Abstract
Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) strains, which often produce Panton-Valentine leucocidin (PVL), are increasingly noted worldwide. In this study, we examined 42 MRSA strains (25 PVL-positive [PVL+] strains and 17 PVL-negative [PVL−] strains) isolated in Taiwan for their molecular characteristics. The PVL+ MRSA strains included CA-MRSA strains with multilocus sequence type (ST) 59 (major PVL+ MRSA in Taiwan), its variants, and worldwide CA-MRSA ST30 strains. The PVL− MRSA strains included the pandemic Hungarian MRSA ST239 strain, the Hungarian MRSA ST239 variant, MRSA ST59 (largely hospital-acquired MRSA strains) and its variants, the pandemic New York/Japan MRSA ST5 strain (Japanese type), and the MRSA ST8 strain. The major PVL+ CA-MRSA ST59 strain possessed a tetracycline resistance-conferring (tetK positive) penicillinase plasmid and a drug resistance gene cluster (a possible composite transposon) for multidrug resistance. Moreover, it carried a novel staphylococcal cassette chromosome mec (SCCmec) with two distinct ccrC genes (ccrC2-C8). This SCCmec (previously named SCCmec type VT) was tentatively designated SCCmec type VII. Sequencing of the PVL genes revealed the polymorphisms, and the PVL+ CA-MRSA ST59 strain possessed the ST59-specific PVL gene sequence. The data suggest that a significant amount of clonal spread is occurring in Taiwan and that the major PVL+ CA-MRSA ST59Taiwan strain exhibits unique genetic characteristics, such as a novel SCCmec type and an ST59-specific PVL gene sequence.
Methicillin-resistant Staphylococcus aureus (MRSA), alternatively called hospital-acquired MRSA (HA-MRSA), has been a major cause of nosocomial infections since the 1960s (6). MRSA is considered to have emerged from S. aureus through the acquisition of staphylococcal cassette chromosome mec (SCCmec), which carries the mecA gene for methicillin resistance (10, 41, 69). Several such pandemic clones have spread worldwide (1), including a Hungarian clone (belonging to multilocus sequence type [ST] 239) that has spread to, e.g., Taiwan, and a New York/Japan (ST5) clone that has spread to the United States and Japan.
In addition, since the period from 1997 to 1999, community-acquired MRSA (CA-MRSA) has also become a major concern worldwide (8, 59, 68). CA-MRSA is associated with skin and soft tissue infections in young, otherwise healthy individuals in the community (68) and also with severe diseases such as necrotizing pneumonia and sepsis (8, 68). CA-MRSA often carries genes for Panton-Valentine leucocidin (PVL) (31, 59, 68), a harmful toxin which destroys bacterium-engulfing immune cells and also respiratory tissue (19, 33). The rates of carriage of the PVL toxin gene are, e.g., >75% for CA-MRSA strains (21) and 67% and 80% for CA-MRSA strains with ST8 and ST1, respectively, in the United States (37).
Some PVL-positive (PVL+) CA-MRSA clones are rather continent specific; e.g., clones with ST1 and ST8 are mostly found in the United States and Canada (20, 38, 59), and clones with ST80 are mostly found in Europe (59). In contrast, PVL+ CA-MRSA strains with ST30 are found worldwide, e.g., in the United States, Europe, Oceania, Japan, and Russia (3, 16, 46, 52, 59). PVL+ CA-MRSA strains with ST59 have been reported from Taiwan and the United States (5, 7, 16). Although in many cases PVL+ CA-MRSA strains are susceptible to non-β-lactam antimicrobial agents, the PVL+ CA-MRSA ST59Taiwan strain is multidrug resistant (5). In this study, we investigated the genetic, virulence, and drug resistance characteristics of PVL+ CA-MRSA ST59Taiwan and compared with them with those of other PVL+ and PVL-negative (PVL−) MRSA clones.
MATERIALS AND METHODS
Bacterial strains.
Forty-two MRSA strains (25 PVL+ strains and 17 PVL− strains) were isolated in National Taiwan University Hospital from 2000 to June 2006; of those, 32 strains were isolated from patients in the pediatric department and the emergency surgery room and from patients with skin and soft tissue infections in other departments and 10 strains were randomly chosen from among isolates recovered in all departments. All PVL− strains examined in this study were isolated from inpatients (48 h after hospitalization), indicating that they were HA-MRSA strains. An additional 10 MRSA strains belonging to the pandemic New York/Japan ST5 clone were also obtained from inpatients at the same hospital in 2006. CA-MRSA ST59 strains TSGH17 from Taiwan (5) and USA1000 from the United States (38) were kindly provided by C. C. Wang and by L. K. McDougal and L. C. McDonald, respectively. The following strains were also employed for PVL gene sequence analysis: PVL+ methicillin-susceptible S. aureus (MSSA) strains GN1, GN3, GN4, and GN5 (isolated in Japan in 2005 [this study]); CA-MRSA ST30 strain EB00449 (isolated in Japan in 2002 [67]); CA-MRSA ST30 strain RS08 (isolated in Russia in 2006 [3]); CA-MRSA ST30 strain ER7 (isolated in Egypt in 2007 [this study]); CA-MRSA ST80 strain HT20030345 (isolated in The Netherlands [67]); and CA-MRSA ST80 strain HT20030442 (isolated in France [67]). The pandemic New York/Japan ST5 clone (strain BK2464) originated in the United States (1) and was kindly provided by H. de Lencastre.
Media and bacterial growth.
For bacterial growth, we used LB broth (Difco, Sparks, MD) as the liquid medium, which was inoculated and incubated at 37°C with agitation until the isolates reached log phase. Nutrient agar (Eiken Chemical, Tokyo, Japan) was used as the solid medium. Mueller-Hinton agar (Difco) was used for susceptibility testing.
Molecular typing.
Multilocus sequence typing was performed by using seven housekeeping genes, as described previously (17). An allelic profile (allele number) was obtained from the multilocus sequence typing website (http://www.mlst.net/), and the ST data were further analyzed by using eBURST software (18) to determine the clonal complex to which each ST belonged. spa (staphylococcal protein A gene) typing was performed as described previously (49). The spa type was determined by using a public spa type database (http://tools.egenomics.com/). Detection of the accessory gene regulator (agr) allele group was done by PCR with the primers described previously (50). The strains were analyzed for their SCCmec types (types I to V) by PCR, as described previously (10, 27, 41, 69), by using reference strains. In the case of SCCmec type IV, four subtypes (subtypes IVa, IVb, IVc, and IVd) were further analyzed by PCR with the primers described previously (26). PCR primers for the detection of SCCmec type VT were designed on the basis of the ccrC2 gene sequence of strain TSGH17 (GenBank accession no. AY894416). They were ccrC2-F2 (5′-ATAAGTTAAAAGCACGACTCA) and ccrC2-R2 (5′-TTCAATCCTATTTTTCTTTGTG), which generate a 257-bp product.
Coagulase typing.
The MRSA and MSSA strains were examined for their coagulase types by using a staphylococcal coagulase antiserum kit (Denka Seiken, Tokyo, Japan), in accordance with the manufacturer's instructions.
