Summary
In this paper we review the associations between maternal and child undernutrition with human capital and risk of adult diseases in low-income and middle-income countries. We analysed data from five long-standing prospective cohort studies from Brazil, Guatemala, India, the Philippines, and South Africa and noted that indices of maternal and child undernutrition (maternal height, birthweight, intrauterine growth restriction, and weight, height, and body-mass index at 2 years according to the new WHO growth standards) were related to adult outcomes (height, schooling, income or assets, offspring birthweight, body-mass index, glucose concentrations, blood pressure). We undertook systematic reviews of studies from low-income and middle-income countries for these outcomes and for indicators related to blood lipids, cardiovascular disease, lung and immune function, cancers, osteoporosis, and mental illness. Undernutrition was strongly associated, both in the review of published work and in new analyses, with shorter adult height, less schooling, reduced economic productivity, and—for women—lower offspring birthweight. Associations with adult disease indicators were not so clear-cut. Increased size at birth and in childhood were positively associated with adult body-mass index and to a lesser extent with blood pressure values, but not with blood glucose concentrations. In our new analyses and in published work, lower birthweight and undernutrition in childhood were risk factors for high glucose concentrations, blood pressure, and harmful lipid profiles once adult body-mass index and height were adjusted for, suggesting that rapid postnatal weight gain—especially after infancy—is linked to these conditions. The review of published works indicates that there is insufficient information about long-term changes in immune function, blood lipids, or osteoporosis indicators. Birthweight is positively associated with lung function and with the incidence of some cancers, and undernutrition could be associated with mental illness. We noted that height-for-age at 2 years was the best predictor of human capital and that undernutrition is associated with lower human capital. We conclude that damage suffered in early life leads to permanent impairment, and might also affect future generations. Its prevention will probably bring about important health, educational, and economic benefits. Chronic diseases are especially common in undernourished children who experience rapid weight gain after infancy.
This is the second in a Series of five papers about maternal and child undernutrition
Introduction
This is the second article in the Lancet Series on maternal and child undernutrition. The previous article emphasised the magnitude of the problem and its short-term consequences in low-income and middle-income countries.1 In this paper we address the potential long-term implications of undernutrition.
We start with an assessment of the long-term effects of undernutrition on adult human capital, including height, school achievement, economic productivity, and birthweight of the offspring. We then discuss the relevance to low-income and middle-income countries of the hypothesis on developmental origins of health and disease2—namely, that early growth patterns might have long-term effects on risk of development of chronic diseases.3 Although these issues are still debated,4,5 published work from high-income countries suggests that intrauterine growth restriction, especially when followed by excessive weight gain in childhood, is associated with increased risk of several chronic diseases. Studies of the developmental origins of adult disease in low-income and middle-income countries are particularly important because adults were born when rates of undernutrition were high and have had to adapt to rapidly changing postnatal diets and environments.
As researchers involved in large cohort studies entailing the long-term follow-up of children born in low-income and middle-income countries, we present a systematic review of published work on the long-term effects of malnutrition, and new analyses of data from cohorts followed up from birth into late adolescence or adult age in Brazil, Guatemala, India, the Philippines, and South Africa.
Key messages.
-
•
Poor fetal growth or stunting in the first 2 years of life leads to irreversible damage, including shorter adult height, lower attained schooling, reduced adult income, and decreased offspring birthweight
-
•
Children who are undernourished in the first 2 years of life and who put on weight rapidly later in childhood and in adolescence are at high risk of chronic diseases related to nutrition
-
•
There is no evidence that rapid weight or length gain in the first 2 years of life increases the risk of chronic disease, even in children with poor fetal growth
-
•
The prevention of maternal and child undernutrition is a long-term investment that will benefit the present generation and their children
New data analyses
The new analyses presented here are based on the cohorts in Brazil,6 Guatemala,7,8 India,9,10 the Philippines,11 and South Africa12 (table 1). Panel 1 shows the exposures that were assessed. Outcomes were measured in late adolescence in South Africa and in adulthood in the other sites (table 1), and will be referred to as adult outcomes (panel 1).
Table 1.
Basic characteristics of the five cohort studies included in the new analyses
| Design | Year of cohort recruitment | Age at recruitment | Initial sample | Age at last visit (years) | Number examined in last visit | Attrition rate | Infant mortality (per 1000) | Comments | |
|---|---|---|---|---|---|---|---|---|---|
| Brazil (Pelotas) | Prospective cohort | 1982 | Birth | 5914 | 21–23 | 4297 | 23%* | 36 | All children born in the city's maternity hospitals (>99% of all births) during 1982 were enrolled. All social classes included |
| Guatemala (four villages) | Community trial | 1969–77 | Birth–7 years | 2392 | 26–41 | 1571 | 23%† | 75 | Intervention trial with two communities receiving high-energy and protein supplement and two control villages. All children younger than 7 years in 1969 and all born during 1969–77 were enrolled and followed up until 7 years of age or until the study ended in 1977. Furthermore, data were obtained for mothers during pregnancy and breast-feeding periods |
| India (New Delhi) | Prospective cohort | 1969–72 | Before pregnancy | 8181 | 26–32 | 1583 | 68%‡ | 47 | All married women living in a defined area of the city were recruited and followed up. Pregnancies were identified, and neonates were enrolled and followed up. Primarily middle-class sample |
| Philippines (Cebu) | Prospective cohort | 1983–84 | Gestation | 3080 | 21·4 | 2032 | 34% | 51§ | Pregnant women living in 33 neighbourhoods selected by random; first data collection at 30 weeks' gestation. All social classes included |
| South Africa (Soweto) | Prospective cohort | 1990 | Gestation | 3273 | 15 | 2100 | 22% | 27 | Pregnant women with a gestational age of 26–32 weeks living in a defined urban geographical area. Predominantly poor, black sample |
Participants known to have died were regarded as having been traced; those who moved out of the study area were regarded as lost to follow-up.
Excludes participants who were no longer living in Guatemala and regards those known to have died as having been traced.
Includes participants known to have died and those migrated from the study area.
Infant mortality rate when survey was initiated, based on 1983 Demographic and Health Survey.
Panel 1. Exposure variables and adult outcomes that were assessed.
Exposure variables
-
•
Maternal height (cm): measured by the study teams during pregnancy or soon after delivery. No data were available for India
-
•
Birthweight (kg): measured by the research teams except in South Africa where they were obtained from reliable birth records.13 In Cebu (the Philippines), data for birthweight include interviewer-measured data (60%) and hospital records
-
•
Intrauterine growth restriction: gestational age was calculated from the date of the last menstrual period except in Cebu, in which the Ballard scores14 were used for all infants with low birthweight or whose mothers had pregnancy complications. Intrauterine growth restriction was defined as a birthweight for gestational age below the tenth percentile of the sex-specific Williams reference curves15
-
•
Height-for-age or length-for-age (Z scores): children were measured by the research teams at around 2 years of age, and their height (in South Africa) or recumbent length (other sites) were converted into Z scores with the WHO Growth Standards.16 Stunting was defined as being less than the Z score cutoff of −2
-
•
Weight-for-age and body-mass-index-for-age (Z scores): defined as above. Underweight and wasting were defined by the Z score cutoff of −2
Adult and adolescent outcomes
-
•
Height (cm): measured by the study teams
-
•
Achieved schooling/education (years): number of years completed with approval
-
•
Income or assets: personal income was measured in local currency in Brazil and Guatemala and expressed in US dollars. Individuals with no income—mostly young adults living with their families or housewives (36% in Brazil and 13% in Guatemala)—were excluded from the analyses. Data from the Philippines and Soweto (South Africa) were not included because few adolescents had independent incomes. In India, household assets were recorded
-
•
Offspring birthweight (g): In Brazil and India, data were obtained from birth records or if unavailable by maternal recall; measured by the research team in Guatemala; and by maternal recall in the Philippines
-
•
Body-mass index (kg/m2): measured by the study teams
-
•
Blood glucose concentration (mmol/L): fasting glucose concentrations were measured in Guatemala and the Philippines. In Brazil, only non-fasting concentrations were available. In India, fasting concentrations and 120-min results from glucose tolerance tests were obtained. This information is not yet available for South Africa. To correct for the skewed distribution in some sites, we used a natural logarithmic transformation
-
•
Systolic blood pressure (mm Hg): measured by the research team in all sites. In Brazil, South Africa, and India, we used the average of two values taken on the same day. In the Philippines and Guatemala, three measures were averaged
Potential confounding variables included the participant's age when the outcome variables were measured, years of schooling completed by the mother (in India, by the father), and a measure of early childhood socioeconomic status. In India, occupation of the father was classified in an ordinal six-point scale, whereas in the other sites wealth quintiles were calculated through principal component analysis of household assets.
Unless otherwise stated, we present only confounder-adjusted estimates. For glucose concentrations and blood pressure, further analyses incorporated control for adult height and body-mass index, which are potential mediating factors in the association between undernutrition and adult outcomes. Such adjustment is commonly used in published work and might help understand the role of later growth. All analyses were stratified by sex.
In every analysis, we included only participants with full information in the exposure, outcome, confounding, and stratification variables. With the exception of the dichotomous variable for intrauterine growth restriction, all other exposures were initially coded in four categories and tested for linearity with analysis of variance. Because there was no consistent evidence of non-linearity, we used multiple linear regression analyses.
Results from the five studies were combined with a meta-analytic procedure that included examination of heterogeneity with use of the Q test;17 if results were significant, we used a random-effects model.18
Findings
We have organised our results according to the 14 outcomes studied. For every outcome, we considered maternal size and nutrition, newborn infant size, infant and child size, and growth. For eight outcomes, new information is provided from our cohorts. Table 2 shows descriptive statistics for exposure and outcome variables by study site. Brazil tended to show the lowest prevalence of undernutrition, and Guatemala the highest. Birthweight was lowest and intrauterine growth restriction highest in India. When adult outcomes are compared, it should be noted that South African participants are still in their late teens.
Table 2.
Exposure and outcome variables, by study site and sex
|
Brazil (Pelotas) |
Guatemala (4 villages) |
India (New Delhi) |
Philippines (Cebu) |
South Africa (Soweto) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | Males | Females | Males | Females | |
| Exposure variables | ||||||||||
| Maternal height (cm) | 156·5 (6·2) | 156·4 (5·9) | 149·1 (5·3) | 149·0 (5·2) | N/A | N/A | 150·6 (5·0) | 150·5 (5·0) | 158·7 (11·2) | 158·5 (6·6) |
| Birthweight (kg) | 3·25(0·57) | 3·13(0·55) | 3·10(0·51) | 3·00(0·50) | 2·89(0·44) | 2·79(0·38) | 3·03(0·43) | 2·98(0·41) | 3·13(0·51) | 3·04(0·50) |
| Birthweight (<2500 g) | 8·0% (7·0–9·0) | 10·1% (9·0–11·2) | 8·8% (6·4–11·2) | 10·6% (7·8–13·4) | 16·6% (14·0–19·2) | 19·9% (16·7–23·3) | 10·8% (7·4–10·9) | 9·2% (8·8–12·8) | 9·1% (7·5–10·7) | 11·4% (9·6–13·2) |
| IUGR (%) | 15·3% (13·9–16·7) | 14·4% (13·0–15·8) | 34·0% (29·4–38·6) | 27·9% (23·2–32·6) | 39·8% (36·2–43·4) | 40·0% (35·7–44·3) | 22·3% (20·2–24·3) | 20·1% (18·0–22·2) | 15·1% (13·1–17·1) | 12·7% (10·9–14·5) |
| Weight-for-age* (Z scores) | 0·05(1·13) | 0·14(1·03) | −1·73(0·98) | −1·73(1·01) | −1·48(1·07) | −1·41(1·08) | −1·68(0·96) | −1·68(0·99) | −0·54(1·32) | −0·34(1·19) |
| Weight-for-age* (<−2 Z scores) | 3·6% (2·9–4·3) | 2·6% (2·0–3·2) | 35·6% (31·4–39·8) | 37·9% (33·3–42·5) | 32·3% (29·1–35·5) | 27·0% (23·4–30·6) | 35·3% (32·4–38·2) | 36·6% (33·5–39·8) | 12·2% (9·5–14·9) | 8·0% (5·9–10·2) |
| Height-for-age* (Z scores) | −0·78(1·28) | −0·61(1·20) | −3·26(1·10) | −3·15(1·01) | −1·97(1·18) | −1·90(1·12) | −2·59 (1·12) | −2·50 (1·10) | −1·43 (1·31) | −1·16 (1·19) |
| Height-for-age* (<−2Z scores) | 16·3% (14·9–17·7) | 11·6% (10·3–12·9) | 87·4% (84·4–90·4) | 86·3% (83·0–89·6) | 49·4% (46·0–52·8) | 43·4% (39·4–47·4) | 69·0% (66·2–68·9) | 65·6% (62·5–68·9) | 29·9% (26·1–33·7) | 22·9% (19·5–26·3) |
| Outcomes (adolescents and adults) | ||||||||||
| Height (cm) | 173·7 (6·9) | 160·7 (6·2) | 162·8 (6·1) | 150·7 (5·6) | 169·7 (6·3) | 154·9 (5·7) | 163·1 (5·9) | 151·2 (5·5) | 166·3 (8·1) | 158·7 (6·2) |
| Attained schooling (years) | 9·0 (3·2) | 9·8 (3·1) | 5·4 (3·5) | 4·5 (3·2) | 13·1 (3·4) | 13·9 (3·1) | 9·9 (3·4) | 11·2 (2·8) | 9·6 (3·7) | 10·1 (0·9) |
| Monthly income (log US$)† | 5·1 (0·7) | 4·8 (0·7) | 5·3 (0·9) | 3·7 (1·8) | 17·2%‡ | 18·7%‡ | N/A | N/A | N/A | N/A |
| Offspring birthweight (kg) | 3·16(0·63) | 3·09(0·56) | 2·94(0·45) | 2·93(0·45) | 2·82(0·50) | 2·85(0·55) | N/A | 2·95(0·52) | N/A | N/A |
| BMI (kg/m2) | 23·8 (4·1) | 23·5 (4·6) | 24·7 (3·6) | 26·9 (4·8) | 24·9 (4·3) | 24·6 (5·1) | 21·0 (3·1) | 20·2 (3·1) | 19·7 (3·4) | 22·1 (4·4) |
| BMI (≥25 kg/m2) | 30·6% (28·7–32·5) | 27·0% (25·1–28·9) | 41·1% (37·2–45·0) | 62·3% (58·7–65·9) | 47·0% (43·7–50·3) | 45·4% (41·6–49·3) | 7·5% (5·9–9·1) | 9·7% (8·0–11·5) | 12·8% (10·4–15·2) | 29·5% (26·4–32·6) |
| BMI (≥30 kg/m2) | 7·5% (6·4–8·6) | 9·1% (7·9–10·3) | 8·9% (6·7–11·1) | 23·9% (20·7–27·1) | 9·5% (7·6–11·4) | 13·1% (10·5–15·8) | 1·1% (0·4–1·7) | 2·0% (1·2–2·9) | 3·6% (2·3–4·9) | 9·1% (7·1–11·1) |
| Plasma glucose (mmol/L)§ | 5·54(0·83) | 5·27(0·79) | 5·17 (0·73) | 5·27 (1·59) | 5·37 (1·21) | 5·29(1·18) | 5·66(0·52) | 5·52 (0·51) | N/A | N/A |
| Systolic blood pressure (mm Hg) | 123·5 (14·4) | 111·3 (13·0) | 116·8 (11·4) | 108·5 (13·0) | 118·3 (11·3) | 106·7 (11·0) | 111·8 (10·8) | 99·3 (9·9) | 113·4 (25·3) | 108·8 (19·7) |
| Number of participants¶ | 445–3035 | 843–2873 | 348–921 | 356–878 | 719–876 | 513–626 | 912–1079 | 762–953 | 558–1184 | 577–1251 |
Data are mean (SD) or prevalence (95% CI).
