Abstract
Anoikis, a Bax dependent apoptosis triggered by detachment from the extracellular matrix, is often dysfunctional in metastatic cancer cells. Using wild type and c-Src transformed NIH3T3 cells as a model we identified Mcl-1 degradation and Bim upregulation as a critical determinant of anoikis initiation. Detachment rapidly degraded Mcl-1 via a GSK-3β-dependent proteasomal pathway and transcriptionally upregulated Bim expression. Mcl-1 degradation in the presence of Bim was sufficient to induce anoikis. By analyzing non-metastatic Saos-2 and metastatic derivative LM7 cells, we confirmed that dysregulation of Mcl-1 degradation and Bim induction during detachment contributes to decreased anoikis sensitivity of metastatic cells. Furthermore, knockdown of Mcl-1 or pharmacological inhibition of the PI3K/Akt and MAPK pathways that suppress Mcl-1 degradation and Bim expression could markedly sensitize metastatic breast cancer cells to anoikis and prevent metastases in vivo. Therefore, Mcl-1 degradation primes the cell for Bax activation and anoikis, which can be blocked by oncogenic signaling in metastatic cells.
Keywords: Anoikis, Bim, Mcl-1, Metastasis, Src
Introduction
The extracellular matrix (ECM) provides adhesive support to normal tissues and controls numerous signals that regulate diverse cellular processes such as survival, growth, and differentiation (1). When normal cells are detached from the ECM they die by a process termed anoikis (2). Anoikis is essential for common biological processes involved in homeostasis, morphogenic changes, and inhibition of cancer metastasis. Aggressive breast, colon, and lung malignancies with a propensity to metastasize have been shown to lack the normal apoptotic response after detachment from the supporting matrix (3–5). Therefore, cancer cells more apt to resist anoikis due to genetic mutation or overactive survival signaling are increasingly likely to initiate distal metastases (6).
Anoikis relies heavily on the mitochondrial pathway of apoptosis and Bax has been found to be a key effector of this process (7, 8). Bax is an apical regulator of the intrinsic apoptotic pathway and its activation status is determined by its conformation (9, 10). The Bcl-2 family of proteins is composed of both pro- and anti-apoptotic members and the balance of life and death for the cell is dependent on the prevalence and potency of these proteins. The BH3-only proteins Bim, tBid and Puma are pro-apoptotic because of their ability to activate Bax. However, on the opposing side of the equation are proteins such as Bcl-2, Bcl-XL, and Mcl-1 which can repress Bax activation (11). It is known that sequestering of BH3-only proteins such as Bim and tBid to the mitochondria by Mcl-1 prevents apoptosis (12). Mcl-1 is a short-lived protein in comparison to Bcl-2 or Bcl-XL with a half-life as short as 40 minutes (13). Mcl-1 is regulated post-translationally by Mule, Mcl-1 ubiquitin-ligase E3, which ubiquitinates Mcl-1 to promote its degradation by the proteasome (14). This ubiquitination can be directed by phosphorylation events involving the kinase GSK-3β at Ser159 of Mcl-1 (15). In relation to metastasis, it was recently determined in a Lewis lung carcinoma model that hypoxia-induced clonal selection of cells overexpressing Mcl-1 correlated with a higher metastatic potential of the tumor (16).
In this study, we identified the stabilization of Mcl-1 and suppression of Bim as critical events during oncogenic suppression of anoikis. The transition of cells to a metastatic phenotype correlates not only with increased Mcl-1 expression, but also to the altered regulation of its degradation profile in response to detachment. Inhibition of survival signals mediated by active Src or downstream Akt and Erk1/2 kinases that control Mcl-1 degradation and Bim induction is able to restore Bax activation and anoikis susceptibility. Furthermore, Mcl-1 repression, but not Bcl-2 or Bcl-XL inhibition, is capable of initiating anoikis in metastatic cancer cells. This study is the first to characterize Mcl-1 degradation in anoikis and the oncogenic signaling that can disrupt this essential mechanism in human cancers.
Materials and Methods
Reagents
Poly(2-hydroxyethyl methacrylate) (polyHEMA), caspase-3 assay kit, cycloheximide, MG132, oligonucleotides for shRNA constructs, and monoclonal antibodies specific for Bax (clone 6A7), α-tubulin and β-actin were purchased from Sigma. 3-[3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) was purchased from MP Biomedical. Anti-mouse Mcl-1 was purchased from Rockland. Monoclonal anti-Akt and polyclonal anti-p473 Akt antibodies were purchased from R&D Systems. Anti-p202/204 Erk1/2 and Anti-Erk1/2 polyclonal antibodies and U0126 were purchased from Cell Signaling. Polyclonal Bax (N20) and Goat anti-rabbit IgG-HRP were purchased from Santa Cruz. Polyclonal anti-Bim antibody, TDZD-8, and the fluorogenic chymotrypsin substrate III were purchased from Calbiochem. The anti-human Mcl-1 antibody was purchased from BD Biosciences. LY294002 was purchased from Alexis.
Plasmids
The pcDNA3-BimEL vector was described previously (9). The pcDNA3.1/V5-His-TOPO plasmids encoding wild type and mutant Mcl-1 were described previously (15). Oligos for shRNAs targeting Bim 5′-GTTCTGAGTGTGACAGAGA-3′ and Mcl-1 5′-GAGGACGACCTATACCGCC-3′ (c-1) and 5′-GCCCTAATTAACAACGTTG-3′ (c-3) were synthesized and cloned into the Bgl II and Sal I restriction sites of LTRH1-puro (Ken Watanabe, National Center for Geriatrics & Gerontology, Aichi, Japan). The retroviral constructs expressing Bcl-2-IRES-Bim, Bcl-XL-IRES-Bim, and Mcl-1-IRES-Bim were described previously (17). The pLKO.1-based lentiviral shRNA targeting human Mcl-1, TRCN0000005517 was purchased from Open Biosystems. The pLKO.1-based scrambled control shRNA vector was purchased from Sigma.
Cell culture and transfection
Wild type, v-Src, and c-Src (527F) NIH3T3 cells were previously described (18). GSK-3β−/− MEF cells were kindly provided by James Woodgett (19). NIH3T3 and MEF cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Saos-2, LM7, and MDA-MB-231 cell lines were maintained in MEM medium supplemented with 10% FBS, 1% penicillin/streptomycin, 1 mM NaPyruvate, and 1x MEM non-essential amino acids. To induce anoikis, cells were maintained in the same media supplemented with 1% (for mouse cell lines) or 10% (for human cell lines) FBS in polyHEMA coated plates. Transfection was completed with Lipofectamine2000 (Invitrogen) according to the manufacturer’s recommendations. Recombinant retrovirus and lentivirus were produced in Amphotropic 293T packaging cells and 293FT cells with ViraPower™ Packaging Mix (Invitrogen), respectively.