Virulence gene analysis.
Forty-one staphylococcal virulence genes (except the lukE-lukD genes) were detected by PCR by using the primers reported previously. The targeted genes were 3 leucocidin genes (29, 40), 5 hemolysin genes (29), 17 staphylococcal enterotoxin (SE) genes (4, 14, 23, 28, 43, 70), 1 putative staphylococcal enterotoxin gene (34), 3 exfoliative toxin genes (4, 66), an exotoxin-like gene cluster (63), the epidermal cell differentiation inhibitor gene (29), and 14 adhesin genes (39, 44, 55, 58, 60). The lukE-lukD genes were assayed with primers LUKED-F1 (5′-CAGATGTGAAGGGTAGTGGA) and LUKED-R5 (5′-TCATTATCAGATGTTGCTGTTG), designed in this study.
PVL gene diversity analysis.
The PVL genes were sequenced as described previously (40), and the phylogenetic diversity of the PVL genes was analyzed by use of the CLUSTAL W program (http://clustalw.ddbj.nig.ac.jp/top-j.html).
Drug resistance gene analysis.
Resistance genes were detected by PCR. They included genes for penicillin resistance (53), tetracycline resistance (56), aminoglycoside resistance (9, 11), macrolide and lincosamide resistance (51), chloramphenicol resistance (32), and cadmium resistance (cadDX [36]); for the detection of the other cadmium resistance genes (cadA and cadC), primer set cadA-F1 (5′-GTTCGATTGTAATTGGCGG) and cadA-R1 (5′-TTTCCTGACCATTCCGC) and primer set cadC-F1 (5′-GAAGATAAGGTAAACAGGGCT) and cadC-R1 (5′-CAAGCTGTTTAACATGCTC), respectively, were designed in this study on the basis of the gene sequences of MRSA strain 252 (GenBank accession no. BX571856). Stock strains in our laboratory were used as positive controls for PCR analysis for resistance genes. For fluoroquinolone resistance, mutations in gyrA (for DNA gyrase) and grlA (for topoisomerase IV) were determined as described previously (24, 61). In order to detect Tn551, which hopped onto a plasmid, PCR primer sets were designed on the basis of the sequence of Tn551 (GenBank accession no. Y13600): primer set tnpR-F (5′-ATGATTTTTGGCTATGCTCG) and tnpR-R (5′-TAAGACCAGAGTTAGTTCGTTC), which generated a 382-bp product for the resolvase gene (tnpR), and primer set tnpA-F (5′-ACAACTTCTTTCTGTAGACCAC) and tnpA-R (5′-GTCTTTTAGCCAAGCGAG), which generated a 604-bp product for the transposase gene (tnpA). PCR was conducted for the detection of three genes of Tn551 (ermB, tnpR, and tnpA).
Plasmid analysis.
Plasmid DNA was isolated by using a plasmid midi kit (Qiagen, Hilden, Germany) and lysostaphin (Wako Pure Chemicals, Osaka, Japan), according to the manufacturers’ instructions.
PCase plasmid transfer.
Penicillinase (PCase) plasmid DNA was introduced into S. aureus RN2677 (52, 67) by electroporation by using a Gene Pulser II electroporator (Bio-Rad, Tokyo, Japan), according to the manufacturer's instructions, or by the filter mating method. In the latter method, transconjugants were selected for both the donor resistance (PCase plasmid) marker (with cadmium acetate at 10 μg/ml) and the recipient marker (with novobiocin at 5 μg/ml or rifampin at 1 μg/ml).
Susceptibility testing.
Susceptibility testing of the bacterial strains was done by the agar dilution method with Mueller-Hinton agar according to previously described procedures (12). The antimicrobial agents were gifts from their manufacturers.
Nucleotide sequence accession numbers.
The GenBank accession no. for the rgc region is AB300568. The GenBank accession no. for the novel SCCmec sequence is AB353125. The GenBank accession numbers for the PVL gene are AB303648 for strain EB00449, AB256036 for strain GN1, AB256038 for strain GN3, AB256039 for strain GN4, AB256037 for strain GN5, AB303647 for strain PM7, AB303649 for strain RS08, AB353126 for strain ER7, AB295470 for strain PM1, AB295471 for strain PM4, AB295472 for strain PM35, AB303645 for strain PM13, AB303646 for strain PM17, AB295473 for strain USA1000, AB331244 for strain HT20030345, and AB331245 for strain HT20030442.
RESULTS
Characterization and identification of MRSA strains.
Forty-two MRSA strains (25 PVL+ strains and 17 PVL− strains) from Taiwan were examined for their genetic types, toxin and adhesin gene patterns, and oxacillin resistance. The data for these strains and their identities are summarized in Table 1.
TABLE 1.
Characteristics and identification of MRSA strains from Taiwan
| Group | No. of strains | Type
|
Presence of toxin gene
|
Adhesin genec | Oxacillin MIC(s) (μg/ml [% of strains]) | Isolation site (% of strains) | Identification of MRSA | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ST (clonal complex) | SCCmec typea | Other | lukPVSF (PVL) | Otherb (% of strains) | ||||||
| 1 | 20 | 59 (59) | VT | spa143, agr1, CoaVII | + | hlb, hlg-v, seb (80) | sdrE (85%) | ≤32 (60); ≥64 (40) | Abscess (85), bacteremia (5), RTId (5), surgical wound (5) | Major PVL+ MRSA clone in Taiwan, ST59 |
| 1-v1 | 1 | 59 (59) | V | spa143, agr1, CoaVII | + | hlb, hlg-v, seb | sdrE | 64 | Surgical wound | PVL+ ST59 variant |
| 1-v2 | 1 | 59 (59) | X | spa143, agr1, CoaVII | + | hlb, hlg-v, seb | sdrE | 64 | RTI | PVL+ ST59 variant |
| 1-v3 | 1 | 59 (59) | V | spa775,eagr1, CoaVII | + | hlb, hlg-v, seb | sdrE | 4 | UTIf | PVL+ ST59 variant |
| 1-v4 | 1 | 59 (59) | VT | spa776,eagr1, CoaVII | + | hlb, hlg-v, seb | sdrE | 4 | Abscess | PVL+ ST59 variant |
| 2 | 1 | 30 (30) | IVc | spa19, agr3, CoaIV | + | egc,gseu | cna, bbp | 32 | UTI | PVL+ MRSA clone, worldwide ST |
| 3 | 7 | 239 (8) | III | spa3, agr1, CoaIV | − | lukE-lukD, hlb (14.3), hlg-v, sea | cna, sdrD, sdrE | ≥128 | Abscess (14.3), bacteremia (85.7) | Major PVL− MRSA clone in Taiwan, Hungarian clone |
| 3-v1 | 1 | 900h (8) | III | spa3, agr1, CoaIV | − | lukE-lukD, hlg-v, sea | cna, sdrD, sdrE | ≥128 | Bacteremia | Hungarian clone variant |
| 4 | 4 | 59 (59) | IVx | spa143, agr1, CoaVII | − | hlb, hlg-v, seb | sdrE | ≥128 | Bacteremia (75), RTI (25) | Major PVL− MRSA clone in Taiwan, ST59 |
| 4-v1 | 1 | 59 (59) | X | spa143, agr1, CoaVII | − | hlb, hlg-v, seb | sdrE | 32 | Bacteremia | PVL− ST59 variant |
| 4-v2 | 1 | 59 (59) | IVx | spa777,eagr1, CoaVII | − | hlb, hlg-v, seb | sdrE | 64 | Abscess | PVL− ST59 variant |
| 4-v3 | 1 | 59 (59) | IVc | spa778,eagr1, CoaVII | − | hlb, hlg-v, seb | sdrE | 32 | Abscess | PVL− ST59 variant |
| 5 | 1 | 5 (5) | II | spa2, agr2, CoaII | − | lukE-lukD, hlb, hlg-v, tst, sec, egc | sdrD, sdrE | ≥128 | Bacteremia | New York/Japan clone (Japanese type) |
| 6 | 1 | 8 (8) | IVx | spa779,eagr1, CoaIII | − | lukE-lukD, hlb, hlg-v | sdrD, sdrE | 32 | Bacteremia | MRSA clone, worldwide ST |
SCCmec type VT was detected by PCR with primers ccrC2-F2 and ccrC2-R2, designed in this study. SCCmec type V was negative by PCR with the SCCmec type VT-specific primers. SCCmec type X is an unknown type (i.e., it is a type other than type I, II, III, IV, or V). SCCmec type IVx is type IV with unknown subtypes (a subtype other than subtype IVa, IVb, IVc, or IVd).