IUGR=intrauterine growth restriction. BMI=body-mass index. N/A=not available.
At roughly 2 years of age.
Geometric mean and SD.
Percentage in the highest quintile of income based on assets score for the Delhi cohort.
Geometric mean and SD of fasting glucose, except for Brazil where a non-fasting sample was obtained.
Smallest and largest number of participants with available data for the variables under study. For the Delhi cohort, these sample sizes refer to participants for whom complete information is available about occupation of father when the participant was a child, and adult age and BMI. First offspring birthweight is excluded from these values; the sample sizes for this variable are 231 for men and 295 for women.
Height
Attained height is affected by genetic and environmental factors throughout the growth period. Linear growth failure is largely confined to the intrauterine period and the first few years of life, and is caused by inadequate diets and frequent infections.19
Short stature of the mother and poor maternal nutrition stores are associated with increased risk of intrauterine growth retardation.1,20 Several studies from low-income and middle-income countries report that adult height is positively associated with birthweight9,21–24 and length.9,21,23 A 1 cm increase in birthlength is associated with a 0·7–1 cm increase in adult height.9,21–24
Early growth failure will lead to reduced adult stature unless there is compensatory growth (so-called catch-up growth) in childhood, which is partly dependent on the extent of maturational delay that lengthens the period of growth. Because maturational delays in low-income and middle-income countries are usually shorter than 2 years,25 only a small part of the growth failure is compensated for. In Guatemala,26 there was little evidence of catch-up growth after 3 years of age but in Senegal, where maturational delays were substantial, adults heights were only about 2 cm shorter than the reference despite severe stunting in childhood.27 In both countries, differences in height between stunted and non-stunted children younger than 5 years remained largely unchanged into adulthood. People who remain in the setting in which they developed childhood undernutrition tend to become short adults.9,24,25 Improvements in living conditions such as those brought on through adoption can trigger catch-up growth but do so more effectively in very young children.25,28
Data from Guatemala suggest that the contributions of the intrauterine and early postnatal period to reduced adult stature are about equal.29 Because nutrition interventions during pregnancy and early life reduce stunting,26,30 these interventions might be expected to lead to increased adult stature.31
Analyses of height data from our five cohorts are shown in webtables 1a and b and webfigures 1a–f, and are summarised in table 3.
Table 3.
Summary of the pooled adjusted results from the five cohort studies: height, schooling, income/assets, offspring birthweight, body-mass index, blood pressure, and glucose concentration¶
|
Males |
Females |
Both sexes |
||||
|---|---|---|---|---|---|---|
| Pooled estimate (range) | p* | Pooled estimate (range) | p* | Pooled estimate | p* | |
| Height (cm) | ||||||
| Maternal height† (cm) | 0·47 (0·16 to 0·59) | <0·0001 | 0·51 (0·46 to 0·54) | <0·0001 | 0·50 | <0·0001 |
| Birthweight (kg) | 3·25 (2·72 to 4·19) | <0·0001 | 3·25 (3·06 to 3·64) | <0·0001 | 3·25 | <0·0001 |
| IUGR (yes/no) | −2·17 (−3·32 to −1·44) | <0·0001 | −2·32 (−2·60 to −1·78) | <0·0001 | −2·24 | <0·0001 |
| WAZ at 2 years (Z score) | 2·75 (1·83 to 2·86) | <0·0001 | 2·63 (1·73 to 2·81) | <0·0001 | 2·69 | <0·0001 |
| HAZ at 2 years (Z score) | 3·26 (3·13 to 3·31) | <0·0001 | 3·22 (2·92 to 3·50) | <0·0001 | 3·24 | <0·0001 |
| Schooling (years) | ||||||
| BAZ at 2 years (Z score) | 0·20 (−1·39 to 0·51) | 0·03 | 0·17 (−0·39 to 0·40) | 0·08 | 0·18 | 0·06 |
| Maternal height† (cm) | 0·02 (0·00 to 0·07) | 0·04 | 0·02 (0·01 to 0·06) | <0·0001 | 0·02 | <0·0001 |
| Birthweight (kg) | 0·39 (0·16 to 0·48) | <0·0001 | 0·25 (−0·06 to 0·68) | <0·0001 | 0·30 | <0·0001 |
| IUGR (yes/no) | −0·18 (−0·39 to 0·16) | 0·10 | −0·25 (−0·52 to 0·28) | 0·003 | −0·23 | 0·001 |
| WAZ at 2 years (Z score) | 0·51 (0·21 to 0·59) | <0·0001 | 0·52 (−0·02 to 0·57) | <0·0001 | 0·52 | <0·0001 |
| HAZ at 2 years (Z score) | 0·48 (0·32 to 0·51) | <0·0001 | 0·53 (0·03 to 0·56) | <0·0001 | 0·50 | <0·0001 |
| BAZ at 2 years (Z score) | 0·09 (−0·57 to 0·37) | 0·51 | 0·16 (−0·03 to 0·33) | <0·0001 | 0·16 | 0·02 |
| Birthweight of the first offspring‡(g) | ||||||
| Maternal height† (cm) | 7·0 (2·5 to 11·9) | 0·02 | ||||
| Birthweight (kg) | 208·0 (190 to 294) | <0·0001 | ||||
| IUGR (yes/no) | −126·7(−174 to −44) | 0·002 | ||||
| WAZ at 2 years (Z score) | 74·7 (31 to 94) | <0·0001 | ||||
| HAZ at 2 years (Z score) | 78·5 (43 to 98) | <0·0001 | ||||
| BAZ at 2 years (Z score) | 14·5 (−2 to 39) | 0·37 | ||||
| Body-mass index (kg/m2) | ||||||
| Maternal height† (cm) | 0·01 (−0·01 to 0·05) | 0·15 | 0·01 (0·00 to 0·02) | 0·39 | 0·01 | 0·09 |
| Birthweight (kg) | 0·71 (0·01 to 1·02) | <0·0001 | 1·13 (0·91 to 2·21) | <0·0001 | 0·87 | <0·0001 |
| IUGR (yes/no) | −0·50 (−0·82 to 0·03) | <0·0001 | −0·76 (−1·42 to −0·38) | <0·0001 | −0·60 | <0·0001 |
| WAZ at 2 years (Z score) | 0·90 (0·39 to 1·26) | <0·0001 | 0·95 (0·66 to 1·35) | <0·0001 | 0·92 | <0·0001 |
| HAZ at 2 years (Z score) | 0·42 (0·06 to 0·63) | <0·0001 | 0·38 (0·13 to 0·84) | <0·0001 | 0·40 | <0·0001 |
| BAZ at 2 years (Z score) | 0·74 (0·33 to 1·13) | <0·0001 | 0·93 (0·68 to 1·14) | <0·0001 | 0·81 | <0·0001 |
| Glucose concentration‡(log/mmol/L) | ||||||
| Maternal height† (cm) | 0·067 (0·033 to 0·182) | 0·11 | −0·011 (−0·113 to 0·082) | 0·81 | 0·031 | 0·31 |
| Adjusted maternal height§ (cm) | 0·034 (−0·019 to 0·184) | 0·45 | 0·005 (−0·109 to 0·104) | 0·92 | 0·020 | 0·56 |
| Birthweight (kg) | −0·001 (−0·018 to 0·004) | 0·84 | −0·009 (−0·012 to −0·005) | 0·08 | −0·004 | 0·18 |
| Adjusted birthweight§ (kg) | −0·005 (−0·028 to−0·002) | 0·28 | −0·013 (−0·024 to −0·010) | 0·01 | −0·009 | 0·01 |
| IUGR (yes/no) | −0·006 (−0·016 to −0·003) | 0·30 | −0·005 (−0·028 to 0·006) | 0·36 | −0·005 | 0·17 |
| Adjusted IUGR§ (yes/no) | −0·004 (−0·020 to 0·000) | 0·43 | −0·003 (−0·020 to 0·014) | 0·65 | −0·004 | 0·38 |
| WAZ at 2 years (Z score) | 0·001 (−0·007 to 0·014) | 0·65 | −0·002 (−0·011 to 0·000) | 0·38 | 0·000 | 0·79 |
| Adjusted WAZ at 2 years§ (Z score) | −0·005(−0·010 to 0·006) | 0·05 | −0·005 (−0·019 to−0·002) | 0·05 | −0·005 | 0·005 |
| HAZ at 2 years (Z score) | 0·003 (−0·004 to 0·015) | 0·15 | −0·002 (−0·010 to 0·005) | 0·26 | 0·000 | 0·76 |
| Adjusted HAZ at 2 years§ (Z score) | −0·002 (−0·008 to 0·010) | 0·50 | −0·004 (−0·016 to −0·002) | 0·13 | −0·003 | 0·13 |
| BAZ at 2 years (Z score) | −0·001 (−0·003 to 0·005) | 0·69 | 0·000 (−0·013 to 0·003) | 0·92 | −0·001 | 0·72 |
| Adjusted BAZ at 2 years§ (Z score) | −0·003 (−0·006 to 0·005) | 0·16 | −0·004 (−0·018 to −0·001) | 0·09 | −0·004 | 0·03 |
| Systolic blood pressure (mm Hg) | ||||||
| Maternal height† (cm) | 0·10 (0·04 to 0·19) | 0·03 | 0·06 (0·02 to 0·24) | 0·10 | 0·08 | 0·07 |
| Adjusted maternal height§ (cm) | −0·01 (−0·10 to 0·04) | 0·85 | −0·04 (−0·09 to 0·10) | 0·38 | −0·02 | 0·45 |
| Birthweight (kg) | −0·54 (−0·86 to 1·24) | 0·18 | 0·11 (−0·51 to 3·16) | 0·79 | −0·22 | 0·43 |
| Adjusted birthweight§ (kg) | −2·04 (−3·81 to −0·28) | <0·0001 | −1·46 (−1·82 to 0·03) | <0·0001 | −1·76 | <0·0001 |
| IUGR (yes/no) | −0·26 (−1·76 to 3·28) | 0·58 | −0·03 (−1·40 to 1·17) | 0·95 | −0·16 | 0·65 |
| Adjusted IUGR§ (yes/no) | 0·68 (−0·57 to 6·77) | 0·13 | 0·93 (−0·87 to 1·78) | 0·05 | 0·79 | 0·02 |
| WAZ at 2 years (Z score) | 0·98 (0·55 to 1·86) | <0·0001 | 0·68 (−0·07 to 1·70) | <0·0001 | 0·83 | <0·0001 |
| Adjusted WAZ at 2 years§ (Z score) | −0·72 (−1·10 to −0·30) | 0·001 | −0·45 (−0·92 to 0·72) | 0·039 | −0·59 | <0·0001 |
| HAZ at 2 years (Z score) | 0·96 (0·62 to 2·93) | <0·0001 | 0·61 (−0·17 to 2·09) | 0·001 | 0·79 | <0·0001 |
| Adjusted HAZ at 2 years§ (Z score) | −0·08 (−0·96 to 0·53) | 0·68 | −0·02 (−1·67 to 2·67) | 0·92 | −0·05 | 0·71 |
| BAZ at 2 years (Z score) | 0·27 (−1·02 to 0·98) | 0·16 | 0·30 (−0·02 to 0·65) | 0·13 | 0·29 | 0·04 |
| Adjusted BAZ at 2 years§ (Z score) | −0·76 (−1·26 to −0·22) | <0·0001 | −0·48 (−1·19 to 0·11) | 0·01 | −0·63 | <0·0001 |
WAZ=weight-for-age Z score. HAZ=height-for-age Z score. BAZ=body-mass-index-for-age Z score. IUGR=intrauterine growth restriction.
Data are adjusted coefficients from linear regression analyses, using all exposures as continuous variables, except IUGR, which was included in the model as a dummy variable (0=no; 1=yes).
Information about maternal height not available for India.
Glucose and offspring birthweight not available for South Africa.
Additional adjustment for adult BMI and height.
Adjusted for individual's age when the outcome variables were measured, years of schooling completed by the mother (in India, by the father), and a measure of early childhood socioeconomic status.
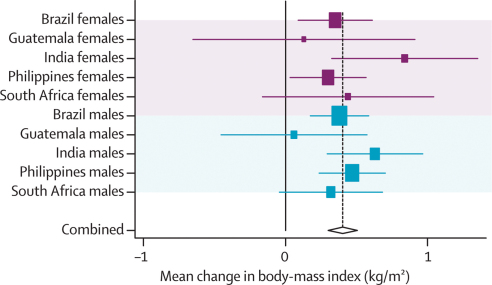
Crude and adjusted results were much the same (webtables 1a and b). Every 1 cm of maternal height was associated with an increase of about 0·5 cm in adult offspring; the only outliers were male adolescents from South Africa, who had not yet attained adult height, which possibly attenuated the association (coefficient 0·16 cm). Highly consistent cross-site effects were noted for birthweight (3·3 cm per kg), intrauterine growth restriction (2·2 cm shorter), weight-for-age at 2 years (2·7 cm per Z score), and height-for-age at 2 years (3·2 cm per Z score). When effect sizes are compared, it is noteworthy that the standard deviation for birthweight is roughly 500 g (table 2), so that a difference of 1 kg is quite large. The association with body-mass-index-for-age at 2 years was much weaker (0·2 cm per Z score) and—for reasons that are unclear—varied substantially among countries, with Guatemala showing a negative coefficient.
