Abstract
Ca2+ oscillations have been considered to obey deterministic dynamics for almost two decades. We show for four cell types that Ca2+ oscillations are instead a sequence of random spikes. The standard deviation of the interspike intervals (ISIs) of individual spike trains is similar to the average ISI; it increases approximately linearly with the average ISI; and consecutive ISIs are uncorrelated. Decreasing the effective diffusion coefficient of free Ca2+ using Ca2+ buffers increases the average ISI and the standard deviation in agreement with the idea that individual spikes are caused by random wave nucleation. Array-enhanced coherence resonance leads to regular Ca2+ oscillations with small standard deviation of ISIs.
INTRODUCTION
How are random molecular events orchestrated into reliable cellular behavior like global intracellular Ca2+ oscillations? If many molecules are involved, it is generally assumed that the cell behaves like a continuously stirred reactor and the law of large numbers establishes predictable behavior (1). Most models of the dynamics of intracellular Ca2+ release adopt this idea (2–4), but it is not consistent with experimental analyses showing that global oscillations arise from Ca2+ waves initiated locally (5–9). Such a local mechanism is predicted to lead to stochastic oscillations because although each cell has many IP3Rs and Ca2+ ions, the law of large numbers does not apply to the initiating event (3,10), which is restricted to very few IP3Rs by steep Ca2+ concentration gradients (7,9,11). Indeed, the law of large numbers may not apply either to other signaling pathways that generate steep intracellular concentration gradients (12).
Oscillations of intracellular Ca2+ dynamics exhibit spike trains which appear rather regular by casual inspection. This led to formulation of models with an oscillatory deterministic regime (2–4), but cells might exhibit stochastic dynamics which produce very similar global signals. That type of dynamics, often described as a stochastic medium (10,13–16), was predicted theoretically for reaction-diffusion systems with discrete stochastic sources of the diffusing species. Intracellular Ca2+ dynamics may be perceived as a stochastic medium: the diffusing species is cytosolic Ca2+ and the discrete stochastic sources are ion channels. Such systems generate spatiotemporal structures by wave nucleation due to thermal noise. In general, that leads to random spike sequences. The randomness does not arise from small numbers of molecules in the system, but rather from the fact that global events are initiated locally. Stochastic media exhibit almost regular oscillations in the regime of array enhanced coherence resonance (AECR) (10,13–16). Herein wave initiation is frequent enough to ensure that waves emerge as soon as the refractory period has passed. While there is experimental and theoretical evidence for the nucleation of global Ca2+ release events by local events (7,9,10), we investigate here whether spike sequences are random.
Transient changes in cytosolic Ca2+ concentration are used with great versatility to signal within cells (17). Under appropriate conditions and in many cell types, these transients form oscillations that encompass the entire cell (6,7,9,17). The interspike interval (ISI) of these oscillations varies from a few seconds to many minutes and their temporal profiles include spikes, sinusoidal oscillations, and bursts (2,3,7).
In many cells, including those examined in this study, oscillatory changes in free cytosolic Ca2+ concentration ([Ca2+]i) result from the periodic release of Ca2+ from intracellular stores within the endoplasmic reticulum (ER). Release of Ca2+ occurs via intracellular Ca2+ channels, notably inositol 1,4,5-trisphosphate receptors (IP3Rs), and Ca2+ is then resequestered into the ER by Ca2+ pumps (SR/ER Ca2+-ATPases). IP3Rs are regulated by the Ca2+ they conduct, such that fast activation by cytosolic Ca2+ and slower inhibition lead to a bell-shaped Ca2+-dependence of the stationary open probability of the IP3R (18–20). Within the membrane of the ER, clusters of IP3Rs are separated by perhaps 1–7 μm (8,9,21), and clustering is also dynamically regulated (22,23). These clusters of channels generate global concentration spikes via a hierarchy of Ca2+ release events (5–7) that depend upon Ca2+ diffusing between IP3Rs to ignite the activity of successive clusters (3,17). The smallest Ca2+ release events, blips (5), probably reflect random openings of single IP3Rs . Larger events, puffs, lasting tens of milliseconds and restricted to a volume of <0.5 fl, reflect the almost simultaneous opening of a few IP3Rs within a cluster (5,8,9,21). Many coordinated puffs form oscillations and waves (3,24,25). Ca2+ oscillations therefore depend upon both the spatial organization of IP3Rs and their regulation by Ca2+ (19,20,26).
The links between IP3R behavior and Ca2+ oscillations are poorly understood. Is oscillatory behavior a feature of individual IP3Rs and their local interaction with Ca2+ and IP3, with channels opening and closing in oscillatory fashion? Or do oscillations arise from properties that depend upon a higher level of organization involving many IP3Rs? Is AECR the new quality added by the more complex organization that would allow oscillations to emerge even from nonoscillatory elements?