Other than the common four genes, which consist of three hemolysin genes (hla, hlg, hld) and the SE gene (set).
Other than the 10 genes icaA, icaD, eno, fnbA, fnbB, ebpS, clfA, clfB, fib, and sdrC.
RTI, respiratory tract infection.
Novel spa types.
UTI, urinary tract infection.
egc, enterotoxin gene cluster, which carries seg, sei, sem, sen, and seo (30).
Single-locus variant of ST239.
The major PVL+ ST59 clone (group 1; which accounted for 96% of the PVL+ MRSA strains) exhibited SCCmec type VT, spa143, agr-1, and coagulase type VII (CoaVII) and was positive for all hemolysin genes examined; and most of the strains of this clone (80%) were positive for the SEB gene (seb). It was positive for 10 (common) adhesin genes, and most of the strains of this clone (85%) were positive for the fibrinogen adhesin gene (adrE) but negative for the collagen adhesin gene (cna) and the bone sialoprotein adhesin gene (bbp). The oxacillin resistance levels of the strains of the major PVL+ ST59 clone were relatively low (MICs, ≤32 μg/ml), and most strains of this clone (85%) were isolated from abscesses, in good agreement with the findings for CA-MRSA strains. There were several other related strains with minor differences (groups 1-v1 to 1-v4). The remaining PVL+ MRSA clone (group 2; which accounted for 4% of the PVL+ MRSA strains) was CA-MRSA ST30, which has a worldwide distribution. The oxacillin resistance levels of these strains were also relatively low (MICs, 32 μg/ml).
One major PVL− clone (group 3; which accounted for 41.2% of the PVL− MRSA strains) was the pandemic Hungarian MRSA ST239 clone, which is the predominant HA-MRSA clone in Taiwan. Strains of this clone exhibited high-level oxacillin resistance (MICs, ≥256 μg/ml) and were mostly (85.7%) isolated from blood. A variant (group 3-v1) of the Hungarian MRSA ST239 clone exhibited a novel ST type, ST900, and is a single-locus variant of ST239.
Another major PVL− clone (group 4; which accounted for 23.5% of the PVL− MRSA strains) was MRSA ST59, which closely resembled the major PVL+ ST59 clone (Table 1), except that it exhibited SCCmec type IVx and had high levels of oxacillin resistance (MICs, ≥256 μg/ml), and most of the strains of this clone (75%) were isolated from blood. Since these strains were isolated from inpatients, they were classified as HA-MRSA. There were several other related strains with minor differences (groups 4-v1 to 4-v3).
Minor PVL− MRSA clones (which were isolated infrequently) included the pandemic New York/Japan ST5 clone (group 5; which accounted for 5.9% of the PVL− MRSA strains). These strains were positive for hlb (which codes for β-hemolysin) and the mobile pathogenicity island (SaPI1 [48]), which carries tst (which codes for toxic shock syndrome toxin 1), sec (which codes for SEC), and sel (which codes for SEL), like Japanese strains and unlike strain BK2464 from the United States (data not shown). This indicates that the Taiwanese New York/Japan clone is of the Japanese type. PVL− clone ST8 (group 6) was also isolated infrequently.
Drug resistance of MRSA strains.
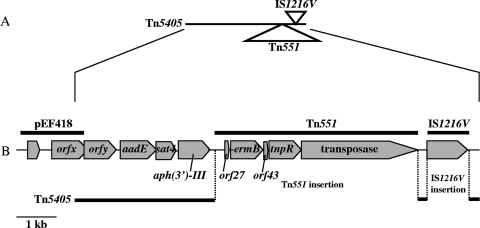
The drug resistance and the resistance genes of the MRSA strains belonging to each ST type are shown in Table 2. Strains of the major PVL+ MRSA ST59 clone were mostly (75%) positive for tetK, which codes for tetracycline resistance. The tetK gene was carried on a PCase (blaZ) plasmid (designated pTPC1 [42 kb] in strain PM1); strain PM1 carried only this plasmid. pTPC1 also carried cadDX, which codes for cadmium resistance. Moreover, most strains of this clone (90%) were positive for aph(3′)-IIIa, which codes for kanamycin resistance; aadE, which codes for streptomycin resistance; and ermB, which codes for erythromycin and clindamycin resistance. The three genes constituted a resistance gene cluster (rgc) and were parts of Tn5405 and Tn551 (Fig. 1), indicating the possibility of a novel composite transposon. Of those structures, Tn551 occasionally translocated onto pTPC1; the ermB gene on the Tn551-containing plasmid (pTPC1-EC1) encoded (and expressed constitutively) resistance to both erythromycin and clindamycin (data not shown). Strains of the major PVL+ MRSA ST59 clone were mostly (75%) positive for cat, which codes for chloramphenicol resistance; the cat gene was located independently of tetK and, probably, rgc. The characteristics of PVL+ MRSA ST59 strain TSGH17, described previously, were the same as those of strain PM1 (data not shown).
TABLE 2.
