepulse/pulse latency.
Experiment #1: Baseline characterization of pulse alone startle amplitude and PPI in male and female rats
Upon arrival at the animal facility animals were housed in adjacent rooms (5 males and 20 females per room) and maintained on 12h:12h light/dark cycles with the lights either on or off at 10:00 hrs. The design of this experiment is shown in Figure 1B. The estrous cycle stage of the 40 female rats was determined daily between 06:30 and 06:50 hrs. Each female rat was tested for PPI a total of 4 times: at 07:00 during D1 and P and at 13:00 hrs during D1 and P. There was at least a 3 day interval between test sessions. PPI testing was completed within a one-hour time frame. All male rats were tested for PPI a total of 6 times, 3 times at 07:00 hrs and 3 times at 13:00 hrs, with at least a 3 day interval between test sessions. For graphical purposes, time of day relative to lights on at 10:00 hrs is used (testing done at 07:00 hrs with lights off =07:00 hrs, 13:00 hrs with lights on =13:00 hrs, 07:00 hrs with lights on=19:00 hrs, 13:00 hrs with lights off =01:00 hrs). Both time of day and estrous cycle stage were within subject factors in this experiment.
Experiment #2: Examination of the effects of the neurotensin receptor antagonist SR 142948A and haloperidol on pulse alone startle amplitude and PPI in P and D1 female rats
Upon arrival at the animal facility animals were housed in adjacent rooms (20 females per room) in groups of 3 and maintained on 12h:12h light/dark cycles with the lights either on or off at 10:00 hrs. The design of this experiment is shown in Figure 1C. The estrous cycle stage of the forty female rats was determined daily between 09:00 and 10:00 hrs. All PPI testing was completed within a one-hour time frame around 13:00 hrs. In contrast to experiment #1 in which time of day and estrous cycle stage were within subject factors, females in experiment #2 were assigned to a specific group (ON-D1, ON-P, OFF-D1 or OFF-P). Each animal within a group was PPI-tested a total of 4 times with all animals receiving the same drug treatments. The test sessions consisted of 1) baseline determination of PPI (single injection of 0.9% NaCl + drops Tween 20 (SR 142948A vehicle), 2) a single injection of haloperidol (0.1 mg/kg, i.p.) 30 minutes before PPI testing, 3) baseline determination of PPI (single injection of 0.9% NaCl + drops Tween 20 (SR 142948A vehicle) and 4) a single injection of the neurotensin receptor antagonist SR 142948A (0.1 mg/kg, i.p.) 1 hour before PPI testing. In this experiment, drug treatment was a within subject factor and time of day and estrous cycle stage were between subject factors.
Data Analysis
Data are expressed as mean ± SEM. Multivariate repeated measures analyses of variance (ANOVA) were performed where appropriate. Male rats were tested 3 times at two time points (07:00 hrs and 13:00 hrs). Pulse alone startle amplitude in male rats was first analyzed by two-way repeated measures ANOVA (test session × light cycle) with test session the within subject factor and light cycle the between subjects factor. In the absence of a significant effect of test session, the pulse alone startle amplitudes from the three test sessions at each time point were averaged in order to generate two pulse alone startle values for each rat (one at 07:00 and one at 13:00 hrs). Similar analyses were run for pulse alone startle amplitudes of trials #1–4 and #5–11 and PPI.
For pulse-alone startle amplitude, onset latency and peak latency, main effects of sex, estrous cycle stage, treatment, time of day, and interactions were examined where appropriate. For PPI, the main effects of sex, estrous cycle stage, treatment, time of day, prepulse intensity, and interactions between the factors were examined as appropriate. In studies examining the effects of haloperidol and the NTR antagonist on PPI, differences between baseline PPI in test weeks 1 and 3 were first examined. In the absence of significant differences between baseline values within estrous cycle stage in test weeks 1 and 3, data from these two test sessions were averaged in order to generate one control value for each rat. The Student-Newman-Keuls multiple comparisons test was used for all post hoc analyses. α level was 0.05 (SPSS for Windows, SPSS Inc., Chicago, Illinois).