Caspase-3, chymotrypsin, and LDH release
Caspase-3 activation was assayed as DEVDase activity with the Caspase-3 fluorescence assay kit (Sigma). Cell death was measured using the LDH cytotoxicity assay (Biovision) according to the manufacturer’s recommendations. The fluorogenic chymotrypsin substrate III (Calbiochem) was used to measure chymotrypsin-like activity.
Semi-quantitative RT-PCR
Semi-quantitative RT-PCR was completed using the Qiagen OneStep RT-PCR system according to the manufacturer’s recommendations. Primers used for the reactions are: 5′-TGCAGGGTTATGGAATCCTC-3′ and 5′-GCCCCTACCTCCCTACAGAC-3′ for Bim; 5′-GCAGCTTCAAGTCCACCTTC-3′ and 5′-AGATGGCGTAACAAACTGGG-3′ for Mcl-1; 5′-AATGTGTCCGTCGTGGATCT-3′ and 5′-CCCTGTTGCTGTAGCCGTAT-3′ for GAPDH.
In vivo metastasis model
MDA-MB-231-luc-D3H2LN cells were obtained from Xenogen and infected with lentiviral Mcl-1 shRNA (shMcl-1) or control scrambled shRNA (shScr). After 10 days selection with 0.5 μg/mL puromycin, 1 × 106 cells were injected into 10–12 week-old Harlan nude mice via the tail vein. Three weeks post-injection mice were imaged using the IVIS200 system (Xenogen) as per the manufacturer’s recommendations. Statistical significance was determined using Student’s t-test.
Results
Src signaling ablates the anoikis response due to an inhibition of Bax activation
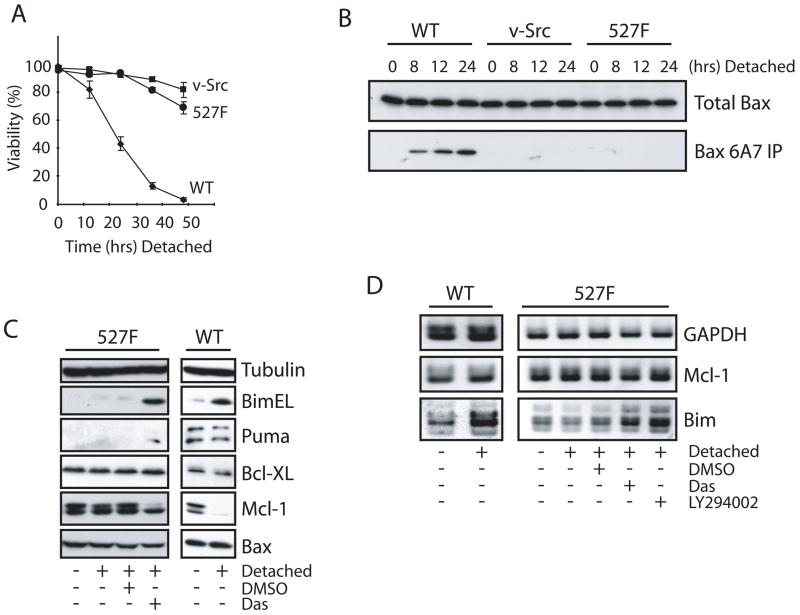
The anoikis response is known to be inhibited in cells expressing the Src oncogene (20), but the mechanisms involved are poorly understood. To determine the characteristics of the anoikis response, parental and Src transformed NIH3T3 cells were forcibly detached on polyHEMA coated plates for varying lengths of time. Wild type cells were observed to rapidly loose viability in a time dependent manner while cells with active c-Src (527F) and v-Src exhibited limited cell death as determined by a trypan blue exclusion assay (Fig. 1A). The loss of viability in wild type cells correlated to the activation of caspase-3 as measured by the DEVDase activity (Supplementary Fig. S1A). The activation of Bax is known to be an initiating event in the activation of the caspase cascade during anoikis (21). Therefore, we assessed the activation status of Bax by immunoprecipitation (Fig. 1B) with the Bax 6A7 monoclonal antibody and immunostaining (Supplementary Fig. S1B) with the Bax N20 polyclonal antibody. Both of these antibodies specifically recognize conformationally active Bax. Figure 1B illustrates that Bax activation is completely inhibited in 527F and v-Src cells while wild type cells exhibit a time dependent increase in the conformationally changed Bax protein beginning as early as 8 hours post detachment. Similarly, immunofluorescence staining (Supplementary Fig. S1B & C) demonstrates that Bax becomes active in wild type cells but not 527F expressing cells in response to cell detachment. However, treatment with the Src family kinase inhibitor dasatinib clearly restored detachment-induced Bax activation in 527F cells (Supplementary Fig. S1B & C).
Figure 1.
Constitutively active Src deregulates cell death, the activation of Bax, and expression of Bim and Mcl-1. (A, B) Wild type, v-Src, and c-Src (527F) NIH3T3 cells were detached on polyHEMA-coated plates for the indicated times. Cell viability was determined by trypan blue dye exclusion assay. Conformational activation of Bax was determined by immunoprecipitation with anti-Bax 6A7 antibody. (C, D) Wild type and c-Src (527F) NIH3T3 cells were cultured in normal conditions or detached on polyHEMA-coated plates with DMSO, 50 nM dasatinib (Das) or 50 μM LY294002 for 8 hours and subjected to Western blot (C) and semi-quantitative RT-PCR (D) analyses.
Mcl-1 and Bim are critical regulators of anoikis
To determine the role of protein neogenesis in the activation of Bax during anoikis, cycloheximide (CHX) was used to block de novo protein synthesis in both wild type and 527F cells detached on polyHEMA. CHX markedly reduced detachment-induced Bax activation and cellular caspase-3 activity in wild type cells as well as 527F cells treated with dasatinib (Supplementary Fig. S2A & B). This prompted us to examine the gene expression profiles associated with anoikis in 527F cells treated with or without dasatinib by microarray (Supplementary Fig. S2C). There was a clear increase in the induction of Bim and Puma, both known activators of Bax. There was also a slight decrease in transcription of the anti-apoptotic proteins Mcl-1 and Bcl-XL. Members of the caspase family were generally unchanged; however, their involvement in Bax activation was ruled out through the use of the pan caspase inhibitor z-VAD-fmk which was unable to inhibit Bax conformational change during detachment (data not shown).
