Abstract
The effects of the 9-cis and 13-cis isomers of zeaxanthin on the molecular organization and dynamics of dimyristoylphosphatidylcholine (DMPC) membranes were investigated using conventional and saturation-recovery EPR observations of the 1-palmitoyl-2-(14-doxylstearoyl)phosphatidylcholine (14-PC) spin label. The results were compared with the effects caused by the all-trans isomer of zeaxanthin. Effects on membrane fluidity, order, hydrophobicity, and the oxygen transport parameter were monitored at the center of the fluid phase DMPC membrane. The local diffusion-solubility product of oxygen molecules (oxygen transport parameter) in the membrane center, studied by saturation-recovery EPR, decreased by 47% and 27% by including 10 mol% 13-cis and 9-cis zeaxanthin, respectively; whereas, incorporation of all-trans zeaxanthin decreased this parameter by only 11%. At a zeaxanthin-to-DMPC mole ratio of 1:9, all investigated isomers decreased the membrane fluidity and increased the alkyl chain order in the membrane center. They also increased the hydrophobicity of the membrane interior. The effects of these isomers of zeaxanthin on the membrane properties mentioned above increase as: all-trans < 9-cis ≤ 13-cis. Obtained results suggest that the investigated cis-isomers of zeaxanthin, similar to the all-trans isomer, are located in the membrane interior, adopting transmembrane orientation with the polar terminal hydroxyl groups located in the opposite leaflets of the bilayer. However, the existence of the second pool of cis-zeaxanthin molecules located in the one leaflet and anchored by the terminal hydroxyl groups in the same polar headgroup region cannot be completely ruled out.
Keywords: cis-zeaxanthin, xanthophylls, carotenoids, lipid bilayer, spin labeling, EPR
1. Introduction
The heterogeneity of carotenoids is greatly increased by the existence of their geometrical isomers. The polyene-chain double-bonds present in carotenoids can exist in mono-cis, poly-cis, or all-trans configurations; however, the vast majority of naturally occurring carotenoids exist in all-trans conformations. In organisms that can synthesize carotenoids (lower organisms and plants), the geometrical isomers of carotenoids have specific distributions and functions in their photobiology. For example, for Rhodospirillum rubrum the all-trans isomer of spirilloxanthin is selected by the light-harvesting complexes, whereas the 15-cis isomer is selected by the reaction centers [1]. In the photosynthetic tissues of plants, the 9′-cis form of neoxanthin (but not the all-trans form) was found in the chloroplasts of seed plants, ferns, mosses and green algae, which all contain chlorophylls a and b; whereas, the all-trans form of neoxanthin was found only in non-photosynthetic organs [2]. It is uncertain, however, if similar specific distributions and functions of geometrical isomers exists in animals, including humans.
Animals cannot synthesize carotenoids, but obtain them from diet. Dietary carotenoids exist mostly as all-trans isomers, but cis-isomers (5-, 9-, 13-, and 15-cis) are also present in food in significant quantities. These cis-isomers of carotenoids have also been identified in human plasma [3,4]. Some papers suggest a different intestinal uptake of trans- and cis-isomers of β-carotene [5,6]. It also has been shown that cis-isomers of lycopene are better absorbed than all-trans isomers, and it has been suggested that this is because cis-isomers of lycopene are more soluble (more readily incorporated) in chylomicrons than all-trans isomers [7,8]. A significant difference between the amount of cis-isomers of xanthophylls in both a monkey’s diet and a monkey’s plasma also has been reported [9]. It should be noted that the zeaxanthin-to-lutein ratio in the plasma of monkeys is very similar to that of humans. The 9-cis isomer of both lutein and zeaxanthin is generally present at a very low level the plasma of monkeys, much lower than is present in their diet; however, for the 13-cis isomer, the situation is reversed.
We are especially interested in the localization of macular xanthophylls in lipid bilayer membranes and their interaction with the membrane. All major macular xanthophylls—lutein, zeaxanthin, and meso-zeaxanthin—are dipolar, terminally dihydroxylated carotenoids. In the lipid bilayer membranes, all-trans isomers of these xanthophylls adopt transmembrane orientation with the polar terminal hydroxyl groups anchored in the polar headgroup region of the opposite leaflets of the bilayer [10,11,12,13,14,15,16]. The data demonstrate that lutein molecules can exists in two pools, one in which they accept transmembrane orientation, and the other in which they accept orientation parallel to the membrane surface, with the polar terminal hydroxyl groups anchored in the polar headgroup region of the same leaflet of the bilayer [11]. Only lutein and zeaxanthin are selectively accumulated in membranes of the eye retina from blood plasma. Another macular xanthophyll, meso-zeaxanthin, is presumably formed from lutein in the retina [17,18].
In addition to the all-trans isomers, the cis-isomers of macular xanthophylls also were isolated from the human eye retina and characterized [19]. The 9-cis and 13-cis isomers of zeaxanthin were present in the greatest amounts [19]. It is commonly accepted that these cis-isomers are mainly produced directly in the eye retina under the intensive light exposure from the all-trans isomers already selectively accumulated there from the blood plasma [20]. It is unknown if the cis-isomers are serving any function in the eye retina, or if they are only the products of light-induced isomerization. Also, their localization and orientation in the membrane were not investigated. Based on the molecular structure of the cis-isomers, localization of polar and hydrophobic parts of the molecule, and the “fit” to the membrane hydrophobic thickness, a model was proposed that placed the cis-isomers of zeaxanthin horizontally with respect to the plane of the membrane and with polar hydroxyl groups anchored in the same polar headgroup region (the same leaflet) of the bilayer (see Review [21]). However, there are no data that confirm or reject this model, and because of this we have undertaken measurements, using different EPR spin labeling approaches, to look at the effects of cis-isomers of zeaxanthin on different properties of the central region of the DMPC bilayer and compared them with those effects caused by the trans-isomer of zeaxanthin.
Zeaxanthin was chosen for these investigations because its interaction with membranes has been investigated in great details [10,22,23,24,25]. Additionally, zeaxanthin adopts only transmembrane orientation in lipid bialyers [10,11,15,16] (including the DMPC bilayer [10]), which makes the interpretation of our results much simpler. The saturated phospholipid DMPC was chosen because the interaction of different carotenoids (especially macular xanthophylls) with the DMPC bilayer membrane also has been extensively investigated [10,22,23,24,25,26]. It is also convenient that the DMPC bilayer has the main phase transition at 23.6°C, and, thus it exists at physiological temperatures in the biologically relevant fluid phase. Additionally, the effects of xanthophylls on membrane properties, observed by the use of EPR spin labeling methods in saturated membranes, are much greater than those observed in unsaturated membranes [24]. Also, we think the measurements in the membrane center, not at or close to the membrane surface, can unequivocally confirm which orientation of the cis-isomers of zeaxanthin in the membrane is the most probable, horizontal or transmembrane (see also Discussion). Because of this we chose the 14-PC to place the nitroxide moiety of the spin label exactly at the center of the DMPC bilayer, possessing 14 carbon atoms in the alkyl chains. Our measurements indicate that in the DMPC bilayer the transmembrane orientation of cis-isomers of zeaxanthin is prevalent; however, we cannot completely exclude the existence of the horizontal orientation.