Drug resistance of MRSA strains from Taiwan
| Drug, resistance gene | Presence of gene or gene mutation in the following MRSA strains (% of strains)a:
|
|||||
|---|---|---|---|---|---|---|
| PVL+ ST59 (n = 24) | PVL+ ST30 (n = 1) | PVL− ST239/ST900 (n = 8) | PVL− ST59 (n = 7) | PVL− ST5 (n = 1) | PVL− ST8 (n = 1) | |
| Tetracycline | ||||||
| tetK | + (75)b | − | + (63) | + (71)c | − | − |
| tetM | − | − | + | + (14) | − | − |
| Gentamicin-kanamycin, aac(6′)-aph(2″) | − | − | + | + (71) | + | − |
| Kanamycin | ||||||
| aph(3′)-IIIa | + (92)d | − | + | + (71) | − | − |
| ant(4′)-Ia | − | − | − | − | + | − |
| Streptomycin | ||||||
| aadE | + (92)d | − | + (25) | + (14) | − | − |
| spc | − | − | + | − | + | − |
| Erythromycin-clindamycin | ||||||
| ermA | − | − | + | − | + | + |
| ermB | + (92)d | − | − | + | − | − |
| ermC | − | − | − | + (14) | − | − |
| Erythromycin, msrA/msrB | − | − | − | − | − | − |
| Chloramphenicol, cat | + (75) | − | − | + (57) | − | − |
| Fluoroquinolonese | ||||||
| gyrA mutation | − | − | S84L (88), E88K (12) | − | S84L | − |
| grlA mutation | − | − | S80F | − | S80Y | − |
| Ampicillin, blaZ | + (96)b | + | + | + (86)c | +f | + |
| Cadmium | ||||||
| cadDX | + (96)b | + | + (63) | + (86)c | +f | − |
| cadA | − | − | + (50) | − | − | − |
| cadC | − | − | + (50) | − | − | − |
Strains are from Table 1. PVL+ ST59 includes groups 1 and groups 1-v1 to 1-v4. PVL+ ST30 corresponds to group 2. PVL− ST239/ST900 includes group 3 and group 3-v1. PVL− ST59 includes group 4 and groups 4-v1 to 4-v3. PVL− ST5 and PVL− ST8 correspond to groups 5 and 6, respectively.
The PCase plasmids of strains PM1 and PM9 code for tetracycline resistance (tetK), ampicillin resistance (blaZ), and cadmium resistance (cadDX).
The PCase plasmids of strains PM26 and PM34 code for tetracycline resistance (tetK), ampicillin resistance (blaZ), and cadmium resistance (cadDX).
The resistance to kanamycin, streptomycin, and erythromycin-clindamycin of strain PM1 was encoded by rgc (drug resistance gene cluster), shown in Fig. 1.
+, fluoroquinolone resistance with gyrA and grlA mutations; −, fluoroquinolone susceptible.
The PCase plasmid of strain PM29 codes only for ampicillin resistance (blaZ) and cadmium resistance (cadDX).
FIG. 1.
Schematic structure of the drug resistance gene cluster (rgc) of PVL+ CA-MRSA ST59Taiwan strain PM1. Two mobile genetic elements, transposon Tn551 (which carries ermB, which encodes constitutive resistance to erythromycin and clindamycin [65]) and insertion sequence IS1216V, were inserted into transposon Tn5405, which carries aadE (which encodes streptomycin resistance) and aph(3′)-III (which encodes kanamycin resistance). Occasionally, Tn551 transposed onto pTPC1 (PCase plasmid) in strain PM1.
PVL− MRSA ST59 strain PM34 also carried a tetK-positive PCase plasmid (designated pTPC34 [46 kb]), in addition to a 4.5-kb plasmid. All strains (including the MRSA ST8 strains) listed in Tables 1 and 2 were negative for macrolide resistance genes (msrA/msrB), which is characteristic of many CA-MRSA strains, such as USA300 (ST8).
Novel structure of SCCmec type VT.
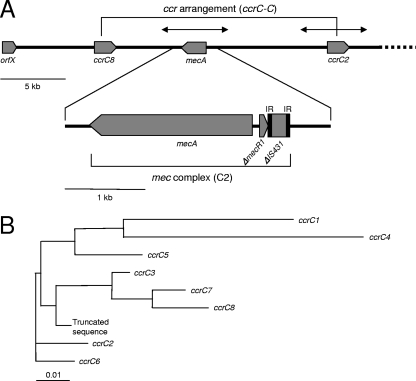
Analysis of the SCCmec type VT sequence of CA-MRSA ST59Taiwan (strain PM1) revealed two distinct ccrC genes within the SCCmec region, one of which, ccrC2, has been reported previously and the other one of which was a novel ccrC gene (designated ccrC8) (Fig. 2). The sequences of the ccrC2 region and the mec complex C2 (mecA-ΔmecR1-ΔIS431) region were the same as those of previously described strain TSGH17. The ccrC2 gene was 1,680 bp (560 amino acids) long, and the ccrC8 gene was 1,677 bp (559 amino acids) long, and they showed 92.5% and 93.7% homologies at the nucleotide and amino acid levels, respectively. SCCmec type VT, which contains a novel ccr arrangement (ccrC-C) and mec complex (mec complex C2), is a novel class of SCCmec rather than a variant of SCCmec type V. This novel SCCmec type was tentatively designated SCCmec type VII.
FIG. 2.
Structure of the novel SCCmec (previously named SCCmec type VT) of PVL+ CA-MRSA ST59Taiwan strain PM1 (A) and the phylogenetic diversity of the ccrC genes (B). (A) Arrows above the novel SCCmec structure indicate the sequence region of strain TSGH17, determined by Boyle-Vavra et al. (5). IR, inverted repeats. (B) Phylogenetic diversity was analyzed by use of the CLUSTAL W program (http://clustalw.ddbj.nig.ac.jp/top-j.html), and the graph was constructed by using the Tree View X program. GenBank accession nos. for the previously described ccrC gene sequences are AB121219 for ccrC1, AY894416 for ccrC2, AB037671 for ccrC3, U10927 for ccrC4, AP006716 for ccrC5, EF190467 for ccrC6, EF190468 for ccrC7, and AB047089 for the truncated sequence. ccrC8 (GenBank accession no. AB353125) is a novel ccrC found in the SCCmec of strain PM1 (A) and showed 97.5% homology to ccrC7. The scale bar represents nucleotide differences.
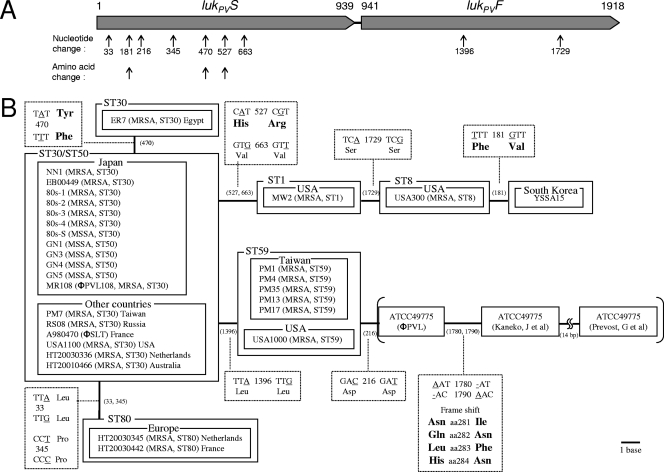
Polymorphisms and ST type dependency of the PVL gene sequences.