Results
Baseline measurement of pulse alone amplitude in male and female rats
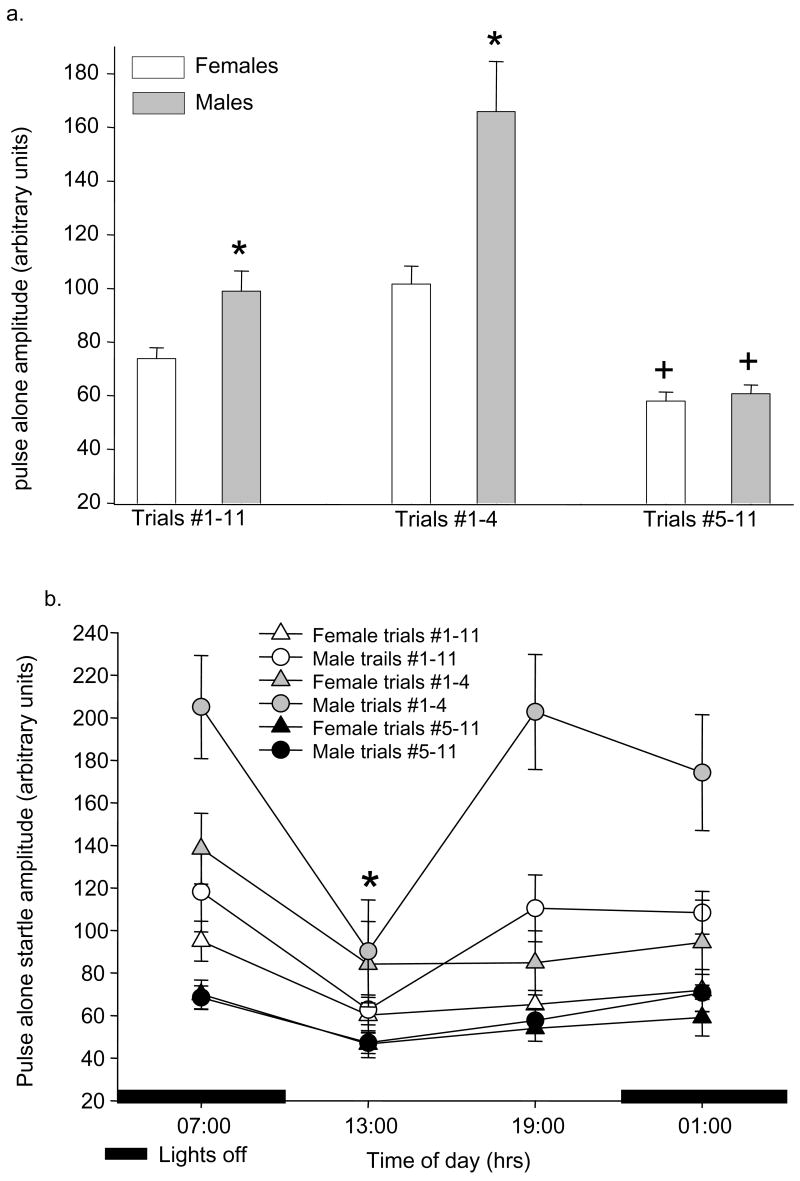
In each PPI test session there are 11 pulse alone trials. At the end of the 5 minute acclimation period, the pulse trials begin with 4 pulse alone trials followed by 7 additional pulse alone trials, pseudorandomly distributed throughout the prepulse-pulse trials. Analysis of all 11 pulse alone trials demonstrated a significant effect of sex (F1,168=18.4, p<0.001) on pulse alone startle amplitude with males having significantly greater pulse alone startle than females (figure 2a). When pulse alone trials were grouped by trials #1–4 vs. trials #5–11, there was a significant effect of trial # (F1,337=81.6, p<0.001), and significant interactions between trial # and sex (F1,337=14.8, p<0.001) and trial # and time of day (F3,337=2.7, p<0.05). Males had significantly higher pulse alone startle amplitudes than females in trials #1–4, but not trials #5–11 (Figure 2a). In both male and female rats, there was a significant decrease in pulse alone startle amplitude (trials #1–11, trials #1–4 and trials #5–11) 3 hours after lights on (13:00 time point, Figure 2b). There were no significant effects of estrous cycle stage on any measure of pulse alone startle amplitude.
Figure 2.
Baseline pulse alone startle amplitude in male and female rats. All data shown are mean ± SEM. (2a) Startle amplitudes are shown for pulse alone trials #1–11 (all trials), trials #1–4 and trials #5–11. * p< 0.001 compared to females in same trials. + p<0.05 compared to same sex in trials #1–4. (2b) Pulse alone startle amplitude across the day in male and female rats. * p<0.05 compared to same sex at 07:00 hrs.