The microarray analysis allowed us to form a short list of Bcl-2 family proteins that are differentially transcribed between anoikis responsive and unresponsive cells. However, the prevalence or absence of transcripts does not always coincide with the expression of the protein product. To this end, we examined the protein expression profiles of BimEL, Puma, Bcl-XL, Mcl-1 and Bax in 527F cells detached and treated with dasatinib or DMSO and compared them to wild type cells (Fig. 1C). Several reports have suggested that Bcl-XL is induced as the result of Src signaling and that this provides resistance to anoikis (22, 23). However, we observed little or no decrease in Bcl-XL at the time of Bax activation in either dasatinib treated 527F or wild type cells. Similarly, there was no significant increase in the protein levels of Bax in either of the two cell types. We did find that Mcl-1 and Bim were the most dynamically regulated proteins analyzed. In particular, Mcl-1 expression was substantially reduced while Bim was increased during anoikis; this response was also found in detached 527F cells treated with dasatinib indicating the relevance to a restored anoikis response by Src inhibition. Puma was moderately induced in dasatinib treated 527F but not in wild type cells, suggesting that Puma may not be a key regulator of anoikis.
The results from the microarray in relation to Mcl-1 and Bim were validated using semi-quantitative RT-PCR in samples of wild type and 527F cells (Fig. 1D). Transcripts of Mcl-1 were marginally decreased in dasatinib treated 527F cells. Interestingly, there did not appear to be any decrease in the transcription of Mcl-1 in the wild type samples, indicating that post-transcriptional regulation was responsible for the observed decrease in protein levels. Contrastingly, Bim transcripts were increased dramatically by detachment in wild type and dasatinib treated 527F cells. Bim transcription is known to be positively regulated through the transcription factor Foxo3a which is negatively regulated by Akt (24). Therefore, the ability of Akt signaling in 527F cells to inhibit Bim expression was assessed through the use of LY294002, a PI3K inhibitor. Indeed, this inhibitor restored Bim induction, indicating that the Src/Akt/Foxo3a pathway is likely involved in the transcriptional suppression of this pro-apoptotic protein.
Mcl-1 and Bim regulate detachment induced Bax activation
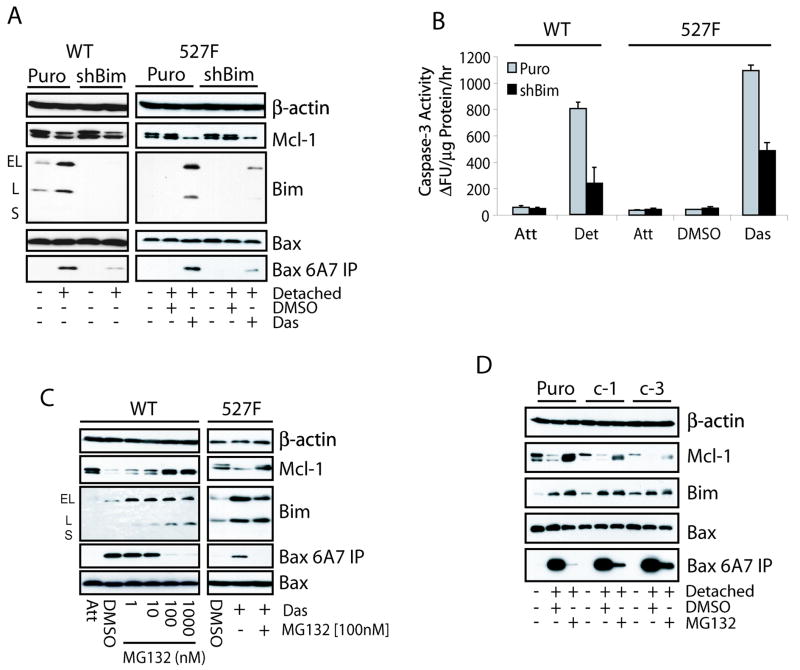
The functional importance of increased Bim expression in the anoikis response was assessed by shRNA mediated targeted knockdown. Both wild type and 527F cells were infected with Bim shRNA (shBim) or control retrovirus. Stable pools were detached on polyHEMA plates and their ability to initiate Bax activation and anoikis was assayed. Decreased Bim expression led to a similar decrease in the activation of Bax (Fig. 2A) as well as the caspase-3 activity (Fig. 2B) in cells cultured on polyHEMA plates. These results suggest that Bim is the major activator of Bax during anoikis. This also implies that Puma induction seen in Figure 1C is of little functional relevance.
Figure 2.
Mcl-1 and Bim expression regulates anoikis. (A, B) Wild type and c-Src (527F) NIH3T3 cells were infected with control (Puro) or Bim shRNA (shBim) retroviruses and selected for 14 days on puromycin. The resulting puromycin-resistant transfectants were maintained in normal culture (Att) or detached (Det) on polyHEMA-coated plates with or without DMSO or 50 nM dasatinib for 8 hours and subjected to immunoprecipitation with anti-Bax 6A7 antibody (A) and caspase-3 activity (B) assays. (C) Wild type NIH3T3 cells were maintained as attached on normal plates or detached on polyHEMA-coated plates containing DMSO or increasing amounts of MG132 while c-Src (527F) NIH3T3 cells were detached and treated with DMSO or 50 nM dasatinib with or without 100 nM MG132 for 8 hours. Cells were then subjected to anti-Bax 6A7 immunoprecipitation/immunoblot analysis. (D) NIH3T3 cells stably expressing Mcl-1 shRNA (c-1 or c-3) or control vector (Puro) were left in normal culture or detached on polyHEMA-coated plates in the presence of DMSO or 100 nM MG132 for 8 hours and subjected to anti-Bax 6A7 immunoprecipitation/immunoblot analysis.
To test the functional significance of Mcl-1 repression during detachment, increasing amounts of the Mcl-1 construct were transfected into wild type cells along with the pGL3 Luciferase reporter construct. By measuring luciferase activity, we found that overexpression of Mcl-1 led to a dose dependent suppression of cell death in detached as compared to attached conditions (Supplementary Fig. S3A). Bim alone was able to induce an apoptotic response in 527F cells as transfection of increasing amounts of the Bim construct led to a dose dependent decrease in cell viability (Supplementary Fig. S3B). This demonstrates that decreased Mcl-1 expression is vital to the induction of anoikis and that overexpression of Bim can induce apoptosis in 527F expressing cells, presumably through a mechanism that activates Bax once anti-apoptotic Bcl-2 members are saturated by Bim.
