Abstract
The proton–sucrose symporter mediates the key transport step in the resource distribution system that allows many plants to function as multicellular organisms. In the results reported here, we identify sucrose as a signaling molecule in a previously undescribed signal-transduction pathway that regulates the symporter. Sucrose symporter activity declined in plasma membrane vesicles isolated from leaves fed exogenous sucrose via the xylem transpiration stream. Symporter activity dropped to 35–50% of water controls when the leaves were fed 100 mM sucrose and to 20–25% of controls with 250 mM sucrose. In contrast, alanine symporter and glucose transporter activities did not change in response to sucrose treatments. Decreased sucrose symporter activity was detectable after 8 h and reached a maximum by 24 h. Kinetic analysis of transport activity showed a decrease in Vmax. RNA gel blot analysis revealed a decrease in symporter message levels, suggesting a drop in transcriptional activity or a decrease in mRNA stability. Control experiments showed that these responses were not the result of changing osmotic conditions. Equal molar concentrations of hexoses did not elicit the response, and mannoheptulose, a hexokinase inhibitor, did not block the sucrose effect. These data are consistent with a sucrose-specific response pathway that is not mediated by hexokinase as the sugar sensor. Sucrose-dependent changes in the sucrose symporter were reversible, suggesting this sucrose-sensing pathway can modulate transport activity as a function of changing sucrose concentrations in the leaf. These results demonstrate the existence of a signaling pathway that can control assimilate partitioning at the level of phloem translocation.
Resource allocation between tissues is a fundamental process in all multicellular organisms. In higher plants, leaves function as the principle site of resource acquisition by utilizing the free energy captured in photosynthesis for the reductive assimilation of oxidized forms of carbon and nitrogen into carbohydrates and amino acids, respectively. These resources are subsequently distributed to the many heterotrophic tissues of the plant. Indeed, as much as 80% of the carbon acquired in photosynthesis is transported in the plant’s vascular system to the import-dependent organs. Sucrose is arguably the most important metabolite in this system of resource allocation because it is generally the major end product of photosynthetic carbon metabolism and, in most plants, it is the predominant form of carbon transported to the heterotrophic tissues (1, 2). Moreover, in many plants energy-dependent sucrose accumulation in the phloem generates the high hydrostatic pressure that drives the long-distance flow of resources. The systemic distribution of photosynthate is known as “assimilate partitioning,” and it is a major determinant of plant growth and productivity (3).
Our understanding of assimilate partitioning has advanced considerably over the last 10 years with the successful biochemical and molecular descriptions of several proteins that participate in this essential process. One of the key proteins described was the protonmotive-force-driven sucrose symporter that mediates sucrose accumulation in plants that utilize apoplastic phloem loading (4–6). The symporter is an electrogenic, secondary active transporter that couples sucrose transport to the proton electrochemical potential difference that exists across the plasma membrane of plant cells (7). The gene encoding this symporter has been cloned from many plant species (refs. 8–11; J. M.-Y. Lu and D.R.B., data not shown), and immunolocalization has demonstrated that it is found in the plasma membrane of phloem cells (12–15). Moreover, antisense expression of the symporter in transgenic plants inhibits assimilate partitioning. Those plants exhibit elevated levels of soluble carbohydrates and starch in their leaves, reduced sucrose transport activity, and impaired growth (6, 16). These data support the conclusion that the sucrose symporter mediates a pivotal step in assimilate partitioning.
In spite of these important advances, however, vanishingly little is known about the control pathways that regulate assimilate partitioning. Yet innumerable studies have shown that this is a regulated process and that plants have the ability to redirect resources in response to environmental or developmental changes (17–19). For example, many plants respond to nutrient deficiency and water deficits by directing a larger percentage of assimilated carbon to root growth as a mechanism for enhancing their capacity for nutrient and water uptake (18, 19). In the results reported here, we describe a sucrose-dependent signaling pathway that alters the transport activity and expression level of the proton–sucrose symporter. Significantly, with this pathway we have found an example of a regulatory system that has a direct impact on assimilate partitioning at the level of phloem transport.