Baseline measurement of prepulse inhibition in male and female rats
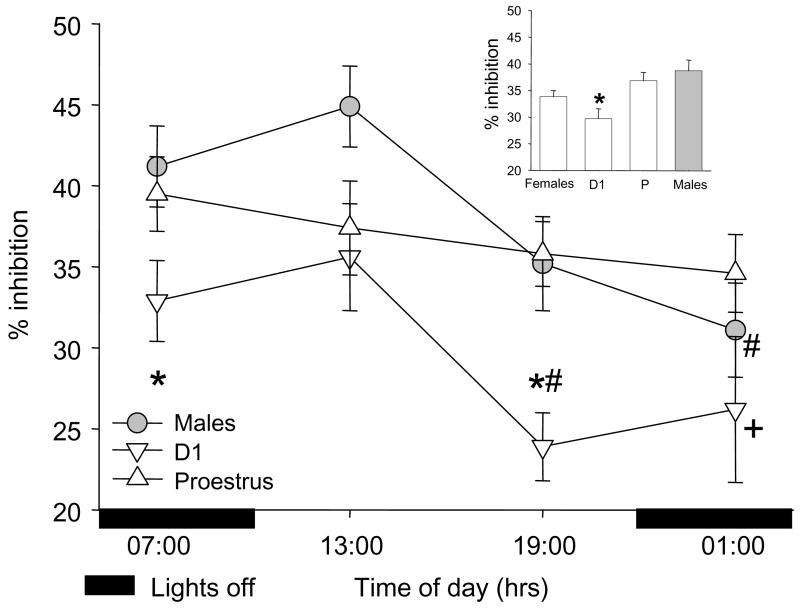
There was a significant effect of prepulse intensity (F2,506=46.1, p<0.001) on PPI in all analyses, but no significant interactions between prepulse intensity and any other factor. Because a large degree of habituation to the pulse alone startle occurs in the initial pulse alone trials, the initial pulse alone trials (#1–4) were not used in computation of PPI and because all of the sex differences in pulse alone startle amplitude were due to higher male pulse alone startle in trials #1–4, % PPI was calculated and analyzed using the average amplitude of pulse alone trials #5–11. The effect of sex (F1,506=3.5, p=.06) on PPI approached significance with males having greater PPI than females. The sex effect was due to PPI being significantly lower in D1 females than P females or males (cycle F2,506=5.1, p<0.05). There was a significant time of day effect on PPI (F3,506=3.8, p<0.05) with both males and D1 females demonstrating a significant decrease in PPI across the day (Figure 3). Because of previous studies reporting a lack of circadian regulation of PPI in male rats, the effect of light cycle (lights on or off without regard to time of day) on PPI was also examined. In agreement with these previous studies, there were no significant differences between PPI in males with lights on (overall PPI=40.0 ± 3.0) compared to lights off (overall PPI 36.0 ± 3.1).
Figure 3.
The effect of time of day on prepulse inhibition of acoustic startle in male and female rats. Because there were no significant interactions between prepulse intensity and any other factor, data shown are mean ± SEM collapsed across prepulse intensity. (4 inset) PPI is significantly decreased in diestrus 1 (D1) females compared to proestrus (P) and males. * p<0.05 D1 compared to P and males. + p<0.05 D1 compared to P. #p<0.05 compared to 13:00 hrs for same sex or estrous cycle stage.
Baseline measurement of onset latency, peak latency and latency facilitation in male and female rats
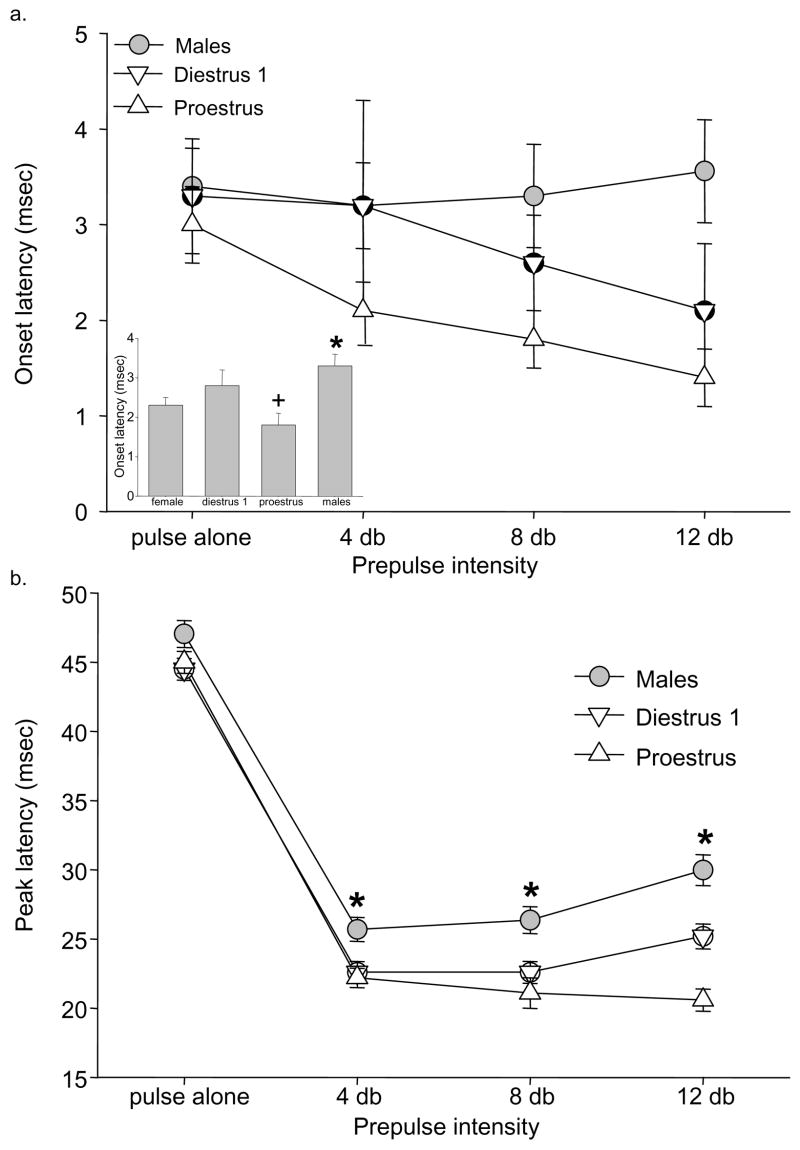