2. Materials and methods
2.1. Materials
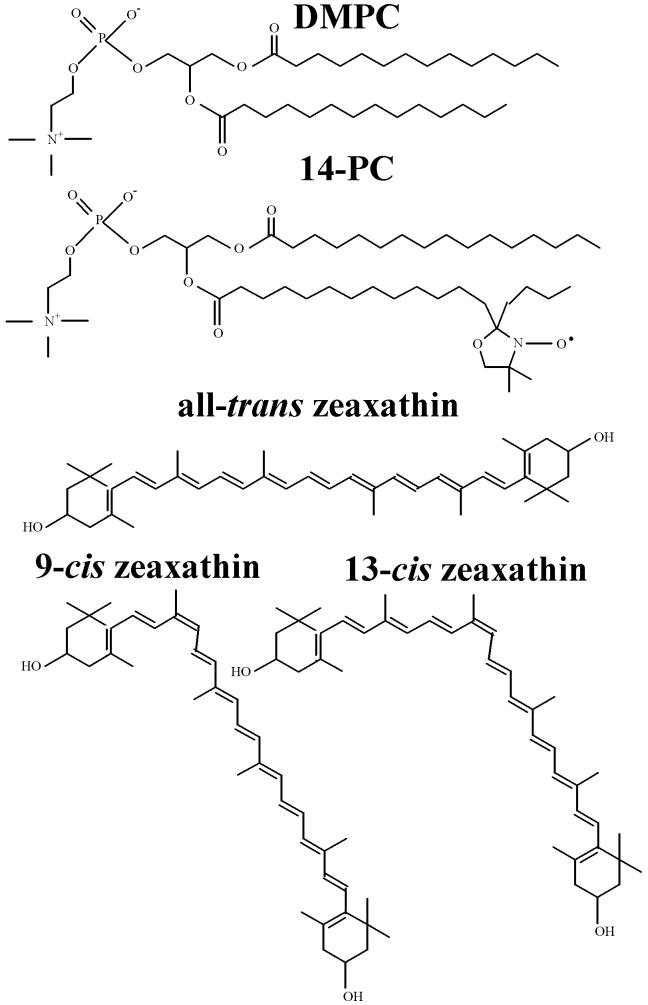
Synthetic crystalline all-trans zeaxanthin ((3R,3′R)-β,β-carotene-3,3′-diol) was purchased from CarotenNature, (Lupsingen, Switzerland). 9-cis and 13-cis isomers of zeaxanthin were obtained as a product of iodine-catalyzed photo-conversion of the all-trans form following the procedure described by Molnar et al. [27]. Isomeric forms of zeaxanthin were separated chromatographically on C-30-coated high-performance liquid chromatography (ProntSIL, length 250 mm, internal diameter: 4.6 mm). Solvent mixture of methyl tertiary butyl ether/methanol (5:95, v/v) was used as a mobile phase. The efficiency of this method was rather low, and amounts of available cis-isomers were limited. Dimyristoylphosphatidylcholine (DMPC), 1-palmitoyl-2-(14-doxylstearoyl)phosphatidylcholine (14-PC), and 1-palmitoyl-2-(5-doxylstearoyl)phosphatidylcholine (5-PC) were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL). Other chemicals of at least reagent grade were purchased from Aldrich (Milwaukee, WI). Chemical structures of geometrical isomers of zeaxanthin, DMPC and 14-PC, are presented in Fig. 1.
Fig. 1.
The structures of all-trans, 9-cis, and 13-cis geometrical isomers of zeaxanthin together with the structure of DMPC and 14-PC spin label.
2.2. Preparation of liposomes
The membranes used in this work were multilamellar dispersions of DMPC containing 1 mol% 14-PC and 10 mol% 9-cis, 13-cis, or all-trans zeaxanthin added to the sample during preparation. Briefly, these liposomes were prepared by the following method [23]: chloroform solutions of lipids, isomers of zeaxanthin, and 14-PC were mixed (containing ∼0.5 μmol of total lipids); the chloroform was evaporated with a stream of nitrogen gas; and the lipid film on the bottom of the test tube thoroughly dried under reduced pressure (about 0.1 mmHg) for 12 h. Preheated buffer solution (0.25 mL of 10 mM PIPES and 150 mM NaCl, pH 7.0) was added to the dried film and vortexed at 40°C for ∼15 minutes. The multilamellar liposomes were centrifuged briefly (15 min at 4°C with an Eppendorf bench centrifuge at 16,000 g), and the loose pellet (about 20% lipid, w/w) was used for EPR measurements.
2.3. Conventional EPR
For conventional EPR measurements, the sample was placed in a 0.6 mm i.d. capillary made of gas-permeable methylpentene polymer, called TPX, and the capillary was placed inside the EPR dewar insert. It was than equilibrated with nitrogen gas used for temperature control [28,29]. The sample was thoroughly deoxygenated. EPR spectra were obtained with an X-band Bruker EMX spectrometer with temperature-control accessories. Modulation amplitude of 0.5 G and an incident microwave power of 5.0 mW were used for measurement at 35°C. To measure the hydrophobicity at the membrane center, the z-component of the hyperfine interaction tensor of the 14-PC, AZ, was determined from the EPR spectra of 14-PC for samples frozen at -163°C, recorded with modulation amplitude of 2 G and an incident microwave power of 2 mW [30]. Figure 2 shows the typical conventional EPR spectra of 14-PC in investigated membranes recorded at 35°C and -163°C with the indication of the measured spectral parameters.
Fig. 2.
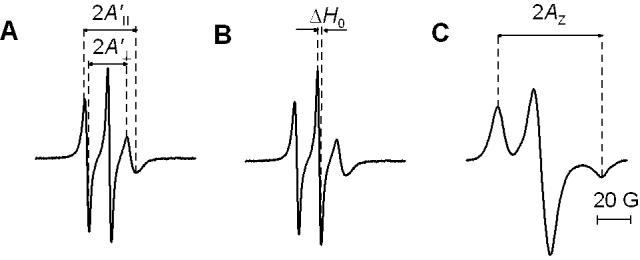
EPR spectra of 14-PC in DMPC membranes containing 10 mol% 9-cis zeaxanthin recorded at 35°C (A,B) and -163°C (C). Measured values for evaluating order parameters (A’II and A’⊥) are indicated in (A). In (B) the measured ΔH0 value (peak-to-peak central line width) is indicated. In (C) the measured 2AZ value (z-component of the hyperfine interaction tensor) is indicated.
2.5. Saturation-recovery EPR
The spin-lattice relaxation times (T1s) of the 14-PC were determined by analyzing the saturation-recovery signal of the central line obtained by short-pulse saturation-recovery EPR at X-band [31,32]. Accumulation of the saturation-recovery signals was carried out with 2048 data points on each decay. Details for measurements with carotenoids are described in our earlier paper [33] (see also the caption to Fig. 3A).
Fig. 3.
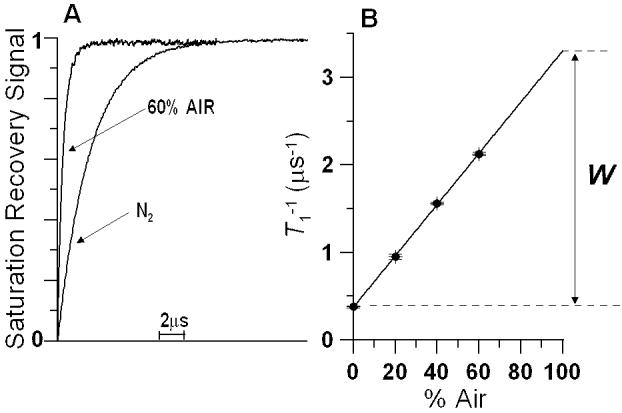
(A)Representative saturation-recovery signals of 14-PC in DMPC membranes containing 10 mol% 9-cis zeaxanthin at 35°C for samples equilibrated at 35°C with nitrogen gas and with a gas mixture of 60% air and 40% nitrogen. The fits to the single exponential curves with recovery times of 2.64 μs (N2) and 0.47 μs (60% air) were satisfactory. The decay time constant can be determined with the accuracy of ±3%. (B) T1-1 for 14-PC in DMPC membranes containing 10 mol% 9-cis zeaxanthin at 35°C plotted as % air in the equilibrating gas mixture. Experimental points show a linear dependence up to 60% air, and extrapolation to 100% is performed to indicate a way of calculating the oxygen transport parameters, W. The W values can be evaluated with the accuracy better than ±10%.
