Abstract
Adeno-associated virus (AAV) vectors are associated with relatively mild host immune responses in vivo. Although AAV induces very weak innate immune responses, neutralizing antibodies against the vector capsid and transgene still occur. To understand further the basis of the antiviral immune response to AAV vectors, studies were performed to characterize AAV interactions with macrophages. Primary mouse macrophages and human THP-1 cells transduced in vitro using an AAV serotype 2 (AAV2) vector encoding green fluorescent protein did not result in measurable transgene expression. An assessment of internalized vector genomes showed that AAV2 vector uptake was enhanced in the presence of normal but not heat-inactivated or C3-depleted mouse/human serum. Enhanced uptake in the presence of serum coincided with increased macrophage activation as determined by the expression of NF-κB-dependent genes such as macrophage inflammatory protein 2 (MIP-2), interleukin-1β (IL-1β), IL-8, and MIP-1β. AAV vector serotypes 1 and 8 also activated human and mouse macrophages in a serum-dependent manner. Immunoprecipitation studies demonstrated the binding of iC3b complement protein to the AAV2 capsid in human serum. AAV2 did not activate the alternative pathway of the complement cascade and lacked cofactor activity for factor I-mediated degradation of C3b to iC3b. Instead, our results suggest that the AAV capsid also binds complement regulatory protein factor H. In vivo, complement receptor 1/2- and C3-deficient mice displayed impaired humoral immunity against AAV2 vectors, with a delay in antibody development and significantly lower neutralizing antibody titers. These results show that the complement system is an essential component of the host immune response to AAV.
Adeno-associated virus (AAV) vectors are generally associated with low toxicity, resulting in vector persistence and long-term transgene expression (29, 34, 70). The inability of AAV vectors to efficiently transduce or activate antigen-presenting cells may account for their decreased immunogenicity (74). However, AAV vectors can induce cellular and humoral responses to the transgene product (15, 21, 22, 41, 43, 49, 71) and AAV-mediated gene therapy leads to the development of antibodies against the vector capsid, confirming that a significant interaction with the immune system exists (9, 28, 55). Anti-AAV antibodies have neutralizing effects that decrease the efficiency of in vivo gene therapy and can prevent vector readministration (13, 52). Furthermore, AAV serotype 2 (AAV2) vectors induce transient innate immune responses in mice (72) and in a recent clinical trial unexpected AAV-induced liver toxicity was noted in two patients following intrahepatic administration of AAV2 (44). It is therefore important to understand the mechanisms that lead to the induction of immune responses directed against AAV.
The serum complement system represents a chief component of innate immunity. Activation of the complement system leads to opsonization of microorganisms, lysis of target cells, and release of inflammatory mediators from leukocytes. Complement components are inactive proenzymes circulating in serum that are activated through highly regulated enzymatic cascades. Complement activation occurs via three different mechanisms: the lectin, the alternative, and the classical pathways. All pathways result in the formation of the C3 convertases, which cleave C3 into C3a and C3b. The fate of C3b is critical to the regulation of the complement cascade. Persistence of C3b allows further binding of factor B and hence amplified C3 cleavage. C3b is necessary to activate downstream complement proteins and effector mechanisms. Catabolism of C3b into iC3b inhibits amplification of C3 cleavage and results in downregulation of the complement system (42). Complement regulatory proteins such as factor H in plasma can limit complement activation through a function as a cofactor for factor I-mediated cleavage of C3b into iC3b. Many pathogens have evolved evasion strategies to avoid complement activation. Vaccinia virus, for example, encodes a secretory protein (complement control protein, VPC) which is homologous to human complement control proteins and acts as a cofactor for factor I-mediated C3b degradation (37). Other pathogens recruit factor H to their surface to evade complement neutralization (62).
Deposition of C3 fragments such as C3b and iC3b on pathogen surfaces leads to opsonization, enhanced phagocytosis, immune complex clearance, adhesion, and cytokine production (24). Most such activities depend upon the engagement of specific complement receptors. These include complement receptor 1 (CR1, CD35), complement receptor 2 (CR2, CD21), and the beta-integrins CR3 (CD11b/CD18), CR4 (CD11c/CD18), and the recently discovered immunoglobulin superfamily receptor, CRIg (27). All complement receptors bind iC3b. CR1 and CR2 are thought to participate mainly in particle binding. CR3 and CR4 are involved in phagocytosis of C3b- and iC3b-opsonized pathogens (3, 16, 38, 51, 56).
The complement system evolutionarily predates the adaptive immune response but has adapted to mediate cross talk between the adaptive and innate responses. In addition to its role in inflammation, increasing evidence supports the role of complement in regulating B lymphocytes and in contributing to the development of humoral immunity (4-6, 19, 23). On B cells, CR1 (CD21) forms a coreceptor with the signaling molecule CD19 and receptor CD81. Coengagement of the CD21/CD19/CD81 receptor complex with the B-cell antigen receptor (BCR) enhances B-cell responses by lowering substantially the threshold for B-cell activation (45). Corecognition of the BCR and CD21 leads to increased cell proliferation and differentiation and enhanced antibody production. Engagement of CR1 is especially critical when suboptimal doses of antigen are present or the affinity of the antibody is low, as is the case during primary immune responses. Recent research has shown that complement can also modulate T-cell responses, both through direct modulation of the T cell and indirectly through antigen-presenting cells (5, 33). For example, in the absence of C3, CD4+ and CD8+ T-cell priming was impaired in an influenza virus model (36), suggesting a more general role of the complement system in adaptive immunity.
We have shown previously that AAV vectors can elicit transient inflammatory responses in vivo but that these responses are entirely dependent on resident macrophages (72). To understand further the biology of the host immune response to AAV vectors, we examined the basis and functional consequence of AAV vector interactions with macrophages, an important effector leukocyte of the innate immune system. In this study, we show that AAV capsid binds C3 complement proteins, which enhances AAV uptake into macrophages and macrophage activation. In vivo studies of C3- and complement receptor 1/2 (CR1/2)-deficient mice show that the complement system is essential in the humoral immune response against AAV vectors.
MATERIALS AND METHODS
Virus vectors.
AAV serotype 1, 2, and 8 vectors encoding green fluorescent protein (GFP) or LacZ were produced from producer cell lines by using wild-type adenovirus type 5 (Ad5) as described by Clark et al. (12). Vectors were purified by high-pressure liquid chromatography with a Poros HE/M heparin column. Final vector preparations were dialyzed against 4 liters of dialysis buffer containing 3% sucrose, 150 mM NaCl, 10 mM Tris (pH 7.4), and 1 mM MgCl2 for 4 h at 4°C and stored at −70°C. The absence of replication-competent adenovirus in the AAV preparations was assayed by passing 1% of the purified vector stock onto 293 cells and scoring for adenovirus cytopathic effect after 7 days. Adenovirus contamination was consistently less than 1 infectious adenovirus particle per 1012 AAV particles, when evidenced at all. DNase-resistant particle values were determined using a Perkin-Elmer Applied Biosystems Prism 7700 sequence detector as described previously (12).
The type 5, E1-deleted, E3-defective adenovirus encoding the GFP transgene under the control of a cytomegalovirus promoter was generated using the AdEasy system (Stratagene), plaque purified, and then propagated on human embryonic kidney cells (293 cells). Vectors were purified on cesium chloride gradients as previously described (2). After purification, vectors were dialyzed against 4 liters of dialysis buffer as described above and stored at −70°C. The particle titer was determined by measuring the optical density at 260 nm and described as particle per cell (part/cell). Plaque assay on HeLa cells and PCR were used to test for replication-competent adenovirus. The concentration remained consistently <1 per 1010 particles. Low-endotoxin tissue culture reagents and buffers were used for vector production and experiments. AAV and adenovirus vectors were routinely tested for the presence of endotoxin by use of a Limulus amebocyte lysate kit. All vectors contained less then 0.1 endotoxin units/ml. Purified herpes simplex virus type 1 (HSV-1) was purchased from Advanced Biotechnologies (MacIntyre strain).