With oscillatory dynamics arising from local interactions between individual IP3Rs and their ligands, each IP3R cluster is also an oscillator because channels within a cluster are synchronized by Ca2+ diffusion (11). Characteristics of the global oscillation, like period and amplitude, would be determined by local parameters such as channel state dynamics. Spatial coupling of channel clusters by Ca2+ diffusion would serve only to synchronize the oscillations. The global oscillation is deterministic: it has regular ISI and the timing of each spike is predictable. But recent theoretical studies suggest that IP3R clusters are not deterministic oscillators (10,27). A deterministic model generates oscillations only if IP3Rs are exposed to Ca2+ concentrations similar to those that cause their half-maximal regulation (∼0.1–10 μM) (18–20,26). Local Ca2+ concentrations near open IP3Rs are much higher, and therefore do not allow for deterministic oscillations (11,27–29).
Another possibility is that stochastic fluctuations render IP3R clusters oscillatory. Stochastic behavior of IP3R clusters is observed experimentally as Ca2+ puffs (6,9). Simulations show that random binding and dissociation of Ca2+ and IP3 to IP3Rs are sufficient sources of stochastic fluctuations to explain the random generation of Ca2+ puffs (10,30,31). However, these fluctuations alone cannot generate the observed timescale of global oscillations because puff durations and frequencies are 3–100 times faster than the ISI in cells (6). Processes occurring within a single IP3R cluster do not, therefore, transform random molecular behavior into oscillations. Local processes are not oscillatory, and so the idea that channel clusters are oscillators is untenable. A higher level of spatial organization is required to generate oscillations. The next spatial level is the cell, with its array of IP3R clusters each capable of generating Ca2+ puffs.
That suggests that intracellular Ca2+ dynamics behave as a stochastic medium. Experimental and theoretical studies show that Ca2+ puffs can initiate global Ca2+ spikes (3,6,7,9,10). But theoretical analysis suggests that cells would not then oscillate deterministically; instead, they would produce random sequences of global Ca2+ release events (3,10,27,32). In particular, factors that modify spatial coupling would affect both frequency and the ability to oscillate (3,10).
METHODS
Measurements of [Ca2+]i in glia
Glial cell cultures were prepared from brains of newborn NMRI mice (33) using whole brains for microglia, but only cortex for astrocyte cultures. Cells were loaded with fluo4-AM (5 μM) for 30 min at 20°C in glial medium (148.9 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 10 mM CaCl2, 5 mM HEPES, and 10 mM D-glucose, at pH 7.4). Cultures were fixed within the microscope chamber by a U-shaped Pt wire and superfused with glial medium at 20°C. Cells were illuminated (495 nm) from a monochromator (TILL Photonics, München, Germany) and fluorescent images (515–545 nm) collected every 3 s with a 12-bit camera (SensiCam, Cooke, Romulus, MI) on an upright microscope and visualized with ImagingCellsEasily software (Max Dulbrück Center, Berlin, Germany).
Measurements of [Ca2+]i in human embryonic kidney (HEK) cells
HEK293 cells were cultured in Dulbecco's modified Eagle's medium/Ham's F-12 supplemented with fetal bovine serum (10 %) and L-glutamine (2 mM), in a humidified atmosphere (95% air, 5% CO2, 37°C). Medium was replaced every third day, and cells were passaged when they reached 80% confluence. For single cell imaging, cells were plated onto 22-mm round glass coverslips coated with 0.01% poly-L-lysine and used after two days. They were loaded with 2 μM Fura-2-AM in HEK medium (135 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 1.5 mM CaCl2, 11.6 mM HEPES, 11.5 mM D-glucose, and pH 7.3) supplemented with 1 mg/ml bovine serum albumin and 0.02% Pluronic F-127. Cells were loaded for 45 min at 20°C and then washed for 45 min in HEK medium to allow deesterification of the Fura-2-AM. Measurements of [Ca2+]i in single cells were performed as previously described (34), with fluorescence collected at intervals of 5 s. Fluorescence ratios were calculated after correction for autofluorescence.
Measurements of [Ca2+]i in processed lipoaspirate (PLA) cells
The study conforms to the Declaration of Helsinki and all cell donors gave their informed written consent to use part of their fat tissue for the generation of processed lipoaspirate (PLA) cells.