In these experiments, the bimolecular collision rate between oxygen (a fast-relaxing species) and the nitroxide moiety of 14-PC (a slow-relaxing species) was evaluated in terms of an oxygen transport parameter (W(x)). W(x) was defined as
| (1) |
where the T1s are the spin-lattice relaxation times of the nitroxide in samples equilibrated with atmospheric air and nitrogen, respectively [34]. W(x) is proportional to the product of the local translational diffusion coefficient D(x) and the local concentration C(x) of oxygen at a “depth” x in a lipid bilayer that is equilibrated in the atmospheric air (at C14 position in present experiments):
| (2) |
For measurements of the oxygen transport parameter, the concentration of oxygen in the sample was controlled by equilibration with the same gas that was used for the temperature control (i.e., a controlled mixture of nitrogen and dry air adjusted with flowmeters (Matheson Gas Products model 7631H-604) [28,29]) (see also Fig. 3B for more explanation).
3. Results
3.1. Saturation-recovery measurements
To get more insight into the interactions of different isomers of zeaxanthin with the lipid bilayer in the bilayer center, we applied the saturation-recovery spin-label oximetry method, which is a dual-probe saturation-recovery EPR approach in which the observable parameter is the spin-lattice relaxation time (T1) of lipid spin labels and the measured value is the bimolecular collision rate between molecular oxygen and the nitroxide moiety of spin labels. This method has proven to be extremely sensitive to changes in the local oxygen diffusion-concentration product (around the nitroxide moiety) because of the long T1 of lipid spin labels (1-10 μs) and also because molecular oxygen is a unique probe molecule. Molecular oxygen is paramagnetic, small, and has an appropriate level of hydrophobicity that allows it to enter the small vacant pockets that are transiently formed in the lipid bilayer membrane. Therefore, collision rates between molecular oxygen and nitroxide spin labels at specific locations in the membrane are sensitive to the dynamics of gauche-trans isomerization of lipid hydrocarbon chains and to the structural nonconformability on neighboring lipids [31,35,36,37]. The free volume in the lipid bilayer may be very small, just sufficient to contain a single molecule of oxygen. Kusumi et al. [34] concluded, that the oxygen transport parameter (see Eq. 1) is a useful monitor of membrane fluidity that reports on translational diffusion of small molecules. We used this parameter to monitor effects of different isomers of zeaxanthine in the central region of the DMPC bilayer.
Figure 3A shows typical saturation-recovery curves for 14-PC in the DMPC membrane containing 10 mol% 9-cis zeaxanthin at 35°C in the presence and absence of oxygen. The recovery curves were fitted by single, double, and triple exponentials and compared. For all of the recovery curves obtained in this work, the results indicated that no substantial improvement in the fitting was observed when the number of exponentials was increased from one, suggesting that these recovery curves can be analyzed as single exponentials. The decay time constants were determined with an accuracy of ±3%.
The spin-lattice relaxation times of 14-PC recorded for DMPC samples containing cis-isomers of zeaxanthin (in the absence of oxygen) were always significantly greater than the spin-lattice relaxation time recorded for the pure DMPC bilayer (without zeaxanthin). This result, in agreement with the theoretical development on spin-lattice relaxation of nitroxides [38], indicates that the translational motion of 14-PC is suppressed. In our earlier work [33], we showed that the macular xanthophyll lutein increases T1 of lipid spin labels at all depths in the membranes, while cholesterol increases T1 in and near the polar headgroup region and decreases it in the membrane center. The major conclusion from this work was that cholesterol increases frequency of chain-bending in the membrane center, while lutein decreases this frequency. Comparison of the effects of xanthophylls and cholesterol in the membrane center should help to determine position and orientation of cis-isomers in the lipid bilayer (see also Discussion).
3.2. Oxygen transport parameter at the membrane center
Saturation-recovery measurements were carried out systematically as a function of the partial pressure of oxygen in the equilibrating gas mixture for all investigated isomers of zeaxanthin present in the DMPC bilayer. Previously, we proved that lipid spin labels are able to partition between different membrane domains, and that the presence of two recovery constants in the saturation-recovery curve (which indicates the presence of two membrane environments) can be found more readily in the presence of oxygen [32,35,39,40]. However, in this work, single exponential recovery was constantly observed, both in the absence and presence of oxygen. This indicates the presence of a single homogenous membrane when averaged over 0.3 μs (the shortest recovery time observed here), and that, in the membranes, the rate of lipid exchange among the purported membrane domains is greater than the T1 relaxation rate (greater than 3.3 × 106 s-1, see Refs. [32,35] for more details).
In Fig. 3B, T1-1 values measured for 14-PC in the DMPC membranes containing 10 mol% 9-cis zeaxanthin at 35°C are plotted as a function of oxygen concentration (in % air) in the equilibrating gas mixture. All plots of T1-1 for investigated membranes show a linear dependence on the oxygen concentration between 0 and 60% air. For each sample, the oxygen transport parameter was obtained by extrapolating the linear plot to the sample equilibrated with atmospheric air (100% air, see Eq. (1)) as shown in Fig. 3B. This process is required because accurate observation of saturation recovery becomes increasingly difficult as the oxygen partial pressure is increased due to fast relaxations. Not less than three decay measurements were performed for each point in the plot with accuracy of the evaluation of W(x) better than ±10%.
The oxygen transport parameter at 35°C for DMPC membranes with and without the addition of 10 mol% isomers of zeaxanthin obtained using 14-PC spin labels are shown in Fig. 4. Significant decreases in W(x) at the membrane center of the DMPC bilayer were induced by incorporating all of the isomers of zeaxanthin in the membrane. The strongest effect was observed for the 13-cis isomer, a weaker effect for the 9-cis isomer, and the weakest for the all-trans isomer. The very strong effect of cis-isomers on the oxygen transport parameter in the membrane center suggests that the investigated cis-isomers of zeaxanthin, similarly to the all-trans isomers, are located in the membrane center, adopting transmembrane orientation with the polar terminal hydroxyl groups anchored in the opposite leaflets of the bilayer. This was a rather unexpected result in light of the model appearing in the literature, which placed cis-isomers of zeaxanthin horizontally with respect to the plane of the membrane and with polar hydroxyl groups anchored in the same polar headgroup region (the same leaflet) of the bilayer (see [21,41]). Because of this we have undertaken additional measurements to see how these cis-isomers affect other membrane properties including alkyl chain order, fluidity, and hydrophobicity.
Fig. 4.
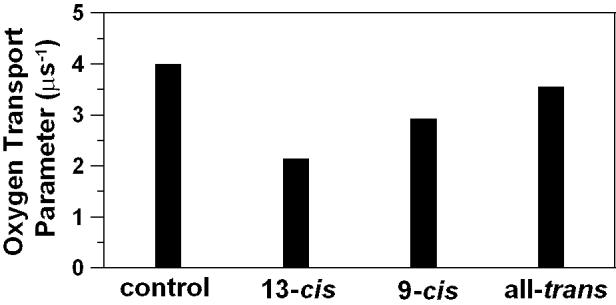
The oxygen transport parameter measured at 35°C with the use of 14-PC in the center of the pure DMPC membrane (Contral) and DMPC membranes containing 10 mol% 13-cis, 9-cis, or all-trans isomer of zeaxanthin. The accuracy of the evaluation of the oxygen transport parameter is better than ±10%.
3.4. Order of membrane interior
One of the features of conventional EPR spectra of 14-PC for samples containing cis- and trans-isomers of zeaxanthin is that none show any indication of the presence of two components (weakly and strongly immobilized). We can conclude that zeaxanthin is well dispersed in the membrane on a time scale determined by the anisotropy of the hyperfine interaction of the nitroxide (10 ns). This result is consistent with the oxygen transport data, in which all of the saturation-recovery curves were single-exponential curves. We should mention here that the use of a spin probe attached to the C14 position of the alkyl chain was first employed by Marsh and his colleagues [42,43]. Their results indicate that the presence of two components in a conventional EPR spectrum is visually clearer with phospholipids labeled at C14 than at other positions in the alkyl chain.