Cell culture.
Human embryonic kidney cells (HEK 293 cells) were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Gibco BRL). Human THP-1 monocytic leukemia cells were grown in RPMI 1640 medium supplemented with 10% FBS, 1% penicillin-streptomycin, 1% sodium pyruvate (100 mM), and 0.01% β-mercaptoethanol. THP-1 cell differentiation was achieved by resuspending the cells in growth medium containing 10−8 M phorbol-12-myristate-13-acetate (63, 67). Experiments were performed 24 h after the addition of differentiation medium in normal growth medium. Cells were tested periodically for mycoplasma contamination. For selected experiments, the FBS in the cell culture medium was replaced by human serum (HS). HS was collected from 10 healthy donors, pooled, and filtered through a sterile 0.45-μm mesh filter. For inhibition of the complement system, HS was heat inactivated for 30 min at 56°C (referred to as HI-HS). For depletion of immunoglobulin G (IgG), serum aliquots were treated with equal volumes of protein G-Sepharose beads as previously described (18) (referred to as HS-IgG). C3-depleted HS was purchased from CompTech (Tyler, TX).
Primary mouse bone marrow macrophages were obtained by isolating bone marrow stem cells from the tibiae and femurs of C57BL/6 mice (8 to 14 weeks of age), followed by differentiation into macrophages for 7 days in tissue culture. Briefly, mice were euthanized, the hind legs were removed, and the bones were carefully separated intact. Bones were flushed out by inserting a 26-gauge needle attached to a syringe with bone marrow medium into the bone marrow cavity. The cells were washed and seeded at 1.4 × 106 cells per well of a six-well plate or 2.5 × 105 cells per well of a 24-well plate in bone marrow medium with a total volume of 3 ml or 0.8 ml per well, respectively. The plates were incubated for 5 days in a humidified atmosphere with 10% CO2 at 37°C. The medium was replaced with fresh bone marrow medium on day 5, and the cells were used for experiments over the following two to three days. Cells expressed the murine macrophage differentiation marker F4/80 (data not shown). Bone marrow medium consists of DMEM supplemented with 2% penicillin-streptomycin, 10% FBS, and 10% L-cell conditioned medium. L-cell conditioned medium was produced by culturing L929 cells in T-150 tissue culture flasks at 0.24 × 106 cells per flask in 50 ml culture medium (DMEM supplemented with 1% penicillin-streptomycin and 10% FBS). After 7 days the medium was collected, filtered through a 0.2-μm filter, and stored at −20°C. Fresh medium was added onto the cells (50 ml per T-150 flask), and the cells were incubated for another 7 days. At the end of the second week, the medium was again collected and stored as described above. The conditioned medium was used as a 50/50 mix of week 1 and week 2 at 10% in bone marrow medium to provide the correct growth rate of the bone marrow-derived macrophages. In some experiments the FBS in the bone marrow medium was replaced with mouse plasma (MP). MP was obtained from naïve or AAV2-immunized C57BL/6 mice (8 to 12 weeks of age) placed under anesthesia by cardiac puncture using a heparinized needle and syringe. Plasma was isolated from collected blood by centrifugation at 900 × g for 5 min, pooled, and stored at −80°C. For complete complement inhibition, plasma was heat inactivated for 30 min at 56°C.
Viral transductions were performed in six-well plates or 24-well plates with 1 × 106 to 1.4 × 106 cells/well or 2 × 105 cells/well, respectively. Cells were incubated at 37°C with 1 ml of medium per six-well plate or 0.3 ml of medium per 24-well plate containing viral vectors. AAV-transduced cells were coinfected with wild-type Ad5 (multiplicity of infection of 1). To assess GFP transgene expression, cells were analyzed using excitation/emission filter cubes of 490/528 nm of an Olympus IX70 inverted epifluorescence microscope attached to a camera. Images were obtained with Openlab software.
Animal studies.
All animal studies were performed in accordance with the guidelines of the Animal Care Committee at the University of Calgary. Male or female C57BL/6 mice, 6 to 8 weeks old, were purchased from Charles River Laboratories and housed under single-barrier conditions. Mice were used for experiments at 7 to 12 weeks of age (20 to 30 g). The murine Cr2 gene codes for both complement receptor 1 (CR1) and complement receptor 2 (CR2) transcripts by alternative splicing. Mice genetically deficient of the Cr2 gene (CR1/2−/−) on a C57BL/6 background were described previously (50). Mice genetically deficient of complement component C3 (C3−/−) were obtained from Jackson Laboratories. CR1/2−/− or C3−/− and C57BL/6 wild-type control mice were age and sex matched for the experiments. AAV vectors (2 × 1011 part/mouse) were injected under general anesthesia via the femoral vein in a total volume of 100 μl (vector plus vehicle). Control animals received vehicle alone. Animals were allowed to recover and then sacrificed at predetermined time points, and the livers and sera were harvested for analysis.
Antibody titration.
To monitor the development of neutralizing AAV capsid antibodies in CR1/2−/−, C3−/−, or wild-type control mice, animals were immunized by intravenous injection of 2 × 1011 particles of AAV vector and blood samples were collected over time by lateral saphenous vein puncture. Serum was obtained from blood by centrifugation at 1,000 × g for 5 min and stored at −80°C until the time of analysis.
Neutralizing antibody titers were determined in AAV transduction assays and GFP transgene expression in HEK 293 cells. Murine serum samples were serially diluted in serum-free tissue culture medium and incubated with 8 × 109 particles of AAV2-GFP for 10 min at 37°C in a total volume of 100 μl. The AAV-serum mix was then applied to wells of a 24-well plate seeded with 293 cells (∼90% confluent) in addition to wild-type Ad5 (multiplicity of infection of 1). Cells were collected at 48 h, and GFP expression was quantified by flow cytometry using a FACScan instrument (Becton Dickinson) and plotted against the total serum dilution. The antibody titer was determined as the highest serum dilution which inhibited virus transduction by 50%.
DNA isolation and Southern blotting.
To obtain total DNA, transduced cells were trypsinized for 5 min and washed several times with phosphate-buffered saline to remove bound virus from the cell surface. The cell pellet was subjected to processing for total DNA by use of a DNeasy kit (Qiagen) according to the manufacturer's protocol. For Southern analysis, 1 to 3 μg of DNA was digested with HindIII and separated on a 1% agarose gel. The gel was washed in 0.25 M HCl, followed by another wash in a 0.4 M NaOH solution. The DNA was then alkaline transferred onto a positive-charged Hybond XL nylon membrane (Amersham Pharmacia) overnight. A full-length GFP cDNA fragment was labeled with [32P]dCTP by use of a Redprime random prime labeling system (Amersham Pharmacia). Hybridization was performed by using 2 ng of the labeled DNA probe per 5 ml of ExpressHyb solution (Clontech) at 60°C for 1 h. The membrane was then washed three times for 15 min in 2× SSC (1× SSC is 0.15 M NaCl, 0.015 M sodium citrate) and 0.1% sodium dodecyl sulfate (SDS) at 60°C and exposed to radiographic film for visualization. For quantification, the blots were subjected to phosphorimager analysis by use of a personal F/X molecular imager (Bio-Rad), and the signal intensities were analyzed with Quantity One software (Bio-Rad). To correct for DNA loading, intensity scores were normalized to the density of total DNA in the agarose gel prior to membrane transfer and expressed as phosphorimager units.
RNase protection assays.
Total RNA from liver tissues or tissue culture cells was isolated using an RNeasy kit (Qiagen) according to the manufacturer's protocol. RNase protection assays were carried out using a RiboQuant MultiProbe RNase protection assay system, with mouse mCK5c/mCK2b and human hCK5/hCK2b multiprobe template sets, according to the manufacturer's protocol (BD Pharmingen). For quantification, the gels were subjected to phosphorimager analysis by use of a personal F/X molecular imager (Bio-Rad), and the signal intensities were analyzed with Quantity One software (Bio-Rad). Each intensity score was normalized to the intensity of the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene and expressed as phosphorimager units relative to the percentage of GAPDH.