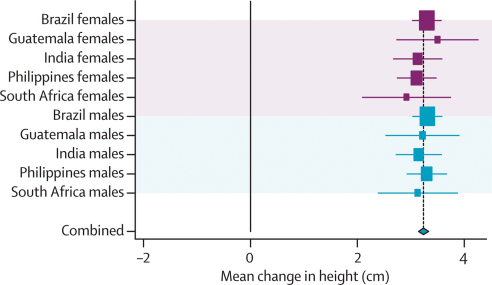
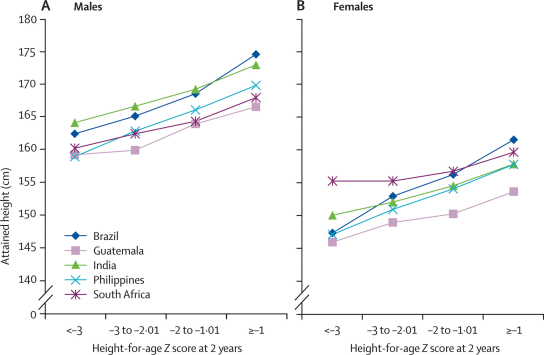
Pooled results for all exposures are shown in the webfigures, and those for height-for-age in figure 1. Figure 2 shows the corresponding categorical analyses. There is remarkable similarity across studies, and between males and females. 1 Z score of length-for-age or height-for-age at 2 years equals 3·1 cm for boys and 3·2 cm for girls. Because the adult height difference associated with a difference of 1 Z score at 2 years is also 3·2 cm, our results suggest that differences observed in the first 2 years will on average remain until adulthood. This finding is consistent with studies on the secular trend in increasing height recorded in all societies as child undernutrition is reduced.32
Figure 1.
Forest plot for effect of height-for-age at 2 years on height
Mean change per unit change in height-for-age Z score at 2 years.
Figure 2.
Attained height according to height-for-age at 2 years in the five cohorts, for males (A) and females (B)
Achieved schooling and educational performance
Undernutrition can affect cognitive development by causing direct structural damage to the brain and by impairing infant motor development33 and exploratory behaviour.34 Long-term effects can arise through structural and functional adaptation; the persistence of early deficits, partly because of the absence of opportunities for remediation in deprived environments; and by altering the way in which individuals deal with learning.35
Birthweight is positively associated with cognitive skills in children, but the effect of environmental factors weakens this association over time.36 A review of studies from high-income countries—nine investigating adolescent outcomes and six adult outcomes—showed that intrauterine growth restriction had little or no measurable effect on cognitive performance.37
Studies from Guatemala38,39 and Zimbabwe40 report long-term associations between early child growth and education. In Guatemala, height and head circumference at 2 years (but not birthsize) were inversely associated with educational achievement in adult women.29 In Cebu, stunting at 2 years was associated with delayed school entry, greater grade repetition and dropout rates, decreased graduation rates from primary and secondary school, and lower school performance.41 In Guatemala, food supplementation during early childhood improved schooling in women by 1·2 years, and tests scores in men and women.42 In Zimbabwe, a difference of 3·4 cm in height-for-age at 3 years was associated with almost an additional grade of achieved schooling.40
Although there are few follow-up studies from childhood to adult age, substantial evidence suggests an association between stunting and present or later cognitive ability or school performance in children from low-income and middle-income countries. Of 18 cross-sectional studies reviewed, only three did not report significant associations with height-for-age. Excluding studies of children admitted for severe malnutrition, four of five longitudinal studies report that height-for-age predicts school or cognitive test performance in later life. A re-analysis of longitudinal data from the Philippines, Jamaica, Peru, and Indonesia, together with new data from Brazil and South Africa, showed that stunting between 12 and 36 months of age predicted poorer cognitive performance and/or lower school grades attained in middle childhood.43
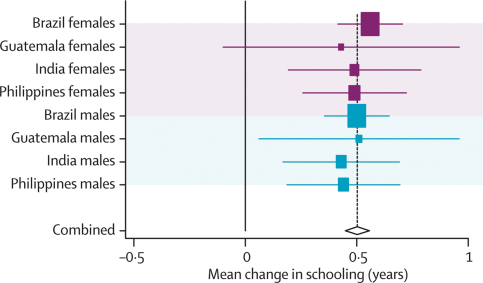
Analyses of attained schooling are shown in webtables 2a and b and webfigures 2a–f, and summarised in table 3. Data from South Africa were not included in the pooled estimates because most 15-year-old children were still at school. Almost all undernutrition indicators were associated with lower educational achievement.
In several analyses (webtables 2a and b), adjustment for confounding reduced the magnitude of the crude effects. After adjustment, the strongest positive predictors of schooling were height-for-age (about 0·50 years of schooling per Z score; figure 3) and weight-for-age (0·52 years per Z score). For birthweight, every 1 kg (roughly 2 Z scores) was associated with an additional 0·30 years of schooling. Intrauterine growth restriction showed an inverse association, whereas associations with child body-mass-index-for-age and maternal height were much weaker. These results are consistent with recent analyses.43,44
Figure 3.
Forest plot for effect of height-for-age at 2 years on attained schooling
Mean change per unit change in height-for-age Z score at 2 years.
Income and assets
Poverty is both a cause and an outcome of poor human development, and investments in child nutrition are being promoted as a strategy for economic development.40,45–47 Better child nutrition improves cognition and schooling, as discussed. It can also affect adult earnings through reduced lean body mass (including shorter height) and decreased productivity in jobs requiring manual labour. In the Guatemala trial, the nutrition intervention led to increased body size and improved work capacity.31,48
These indicators of physical and intellectual human capital, in turn, have been associated with increased earnings. Adult height is positively associated with income, even in urban settings and even after adjustment for education.49 The economic returns to schooling are substantial; for central America, 1 additional year of schooling is associated with 12–14% increased lifetime earnings,50 and much the same effects were estimated in Brazil.51 Exposure to famine in early life in China was associated with shorter stature and lower incomes.52 Exposure to improved nutrition before, but not after, 3 years of age was associated with higher hourly wages in Guatemalan men. For exposure from 0 to 2 years, the increase was US$0·67 per h (95% CI 0·16–1·17), which equates to a 46% increase in average wages.53
The only study showing a direct association between growth in infancy and adult income was undertaken in a high-income country, Finland.54 Data for adult income levels are available for Brazil and Guatemala, and for household assets from India (webtable 3). We made no attempt to do pooled analyses. Individuals from Brazil and Guatemala who had no independent income were excluded, but similar results were obtained when they were included.
Income levels were much the same in Guatemala and Brazil, although the cohort in Brazil was just entering the labour market (table 2). Confounder adjustment led in most cases to reduced effect estimates (webtables 3a and b). Most indicators of undernutrition were associated with lower income in Brazil and fewer assets in India, but in Guatemala few associations were significant. The most consistent results for men were for height-for-age: 1 Z score was associated with an 8% increase in income in Brazil (p<0·0001) and Guatemala (p=0·07), as well as with an increase of 0·27 household assets in India (p<0·0001). Associations with weight were less consistent.
Salaries were substantially lower for women than for men (table 2). The only significant associations for women were between height-for-age and income in both Brazil and Guatemala (8% and 25%, respectively), and with number of assets in India. In Brazil and India, there were also positive associations with weight-for-age. Body-mass index was not associated with income or assets in any of the three countries.
Birthweight in the next generation
Maternal bodysize is strongly associated with size of newborn children.55 As shown above, undernourished girls tend to become short adults, and thus are more likely to have small children. However, evidence from low-income and middle-income countries for the intergenerational effects of undernutrition is scarce.
Ramakrishnan and colleagues56 have shown that for every 100 g increase in maternal birthweight, her child's birthweight increased by 10–20 g, but studies were primarily undertaken in high-income countries. In Guatemala, birthweight rose by 29 g per 100 g increase in maternal birthweight, and birthlength rose by 0·2 cm for every 1 cm increase in mother's birthlength.56 In India, maternal birthweight is a strong predictor of offspring birthweight, even after adjustment for maternal adult size.20
In Guatemala, weight and height were assessed in children younger than 3 years whose mothers participated in the nutrition supplementation trial as children.57 Children born to women who had received a protein-energy supplement were on average 0·8 cm taller (95% CI 0·16–1·44) than were those whose mothers received a low-energy supplement.
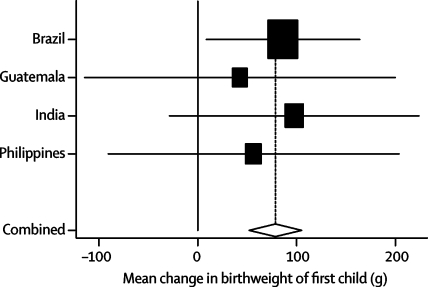
Data for birthweight of firstborn offspring were available for all sites except South Africa (webtable 4, figure 4, and table 3). Undernutrition was associated with lower birthweight in the next generation. Adjustment for confounding led to reductions of about 10–20% in the crude effect sizes. Every 1 kg of birthweight was associated with a 208 g increase in offspring birthweight, roughly to 100 g per Z score of maternal birthweight. Maternal intrauterine growth restriction was associated with a pooled reduction of 127 g, and both weight-for-age and height-for-age—but not body-mass-index-for-age—were associated with increases of about 70–80 g per Z score (figure 5). There was a small association between maternal height and birthweight of their grandchildren.
Figure 4.
Forest plot for effect of height-for-age at 2 years on offspring birthweight (females only)
Mean change per unit change in height-for-age Z score at 2 years.
Figure 5.
Forest plot for effect of height-for-age at 2 years on body-mass index
Mean change per unit change in height-for-age Z score at 2 years.
Body-mass index, body composition, and obesity
Maternal nutritional status during pregnancy can affect offspring bodysize and composition by production of long-term deficits in fetal lean body mass,58 altering sensitivity of the hypothalamic-pituitary-adrenal axis59 which affects appetite and physical activity,60,61 or through the action of specific components of the maternal diet on gene expression.62,63
Four studies from low-income and middle-income countries showed that impaired fetal nutrition results primarily in long-term deficits in lean rather than in fat mass.64 In New Delhi (India)9 and Guatemala,29 birthweight was positively related to adult lean mass in both men and women, but with fat mass or sum of skinfolds9 only in women. Birthlength was positively related to adult lean mass and sum of skinfolds (Indian men and women), fat mass (Guatemalan men and women), fat-free mass (Guatemalan men), and percentage of body fat (Guatemalan women). In Brazil, birthweight was associated with lean mass in 18-year-old men.65 In Shanghai adults, waist circumference was higher in those who weighed less than 2·5 kg or more than 3·5 kg than it was in those in the middle of the distribution.66 This bimodal distribution is consistent with two different pathways—undernutrition and maternal obesity or gestational diabetes affecting the risk of bigger babies,67,68 which is not part of this review.
There are few data for associations between birthweight and fat distribution. A review of evidence mainly from European studies reports that with adjustment for adult body-mass index, lower birthweight relates to central adiposity represented by the subscapular to triceps skinfold ratio and in some cases, waist to hip ratio.64 In men and women from New Delhi9 and in men from Cebu,21 birthweight and birthlength were inversely related to skinfold ratio. In adults from New Delhi, birthweight was not associated with the waist to hip ratio, but by contrast, birthweight was positively associated with adult waist to hip ratio in women from Guatemala.9,29
Recent reviews of how infant size and growth relate to later risk of obesity69–71 focused on heavy rather than undernourished children, and included very few studies of adults or people from low-income and middle-income countries. In nearly all studies, larger size and growth rates were directly associated with increased risk of obesity in later life. By contrast, linear growth retardation in the first 2 years of life is associated with lower lean body mass in adulthood. In Guatemala, a difference of 1 standard deviation in length at 2 years of age was associated with an effect size of nearly 0·5 standard deviations for adult fat-free mass.29 In Delhi, body-mass index gain in infancy was more strongly associated with adult lean than fat mass.9 However, in Brazil weight gain in the first 2 years of life was associated with lean mass in 18-year-old men, whereas later weight gain was more strongly associated with fat mass.65
Early childhood stunting was associated with lower adult body-mass index but greater central adiposity in Guatemalan adults after adjustment for overall fatness and confounders.72 However, previous stunting was not associated with total or central adiposity in adults from New Delhi9 or in Jamaican 17–18-year-olds.73
Webtables 5a and b, figure 5, and webfigures 3a–f show analyses of body-mass index, and table 3 provides a summary. Crude and adjusted results were much the same. There was an inverse association with intrauterine growth restriction and no association with maternal height. Adult body-mass index was strongly and positively associated with birthweight, weight-for-age, and body-mass index at 2 years of age, and less strongly associated with height-for-age (figure 5). Guatemalan men showed a different pattern for most exposures.
Blood lipids
Intrauterine malnutrition and early life growth patterns can result in metabolic and physiological programming, with lifelong effects on the risk of cardiovascular disease. Unhealthy lipid profiles can be a potential mechanism underlying these associations,74 and animal studies have supported this notion.75 Changes in liver microstructure can mediate this effect.76
Three systematic reviews on newborn size and lipid concentrations are available from high-income countries.77–79 The most recent study79 reported a pooled estimate of −1·39 mg/L total cholesterol (95% CI −1·81 to −0·97 mg) per kg of birthweight. Stronger associations were noted in small studies and in infancy. Several studies reported fractions and triglycerides, but most of these associations were null and, as for total cholesterol, inverse associations were most common in small studies.79 Abdominal circumference, showing liver size, could be a better predictor of adult lipid concentrations than could birthweight,74 but data for this association are scarce.
Five studies from low-income and middle-income countries are available. In South Africa, intrauterine growth restriction was not associated with lipid concentrations at 20 years of age.80 In people aged 45 years from Beijing, low birthweight was related to raised triglycerides and reduced HDL cholesterol, after adjustment for sex and adult body-mass index.81 In Guatemala, birthweight was not associated with serum lipids at 24 years of age; in men, there were inverse trends with total cholesterol and LDL cholesterol, with borderline significance.82 A Gambian study83 assessed lipid concentrations in men (mean age 36 years) born in the season of nutritional deprivation (known as the hungry season) and in the harvest seasons, and reported no differences in total, HDL, or LDL cholesterol, or triglycerides. In Brazil, birthweight was not associated with total cholesterol, its fractions, or triglycerides, in 18-year-old men (Horta BL, Universidade Federal de Pelotas, personal communication).