MATERIALS AND METHODS
Plant Material and Sugar Treatments.
Sugar beets (Beta vulgaris Linnaeus) were grown hydroponically in a growth chamber with a 12 h light/dark cycle as described previously (20). Fully expanded leaves from 4- to 8-month-old plants were used in all experiments. The petioles of detached leaves were recut while submerged in their water or treatment solutions to maintain continuous water columns in the xylem. Treated leaves were returned to the growth chamber for the desired treatment period. For experiments using leaf discs, 1.2-cm-diameter discs were cut with a cork borer, and vacuum infiltration was used to soak the intercellular space with the desired treatment solutions. Leaf discs were incubated at room temperature in the light with gentle shaking for 16 h. All experiments were repeated several times, and representative data from a single experiment are shown.
Plasma Membrane Vesicle Isolation.
Sugar beet leaves were homogenized with a Brinkmann Polytron in 240 mM sorbitol/50 mM Hepes/10 mM KCl/3 mM EGTA/3 mM DTT/0.5% BSA; pH was adjusted to 8.0 with solid Bistris propane (21). The homogenate was filtered through four layers of cheesecloth and centrifuged at 10,000 × g for 8 min to remove tissue debris and organelles. Microsome membranes were pelleted from the supernatant with a 60-min centrifugation at 50,000 × g. Plasma membrane vesicles were purified by using the phase partitioning method as previously described (21). The purified plasma membrane vesicles were washed in 25 ml of resuspension buffer (330 mM sorbitol/2 mM Hepes/10 mM KCl/0.1 mM DTT, pH to 8.0 with Bistris propane), repelleted with a 60-min 50,000 × g centrifugation, and resuspended at 3–5 mg of protein per ml of resuspension buffer. Plasma membrane ATPase activity was measured as described (21).
Transport Assays.
Transport assays were conducted at 12°C as previously described (21). Briefly, 20 μl of membrane vesicles was added to 180 μl of resuspension buffer (pH adjusted to 6.0 with solid Mes) that contained 5 μM valinomycin and 14C-labeled transport substrate [0.5 mM sucrose, 0.2 μCi; 0.1 mM alanine, 0.05 μCi; or 0.5 mM glucose, 0.2 μCi (1 μCi = 37 kBq)]. After 4 min, the membrane vesicles were collected on a micropore filter and extravesicular transport solution was removed with repeated rinses. Protonmotive-force-coupled transport was defined as the difference in substrate accumulation between samples in the absence and those in the presence of 10 μM carbonyl cyanide m-chlorophenylhydrazone. Proteins were measured with the Markwell method (22) after solubilization in 0.02% sodium deoxycholate and precipitation in 6.2% trichloroacetic acid.
RNA Gel Blot Analysis.
Total RNA was isolated by the hot borate method (23). Twenty-five micrograms of total RNA of each sample was run on a formaldehyde gel (24) and blotted onto a nylon membrane. The membrane was blocked in a solution containing 7% SDS, 1% BSA, 1 mM EDTA, and 250 mM Na2HPO4 (pH 7.4) and then hybridized overnight with 32P-labeled probe. The full-length cDNA of the proton–sucrose symporter from sugar beet was used as the probe. This cDNA is 84% identical (at the amino acid level) to the spinach clone, it is expressed maximally in mature leaf tissue, and sucrose transport activity has been demonstrated in Xenopus oocytes (J. M.-Y. Lu and D.R.B., unpublished results). Hybridization and wash were done in a standard procedure (25). The same membrane was stripped and rehybridized with an Arabidopsis actin [ACT2, expressed sequence tag (EST) accession number 40F11T7] probe as a loading control. A PhosphorImager (Molecular Dynamics) was used to quantify the messages and normalize them relative to the actin controls.