C3a-desArg complement assay.
C3a protein in HS was quantified using an enzyme-linked immunosorbent assay (ELISA) against C3a-desArg according to the manufacturer's instructions (BD OptEIA human C3a ELISA; BD Biosciences, San Diego, CA). Briefly, 96-well ELISA plates coated with anti-human C3a-desArg capture antibody were incubated with HS samples for 2 h at room temperature. After being washed extensively, plates were incubated with biotinylated polyclonal anti-human C3a detection antibody and horseradish peroxidase-conjugated streptavidin. Plates were washed again and developed after addition of tetramethyl benzidine and hydrogen peroxide substrate for 30 min at room temperature, and the reaction was stopped by adding 1 M H3PO4 as the stop solution. Optical densities were determined spectrophotometrically at 450 nm (Benchmark microplate reader; Bio-Rad). Purified recombinant C3a protein in serial dilutions served as the standard.
Immunoprecipitation and immunoblotting.
To study the binding of complement component C3 to AAV capsids in HS, 8 × 109 particles of AAV were incubated with 10 μl pooled HS for 10 min at 37°C to allow binding of complement and neutralizing serum IgG to the virus capsid. Antibody-capsid complexes were immunoprecipitated using protein G-Sepharose beads (Amersham Bioscience). The AAV serum mix (10 μl), Sepharose beads (10 μl), and 400 μl of wash buffer (Tris-buffered saline, 1% Triton X-100) were combined and mixed at 4°C overnight. Sepharose beads were then washed three times with wash buffer, and the precipitates were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and then transferred onto nitrocellulose membranes. The membranes were blocked with 5% milk proteins and immunoblotted with a polyclonal goat anti-C3 antibody (CompTech, Tyler, TX) for 45 min at room temperature or a polyclonal rabbit anti-AAV antibody (American Research Products). Blots were washed, incubated with horseradish peroxidase-conjugated donkey anti-goat IgG or goat anti-rabbit secondary antibody, and visualized using ECL Plus chemiluminescent substrate (Amersham).
To test AAV binding with C3, C3b, or iC3b directly in the absence of HS Ig, 4 × 1010 particles of AAV were incubated with 200 ng of purified C3, C3b, or C3b plus factor I (1 μg) and factor H (1 μg) in 30 μl phosphate buffer (20 mM, pH 6.0) for 1 h at 37°C. The reaction mix was precleared with Sepharose B (10 μl in 200 μl wash buffer) for 1 h at 4°C. C3 in the reaction mix was then immunoprecipitated with a polyclonal goat anti-C3 antibody (0.5 μg) overnight at 4°C and analyzed by Western blotting as described above. AAV without C3 (or C3b) served as the negative control.
To test AAV binding with factor H, 4 × 1010 particles of AAV were incubated with purified factor H (1 μg) in 30 μl phosphate buffer (20 mM, pH 6.0) for 1 h at 37°C. After the reaction mix was precleared with Sepharose B, factor H was immunoprecipitated with a polyclonal goat anti-human factor H antibody (CompTech, Tyler, TX) and analyzed by Western blotting as described above. AAV without factor H served as the negative control.
Cofactor assay.
The cofactor activities of viral vectors and HSV-1 were tested using a modified but previously described protocol (25). Particles of AAV (4 × 1010), adenovirus (4 × 1010), or HSV-1 (4 × 108) were incubated in 20 mM phosphate buffer (pH 6.0) in a final volume of 30 μl containing 200 ng of C3b and 1 μg of factor I for 1 h at 37°C. Reaction mixes with factor I and vehicle or with factor I and factor H (1 μg) alone served as negative and positive controls, respectively. The samples were then separated under reducing conditions using 10% SDS-PAGE and analyzed by immunoblotting as described above. C3b, factor I, factor H, and the polyclonal anti-C3 antibody were commercially obtained from CompTech, Tyler, TX.
Statistics.
Statistical analysis was performed using GraphPad Instat, version 3.01. Values are expressed as the means ± standard derivations of individual samples. Results were analyzed for statistical variance using an unpaired Student t test or one-way analysis of variance followed by pairwise comparison using either Tukey's honestly significant difference test or Dunnett's multiple comparison test as appropriate.
RESULTS
AAV vector-macrophage interactions are enhanced by serum components.
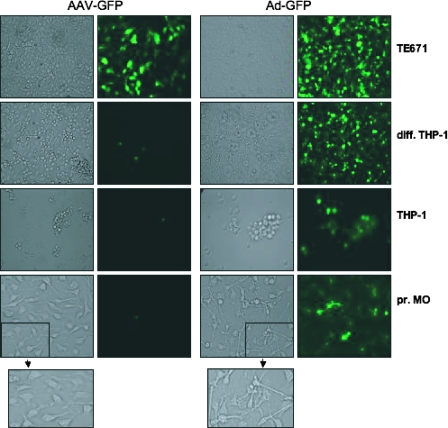
We have shown previously that AAV vectors induce a transient Kupffer cell-dependent upregulation of inflammatory gene expression and leukocyte recruitment in mouse liver in vivo (72). These results showed that macrophages play a key role in initiating the innate immune responses to AAV vectors in vivo. To analyze AAV vector-macrophage interactions directly, experiments with primary mouse bone marrow macrophages and differentiated human THP-1 cells were performed. Transduction of primary mouse bone marrow macrophages and THP-1 cells (in FBS) with 1 × 104 part/cell of an AAV2 vector encoding GFP (AAV2-GFP) did not result in significant GFP transgene expression (Fig. 1). TE671 cells, a human muscle cell line (rhabdomyosarcoma), transduced side by side with the same titer of AAV2-GFP resulted in strong GFP expression, confirming that the vector was functional. In contrast to AAV2-GFP, 1 × 103 part/cell of an adenovirus vector encoding the same transgene (Ad-GFP) efficiently transduced and expressed GFP in all cell types tested (Fig. 1). Furthermore, Ad-GFP, but not AAV2-GFP, significantly changed the phenotype of primary mouse bone marrow macrophages upon transduction at 24 h. After Ad-GFP transduction, primary mouse bone marrow macrophages assumed a “dendritic” net-like morphology consistent with an activated phenotype (Fig. 1, insets).
FIG. 1.
Transduction of macrophage lines and primary macrophages by AAV and adenovirus vectors. Transduction of human muscle TE671 cells, primary mouse bone marrow macrophages (pr. MO), the human monocytic cell line THP-1, or differentiated THP-1 cells (diff. THP-1) with an AAV2 vector (1 × 104 part/cell) or an adenovirus vector encoding GFP (1 × 103 part/cell). In contrast to Ad-GFP, AAV-GFP does not efficiently transduce macrophage lines or primary macrophages and does not induce any phenotypic change (insets). Data are representative samples of at least four independent vector transductions.
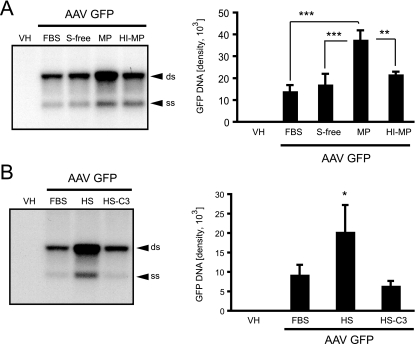
To further characterize the interaction of AAV vectors with primary macrophages and differentiated THP-1 cells, intracellular AAV2-GFP vector genomes were analyzed to determine whether the lack of transgene expression was due to a lack of viral entry into these cells. Primary bone marrow macrophages and differentiated THP-1 cells were incubated with AAV2-GFP (1 × 104 part/cell) under different serum conditions. At 6 h, AAV vector genomes were analyzed by Southern blotting, probing for the GFP transgene. Two prominent bands representing the single- and double-stranded AAV DNA genomes were detected from total cellular DNA extracted from primary macrophages and THP-1 cells (Fig. 2A and B), confirming that macrophages interact with and take up AAV vectors. Interestingly, AAV2-GFP genomes were increased more than twofold in primary macrophages incubated in MP compared to levels in heat-inactivated plasma, in FBS, or under serum-free conditions. These results suggested a possible role for complement in virus-macrophage interactions (Fig. 2A). To specifically determine the effect of complement, the entry of AAV vectors into differentiated THP-1 cells was analyzed in the presence of standard tissue culture medium (FBS), HS, or HS deprived of complement component C3 (HS-C3). C3 is a key component of the complement cascade; in the absence of C3, any complement activation is blocked. Similarly to the effect seen in bone marrow macrophages, AAV vector genomes were significantly higher in the presence of complete HS than in the presence of FBS or HS-C3 (Fig. 2B). In summary, these data show that complement enhances AAV vector internalization in macrophages. Despite this internalization, macrophages are poorly transduced by AAV2-based vectors.