Anoikis is regulated by proteasomal degradation of Mcl-1
Mcl-1 is an ephemeral protein that is degraded in a proteasome dependent manner. This regulatory mechanism allowed us to study if stabilized endogenous Mcl-1 contributes to inhibition of anoikis. As shown in Figure 2C, the proteasome inhibitor MG132 was able to stabilize Mcl-1 in wild type NIH3T3 cells detached on polyHEMA in a dose dependent manner with maximal stabilization occurring at 100 and 1000 nM. This stabilization of Mcl-1 correlated with a similar dose dependent decrease in Bax activation. We also observed the stabilization of Mcl-1 and the complete inhibition of Bax activation in detached 527F cells treated with the combination of dasatinib and MG132 (Fig. 2C). The addition of MG132 to the lysate of detached wild type cells had no effect on the ability to immunoprecipitate active Bax (data not shown). Samples from the wild type cells detached and exposed to the MG132 gradient were also assayed for chymotrypsin-like activity and caspase-3 activity through in vitro protease assays. In concordance with the above results, caspase-3 activity was decreased similar to the chymotrypsin activity inhibited by MG132 (Supplementary Fig. S3C). The repression of Bax activation and anoikis was also observed in a similar manner using Velcade, a proteasome inhibitor currently in clinical trials (data not shown). This study identifies the ability of proteasome inhibitors to prevent Bax activation and anoikis in response to detachment.
To determine whether MG132 mediated inhibition of Bax activation was due to the stabilization of Mcl-1 but not another unidentified protein(s), retroviral shRNA targeting Mcl-1 was infected into wild type cells to decrease its accumulation upon MG132 treatment. Three shRNA constructs were designed, of which constructs 1 and 3 (c-1 and c-3) were the most effective at inhibiting Mcl-1 expression (Fig. 2D). Treatment with MG132 led to the nearly complete inhibition of Bax activation in the Puro control line, but was less effective in suppressing the Bax conformational change when there was less Mcl-1 stabilized. An inverse correlation was found between the amount of Mcl-1 accumulated in MG132 treated samples and the activation of Bax. Similarly, there is decreased repression by MG132 on caspase-3 activation in Mcl-1 knockdown cells compared to control (Supplementary Fig. S3D). These observations strongly suggest that anoikis is dependent on the proteasomal depletion of Mcl-1.
Mcl-1 degradation elicits a robust anoikis response
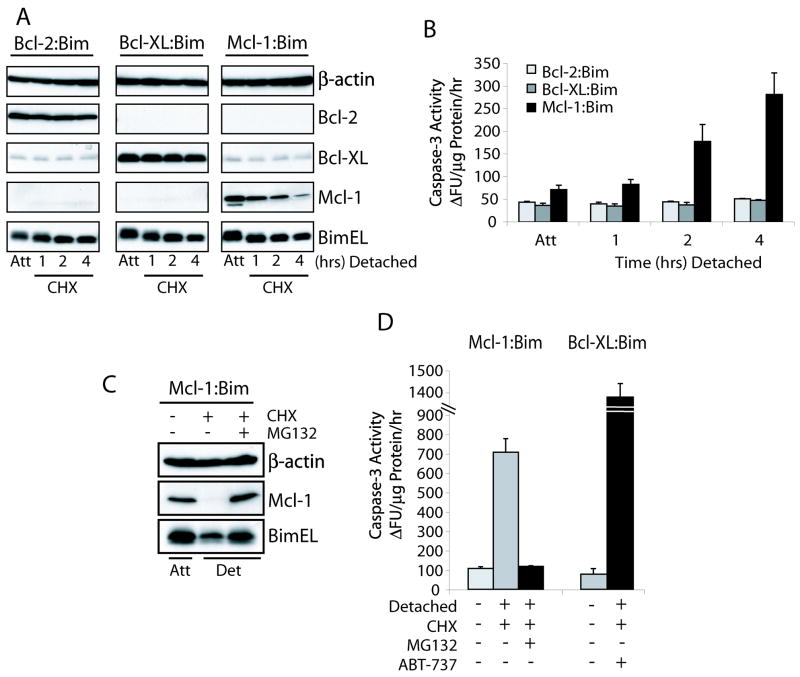
Thus far it has been evident that both Mcl-1 degradation and Bim induction are key regulators of the normal anoikis response. While overexpression of Mcl-1 can undoubtedly protect cells from anoikis (Supplementary Fig. S3A), other groups have shown that overexpression of other anti-apoptotic proteins can also have the same effect (25). Therefore, the vitality of Mcl-1 degradation for anoikis induction was evaluated using retrovirally transduced NIH3T3 cells co-expressing Bcl-2, Bcl-XL, or Mcl-1 with the pro-apoptotic BimEL counterpart via a bicistronic message (Fig. 3A). Repression of protein translation using CHX illustrated that the instability of Mcl-1 is essential for the induction of anoikis, while the co-expression of Bcl-2:Bim or Bcl-XL:Bim had no effect on cell death due to the maintenance of the anti- and pro-apoptotic balance (Fig. 3A & B). The anoikis response to Mcl-1 degradation could be completely resolved by MG132 mediated proteasome inhibition (Fig. 3C & D). We confirmed that releasing Bim from the Bcl-XL:Bim complex by ABT-737, a specific Bcl-2/Bcl-XL inhibitor, was also capable of inducing an apoptotic response (Fig. 3D). The uniquely short half-life of Mcl-1, compared to other anti-apoptotic proteins such as Bcl-2 and Bcl-XL, makes this an essential element of anoikis initiation.
Figure 3.
Mcl-1 degradation alone is sufficient to induce anoikis in the presence of Bim. (A, B) NIH3T3 cells were retrovirally transduced with constructs expressing Bcl-2-IRES-Bim, Bcl-XL-IRES-Bim or Mcl-1-IRES-Bim. Cells were untreated or pretreated with 10 μM CHX for 30 minutes then detached for the indicated times in the presence of CHX and analyzed for expression of the proteins by immunoblot (A) and caspase-3 activation (B). (C, D) NIH3T3 cells expressing Mcl-1-IRES-Bim or Bcl-XL-IRES-Bim were untreated or pretreated for 30 minutes with CHX or CHX plus MG132 or ABT-737 then detached for 5 hours in the presence of their respective inhibitors and analyzed for protein expression and caspase-3 activation.