PLA cells were isolated from fat tissue (35) and cultured in 25 cm2 flasks in 5% CO2, humidified air at 37°C in Ham's F-10 medium (Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum (Sigma, St. Louis, MO), 2 mM glutamine, 0.1 mM β-mercaptoethanol, 1–100 nonessential amino acids (Biochrom), 100 units/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Subconfluent cell cultures were dissociated with a 0.2% trypsin-0.05% EDTA solution and subcultured onto 12 mm coverslips. For Ca2+ measurements, subconfluent cells on coverslips were incubated for 30 min with 10 μM fluo3-AM (Invitrogen) and then washed once in culture medium. Fluo-3 fluorescence was recorded in single cells by confocal laser-scanning microscopy (laser scanning microscope model No. 510 Meta; Zeiss, Jena, Germany) using a 25× objective with a numerical aperture of 0.8 (Plan Neofluor; Zeiss). Excitation was at 488 nm and emission was recorded using a 515 nm longpass filter.
DATA ANALYSIS
All fluorescence signals, whether derived from single wavelength or ratiometric indicators, are expressed relative to the baseline fluorescence (ΔF = signal/basal). Because we analyze only interspike intervals (ISIs), there is no need to calibrate ΔF to [Ca2+]i. For each single-cell time series of fluorescence, the time points of the maxima above a minimal level were determined resulting in a series of spike times, which was used to determine the ISIs. The calculations of statistical characteristics require a stationary ISI series. However, most experimental records of HEK cells have a slight trend and eventually stop oscillating. We reduced that trend by leaving out parts of the ISI series in the beginning and end showing very obvious trends and removed linear trend from the remaining series before we calculated the average period Tav, the standard deviation σ, and correlations. This is illustrated in Supplementary Material Fig. S7.
Correlation coefficients ρk between an ISI and its kth successor for the lth ISI-series were calculated according to
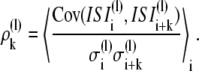 |
(1) |
With the  we formed the population average across n cells by
we formed the population average across n cells by 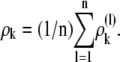
Tav-σ relation for time-dependent global nucleation rate
We show that a global nucleation probability increasing by a relaxation to an asymptotic value provides Tav-σ relations compatible with our findings. We choose a time-dependent nucleation rate Λ like
 |
(2) |
with the regeneration rate ξ. The value ξ could describe, e.g., the recovery from inhibition (36). The probability of observing a global wave at time t is given by
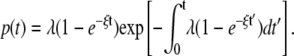 |
(3) |
With this expression we can calculate the first two moments as
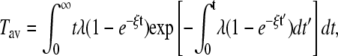 |
(4) |
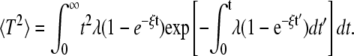 |
(5) |
That can be integrated by parts,
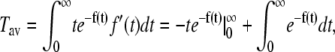 |
(6) |
where the first term vanishes, leading to
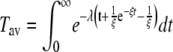 |
(7) |
for Tav. 〈T2〉 is given by
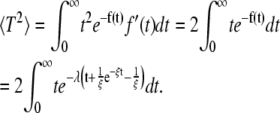 |
(8) |
Integration yields
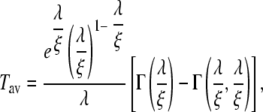 |
(9) |
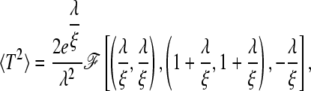 |
(10) |
where Γ(x) denotes the Euler Γ-function, Γ(x, y) the incomplete γ-function, and F[x] is the generalized hypergeometric function 2F2(x). With the relation for the standard deviation
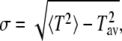 |
(11) |
we obtain the Tav-σ relations shown in Fig. 1.
FIGURE 1.
Tav-σ relation resulting from Eqs. 7 and 8. The lines ξ = constant arise by varying λ. They start at (0,0) with λ = ∞ and converge to Tav = σ with increasing ξ. The lines λ = constant start on Tav = σ = λ−1 with ξ ≫ λ very large. Note that the Tav-σ relation becomes linear for λ/ξ → 0 or Tav → ∞ and σ → ∞ and that the slope decreases from one for ξ → 0. The values ξ and λ are measured in s−1.
We did not consider a deterministic part Td of the ISI in the above calculations, which represents an absolute refractory part of the ISI. Such a deterministic part simply moves the curves in Fig. 1 to larger values of Tav. The complete distribution including Td is
 |
(12) |
The analysis of experimental data showed that Tav, σ, and Td vary between individual cells of the same cell type.
RESULTS AND DISCUSSION
To establish whether intracellular Ca2+ oscillations are deterministic or stochastic, we performed comprehensive measurements of ISI series in different cell types. In each case, IP3Rs evoke the Ca2+ oscillations (37–39) (Fig. S8). Fig. 2 shows representative time series of global oscillations in astrocytes, microglia, PLA stem cells from human adipose tissue, and human embryonic kidney (HEK) cells. These examples show that ISIs are not regular. The length of the ISI changes randomly. Records like these were used to determine the serial correlation coefficients ρk, mean values Tav, and standard deviations σ of ISI. For all cell types and with all degrees of stimulation, σ increases with Tav (Fig. 3).