Effects of 10 mol% geometrical isomers of zeaxanthin added to the sample on the order parameter of 14-PC are summarized in Fig. 5. The order parameter was calculated according to Marsh [44] from parameters measured directly from the EPR spectra as indicated in Fig. 2. It can be seen that the effect of cis-isomers of zeaxanthin is greater than the effect of the all-trans isomer. However, no significant difference in the effect of 9-cis and 13-cis zeaxanthin was indicated. Results presented in Fig. 5 confirm our conclusion made in Section 3.2 about transmembrane localization of cis-isomers of zeaxanthin in the DMPC bilayer.
Fig. 5.
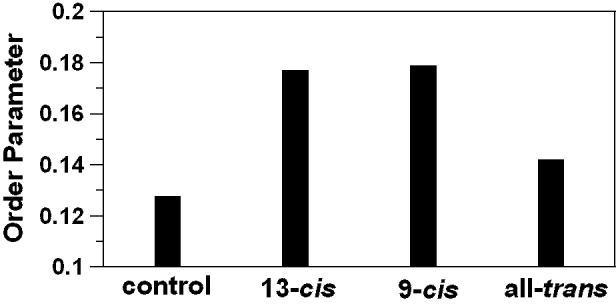
Changes of the molecular order parameter of 14-PC in DMPC membranes induced by the addition of 10 mol% 13-cis, 9-cis, or all-trans isomer of zeaxanthin and measured at 35°C. Control indicates order parameter measured without additions. Because of the sharpness of the EPR lines for 14-PC (see Fig. 2), A’II and A’⊥ values can be measured with the accuracy of ±0.1 G, and the order parameter can be evaluated with the accuracy of ±0.015.
In the membrane, the alkyl chain of 14-PC with the nitroxide moiety attached at the C14 position (see Fig. 1) undergoes rapid anisotropic motion about the long axis of the spin label and wobbling motion of the long axis within the confines of a cone imposed by the membrane environment. Increase in an order parameter indicates that the cone angle of the cone for the wobbling motion of the alkyl chain decrease. Because cis- and trans-isomers of zeaxanthin exert their effects by the steric contact with alkyl chains and introducing the “rigid walls” for wobbling motion, it can be inferred that the rigid, bar-like portion of both isomers is located in the membrane center. Thus, obtained results suggest that the investigated cis-isomers of zeaxanthin, similarly to the all-trans isomer, are located in the membrane interior, adopting transmembrane orientation with the polar terminal hydroxyl groups located in the opposite leaflets of the bilayer.
3.3. Fluidity of membrane interior
Membrane fluidity can be evaluated directly from the EPR spectra of 16-PC (or 16-SASL) by measuring the effective rotational correlation time of the free radical moiety of this lipid spin label, assuming its isotropic rotational diffusion [45]. The rotational correlation time can be calculated from the linear and the quadratic term of the line width parameter. As was indicated in Ref. [45] the motional model is fairly good and the motion is isotropic when both calculated correlation times are similar and correlation time is smaller than 2 ns. Here we used 14-PC because of the best match with the thickness of the DMPC bilayer (14 carbon atoms in the hydrocarbon chain). The nitroxide moiety of 14-PC shows less motion in the DMPC bilayer than 16-PC, and evaluated rotational correlation times are close to 2 ns. Additionally, rotational correlation times evaluated from the linear and the quadratic term of the line width parameter are very different, which indicate onset of anisotropic motion and the invalidity of using the above-mentioned model. Because of this we used the peak-to-peak width of the central line of the EPR spectrum as a conventional experimental parameter related to the rotational motion of the nitroxide moiety of 14-PC (see also Refs. [24,46] for the use of this parameter). Bigger peak-to-peak width indicates slower rotational motion. Figure 6 shows the peak-to-peak line width of 14-PC in the DMPC bilayer in the absence and presence of 10 mol% geometrical isomers of zeaxanthin. All isomers increase the line width of the EPR spectrum, indicating that they not only decrease the cone angle of the cone inside of which wobbling motion occurs, but also decrease the rate of rotation inside the cone for flexible hydrocarbon chains. As in earlier observations, the effect of the 13-cis isomer is the greatest and the effect of the trans-isomer is the smallest.
Fig. 6.
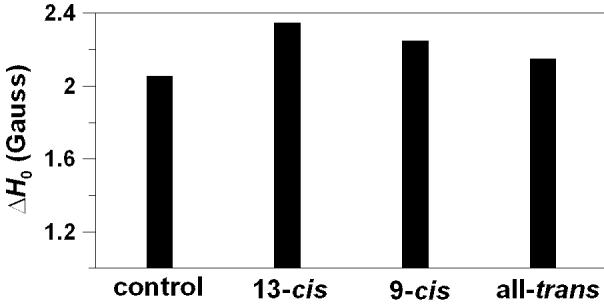
Changes of the peak-to-peak central line width, ΔH0, of 14-PC in DMPC membranes induced by the addition of 10 mol% 13-cis, 9-cis, or all-trans isomer of zeaxanthin and measured at 35°C. Control indicates ΔH0 measured without additions. Because of the sharpness of the central EPR lines for 14-PC (see Fig. 2), the ΔH0 value can be measured with the accuracy better than ±0.05 G.
We would like to point out that an order parameter indicates the static property of the lipid bilayer, whereas the rotational motion and the oxygen transport parameter characterize membrane dynamics (membrane fluidity) that report on rotational diffusion of alkyl chains and translational diffusion of oxygen molecules, respectively. The EPR spin-labeling approach also makes it possible to monitor another bulk property of the lipid bilayer membrane, namely local membrane hydrophobicity.
3.5. Hydrophobicity of membrane interior
To compare effects of 9-cis and 13-cis isomers of zeaxanthin with the all-trans isomer on the hydrophobicity of the membrane center, we used, as a conventional parameter, the z-component of the hyperfine interaction tensor (AZ) of 14-PC [23,30]. With the increase of the local hydrophobicity around the nitroxide moiety of 14-PC, AZ decreases. Results presented in Fig. 7 indicate that all isomers significantly increase the hydrophobicity of the membrane interior, with the effects of cis-isomers being greater than those of the trans-isomer. The difference between the effects of 9-cis and 13-cis isomers is negligible. Usually, we relate the local hydrophobicity in the lipid bilayer as observed by AZ to the hydrophobicity (or ε) of bulk organic solvent by referring to Fig. 2 in Ref. [30]. After addition of 10 mol% zeaxanthin the hydrophobicity of the DMPC membrane center increases from the level close to that of 2-propanol (ε ≈ 20) for pure DMPC to the level close to that of 1-decanol and ethyl acetate (ε ≈ 6-9) for trans-zeaxanthin, and to the level close to that of dipropylamine and N-butylamine (ε ≈ 3-4) for 9-cis and 13-cis zeaxanthin. The induced increase of hydrophobicity is significant, causing decrease of the dielectric constant from the value of ∼20 to close to 3. Griffith et al. [47,48] demonstrated that the hydrophobicity in the membrane interior is largely determined by the extent of water penetration into the membrane, since the dehydration abolishes the hydrophobicity gradient in liposome samples. The greater effect of the cis-isomers in the membrane center is probably a result of the introduction of a larger number of conjugated double bonds into that region (due to their bent structure), as compared with the trans-isomer (see Fig. 1 and scheme presented in Fig. 8). Lipid bilayers containing unsaturated alkyl chains (double bonds) demonstrate less water penetration into the hydrocarbon region than saturated bilayers [30,49]. It is also known that unsaturated hydrocarbons are less hygroscopic than saturated hydrocarbons.
Fig. 7.
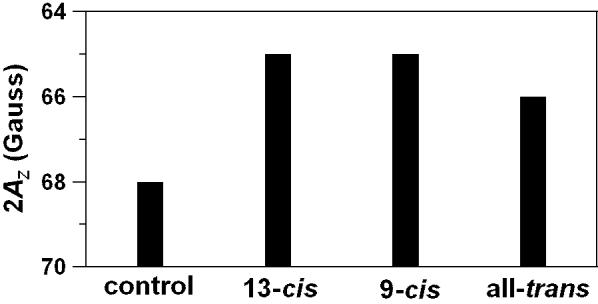
2AZ values (in gauss) for DMPC membranes in the absence and presence of 10 mol% 13-cis, 9-cis, or all-trans isomer of zeaxanthin measured with 14-PC. Taller bars indicate higher hydrophobicity. 2AZ values can be measured with the accuracy of ±0.25 G.