Sequencing of the PVL genes revealed polymorphisms. The phylogenetic diversity of the PVL genes of strains of various STs (31 strains) is summarized in Fig. 3. The PVL gene sequences were ST dependent (except for those of ST30 and ST50). Thus, the PVL gene sequence of CA-MRSA ST59 had a unique nucleotide substitution which was distinct from those of strains of the other STs (Fig. 3B). Nonsynonymous substitutions (which cause amino acid changes) were found in three cases (CA-MRSA ST30Egypt strain ER7, CA-MRSA ST1 [USA400, MW2], and strain YSSA15) and were found only in the lukPVS gene region (Fig. 3A and B).
FIG. 3.
Phlogenetic diversity of the PVL genes. (A) The structure of the PVL gene for lukPVS and lukPVF is from PVL+ CA-MRSA ST30Japan strain NN1 (GenBank accession no. AB186917). The numbering starts at the 5′ end of lukPVS (1). Arrows below the PVL gene structure indicate the positions of nucleotide changes or the amino acid changes observed with the PVL variants shown in panel B; the data for strain ATCC 49775 are not shown. (B) A bar represents 1 base substitution (distance), and the position (nucleotide number) of substitutions corresponds to that in panel A. An amino acid for a codon in a nonsynonymous substitution is shown in boldface. The PVL genes of PVL+ MSSAJapan strains (GN1, GN3, GN4, and GN5), PVL+ CA-MRSA ST30Taiwan strain (PM7; C6 in Table 1), PVL+ CA-MRSA ST30Japan strain (EB00449; [67]), PVL+ CA-MRSA ST30Russia strain (RS08 [3]), PVL+ CA-MRSA ST30Egypt strain (ER7), PVL+ CA-MRSA ST59Taiwan strains (PM1, PM4, PM13, PM17, and PM35; C1, C4, C3, C2, and C5 in Table 1, respectively), PVL+ CA-MRSA ST59USA strain (USA1000 [54]), the PVL+ CA-MRSA ST80Netherlands strain (HT20030345), and the PVL+ CA-MRSA ST80France strain (HT20030442) were determined in this study. The GenBank accession nos. are AB186917 for strain NN1; AB303648 for strain EB00449; AB245449 for strain 80s-1; AB245450 for strain 80s-2; AB245451 for strain 80s-3; AB245452 for strain 80s-4; AB245448 for strain 80s-S; AB256036 for strain GN1; AB256038 for strain GN3; AB256039 for strain GN4; AB256037 for strain GN5; AB243556 for strain MR108 (ϕ108); AB303647 for strain PM7; AB303649 for strain RS08; AB045978 for strain A980470 (ϕSLT); AB245453 for strain USA1100; AB245454 for strain HT20030336; AB245455 for strain HT20010466; AB353126 for strain ER7; BA000033 for strain MW2; CP000255 for strain USA300; DQ993352 for strain YSSA15; AB295470 for strain PM1; AB295471 for strain PM4; AB295472 for strain PM35; AB303645 for strain PM13; AB303646 for strain PM17; AB295473 for strain USA1000; AB009866, AB006796, and X72700 for strain ATCC49775; AB331244 for strain HT20030345; and AB331245 for strain HT20030442.
DISCUSSION
In Taiwan, the macrolide-resistant (ermB-positive), PVL+ CA-MRSA ST59 clone was reported by Wang et al. (62) in 2004. The SCCmec type of most PVL+ CA-MRSA ST59 strains was reported to be untypeable (62); and in 2005, it was classified as SCCmec type VT, which contains a ccrC recombinase gene variant (ccrC2) and mec complex C2, by Boyle-Vavra et al. (5). Boyle-Vavra et al. (5) also designed a particular primer to detect SCCmec type VT and proposed strain TGSH17 as a prototype of the PVL+ CA-MRSA ST59 clone from Taiwan, which shows resistance to four non-β-lactam antimicrobial agents (erythromycin, clindamycin, tetracycline, and chloramphenicol). They also speculated that the PVL+ CA-MRSA ST59 clone in the United States (the San Francisco clone) might have originated from Taipei. Lo et al. described in 2006 that PVL+ CA-MRSA, isolated from patients who lived in Taipei City or Taipei County, Taiwan, was SEB positive, and the majority of the strains exhibited SCCmec type VT, while some were SCCmec type IV (35).
Aires de Sousa et al. reported in 2003 that the majority of strains of the PVL− MRSA clone in Taiwan were the pandemic Hungarian clone (ST239, spa3, SCCmec type III) or an ST241 clone (a single-locus variant of ST239) (2). Huang et al. (25) described in 2006 that a new epidemic clone (ST5, SCCmec type II) had increased in prevalence and also that the PVL+ CA-MRSA ST59 clone had a link to health care settings and had not previously been detected.
In this study, we found that SCCmec type VT possessed two distinct ccrC genes (ccrC2 and a novel ccrC gene designated ccrC8), demonstrating the first case of ccr arrangement (ccrC2-C8). ccrC8 was found in a region that Boyle-Vavra et al. (5) had not examined for the SCCmec type VT sequence.
The previously described ccr complexes include ccrAB1, ccrAB2, ccrAB3, ccrAB4, and ccrC (22); and ccrC is carried only by SCCmec type III (which has a ccr arrangement with a ccrA3B3 complex and ccrC3) and by SCCmec type V (which has a ccr arrangement with ccrC1) (10, 22). SCCmec type VT (of CA-MRSA ST59Taiwan strain PM1), which has a ccr arrangement (ccrC2-C8), should be a new class of SCCmec. This class was tentatively designated SCCmec type VII, following SCCmec type VI, which possesses a ccrAB complex (42).
We also demonstrated the polymorphisms of the PVL gene sequences by the exhaustive sequencing of the PVL genes. The PVL genes were divergent in many cases (as we have described previously [40]) and were ST dependent, except in two cases. PVL consists of S and F proteins (31, 45), and it is considered that the modification of the S protein to the phosphorylated version by the protein kinase of human polymorphonuclear leukocytes (PMNs) after its initial binding to PMNs (probably GM1), followed by the subsequent binding of the F protein, is essential for the induction of cell lysis (31). Interestingly, amino acid changes were observed only in the S-protein region, indicating the possibility that PVL variants (with amino acid changes) show a different host (or tissue) specificity. Further studies are necessary to elucidate this possibility. It has been considered that PVL could be a serious toxin (33, 68). However, since the description of the PVL destruction of white blood cells and lung tissue is based only on in vitro and animal studies, its role in human infections remains to be defined.
The tetK gene is usually carried on small plasmids (4.3 to 4.4 kb) in CA-MRSA strains (15, 54). In contrast, PVL+ CA-MRSA ST59Taiwan (strain PM1) as well as PVL− MRSA ST59Taiwan (strain PM34) carried the tetK gene on a large PCase plasmid (42 or 46 kb). Such multidrug-resistant PCase plasmids have been found in PVL+ MRSA strains derived from MRSA outbreaks in the 1980s to 1990s in Japan (53). Although strain PM34 carried an additional small (4.5-kb) plasmid, the role of this plasmid remains to be defined.