FIGURE 2.
IP3-mediated Ca2+ spikes in diverse cells. Representative examples of oscillation time series for astrocytes (A), microglia (B), PLA cells (C), and HEK cells stimulated with 30 μM carbamyl choline (CCh) (D). The oscillations in panels A–C are spontaneous. Changes in the fluorescence of the Ca2+ indicator relative to its basal level (ΔF) report the increase in free cytosolic [ Ca2+ ]. Lower panels show the ISI after each spike, with ISI defined as the interval between consecutive fluorescence maxima.
FIGURE 3.
Ca2+ spikes occur randomly. Dependence of the standard deviation σ of ISI on the average ISI Tav for 366 astrocytes (A), 224 microglia (B), 270 PLA cells (C), and 137 HEK cells stimulated with 30 μM CCh (D). σ and Tav were obtained from time series of single cells by temporal averaging. The correlation coefficient ρ shows that σ and Tav are highly correlated in all four cases.
Standard deviations are of the same order of magnitude as averages for most points, and for large Tav, σ and Tav are similar. Hence, the uncertainty in predicting the occurrence of a spike is of the same order as the mean ISI: i.e., Ca2+ spikes are random events. If spikes represented the active phase in a deterministic oscillation, the standard deviation of ISI would instead be in the range of the global interpuff interval, i.e., between a few hundred milliseconds for short Tav and a few seconds for long Tav. The uncertainty in spike timing shows in the correlation coefficients ρk of the ISI time series between the ith and (i + k)th ISI (Fig. 4). Consecutive intervals are not correlated, confirming the randomness of the spike-generating mechanism.
FIGURE 4.
Successive ISI are not correlated. Correlation coefficient ρk between the ith and (i+k)th ISI averaged across n time series obtained in the experiments shown in Fig. 6 before (red) and after (blue) loading with additional Ca2+ buffer. The protocols are shown in the insets (see also caption of Fig. 6). The number of time series measured for the first and the second periods were, respectively: (A) n1 = 27, n2 = 20; (B) n1 = 35, n2 = 18; (C) n1 = 36, n2 = 35; (D) n1 = 33, n2 = 23; (E) n1 = 35, n2 = 35; (F) n1 = 35, n2 = 28; (G) n1 = 31, n2 = 22; and (H) n1 = 29, n2 = 18. Error bars show standard error for averaging over n time series.
We conclude that global oscillations result from a sequence of randomly occurring global Ca2+ spikes. The data show also that σ almost vanishes at the smallest values of Tav, indicating that almost regular oscillations with ISIs close to Td do exist. The results are similar for spontaneous oscillations in astrocytes, microglia, and PLA cells, and for oscillations evoked by different levels of stimulation in HEK cells, suggesting that IP3-evoked Ca2+ oscillations are sequences of stochastic spikes in many cell types.
Our conclusion is compatible with the idea that each Ca2+ spike reflects the passage of a Ca2+ wave across the cell driven by successive activation of IP3R clusters by Ca2+ diffusing between them (6,7,9). This mechanism can generate the spectrum of observed shapes of oscillations (3,10). Our results show that waves initiate randomly. The time of initiation is not set by a deterministic process, such as recovery from Ca2+-inhibition or a progressive sensitization of IP3Rs by Ca2+.
Stochastic models of repetitive waves show that if σ is of the same order as Tav, it is dominated by the probability (Ptrig) of triggering a wave after the cell has recovered from the previous one (10). The smaller the value of Ptrig, the longer it takes on average for the next wave to occur, and the larger is the value of σ. For such repetitive triggering of waves, σ increases linearly with Tav, and σ = 1/Ptrig holds for large values of Tav. Even if Ptrig relaxes exponentially from 0 immediately after a spike to an asymptote, the linear relation between σ and Tav for large Tav still holds and it has a slope <1 (see Fig. 1 and Fig. 3 D). Such a relaxation of Ptrig appears to apply also to wave initiation reported in Marchant and Parker (9). Almost regular oscillations arise when Ptrig is very large, because then as soon as the cell has recovered from one spike, the next one is triggered. We refer to the length of the ISI of these regular oscillations as the deterministic part Td of the ISI. It might be set by a variety of processes depending on cell type, for example: store refilling, IP3R inhibition, or Ca2+-feedback to the IP3 concentration. Each of these processes may also cause a time-dependence of Ptrig such as that described in a simplified way by the relaxation with time constant ξ in Eq. 2.