RESULTS
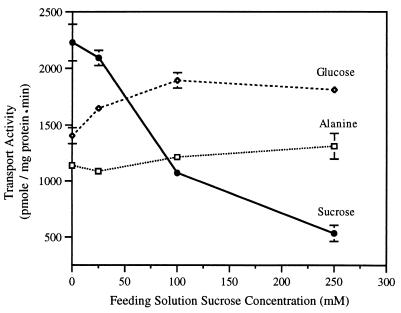
Detached sugar beet leaves were fed increasing concentrations of exogenous sucrose via the xylem transpiration stream and, after 24 h, plasma membranes vesicles were isolated and in vitro transport activities were assayed. Protonmotive-force-driven sucrose transport decreased 50–65% in leaves fed 100 mM sucrose relative to water controls (Fig. 1). Transport activity dropped to 20–25% of control activity when the leaves were treated with 250 mM sucrose (Fig. 1). In contrast, proton-coupled alanine transport and facilitated glucose transport activities were not inhibited by these treatments, suggesting diminished sucrose transport activity was not the result of a general perturbation of the integrity of the plasma membrane. We concluded from these data that decreased protonmotive-force-driven sucrose transport is a specific response to exogenous sucrose in a concentration-dependent reaction.
Figure 1.
Concentration-dependent inhibition of sucrose transport activity in purified plasma membrane vesicles. Sucrose, alanine, and glucose transport activities were assayed in membrane vesicles that were isolated after 24 h of transpirational feeding of solutions containing the indicated concentrations of sucrose. Error bars represent ± standard deviation.
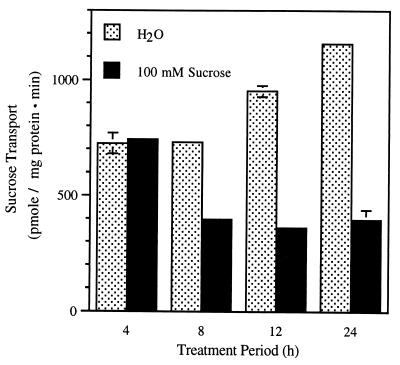
The time course of the sucrose effect was examined to establish the time dependence of the response. Repression of sucrose transport was not detected after 4 h. It was clearly evident at 8 h and reached a maximum at 12–24 h (Fig. 2). Interestingly, we also observed increased transport activity in water-treated controls during the 24-h incubation period. This increase in extractable sucrose transport activity after cutting has been described previously (26). The time dependence of sucrose repression is a complex function between sucrose delivery by the transpiration stream and the response pathway. Nevertheless, these results suggest some level of sucrose accumulation in the leaf is necessary before repression is evident. Indeed, we have measured sucrose concentrations in leaves treated with 100 mM sucrose and shown that sucrose levels increase by 3- to 5-fold relative to water controls (data not shown).
Figure 2.
Symporter-mediated sucrose transport activity in plasma membrane vesicles isolated from sugar beet leaves treated in H2O or in 100 mM sucrose for 4, 8, 12, or 24 h.
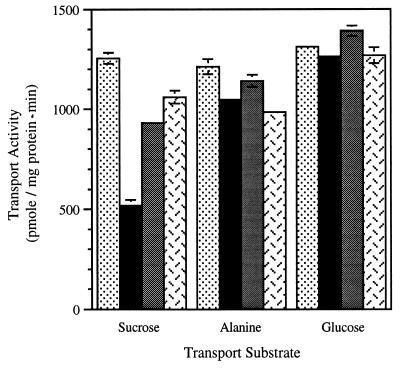
To examine the possibility that decreased sucrose transport is caused by an osmotic effect on leaf water potential, transport activities were assayed after 12-h treatments in osmotically equivalent solutions of sucrose, glucose, or KCl (Fig. 3). Sucrose transport was inhibited by 100 mM sucrose, but neither glucose nor KCl resulted in the same degree of repression. This suggests repression was not due to an osmotic effect. Significantly, neither alanine nor glucose transport was affected by these treatments (Fig. 3). Because this experiment also relied on transpirational feeding, it is conceivable that the composition of the transpiration stream was modified as it passed through the petiole to the target cells in the leaf. Therefore, we also treated isolated leaf discs in water, 100 mM sucrose, 100 mM mannitol, or 100 mM sorbitol to eliminate this potential complication. The results were similar to those observed in transpirational delivery: only the 100 mM sucrose treatment resulted in decreased transport activity (Table 1). Taken together, these results support the conclusion that sucrose repression is not due to an osmotic effect.