The reviews have shown that adjustment for adult nutritional status increased the negative association of birthweight and lipids, suggesting that postnatal growth plays an important part.84 The Brazilian study noted no association with birthweight, but rapid weight gain between 2 and 4 years was associated with increased concentrations of VLDL cholesterol and triglycerides at age 18 years (Horta BL, personal communication). However, the only randomised trial—from Guatemala—did not accord with this finding; children supplemented in utero or up to 36 months of age had lower triglycerides and higher HDL cholesterol (men only) in adulthood. Improved nutrition at any age before 7 years was not associated with total or LDL cholesterol.85 Specific components of the young child's diet, such as breastmilk, might have a role.86
Insulin resistance and type 2 diabetes
Type 2 diabetes results from a combination of insulin resistance and insulin secretory failure. The so-called thrifty phenotype hypothesis87 proposed that undernourished fetuses and infants make changes (reduced lean-tissue growth and insulin sensitivity, up-regulation of the cortisol axis, and impaired pancreatic development) that cause diabetes in later life. There is strong evidence from animals that maternal dietary deprivation leads to diabetes and insulin resistance in offspring.60,88,89
Published work shows inconsistent evidence for the effect of maternal size and nutrition on insulin resistance and type 2 diabetes. Poorer glucose tolerance was associated with higher maternal weight in India90 but lower maternal body-mass index in China.81 Fasting glucose was unrelated to maternal size or nutritional supplementation in the Guatemala trial,39,82,85 or to season of birth in The Gambia.83
All studies from high-income countries show associations of lower birthweight with later type 2 diabetes and insulin resistance.22,91 The risk is greatest for people who became obese, and adjustment for adult body-mass index consistently strengthens the association with low birthweight, suggesting an important effect of weight gain in later life. There is an increased risk of diabetes associated with very high birthweight,22,92 which has been linked to maternal diabetes in pregnancy. Three studies from low-income and middle-income countries (all adjusting for adult weight) showed higher glucose concentrations in people with lower birthweight,10,80,81 whereas the two studies that did not adjust showed no associations.82,93 In four studies of diabetes and impaired glucose tolerance, one (Mysore, India) showed a positive association with ponderal index at birth,90 one (Delhi, India) showed no association with birth size,10 one (China) showed an association with low birthweight,66 and one (South Africa, adjusted for adult size) showed a higher prevalence in small for gestational age births.80 Three studies81,90,91 (all adjusting for adult size) showed inverse associations between birthweight and insulin resistance, whereas two without adjustment showed no relation.80,93 Three studies recorded no associations between birth size and insulin secretion.80,90,94
Two studies in high-income countries showed an increased risk of diabetes in people who had low weight in infancy.95 In studies in low-income and middle-income countries, fasting insulin was positively related to 18-month weight in The Gambia, but there was no association between 18-month weight and fasting glucose.83 In Delhi, India, diabetes and impaired glucose tolerance were associated with low weight at 1 and 2 years of age (adjusted for adult body-mass index).10 This study showed a strong association of accelerated body-mass index gain in childhood, after infancy, with diabetes and impaired glucose tolerance. In the Guatemala trial, supplementation during infancy was associated with a small reduction in adult fasting glucose concentration.85
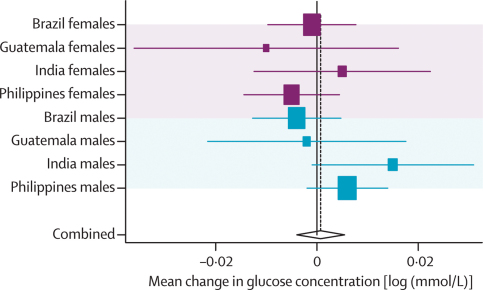
Concentrations of fasting blood glucose were available in Guatemala and the Philippines; in Brazil, we obtained a random glucose measurement, and measured fasting and 120-min concentrations from a glucose tolerance test in Delhi. Because the random measurements in Brazil were close to fasting levels in the other sites (table 2), the pooled analyses included the four sites.
Results are expressed in natural log scale (webtables 6a and b, figure 6, and table 3). Adjustment for socioeconomic confounders, age, and skin colour (in Brazil and South Africa only) did not produce consistent changes in effect sizes. None of the pooled adjusted estimates was significant but after additional adjustment for adult body-mass index and height, birthweight, weight-for-age, and body-mass- index-for-age at 2 years showed significant inverse associations with blood glucose concentrations (table 3).
Figure 6.
Forest plot for effect of height-for-age at 2 years on glucose concentration
Mean change per unit change in height-for-age Z score at 2 years.
Blood pressure
Animal studies provide strong evidence that blood pressure is raised in offspring of mothers who are exposed to diet restriction during pregnancy. Inadequate nutrition is postulated to reduce the size and number of nephrons, thereby restricting adult renal functional capacity.96–98 Early nutrition can also affect the renin-angiotensin system,99,100 exposure to glucocorticoids,101 and arterial distensibility,102,103 and it has indirect effects on blood pressure through body composition.
Evidence for an association of maternal diet during pregnancy with raised blood pressure in later life is sparse. An effect of macronutrient imbalance is suggested in European cohorts104–106 and in one study of adolescents in low-income and middle-income countries,107 but results differ. Maternal calcium supplementation can reduce offspring blood pressure, as shown in a trial in Argentina.108 Low maternal fat stores or inadequate pregnancy weight gain is related to elevated offspring blood pressure in Jamaican children109,110 and Filipino adolescents,107 but there is little evidence for adults in low-income and middle-income countries.
Many reviews and meta-analyses relate birthweight to adult blood pressure.4,111–116 Across a wide range of birthweights, most studies report an inverse association of birthweight to systolic blood pressure and hypertension prevalence in later life as well as weaker, less consistent inverse associations with diastolic blood pressure. Effects can amplify with age.117 Large studies tend to find less significant effects than do small studies.113 The association of birthweight with later blood pressure strengthens when adjusted for adult body-mass index, suggesting a role for postnatal growth.4,84,118,119 Individuals at highest risk are those with intrauterine growth restriction but a high body-mass index in adulthood. A study of blood pressure in adults from Shanghai showed a U-shaped relation with birth weight, emphasising the importance of examining non-linear relations.66
In Beijing adults,81 an increase of 1 kg in birthweight was associated with −2·9 mm Hg systolic and −1·7 mm Hg diastolic blood pressure after adjustment for adult body-mass index. Much the same findings were reported for Australian Aboriginals.120 By contrast, studies from India93,121 and Guatemala82 showed no inverse relation of birthweight to adult blood pressure. In India, birthlength was positively associated with adult systolic blood pressure and left ventricular mass,121 and in the Guatemala trial birthweight was positively associated with systolic and diastolic blood pressure in the daughters, but not sons, of women taking nutrition supplements.82
A systematic review of 23 studies recorded a positive association between rapid postnatal growth or weight gain and later blood pressure, but no studies included adults in low-income and middle-income countries.113 The timing of compensatory growth is important. There is no evidence that rapid infant weight gain increases adult blood pressure. Conversely, adults in Hong Kong with large increases in ponderal index during infancy had lower blood pressure,122 and male Filipino adolescents with higher weight and height velocity in infancy had decreased risk of high blood pressure.123
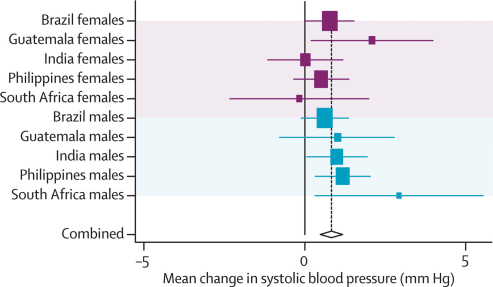
In our new analyses, data for systolic and diastolic blood pressure were available for all five cohorts. Because both sets of results were almost identical, we present only those for systolic blood pressure. Except for South Africa, adjustment for confounders other than adult body-mass index and height did not produce substantial changes in the effect estimates (webtables 7a and b, figure 7). The adjusted results differed across sites. In the pooled analyses, weight, height, and body-mass-index-for-age at 2 years were positively associated with systolic blood pressure.
Figure 7.
Forest plot for effect of height-for-age at 2 years on systolic blood pressure
Mean change per unit change in height-for-age Z score at 2 years.
Further adjustment for adult body-mass index and height led to important changes in the coefficients for birthweight (table 3), which became negative in most countries. This pattern was also noted, albeit to a lesser extent, for anthropometric indices measured at 2 years, especially weight-for-age and body-mass-index-for-age.
Cardiovascular disease
The possible biological mechanisms underlying associations between undernutrition and cardiovascular disease are similar to those involved in the aetiology of high blood pressure, lipids, and diabetes.
Several studies in high-income countries have shown that birthweight is inversely associated with the risk of coronary heart disease124 and stroke.125–127 The hazard ratio for coronary heart disease in the Helsinki cohort study was 3·63 in men who weighed less than 2·5 kg at birth compared with those weighing more than 4·0 kg. Lower birthweight has also been associated with increased carotid intima media thickness, reduced arterial compliance, and impaired endothelial function, which are all considered to be precursors of cardiovascular disease.127 Evidence from low-income and middle-income countries is limited to one study from India;121,128 prevalence of coronary heart disease was inversely related to birthweight after adjustment for adult body-mass index (14% in men and women older than 45 years weighing <5 lbs [2·27 kg] compared with 4% for those weighing >7 lbs [3·18 kg] at birth). Arterial compliance was unrelated to birthweight.
A systematic review concluded that lower infant weight is associated with an increased risk of coronary heart disease in men.95 Shorter childhood height, but accelerated childhood weight gain, are associated with increased risk of cardiovascular disease.125,126 Several studies show an increased risk of cardiovascular disease in shorter men and women; however, no studies were identified from low-income and middle-income countries.
Lung function
Lung architecture develops in utero and during the first 2–3 years of life.129 Early impairment of nutrition or oxygen availability can permanently damage lung structure and function.130 Forced expiratory volume in 1 second (FEV1) and forced vital capacity show pulmonary development and have been used as outcomes in several studies of early determinants.
A meta-analysis of eight studies (six from Europe), showed positive associations between birthweight and FEV1 after adjustment for age, smoking, and height.131 The two non-European studies included a retrospective cohort of men and women aged 38–59 years from India, in whom mean FEV1 and forced vital capacity were positively associated with birthweight irrespective of smoking; however, this study did not adjust for socioeconomic status.132 In the Pelotas cohort in Brazil, both indicators were lowest in 18-year-olds with low birthweight, especially those with intrauterine growth restriction, but the effect disappeared after adjustment for socioeconomic and gestational confounding factors.133
Immune function
Studies from The Gambia note that individuals born in the hungry season show immune system changes—suggesting lower thymic output and reduced cellular and humoral responses—that might lead to long-term programming effects.134,135
In Pakistani adults136 and Filipino adolescents,137 antibody response to selected vaccines was lower in people who were small at birth than in those with a birthweight of 2500 g or more. A Gambian study showed that young adults born in the annual hungry season were substantially more likely to die from infections than were those born the rest of the year.138,139 This finding was not confirmed in other settings with similar seasonal variability and high adult mortality because of infectious diseases,140,141 nor in the Dutch famine study in 1944.142 Further studies are needed to establish the clinical significance of these findings for adults.
Cancers
Unlike other outcomes considered here, cancers are associated with larger size in early life, possibly reflecting increased exposure to growth factors before or after birth, or both.143–146 Most of the published work relates to breast cancer.
In high-income countries, studies have shown consistent positive association between birthweight and premenopausal breast cancer.143–146 The risk typically increases by 20–40% from lowest to highest birthweight categories. Much the same associations were reported for prostate, haemopoietic, and colorectal cancers. Data from low-income and middle-income countries are scarce. A small study in China showed no association between birthweight and breast cancer,147 although in Poland, women of higher birthweight were at increased risk.148
Although data are scarce, no associations have been reported between weight in infancy and cancers.95 Single studies have suggested that higher energy intake in childhood is associated with increased cancer risk, and famine exposure is protective.149 However, there is no convincing evidence that higher bodyweight in childhood predicts cancer in later life; in fact some trials have suggested that the opposite notion could be true.143–145 There are no data from low-income and middle-income countries to date. Studies from high-income countries have shown that taller adults have an increased risk of several cancers; however, the Chinese study showed no association for breast cancer.147
Bone mass, fracture risk, and osteoporosis
Bone mass is a composite measure of skeletal size and mineral density. It peaks in young adulthood and subsequently decreases, resulting in a heightened risk of osteoporosis and bone fractures in old age. Bone mass in elderly people results from the rate of mineral loss and the mass accumulated during skeletal growth, which in turn depends on dietary calcium and vitamin D status.
Maternal calcium intake and vitamin D status in pregnancy are positively related to bone mass in children.150 No studies have examined adult outcomes.
Short birthlength is associated with an increased risk of bone fractures in adults.150 Positive correlations between birthweight or weight in infancy and adult bone-mineral content or density suggest that fetal and infant growth make important contributions to adult bone mass.150 Correlations are stronger for bone-mineral content than for density, and are reduced after adjustment for adult height, indicating that early weight predicts adult bone mass through its effect on skeletal size. A study from Finland showed higher risk of hip fracture in adults who grew rapidly from birth to 7 years and slowly from 7 to 15 years.150
Mental illness
Specific forms of mental illness are thought to be affected by adverse intrauterine experience, including maternal undernutrition. Alterations in brain development, occurring sometime in midgestation, can precipitate evolving malfunction that manifests in early adulthood. The neurodevelopmental hypothesis is supported by significant changes in the size and structure of features of the brain in some adults diagnosed with schizophrenia. Other effects of prenatal undernutrition, such as changes in arousal and sleep waves, are consistent with schizophrenia.151 The strong link between poverty and mental health152 can be partly explained by nutritional factors.
Studies investigating the Dutch Famine report more than a two-fold increase in risk for schizophrenia associated with malnutrition in midgestation.153 A very severe famine in China in 1958–61 indicated a similar level of risk of schizophrenia in a low-income or middle-income setting.154 Studies of the Chinese example suggest that an increased risk of mental illness is robustly associated with prenatal exposure to famine.155 Bennet and Gunn state that “nutritional inadequacy, in one form or another is one of the largest single non-genetic contributors to mental retardation and aberrant neural development”.151
Several cohort studies have reported associations between low birthweight and depression in men, but not in women,36 also between infant size and depression in both men and women, after adjustment for socioeconomic status, and with suicide in men.95 We identified no studies from low-income and middle-income countries.
Discussion
Table 4 summarises the review of published data and our new analyses, restricted to anthropometric indicators of undernutrition (panel 2). We provide strong evidence that adequate nutrition in utero and in the first 2 years of life is essential for formation of human capital. Undernourished children are more likely to become short adults, to have lower educational achievement, and to give birth to smaller infants. Undernutrition is also associated with lower economic status in adulthood. At present our cohorts are too young to assess the association between undernutrition and life expectancy, but in view of the direct association between longevity and schooling,159 such an association will probably become apparent in the long term. Because of the major importance of nutrition for human capital, the amount of research on this issue is remarkably small. Areas in which further research is particularly needed are listed in panel 3.
Table 4.