Because of the possibility that alterations in PPI across the estrous cycle are due to endocrine-mediated changes in acoustic threshold that alter “detectability”, sex differences in onset latency, peak latency and latency facilitation were examined. As previously described (Swerdlow et al., 1997), there was a significant effect of prepulse intensity on peak latency (F2,506=10.3, p<0.001) and peak latency facilitation (F2,506=6.0, p<0.01). There was a significant effect of sex on onset latency (F1,506=6.6, p<0.01) and peak latency (F1,506=34.9, p<0.001) with males having significantly longer onset (figure 4a) and peak latencies (figure 4b) than females. The effect of sex on onset latency was due to onset latency being significantly shorter in P females compared to D1 females and males (cycle F2,506=6.5, p<0.01). Estrous cycle did not significantly affect peak latency. There were no significant effects of sex or cycle on latency facilitation.
Figure 4.
Baseline onset latency and peak latency in male and female rats. (4a) The effect of prepulse intensity on onset latency in males and females in diestrus 1 and proestrus. Males have significantly longer onset latencies than female rats in proestrus (4a inset). All data shown are mean ± SEM. * p<0.05 compared to females. + p<0.05 compared to males and females in diestrus. (4b) The effect of prepulse intensity on peak latency in males and females in diestrus 1 and proestrus. Males have significantly longer onset latencies than female rats in proestrus and diestrus 1. * p<0.05 compared to diestrus 1 and proestrus at same prepulse intensity.
The effect of haloperidol or the neurotensin receptor antagonist SR 142948A on pulse alone startle amplitude in P and D1 female rats
There was no significant difference in pulse alone startle amplitude between test sessions after vehicle administration (test sessions 1 and 3, data not shown). Therefore, for further analysis, pulse alone startle values in these two test sessions were averaged for each rat in order to generate one control pulse alone startle amplitude value for each animal. There was a significant effect of time of day (F1,238=3.9, p<0.05) on pulse alone startle amplitude with amplitude being significantly lower at 01:00 (lights off) compared to 13:00 (lights on, data not shown). There was no significant effect of estrous cycle stage or treatment on any measure of pulse alone startle amplitude.