Figure 3.
Sucrose, alanine, and glucose transport activities in plasma membrane vesicles isolated from leaves fed H2O (stippled bars) or isoosmotic solutions of 100 mM sucrose (solid bars), 100 mM glucose (gray bars), or 50 mM KCl (herringbone bars) for 12 h.
Table 1.
Effect of various osmotically active sugars on sucrose transport activity in plasma membrane vesicles ioslated from sugar beet leaf discs that were incubated for 16 h in water, sucrose, mannitol, or soribitol
| Treatment solution | Sucrose transport activity, pmol/mg protein·min |
|---|---|
| Water | 1,624 ± 18 |
| Sucrose (100 mM) | 806 ± 3 |
| Mannitol (100 mM) | 1,721 ± 25 |
| Sorbitol (100 mM) | 1,976 ± 31 |
Plasma membrane vesicles were isolated after the treatment period and sucrose transport activity was measured in vitro as described in the text.
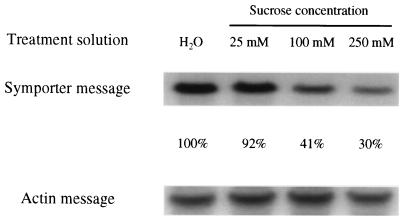
The kinetics of sucrose transport activity were determined in membrane vesicles isolated from control and repressed leaves to learn about the cause of decreased transport activity. The Km for sucrose was not affected by these treatments but Vmax declined in a concentration-dependent reaction (Table 2), and this decline correlated with the decrease in transport activity. Decreased Vmax activity is consistent with fewer symporters in the membrane, although other forms of down-regulation, such as protein modification, cannot be ruled out. If there are decreased levels of symporter in the membrane, one would expect increased rates of protein turnover and/or decreased levels of symporter synthesis. As an initial examination of this question, we determined the impact of these treatments on symporter message levels. Steady-state mRNA for the sucrose symporter decreased in sucrose treatments, and this drop was concentration dependent and paralleled decreased transport activity (Fig. 4). These data suggest sucrose repression is mediated, at least in part, by changes in transcriptional activity or mRNA turnover. We cannot rule out, however, additional contributions by changes in protein turnover or direct modification.
Table 2.
Kinetic analysis of sucrose transport activity in plasma membrane vesicles isolated from leaves fed water or sucrose for 24 h
| Treatment solution | Km, mM | Vmax, μmol/mg protein·min |
|---|---|---|
| Water | 1.06 ± 0.04 | 15.8 ± 0.8 |
| Sucrose (100 mM) | 1.12 ± 0.09 | 7.2 ± 0.6 |
| Sucrose (250 mM) | 1.29 ± 0.15 | 4.1 ± 0.4 |
Km and Vmax of sucrose transport activity are reported ±SD.
Figure 4.
RNA gel blot probed with cDNA of the sugar beet proton–sucrose symporter. Leaves were fed H2O, 25 mM sucrose, 100 mM sucrose, or 250 mM sucrose for 24 h. The Arabidopsis actin gene was used as a control message. The percentage changes of the normalized symporter signals are presented.