Summary of the evidence, particularly from low-income and middle-income settings, on the associations between maternal and child undernutrition and adult exposures
| Maternal size and nutrition | Size of newborn baby | Infant and child size and growth | |
|---|---|---|---|
| Height | |||
| Published work | Insufficient evidence | Strong, positive | Strong, positive |
| New analysis | Consistent association with maternal height | Positive association with birthweight and negative association with IUGR | Strong associations with height for age and weight-for-age; no association with BMI-for-age |
| Achieved schooling and educational performance | |||
| Published work | Insufficient evidence | Weak, positive | Strong, positive |
| New analysis | Weak positive association with maternal height | Strong positive association with birthweight; negative association with IUGR | Strong positive association with height and weight-for-age; weak association with BMI-for-age |
| Income and wealth | |||
| Published work | Insufficient evidence | Indirect evidence, mediated through schooling and adult size | Indirect evidence, mediated through schooling and adult size |
| New analysis | No association with maternal height | Positive association with birthweight; negative association with IUGR in two of three countries. | Positive association with height and weight-for-age; no association with BMI-for-age |
| Bodysize of offspring | |||
| Published work | Insufficient evidence | Strong, positive | Strong, positive |
| New analysis | Weak direct association between maternal height and birthweight of their grandchildren | Strong positive association of maternal and offspring birthweight; inverse association between maternal IUGR and offspring birthweight | Positive associations between weight and height-for-age—but not BMI-for-age—with birthweight of the offspring |
| BMI, body composition, and obesity | |||
| Published work | Insufficient evidence | Strong, positive with lean body mass; no clear association with fat mass | Positive association between large infant size and both lean and fat mass |
| New analysis | No association with maternal height | Strong positive association with birthweight and negative association with IUGR | Strong positive association with BMI-for-age and weight-for-age; weak positive association with height-for-age |
| Insulin resistance and type 2 diabetes | |||
| Published work | Inconsistent | Weak, inverse (low birthweight associated with higher risk)* | Strong evidence that rapid weight gain increases risk of diabetes |
| New analysis | No association with maternal height | No association with birthweight except when adult BMI was adjusted for, when an inverse association became apparent | No association with height-for-age. Negative associations with weight and BMI-for-age after adjustment for adult BMI and height |
| Blood pressure | |||
| Published work | Weak, inconsistent | Moderate, negative | Strong positive, synergistic with small newborn size |
| New analysis | No association | No consistent association with birthweight except when adult BMI was adjusted for, when an inverse association became apparent | Positive association with weight and height-for-age, and to a lesser extent with BMI-for-age. Associations tended to become negative after adjustment for adult BMI and height |
| Cardiovascular disease | |||
| Published work | Insufficient evidence | Little evidence of a negative association after adjustment for adult size† | Evidence for an association between small size—especially when followed by rapid weight gain—and cardiovascular disease, but no studies from low-income and middle-income countries |
| Lung function | |||
| Published work | Insufficient evidence | Strong, positive | Insufficient evidence |
| Immune function | |||
| Published work | Inconsistent | Inconsistent | Insufficient evidence |
| Blood lipids | |||
| Published work | Insufficient evidence | Evidence of no association | Inconsistent |
| Cancers | |||
| Published work | Insufficient evidence | Studies from high-income countries show evidence of a positive association for some cancers, confirmed in one of only two studies identified from low-income and middle-income countries | Inconsistent |
| Bone mass, fracture risk, and osteoporosis | |||
| Published work | Insufficient evidence | Insufficient evidence‡ | Insufficient evidence |
| Mental illness | |||
| Published work | Little evidence between intrauterine exposure to famine and schizophrenia | Little evidence of inverse association between birthweight, depression, and suicide | Insufficient evidence |
BMI=body-mass index. IUGR=intrauterine growth restriction.
In most studies from high-income countries, inverse associations are reported.
Not true for studies from high-income settings, where inverse associations are noted (no adjustment for adult size).
Studies from high-income countries show consistent associations between birthweight and adult bone mass.
Panel 2. What this paper does not cover.
This paper addresses maternal and child undernutrition through the use of anthropometric indicators. There are other dimensions of undernutrition that are equally important. These include micronutrient deficiencies—eg, iodine, iron, vitamin A, and calcium—which might lead to long-term consequences. An earlier Lancet Series addressed the long-term consequences of iodine and zinc deficiency on intellectual development,156 and these deficiencies were incorporated in the estimates of burden of diseases included in the first paper in this Series.1
Breastfeeding can also have long-term health consequences. A recent series of systematic reviews addressed its association with body-mass index, blood pressure, diabetes and related indicators, blood lipids, and schooling.86
Two important studies on the effects of short exposures to malnutrition during famines in Europe—the Dutch famine157,158 and Leningrad siege studies—were cited only when evidence from low-income and middle-income countries was very scarce. Although these investigations provide unique information about crucial periods when undernutrition is likely to have lasting effects, they were deemed not to be representative of the situation in such countries when undernutrition acts throughout longer time periods including pregnancy, infancy, and childhood.
Panel 3. Areas for future research.
-
•
Association between rapid weight and length gain at different age intervals in infancy and childhood with human capital and outcomes related to chronic disease, to define the age after which rapid growth should be avoided
-
•
Long-term effects of weight gain in late childhood stratified in previously stunted and non-stunted children, and for children with and without intrauterine growth restriction
-
•
Long-term effects of micronutrient deficiencies in childhood
-
•
Association between undernutrition and long-term changes in immune function, blood lipids, osteoporosis, and mental illness
-
•
Improved quantification of the economic effect of undernutrition on adult productivity
-
•
Interactions between genes and environmental factors in long-term outcomes
The effect of undernutrition spans at least three generations, as suggested by the small but significant association between grandmother's height and birthweight of children born to women from the five cohorts. Because of their fairly small magnitude, intergenerational effects do not preclude achievement of progress by acting only on the present generation.
The results of outcomes related to chronic disease were not so straightforward (table 4). Adult body-mass index seems to be strongly affected by the childhood indices related to weight, and to a lesser extent by height-for-age. Because body-mass index includes fat and lean mass, its associations with early weight and height might have different biological implications. Glucose concentrations were not associated with any of the exposures, but it should be noted that participants in the five cohorts are fairly young and that a post-glucose-load concentration was available in only one cohort. In high-income countries, the association of lower birthweight with raised glucose concentration is seen mainly with post-glucose-load values. Systolic and diastolic blood pressure were positively associated with childhood weight and height, and to a lesser extent to body-mass-index-for-age, but these associations were small and their clinical relevance is questionable. Taken together, our results show weaker associations between size at birth or in infancy with outcomes related to chronic disease than do those arising from cohorts in developed countries. In addition to the young age of our cohorts, other factors might have a role, including the fact that the causes of low birthweight are different and there is less catch-up growth compared with developed settings, and that some of our cohorts were not undergoing the nutrition transition.
We analysed five long running prospective cohorts in low-income and middle-income countries in a similar way. The consistency of most results is remarkable, considering that study sites are located in South and Central America, sub-Saharan Africa, and south and east Asia. Because analyses were defined a priori, our results are not affected by publication bias.
There were substantial increases in the coefficients after adjustment for adult body-mass index and height. In earlier analyses related to the Barker hypothesis, adjustment for present size was a standard procedure, but this practice has been challenged.84 Our reviews of studies from low-income and middle-income countries on lipid profiles, diabetes, blood pressure, and cardiovascular disease showed that the negative effects of undernutrition often only become apparent—or at least were strengthened—after such adjustment. If an early weight and present weight or body-mass index are included in the same regression equation, the coefficient associated with the early weight measure will become negative whenever weight gain is positively associated with the outcome. For example, a negative association with birthweight that becomes apparent only when adult body-mass index is included in the statistical model does not suggest that low birthweight itself is a risk factor; in fact, postnatal excessive weight gain might have a large role.70,84,95,119
For the outcomes for which no new data are presented, there is insufficient evidence linking undernutrition to long-term changes in immune function or blood lipids, or in indicators related to osteoporosis. Birthweight is positively associated with lung function, and there is some evidence that undernutrition might contribute to mental illness. By contrast with these findings (ie, showing detrimental effects of undernutrition), studies suggest a positive association between birthweight and the incidence of some cancers.
A recent symposium160 addressed the contrasting perspectives of auxology and biomedicine—“poor growth is poor health”—and evolutionary biology and anthropology—“poor growth is adaptive”. The evidence for the biomedical stance is overwhelming and recognised by the symposium participants, but the two approaches are not incompatible. In response to poor nutrient availability at the cellular level, vital functions are preserved, linear growth is stopped, and muscle and fat can be metabolised for continued function. Thus poor growth can be a survival strategy. However, the evidence that growth failure has a huge cost is overwhelming: compared with people who grow well, there is increased susceptibility to infections and greater mortality1 and losses in human capital in survivors. A population of stunted people will indeed have lower nutritional requirements than will a population with unrestricted growth, which might be seen as an adaptation; however, such a population will be less likely to be competitive in the modern world because of reduced human capital.
Rapid weight gain is especially relevant in low-income and middle-income countries that are undergoing rapid transition and facing an epidemic of overweight and obesity. The long-term effects of early undernutrition might be compounded by the adoption of diets and lifestyles of developed countries.134 A baby of low birthweight, who is stunted and underweight in infancy and gains weight rapidly in childhood and adult life, can represent a worst-case scenario for cardiovascular and metabolic disease.10,134,161,162 However, rapid weight gain in infancy is associated with lower morbidity and mortality in low-income and middle-income settings,1,163 and as shown above, bodysize at 2 years of age is clearly associated with enhanced human capital. Although these are sufficient reasons for strong efforts in the prevention of undernutrition, attention should also be given to preventing excessive weight gain after infancy.
To design evidence-based policies, the potential hazards of rapid weight gain in different age ranges should be established. There is growing evidence that a high birthweight64 and weight gain in infancy lead primarily to accumulation of lean body mass, whereas gaining weight later in childhood is more likely to result in accumulation of fat mass.9,22,65,164 A recent meta-analysis centred on studies from high-income countries concluded that “there is insufficient evidence to recommend prevention of adult disease through strategies to alter infant growth.”95 Therefore, present evidence does not accord with limiting weight gain in the first year of life, and suggests that rapid weight gain becomes hazardous only later in childhood. Further research is needed to establish the exact age when rapid weight gain does more harm than good.
The first article in this Series proposed stunting as a better overall indicator of undernutrition than underweight.1 In countries undergoing the nutrition transition, monitoring length-for-age and weight-for-length in young children has been argued to be more appropriate than monitoring weight-for-age,165 because weight gain can reflect children becoming taller, fatter, or both. Our findings support this argument. Height-for-age at 2 years was more closely related to outcomes for human capital than birthweight, weight-for-age, or body-mass-index-for-age. Body-mass-index-for-age was not an important predictor of human capital, although it is highly predictive of adult body-mass index. Countries undergoing the nutrition transition should consider the advantages of assessing height-for-age and body-mass-index-for-age, in view of their different predictive values.
Because of the observational nature of our analyses, the possibility of residual confounding cannot be ruled out. However, that adjustment for confounders, including socioeconomic indicators, made little difference to estimates of effect size is reassuring. In the one trial included in our analysis, exposure to a nutritious supplement during pregnancy and early childhood, compared with exposure to low-energy supplement, led to greater adult height, schooling (women only), improved scores on tests of intelligence and reading, greater income, and better growth of the next generation.31,38,48,57,166,167
Our results strongly suggest that undernutrition leads to long-term impairment. This evidence, combined with the well-known short-term effects of undernutrition, is sufficient for giving the prevention of undernutrition high priority in national health, education, and economic agendas in low-income and middle-income countries.45,168 At the same time as investments are made against undernutrition, middle-income countries undergoing the nutrition transition should also address the negative consequences of rapid weight gain, especially in later childhood.
Search strategy and selection criteria
14 adult outcomes were selected: height; achieved schooling and educational performance; income and assets; birthweight in the offspring; body-mass index, body composition, and obesity; blood lipids; insulin resistance and type 2 diabetes; blood pressure; cardiovascular disease; lung function; immune function; cancers; bone mass, fracture risk, and osteoporosis; and mental illness. Exposure variables were measured during pregnancy (maternal height and weight before pregnancy, weight gain, micronutrient status and diet), at birth (weight, length, ponderal index, intrauterine growth restriction), and at 2 years of age (stunting, wasting, underweight). Searches of published work were undertaken in the Medline, Embase, CINAHL, EconLit, Psychinfo, and PsychArticles databases, with all possible combinations of exposures and outcomes, which identified more than 15 000 original articles and 700 reviews. The search was narrowed down to articles from low-income and middle-income countries in which outcomes were measured in adulthood or late adolescence. We identified 28 relevant articles originating from populations in low-income and middle-income countries. We excluded studies with low statistical power or poor methodological quality. Information from high-income countries was summarised by selecting high-quality review articles, and was complemented by original research articles if necessary. We complemented the search by contacting investigators involved in long-term cohort studies in low-income and middle-income countries to identify relevant original or review articles and book chapters, and by searching our own personal files.
Acknowledgments
Acknowledgments
Funding for the preparation of the Series was provided by the Bill & Melinda Gates Foundation. Meetings were hosted by the UNICEF Innocenti Research Centre and the Rockefeller Foundation Bellagio Conference Center. Analyses in this paper were made possible by a grant by the Wellcome Trust of the UK.The sponsors had no role in the analysis and interpretation of the evidence nor in writing the report and the decision to submit for publication. Jean-Pierre Habicht critically reviewed the manuscript. We thank the following colleagues from each site—Guatemala: Rafael Flores, Usha Ramakrishnan, Aryeh Stein, and Kathryn Yount of Emory University; Ruben Grajeda, Paul Melgar, Manuel Ramirez-Zea, Humberto Mendez, and Luis Fernando Ramirez of INCAP; Jere Behrman of the University of Pennsylvania; John Hoddinott, Agnes Quisumbing, and Alexis Murphy of IFPRI; and John Maluccio of Middlebury College. Cebu: Socorro A Gultiano, Josephine Avila, and Lorna Perez of Office of Population Studies Foundation, University of San Carlos, Cebu, Philippines; Christopher Kuzawa and Thomas W McDade, Northwestern University. Pelotas: Fernando Barros, Bernardo Horta, and Denise Gigante of the Universidade Federal de Pelotas, and Rosangela Lima of the Universidade Católica de Pelotas. India: Santosh K Bhargava of Sunder Lal Jain Hospital. The original cohort study was funded by the US National Center for Health Statistics and the Indian Council of Medical Research. Soweto: John Pettifor and Stella Fleetwood of the University of Witwatesrand. We thank Mario Azevedo (Universidade Federal de Pelotas) for preparation of tables, figures, and meta-analyses; and Shane Norris (University of Witwatersrand), Clive Osmond and Shirin Wadia (University of Southampton), Meng Wang (Emory University) for data analyses. Finally, we would like to acknowledge funding sources of each individual study: Wellcome Trust (Pelotas and Soweto), US National Institutes of Health and the US National Science Foundation (Guatemala), British Heart Foundation, the Medical Research Council UK, and the Indian Council of Medical Research (India), Human Sciences Research Council, South African Medical Research Council, the Mellon Foundation, the South-African Netherlands Programme on Alternative Development and the Anglo American Chairman's Fund (Soweto), Ford Foundation, USAID, The World Bank, Nestle Coordinating Center for Nutrition Research (Cebu).
Contributors
CGV convened the working group, and conceptualised and coordinated the preparation of the paper. PCH led the analyses. Primary responsibility for specific topics were as follows: height and economic productivity (RM); education and mental health (LR); intergenerational effects, lipids, and immunity (CGV); body composition and blood pressure (LA); and diabetes, cardiovascular disease, cancer, and bone health (CF and HSS). All authors provided original data and contributed to the final paper.