PVL+ CA-MRSA ST30 is the worldwide type, being egc positive and highly adhesive (cna and bbp positive). egc is frequently found in S. aureus strains from carriers, suggesting the possibility for a role of egc in bacterial colonization (57).
PVL− MRSA ST5Taiwan was classified as the Japanese type of the New York/Japan clone (52) and not the USA type (14). Strains from Japanese patients with toxic shock syndrome were sea positive, while those from patients with neonatal toxic shock syndrome-like exanthematous disease (NTED) were sea negative (52). Thus, the New York/Japan clone from Taiwan resembled that from patients with NTED. The New York/Japan clone, which has the ability to replace preexisting MRSA clones in hospital settings (13), may be expanding to the western-most areas in Pacific regions.
MRSA ST8Taiwan seems to be different from MRSA ST8, which is found in both Europe and the United States. In the United States, the majority of such strains carry the msrA gene (which codes for macrolide resistance) and not the erm gene (which codes for macrolide-lincosamide-streptogramin B resistance), and some strains carry both the msrA and the erm genes (64). In this study, all MRSA strains, including PVL− MRSA ST8Taiwan, were negative for the msrA gene.
Although the PVL+ CA-MRSA ST59Taiwan and PVL− MRSA ST59Taiwan (identified as HA-MRSA in this study) clones shared many common characteristics, the evolutionary relationship between the two clones remains to be clarified. A previous study demonstrated that descendants of early pandemic PVL+ S. aureus strains have acquired SCCmec and have reemerged as CA-MRSA (47).
As for oxacillin resistance, HA-MRSA strains manifest high levels of resistance (MICs, ≥256 μg/ml), in contrast to those for CA-MRSA strains (MICs, ≤32 μg/ml), in many cases in Japan (52, 53). This seems to be the case even in some instances in Taiwan.
Acknowledgments
We thank H. de Lencastre for the New York/Japan clone (strain BK2464) and H. Zaraket for technical assistance.
This study was supported by a grant from the Interchange Association, Japan, and a grant from the Japan Science and Technology Agency.
Footnotes
Published ahead of print on 17 December 2007.
REFERENCES
- 1.Aires de Sousa, M., T. Conceição, C. Simas, and H. de Lencastre. 2005. Comparison of genetic backgrounds of methicillin-resistant and -susceptible Staphylococcus aureus isolates from Portuguese hospitals and the community. J. Clin. Microbiol. 43:5150-5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aires de Sousa, M., M. I. Crisóstomo, I. S. Sanches, J. S. Wu, J. Fuzhong, A. Tomasz, and H. de Lencastre. 2003. Frequent recovery of a single clonal type of multidrug-resistant Staphylococcus aureus from patients in two hospitals in Taiwan and China. J. Clin. Microbiol. 41:159-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baranovich, T., V. Potapov, and T. Yamamoto. 2007. The first isolation of Panton-Valentine leukocidin (PVL) positive community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) in Russia. Euro. Surveill. 12:4. [DOI] [PubMed] [Google Scholar]
- 4.Becker, K., R. Roth, and G. Peters. 1998. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J. Clin. Microbiol. 36:2548-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle-Vavra, S., B. Ereshefsky, C. C. Wang, and R. S. Daum. 2005. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV. J. Clin. Microbiol. 43:4719-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brumfitt, W., and J. Hamilton-Miller. 1989. Methicillin-resistant Staphylococcus aureus. N. Engl. J. Med. 320:1188-1196. [DOI] [PubMed] [Google Scholar]
- 7.Carleton, H. A., B. A. Diep, E. D. Charlebois, G. F. Sensabaugh, and F. Perdreau-Remington. 2004. Community-adapted methicillin-resistant Staphylococcus aureus (MRSA): population dynamics of an expanding community reservoir of MRSA. J. Infect. Dis. 190:1730-1738. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997-1999. MMWR Morb. Mortal. Wkly. Rep. 48:707-710. [PubMed] [Google Scholar]
- 9.Choi, S. M., S. H. Kim, H. J. Kim, D. G. Lee, J. H. Choi, J. H. Yoo, J. H. Kang, W. S. Shin, and M. W. Kang. 2003. Multiplex PCR for the detection of genes encoding aminoglycoside modifying enzymes and methicillin resistance among Staphylococcus species. J. Korean Med. Sci. 18:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chongtrakool, P., T. Ito, X. X. Ma, Y. Kondo, S. Trakulsomboon, C. Tiensasitorn, M. Jamklang, T. Chavalit, J. H. Song, and K. Hiramatsu. 2006. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob. Agents Chemother. 50:1001-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark, N. C., Ø. Olsvik, J. M. Swenson, C. A. Spiegel, and F. C. Tenover. 1999. Detection of a streptomycin/spectinomycin adenylyltransferase gene (aadA) in Enterococcus faecalis. Antimicrob. Agents Chemother. 43:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2005. Performance standard for antimicrobial susceptibility testing; 15th informational supplement. M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 13.Coombs, G. W., H. Van Gessel, J. C. Pearson, M. R. Godsell, F. C. O'Brien, and K. J. Christiansen. 2007. Controlling a multicenter outbreak involving the New York/Japan methicillin-resistant Staphylococcus aureus clone. Infect. Control Hosp. Epidemiol. 28:845-852. [DOI] [PubMed] [Google Scholar]
- 14.Diep, B. A., H. A. Carleton, R. F. Chang, G. F. Sensabaugh, and F. Perdreau-Remington. 2006. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 193:1495-1503. [DOI] [PubMed] [Google Scholar]
- 15.Diep, B. A., S. R. Gill, R. F. Chang, T. H. Phan, J. H. Chen, M. G. Davidson, F. Lin, J. Lin, H. A. Carleton, E. F. Mongodin, G. F. Sensabaugh, and F. Perdreau-Remington. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731-739. [DOI] [PubMed] [Google Scholar]
- 16.Diep, B. A., G. F. Sensabaugh, N. S. Somboona, H. A. Carleton, and F. Perdreau-Remington. 2004. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J. Clin. Microbiol. 42:2080-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feil, E. J., B. C. Li, D. M. Aanensen, W. P. Hanage, and B. G. Spratt. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genestier, A. L., M. C. Michallet, G. Prévost, G. Bellot, L. Chalabreysse, S. Peyrol, F. Thivolet, J. Etienne, G. Lina, F. M. Vallette, F. Vandenesch, and L. Genestier. 2005. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Investig. 115:3117-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert, M., J. MacDonald, D. Gregson, J. Siushansian, K. Zhang, S. Elsayed, K. Laupland, T. Louie, K. Hope, M. Mulvey, J. Gillespie, D. Nielsen, V. Wheeler, M. Louie, A. Honish, G. Keays, and J. Conly. 2006. Outbreak in Alberta of community-acquired (USA300) methicillin-resistant Staphylococcus aureus in people with a history of drug use, homelessness or incarceration. CMAJ 175:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton, S. M., A. E. Bryant, K. C. Carroll, V. Lockary, Y. Ma, E. McIndoo, L. G. Miller, F. Perdreau-Remington, J. Pullman, G. F. Risi, D. B. Salmi, and D. L. Stevens. 2007. In vitro production of Panton-Valentine leukocidin among strains of methicillin-resistant Staphylococcus aureus causing diverse infections. Clin. Infect. Dis. 45:1550-1558. [DOI] [PubMed] [Google Scholar]
- 22.Hanssen, A. M., and J. U. Sollid. 2007. Multiple staphylococcal cassette chromosomes and allelic variants of cassette chromosome recombinases in Staphylococcus aureus and coagulase-negative staphylococci from Norway. Antimicrob. Agents Chemother. 51:1671-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holtfreter, S., K. Bauer, D. Thomas, C. Feig, V. Lorenz, K. Roschack, E. Friebe, K. Selleng, S. Lövenich, T. Greve, A. Greinacher, B. Panzig, S. Engelmann, G. Lina, and B. M. Broker. 2004. egc-encoded superantigens from Staphylococcus aureus are neutralized by human sera much less efficiently than are classical staphylococcal enterotoxins or toxic shock syndrome toxin. Infect. Immun. 72:4061-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooper, D. C. 2003. Mechanisms of quinolone resistance, p. 41-67. In D. C. Hooper and E. Rubinstein (ed.), Quinolone antimicrobial agents. ASM Press, Washington, DC.