FIG. 2.
AAV vector genome uptake in macrophages in vitro. (A) Southern blot analysis of intracellular vector genomes, blotting for the GFP transgene, 6 h after transduction of primary mouse bone marrow macrophages with AAV2-GFP. Cells were transduced in the presence of normal tissue culture medium containing 10% FBS, serum-free medium (S-free), medium containing 10% MP, or medium containing 10% HI-MP. Vector genome uptake is significantly higher in the presence of MP than in the presence of normal tissue culture medium (FBS), S-free, or HI-MP (means ± standard deviations [SD]) (**, P < 0.01; ***, P < 0.001; n = 3). Differences between FBS, S-free, and HI-MP were not significant (n = 3). (B) Southern blot showing internalized AAV vector genomes in differentiated THP-1 cells blotting for the GFP transgene. Cells were transduced in tissue culture medium supplemented with 10% FBS, 10% HS, or 10% HS-C3. DNA quantities were determined by phosphorimaging (ss, single-stranded vector genome; ds, double-stranded monomer). Vector genome uptake is significantly higher in the presence of HS than in the presence of normal tissue culture medium (FBS) or HS-C3 (means ± SD) (*, P < 0.05; n = 3). Differences between FBS and HS-C3 were not significant (n = 3). VH, vehicle.
AAV vectors induce inflammatory gene expression in macrophages.
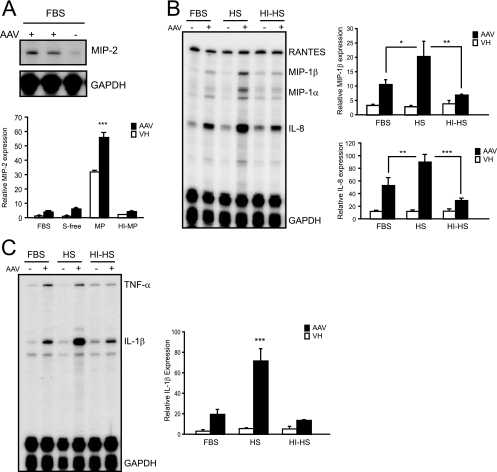
Although macrophages were poorly transduced by AAV2-GFP, ongoing vector internalization suggested that host innate mechanisms may still be activated. To assess the impact of AAV vector internalization on macrophage activation, primary mouse bone marrow macrophages and differentiated THP-1 cells were challenged with AAV vectors and the expression of inflammatory genes was determined. Cells were challenged with AAV vectors in the presence of 10% MP, 10% HS, or heat-inactivated plasma or serum to remove complement activity. At 90 min following AAV2-GFP challenge, macrophage total RNA was analyzed by RNase protection assay. AAV2-GFP induced the expression of the chemokine macrophage inflammatory protein 2 (MIP-2) in primary macrophages (Fig. 3A). Other chemokines such as MIP-1β, MIP-1α, and IP-10 were not elevated significantly over baseline. Although MP alone significantly enhanced baseline MIP-2 expression in primary macrophages, AAV significantly induced MIP-2 mRNA expression further in the presence of 10% MP compared to that in the presence of 10% heat-inactivated MP (HI-MP), serum-free medium, or normal tissue culture medium containing 10% FBS. Similarly, incubation of differentiated THP-1 cells with AAV2-GFP in the presence of medium containing 10% FBS, 10% HS, or 10% HI-HS induced the expression of multiple chemokines and cytokines (Fig. 3B and C). Furthermore, levels of MIP-1β, MIP-1α, interleukin-8 (IL-8), and IL-1β RNA expression in response to the AAV vector were significantly higher in the presence of HS than in the presence of HI-HS or FBS. These results show that, despite poor transduction, AAV2 vector internalization into macrophages correlates with macrophage activation in vitro. The enhanced activation and cellular uptake observed in the presence of intact serum or plasma suggest that complement opsonization of AAV increases the interaction with and activation of macrophages.
FIG. 3.
AAV vector-induced chemokine and cytokine expression in macrophages in vitro. (A) MIP-2 RNA expression 90 min after AAV2-GFP transduction (1 × 105 part/cell) of primary mouse bone marrow macrophages. Cells were transduced in the presence of normal tissue culture medium containing 10% FBS, serum-free medium (S-free), medium containing 10% MP, or medium containing 10% HI-MP. Quantification of MIP-2 expression is based on phosphorimaging of RNase protection assays and normalized against GAPDH. AAV induced the expression of MIP-2 significantly above baseline (vehicle [VH]) under all serum conditions (means ± standard deviations [SD]) (***, P < 0.001). Furthermore, AAV-induced MIP-2 expression is significantly higher in the presence of MP than in the presence of normal tissue culture medium (FBS), S-free, or HI-MP (means ± SD) (***, P < 0.001; n = 3). (B and C) RNase protection assay of differentiated THP-1 cell RNA 90 min after transduction with AAV2-GFP (1 × 105 part/cell). Quantification of chemokine/cytokine expression is based on phosphorimaging of RNA blots and normalized against GAPDH. Cells were transduced in tissue culture medium supplemented with 10% FBS, 10% HS, or 10% HI-HS. Cytokine/chemokine gene expression in the presence of HS was significantly increased compared to that in the presence of FBS (means ± SD) (*, P < 0.05 for MIP-1β; **, P < 0.01 for IL-8; ***, P < 0.001 for IL-1β; n = 3) or HI-HS (means ± SD) (**, P < 0.01 for MIP-1β; ***, P < 0.001 for IL-8 and IL-1β; n = 3).
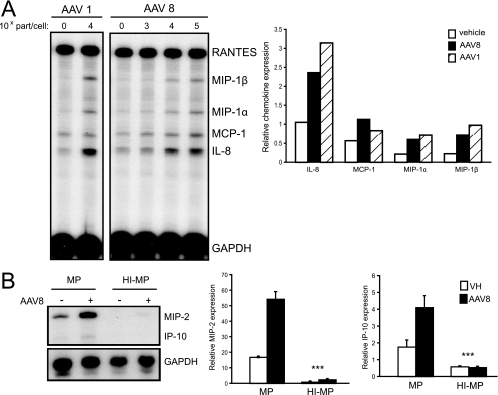
Several new AAV serotypes are being developed and applied to gene therapy. To determine if other AAV vector serotypes interacted with and activated macrophages similarly to AAV2, experiments with AAV1 and AAV8 vectors expressing the GFP transgene were performed. Both AAV1 and AAV8 activated cytokine and chemokine mRNA expression in THP-1 cells and primary murine bone marrow-derived macrophages at 90 min, as determined by the RNase protection assay (Fig. 4A and B). Similarly to the results observed with AAV2, serotype 8 AAV-GFP induction of MIP-2 and IP-10 gene expression was abolished in HI-MP (Fig. 4B). MIP-1β and MIP-1α were not elevated significantly over baseline regardless of the serum conditions in these cells. These results show that complement-dependent AAV interactions with macrophages are not restricted to AAV2 serotypes.
FIG. 4.