Maternal and Child Undernutrition Study Group
Series steering committee—Robert E Black (Johns Hopkins Bloomberg School of Public Health, USA), Zulfiqar A Bhutta (Aga Khan University, Pakistan), Jennifer Bryce (Johns Hopkins Bloomberg School of Public Health, USA), Saul S Morris (London School of Hygiene and Tropical Medicine, UK), Cesar G Victora (Federal University of Pelotas, Brazil).
Other members—Linda Adair (University of North Carolina, USA), Tahmeed Ahmad (ICDDR,B, Bangladesh), Lindsay H Allen (USDA ARS Western Human Nutrition Research Center, USA), Laura E Caulfield (Johns Hopkins Bloomberg School of Public Health), Bruce Cogill (UNICEF, USA), Denise Coitinho (WHO, Switzerland), Simon Cousens (London School of Hygiene and Tropical Medicine, UK), Ian Darnton-Hill (UNICEF, USA), Mercedes de Onis (WHO, Switzerland); Kathryn Dewey (University of California, Davis, USA), Majid Ezzati (Harvard School of Public Health, USA), Caroline Fall (University of Southhampton, UK), Elsa Giugliani (Federal University of Rio Grande de Sul, Brazil), Batool A Haider (Aga Khan University, Pakistan), Pedro Hallal (Federal University of Pelotas, Brazil), Betty Kirkwood (London School of Hygiene and Tropical Medicine, UK), Reynaldo Martorell (Emory University, Rollins School of Public Health, USA), Colin Mathers (WHO, Switzerland), David Pelletier (Cornell University, USA), Per Pinstrup-Andersen (Cornell University, USA), Linda Richter (Human Sciences Research Council, South Africa), Juan A Rivera (Mexico National Institute of Public Health), Harshpal Singh Sachdev (Sitaram Bhartia Institute of Science and Research, India), Meera Shekar (World Bank, USA), Ricardo Uauy (Institute of Nutrition, Chile).
Conflict of interest statement
We declare that we have no conflict of interest.
Web Extra Material
Effect of maternal height on attained height in adulthood – meta-analyses of four studies stratified by sex.
P value <0.001 (random effects)
Effect of birthweight on attained height in adulthood – meta-analyses of five studies stratified by sex.
P value <0.001 (fixed effects)
Effect of intrauterine growth retardation on attained height in adulthood – meta-analyses of five studies stratified by sex.
P value<0.001 (fixed effects)
Effect of weight for age Z-score at 2 years on attained height in adulthood – meta-analyses of five studies stratified by sex.
P value <0.001 (random effects)
Effect of height for age Z-score at 2 years on attained height in adulthood – meta-analyses of five studies stratified by sex.
P value <0.001 (fixed effects)
Effect of body mass index for age Z-score at 2 years on attained height in adulthood – meta-analyses of five studies stratified by sex.
P value = 0.423 (random effects)
Effect of maternal height on schooling in adulthood – meta-analyses of three studies stratified by sex.
P value<0.001 (fixed effects)
Effect of birthweight on schooling in adulthood – meta-analyses of four studies stratified by sex.
P value<0.001 (fixed effects)
Effect of intrauterine growth retardation on schooling in adulthood – meta-analyses of four studies stratified by sex.
P value<0.001 (fixed effects)
Effect of weight for age Z-score at 2 years on schooling in adulthood – meta-analyses of four studies stratified by sex.
P value<0.001 (fixed effects)
Effect of height for age Z-score at 2 years on schooling in adulthood – meta-analyses of four studies stratified by sex.
P value <0.001 (fixed effects)
Effect of body mass index for age Z-score at 2 years on schooling in adulthood – meta-analyses of four studies stratified by sex.
P value = 0.019 (random effects)
Effect of maternal height on body mass index in adulthood – meta-analyses of four studies stratified by sex.
P value = 0.092 (fixed effects)
Effect of birthweight on body mass index in adulthood – meta-analyses of five studies stratified by sex.
P value<0.001 (fixed effects)
Effect of intrauterine growth retardation on body mass index in adulthood – meta-analyses of five studies stratified by sex.
P value<0.001 (fixed effects)
Effect of weight for age Z-score at 2 years on body mass index in adulthood – meta-analyses of five studies stratified by sex.
P value<0.001 (random effects)
Effect of height for age Z-score at 2 years on body mass index in adulthood – meta-analyses of five studies stratified by sex.
P value<0.001 (fixed effects)
Effect of body mass index for age Z-score at 2 years on body mass index in adulthood – meta-analyses of five studies stratified by sex.
P value<0.001 (random effects)
Attained height (cm) for males in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
Attained height (cm) for females in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
Schooling (number of years completed with approval) for males in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
Schooling (number of years completed with approval) for females in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
Annual income (log US$) for males in Brazil and Guatemala, and number of assets in India, according to indicators of maternal and child undernutrition: regression analyses. No information available for Philippines and South Africa, where most study subjects are not yet financially independent.
Annual income (log US$) for females in Brazil and Guatemala, and number of assets in India, according to indicators of maternal and child undernutrition: regression analyses. No information available for Philippines and South Africa, where most study subjects are not yet financially independent.
Birthweight of the first offspring (g) for females in four study sites, according to indicators of maternal and child undernutrition: regression analyses. No information available for South Africa, where the number of births was very small.
Body mass index (kg/m2) for males in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
Body mass index (kg/m2) for females in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
Glucose (loge mmol/L) for males in four study sites, according to indicators of maternal and child undernutrition: regression analyses. No data available from South Africa.
Glucose (loge mmol/L) for females in four study sites, according to indicators of maternal and child undernutrition: regression analyses. No data available from South Africa.
Systolic blood pressure (mm/Hg) for males in four study sites, according to indicators of maternal and child undernutrition: regression analyses.
Systolic blood pressure (mm/Hg) for females in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
References
- 1.Black RE, Allen LH, Bhutta ZA, for the Maternal and Child Undernutrition Study Group Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008 doi: 10.1016/S0140-6736(07)61690-0. published online Jan 17. DOI: 10.1016/S0140-6736(07)61690-0 [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 4.Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? Lancet. 2002;360:659–665. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 5.Joseph KS, Kramer MS. Review of the evidence on fetal and early childhood antecedents of adult chronic disease. Epidemiol Rev. 1996;18:158–174. doi: 10.1093/oxfordjournals.epirev.a017923. [DOI] [PubMed] [Google Scholar]
- 6.Victora CG, Barros FC, Lima RC. The Pelotas birth cohort study, Rio Grande do Sul, Brazil, 1982-2001. Cadernos de Saude Publica. 2003;19:1241–1256. doi: 10.1590/s0102-311x2003000500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martorell R, Behrman JR, Flores R, Stein AD. Rationale for a follow-up study focusing on economic productivity. Food Nutr Bull. 2005;26(suppl 1):S5–S14. doi: 10.1177/15648265050262S102. [DOI] [PubMed] [Google Scholar]
- 8.Grajeda R, Behrman JR, Flores R, Maluccio JA, Martorell R, Stein AD. The human capital study 2002-04: tracking, data collection, coverage, and attrition. Food Nutr Bull. 2005;26(suppl 1):S15–S24. doi: 10.1177/15648265050262S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachdev HS, Fall CH, Osmond C. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–466. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 10.Bhargava SK, Sachdev HS, Fall CH. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Underlying and proximate determinants of child health: the Cebu Longitudinal Health and Nutrition Study. Am J Epidemiol. 1991;133:185–201. [PubMed] [Google Scholar]
- 12.Richter LM, Norris SA, De Wet T. Transition from Birth to Ten to Birth to Twenty: the South African cohort reaches 13 years of age. Paediatr Perinat Epidemiol. 2004;18:290–301. doi: 10.1111/j.1365-3016.2004.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison GT, Richter LM, de Wet T, Harris HE, Griesel RD, McIntyre JA. The reliability of hand-written and computerised records of birth data collected at Baragwanath hospital in Soweto. Curationis. 1997;20:36–40. [PMC free article] [PubMed] [Google Scholar]
- 14.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–423. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 15.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol. 1982;59:624–632. [PubMed] [Google Scholar]
- 16.WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 17.Normand SL. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med. 1999;18:321–359. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Shrimpton R, Victora CG, de Onis M, Lima RC, Blossner M, Clugston G. Worldwide timing of growth faltering: implications for nutritional interventions. Pediatrics. 2001;107:E75. doi: 10.1542/peds.107.5.e75. [DOI] [PubMed] [Google Scholar]
- 20.Leary S, Fall C, Osmond C. Geographical variation in relationships between parental body size and offspring phenotype at birth. Acta Obstet Gynecol Scand. 2006;85:1066–1079. doi: 10.1080/00016340600697306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adair LS. Size at birth and growth trajectories to young adulthood. Am J Hum Biol. 2006;19:327–337. doi: 10.1002/ajhb.20587. [DOI] [PubMed] [Google Scholar]
- 22.Newsome CA, Shiell AW, Fall CH, Phillips DI, Shier R, Law CM. Is birth weight related to later glucose and insulin metabolism?—A systematic review. Diabet Med. 2003;20:339–348. doi: 10.1046/j.1464-5491.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- 23.Haeffner LS, Barbieri MA, Rona RJ, Bettiol H, Silva AA. The relative strength of weight and length at birth in contrast to social factors as determinants of height at 18 years in Brazil. Ann Hum Biol. 2002;29:627–640. doi: 10.1080/03014460210145847. [DOI] [PubMed] [Google Scholar]
- 24.Gigante DP, Nazmi A, Lima RdC, Barros FC, Victora CG. Epidemiology of early and late growth in height, leg and trunk length: findings from a birth cohort of Brazilian males. Eur J Clin Nutr (in press). [DOI] [PubMed]
- 25.Martorell R, Khan LK, Schroeder DG. Reversibility of stunting: epidemiological findings in children from developing countries. Eur J Clin Nutr. 1994;48(suppl 1):S45–S57. [PubMed] [Google Scholar]
- 26.Martorell R. The policy and program implications of research on the long-term consequences of early childhood nutrition: lessons from the INCAP follow-up study. Pan American Health Organization; Washington DC: 2005. [Google Scholar]
- 27.Coly AN, Milet J, Diallo A. Preschool stunting, adolescent migration, catch-up growth, and adult height in young senegalese men and women of rural origin. J Nutr. 2006;136:2412–2420. doi: 10.1093/jn/136.9.2412. [DOI] [PubMed] [Google Scholar]
- 28.Proos LA, Karlberg J, Hofvander Y, Tuvemo T. Pubertal linear growth of Indian girls adopted in Sweden. Acta Paediatr. 1993;82:641–644. doi: 10.1111/j.1651-2227.1993.tb18031.x. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Stein AD, Barnhart HX, Ramakrishnan U, Martorell R. Associations between prenatal and postnatal growth and adult body size and composition. Am J Clin Nutr. 2003;77:1498–1505. doi: 10.1093/ajcn/77.6.1498. [DOI] [PubMed] [Google Scholar]
- 30.Bhutta ZA, Ahmed T, Black RE, for the Maternal and Child Undernutrition Study Group What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008 doi: 10.1016/S0140-6736(07)61693-6. published online Jan 17. DOI: 10.16/S0140-6736(07)61693-6 [DOI] [PubMed] [Google Scholar]
- 31.Rivera JA, Martorell R, Ruel MT, Habicht JP, Haas JD. Nutritional supplementation during the preschool years influences body size and composition of Guatemalan adolescents. J Nutr. 1995;125(suppl):1068S–1077S. doi: 10.1093/jn/125.suppl_4.1068S. [DOI] [PubMed] [Google Scholar]
- 32.Cole TJ. Secular trends in growth. Proc Nutr Soc. 2000;59:317–324. doi: 10.1017/s0029665100000355. [DOI] [PubMed] [Google Scholar]
- 33.Pitcher J, Henderson-Smart D, Robindon J. Prenatal programming of uman motor function. In: Wintour E, Owens J, editors. Early life origins of health and disease. Springer Science and Business Media; New York: 2006. [Google Scholar]
- 34.Brown JL, Pollitt E. Malnutrition, poverty and intellectual development. Sci Am. 1996;274:38–43. doi: 10.1038/scientificamerican0296-38. [DOI] [PubMed] [Google Scholar]
- 35.Pollitt E, Golub M, Gorman K. A reconceptualization of the effects of undernutrition on children's biological, psychosocial and behavioural development. Society for Research in Child Development Social Policy Report. 1996;X:1–31. [Google Scholar]
- 36.Landon J, Davison M, Breier B. The developmental environment: influences on subsequent cognitive function and behaviour. In: Gluckman P, Hanson M, editors. Developmental origins of health and disease. Cambridge University Press; Cambridge: 2006. pp. 370–378. [Google Scholar]
- 37.Hack M. Effects of intrauterine growth retardation on mental performance and behavior, outcomes during adolescence and adulthood. Eur J Clin Nutr. 1998;52(suppl 1):S65–S71. [PubMed] [Google Scholar]
- 38.Li H, Barnhart HX, Stein AD, Martorell R. Effects of early childhood supplementation on the educational achievement of women. Pediatrics. 2003;112:1156–1162. doi: 10.1542/peds.112.5.1156. [DOI] [PubMed] [Google Scholar]
- 39.Conlisk AJ, Barnhart HX, Martorell R, Grajeda R, Stein AD. Maternal and child nutritional supplementation are inversely associated with fasting plasma glucose concentration in young Guatemalan adults. J Nutr. 2004;134:890–897. doi: 10.1093/jn/134.4.890. [DOI] [PubMed] [Google Scholar]
- 40.Alderman H, Hoddinott J, Kinsey B. Long term consequences of early childhood malnutrition. Oxf Econ Pap. 2006;58:450–474. [Google Scholar]
- 41.Daniels MC, Adair LS. Growth in young Filipino children predicts schooling trajectories through high school. J Nutr. 2004;134:1439–1446. doi: 10.1093/jn/134.6.1439. [DOI] [PubMed] [Google Scholar]
- 42.Maluccio JA, Hoddinott J, Behrman JR, Martorell R, Quisumbing AR. The impact of nutrition during early childhood on education among Guatemalan adults. Middlebury College Economics Discussion Paper number 06-14. Middlebury College, VT, 2006.