- 25.Huang, Y. C., L. H. Su, T. L. Wu, and T. Y. Lin. 2006. Changing molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream isolates from a teaching hospital in Northern Taiwan. J. Clin. Microbiol. 44:2268-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito, T., K. Kuwahara, K. Hisata, K. Okuma, L. Cui, and K. Hiramatsu. 2004. Community-associated methicillin-resistant Staphylococcus aureus: current status and molecular epidemiological perspective. Kansenshogaku Zasshi 78:459-469. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 27.Ito, T., X. X. Ma, F. Takeuchi, K. Okuma, H. Yuzawa, and K. Hiramatsu. 2004. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob. Agents Chemother. 48:2637-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarraud, S., G. Cozon, F. Vandenesch, M. Bes, J. Etienne, and G. Lina. 1999. Involvement of enterotoxins G and I in staphylococcal toxic shock syndrome and staphylococcal scarlet fever. J. Clin. Microbiol. 37:2446-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarraud, S., C. Mougel, J. Thioulouse, G. Lina, H. Meugnier, F. Forey, X. Nesme, J. Etienne, and F. Vandenesch. 2002. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 70:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarraud, S., M. A. Peyrat, A. Lim, A. Tristan, M. Bes, C. Mougel, J. Etienne, F. Vandenesch, M. Bonneville, and G. Lina. 2001. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 166:669-677. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko, J., and Y. Kamio. 2004. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 68:981-1003. [DOI] [PubMed] [Google Scholar]
- 32.Kim, T. J., Y. R. Na, and J. I. Lee. 2005. Investigations into the basis of chloramphenicol and tetracycline resistance in Staphylococcus intermedius isolates from cases of pyoderma in dogs. J. Vet. Med. B Infect. Dis. Vet. Public Health 52:119-124. [DOI] [PubMed] [Google Scholar]
- 33.Labandeira-Rey, M., F. Couzon, S. Boisset, E. L. Brown, M. Bes, Y. Benito, E. M. Barbu, V. Vazquez, M. Höök, J. Etienne, F. Vandenesch, and M. G. Bowden. 2007. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 315:1130-1133. [DOI] [PubMed] [Google Scholar]
- 34.Letertre, C., S. Perelle, F. Dilasser, and P. Fach. 2003. Identification of a new putative enterotoxin SEU encoded by the egc cluster of Staphylococcus aureus. J. Appl. Microbiol. 95:38-43. [DOI] [PubMed] [Google Scholar]
- 35.Lo, W. T., W. J. Lin, M. H. Tseng, S. R. Wang, M. L. Chu, and C. C. Wang. 2006. Community-acquired methicillin-resistant Staphylococcus aureus in children, Taiwan. Emerg. Infect. Dis. 12:1267-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massidda, O., M. Mingoia, D. Fadda, M. B. Whalen, M. P. Montanari, and P. E. Varaldo. 2006. Analysis of the beta-lactamase plasmid of borderline methicillin-susceptible Staphylococcus aureus: focus on bla complex genes and cadmium resistance determinants cadD and cadX. Plasmid 55:114-127. [DOI] [PubMed] [Google Scholar]
- 37.McAleese, F., E. Murphy, T. Babinchak, G. Singh, B. Said-Salim, B. Kreiswirth, P. Dunman, J. O'Connell, S. J. Projan, and P. A. Bradford. 2005. Use of ribotyping to retrospectively identify methicillin-resistant Staphylococcus aureus isolates from phase 3 clinical trials for tigecycline that are genotypically related to community-associated isolates. Antimicrob. Agents Chemother. 49:4521-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mongodin, E., O. Bajolet, J. Cutrona, N. Bonnet, F. Dupuit, E. Puchelle, and S. de Bentzmann. 2002. Fibronectin-binding proteins of Staphylococcus aureus are involved in adherence to human airway epithelium. Infect. Immun. 70:620-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagawa, S., I. Taneike, D. Mimura, N. Iwakura, T. Nakayama, T. Emura, M. Kitatsuji, A. Fujimoto, and T. Yamamoto. 2005. Gene sequences and specific detection for Panton-Valentine leukocidin. Biochem. Biophys. Res. Commun. 328:995-1002. [DOI] [PubMed] [Google Scholar]
- 41.Oliveira, D. C., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliveira, D. C., C. Milheiriço, and H. de Lencastre. 2006. Redefining a structural variant of staphylococcal cassette chromosome mec, SCCmec type VI. Antimicrob. Agents Chemother. 50:3457-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orwin, P. M., D. Y. Leung, H. L. Donahue, R. P. Novick, and P. M. Schlievert. 2001. Biochemical and biological properties of staphylococcal enterotoxin K. Infect. Immun. 69:360-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otsuka, T., K. Saito, S. Dohmae, T. Takano, W. Higuchi, Y. Takizawa, T. Okubo, N. Iwakura, and T. Yamamoto. 2006. Key adhesin gene in community-acquired methicillin-resistant Staphylococcus aureus. Biochem. Biophys. Res. Commun. 346:1234-1244. [DOI] [PubMed] [Google Scholar]
- 45.Pédelacq, J. D., L. Maveyraud, G. Prévost, L. Baba-Moussa, A. González, E. Courcelle, W. Shepard, H. Monteil, J. P. Samama, and L. Mourey. 1999. The structure of a Staphylococcus aureus leucocidin component (LukF-PV) reveals the fold of the water-soluble species of a family of transmembrane pore-forming toxins. Structure 7:277-287. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro, A., C. Dias, M. C. Silva-Carvalho, L. Berquó, F. A. Ferreira, R. N. Santos, B. T. Ferreira-Carvalho, and A. M. Figueiredo. 2005. First report of infection with community-acquired methicillin-resistant Staphylococcus aureus in South America. J. Clin. Microbiol. 43:1985-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson, D. A., A. M. Kearns, A. Holmes, D. Morrison, H. Grundmann, G. Edwards, F. G. O'Brien, F. C. Tenover, L. K. McDougal, A. B. Monk, and M. C. Enright. 2005. Re-emergence of early pandemic Staphylococcus aureus as a community-acquired meticillin-resistant clone. Lancet 365:1256-1258. [DOI] [PubMed] [Google Scholar]