The effect of haloperidol or the neurotensin receptor antagonist SR 142948A on % prepulse inhibition of acoustic startle in female rats during diestrus 1 or proestrus
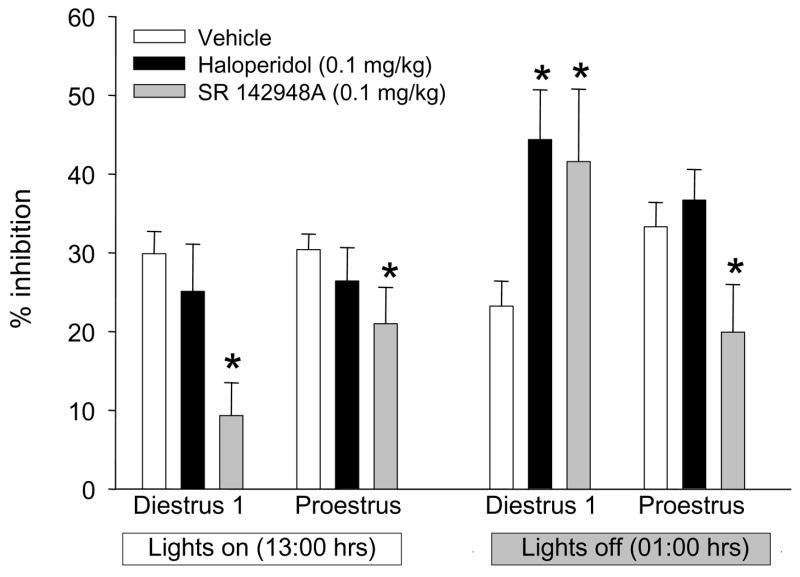
There was a significant effect of prepulse intensity (F2,716=25.5, p<0.001) on PPI in all analyses, but no significant interactions between prepulse intensity and any other factor. There was a significant effect of time of day (F1,716=12.2, p<0.001) on PPI. The NTR antagonist significantly decreased PPI during D1 and P at 13:00 hrs, and decreased PPI during P at 01:00 hrs (Figure 5). In contrast, both haloperidol and the NTR antagonist significantly increasing PPI during D1 at 01:00 hrs.
Figure 5.
The effect of haloperidol (0.1 mg/kg) or the neurotensin receptor antagonist SR 142948A (0.1 mg/kg) on prepulse inhibition of the acoustic startle response 2–3 hours after lights on (13:00) or off (01:00) in diestrus 1 and proestrus female rats. Because there were no significant interactions between prepulse intensity and any other factor, data shown are mean ± SEM collapsed across prepulse intensity. * p < 0.05 compared to vehicle (0.9 % NaCl + drops Tween 20) in same estrous cycle stage and light cycle.
Discussion
The present studies demonstrate significant effects of estrous cycle stage and time of day on PPI. Our original hypothesis based on the rapidly changing hormone levels during P, was that PPI during D1 and in males would be stable across the day and PPI during P would fluctuate with changing hormone levels. Surprisingly, the reverse was found, with stable PPI across the day of P, and PPI decreasing significantly across the day of D1 and in males. The similar pattern of PPI across the day in males and D1 females suggest diurnal regulation of PPI unrelated to fluctuations in ovarian hormones.
In agreement with previous studies we found enhanced acoustic startle response during the dark compared to the light cycle (Chabot and Taylor, 1992a; b; Frankland and Ralph, 1995; Ison et al., 1991) and in male compared to female rats (Melnick et al., 2002; Plappert et al., 2005). Although it is possible that the circadian variation in PPI seen in males and D1 females is due in part to circadian regulation of acoustic startle, it is unlikely that the stabilization of PPI during P is due to the effects of ovarian hormones on the acoustic startle response as significant effects of estrous cycle stage on PPI are present in the absence of any difference in pulse alone startle amplitudes in either D1 or P female rats at any given time point.
Despite the prediction of circadian regulation of PPI in male rats based on the activity dependent reduction in the ability of a prepulse to inhibit a subsequent startle response as reported by Wecker and Ison (1986), circadian regulation of PPI in male rats was not found when PPI was measured in a 4 hour time frame between 2–6 hours after lights on or off (Weiss et al., 1999). In accordance with these results, we report no significant overall effect of light status (i.e. on or off) on PPI in male rats. However, we do find a significant effect of time of day on PPI testing in the current study when PPI testing is completed within a 1 hr window around the specified time of testing. The significant difference in PPI in male rats 3 hours after lights on compared to lights off demonstrates the necessity of examining circadian regulation of PPI within shorter time frames.
Men are reported to be more inhibited by weak prepulses than women (Abel et al., 1998; Rahman et al., 2003; Swerdlow et al., 1993; Swerdlow et al., 1997). In the current study, there was a non-significant trend (p=0.06) for PPI in males to be greater than females. Previous studies in rodents report either no difference in PPI between males and females (Bubeníková et al., 2005; Plappert et al., 2005; Swerdlow et al., 1993) or PPI in males greater than females (Melnick et al., 2002). Conflicting results are most likely due to the unknown estrous cycle stage of female rats at the time of testing (e.g. more females in P than D1 would lead to no significant difference in PPI between males and females) and the time of day of testing. The near significant difference in PPI between male and female rats in the current study was due to the significant decrease in PPI across the day in D1 females compared to P females. At 13:00 hrs (3 hours after lights on), there was no significant difference in PPI between males, D1 females or P females. These results are in agreement with previous studies most likely conducted with lights on (time frame unreported except for Gulinello et al. 2003) in which PPI during D1 and P were not found to be significantly different (Bubeníková et al., 2005; Gulinello et al., 2003; Plappert et al., 2005) or PPI in D1 females was greater than P females (Koch, 1998). In contrast, at 01:00 hrs (3 hrs after lights off) in the current study, PPI in males and P females was significantly greater than PPI in D1 females.