Mcl-1 degradation is mediated by GSK-3β
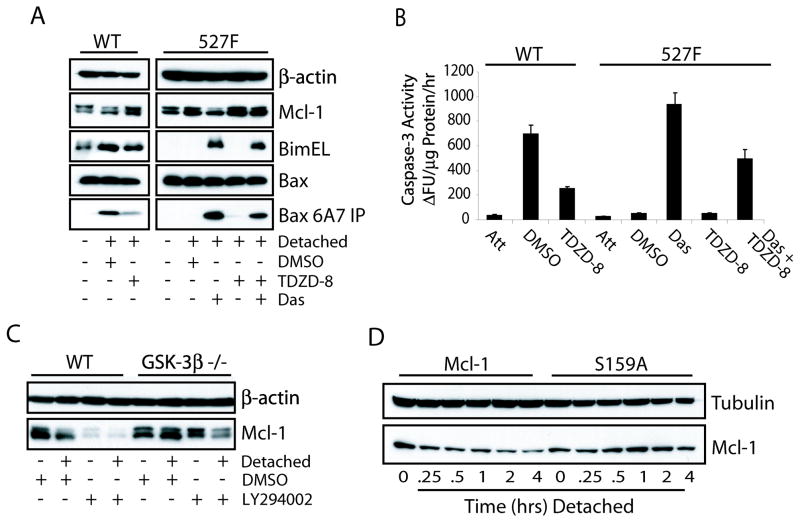
The phosphorylation of Mcl-1 by GSK-3β at Ser159 is known to promote an increased turnover of Mcl-1 (15). To determine if this phosphorylation controls Mcl-1 degradation during anoikis, the GSK-3β inhibitor TDZD-8, a non-ATP competitive inhibitor, was used to treat cells beginning immediately with detachment. As shown in Figure 4A, TDZD-8 was able to stabilize Mcl-1 in both wild type and 527F cells when detached or sensitized with dasatinib, respectively. This stabilization was once again associated with a decrease in Bax activation and inhibition of the apoptotic response (Fig. 4A & B). To further validate the role of GSK-3β in the degradation of Mcl-1 induced by detachment, wild type and GSK-3β knockout MEFs were either attached or detached on polyHEMA plates for 12 hours in the presence of DMSO or LY294002. Detached wild type MEFs showed a decrease in the protein levels of Mcl-1 as did treatment with LY294002 (Fig. 4C). However, the GSK-3β null cells did not exhibit a decrease in Mcl-1 protein levels in response to detachment, and treatment with LY294002 resulted in only a slight decrease of Mcl-1; this decrease is likely due to the functional redundancy of GSK-3α.
Figure 4.
GSK-3β phosphorylation of Mcl-1 promotes its degradation after detachment. (A, B) Wild type and c-Src (527F) NIH3T3 cells were maintained in normal culture or detached on polyHEMA-coated plates containing DMSO, 25 μM TDZD-8, 50 nM dasatinib, or both inhibitors for 8 hours and subjected to anti-Bax 6A7 immunoprecipitation/immunoblot (A) and caspase-3 activity (B) assays. (C) Wild type and GSK-3β null MEFs were treated with DMSO or 25 μM LY294002 in normal or polyHEMA-coated plates for 12 hours and Mcl-1 expression was determined by Western blot. (D) NIH3T3 cells transfected with wild type or S159A mutant Mcl-1 were detached on polyHEMA plates for the indicated times in the presence of CHX to determine the half-life of the Mcl-1 proteins during anoikis by immunoblot analysis.
To determine if phosphorylation of Ser159 is required for Mcl-1 degradation during anoikis, constructs encoding the human Mcl-1 wild type and S159A mutant were transfected into wild type NIH3T3 cells. These cells were then detached and treated with CHX to observe the degradation of Mcl-1. Figure 4D shows that the half-life of wild type Mcl-1 is very short when compared to that of the S159A mutant. This suggests that Mcl-1 is degraded in response to a phosphorylation priming event that in turn targets the protein for ubiquitination and degradation. Moreover, detachment was found to decrease the levels of phospho-Akt in wild type NIH3T3 cells (data not shown), which may explain the increased degradation of Mcl-1 regulated by GSK-3β.
Src regulated Akt and Erk signaling control Mcl-1 and Bim expression
Src is known to promote survival signaling through multiple pathways. Two of the most well defined pathways controlled by Src are Akt and Erk, both of which have been implicated in anti-anoikis but debate remains over their involvement (26, 27). We therefore explored the consequences of Src, Akt, and Erk inhibition on Mcl-1 and Bim protein levels in 527F cells treated with dasatinib, LY294002, U0126, or the combination of LY294002 and U0126 during detachment (Supplementary Fig. S4A). Dasatinib inhibited signaling through both Akt and Erk, while treatment with LY294002 or U0126 specifically inhibited their target’s pathways. Inhibition of Akt but not Erk1/2 resulted in a significant decrease in Mcl-1 levels but did not extend to the levels observed in dasatinib treated cells. Also, Akt inhibition led to the appearance of multiple bands of Bim that are presumed to be Erk phosphorylated because U0126 inhibited these slower migrating bands and stabilized the Bim protein. Unlike dasatinib, however, treatment with LY294002, U0126 or the combination did not lead to the activation of Bax and caspase-3 (Supplementary Fig. S4A & B). It is possible that the decreasing Mcl-1 and increasing Bim by LY294002 and/or U0126 could not reach the threshold to shift the anti- and pro-apoptotic balance of the cell towards anoikis. To test this possibility, we used the c-3 shMcl-1 construct (Fig. 2D) to knockdown Mcl-1 expression in detached 527F cells treated with dasatinib, LY294002, U0126, or the combination of LY294002 and U0126. Knockdown of Mcl-1 led to a more anoikis sensitive phenotype in the presence of these kinase inhibitors determined by casapse-3 activity (Supplementary Fig. S4C). Importantly, the activation of Bax was also found to be enhanced in Mcl-1 knockdown cells over that of control (Supplementary Fig. S4D). This not only indicates that the level of Mcl-1 is critical for the initiation of Bax activation in response to Akt and Erk inhibition, but also illustrates the multi-functional ability of Src to suppress anoikis.