Chemokine and cytokine expression in macrophages transduced with AAV1 and AAV8 in vitro. (A) RNase protection assay of THP-1 cell RNA 90 min after transduction with AAV1-GFP (1 × 105 part/cell) or AAV8-GFP (1 × 103, 1 × 104, or 1 × 105 part/cell). Quantification of chemokine expression following transduction with AAV8-GFP (1 × 105 part/cell) or AAV1-GFP (1 × 105 part/cell) by phosphorimaging normalized against GAPDH. (B) RNase protection assay of primary mouse bone marrow macrophage RNA 90 min after transduction with AAV8-GFP (1 × 105 part/cell). Quantification of MIP-2 and IP-10 expression by phosphorimaging normalized against GAPDH. Cells were transduced in medium supplemented with either 10% MP or 10% HI-MP. In the presence of MP, AAV8-GFP induced the expression of MIP-2 and IP-10 RNA significantly above baseline (means ± standard deviations) (***, P < 0.001; n = 3). Furthermore, AAV-induced MIP-2 and IP-10 expression was significantly increased in the presence of MP compared to that in the presence of HI-MP (***, P < 0.001; n = 3). VH, vehicle.
AAV vectors interact with complement proteins.
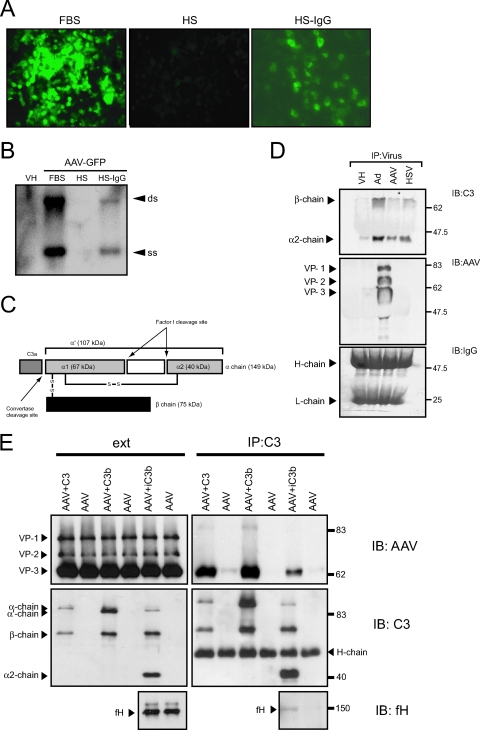
Results from the previous experiments demonstrated that AAV vector-macrophage interactions were significantly enhanced by complement. To determine whether complement components interacted directly with the viral capsid, coimmunoprecipitation studies using AAV2-GFP were performed. The AAV2 vector was incubated with HS and immunoprecipitated, followed by immunoblotting for C3 complement components. The prevalence of serum antibodies against AAV in the population is between 35 and 80%, according to age group and geographical location (8, 17, 52). Consistent with this, pooled HS abrogated AAV2-GFP transduction in 293 cells; this process was reversed following IgG depletion using protein G-Sepharose (Fig. 5A). Southern blot analysis of internalized vector genomes confirmed that the abrogated transgene expression was a result of neutralization of virus particles and reduced entry into these cells (Fig. 5B).
FIG. 5.
AAV interaction with complement. (A) Pooled HS contains neutralizing anti-AAV IgG antibodies. Transduction of HEK 293 cells with AAV2-GFP in the presence of tissue culture medium supplemented with 10% FBS, 10% pooled HS, or 10% HS-IgG. Pooled HS abrogates GFP transgene expression. (B) Southern blot of internalized AAV vector genomes blotting for the GFP transgene. HEK 293 cells were transduced with AAV2-GFP, and total DNA was harvested and analyzed for AAV vector genomes at 6 h. Pooled HS abrogates viral vector entry into HEK 293 cells. Representative Southern blot of at least three independent experiments. VH, vehicle; ss, single-stranded vector genome; ds, double-stranded monomer. (C) Diagram of C3 structure. C3 is composed of two chains, α and β. Upon activation C3a is released, leaving C3b, consisting of the truncated α′ chain (107 kDa) and the β chain (75 kDa). Factor I and a cofactor cleave the α′ chain at two locations, thereby creating iC3b, which consists of β, α1, and α2 chains (75, 67, and 40 kDa, respectively). (D) Viral complement coimmunoprecipitation. AAV2-GFP, Ad-GFP, and HSV-1 were immunoprecipitated from HS and analyzed by immunoblotting C3 complement components or AAV capsid proteins. Staining for human IgG heavy (H-chain) and light (L-chain) chains served as the loading control. Representative blot from three independent experiments. (E) Coimmunoprecipitation of AAV and complement in the absence of HS. AAV was incubated with complement component C3, C3b, or C3b mixed with factor H (fH) and factor I to generate iC3b, followed by immunoprecipitation of the complement components with a polyclonal anti-C3 antibody. Precipitate (IP) and starting material (ext) were analyzed by immunoblotting (IB) for AAV capsid proteins, C3 complement components, and fH.
To immunoprecipitate AAV, AAV2-GFP was incubated with pooled HS to bind neutralizing IgG and then incubated with protein G-Sepharose. The beads were collected and the recovered proteins analyzed by immunoblotting for C3 complement and AAV2 capsid proteins (Fig. 5D). The adenovirus capsid and the glycoproteins C of HSV-1 have been shown to bind C3-derived fragments (31, 47, 64, 66). Adenovirus vector and HSV-1 therefore were included as positive controls. Immunoprecipitation of AAV2-GFP was effective as determined by immunoblotting for AAV2 capsid proteins. Two C3 cleavage products of ∼70 kDa and ∼40 kDa corresponding to the C3 β chains (75 and 67 kDa) and the α2 chain (40 kDa), respectively, coimmunoprecipitated with AAV2-GFP (Fig. 5D), consistent with the deposition of iC3b (iC3b is generated when the α′ chain of C3b is cleaved) (Fig. 5C) on the AAV capsid. As expected, iC3b also coimmunoprecipitated with adenovirus and HSV-1. These data suggest that the complement component iC3b is associated with AAV in HS. Coimmunoprecipitated iC3b might bind to the Ig-AAV complex or alternatively bind to the AAV capsid directly. To determine whether C3 complement components bind directly to the AAV capsid, AAV was incubated with C3, C3b, or C3b incubated with factor H and factor I (to cleave C3b to iC3b) in the absence of serum. Viral-protein mixes were then immunoprecipitated using a polyclonal anti-C3 antibody and analyzed by Western blotting (Fig. 5E). AAV was coimmunoprecipitated with C3, C3b, and iC3b, as shown by immunoblotting for AAV capsid proteins (Fig. 5E). No AAV was immunoprecipitated in the absence of C3 components, confirming that antibodies or beads alone do not bind to the virus nonspecifically. Interestingly, the complement regulatory factor H also coimmunoprecipitated with the AAV-complement complex, suggesting that iC3b formation occurs on the AAV capsid. These results show that C3-AAV capsid interactions are direct and can occur independently of anti-AAV antibodies.