The time-dependence of the global nucleation rate differs between the different cell types as revealed by the slopes of the relationship between σ and Tav. Fig. 5 shows values of the parameters of the time-dependent global nucleation rate (Eq. 2) for the different cell types. The spontaneous oscillations in astrocytes and microglia have a slope of the σ-Tav-relation close to 1 and an asymptotic nucleation rate λ much smaller than the relaxation rate ξ. Despite the relatively fast recovery from the previous spike, these oscillations are neither fast nor regular, since the asymptotic nucleation rate λ of these cells is small. The stimulated oscillations in HEK cells show the inverse relation between λ and ξ. That is correlated with the information content of stimulated and spontaneous oscillations which increases with increasing λ/ξ (40).
FIGURE 5.
Values of the parameters λ and ξ of the time-dependent global nucleation rate Eq. 2 for the different cell types. The values were obtained by fitting σ and Tav of individual time series to Eqs. 10 and 11 to obtain λ and ξ, which were then averaged across all cells of the same type. We have approximated Td by the smallest value of Tav observed for the corresponding cell type in the fitting procedure. Error bars show standard error.
Recovery from the previous spike is relatively slow in HEK cells. That corresponds to the initial decline in spike amplitudes in Fig. 2 during which the cell reaches a state corresponding to incomplete recovery from a spike during each ISI. One possibility is that insufficient refilling of the ER causes the decline. We might then suggest that immediately after stimulation, Ca2+ reuptake during the ISI fails to keep pace with release during a spike and successive Ca2+ transients decrease in amplitude. But during the stationary phase of oscillations, the two fluxes come into balance such that Ca2+ uptake by the stores during the ISI matches the amount released during the preceding spike. The time-dependent nucleation rate Λ(t) reaches only 60% of λ during an average ISI due to the small value of ξ. However, since the asymptotic nucleation rate λ is rather large, the oscillations in HEK cells have smaller σ than astrocyte oscillations. The values for λ and ξ of PLA cells lie between those for astrocytes and HEK cells.
We can further examine the stochastic component of wave triggering by manipulating the probability of wave initiation, for example by increasing the Ca2+-buffering capacity of the cytosol (10,11). Decreasing Ptrig increases the average ISI, but if wave triggering is random, the standard deviation σ should also increase. Ptrig is actually the probability of two sequential events: that a puff occurs (Ppuff) and then that the puff ignites a wave (Pwave). The latter turns the puff frequency into the smaller wave frequency (Ptrig = PpuffPwave) and the standard deviation of interpuff intervals into the standard deviation of interspike intervals. Pwave is expected to decrease when diffusion of free Ca2+ between clusters (the spatial coupling) is reduced by buffers (11). The precise effect depends on the dynamic regime of the cell. With slow irregular waves, a slight decrease in coupling is predicted to increase Tav and σ, while larger decreases are predicted to abolish the self-amplifying mechanism required for oscillations leaving only moderate puff activity (10,11). Oscillations are predicted to be very sensitive to addition of Ca2+ buffers with fast binding and dissociation rates, and with 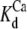 similar to resting cytosolic Ca2+ concentration because such buffers effectively reduce diffusion of free Ca2+ at distances similar to the likely spacings of IP3R clusters (11). Because the buffers we use do not change the resting cytosolic free Ca2+ concentration, the IP3 concentration, or the interactions between IP3Rs within clusters (41), we do not expect them to affect Ppuff.
similar to resting cytosolic Ca2+ concentration because such buffers effectively reduce diffusion of free Ca2+ at distances similar to the likely spacings of IP3R clusters (11). Because the buffers we use do not change the resting cytosolic free Ca2+ concentration, the IP3 concentration, or the interactions between IP3Rs within clusters (41), we do not expect them to affect Ppuff.
We measured the effect of Ca2+-buffering on oscillations in astrocytes, HEK cells, and PLA cells. Oscillations were first recorded to establish Tav and σ. The buffering capacity of the cells was then increased by incubating the cells with BAPTA-AM or EGTA-AM, before continuing measurements of [Ca2+]i. BAPTA loading caused most of the HEK cells to stop oscillating, and for the cells that resumed oscillations (∼50% for cells loaded with 0.5 or 1 M BAPTA-AM), Tav and σ were both increased in a concentration-dependent way (Fig. 6, D–F). EGTA loading of PLA cells and astrocytes also caused oscillations to terminate in ∼80% of cells, and increased both Tav and σ in the cells that resumed oscillations (Fig. 6, A–C, G–I).
FIGURE 6.
Ca2+ buffers render spike times even less predictable. Results show Ca2+ spikes in astrocytes (A–C), HEK cells stimulated with 30 μM CCh (D–F), and PLA cells (G–I) before (red) and after (blue) loading with additional Ca2+ buffer. The protocols and typical results are shown in panels A, D, and G; the durations of the loading and subsequent hydrolysis times are shown in the triangles. Changes to concentrations of EGTA-AM or BAPTA-AM are shown in the histograms. For the 50% of cells that resumed Ca2+ spiking, changes dTav and dσ in Tav and σ are shown relative to the values obtained before incubation with the Ca2+ buffer. Error bars show standard error from different cells and experiments.