- 43.Grantham-McGregor S, Cheung Y, Cueto S, Glewwe P, Richter L, Strupp L. Developmental pontential in the first 5 years for child in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engle PL, Black MM, Behrman JR. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369:229–242. doi: 10.1016/S0140-6736(07)60112-3. [DOI] [PubMed] [Google Scholar]
- 45.World Bank . Repositioning nutrition as central to development: a strategy for large-scale action. The World Bank; Washington DC: 2006. [Google Scholar]
- 46.Behrman JR, Rosenzweig MR. Returns to Birthweight. Rev Econ Stat. 2004;86:586–601. [Google Scholar]
- 47.McGregor S, Cheung Y, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years or children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haas JD, Martinez EJ, Murdoch S, Conlisk E, Rivera JA, Martorell R. Nutritional supplementation during the preschool years and physical work capacity in adolescent and young adult Guatemalans. J Nutr. 1995;125(suppl):1078S–1089S. doi: 10.1093/jn/125.suppl_4.1078S. [DOI] [PubMed] [Google Scholar]
- 49.Thomas D, Strauss J. Health and wages: evidence on men and women in urban Brazil. J Econom. 1997;77:159–185. doi: 10.1016/s0304-4076(96)01811-8. [DOI] [PubMed] [Google Scholar]
- 50.Psacharopoulos G, Patrinos HA. Returns to investment in education: a further update. Educ Econ. 2004;12:111–134. [Google Scholar]
- 51.Victora CG, Barros FC, Horta BL, Lima RC. Breastfeeding and school achievement in Brazilian adolescents. Acta Paediatr. 2005;94:1656–1660. doi: 10.1080/08035250500252658. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Zhou LA. The long-term health and economic consequences of the 1959-1961 famine in China. J Health Econ. 2007;26:659–681. doi: 10.1016/j.jhealeco.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. Effect of a nutritional intervention during early childhood on economic productivity in Guatemalan adults. Lancet (in press). [DOI] [PubMed]
- 54.Barker DJ, Eriksson JG, Forsen T, Osmond C. Infant growth and income 50 years later. Arch Dis Child. 2005;90:272–273. doi: 10.1136/adc.2003.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 56.Ramakrishnan U, Martorell R, Schroeder DG, Flores R. Role of intergenerational effects on linear growth. J Nutr. 1999;129(suppl):544S–549S. doi: 10.1093/jn/129.2.544S. [DOI] [PubMed] [Google Scholar]
- 57.Stein AD, Barnhart HX, Hickey M, Ramakrishnan U, Schroeder DG, Martorell R. Prospective study of protein-energy supplementation early in life and of growth in the subsequent generation in Guatemala. Am J Clin Nutr. 2003;78:162–167. doi: 10.1093/ajcn/78.1.162. [DOI] [PubMed] [Google Scholar]
- 58.Yajnik CS, Fall CH, Coyaji KJ. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27:173–180. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 59.Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- 60.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 61.Vickers MH, Breier BH, McCarthy D, Gluckman PD. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am J Physiol Regul Integr Comp Physiol. 2003;285:R271–R273. doi: 10.1152/ajpregu.00051.2003. [DOI] [PubMed] [Google Scholar]
- 62.Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20:63–68. doi: 10.1016/j.nut.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogers I. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27:755–777. doi: 10.1038/sj.ijo.0802316. [DOI] [PubMed] [Google Scholar]
- 65.Victora CG, Sibbritt D, Horta BL, Lima Rda C, Cole TJ, Wells J. Weight gain in childhood and body composition at 18 years of age in Brazilian males. Acta Paediatrica. 2007;96:296–300. doi: 10.1111/j.1651-2227.2007.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian JY, Cheng Q, Song XM. Birth weight and risk of type 2 diabetes, abdominal obesity and hypertension among Chinese adults. Eur J Endocrinol. 2006;155:601–607. doi: 10.1530/eje.1.02265. [DOI] [PubMed] [Google Scholar]
- 67.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–2292. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 68.Malcolm JC, Lawson ML, Gaboury I, Lough G, Keely E. Glucose tolerance of offspring of mother with gestational diabetes mellitus in a low-risk population. Diabet Med. 2006;23:565–570. doi: 10.1111/j.1464-5491.2006.01840.x. [DOI] [PubMed] [Google Scholar]
- 69.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: Systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 70.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life–a systematic review. Obes Rev. 2005;6:143–154. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 72.Schroeder DG, Martorell R, Flores R. Infant and child growth and fatness and fat distribution in Guatemalan adults. Am J Epidemiol. 1999;149:177–185. doi: 10.1093/oxfordjournals.aje.a009784. [DOI] [PubMed] [Google Scholar]
- 73.Walker SP, Chang SM, Powell CA. The association between early childhood stunting and weight status in late adolescence. Int J Obes (Lond) 2006;31:347–352. doi: 10.1038/sj.ijo.0803383. [DOI] [PubMed] [Google Scholar]
- 74.Barker DJ. Commentary: Developmental origins of raised serum cholesterol. Int J Epidemiol. 2003;32:876–877. doi: 10.1093/ije/dyg293. [DOI] [PubMed] [Google Scholar]
- 75.Lucas A, Baker BA, Desai M, Hales CN. Nutrition in pregnant or lactating rats programs lipid metabolism in the offspring. Br J Nutr. 1996;76:605–612. doi: 10.1079/bjn19960066. [DOI] [PubMed] [Google Scholar]
- 76.Ozanne SE. Metabolic programming in animals. Br Med Bull. 2001;60:143–152. doi: 10.1093/bmb/60.1.143. [DOI] [PubMed] [Google Scholar]
- 77.Owen CG, Whincup PH, Odoki K, Gilg JA, Cook DG. Birth weight and blood cholesterol level: a study in adolescents and systematic review. Pediatrics. 2003;111:1081–1089. doi: 10.1542/peds.111.5.1081. [DOI] [PubMed] [Google Scholar]
- 78.Lauren L, Jarvelin MR, Elliott P. Relationship between birthweight and blood lipid concentrations in later life: evidence from the existing literature. Int J Epidemiol. 2003;32:862–876. doi: 10.1093/ije/dyg201. [DOI] [PubMed] [Google Scholar]
- 79.Huxley R, Owen CG, Whincup PH, Cook DG, Colman S, Collins R. Birth weight and subsequent cholesterol levels: exploration of the “fetal origins” hypothesis. JAMA. 2004;292:2755–2764. doi: 10.1001/jama.292.22.2755. [DOI] [PubMed] [Google Scholar]
- 80.Levitt NS, Lambert EV, Woods D, Hales CN, Andrew R, Seckl JR. Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young south african adults: early programming of cortisol axis. J Clin Endocrinol Metab. 2000;85:4611–4618. doi: 10.1210/jcem.85.12.7039. [DOI] [PubMed] [Google Scholar]
- 81.Mi J, Law C, Zhang KL, Osmond C, Stein C, Barker D. Effects of infant birthweight and maternal body mass index in pregnancy on components of the insulin resistance syndrome in China. Ann Intern Med. 2000;132:253–260. doi: 10.7326/0003-4819-132-4-200002150-00002. [DOI] [PubMed] [Google Scholar]
- 82.Stein AD, Conlisk A, Torun B, Schroeder DG, Grajeda R, Martorell R. Cardiovascular disease risk factors are related to adult adiposity but not birth weight in young guatemalan adults. J Nutr. 2002;132:2208–2214. doi: 10.1093/jn/132.8.2208. [DOI] [PubMed] [Google Scholar]
- 83.Moore SE, Halsall I, Howarth D, Poskitt EM, Prentice AM. Glucose, insulin and lipid metabolism in rural Gambians exposed to early malnutrition. Diabet Med. 2001;18:646–653. doi: 10.1046/j.1464-5491.2001.00565.x. [DOI] [PubMed] [Google Scholar]
- 84.Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease-the hypothesis revisited. BMJ. 1999;319:245–249. doi: 10.1136/bmj.319.7204.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stein AD, Wang M, Ramirez-Zea M. Exposure to a nutrition supplementation intervention in early childhood and risk factors for cardiovascular disease in adulthood: evidence from Guatemala. Am J Epidemiol. 2006;164:1160–1170. doi: 10.1093/aje/kwj328. [DOI] [PubMed] [Google Scholar]
- 86.Horta B, Bahl R, Martines J, Victora CG. Evidence on the long-term effects of breastfeeding: systematic reviews and meta-analyses. Study commissioned by WHO/CAH; 2006. [Google Scholar]
- 87.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 88.Ozanne SE, Hales CN. The long-term consequences of intra-uterine protein malnutrition for glucose metabolism. Proc Nutr Soc. 1999;58:615–619. doi: 10.1017/s0029665199000804. [DOI] [PubMed] [Google Scholar]
- 89.Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fall CH, Stein CE, Kumaran K. Size at birth, maternal weight, and type 2 diabetes in South India. Diabet Med. 1998;15:220–227. doi: 10.1002/(SICI)1096-9136(199803)15:3<220::AID-DIA544>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 91.Boyko EJ. Proportion of type 2 diabetes cases resulting from impaired fetal growth. Diabetes Care. 2000;23:1260–1264. doi: 10.2337/diacare.23.9.1260. [DOI] [PubMed] [Google Scholar]
- 92.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165:849–857. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- 93.Krishnaswamy K, Naidu AN, Prasad MP, Reddy GA. Fetal malnutrition and adult chronic disease. Nutr Rev. 2002;60:S35–S39. doi: 10.1301/00296640260130713. [DOI] [PubMed] [Google Scholar]
- 94.Choi CS, Kim C, Lee WJ. Association between birth weight and insulin sensitivity in healthy young men in Korea: role of visceral adiposity. Diabetes Res Clin Pract. 2000;49:53–59. doi: 10.1016/s0168-8227(00)00131-5. [DOI] [PubMed] [Google Scholar]
- 95.Fisher D, Baird J, Payne L. Are infant size and growth related to burden of disease in adulthood? A systematic review of literature. Int J Epidemiol. 2006;35:1196–1210. doi: 10.1093/ije/dyl130. [DOI] [PubMed] [Google Scholar]
- 96.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 97.Mackenzie HS, Brenner BM. Fewer nephrons at birth: a missing link in the etiology of essential hypertension? Am J Kidney Dis. 1995;26:91–98. doi: 10.1016/0272-6386(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 98.Mackenzie HS, Lawler EV, Brenner BM. Congenital oligonephropathy: the fetal flaw in essential hypertension? Kidney Int Suppl. 1996;55:S30–S34. [PubMed] [Google Scholar]
- 99.Woods LL. Fetal origins of adult hypertension: a renal mechanism? Curr Opin Nephrol Hypertens. 2000;9:419–425. doi: 10.1097/00041552-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 100.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 101.Dodic M, Moritz K, Wintour EM. Prenatal exposure to glucocorticoids and adult disease. Arch Physiol Biochem. 2003;111:61–69. doi: 10.1076/apab.111.1.61.15144. [DOI] [PubMed] [Google Scholar]
- 102.Cheung YF, Taylor MJ, Fisk NM, Redington AN, Gardiner HM. Fetal origins of reduced arterial distensibility in the donor twin in twin-twin transfusion syndrome. Lancet. 2000;355:1157–1158. doi: 10.1016/s0140-6736(00)02068-7. [DOI] [PubMed] [Google Scholar]
- 103.aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet. 1997;350:953–955. doi: 10.1016/s0140-6736(96)10508-0. [DOI] [PubMed] [Google Scholar]
- 104.Roseboom TJ, van der Meulen JH, van Montfrans GA. Maternal nutrition during gestation and blood pressure in later life. J Hypertens. 2001;19:29–34. doi: 10.1097/00004872-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 105.Campbell DM, Hall MH, Barker DJ, Cross J, Shiell AW, Godfrey KM. Diet in pregnancy and the offspring's blood pressure 40 years later. Br J Obstet Gynaecol. 1996;103:273–280. doi: 10.1111/j.1471-0528.1996.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 106.Shiell AW, Campbell-Brown M, Haselden S, Robinson S, Godfrey KM, Barker DJ. High-meat, low-carbohydrate diet in pregnancy: relation to adult blood pressure in the offspring. Hypertension. 2001;38:1282–1288. doi: 10.1161/hy1101.095332. [DOI] [PubMed] [Google Scholar]
- 107.Adair LS, Kuzawa CW, Borja J. Maternal energy stores and diet composition during pregnancy program adolescent blood pressure. Circulation. 2001;104:1034–1039. doi: 10.1161/hc3401.095037. [DOI] [PubMed] [Google Scholar]
- 108.Belizan JM, Villar J, Bergel E. Long-term effect of calcium supplementation during pregnancy on the blood pressure of offspring: follow up of a randomised controlled trial. BMJ. 1997;315:281–285. doi: 10.1136/bmj.315.7103.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clark PM, Atton C, Law CM, Shiell A, Godfrey K, Barker DJ. Weight gain in pregnancy, triceps skinfold thickness, and blood pressure in offspring. Obstet Gynecol. 1998;91:103–107. doi: 10.1016/s0029-7844(97)00581-4. [DOI] [PubMed] [Google Scholar]
- 110.Godfrey KM, Forrester T, Barker DJ. Maternal nutritional status in pregnancy and blood pressure in childhood. BJOG. 1994;101:398–403. doi: 10.1111/j.1471-0528.1994.tb11911.x. [DOI] [PubMed] [Google Scholar]
- 111.Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14:935–941. [PubMed] [Google Scholar]
- 112.Leon D, Koupilova I. Birthweight, blood pressure and hypertension: epidemiologic studies. In: Barker D, editor. Fetal origins of cardiovascular and lung disease. Marcel Dekker; New York: 1999. [Google Scholar]
- 113.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 114.Lawlor DA, Smith GD. Early life determinants of adult blood pressure. Curr Opin Nephrol Hypertens. 2005;14:259–264. doi: 10.1097/01.mnh.0000165893.13620.2b. [DOI] [PubMed] [Google Scholar]
- 115.Adair L, Dahly D. Developmental determinants of blood pressure in adults. Annu Rev Nutr. 2005;25:407–434. doi: 10.1146/annurev.nutr.25.050304.092538. [DOI] [PubMed] [Google Scholar]
- 116.Hardy R, Sovio U, King VJ. Birthweight and blood pressure in five European birth cohort studies: an investigation of confounding factors. Eur J Public Health. 2006;16:21–30. doi: 10.1093/eurpub/cki171. [DOI] [PubMed] [Google Scholar]
- 117.Lawlor DA, Ebrahim S, Davey Smith G. Is there a sex difference in the association between birth weight and systolic blood pressure in later life? Findings from a meta-regression analysis. Am J Epidemiol. 2002;156:1100–1104. doi: 10.1093/aje/kwf154. [DOI] [PubMed] [Google Scholar]
- 118.Tu YK, Gilthorpe MS, Ellison GT. What is the effect of adjusting for more than one measure of current body size on the relation between birthweight and blood pressure? J Hum Hypertens. 2006;20:646–657. doi: 10.1038/sj.jhh.1002044. [DOI] [PubMed] [Google Scholar]
- 119.Tu YK, West R, Ellison GT, Gilthorpe MS. Why evidence for the fetal origins of adult disease might be a statistical artifact: the “reversal paradox” for the relation between birth weight and blood pressure in later life. Am J Epidemiol. 2005;161:27–32. doi: 10.1093/aje/kwi002. [DOI] [PubMed] [Google Scholar]
- 120.Singh GR, Hoy WE. The association between birthweight and current blood pressure: a cross-sectional study in an Australian Aboriginal community. Med J Aust. 2003;179:532–535. doi: 10.5694/j.1326-5377.2003.tb05679.x. [DOI] [PubMed] [Google Scholar]