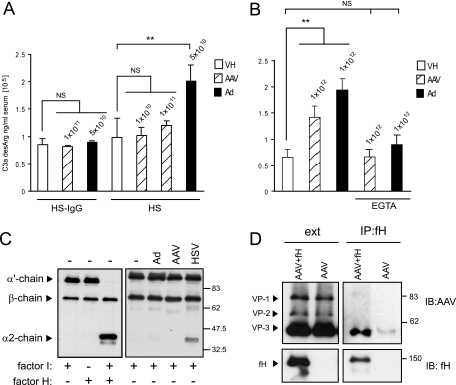
Studies were next performed to determine the role of AAV on complement activation. During the early phase of complement activation, the anaphylotoxin C3a is generated as a cleavage product of C3. C3a is unstable and quickly converted into C3a-desArg, which can be measured by ELISA. To determine if AAV vectors could directly activate the complement cascade, HS samples were challenged with 1 × 1010 or 1 × 1011 part/ml AAV2-GFP at 37°C for 90 min and the generation of C3a-desArg was determined by ELISA (Fig. 6A). Complement activation can occur by antigen-antibody complexes (classical pathway) or directly after serum contact with the pathogen surface (alternative pathway). Since HS contains neutralizing AAV antibodies (Fig. 5A), AAV-induced complement activation was also analyzed in the absence of antibodies by use of HS-IgG. Adenovirus vectors have been shown to activate complement (11, 31, 35); therefore, Ad-GFP in a concentration of 5 × 1010 part/ml was included as a positive control and vehicle served as a negative control. In HS-IgG, AAV and adenovirus vectors did not generate a significant increase of C3a above baseline (Fig. 6A). In pooled HS, 5 × 1010 part/ml of adenovirus vectors increased C3a significantly over baseline (2.01 × 105 ± 0.30 × 105 ng/ml versus 0.98 × 105 ± 0.34 × 105 ng/ml) (P < 0.01). In contrast, 1 × 1011 part/ml of AAV did not generate a significant increase of C3a above baseline (1.21 × 105 ± 0.08 × 105 ng/ml versus 0.98 × 105 ± 0.34 × 105 ng/ml) (P > 0.05) (Fig. 6A). To test whether the lack of AAV-induced complement activation could be circumvented by using higher doses, 10-fold-higher titers (1 × 1012 part/ml) of AAV and adenovirus vectors were used and the generation of C3a was analyzed by ELISA (Fig. 6B). At these very high titers, AAV activated complement significantly over baseline (P < 0.001) but less than adenovirus (Fig. 6B). Furthermore, consistent with the results observed for IgG-depleted serum, the response was blocked by EGTA, confirming that any complement activation by AAV is primarily antibody dependent (classical pathway).
FIG. 6.
AAV-induced complement activation. (A) Complement activation in HS after stimulation with adenovirus or AAV vectors in the presence or absence of neutralizing antibodies. Values above bars represent the concentrations of vector particles per milliliter of HS. Vehicle (VH) or vector was incubated with HS at the indicated concentration for 90 min at 37°C. C3a-desArg levels were determined by ELISA. EGTA, HS pretreated with 1/10 volume of 0.1 M EGTA to inhibit the classical pathway of complement activation. Values represent mean C3a concentrations ± standard deviations (n = 3). In IgG-depleted or EGTA-treated HS, AAV and adenovirus vectors did not generate a significant increase of C3a above baseline (NS, P > 0.05). In pooled HS, adenovirus vectors increased C3a significantly over baseline (**, P < 0.01). At 1 × 1011 part/ml, AAV did not generate a significant increase of C3a above baseline (NS, P > 0.05). (B) Complement activation in HS after stimulation with high titers of adenovirus or AAV vectors. At 1 × 1012 part/ml, AAV induced a measurable increase of C3a over baseline but less than the equivalent titer of adenovirus. (C) Analysis of factor I cofactor activity of AAV for C3b. C3b (200 ng) was incubated with 1 μg of factor I and AAV (4 × 1010 particles), adenovirus vectors (4 × 1010 particles), or HSV-1 (4 × 108 particles) for 1 h. C3b cleavage was analyzed by Western blotting. Reaction mixes without virus vectors and with or without fI and factor H (fH) were included as controls. Cofactor activity is demonstrated by the appearance of the α′ chain cleavage fragments at 67 and 40 kDa. HSV-1, but not AAV or adenovirus, has cofactor activity. (D) Coimmunoprecipitation of AAV and human fH using anti-human fH antibody. Starting material (ext) and immunoprecipitates (IP) were immunoblotted (IB) for AAV capsid proteins and fH. NS, not significant; **, P < 0.01.
The cleavage of plasma C3 occurs spontaneously via the alternative or the classical complement pathway to generate C3b, which continuously deposits on all surfaces in contact with blood. In this process, C3b does not have the ability to discriminate between self and nonself; whether amplification or inactivation occurs depends on the nature of the surface to which the C3b is attached (61). To avoid excess complement activation and to protect the host, the complement system is tightly controlled by complement regulators, which are present in the fluid phase and on cell membranes (40). The host factor I protects host cells from complement lysis by cleaving C3b into iC3b in the presence of a regulatory cofactor molecule. Many pathogens have the ability to bind complement regulators or have cofactor abilities themselves in order to evade complement activation (20, 39). Therefore, to further analyze how AAV-associated iC3b was generated, a C3b fluid-phase cofactor assay was performed. In this assay C3b was incubated with factor I and AAV2-GFP or controls in a physiological ionic strength buffer. The reaction mix was separated by SDS-PAGE, and cleavage of the α′ chain of C3b was monitored by immunoblotting for the appearance of the ∼40-kDa cleavage product. Figure 6C shows a comparison of cofactor activities of AAV, adenovirus, HSV-1, and controls. C3b is composed of an α′ chain (107 kDa) and a β chain (75 kDa), and as expected, the α′ chain was cleaved at two sites to generate fragments of 67 and 40 kDa only in the presence of both serum factor I and factor H. Interestingly, like factor H, HSV-1 exhibited cofactor activity for factor I-dependent cleavage of C3b to iC3b, as evident by the appearance of the ∼40-kDa cleavage product. Adenovirus and AAV vectors did not display direct cofactor activity, suggesting that in contrast to HSV-1, the host complement regulatory factor H was likely required to cleave C3b associated with AAV and adenovirus capsids.
To further evaluate the role of factor H in the formation of AAV-associated iC3b, factor H-AAV coimmunoprecipitation was performed. AAV and factor H were incubated following immunoprecipitation using a polyclonal anti-factor H antibody. AAV coimmunoprecipitated with factor H as indicated by immunoblotting for AAV capsid proteins (Fig. 6D). Beads and anti-factor H antibodies alone failed to immunoprecipitate AAV, confirming that the interaction was specific. Taken together, these results show that AAV-bound iC3b is generated by factor H on the AAV capsid.
Complement is required for the host humoral response to AAV vectors.
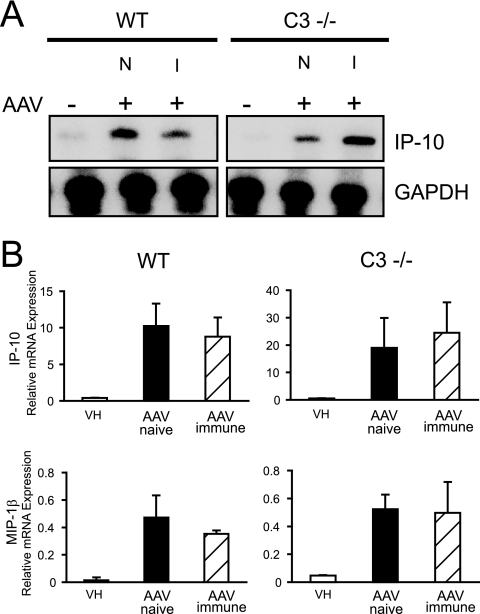
To determine the biological significance of complement-AAV interactions in vivo, experiments with mice deficient in the complement receptors 1/2 (CR1/2−/−) and the complement component C3 (C3−/−) were next performed. AAV vectors induce a mild innate immune response and a prominent adaptive response in the form of neutralizing antibodies to the virus capsid (9, 52, 73). First, innate responses to AAV vectors were determined. Wild-type, C3−/−, and CR2−/− mice were injected intravenously with 2.5 × 1011 particles of AAV2-GFP. Liver cytokine and chemokine expression was determined at 1 h. AAV induced the expression of multiple chemokines, as described previously (72). Surprisingly, no significant differences in early inflammatory response were observed in the different mouse strains, as shown for IP-10 and MIP-1β (Fig. 7A and B) (data not shown for CR2−/− mice). Since the in vitro results suggested that any AAV-induced complement activation might be antibody dependent, the innate immune response in mice preimmunized with AAV was also studied. Similarly to naïve animals, the innate immune response to AAV2-GFP was not enhanced but still occurred at 1 h in immunized wild-type mice. Similarly, innate immune responses in C3−/− and CR2−/− mice previously exposed to AAV were unchanged compared to responses in naïve and wild-type mice at 1 h (Fig. 7A and B) (data not shown for CR2−/− mice). Unlike the observations in vitro, these results imply that complement-AAV interactions may not be required for the innate immune response to AAV in vivo.