The results demonstrate both the exquisite sensitivity of Ca2+ oscillations to Ca2+ buffering, whether provided by fast (BAPTA) or slow (EGTA) Ca2+ buffers, and the increase in both Tav and σ predicted for a stochastic mechanism dependent on repetitive Ca2+ waves (10).
We have shown that cells use a stochastic mechanism to generate global Ca2+ spikes. With ∼104 IP3Rs in each HEK cell (unpublished) and far more Ca2+ ions, why does random behavior play such an important part in Ca2+ spiking? The reason is that, as for any stochastic medium, global events are initiated by local processes. A puff changes the local free Ca2+ concentration by 100–1000-fold (11) and the gradients are so steep that only neighboring IP3R clusters experience the change. Each puff, occurring within only a small part of the cell, is a potential wave initiation site, and the few IP3R clusters that participate in the initiation of each wave are too few for the process to become deterministic. These properties identify intracellular Ca2+ dynamics as a random medium. The existence of so-called hot-spots (5) does not change these conclusions; it simply means that Ppuff is not spatially homogeneous.
Intracellular Ca2+ spikes occur randomly. But a regular regime exists if Ptrig is high due to the existence of a deterministic part of the ISI. The mean and the standard deviation of the ISI distribution depend on spatial coupling. The regular Ca2+ spikes are thus an example of array enhanced coherence resonance, an intrinsically stochastic phenomenon (10,13,15,16,42). This is consistent with hierarchical recruitment of Ca2+ release events (6,7). Hence, oscillations are an emergent property of arrays of IP3R clusters and not a property of single IP3Rs. Ca2+ oscillations are the first example of the constructive use of noise in cell signaling and the first experimental verification of AECR in a natural system.
These conclusions are based on the data presented here, previous measurements on the initiation of spikes and waves (6,7,9,43) and on theoretical studies investigating the role of noise and spatial coupling for global signals (10,16,42,44,45). It is accepted that elemental events like Ca2+ puffs and sparks are random (3,30,31,46–53), but this has not previously been shown for global events. Indeed, and despite the observation that over a large area of the cytosol (8 μm × 8 μm) of a Xenopus oocyte σ increases with Tav (9), it has been assumed that global signals are deterministic (2).
The probability of initiating a wave (Ptrig) is determined by local properties, such as channel state dynamics, numbers of channels in a cluster, release currents, and by diffusion of free Ca2+ between clusters. Ptrig sets σ of the ISI distribution. Information on local properties, like the open probability in vivo, can thus, in principle, be obtained from the fluctuations of the global Ca2+ signal.
We have shown that in many different cell types IP3-evoked Ca2+ spikes are caused by random wave nucleation with a regular regime arising from AECR. The randomness of spike trains does not prevent Ca2+ oscillations from transmitting information. As long as the ISI distribution of stimulated spike trains is sufficiently different from the ISI distribution of spontaneous spiking (40), cells can distinguish the message from the noise.
SUPPLEMENTARY MATERIAL
To view all of the supplemental files associated with this article, visit www.biophysj.org.
Acknowledgments
A.S. is supported by the International Research Training Group of the Deutschen Forschungsgemeinschaft (grant No. GRK 1360). C.W.T. and S.C.T. are supported by the Wellcome Trust.
Editor: Arthur Sherman.
References
- 1.van Kampen, N. G. 2001. Stochastic Processes in Physics and Chemistry. North-Holland, Amsterdam, The Netherlands.
- 2.Schuster, S., M. Marhl, and T. Höfer. 2001. Modeling of simple and complex calcium oscillations. Eur. J. Biochem. 269:1333–1355. [DOI] [PubMed] [Google Scholar]
- 3.Falcke, M. 2004. Reading the patterns in living cells—the physics of Ca2+ signaling. Adv. Phys. 53:255–440. [Google Scholar]
- 4.Goldbeter, A. 1996. Biochemical Oscillations and Cellular Rhythms. Cambridge University Press, Cambridge, UK.