An alternate hypothesis to ovarian hormone regulation of PPI is provided by Swerdlow et al, (1997). It is possible that alterations in PPI across the estrous cycle are due to endocrine-mediated changes in acoustic threshold at a sensorineural level that alters “detectability”. It is posited that a reduction in auditory sensitivity would blunt the effectiveness of a weak prepulse in inhibiting reflex amplitude and the effectiveness of the prepulse in reducing reflex latency, leading to a change in latency facilitation. In the current study, males had significantly delayed onset and peak latencies compared to females, with no significant difference between the sexes in peak latency facilitation. These data would argue against the PPI effects of ovarian hormones being due to diminished auditory sensitivity in females. The effect of sex on onset latency was due to onset latency being significantly reduced in P females compared to D1 females and males. This estrous cycle effect on onset latency was not associated with the magnitude of the startle response as there was no difference in startle response amplitude between D1 and P female rats. In contrast to the onset latency, peak latency is not associated with startle magnitude (Cadenhead et al., 1999). In addition to males having significantly delayed peak latencies compared to females, peak latency in males (but not females) was reduced when lights were off compared to lights on. Although there were no significant differences in peak latency between P and D1 females, it is possible that the changes in peak latency across the day in males are related to the circadian regulation of both pulse alone startle amplitude and PPI seen in males and D1 females.
The neuropeptide NT has been posited to be an endogenous antipsychotic (Nemeroff, 1980). The ability of peripherally administered NT receptor agonists to increase baseline levels of PPI (Feifel et al., 2004; Shilling et al., 2003) and data demonstrating estrous cycle regulation of the NT system (Kinkead et al., 2000) led to the hypothesis that NT may be involved in estrous cycle regulation of PPI. Elevated levels of PPI in female rats were found to be NT dependent because administration of the NT receptor antagonist SR 142948A decreased elevated PPI in both D1 and P rats. In contrast, at a time point when PPI was significantly lower in D1 rats compared to P, the NT receptor antagonist significantly increased PPI in D1 females. Similar to pharmacologic, genetic or environmental disruption of PPI, haloperidol increased low levels of PPI in D1 but had no effect on baseline levels of PPI at any other time point.
The finding that the NT receptor antagonist both increases low levels of PPI and decreases high levels of PPI indicates a dual role of endogenous NT in the regulation of PPI. One possible explanation is differential estrous cycle regulation of endogenous NT peptide levels within multiple brain regions involved in regulation of PPI, including the nucleus accumbens and the ventral tegmental area (Swerdlow et al., 2001). NT is closely associated with central nervous system dopamine systems and in general acts to decrease dopaminergic activity at D2 dopamine receptors (for review see Binder et al, 2001). Although injection of NT into the ventral tegmental area does not significantly affect baseline PPI in male rats (Feifel and Reza, 1999), it is possible that elevated levels of NT in this brain region during D1 (Kinkead et al., 2000) and consequent increased NT stimulation of dopaminergic projections to the nucleus accumbens contribute to the decrease in PPI levels across the day of D1. Alternately, the role of NT in estrous cycle regulation of PPI may not be dependent on modulation of NT levels, but may be due to the effects of comparable levels of NT in the presence of estrous cycle related regulation of other neurotransmitter systems, including dopamine (Morissette and Di Paolo, 1993; Thompson and Moss, 1997; Xiao and Becker, 1994).
In summary, circadian fluctuations in PPI across the day of P are reduced compared to males and D1 females. Elevated PPI levels during P and D1 are dependent on NT neurotransmission supporting a PPI enhancing role of NT. However, the finding that blockade of NT receptors during low D1 PPI increases PPI (similar to the antipsychotic drug haloperidol) indicates that the role of NT in regulation of PPI in female rats is not straightforward and most likely reflect brain region specific regulation of the NT system. Based on the present findings, sex, hormone status and time of day of PPI testing are important variables in studies examining sensorimotor gating in female rats.