FIG. 7.
Innate immune response to AAV in C3−/− mice. RNase protection assay of mouse liver RNA from C3−/− mice or wild-type (WT) C57BL/6 mice 1 h following an intravenous injection of AAV2-GFP (2.5 × 1011 particles). Mice either were naïve (N) or had been immunized (I) 2 weeks earlier with AAV2-GFP. Quantification of IP-10 and MIP-1β expression is based on phosphorimaging of RNA blots and normalized against GAPDH. AAV-induced chemokine and cytokine mRNA expression in C3−/− mice is not significantly different from that in wild-type mice (means ± standard deviations). VH, vehicle.
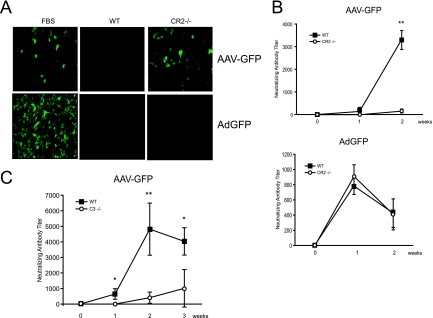
Next, to determine if complement and complement receptors played a role in the adaptive immune response to AAV, the production of AAV-specific neutralizing antibodies in wild-type, CR1/2−/−, and C3−/− mice following the intravenous administration of 2.5 × 1011 particles of AAV2-GFP was determined. Serum from immunized C57BL/6 wild-type mice at 7 days completely abrogated AAV2-GFP transduction of HEK 293 cells (Fig. 8A). In contrast, naïve mouse serum or serum from CR1/2−/− mice that received AAV2-GFP did not affect transgene expression in these cells. These results show that wild-type mice but not mice lacking the complement receptors CR1/2 developed neutralizing AAV2 antibodies. In contrast to the results observed with AAV2-GFP, serum from wild-type mice and CR1/2−/− mice immunized with Ad-GFP effectively inhibited adenovirus transduction in TE671 cells, suggesting that CR1/2 play little role in the humoral response to adenovirus (Fig. 8A). The kinetics of neutralizing antibody titers in wild-type and CR1/2−/− mice were also assessed. AAV-specific neutralizing antibodies were first detected at day 7 following AAV2-GFP injection of wild-type mice (mean titer of 1:136.2), rising to a mean titer of 1:3,423 by day 14 (Fig. 8B). CR1/2−/− mice demonstrated a 1-week delay in the onset of antibody development, and the titers were significantly lower (1:154 at day 14) than those of wild-type controls. In contrast, wild-type and CR1/2−/− mice immunized with adenovirus showed no difference in neutralizing antibody titers within 2 weeks of vector administration. Similar results were obtained with C3−/− mice (Fig. 8C). Following the administration of AAV2-GFP, C3−/− mice demonstrated a significant delay and reduction in neutralizing AAV antibody titers at 1 to 3 weeks compared to results for wild-type controls (mean titer of 1:4,818.5 for the wild type versus 1:408.7 for C3−/− mice at day 14; mean titer of 1:4,032.7 for the wild type versus 1:1,012.2 for C3−/− mice at day 21). These data show that complement-AAV interactions are biologically significant. Complement C3 and complement receptor 1/2 are essential for the host humoral but not the innate immune response to AAV in vivo.
FIG. 8.
Humoral immune response to AAV vectors in CR1/2−/− and C3−/− mice. (A) AAV2-GFP transduction of HEK 293 cells and Ad-GFP transduction of TE671 cells in the presence of tissue culture medium supplemented with 5% naïve mouse serum or 5% serum from AAV2-GFP-immunized C57BL/6 wild-type (WT) or CR1/2−/− mice. Serum from immunized WT but not CR1/2−/− mice abrogates AAV transgene expression in HEK 293 cells. Adenovirus transgene expression is abrogated by serum from both WT and CR1/2−/− mice. Representative images of at least three independent transductions are shown. (B) AAV and adenovirus neutralizing antibody titer development over time in WT and CR1/2−/− mice following AAV2-GFP administration. The antibody titer is displayed as the level that inhibited transgene expression 50% in tissue culture as determined by serial dilutions. Neutralizing antibody titers were significantly higher for AAV2-GFP-injected WT mice than for CR1/2−/− mice at 2 weeks (means ± standard deviations [SD]) (**, P < 0.01; n = 3). Ad-GFP-injected WT and CR1/2−/− animals displayed no significant difference in neutralizing antibody titer at 2 weeks. (C) AAV neutralizing antibody titer in WT and C3−/− mice receiving AAV2-GFP. Neutralizing antibody titers were significantly higher for WT mice than for C3−/− mice at 1, 2, and 3 weeks (means ± SD) (*, P < 0.05; **, P < 0.01; n = 3 to 5).
DISCUSSION
Inflammatory gene expression in vivo following AAV administration is significantly reduced in the absence of macrophages, suggesting that these cells are key effectors of the host immune response to AAV vectors (72). In this study we characterized further the relationship between macrophages and AAV vectors in vitro and in vivo. Our data show that AAV vector-macrophage interactions occur, resulting in cellular activation. Furthermore, our results demonstrate for the first time a significant role for complement in the host response to AAV vectors. Although unable to efficiently activate the complement cascade in comparison to adenovirus vectors, AAV vectors interact with the complement components C3, C3b, iC3b, and complement regulatory factor H. This is a significant event since a deficiency in the complement protein C3 or complement receptors CR1/2 impairs the humoral response to AAV.
Our studies showed that while AAV vectors could internalize into macrophages, AAV did not express transgene in primary macrophages or differentiated THP-1 cells in vitro. Impaired transduction by AAV vectors has also been reported to occur in dendritic cells and in hematopoietic progenitor cells, due to a failure of the virus to efficiently perform second-strand DNA synthesis (26, 32, 57, 75). In our studies, Southern blots of DNA from primary macrophages and differentiated THP-1 cells transduced with AAV vectors demonstrated double-stranded AAV genome species similar to that observed with nonhematopoietic cell types. Thus, the reduced transgene expression observed in hematopoietic lineages cannot simply be explained by the failure of double-stranded DNA conversion. It is likely that other factors specific to macrophages play a role in limiting the expression of viral delivered genes.
Macrophages were activated by AAV vectors to express NF-κB-dependent genes. AAV vector transduction of primary mouse bone marrow macrophages increased predominantly the RNA expression of MIP-2, a potent chemotactic factor for neutrophils, which is secreted during the early stages of innate immunity (14). Both AAV vector uptake and activation of inflammatory genes in macrophage lines and primary macrophages were enhanced in the presence of serum but returned to baseline when the serum was heat inactivated or depleted of complement component C3, demonstrating that AAV interacts with macrophages in an opsonin-dependent manner. This was confirmed in coimmunoprecipitation studies that demonstrated iC3b binding to AAV2 capsids in serum. The complement receptor most likely involved in AAV uptake and induction of inflammatory responses is currently unknown. We were unable to identify a specific complement receptor expressed on macrophages in blocking studies, suggesting that complement-mediated interactions involve redundant receptors (data not shown). Although CR3 (CD11b/CD18, or Mac-1) is the primary receptor for iC3b-opsonized particles and is expressed on naïve and phorbol-12-myristate-13-acetate-stimulated macrophages (59, 68), CR1/2, CR4, and CRIg can all bind iC3b-opsonized particles. The presence of redundant innate mechanisms was also exemplified by the intact inflammatory responses to AAV in the livers of CR1/2−/− and C3−/− mice. This result was unexpected yet not surprising. While both CR3 and CRIg receptors on differentiated macrophages contribute to the uptake of opsonized particles in vitro, CR3 does not seem to participate in the initial recognition of iC3b-coated pathogens in vivo (27). Instead, CRIg mediates the complement-dependent clearance of pathogens by Kupffer cells in the liver without inducing an inflammatory response (27, 68). Nevertheless, our results suggest that the initial inflammatory responses to AAV, although macrophage dependent, might be induced by complement-independent mechanisms in vivo. Interestingly, innate immune responses to AAV were also not abolished in immunized wild-type mice. While antibodies inhibit viral entry in epithelium-derived target cells, Ig-opsonized virus is targeted to and taken up by innate immune cells through Fc receptors. Fc receptor cross-linking or the targeting of opsonized virus particles to innate immune cells and the engagement of innate receptors may induce signal transduction events that can lead to inflammatory gene expression.