- 5.Parker, I., J. Choi, and Y. Yao. 1996. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium. 20:105–121. [DOI] [PubMed] [Google Scholar]
- 6.Marchant, J., N. Callamaras, and I. Parker. 1999. Initiation of IP3-mediated Ca2+ waves in Xenopus oocytes. EMBO J. 18:5285–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bootman, M. D., M. Berridge, and P. Lipp. 1997. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 91:367–373. [DOI] [PubMed] [Google Scholar]
- 8.Thomas, D., P. Lipp, M. Berridge, and M. D. Bootman. 1998. Hormone-evoked elementary Ca2+ signals are not stereotypic, but reflect activation of different size channel clusters and variable recruitment of channels within a cluster. J. Biol. Chem. 273:27130–27136. [DOI] [PubMed] [Google Scholar]
- 9.Marchant, J., and I. Parker. 2001. Role of elementary Ca2+ puffs in generating repetitive Ca2+ oscillations. EMBO J. 20:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falcke, M. 2003. On the role of stochastic channel behavior in intracellular Ca2+ dynamics. Biophys. J. 84:42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thul, R., and M. Falcke. 2004. Release currents of IP3 receptor channel clusters and concentration profiles. Biophys. J. 86:2660–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaccolo, M., and T. Pozzan. 2002. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 295:1711–1715. [DOI] [PubMed] [Google Scholar]
- 13.Pikovsky, A. S., and J. Kurths. 1997. Coherence resonance in a noise-driven excitable system. Phys. Rev. Lett. 78:775–778. [Google Scholar]
- 14.Jung, P., and G. Mayer-Kress. 1995. Spatiotemporal stochastic resonance in excitable media. Phys. Rev. Lett. 74:2130–2133. [DOI] [PubMed] [Google Scholar]
- 15.Lindner, B., J. Garcia-Ojalvo, A. Neimann, and L. Schimansky-Geier. 2004. Effects of noise in excitable systems. Phys. Rep. 392:321–424. [Google Scholar]
- 16.Coombes, S., and Y. Timofeeva. 2003. Sparks and waves in a stochastic fire-diffuse-fire model of Ca2+ release. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 68:021915. [DOI] [PubMed] [Google Scholar]
- 17.Berridge, M. J., P. Lipp, and M. D. Bootman. 2000. The versatility and universality of calcium signaling. Nat. Rev. Mol. Cell Biol. 1:11–22. [DOI] [PubMed] [Google Scholar]
- 18.Bezprozvanny, I., J. Watras, and B. E. Ehrlich. 1991. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 351:751–754. [DOI] [PubMed] [Google Scholar]
- 19.Taylor, C. W., and A. Laude. 2002. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+. Cell Calcium. 32:321–334. [DOI] [PubMed] [Google Scholar]
- 20.Mak, D., S. McBride, and J. Foskett. 1998. Inositol 1,4,5-tris-phosphate activation of inositol tris-phosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl. Acad. Sci. USA. 95:15821–15825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suhara, W., M. Kobayashi, H. Sagara, K. Hamadad, T. Goto, I. Fujimoto, K. Torimitsu, and K. Mikoshiba. 2006. Visualization of inositol 1,4, 5-trisphosphate receptor by atomic force microscopy. Neurosci. Lett. 391:102–107. [DOI] [PubMed] [Google Scholar]
- 22.Tateishi, Y., M. Hattori, T. Nakayama, M. Iwai, H. Bannai, T. Nakamura, T. Michikawa, T. Inoue, and K. Mikoshiba. 2004. Cluster formation of inositol 1,4,5-trisphosphate receptor requires its transition to open state. J. Biol. Chem. 280:6816–6822. [DOI] [PubMed] [Google Scholar]
- 23.Chalmers, M., M. J. Schell, and P. Thorn. 2006. Agonist-evoked inositol trisphosphate receptor (IP3R) clustering is not dependent on changes in the structure of the endoplasmic reticulum. Biochem. J. 394:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rooney, T. A., S. K. Joseph, C. Queen, and A. P. Thomas. 1996. Cyclic GMP induces oscillatory calcium signals in rat hepatocytes. J. Biol. Chem. 271:19817–19825. [DOI] [PubMed] [Google Scholar]
- 25.Charles, A. C., E. R. Dirksen, J. E. Merrill, and M. J. Sanderson. 1991. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 6:983–992. [DOI] [PubMed] [Google Scholar]
- 26.Adkins, C. E., and C. W. Taylor. 1999. Lateral inhibition of inositol 1,4,5-trisphosphate receptors by cytosolic Ca2+. Curr. Biol. 9:1115–1118. [DOI] [PubMed] [Google Scholar]
- 27.Thul, R., and M. Falcke, 2004. Stability of membrane-bound reactions. Phys. Rev. Lett. 93:188103-1–4. [DOI] [PubMed] [Google Scholar]
- 28.Shuai, J., H. J. Rose, and I. Parker. 2006. The number and spatial distribution of IP3 receptors underlying calcium puffs in Xenopus oocytes. Biophys. J. 91:4033–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bentele, K., and M. Falcke. 2007. Quasi-steady approximation for ion channel currents. Biophys. J. In press. 10.1529/biophysj.107.104299. [DOI] [PMC free article] [PubMed]