Acknowledgments
Funding for this study was provided by NIMH grants MH-39415 and MH-63400.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aasen I, Kolli L, Kumari V. Sex effects in prepulse inhibition and facilitation of the acoustic startle response: implications for pharmacological and treatment studies. Journal of Psychopharmacology. 2005;19:39. doi: 10.1177/0269881105048890. [DOI] [PubMed] [Google Scholar]
- Abel K, Waikar M, Pedro B, Hemsley D, Geyer M. Repeated testing of prepulse inhibition and habituation of the startle reflex: a study in healthy human controls. Journal of Psychopharmacology. 1998;12:330. doi: 10.1177/026988119801200402. [DOI] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacological Reviews. 2001;53:453. [PubMed] [Google Scholar]
- Bubeníková V, Votava M, Horácek J, Pálenícek T. Relation of sex and estrous phase to deficits in prepulse inhibition of the startle response induced by ecstasy (MDMA) Behavioural Pharmacology. 2005;16:127. doi: 10.1097/00008877-200503000-00009. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Carasso BS, Swerdlow NR, Geyer MA, Braff DL. Prepulse inhibition and habituation of the startle response are stable neurobiological measures in a normal male population. Biological Psychiatry. 1999;45:360. doi: 10.1016/s0006-3223(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Taylor DH. Circadian modulation of the rat acoustic startle response. Behavioral Neuroscience. 1992a;106:846. doi: 10.1037//0735-7044.106.5.846. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Taylor DH. Daily rhythmicity of the rat acoustic startle response. Physiology & Behavior. 1992b;51:885. doi: 10.1016/0031-9384(92)90131-k. [DOI] [PubMed] [Google Scholar]
- Della Casa V, Hofer I, Weiner I, Feldon J. The effects of smoking on acoustic prepulse inhibition in healthy men and women. Psychopharmacology. 1998;137:362. doi: 10.1007/s002130050631. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. A systemically administered neurotensin agonist blocks disruption of prepulse inhibition produced by a serotonin-2A agonist. Neuropsychopharmacology. 2003;28:651. doi: 10.1038/sj.npp.1300083. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. Reversal of sensorimotor gating deficits in Brattleboro rats by acute administration of clozapine and a neurotensin agonist, but not haloperidol: a potential predictive model for novel antipsychotic effects. Neuropsychopharmacology. 2004;29:731. doi: 10.1038/sj.npp.1300378. [DOI] [PubMed] [Google Scholar]
- Feifel D, Minor KL, Dulawa S, Swerdlow NR. The effects of intra-accumbens neurotensin on sensorimotor gating. Brain Research. 1997;760:80. doi: 10.1016/s0006-8993(97)00306-5. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza TL. Effects of neurotensin administered into the ventral tegmental area on prepulse inhibition of startle. Behavioural Brain Research. 1999;106:189. doi: 10.1016/s0166-4328(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza TL, Wustrow DJ, Davis MD. Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. Journal of Pharmacology & Experimental Therapeutics. 1999;288:710. [PubMed] [Google Scholar]
- Frankland PW, Ralph MR. Circadian modulation in the rat acoustic startle circuit. Behavioral Neuroscience. 1995;109:43. doi: 10.1037//0735-7044.109.1.43. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Molecular Psychiatry. 2002;7:1039. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Gogos A, Van den Buuse M. Estrogen and progesterone prevent disruption of prepulse inhibition by the serotonin-1A receptor agonist 8-hydroxy-2-dipropylaminotetralin. Journal of Pharmacology & Experimental Therapeutics. 2004;309:267. doi: 10.1124/jpet.103.061432. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Mennin SP, Kubo K. The neural and hormonal bases of the reproductive cycle of the rat. Advances in Experimental Medicine & Biology. 1975;54:115. doi: 10.1007/978-1-4684-8715-2_6. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Orman R, Smith SS. Sex differences in anxiety, sensorimotor gating and expression of the alpha4 subunit of the GABAA receptor in the amygdala after progesterone withdrawal. European Journal of Neuroscience. 2003;17:641. doi: 10.1046/j.1460-9568.2003.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Bowen GP, Kellogg C. Potentiation of acoustic startle behavior in the rat (Rattus norvegicus) at the onset of darkness. Journal of Comparative Psychology. 1991;105:3. doi: 10.1037/0735-7036.105.1.3. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Szilagyi S, Chakravorty S, Fiallos AM, Lewison BJ, Parwani A, Schwartz MP, Gonzenbach S, Rotrosen JP, Duncan EJ. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology. 2004;41:401. doi: 10.1111/1469-8986.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Kinkead B, Lorch LM, Owens MJ, Nemeroff CB. Sex- and estrous cycle-related differences in the effects of acute antipsychotic drug administration on neurotensin-containing neurons in the rat brain. Journal of Pharmacology and Experimental Therapeutics. 2000;295:205. [PubMed] [Google Scholar]