Many studies have described sugar-mediated changes in gene expression, and recent research has provided convincing evidence that hexoses and hexokinases play an important role in this pathway (27–29). Significantly, several studies have claimed sucrose-dependent signaling. However, these can often be attributable to hexoses derived from hydrolyzed sucrose (29–31). Therefore, we investigated the impact of glucose and fructose on sucrose transport activity to address whether the sucrose-mediated changes in transport activity described here are mediated by sucrose or its hydrolysis products. Although equilmolar treatments with these hexoses resulted in a small degree of decreased transport activity, this effect was not as robust as the sucrose-dependent decrease (Table 3, experiment A). Even treatment with 100 mM glucose plus 100 mM fructose, which was used to imitate complete hydrolysis of 100 mM sucrose, did not mimic the sucrose effect. Tracer experiments linked with TLC analysis showed that about 50% of the applied sucrose was hydrolyzed during the treatment period (data not shown). We also showed that feeding 10 mM mannoheptulose, a hexokinase inhibitor that blocks hexose mediated-signaling (29), did not have an impact on the sucrose response (Table 3, experiment B). These data show that the sucrose-specific response pathway described here is not mediated by hexokinase as the sugar sensor.
Table 3.
Effect of hexoses and mannoheptulose on sucrose transport activity
| Exp. | Treatment solution | Transport activity, pmol/mg protein·min |
|---|---|---|
| A | Water | 1,161 ± 12 |
| Sucrose (100 mM) | 416 ± 3 | |
| Glucose (100 mM) | 818 ± 15 | |
| Fructose (100 mM) | 820 ± 15 | |
| Glucose + fructose | 828 ± 16 | |
| B | Water | 1,071 ± 70 |
| Mannoheptulose (10 mM) | 972 ± 43 | |
| Sucrose (100 mM) | 355 ± 24 | |
| Sucrose + mannoheptulose | 437 ± 25 |
In experiment A, sugar beet leaves were fed water, 100 mM sucrose, 100 mM glucose, 100 mM fructose, or 100 mM glucose plus 100 mM fructose for 10 h. Plasma membrane vesicles were isolated from the treated leaves and sucrose transport activity was assayed in vitro. In experiment B, leaves were fed water or 100 mM sucrose in the presence or absence of 10 mM mannoheptulose for 11 h. Plasma membrane vesicles were isolated from the treated leaves and sucrose transport activity was assayed in vitro.
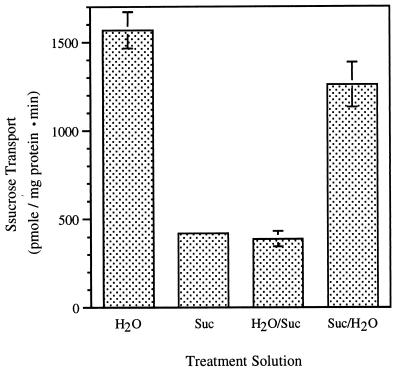
The effect of sucrose feeding on the sucrose transporter was reversed when treated leaves were placed in water for an additional 24 h (Fig. 5) Likewise, water-treated leaves responded to sucrose when transferred into sucrose solutions for an additional 24 h. Reversibility suggests this signaling pathway responds to dynamic changes in sucrose concentration and further supports the idea that it plays an important role in regulating phloem loading.
Figure 5.
Sucrose-mediated changes in symporter activity were reversible. Four leaves were cut from an individual plant, and two of them were placed in H2O and the other two in 100 mM sucrose. Plasma membranes were purified from one leaf of each treatment after 24-h transpirational feeding. At the same time, petioles of the other two leaves were recut, and those leaves were placed in the opposite treatment solution [i.e., H2O-treated leaves into 100 mM sucrose (H2O/Suc), and sucrose-treated leaves into H2O (Suc/H2O)] for another 24 h before isolating plasma membrane vesicles.
DISCUSSION
The proton-coupled sucrose symporter mediates phloem loading, the key transport step in assimilate partitioning for many plants (4–6). In the results reported here, we provide evidence that this pivotal activity is regulated by a response pathway that is sensitive to sucrose levels in the leaf. Regulation of symporter activity was specific for sucrose, concentration dependent, linked to changes in transcript abundance, and reversible. These characteristics are consistent with a sucrose-dependent signaling pathway that dynamically regulates phloem loading by responding to the sucrose levels in the phloem.