- 121.Kumaran K, Fall CH, Martyn CN, Vijayakumar M, Stein C, Shier R. Blood pressure, arterial compliance, and left ventricular mass: no relation to small size at birth in south Indian adults. Heart. 2000;83:272–277. doi: 10.1136/heart.83.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheung YB, Low L, Osmond C, Barker D, Karlberg J. Fetal growth and early postnatal growth are related to blood pressure in adults. Hypertension. 2000;36:795–800. doi: 10.1161/01.hyp.36.5.795. [DOI] [PubMed] [Google Scholar]
- 123.Adair LS, Cole TJ. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension. 2003;41:451–456. doi: 10.1161/01.HYP.0000054212.23528.B2. [DOI] [PubMed] [Google Scholar]
- 124.Huxley R, Owen CG, Whincup PH. Is birth weight a risk factor for ischemic heart disease in later life? Am J Clin Nutr. 2007;85:1244–1250. doi: 10.1093/ajcn/85.5.1244. [DOI] [PubMed] [Google Scholar]
- 125.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 126.Barker DJ, Clark PM. Fetal undernutrition and disease in later life. Rev Reprod. 1997;2:105–112. doi: 10.1530/ror.0.0020105. [DOI] [PubMed] [Google Scholar]
- 127.Fall CH. Developmental origins of cardiovascular disease, type 2 diabetes and obesity in humans. In: Wintour E, Owens J, editors. Early life origins of health and disease. Springer Science; New York: 2006. [Google Scholar]
- 128.Stein CE, Fall CH, Kumaran K, Osmond C, Cox V, Barker DJ. Fetal growth and coronary heart disease in south India. Lancet. 1996;348:1269–1273. doi: 10.1016/s0140-6736(96)04547-3. [DOI] [PubMed] [Google Scholar]
- 129.Shaheen S. The beginnings of chronic airflow obstruction. Br Med Bull. 1997;53:58–70. doi: 10.1093/oxfordjournals.bmb.a011606. [DOI] [PubMed] [Google Scholar]
- 130.Harding R, Cock ML, Maritz GS. The developmental environment: effects on lung structure and function. In: Gluckman P, Hanson M, editors. Developmental origins of health and disease. Cambridge University Press; Cambridge, UK: 2006. pp. 336–348. [Google Scholar]
- 131.Lawlor DA, Ebrahim S, Davey Smith G. Association of birth weight with adult lung function: findings from the British Women's Heart and Health Study and a meta-analysis. Thorax. 2005;60:851–858. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stein CE, Kumaran K, Fall CH, Shaheen SO, Osmond C, Barker DJ. Relation of fetal growth to adult lung function in south India. Thorax. 1997;52:895–899. doi: 10.1136/thx.52.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lima Rda C, Victora CG, Menezes AM, Barros FC. Respiratory function in adolescence in relation to low birth weight, preterm delivery, and intrauterine growth restriction. Chest. 2005;128:2400–2407. doi: 10.1378/chest.128.4.2400. [DOI] [PubMed] [Google Scholar]
- 134.Prentice AM, Moore SE. Early programming of adult diseases in resource poor countries. Arch Dis Child. 2005;90:429–432. doi: 10.1136/adc.2004.059030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moore SE, Collinson AC, Tamba N'gom P, Aspinall R, Prentice AM. Early immunological development and mortality from infectious disease in later life. Proc Nutr Soc. 2006;65:311–318. doi: 10.1079/pns2006503. [DOI] [PubMed] [Google Scholar]
- 136.Moore SE, Jalil F, Ashraf R, Szu SC, Prentice AM, Hanson LA. Birth weight predicts response to vaccination in adults born in an urban slum in Lahore, Pakistan. Am J Clin Nutr. 2004;80:453–459. doi: 10.1093/ajcn/80.2.453. [DOI] [PubMed] [Google Scholar]
- 137.McDade TW, Beck MA, Kuzawa C, Adair LS. Prenatal undernutrition, postnatal environments, and antibody response to vaccination in adolescence. Am J Clin Nutr. 2001;74:543–548. doi: 10.1093/ajcn/74.4.543. [DOI] [PubMed] [Google Scholar]
- 138.Moore SE, Cole TJ, Poskitt EM. Season of birth predicts mortality in rural Gambia. Nature. 1997;388:434. doi: 10.1038/41245. [DOI] [PubMed] [Google Scholar]
- 139.Moore SE, Cole TJ, Collinson AC, Poskitt EM, McGregor IA, Prentice AM. Prenatal or early postnatal events predict infectious deaths in young adulthood in rural Africa. Int J Epidemiol. 1999;28:1088–1095. doi: 10.1093/ije/28.6.1088. [DOI] [PubMed] [Google Scholar]
- 140.Moore SE, Fulford AJ, Streatfield PK, Persson LA, Prentice AM. Comparative analysis of patterns of survival by season of birth in rural Bangladeshi and Gambian populations. Int J Epidemiol. 2004;33:137–143. doi: 10.1093/ije/dyh007. [DOI] [PubMed] [Google Scholar]
- 141.Kynast-Wolf G, Hammer GP, Muller O, Kouyate B, Becher H. Season of death and birth predict patterns of mortality in Burkina Faso. Int J Epidemiol. 2006;352:427–435. doi: 10.1093/ije/dyi150. [DOI] [PubMed] [Google Scholar]
- 142.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Adult survival after prenatal exposure to the Dutch famine 1944-45. Paediatr Perinat Epidemiol. 2001;15:220–225. doi: 10.1046/j.1365-3016.2001.00336.x. [DOI] [PubMed] [Google Scholar]
- 143.Okasha M, Gunnell D, Holly J, Davey Smith G. Childhood growth and adult cancer. Best Pract Res Clin Endocrinol Metab. 2002;16:225–241. doi: 10.1053/beem.2002.0204. [DOI] [PubMed] [Google Scholar]
- 144.Okasha M, McCarron P, Gunnell D, Smith GD. Exposures in childhood, adolescence and early adulthood and breast cancer risk: a systematic review of the literature. Breast Cancer Res Treat. 2003;78:223–276. doi: 10.1023/a:1022988918755. [DOI] [PubMed] [Google Scholar]
- 145.Forman MR, Cantwell MM, Ronckers C, Zhang Y. Through the looking glass at early-life exposures and breast cancer risk. Cancer Invest. 2005;23:609–624. doi: 10.1080/07357900500283093. [DOI] [PubMed] [Google Scholar]
- 146.Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119:2007–2025. doi: 10.1002/ijc.22004. [DOI] [PubMed] [Google Scholar]
- 147.Sanderson M, Shu XO, Jin F. Weight at birth and adolescence and premenopausal breast cancer risk in a low-risk population. Br J Cancer. 2002;86:84–88. doi: 10.1038/sj.bjc.6600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Park SK, Garcia-Closos M, Lissowska J. Intrauterine environment and breast cancer risk in a population-based case-control study in Poland. Int J Cancer. 2006;119:2136–2141. doi: 10.1002/ijc.21974. [DOI] [PubMed] [Google Scholar]
- 149.Uauy R, Solomons N. Diet, nutrition, and the life-course approach to cancer prevention. J Nutr. 2005;135(suppl):2934S–2945S. doi: 10.1093/jn/135.12.2934S. [DOI] [PubMed] [Google Scholar]
- 150.Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. Review: developmental origins of osteoporotic fracture. Osteoporos Int. 2006;17:337–347. doi: 10.1007/s00198-005-2039-5. [DOI] [PubMed] [Google Scholar]
- 151.Bennet L, Gunn A. The fetal origins of adult mental illness. In: Wintour E, Owens J, editors. Early life origins of health and disease. Springer Science and Bussiness Media; New York: 2006. pp. 204–217. [Google Scholar]
- 152.WHO . The world health report 2001—mental health: new understanding, new hope. World Health Organization; Geneva: 2001. [Google Scholar]
- 153.Hulshoff Pol HE, Hoek HW, Susser E. Prenatal exposure to famine and brain morphology in schizophrenia. Am J Psychiatry. 2000;157:1170–1172. doi: 10.1176/appi.ajp.157.7.1170. [DOI] [PubMed] [Google Scholar]
- 154.St Clair D, Xu M, Wang P. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA. 2005;294:557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- 155.McClellan JM, Susser E, King MC. Maternal famine, de novo mutations, and schizophrenia. JAMA. 2006;296:582–584. doi: 10.1001/jama.296.5.582. [DOI] [PubMed] [Google Scholar]
- 156.Walker SP, Wachs TD, Gardner JM. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 157.Lumey LH, Ravelli AC, Wiessing LG, Koppe JG, Treffers PE, Stein ZA. The Dutch famine birth cohort study: design, validation of exposure, and selected characteristics of subjects after 43 years follow-up. Paediatr Perinat Epidemiol. 1993;7:354–367. doi: 10.1111/j.1365-3016.1993.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 158.Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82:485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 159.Crimmins EM, Saito Y. Trends in healthy life expectancy in the United States, 1970–1990: gender, racial, and educational differences. Soc Sci Med. 2001;52:1629–1641. doi: 10.1016/s0277-9536(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 160.Bailey SM, Schell L. Introduction. Am J Hum Biol. 2007;19:603–605. [Google Scholar]
- 161.Lawlor DA, Leon DA, Rasmussen F. Growth trajectory matters: interpreting the associations among birth weight, concurrent body size, and systolic blood pressure in a cohort study of 378 707 Swedish men. Am J Epidemiol. 2007;165:1405–1412. doi: 10.1093/aje/kwm028. [DOI] [PubMed] [Google Scholar]
- 162.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 163.Victora CG, Barros FC, Horta BL, Martorell R. Short-term benefits of catch-up growth for small-for-gestational-age infants. Int J Epidemiol. 2001;30:1325–1330. doi: 10.1093/ije/30.6.1325. [DOI] [PubMed] [Google Scholar]
- 164.Wells JC, Hallal PC, Wright A, Singhal A, Victora CG. Fetal, infant and childhood growth: relationships with body composition in Brazilian boys aged 9 years. Int J Obes (Lond) 2005;29:1192–1198. doi: 10.1038/sj.ijo.0803054. [DOI] [PubMed] [Google Scholar]
- 165.Uauy R, Kain J. The epidemiological transition: need to incorporate obesity prevention into nutrition programmes. Public Health Nutr. 2002;5:223–229. doi: 10.1079/PHN2001297. [DOI] [PubMed] [Google Scholar]
- 166.Maluccio JA, Hoddinott J, Behrman J, Martorell R, Quisumbing A, Stein AD. The impact of an experimental nutritional intervention in childhood on education among Guatemalan adults. International Food Policy Research Institute; Washington DC: 2006. [Google Scholar]
- 167.Stein AD, Wang M, Ramirez-Zea M. Exposure to a nutrition supplementation intervention in early childhood and risk factors for cardiovascular disease in adulthood: evidence from Guatemala. Am J Epidemiol. 2006;164:1160–1170. doi: 10.1093/aje/kwj328. [DOI] [PubMed] [Google Scholar]
- 168.Fogel RW. Nutrition, physiological capital and economic growth. Pan American Health Organization/Inter American Development Bank; Washington DC: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of maternal height on attained height in adulthood – meta-analyses of four studies stratified by sex.
P value <0.001 (random effects)
Effect of birthweight on attained height in adulthood – meta-analyses of five studies stratified by sex.
P value <0.001 (fixed effects)
Effect of intrauterine growth retardation on attained height in adulthood – meta-analyses of five studies stratified by sex.
P value<0.001 (fixed effects)
Effect of weight for age Z-score at 2 years on attained height in adulthood – meta-analyses of five studies stratified by sex.
P value <0.001 (random effects)
Effect of height for age Z-score at 2 years on attained height in adulthood – meta-analyses of five studies stratified by sex.
P value <0.001 (fixed effects)
Effect of body mass index for age Z-score at 2 years on attained height in adulthood – meta-analyses of five studies stratified by sex.
P value = 0.423 (random effects)
Effect of maternal height on schooling in adulthood – meta-analyses of three studies stratified by sex.
P value<0.001 (fixed effects)
Effect of birthweight on schooling in adulthood – meta-analyses of four studies stratified by sex.
P value<0.001 (fixed effects)
Effect of intrauterine growth retardation on schooling in adulthood – meta-analyses of four studies stratified by sex.
P value<0.001 (fixed effects)
Effect of weight for age Z-score at 2 years on schooling in adulthood – meta-analyses of four studies stratified by sex.
P value<0.001 (fixed effects)
Effect of height for age Z-score at 2 years on schooling in adulthood – meta-analyses of four studies stratified by sex.
P value <0.001 (fixed effects)
Effect of body mass index for age Z-score at 2 years on schooling in adulthood – meta-analyses of four studies stratified by sex.
P value = 0.019 (random effects)
Effect of maternal height on body mass index in adulthood – meta-analyses of four studies stratified by sex.
P value = 0.092 (fixed effects)
Effect of birthweight on body mass index in adulthood – meta-analyses of five studies stratified by sex.
P value<0.001 (fixed effects)
Effect of intrauterine growth retardation on body mass index in adulthood – meta-analyses of five studies stratified by sex.
P value<0.001 (fixed effects)
Effect of weight for age Z-score at 2 years on body mass index in adulthood – meta-analyses of five studies stratified by sex.
P value<0.001 (random effects)
Effect of height for age Z-score at 2 years on body mass index in adulthood – meta-analyses of five studies stratified by sex.
P value<0.001 (fixed effects)
Effect of body mass index for age Z-score at 2 years on body mass index in adulthood – meta-analyses of five studies stratified by sex.
P value<0.001 (random effects)
Attained height (cm) for males in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
Attained height (cm) for females in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
Schooling (number of years completed with approval) for males in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
Schooling (number of years completed with approval) for females in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
Annual income (log US$) for males in Brazil and Guatemala, and number of assets in India, according to indicators of maternal and child undernutrition: regression analyses. No information available for Philippines and South Africa, where most study subjects are not yet financially independent.
Annual income (log US$) for females in Brazil and Guatemala, and number of assets in India, according to indicators of maternal and child undernutrition: regression analyses. No information available for Philippines and South Africa, where most study subjects are not yet financially independent.
Birthweight of the first offspring (g) for females in four study sites, according to indicators of maternal and child undernutrition: regression analyses. No information available for South Africa, where the number of births was very small.
Body mass index (kg/m2) for males in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
Body mass index (kg/m2) for females in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.
Glucose (loge mmol/L) for males in four study sites, according to indicators of maternal and child undernutrition: regression analyses. No data available from South Africa.
Glucose (loge mmol/L) for females in four study sites, according to indicators of maternal and child undernutrition: regression analyses. No data available from South Africa.
Systolic blood pressure (mm/Hg) for males in four study sites, according to indicators of maternal and child undernutrition: regression analyses.
Systolic blood pressure (mm/Hg) for females in the five study sites, according to indicators of maternal and child undernutrition: regression analyses.