Metastatic cancers exhibit reduced Mcl-1 degradation and Bim induction
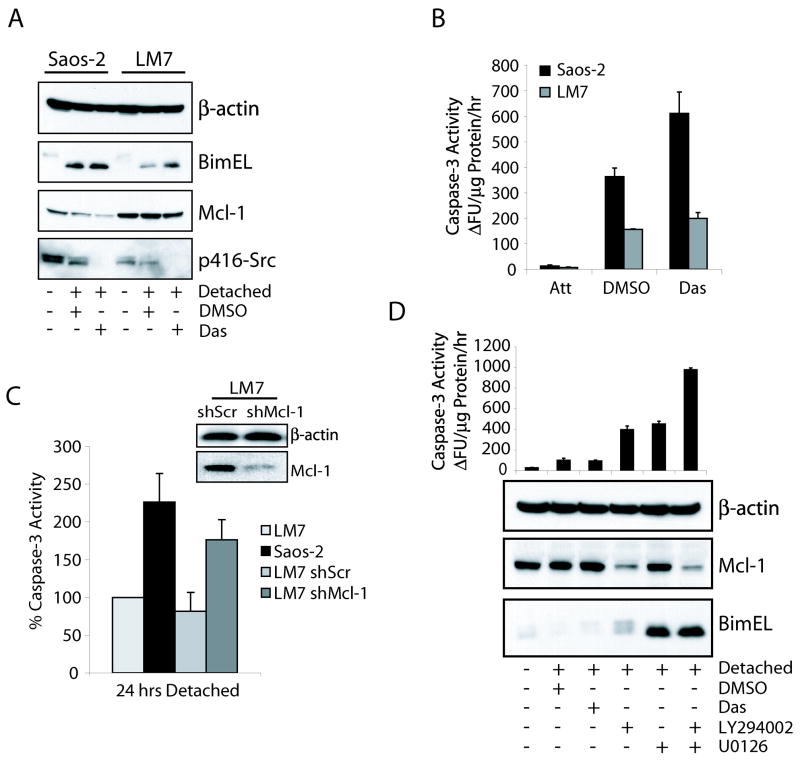
Resistance to anoikis is known to enhance the metastatic potential of cancer cells by affording them increased survival potential once detached from the ECM. Using the non-metastatic osteosarcoma cell line Saos-2 and its metastatic derivative LM7 (28), we found that parental Saos-2 cells were sensitive to anoikis which correlated with Mcl-1 degradation and Bim induction (Fig 5A & B). However, LM7 cells exhibited increased basal levels of Mcl-1 and maintained this expression during detachment, as well as decreased induction of Bim. Inhibition of Src signaling using dasatinib in both cell lines caused Bim induction and Mcl-1 degradation and increases in caspase-3 activity. The relatively slight increase in dasatinib sensitivity in LM7 cells may be the result of increased oncogenic signaling other than Src. To determine if the stabilization of Mcl-1 in LM7 cells was required for their resistance to anoikis, we inhibited Mcl-1 expression using lentiviral delivered shRNA. Knockdown of Mcl-1 was able to restore caspase-3 activation to levels similar to parental Saos-2 cells, while non-targeting scrambled shRNA had no effect on anoikis compared to uninfected LM7 cells (Fig. 5C).
Figure 5.
Mcl-1 and Bim expression is deregulated in human metastatic cancers. (A, B) Saos-2 and LM7 cells were untreated or detached on polyHEMA in the presence of DMSO or 50 nM dasatinib for 24 hours. Protein expression profiles were assayed by immunoblot and apoptotic index was measured by caspase-3 activity. (C) LM7 cells were infected with lentivirus containing control scrambled (shScr) or Mcl-1 (shMcl-1) shRNA constructs. Stably infected cells were analyzed for Mcl-1 expression by immunoblot (insert). The anoikis response of the transfectants was measured by the activation of caspase-3 in response to detachment and compared to the anoikis resistant parental LM7 cells. (D) MDA-MB-231 cells were untreated or detached on polyHEMA-coated plates containing DMSO, 50 nM dasatinib, 25 μM LY294002, 10 μM U0126, or the combination of LY294002 and U0126 for 24 hours and subjected to immunoblot analysis with the indicated antibodies (lower panel) and caspase-3 activity assay (upper panel).
Similarly, the highly metastatic breast cancer MDA-MB-231 cells were also observed to be resistant to anoikis. This was correlated with the maintenance of Mcl-1 expression and repressed Bim induction (Fig. 5D, lower panel), indicating the dysregulation of the normal response of these proteins to detachment is a contributing factor to metastatic potential. To decipher the signaling pathways involved in the observed expression profiles of Mcl-1 and Bim during detachment, we treated the cells with dasatinib, LY294002, U0126, or the combination of LY294002 and U0126. Inhibition of MEK or PI3K individually resulted in similar apoptotic indices as measured by caspase-3 activation (Fig. 5D, upper panel). Similar to results seen in Supplementary Figure S4A, PI3K inhibition caused a decrease in Mcl-1 expression, likely through the derepression of GSK-3β, as well as a limited increase in Bim expression, albeit the Bim protein was highly phosphorylated and likely rapidly turned over. Inhibition of MEK with U0126 had no effect on Mcl-1 expression but did confirm an earlier report of increased Bim expression upon Erk1/2 inhibition (27). Importantly, the combinational treatment of LY294002 and U0126 led to a synergistic increase in anoikis that correlated with Mcl-1 degradation and Bim induction. The failure of dasatinib to sensitize these cells to anoikis is likely the result of independent downstream oncogenic signaling mediated by previously identified mutations in K-RAS and BRAF (29) which activate both the PI3K/Akt and MEK/Erk pathways (30).
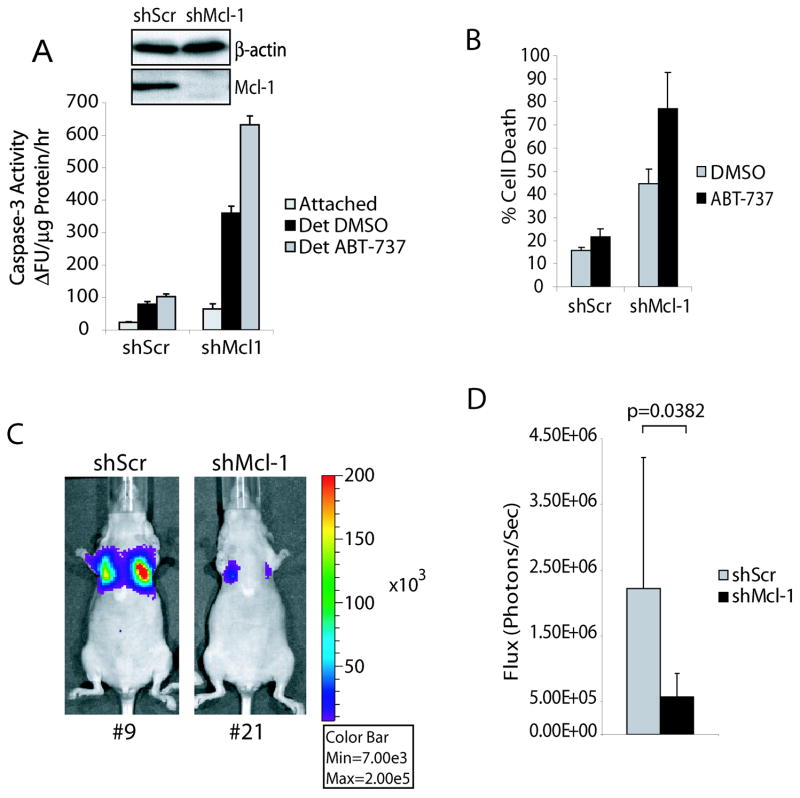
To determine if Mcl-1 degradation alone was able to promote anoikis in the MDA-MB-231 cells similar to PI3K inhibition, we again knocked down Mcl-1 (Fig. 6A, insert) and assayed the response to detachment. After only 24 hours of detachment, cells with depleted Mcl-1 displayed markedly increased caspase-3 activity and by 48 hours contained a much higher fraction of dead cells (Fig. 6A & B). To further confirm that the loss of Mcl-1 function but not other anti-apoptotic Bcl-2 members is essential for initiation of anoikis in metastatic cancer cells, we tested the ability of ABT-737, which targets Bcl-2, Bcl-XL and Bcl-w but not Mcl-1 (31), to induce apoptosis of detached MDA-MB-231 cells. As expected, treatment with ABT-737 was unable to trigger anoikis in control cells, although it significantly enhanced detachment induced apoptosis of Mcl-1 knockdown cells (Fig. 6A & B). Furthermore, in vivo experiments demonstrated that knockdown of Mcl-1 alone can significantly reduce tumor establishment of metastatic MDA-MB-231 cells in lungs of nude mice (Fig. 6C & D). These results strongly imply that inhibition of Mcl-1 degradation during detachment is vital to the establishment of metastatic cells.