Sucrose symporter activity was down-regulated in a concentration-dependent reaction. Transport activity dropped to 35–50% of water controls in leaves fed 100 mM sucrose and to 20–25% of controls in 250 mM sucrose. In contrast, alanine symporter activity and facilitated glucose transport were not affected (Fig. 1). The fact that alanine and glucose transport were not influenced by sucrose feeding suggests that this is a specific effect on the sucrose symporter. Their insensitivity to sucrose treatments also suggests that generic changes in membrane integrity cannot account for altered sucrose transport activity. That conclusion was further supported by the observation that plasma membrane H+-ATPase activity was also unchanged by these treatments and no differences in plasma membrane protein patterns were observed in SDS/PAGE gels (data not shown). Although we have emphasized the impact of this response pathway on the sucrose symporter, we would not be surprised if additional proteins associated with resource allocation are also regulated by this system.
Sucrose delivery via the transpiration stream increases the concentration of osmotically active solutes in the leaf vascular tissue. Therefore, it is possible the symporter is responding to changes in water potential. Several lines of evidence, however, showed that sucrose-dependent decreases in transport activity were not a function of oscillations in osmotic potential. Isoosmotic solutions of glucose or KCl did not elicit the same response in feeding experiments (Fig. 3), nor did isoosmotic solutions of mannitol or sorbitol affect symporter activity in treated leaf discs (Table 1). These results support the conclusion that this is a sucrose-specific response pathway.
Many examples of sugar-dependent changes in gene expression have been recently described, and it seems clear that this type of regulation integrates a variety of cellular responses to changing patterns of resource utilization and allocation (30–32). In the case of assimilate partitioning, sugar-mediated regulation of photosynthetic gene expression in leaf tissue provides a mechanism for linking “sink” metabolism to photosynthesis (27, 33–35). Because sucrose transport activity is linked directly to photosynthetic activity, it is not surprising that the symporter is dynamically regulated by sucrose. Significantly, many investigations have identified hexoses as the active signal molecule in sugar sensing in higher plants, and convincing evidence has been presented that substrate flux through hexokinase is a key step in transducing the sugar signal (29, 30, 36). Indeed, many genes that respond to changes in sucrose levels are also regulated by hexoses, and current thinking suggests that sucrose is hydrolyzed and the released hexoses are responsible for altered patterns of gene expression in many instances of reported “sucrose” regulation. However, there are a few examples of sucrose-dependent changes in gene expression that are not mediated by hexoses. For example, sucrose induces expression of the class I patatin gene in cultured tobacco cells (37), whereas it blocks the transient expression of the plastocyanin gene during seedling development in Arabidopsis (38). Likewise, the sucrose-dependent changes in symporter activity described here are not elicited in the presence of hexoses or blocked by mannoheptulose, thereby demonstrating that this is a sucrose-sensitive transduction pathway.
Glucose and sucrose regulation of an Arabidopsis sucrose symporter gene (SUC2) was previously investigated by using a SUC2-promoter-GUS (β-glucuronidase) gene construct to visualize the expression pattern of the symporter in excised leaves and young seedlings (39). In that system, no sugar-dependent changes in gene expression were observed after 24-h incubations of excised tissues on sugar-containing medium. Thus, the Arabidopsis promoter may not be sensitive to sucrose regulation. However, the sensitive cells may not have been exposed to the elevated levels of sugar (those tissues were not fed sugars via the transpiration stream) and, moreover, the stability of the GUS gene product may have masked changes in transcriptional activity when assayed as a function of GUS enzyme activity. In contrast, high concentrations of either sucrose or glucose decreased sucrose symporter transcript levels in sugar-fed cotyledons of developing fava bean seeds (40). Because glucose elicited the same response, this observation appears to reflect the impact of the hexose signaling pathway on a developmentally regulated pattern of gene expression.