Fig. 8.
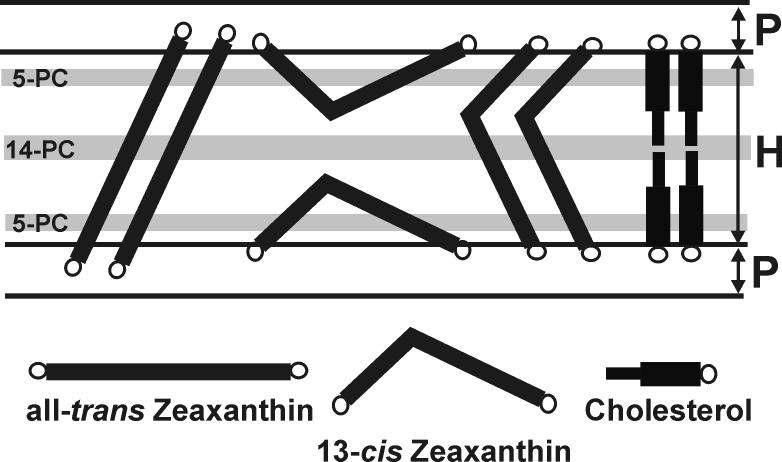
A schematic drawing of the localization of different isomers of zeaxanthin in the DMPC bilayer. The horizontally orientated cis-isomers of zeaxanthin should create more vacant pockets in the membrane center and increase membrane dynamics in that region. Their effects should be very similar to those caused by cholesterol molecules. The transmembrane orientated cis-somers of zeaxanthin should decrease membrane dynamics in the membrane center. Their effects should be very similarly to those caused by all-trans zeaxanthin. Hatched areas indicate regions of the membrane probed by 14-PC and 5-PC.
In our earlier paper, we showed that the trans-isomers of dipolar xanthophylls increase the hydrophobicity of the saturated membrane interior and the effect monitored in the membrane center is independent of the membrane thickness [23]. However, the effects in and near the polar headgroup regions strongly depend on the membrane thickness, more strictly, on its relation to the length of the xanthophyll molecule (distance between the polar hydroxyl groups at both ends of the molecule). In thin membranes (DLPC, DMPC), these dipolar xanthophylls are tilted with respect to the bilayer normal [10,23,24]. In thick membranes (DSPC, DBPC), the polar groups of dipolar xanthophylls sink deeper into the hydrocarbon region, preserving, however, their transmembrane orientation (for DBPC, see Ref. [24]). In our present work, the thickness of the bilayer is fixed (the same DMPC was used in all measurements); however, the distance between the polar hydroxyl groups in different xanthophyll isomers decreases as all-trans > 9-cis > 13-cis. We think the above explanation should also be valid in this case, supporting our conclusion about transmembrane orientation of cis-isomers in the lipid bilayer.
3.6. Effects at C5 position
The amount of available cis-isomers of zeaxanthin was very limited; however, we were able to perform a few measurements at a depth close to the membrane surface. We measured effects of the inclusion 10 mol% 13-cis isomer of zeaxanthin on the order, hydrophobicity, and oxygen transport parameter at the C5 position in fluid phase DMPC membranes with the use of 5-PC spin label. Observed effects were compared with those caused by the all-trans isomer of zeaxanthin. Both isomers increased alkyl chain order and hydrophobicity, and decreased the oxygen transport parameter (data not shown). The effects caused by the 13-cis isomer were greater than those caused by the all-trans isomer. Obtained results can be explained by the transmembrane and/or horizontal orientation of the 13-cis isomer of zeaxanthin in the DMPC bilayer. At the transmembrane orientation, both ends of the 13-cis isomer should sink deeper into the lipid bilayer and ionone rings should be closer to the nitroxide at the C5 position, strongly influencing lipid bilayer properties at this depth. In a similar way, the all-trans xanthophylls affected properties of thick membranes at C5 position more strongly than property of thin membranes because ionone rings sink deeper in thick membranes [23,24]. It is also probable that at the horizontal orientation, when the 13-cis zeaxanthin molecule is located in one leaflet and anchored by the terminal hydroxyl groups at the same polar headgroup region, the effect of the 13-cis isomer on membrane properties measured at the C5 position is stronger than the effect of the trans-isomer. As expected, measurements at the C5 position cannot distinguish between these two orientations of cis-zeaxanthin molecules.
Discussion
Previously, we investigated the interaction of trans-isomers of xanthophylls (including trans-zeaxanthin) with model membranes, looking at alkyl chain order and rotational motion, membrane phase transition, and hydrophobicity of the membrane interior [22,23,24,25,26,33,50]. Our results were in principal agreement with those obtained for biological membranes [51,52,53]. In our investigations, special attention was paid to the effects of carotenoids on the profiles of the oxygen transport parameter across the lipid bilayer [25,50]. We have also investigated the same membrane properties in the presence of cholesterol [30,31,33,36,39,54,55]. This work has shown that the effects of trans-xanthophylls (trans-zeaxanthin) on the structure and dynamics of lipid bilayer membranes are similar to the effects of cholesterol in many aspects. Both increase the order and decrease the alkyl chain motion (observed with a conventional EPR spin-labeling method) in fluid-phase membranes and both are known to broaden the gel-to-fluid phase transition and increase the mobility of polar head groups. As a rule, the presence of unsaturated alkyl chains moderates the effect of trans-xanthophylls and cholesterol. A quantity of 10 mol% trans-isomers of dipolar xanthophylls added to the sample exerts an effect similar to that of 15-20 mol% cholesterol. Both modifiers also increase the hydrophobicity of the membrane center. However, trans-isomers of dipolar xanthophylls decrease the oxygen transport parameter (oxygen diffusion-concentration product) in saturated and unsaturated membranes, with the effect strongest in the membrane center [25,50], whereas cholesterol does not change or increase the oxygen transport parameter in the membrane center [31,36,39,55]. Furthermore, trans-isomers of dipolar xanthophylls decrease the frequency of alkyl chain bending, with the effect strongest in the membrane center, whereas cholesterol decreases this frequency near the polar headgroup region and increases it in the membrane center [33]. These effects are manifested not only by changes in frequency of bimolecular collisions between spin-label pairs, but also by changes in the spin-lattice relaxation time of spin labels. Dipolar xanthophylls in all-trans conformations increase the T1 of spin labels in the membrane center, while cholesterol decreases it.
The observed differences in the effects of these modifiers result from differences in the structure of all-trans dipolar xanthophylls and cholesterol and from their different locations within the lipid bilayer membrane (see Figs. 1 and 8). The cholesterol molecule is located in one leaflet of the bilayer, and its rigid plate-like portion extends to the depth of the 7th to 10th carbon atom in a lipid hydrocarbon chain [56]. The cross-section of the isooctyl chain of the cholesterol molecule is much smaller than the cross-section of the rigid steroid ring and, therefore, produces additional possibilities for undulation and trans-gauche transitions of alkyl chains in the membrane center. Thus, the cholesterol molecule creates many vacant pockets in the membrane center that oxygen molecules can occupy, jumping from one pocket to an adjacent one or moving with the movement of the pocket itself due to the rapid gauche-trans isomerization of the phospholipid hydrocarbon chains. In contrast, one dipolar carotenoid molecule influences both halves of the lipid bilayer. With two polar groups interacting with opposite hydrophilic regions of the membrane, this molecule can brace together the two halves of the bilayer like a tie-bar [52]. Therefore, both—the oxygen transport parameter and vertical fluctuations of the ends of alkyl chains—are reduced in those regions of the lipid bilayer membranes to which the rigid portion of molecule of the modifier extends (see scheme in Fig. 8).