Figure 6.
Mcl-1 depletion sensitizes human breast cancer cells to anoikis and reduces metastasis in vivo. (A, B) MDA-MB-231 cells were infected with lentivirus encoding control (shScr) or Mcl-1 (shMcl-1) shRNA constructs and selected by 0.5 μg/mL puromycin for 14 days. The knockdown of Mcl-1 was confirmed by immunoblot (insert). The anoikis response of shScr and shMcl-1 cells cultured in the presence of DMSO or 100 nM ABT-737 was measured by caspase-3 activation (A) and LDH release (B) assays after 24 or 48 hours detachment, respectively. (C, D) Harlan nude mice were injected with 1 × 106 MDA-MB-231-luc-D3H2LN cells expressing control scrambled (shScr) or Mcl-1 (shMcl-1) shRNA constructs via the tail vein and imaged using the IVIS200 system 3 weeks post-injection. Representative images are shown and bioluminescence is quantified in units of photons per second and shown in bar graph (mean ± s.d.; n = 9).
Discussion
Despite recent advances in early detection and new therapeutic options for cancer patients, metastatic progression is attributed to 90% of human cancer fatalities (32). Anoikis is a vital regulatory mechanism that can prevent metastases in a process that requires Bax translocation to mitochondria, which is inhibited by survival kinase signaling found in many human cancers (8). In spite of our current knowledge of apoptosis, how metastatic cancers escape anoikis remains poorly defined. In this report, we are the first to provide evidence of the unique importance of Mcl-1 degradation in the anoikis response. The Mcl-1 protein rapidly undergoes proteasome-dependent degradation in normal NIH3T3 cells at very early time points after detachment, which allows the subsequently induced Bim to activate Bax. Indeed, depletion of Mcl-1 alone is not enough to activate Bax under conditions in which the induction of Bim is blocked. Conversely, stabilization of Mcl-1 by proteasome inhibition suppresses Bax activation during anoikis, whereas overexpression of Bim causes dose dependent apoptosis. Together, these results suggest a model for anoikis where Mcl-1 degradation is required as a priming event to sensitize the cell towards Bax activation, which occurs in a manner that is dependent on the BH3-only protein Bim induction. These events are suppressed by active Src, Akt, and Erk1/2 signaling thereby conferring cancer cells with activating mutations in these pathways a survival advantage upon detachment from the ECM.
The ability of cancers to inhibit anoikis is propagated by the inability of these cells to activate Bax after the loss of adhesion. Bim, a known activator of Bax, is suppressed in active Src expressing cells through a mechanism controlled by Akt and Erk1/2 signaling pathways. The transcriptional repression of Bim in 527F cells is likely through the Akt pathway, which is known to phosphorylate the Bim transcription factor Foxo3a and promote its retention in the cytoplasm by interacting with 14-3-3 (33). BimEL is also regulated post-translationally by phosphorylation of Ser69 by Erk1/2, which promotes its targeting to the proteasome and degradation (34). JNK is also known to control transcription and sequestration of Bim (35, 36), but upon treatment of 527F cells with JNK inhibitors there are no significant changes in the Bim expression or apoptosis (data not shown).
Although Src can directly phosphorylate STAT3 thereby promoting the expression of Mcl-1 (37), our microarray and semi-quantitative RT-PCR analyses indicate that the Mcl-1 transcripts decrease marginally during anoikis in dasatinib sensitized 527F but not wild type cells. At post-translational levels, however, we found that GSK-3β mediated phosphorylation at Ser159 of Mcl-1 in response to detachment promotes its rapid proteasomal degradation. Akt negatively regulates GSK-3β by phosphorylation at Ser9 (38). Consistently, our studies in 527F and human metastatic cancer cells indicate that Mcl-1 expression is mainly regulated post-translationally through inhibition of GSK-3β activity by Akt. However, in 527F cells the lower expression of Mcl-1 and higher sensitivity to anoikis observed when treated with dasatinib compared to LY294002 suggest that other regulatory mechanisms mediated by Src, such as STAT3-dependent transcription, are also involved.
Mcl-1 degradation is required but by itself is insufficient to induce anoikis which requires the subsequent induction of Bim. Once there is an excess of Bim in relation to Mcl-1 the activation of Bax is initiated. These coordinated events determine the anoikis response, which are deregulated in cells with active oncogenic signaling. The comparison of human osteosarcoma cell line Saos-2 and its metastatic derivative LM7 illustrates that stabilization of Mcl-1 during detachment can afford metastatic cells considerable survival advantage. Consistent with this finding, knockdown of Mcl-1 enhances the sensitivity of metastatic LM7 and MDA-MB-231 cells to anoikis and can prevent establishment of metastases. Likewise, repression of the PI3K/Akt and MAPK signaling pathways that control Mcl-1 stability and Bim expression can resensitize metastatic breast cancer cells to anoikis.
The unique role of Mcl-1 in the anoikis response is likely attributed to its short half-life as its depletion coincides with the commitment of the cell to anoikis. The mechanism of Mcl-1 mediated anoikis inhibition appears to be non-redundant with that of Bcl-2 or Bcl-XL as ABT-737 is unable to initiate anoikis whereas loss of Mcl-1 does. However, the failure of initiation of anoikis by inhibition of Bcl-2/Bcl-XL does not exclude the role that these anti-apoptotic Bcl-2 members play in suppression of anoikis. Indeed, treatment with ABT-737 enhances anoikis initiated by knockdown of Mcl-1 in MDA-MB-231 cells. This finding is consistent with others that show an increased apoptotic response when Mcl-1 inhibition is combined with ABT-737 (39). Moreover, there is no significant decrease in the protein levels of Bcl-XL at early timepoints of anoikis where Bax conformational change and caspase activation are observed. Therefore, Bcl-XL overexpression in cancer cells may promote viability by repressing the velocity of the apoptotic insult initiated by Mcl-1 degradation when detached from the ECM. The prolonged viability may give the cells more time to re-attach to the ECM at a distal site or for the accumulation of additive mutations that can disrupt anoikis execution. Due to the unique characteristics of Mcl-1, such as a short half-life, higher affinity to Bim, and verified intricate regulation, we propose that Mcl-1 serves at the convergence point of many resultant signals downstream of detachment from the ECM that mediates the initiation of an anoikis response and the prevention of metastasis.