Both transcriptional regulation and protein turnover may be involved in the sucrose-dependent signaling pathway described here. Symporter message levels decreased in parallel with sucrose transport activity as a function of increasing concentrations of sucrose (Fig. 4). Although this decrease is consistent with decreased transcriptional activity, we cannot rule out changes in mRNA stability to account for this observation. Kinetic analysis showed that Vmax transport activity also decreased in treated tissues, suggesting that the number of symporters in the membrane had dropped. This would be consistent with decreased synthesis in response to decreased message and, indeed, preliminary experiments with cycloheximide showed that symporter activity is very dependent upon protein synthesis (data not shown). Significantly, high rates of symporter turnover were recently reported in potato (15). Although our results point to a complex interplay between symporter gene expression and protein turnover, we cannot differentiate between a direct effect on the symporter versus indirect effects on positive or negative regulators of this transporter. Thus, a detailed dissection of the sucrose-signaling pathway awaits additional tools, such as antibodies directed against the sugar beet symporter.
How might this response pathway modulate photosynthetic activity and resource allocation? Our working hypothesis suggests that high sucrose levels in the vascular tissue, which can result from decreased “sink” demand, down-regulate symporter activity. Because of decreased phloem loading, carbon will back up in the surrounding mesophyll. Carbohydrate accumulation in the mesophyll results in a concomitant down-regulation of photosynthetic activity, such as that described in symporter-antisense plants (6, 16) and glucose-fed plants (34, 35). In contrast, elevated sink demand would decrease sucrose levels in the phloem. This would up-regulate transport activity and, thus, increase the capacity for phloem loading. Enhanced phloem loading would draw down the sucrose levels in the mesophyll, perhaps stimulating photosynthetic activity and also increasing the percentage of recently fixed carbon directed to sucrose synthesis versus starch accumulation.
Assimilate partitioning is a pivotal determinant of plant growth and productivity because the pattern of resource allocation between organs is responsive to both developmental programs and environmental factors (3, 18, 19). Thus, assimilate partitioning is a globally regulated process that integrates across the needs of the plant as a whole. To date, little is known about the regulation and control of this crucial physiological process. In the results presented here, we provided evidence that the sucrose symporter, a key player in apoplastic phloem loading, is regulated by changing levels of sucrose in the leaf. We suggest that this sucrose-dependent transduction pathway is an important regulatory step in resource allocation.
References
- 1.Avigad G. In: Encyclopedia of Plant Physiology. Loewus F A, Tanner W, editors. 13A. Berlin: Springer; 1982. pp. 217–347. [Google Scholar]
- 2.Ziegler H, Zimmermann M H. In: Transport in Plants I: Phloem Transport. Zimmermann M H, Milburn J A, editors. Berlin: Springer; 1975. pp. 480–503. [Google Scholar]
- 3.Gifford R M, Thorne J H, Hitz W D, Giaquinta R T. Science. 1984;225:801–808. doi: 10.1126/science.225.4664.801. [DOI] [PubMed] [Google Scholar]
- 4.Giaquinta R T. Annu Rev Plant Physiol. 1983;34:347–387. [Google Scholar]
- 5.Bush D R. Photosynthesis Res. 1992;32:155–165. doi: 10.1007/BF00034792. [DOI] [PubMed] [Google Scholar]
- 6.Riesmeier J W, Willmitzer L, Frommer W B. EMBO J. 1994;13:1–7. doi: 10.1002/j.1460-2075.1994.tb06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush D R. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:513–542. [Google Scholar]