In our present work, using one spectroscopic technique but different approaches, we were able to obtain a vast amount of information from one membrane location, in this case from the membrane center (C14 position). As indicated above, this position is the most significant if we choose to distinguish between two orientations of cis-isomers of zeaxanthin in the lipid bilayert membrane. If the most accepted model, the horizontal orientation of cis-isomers, is correct [21,41], the effects observed in the membrane center should be similar to those observed for cholesterol molecules. If the transmembrane orientation is prevalent, the effects of cis-isomers should be similar to those caused by the trans-zeaxanthin (see scheme in Fig. 8). Effects observed in membrane regions closer to the membrane surface should be similar for both orientations of the cis-isomers of zeaxanthin. We should mention here that we are measuring averaged effects from both membrane leaflets.
Present results clearly showed that effects of cis-isomers of zeaxanthin on all investigated membrane properties observed in the membrane center and near the polar headgroup region are similar to those caused by the trans-isomer. However, effects of cis-isomers, observed in the membrane center, on the oxygen transport parameter and T1 value of 14-PC are very different from those caused by cholesterol. This allowed us to conclude that most molecules of cis-isomers adopt transmembrane orientation, similar to that adopted by molecules of the trans-isomer. Unexpectedly, effects of cis-isomers were greater than those caused by the trans-isomer. Similar observations were made by Kostecka-Gugola A., Milanowska J., Gruszecki W. I., and Strzalka K. (personal communication), who also detected greater effects of 9-cis and 13-cis isomers of zeaxanthin on thermodynamic characteristics of the main phase transition of the DPPC membrane, measured with the differential scanning calorimetry, than effects caused by the all-trans isomer. This can be explained by the fact that the rigid hydrocarbon portion of transmembran oriented cis-isomers is tilted with respect to the membrane normal (see Fig. 8), and/or that cis-isomers are better soluble in the lipid bilayer than trans-isomers (they do not form higher aggregates).
It was shown that all-trans isomers of dipolar xanthophylls affect the properties of thin membranes (DLPC, DMPC) more strongly than the properties of thick membranes (DPPC, DSPC, DBPC) [22,24], presumably because in thin membranes these dipolar xanthophylls are tilted with respect to the bilayer normal [10,57,58,59,60] and interact with the larger number of alkyl chains. Thus, the effect of the tilted cis-isomer should be stronger than the effect of the less-tilted trans-isomer. However, we should mention here that in the DMPC bilayer trans-zeaxanthin is tilted by ∼25° [10], which somewhat weakens our explanation. Our second explanation was based on the statement that dipolar xanthophylls should affect membrane properties mainly when they are dissolved in the lipid bilayer as monomers. The reported threshold of the solubility for all-trans xanthophylls in lipid membranes is ∼10 mol% [61]; however, lower and higher values were also indicated (see Discussion in Ref. [22]). Organization of cis-zeaxanthin in the membrane is less investigated. We can, however, make some conclusions based on measurements with monomolecular layers on the air-water interface formed from the mixture of DPPC and all-trans, 9-cis, or 13-cis zeaxanthin [41,62]. Authors of these papers showed that the concentration of zeaxanthin in the monomolecular layer at which aggregation starts is ∼5 mol% for trans-, ∼20 mol% for 9-cis, and higher than 20 mol% for 13-cis zeaxanthin. Additionally, cis-isomers of zeaxanthin do not show a tendency to organize into higher aggregates, even in a very polar solvent like ethanol/water mixture (5:95 v/v), where the trans-isomers aggregate easily [41]. Having in mind the above discussion, we can argue that the later explanation is more probable.
The transmembrane orientation of the cis-isomers of zeaxanthin is probably enhanced in thin lipid-bilayer membranes in which the thickness of the hydrophobic core is smaller than the distance between the hydroxyl groups at the 3 and 3′ positions of zeaxanthin. The thicknesses of the hydrocarbon region in the fluid-phase DMPC bilayer (H in Fig. 8) and the polar headgroup and glycerol backbone region (P in Fig. 8) are 24.4 Å and 5.3 Å, respectively (see Ref [63] for evaluating these thicknesses). The distances between polar hydroxyl groups (O-O) in different geometrical isomers of zeaxanthin were obtained using molecular modeling techniques and were provided to us by Ewa Borcowska, a Ph.D. student of Dr. Marta Pasenkiewicz-Gierula. They are as follows: 30.52 Å for all-trans, 26.86 Å for 9-cis, and 24.38 Å for 13-cis. Thus, all isomers are able to span the hydrophobic core of the DMPC membrane. However, in thick membranes, the horizontal orientation of the cis-isomers may be prevalent.
We would like to add that conventional EPR spectra as well as saturation-recovery curves measured in both the presence and absence of molecular oxygen showed that 14-PC detects the existence of a single homogenous environment, indicating that both carotenoids and DMPC are likely to be undergoing fast translational diffusion in DMPC/zeaxanthin membranes. If the zeaxanthin reach domains are formed, the lipid exchange rates between these purported regions should be faster than 10 ns (time scale determined by the anisotropy of the hyperfine interaction of the nitroxide), and/or these regions must be forming and dispersing rapidly on a time scale shorter than 0.3 μs (the shortest spin-lattice relaxation time measured in the presence of oxygen). Although, this does not exclude the possibility of the formation of small, stable aggregates of zeaxanthin (see discussion above).
Acknowledgements
This work was supported by grants EY015526, EB002052, and EB001980 of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Koyama Y, Takatsuka I, Kanaji M, Tomimoto K, Krro M, Shimamura T, Ymashita J, Saiki K, Tsukida K. Configurations of carotenoids in the reaction center and the light-harvesting complex of Rhodospirillum rubrum. Natural selection of carotenoid configurations by pigment protein complexes. Photochem. Photobiol. 1990;51:119–128. [Google Scholar]
- [2].Bouvier F, D’harlingue A, Backhaus RA, Kumagai MH, Camara B. Identification of neoxanthin synthase as a carotenoid cyclase paralog. Eur. J. Biochem. 2000;267:6346–6352. doi: 10.1046/j.1432-1327.2000.01722.x. [DOI] [PubMed] [Google Scholar]
- [3].Krinsky NI, Russett MM, Handelman GJ, Sondderly DM. Structural and geometrical isomers of carotenoids in human plasma. J. Nutr. 1990;120:1654–1662. doi: 10.1093/jn/120.12.1654. [DOI] [PubMed] [Google Scholar]
- [4].Khachik F, Spangler CJ, Smith JC, Jr., Canfield LM, Steck A, Pfander H. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal. Chem. 1997;69:1873–1881. doi: 10.1021/ac961085i. [DOI] [PubMed] [Google Scholar]