Supplementary Material
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org).
Acknowledgments
We thank Abbott Laboratories (Stephen Fesik) for providing ABT-737; Douglas Green, Emily Cheng and Ken Watanabe for plasmids; Richard Jove and Jack Pledger for cell lines; Neha K. Woods for reviewing this manuscript. Microarray was performed in the Moffitt Core Facility. The work is supported by grants from National Institutes of Health, American Cancer Society, and Flight Attendant Medical Research Institute.
References
- 1.Hynes RO. Cell adhesion: old and new questions. Trends Cell Biol. 1999;9(12):M33–7. [PubMed] [Google Scholar]
- 2.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. JCell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanmugathasan M, Jothy S. Apoptosis, anoikis and their relevance to the pathobiology of colon cancer. Pathol Int. 2000;50(4):273–9. doi: 10.1046/j.1440-1827.2000.01047.x. [DOI] [PubMed] [Google Scholar]
- 4.Yawata A, Adachi M, Okuda H, et al. Prolonged cell survival enhances peritoneal dissemination of gastric cancer cells. Oncogene. 1998;16(20):2681–6. doi: 10.1038/sj.onc.1201792. [DOI] [PubMed] [Google Scholar]
- 5.Wei L, Yang Y, Yu Q. Tyrosine kinase-dependent, phosphatidylinositol 3′-kinase, and mitogen-activated protein kinase-independent signaling pathways prevent lung adenocarcinoma cells from anoikis. Cancer Res. 2001;61(6):2439–44. [PubMed] [Google Scholar]
- 6.Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430(7003):1034–9. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 7.Lindsten T, Ross AJ, King A, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6(6):1389–99. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilmore AP, Metcalfe AD, Romer LH, Streuli CH. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J Cell Biol. 2000;149(2):431–46. doi: 10.1083/jcb.149.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi H, Wang HG. Bcl-XL protects BimEL-induced Bax conformational change and cytochrome C release independent of interacting with Bax or BimEL. J Biol Chem. 2002;277(44):41604–12. doi: 10.1074/jbc.M207516200. [DOI] [PubMed] [Google Scholar]
- 10.Hsu Y-T, Wolter KG, Youle RJ. Cytosol-to-membrane redistibution of members of Bax and Bcl-XL during apoptosis. Proc Natl Acad Sci U S A. 1997;94:3668–72. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92(1):57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 12.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426(6967):671–6. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 13.Nijhawan D, Fang M, Traer E, et al. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17(12):1475–86. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121(7):1085–95. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21(6):749–60. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Koshikawa N, Maejima C, Miyazaki K, Nakagawara A, Takenaga K. Hypoxia selects for high-metastatic Lewis lung carcinoma cells overexpressing Mcl-1 and exhibiting reduced apoptotic potential in solid tumors. Oncogene. 2006;25(6):917–28. doi: 10.1038/sj.onc.1209128. [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Rafiuddin-Shah M, Tu HC, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006 doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 18.Yu CL, Meyer DJ, Campbell GS, et al. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269(5220):81–3. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 19.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406(6791):86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 20.Windham TC, Parikh NU, Siwak DR, et al. Src activation regulates anoikis in human colon tumor cell lines. Oncogene. 2002;21(51):7797–807. doi: 10.1038/sj.onc.1205989. [DOI] [PubMed] [Google Scholar]
- 21.Valentijn AJ, Metcalfe AD, Kott J, Streuli CH, Gilmore AP. Spatial and temporal changes in Bax subcellular localization during anoikis. J Cell Biol. 2003;162(4):599–612. doi: 10.1083/jcb.200302154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen K, Coll ML, Li A, Filmus J. Transforming growth factor-alpha prevents detachment-induced inhibition of c-Src kinase activity, Bcl-XL down-regulation, and apoptosis of intestinal epithelial cells. J Biol Chem. 2001;276(40):37273–9. doi: 10.1074/jbc.M106424200. [DOI] [PubMed] [Google Scholar]
- 23.Coll ML, Rosen K, Ladeda V, Filmus J. Increased Bcl-xL expression mediates v-Src-induced resistance to anoikis in intestinal epithelial cells. Oncogene. 2002;21(18):2908–13. doi: 10.1038/sj.onc.1205388. [DOI] [PubMed] [Google Scholar]
- 24.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162(4):613–22. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosen K, Rak J, Leung T, Dean NM, Kerbel RS, Filmus J. Activated Ras prevents downregulation of Bcl-X(L) triggered by detachment from the extracellular matrix. A mechanism of Ras-induced resistance to anoikis in intestinal epithelial cells. J Cell Biol. 2000;149(2):447–56. doi: 10.1083/jcb.149.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin MJ, Melnyk N, Pollard M, et al. The insulin-like growth factor I receptor is required for Akt activation and suppression of anoikis in cells transformed by the ETV6-NTRK3 chimeric tyrosine kinase. Mol Cell Biol. 2006;26(5):1754–69. doi: 10.1128/MCB.26.5.1754-1769.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukazawa H, Noguchi K, Masumi A, Murakami Y, Uehara Y. BimEL is an important determinant for induction of anoikis sensitivity by mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitors. Mol Cancer Ther. 2004;3(10):1281–8. [PubMed] [Google Scholar]
- 28.Jia SF, Worth LL, Kleinerman ES. A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastasis. 1999;17(6):501–6. doi: 10.1023/a:1006623001465. [DOI] [PubMed] [Google Scholar]
- 29.Hollestelle A, Elstrodt F, Nagel JH, Kallemeijn WW, Schutte M. Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol Cancer Res. 2007;5(2):195–201. doi: 10.1158/1541-7786.MCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 30.Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17(11 Reviews):1395–413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 31.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 32.Weigelt B, Peterse JL, van ‘t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 33.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 34.Luciano F, Jacquel A, Colosetti P, et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22(43):6785–93. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 35.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100(5):2432–7. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29(3):629–43. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 37.Bowman T, Broome MA, Sinibaldi D, et al. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci U S A. 2001;98(13):7319–24. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 39.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67(2):782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org).