- 8.Riesmeier J W, Willmitzer L, Frommer W B. EMBO J. 1992;11:4705–4713. doi: 10.1002/j.1460-2075.1992.tb05575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gahrtz M, Stolz J, Sauer N. Plant J. 1994;6:697–706. doi: 10.1046/j.1365-313x.1994.6050697.x. [DOI] [PubMed] [Google Scholar]
- 10.Sauer N, Stolz J. Plant J. 1994;6:67–77. doi: 10.1046/j.1365-313x.1994.6010067.x. [DOI] [PubMed] [Google Scholar]
- 11.Weig A, Komor E. J Plant Physiol. 1996;147:685–690. [Google Scholar]
- 12.Riesmeier J W, Hirner B, Frommer W B. Plant Cell. 1993;5:1591–1598. doi: 10.1105/tpc.5.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stadler R, Brandner J, Schulz A, Gahrtz M, Sauer N. Plant Cell. 1995;7:1545–1554. doi: 10.1105/tpc.7.10.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stadler R, Sauer N. Bot Acta. 1996;109:299–306. [Google Scholar]
- 15.Kühn C, Franceschi V R, Schulz A, Lemoine R, Frommer W B. Science. 1997;275:1298–1300. doi: 10.1126/science.275.5304.1298. [DOI] [PubMed] [Google Scholar]
- 16.Kühn C, Quick W P, Schulz A, Riesmeier J W, Sonnewald U, Frommer W B. Plant Cell Environ. 1996;19:1115–1123. [Google Scholar]
- 17.Ho L C. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:355–378. [Google Scholar]
- 18.Wardlaw I F. New Phytol. 1990;116:341–381. doi: 10.1111/j.1469-8137.1990.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 19.Geiger D R, Servaites J C. In: Response of Plants to Multiple Stresses. Mooney H A, Winner W E, Pell E J, editors. San Diego: Academic; 1991. pp. 103–127. [Google Scholar]
- 20.Bush D R. Plant Physiol. 1990;93:1590–1596. doi: 10.1104/pp.93.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush D R. Plant Physiol. 1989;89:1318–1323. doi: 10.1104/pp.89.4.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markwell M K, Hass S M, Tolbert N E, Bieber L L. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- 23.Hall T C, Ma Y, Buchbinder B U, Pyne J W, Sun S M, Bliss F A. Proc Natl Acad Sci USA. 1978;75:3196–3200. doi: 10.1073/pnas.75.7.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zielinski R E. Plant Physiol. 1987;84:937–943. doi: 10.1104/pp.84.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 26.Sakr S, Lemoine R, Gaillard C, Delrot S. Plant Physiol. 1993;103:49–58. doi: 10.1104/pp.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheen J. Plant Cell. 1990;2:1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheen J. Photosynthesis Res. 1994;39:427–438. doi: 10.1007/BF00014596. [DOI] [PubMed] [Google Scholar]
- 29.Jang J-C, Sheen J. Plant Cell. 1994;6:1665–1679. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang J-C, Sheen J. Trends Plant Sci. 1997;2:208–214. [Google Scholar]
- 31.Smeekens S, Rook F. Plant Physiol. 1997;115:7–13. doi: 10.1104/pp.115.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch K E. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- 33.Foyer C H. Plant Physiol Biochem. 1988;26:483–492. [Google Scholar]
- 34.Krapp A, Hofmann B, Schafer C, Stitt M. Plant J. 1993;3:817–828. [Google Scholar]
- 35.Krapp A, Stitt M. Planta. 1995;195:313–323. [Google Scholar]
- 36.Jang J-C, Leon P, Zhou L, Sheen J. Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenzler H, Mignery G, Fisher L, Park W. Plant Mol Biol. 1989;13:347–354. doi: 10.1007/BF00015546. [DOI] [PubMed] [Google Scholar]
- 38.Dijkwel P P, Kock P A M, Bezemer R, Weisbeek P J, Smeekens S C M. Plant Physiol. 1996;110:455–463. doi: 10.1104/pp.110.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truernit E, Sauer N. Planta. 1995;196:564–570. doi: 10.1007/BF00203657. [DOI] [PubMed] [Google Scholar]
- 40.Weber H, Borisjuk L, Heim U, Sauer N, Wobus U. Plant Cell. 1997;9:895–908. doi: 10.1105/tpc.9.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]