- [5].Stahl W, Schwarz W, Von Laar J, Sies H. All-trans β-carotene preferentially accumulates in human chylomicrons and very low density lipoproteins compared with the 9-cis geometrical isomers. J. Nutr. 1995;125:2128–2133. doi: 10.1093/jn/125.8.2128. [DOI] [PubMed] [Google Scholar]
- [6].Ederman JW, Jr., Thatcher AJ, Hofmann NE, Lederman JD, Block SS, Lee CM, Mokady S. All-trans beta-carotene is absorbed preferentially to 9-cis beta-carotene but the latter accumulates in the tissues of domestic ferrets (Mustela putorius puro) J. Nutr. 1998;128:2009–2013. doi: 10.1093/jn/128.11.2009. [DOI] [PubMed] [Google Scholar]
- [7].Boileau AC, Merchen NR, Wasson K, Atkinson CA, Ederman JW., Jr. Cis-lycopene is more bioavailable than trans-lycopene in vitro an in vivo in lymph-cannulated ferrets. J. Nut. 1999;129:1176–1181. doi: 10.1093/jn/129.6.1176. [DOI] [PubMed] [Google Scholar]
- [8].Stahl W, Schwarz W, Saundquiste AR, Sies H. Cis-trans isomers of lycopene and beta-carotene in human serum and tissues. Arch Biochem. Biophys. 1992;294:173–177. doi: 10.1016/0003-9861(92)90153-n. [DOI] [PubMed] [Google Scholar]
- [9].Max Sondderly D, Russett MD, Land R, Krinsky NI. Palsma carotenoids of Monkeys (Macaca fascicularis and Saimiri sciureus) J. Nutr. 1990;120:1663–1671. doi: 10.1093/jn/120.12.1663. [DOI] [PubMed] [Google Scholar]
- [10].Gruszecki WI, Sielewiesiuk J. Orientation of xanthophylls in phosphatidylcholine multibilayer. Biochim. Biophys. Acta. 1990;1023:405–412. doi: 10.1016/0005-2736(90)90133-9. [DOI] [PubMed] [Google Scholar]
- [11].Sujak A, Gabrielska J, Grudziński W, Borc R, Mazurek P, Gruszecki WI. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structural aspects. Arch. Biochem. Biophys. 1999;371:301–307. doi: 10.1006/abbi.1999.1437. [DOI] [PubMed] [Google Scholar]
- [12].Bone RA, Landrum JT. Macular pigment in Henle fiber membranes: a model for Haidinger’s brushes. Vis. Res. 1984;24:103–108. doi: 10.1016/0042-6989(84)90094-4. [DOI] [PubMed] [Google Scholar]
- [13].Bone RA, Landrum JT, Cains A. Optical density spectra of the macular pigment in vivo and in vitro. Vision Res. 1992;32:105–110. doi: 10.1016/0042-6989(92)90118-3. [DOI] [PubMed] [Google Scholar]
- [14].Bone RA, Landrum JT. Dichroism of lutein: a possible basis for Haidinger’s brushes. Appl. Opt. 1983;22:775–776. doi: 10.1364/ao.22.000775. [DOI] [PubMed] [Google Scholar]
- [15].Gruszecki WI, Sujak A, Strzałka K, Radunz A, Schmid GH. Organisation of xanthophyll-lipid membranes studied by means of specific pigment antisera, spectrophotometry and monomolecular layer technique: Lutein versus zeaxanthin. Z. Naturforsch. C. 1999;54:517–525. doi: 10.1515/znc-1999-7-810. [DOI] [PubMed] [Google Scholar]
- [16].Okulski W, Sujak A, Gruszecki WI. Dipalmitoylphosphatidylcholine membranes modified with zeaxanthin: numeric study of membrane organization. Biochim. Biophys. Acta. 2000;1509:216–228. doi: 10.1016/s0005-2736(00)00298-4. [DOI] [PubMed] [Google Scholar]
- [17].Bone RA, Landrum JT, Hime GW, Cains A. Stereochemistry of the human macular carotenoids. Invest. Ophthalmol. Vis. Sci. 1993;34:2033–2040. [PubMed] [Google Scholar]
- [18].Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001;385:28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- [19].Berstein PS, Khachik F, Carvalho LS, Garth JM, Da-You Z, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of human eye. Exp. Eye Res. 2001;72:215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- [20].Krinsky NI. Possible biologic mechanisms for protective role of xanthophylls. J. Nutr. 2002;132:540S–542S. doi: 10.1093/jn/132.3.540S. [DOI] [PubMed] [Google Scholar]
- [21].Gruszecki WI. Carotenoid orientation: role in membrane stabilization. In: Krinsky NI, Mayne ST, Sies H, editors. Carotenoids in health and disease. Marcel Dekker; New York: 2004. pp. 151–163. [Google Scholar]
- [22].Wisniewska A, Widomska J, Subczynski WK. Carotenoid-membrane interactions in liposomes: effect of dipolar, monopolar, and nonpolar carotenoids. Acta Biochim. Polonica. 2006;53:475–484. [PubMed] [Google Scholar]
- [23].Wisniewska A, Subczynski WK. Effects of polar carotenoids on the shape of the hydrophobic barrier of phospholipid bilayers. Biochim. Biophys. Acta. 1998;1368:235–246. doi: 10.1016/s0005-2736(97)00182-x. [DOI] [PubMed] [Google Scholar]
- [24].Subczynski WK, Markowska E, Sielewiesiuk J. Spin-label studies on phosphatidylcholine-polar carotenoid membranes: effects of alkyl chain length and unsaturation. Biochim. Biophys. Acta. 1993;1150:173–181. doi: 10.1016/0005-2736(93)90087-g. [DOI] [PubMed] [Google Scholar]
- [25].Subczynski WK, Markowska E, Sielewiesiuk J. Effect of polar carotenoids on the oxygen diffusion-concentration product in lipid bilayers. An EPR spin label study. Biochim. Biophys. Acta. 1991;1068:68–72. doi: 10.1016/0005-2736(91)90061-c. [DOI] [PubMed] [Google Scholar]
- [26].Subczynski WK, Markowska E, Gruszecki WI, Sielewiesiuk J. Effects of polar carotenoids on dimyristoylphosphatidylcholine membranes: spin-label studies. Biochim. Biophys. Acta. 1992;1105:97–108. doi: 10.1016/0005-2736(92)90167-k. [DOI] [PubMed] [Google Scholar]
- [27].Molnar P, Szablocs J. (Z/E)-Photoisomerization of C40-carotenoids by iodine. J. Chem. Soc., Perkin Trans. 1993;2:261–266. [Google Scholar]
- [28].Hyde JS, Subczynski WK. Spin-label oximetry. In: Berliner LJ, Reuben J, editors. Biological Magnetic Resonance. Vol. 8. Spin Labeling: Theory and Applications, Plenum Press; New York: 1989. pp. 399–425. [Google Scholar]
- [29].Subczynski WK, Felix CC, Klug CS, Hyde JS. Concentration by centrifugation for gas exchange EPR oximetry measurements with loop-gap resonators. J. Magn. Reson. 2005;176:244–248. doi: 10.1016/j.jmr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- [30].Subczynski WK, Wisniewska A, Yin J-J, Hyde JS, Kusumi A. Hydrophobic barriers of lipid bilayer membranes formed by reduction of water penetration by alkyl chain unsaturation and cholesterol. Biochemistry. 1994;33:7670–7681. doi: 10.1021/bi00190a022. [DOI] [PubMed] [Google Scholar]
- [31].Subczynski WK, Hyde JS, Kusumi A. Oxygen permeability of phosphatidylcholine-cholesterol membranes. Proc. Natl. Acad. Sci. USA. 1989;86:4474–4478. doi: 10.1073/pnas.86.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kawasaki K, Yin J-J, Subczynski WK, Hyde JS, Kusumi A. Pulse EPR detection of lipid exchange between protein-rich raft and bulk domains in the membrane: Methodology development and its application to studies of influenza viral membrane. Biophys J. 2001;80:738–748. doi: 10.1016/S0006-3495(01)76053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yin J-J, Subczynski WK. Effect of lutein and cholesterol on alkyl chain bending in lipid bilayers: a pulse electron paramagnetic resonance spin labeling study. Biophys. J. 1996;71:832–839. doi: 10.1016/S0006-3495(96)79284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kusumi A, Subczynski WK, Hyde JS. Oxygen transport parameter in membranes as deduced by saturation recovery measurements of spin-lattice relaxation times of spin labels. Proc. Natl. Acad. Sci. USA. 1982;79:1854–1858. doi: 10.1073/pnas.79.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ashikawa I, Yin J-J, Subczynski WK, Kouyama T, Hyde JS, Kusumi A. Molecular organization and dynamics in bacteriorhodopsin-rich reconstituted membranes: Discrimination of lipid environments by the oxygen transport parameter using a pulse ESR spin-labeling technique. Biochemistry. 1994;33:4947–4952. doi: 10.1021/bi00182a025. [DOI] [PubMed] [Google Scholar]