- Koch M. Sensorimotor gating changes across the estrous cycle in female rats. Physiology & Behavior. 1998;64:625. doi: 10.1016/s0031-9384(98)00098-5. [DOI] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Sharma T. Sex differences in prepulse inhibition deficits in chronic schizophrenia. Schizophrenia Research. 2004;69:219. doi: 10.1016/j.schres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Melnick SM, Weedon J, Dow-Edwards DL. The effects of perinatal AZT exposure on the acoustic startle response in adult rats. Neurotoxicology & Teratology. 2002;24:773. doi: 10.1016/s0892-0362(02)00317-3. [DOI] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993;58:16. doi: 10.1159/000126507. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurotensin: perchance an endogenous neuroleptic? Biological Psychiatry. 1980;15:283. [PubMed] [Google Scholar]
- Plappert CF, Rodenbucher AM, Pilz PK. Effects of sex and estrous cycle on modulation of the acoustic startle response in mice. Physiology & Behavior. 2005;84:585. doi: 10.1016/j.physbeh.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Kumari V, Wilson GD. Sexual orientation-related differences in prepulse inhibition of the human startle response. Behavioral Neuroscience. 2003;117:1096. doi: 10.1037/0735-7044.117.5.1096. [DOI] [PubMed] [Google Scholar]
- Research IfLA. Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- Rupprecht R, Koch M, Montkowski A, Lancel M, Faulhaber J, Harting J, Spanagel R. Assessment of neuroleptic-like properties of progesterone. Psychopharmacology. 1999;143:29. doi: 10.1007/s002130050916. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Melendez G, Priebe K, Richelson E, Feifel D. Neurotensin agonists block the prepulse inhibition deficits produced by a 5-HT2A and an alpha1 agonist. Psychopharmacology. 2004;175:353. doi: 10.1007/s00213-004-1835-5. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Richelson E, Feifel D. The effects of systemic NT69L, a neurotensin agonist, on baseline and drug-disrupted prepulse inhibition. Behavioural Brain Research. 2003;143:7. doi: 10.1016/s0166-4328(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Auerbach P, Monroe SM, Hartston H, Geyer MA, Braff DL. Men are more inhibited than women by weak prepulses. Biological Psychiatry. 1993;34:253. doi: 10.1016/0006-3223(93)90079-s. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Filion D, Geyer MA, Braff DL. "Normal" personality correlates of sensorimotor, cognitive, and visuospatial gating. Biological Psychiatry. 1995;37:286. doi: 10.1016/0006-3223(94)00138-S. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Hartman PL, Sprock J, Auerbach PP, Cadenhead K, Perry W, Braff DL. Sex differences in sensorimotor gating of the human startle reflex: all smoke? Psychopharmacology. 1999;146:228. doi: 10.1007/s002130051111. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biological Psychiatry. 1997;41:452. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Martinez ZA, Hanlon FM, Platten A, Farid M, Auerbach P, Braff DL, Geyer MA. Toward understanding the biology of a complex phenotype: rat strain and substrain differences in the sensorimotor gating-disruptive effects of dopamine agonists. Journal of Neuroscience. 2000;20:4325. doi: 10.1523/JNEUROSCI.20-11-04325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neuroscience Letters. 1997;229:145. doi: 10.1016/s0304-3940(97)00450-3. [DOI] [PubMed] [Google Scholar]
- Vaillancourt C, Cyr M, Rochford J, Boksa P, Di Paolo T. Effects of ovariectomy and estradiol on acoustic startle responses in rats, Pharmacology. Biochemistry & Behavior. 2002;74:103. doi: 10.1016/s0091-3057(02)00967-x. [DOI] [PubMed] [Google Scholar]
- Van den Buuse M, Eikelis N. Estrogen increases prepulse inhibition of acoustic startle in rats. European Journal of Pharmacology. 2001;425:33. doi: 10.1016/s0014-2999(01)01139-6. [DOI] [PubMed] [Google Scholar]
- Wecker JR, Ison JR. Effects of motor activity on the elicitation and modification of the startle reflex in rats. Animal Learning and Behavior. 1986;14:287. [Google Scholar]
- Weiss IC, Feldon J, Domeney AM. Circadian time does not modify the prepulse inhibition response or its attenuation by apomorphine, Pharmacology. Biochemistry & Behavior. 1999;64:501. doi: 10.1016/s0091-3057(99)00100-8. [DOI] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neuroscience Letters. 1994;180:155. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]