- 48.Ruzin, A., J. Lindsay, and R. P. Novick. 2001. Molecular genetics of SaPI1—a mobile pathogenicity island in Staphylococcus aureus. Mol. Microbiol. 41:365-377. [DOI] [PubMed] [Google Scholar]
- 49.Shopsin, B., M. Gomez, S. O. Montgomery, D. H. Smith, M. Waddington, D. E. Dodge, D. A. Bost, M. Riehman, S. Naidich, and B. N. Kreiswirth. 1999. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 37:3556-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strommenger, B., C. Cuny, G. Werner, and W. Witte. 2004. Obvious lack of association between dynamics of epidemic methicillin-resistant Staphylococcus aureus in central Europe and agr specificity groups. Eur. J. Clin. Microbiol. Infect. Dis. 23:15-19. [DOI] [PubMed] [Google Scholar]
- 51.Sutcliffe, J., T. Grebe, A. Tait-Kamradt, and L. Wondrack. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40:2562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takizawa, Y., I. Taneike, S. Nakagawa, T. Oishi, Y. Nitahara, N. Iwakura, K. Ozaki, M. Takano, T. Nakayama, and T. Yamamoto. 2005. A Panton-Valentine leucocidin (PVL)-positive community-acquired methicillin-resistant Staphylococcus aureus (MRSA) strain, another such strain carrying a multiple-drug resistance plasmid, and other more-typical PVL-negative MRSA strains found in Japan. J. Clin. Microbiol. 43:3356-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taneike, I., T. Otsuka, S. Dohmae, K. Saito, K. Ozaki, M. Takano, W. Higuchi, T. Takano, and T. Yamamoto. 2006. Molecular nature of methicillin-resistant Staphylococcus aureus derived from explosive nosocomial outbreaks of the 1980s in Japan. FEBS Lett. 580:2323-2334. [DOI] [PubMed] [Google Scholar]
- 54.Tenover, F. C., L. K. McDougal, R. V. Goering, G. Killgore, S. J. Projan, J. B. Patel, and P. M. Dunman. 2006. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J. Clin. Microbiol. 44:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tristan, A., L. Ying, M. Bes, J. Etienne, F. Vandenesch, and G. Lina. 2003. Use of multiplex PCR to identify Staphylococcus aureus adhesins involved in human hematogenous infections. J. Clin. Microbiol. 41:4465-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trzcinski, K., B. S. Cooper, W. Hryniewicz, and C. G. Dowson. 2000. Expression of resistance to tetracyclines in strains of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 45:763-770. [DOI] [PubMed] [Google Scholar]
- 57.van Belkum, A., D. C. Melles, S. V. Snijders, W. B. van Leeuwen, H. F. Wertheim, J. L. Nouwen, H. A. Verbrugh, and J. Etienne. 2006. Clonal distribution and differential occurrence of the enterotoxin gene cluster, egc, in carriage- versus bacteremia-associated isolates of Staphylococcus aureus. J. Clin. Microbiol. 44:1555-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vancraeynest, D., K. Hermans, and F. Haesebrouck. 2004. Genotypic and phenotypic screening of high and low virulence Staphylococcus aureus isolates from rabbits for biofilm formation and MSCRAMMs. Vet. Microbiol. 103:241-247. [DOI] [PubMed] [Google Scholar]
- 59.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasudevan, P., M. K. Nair, T. Annamalai, and K. S. Venkitanarayanan. 2003. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet. Microbiol. 92:179-185. [DOI] [PubMed] [Google Scholar]
- 61.Vickers, A. A., A. J. O'Neill, and I. Chopra. 2007. Emergence and maintenance of resistance to fluoroquinolones and coumarins in Staphylococcus aureus: predictions from in vitro studies. J. Antimicrob. Chemother. 60:269-273. [DOI] [PubMed] [Google Scholar]
- 62.Wang, C. C., W. T. Lo, M. L. Chu, and L. K. Siu. 2004. Epidemiological typing of community-acquired methicillin-resistant Staphylococcus aureus isolates from children in Taiwan. Clin. Infect. Dis. 39:481-487. [DOI] [PubMed] [Google Scholar]
- 63.Williams, R. J., J. M. Ward, B. Henderson, S. Poole, B. P. O'Hara, M. Wilson, and S. P. Nair. 2000. Identification of a novel gene cluster encoding staphylococcal exotoxin-like proteins: characterization of the prototypic gene and its protein product, SET1. Infect. Immun. 68:4407-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Witte, W., B. Strommenger, C. Cuny, D. Heuck, and U. Nuebel. 2007. Methicillin-resistant Staphylococcus aureus containing the Panton-Valentine leucocidin gene in Germany in 2005 and 2006. J. Antimicrob. Chemother. 60:1258-1263. [DOI] [PubMed] [Google Scholar]
- 65.Wu, S. W., H. de Lencastre, and A. Tomasz. 1999. The Staphylococcus aureus transposon Tn551: complete nucleotide sequence and transcriptional analysis of the expression of the erythromycin resistance gene. Microb. Drug Resist. 5:1-7. [DOI] [PubMed] [Google Scholar]
- 66.Yamaguchi, T., K. Nishifuji, M. Sasaki, Y. Fudaba, M. Aepfelbacher, T. Takata, M. Ohara, H. Komatsuzawa, M. Amagai, and M. Sugai. 2002. Identification of the Staphylococcus aureus etd pathogenicity island which encodes a novel exfoliative toxin, ETD, and EDIN-B. Infect. Immun. 70:5835-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto, T., S. Dohmae, K. Saito, T. Otsuka, T. Takano, M. Chiba, K. Fujikawa, and M. Tanaka. Molecular characteristics and in vitro susceptibility to antimicrobial agents, including the des-fluoro(6) quinolone DX-619, of Panton-Valentine leucocidin-positive methicillin-resistant Staphylococcus aureus isolates from the community and hospitals. Antimicrob. Agents Chemother. 50:4077-4086. [DOI] [PMC free article] [PubMed]
- 68.Zetola, N., J. S. Francis, E. L. Nuermberger, and W. R. Bishai. 2005. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect. Dis. 5:275-286. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, K., J. A. McClure, S. Elsayed, T. Louie, and J. M. Conly. 2005. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5026-5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang, S., J. J. Iandolo, and G. C. Stewart. 1998. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej). FEMS Microbiol. Lett. 168:227-233. [DOI] [PubMed] [Google Scholar]