- 30.Swillens, S., G. Dupont, and P. Champeil. 1999. From calcium blips to calcium puffs: theoretical analysis of the requirements for interchannel communication. Proc. Natl. Acad. Sci. USA. 96:13750–13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shuai, J., and P. Jung. 2002. Stochastic properties of Ca2+ release of inositol 1,4,5-trisphosphate receptor clusters. Biophys. J. 83:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thul, R., and M. Falcke. 2006. Frequency of elemental events of intracellular Ca2+ dynamics. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 73:061923. [DOI] [PubMed] [Google Scholar]
- 33.Lyon, S., and H. Kettenmann. 1998. Oligodendrocytes and microglia are selectively vulnerable to combined hypoxia and hypoglycemia injury in vitro. J. Cereb. Blood Flow Metab. 18:521–530. [DOI] [PubMed] [Google Scholar]
- 34.Short, A. D., and C. W. Taylor. 2000. Parathyroid hormone controls the size of the intracellular Ca2+ stores available to receptors linked to inositol trisphosphate formation. J. Biol. Chem. 275:1807–1813. [DOI] [PubMed] [Google Scholar]
- 35.Zuk, P. A., M. Zhu, P. Ashjian, D. A. De Ugarte, J. I. Huang, H. Mizuno, Z. C. Alfonso, J. K. Fraser, P. Benhaim, and M. H. Hedrick. 2002. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 13:4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falcke, M. 2004. Period distributions of intracellular Ca2+ oscillations. Proceedings of the Fourth International Workshop on Bioinformatics and Systems Biology, May 31-June 3, 2004, Kyoto, Japan. Short papers: IBSB 2004. Poster Abstracts: 5–6.
- 37.Fiacco, T. A., and K. D. McCarthy. 2006. Astrocyte calcium elevations: properties, propagation, and effects on brain signaling. Glia. 54:676–690. [DOI] [PubMed] [Google Scholar]
- 38.Färber, K., and H. Kettenmann. 2006. Functional role of calcium signals for microglial function. Glia. 54:656–665. [DOI] [PubMed] [Google Scholar]
- 39.Short, A. D., G. Winston, and C. W. Taylor. 2000. Different receptors use inositol trisphosphate to mobilize Ca2+ from different intracellular pools. Biochem. J. 351:683–686. [PMC free article] [PubMed] [Google Scholar]
- 40.Skupin, A., and M. Falcke. 2008. Statistical properties and information content of calcium oscillations. Genome Inform. 18. [PubMed]
- 41.Dargan, S., and I. Parker. 2003. Buffer kinetics shape the spatiotemporal patterns of IP3-evoked Ca2+ signals. J. Physiol. 553:775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shuai, J., and P. Jung. 2003. Optimal ion channel clustering for intracellular calcium signaling. Proc. Natl. Acad. Sci. USA. 100:506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupont, G., S. Swillens, C. Clair, T. Tordjmann, and L. Combettes. 2000. Hierarchical organization of calcium signals in hepatocytes: from experiments to models. Biochim. Biophys. Acta. 1498:134–152. [DOI] [PubMed] [Google Scholar]
- 44.Falcke, M. 2003. Buffers and oscillations in intracellular Ca2+ dynamics. Biophys. J. 84:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Falcke, M. 2003. Building a wave—models of the puff-to-wave transition. In Understanding Calcium Dynamics: Experiments and Theory. Springer, New York, NY.
- 46.Parker, I., and Y. Yao. 1995. Calcium puffs in Xenopus oocytes. In Calcium Waves, Gradients and Oscillations, Ciba F. Symposium. Wiley, Chichester, England. [DOI] [PubMed]
- 47.Yao, Y., and I. Parker. 1995. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J. Physiol. 482:533–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipp, P., D. Thomas, M. Berridge, and M. Bootman. 1997. Nuclear calcium signaling by individual cytoplasmic calcium puffs. EMBO J. 16:7166–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heemskerk, J., G. Willems, M. Rook, and S. Sage. 2001. Ragged spiking of free calcium in ADP-stimulated human platelets: regulation of puff-like calcium signals in vitro and ex vivo. J. Physiol. 535:625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng, H., W. Lederer, and M. Cannel. 1993. Calcium-sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 262:740–744. [DOI] [PubMed] [Google Scholar]
- 51.Smith, G., J. Keizer, M. Stern, W. Lederer, and H. Cheng. 1998. A simple numerical model of calcium spark formation and detection in cardiac myocytes. Biophys. J. 75:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lukyanenko, V., and S. Györke. 1999. Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. J. Physiol. 521:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rengifo, J., R. Rosales, A. González, H. Cheng, M. Stern, and E. Ríos. 2002. Intracellular Ca2+ release as irreversible Markov process. Biophys. J. 83:2511–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]