- [36].Subczynski WK, Hyde JS, Kusumi A. Effect of alkyl chain unsaturation and cholesterol intercalation on oxygen transport in membranes: a pulse ESR spin labeling study. Biochemistry. 1991;30:8578–8590. doi: 10.1021/bi00099a013. [DOI] [PubMed] [Google Scholar]
- [37].Pace RJ, Chan SI. Molecular motions in lipid bilayers. III. Lateral and transversal diffusion in bilayers. J. Chem. Phys. 1982;76:4241–4247. [Google Scholar]
- [38].Robinson BH, Hass DA, Mailer C. Molecular dynamics in liquid: spin lattice relaxation of nitroxide spin labels. Science. 1994;263:490–493. doi: 10.1126/science.8290958. [DOI] [PubMed] [Google Scholar]
- [39].Subczynski WK, Wisniewska A, Hyde JS, Kusumi A. Three-dimensional dynamic structure of the liquid-ordered domain in lipid membranes as examined by pulse-EPR oxygen probing. Biophys. J. 2007;92:1573–1584. doi: 10.1529/biophysj.106.097568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Subczynski WK, Widomska J, Wisniewska A, Kusumi A. Saturation-recovery electron paramagnetic resonance discrimination by oxygen transport (DOT) method for characterizing membrane domains. In: McIntosh TJ, editor. Methods in Molecular Biology. Vol. 398. Lipid Rafts, Humana Press; Totowa: 2007. pp. 145–159. [DOI] [PubMed] [Google Scholar]
- [41].Milanowska J, Polit A, Wasylewski Z, Gruszecki WI. Interaction of isomeric forms of xanthophyll pigment zeaxanthin with dipalmitoylphosphatidylcholine studied in monomolecular layers. J. Photochem. Photobiol. B Biol. 2003;72:1–9. doi: 10.1016/j.jphotobiol.2003.08.009. [DOI] [PubMed] [Google Scholar]
- [42].Knowles PF, Watts A, Marsh D. Spin-label studies of lipid immobilization in dimyristoylphosphadtidylcholine-substitiudted cytochrome oxidase. Biochemistry. 1979;18:4480–4487. doi: 10.1021/bi00588a005. [DOI] [PubMed] [Google Scholar]
- [43].Marsh D, Watts A, Pates R, Uhl R, Knowles PF, Esmann M. ESR spin label studies of lipid-protein interaction in membranes. Bipohys. J. 1982;37:265–274. doi: 10.1016/S0006-3495(82)84675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Marsh D. Electron spin resonance: spin labels. In: Grell E, editor. Membrane Spectroscopy. Springer-Verlag; Berlin: 1981. pp. 51–142. [DOI] [PubMed] [Google Scholar]
- [45].Berliner LJ. Spin labeling in enzymology: spin-labeled enzymes and proteins. Rotational correlation times calculation. Methods Enzymol. 1978;49:466–470. doi: 10.1016/s0076-6879(78)49020-2. [DOI] [PubMed] [Google Scholar]
- [46].Pistolesi S, Pogni R, Feix JB. Membrane insertion and bilayer perturbation by antimicrobial peptide CM15. Biophys. J. doi: 10.1529/biophysj.107.104034. (published ahead of print on May 11, 2007 as doi:10.1529/biophysj.107.104034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Griffith OH, Dehlinger PJ, Van SP. Shape of the hydrophobic barrier of phospholipids bilayers (Evidence for water penetration into biological membranes) J. Membr. Biol. 1974;15:159–192. doi: 10.1007/BF01870086. [DOI] [PubMed] [Google Scholar]
- [48].Griffith OH, Jost PC. Lipid spin labels in biological membranes. In: Berliner LJ, editor. Spin labeling. Theory and application, Academic Press; New York: 1976. pp. 453–523. [Google Scholar]
- [49].Hiff T, Kevan L. Electron spin echo modulation studies of doxylstearic acid spin probes in frozen vesicles: interaction of the spin probe with water-d2 and effects of cholesterol addition. J. Phys. Chem. 1989;93:1572–1575. [Google Scholar]
- [50].Subczynski WK, Markowska E. Effect of carotenoids on oxygen transport within and across model membranes. Curr. Top. Biophys. 19992;16:62–68. [Google Scholar]
- [51].Huang L, Haug A. Regulation of membrane lipid fluidity in Acholeplasma-laidlawii - effect of carotenoid pigment content. Biochim. Biophys. Acta. 1974;352:361–370. doi: 10.1016/0005-2736(74)90228-4. [DOI] [PubMed] [Google Scholar]
- [52].Rohmer M, Bouvier P, Ourisson G. Molecular evolution of biomembranes: structural equivalents and phylogenetic precursors of sterols. Proc. Natl. Acad. Sci. USA. 1979;76:847–851. doi: 10.1073/pnas.76.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gruszecki WI, Strzalka K. Does the xanthophyll cycle take part in the regulation of fluidity of the thylakoid membrane. Biochim. Biophys. Acta. 1991;1060:310–314. [Google Scholar]
- [54].Kusumi A, Subczynski WK, Pasenkiewicz-Gierula M, Hyde JS, Merkle H. Spin-label studies on phosphatidylcholine-cholesterol membranes: effects of alkyl chain length and unsaturation in the fluid phase. Biochim. Biophys. Acta. 1986;854:307–317. doi: 10.1016/0005-2736(86)90124-0. [DOI] [PubMed] [Google Scholar]
- [55].Widomska J, Raguz M, Dillon J, Gaillard ER, Subczynski WK. Physical properties of the lipid bilayer membrane made of calf lens lipids: EPR spin labeling studies. Biochim. Biophys. Acta. 2007;1768:1454–1465. doi: 10.1016/j.bbamem.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McIntosh TJ. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim. Biophys. Acta. 1978;513:43–58. doi: 10.1016/0005-2736(78)90110-4. [DOI] [PubMed] [Google Scholar]
- [57].Gruszecki WI, Sielewiesiuk J. Galactolipide multibilayers modified with xanthophyls: orientational and diffractometric studies. Biochim. Biophys. Acta. 1991;1069:21–26. doi: 10.1016/0005-2736(91)90099-t. [DOI] [PubMed] [Google Scholar]
- [58].Gruszecki WI, Smal A, Szymczuk D. The effect of zeaxanthin on the thickness of dimyristoylphosphatidylcholine bilayer: X-ray diffraction study. J. Biol. Physics. 1992;18:271–280. [Google Scholar]
- [59].Sujak A, Mazurek P, Gruszecki WI. Xanthophyll pigments lutein and zeaxanthin in lipid multibilayers formed with dimyristoylphosphatidylcholine. J. Photochem. Photobiol. B Biol. 2002;68:39–44. doi: 10.1016/s1011-1344(02)00330-5. [DOI] [PubMed] [Google Scholar]
- [60].Suwasky M, Hidalgo P, Strzalka K, Kostecka-Gugala A. Comperative X-Ray studies on the interaction of carotnoids with model phosphatidylcholine membranes. Z, Naturforsch, C. 2002;57:129–134. doi: 10.1515/znc-2002-1-222. [DOI] [PubMed] [Google Scholar]
- [61].Gabrielska J, Gruszecki WI. Zeaxanthin (dihydroxy-β-carotene) but not β-carotene rigidifies lipid membranes: a 1H-NMR study of carotenoid-egg phosphatidylcholine liposomes. Biochim. Biophys. Acta. 1996;1285:167–174. doi: 10.1016/s0005-2736(96)00152-6. [DOI] [PubMed] [Google Scholar]
- [62].Sujak A, Gruszecki WI. Organization of zeaxanthin and lutein in two-component monomolecular layers in dipalmitoylphosphatidylcholine. J. Photochem. Photobiol. B Biol. 2000;59:42–47. doi: 10.1016/s1011-1344(00)00133-0. [DOI] [PubMed] [Google Scholar]
- [63].Widomska J, Ragiz M, Subczynski WK. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim. Biophys. Acta. 2007 doi: 10.1016/j.bbamem.2007.06.018. doi:10.1016/j.bbamem.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]