We also determined whether AAV and adenovirus vectors are able to activate the complement system in HS by analyzing the release of C3a peptides. Adenovirus induced significantly increased C3a levels at a particle titer of 5 × 1010 part/ml, whereas AAV failed to increase serum C3a above baseline, even at fivefold-higher particle concentrations. AAV-induced complement activation as determined with this assay was observed only at the highest dose tested (1 × 1012 part/ml), suggesting that AAV displays a somewhat blunted ability to activate complement compared to adenovirus. Both adenovirus- and AAV-induced activations of complement were observed only in the presence of Ig, suggesting that both vectors activate the classical but not the alternative pathway of complement in vitro. Cichon et al. determined the level of C3a generated in isolated citrate plasma of healthy individuals after challenge with recombinant and wild-type adenoviruses and showed a substantial, dose-dependent generation of C3a. The result also illustrated the strict antibody dependence of adenovirus-mediated complement activation in human plasma, consistent with our findings (11).
C3b generated either through spontaneous hydrolysis of C3 by the alternative pathway or by activation of the classical pathway is deposited at specific surfaces and has two possible fates. In the presence of a membrane-bound or soluble cofactor of the regulator of complement activation family (RCA), such as factor H, factor I is recruited and C3b is rapidly converted to iC3b, which abrogates further complement activation. In the absence of a cofactor, factor B is recruited, resulting in the formation of the C3 convertase and proceeding to complement activation (40). Immunoprecipitation studies suggested that the AAV vector capsid is associated with iC3b in HS. AAV, however, did not demonstrate direct cofactor activity. Since factor H coimmunoprecipitated with AAV-C3 complexes, our data suggest that iC3b is generated on the viral capsid. Many pathogens have evolved mechanisms to evade direct activation of the complement system by mimicking host surfaces, thereby recruiting factor H (53, 54). Others have cofactor activity themselves. For example, vaccinia virus-encoded complement control protein (VCP) binds to C3b and cleaves it by acting itself as a cofactor for factor I (46, 60). Our results show that HSV-1 also has cofactor activity, as evident by the cleavage of C3b in the absence of factor H. Direct binding of AAV capsid with factor H might explain the reduced ability of AAV to activate complement. Binding of factor H as an immune complement evasion mechanism is quite common in bacteria (1, 7, 30, 58) but has been shown for the first time for a virus only recently (10). West Nile virus nonstructural protein NS1 binds factor H, thereby inhibiting complement activation (10). Similarly to our results with AAV, West Nile virus-encoded NS1 has no direct cofactor activity.
Although less relevant to the innate response in vivo, complement played a substantial role in the development of AAV-specific humoral immunity. The antiviral humoral response to AAV in CR1/2−/− and C3−/− mice was delayed in its onset and resulted in titers of neutralizing antibodies significantly lower than those of wild-type controls. Complement enhances B-cell responses. Complement bound to viral antigen can induce cross-linking of the BCR with CR2 on the B-cell surface, which substantially lowers the threshold for B-cell activation (45). In contrast to AAV, neutralizing antibody titers to adenovirus vectors were not impaired in CR1/2−/− or C3−/− mice. Adenovirus vectors induce potent inflammatory responses in vivo, probably providing enough costimulation for B-cell activation even in the absence of complement. In contrast, the adjuvant effect of complement might be crucial for the initial B-cell activation by the less immunogenic AAV, which would explain the delay in antibody development to AAV in the absence of complement. A similar role of complement in the development of a humoral immune response to viral infections has been observed by others. For example, CR1/2−/− mice showed an impaired humoral response to infectious HSV-1 (69) and antibody responses to polyomavirus infection in CR1/2−/− mice showed 40 to 80% reduced antiviral IgG responses compared to those for wild-type mice (65). Equivalent to the results obtained in our studies, the addition of complement enhanced West Nile virus infection in macrophages and was necessary for the efficient induction of a protective antibody response in mice. The neutralizing antibody titer against West Nile virus reached 1:2,000 within 8 to 10 days, compared to only 1:200 for C3−/− mice. In CR1/2−/− mice, antiviral antibodies were not detectable within the first 10 days after infection (48).
In summary, our studies show that complement plays an important role in mediating the immune response to AAV. Increased insight into the mechanism of immune induction by AAV vectors may allow for strategies to minimize the humoral adaptive response to AAV in vivo.
Acknowledgments
We thank Elizabeth Long for her technical support.
This research was funded by operating and group grants from the Canadian Institutes of Health Research (CIHR). A.K.Z. is the recipient of a doctoral research award from the Heart and Stroke Foundation of Canada (HSFC). M.J.C. was supported by an Alberta Cancer Board postdoctoral fellowship. D.A.M. is the recipient of an Alberta Heritage Foundation for Medical Research (AHFMR) scholar award.
Footnotes
Published ahead of print on 16 January 2008.
REFERENCES
- 1.Alitalo, A., T. Meri, T. Chen, H. Lankinen, Z. Z. Cheng, T. S. Jokiranta, I. J. Seppala, P. Lahdenne, P. S. Hefty, D. R. Akins, and S. Meri. 2004. Lysine-dependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface protein E to the C terminus of factor H. J. Immunol. 1726195-6201. [DOI] [PubMed] [Google Scholar]
- 2.Becker, T. C., R. J. Noel, W. S. Coats, A. M. Gomez-Foix, T. Alam, R. D. Gerard, and C. B. Newgard. 1994. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 43161-189. [DOI] [PubMed] [Google Scholar]
- 3.Cabanas, C., and F. Sanchez-Madrid. 1999. CD11c (leukocyte integrin CR4 alpha subunit). J. Biol. Regul. Homeost. Agents 13134-136. [PubMed] [Google Scholar]
- 4.Carroll, M. C. 2004. The complement system in B cell regulation. Mol. Immunol. 41141-146. [DOI] [PubMed] [Google Scholar]
- 5.Carroll, M. C. 2004. The complement system in regulation of adaptive immunity. Nat. Immunol. 5981-986. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Z., S. B. Koralov, and G. Kelsoe. 2000. Regulation of humoral immune responses by CD21/CD35. Immunol. Rev. 176194-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.China, B., M. P. Sory, B. T. N′Guyen, M. De Bruyere, and G. R. Cornelis. 1993. Role of the YadA protein in prevention of opsonization of Yersinia enterocolitica by C3b molecules. Infect. Immun. 613129-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 61574-1583. [DOI] [PubMed] [Google Scholar]
- 9.Chirmule, N., W. Xiao, A. Truneh, M. A. Schnell, J. V. Hughes, P. Zoltick, and J. M. Wilson. 2000. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J. Virol. 742420-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung, K. M., M. K. Liszewski, G. Nybakken, A. E. Davis, R. R. Townsend, D. H. Fremont, J. P. Atkinson, and M. S. Diamond. 2006. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc. Natl. Acad. Sci. USA 10319111-19116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cichon, G., S. Boeckh-Herwig, H. H. Schmidt, E. Wehnes, T. Muller, P. Pring-Akerblom, and R. Burger. 2001. Complement activation by recombinant adenoviruses. Gene Ther. 81794-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark, K. R., X. Liu, J. P. McGrath, and P. R. Johnson. 1999. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum. Gene Ther. 101031-1039. [DOI] [PubMed] [Google Scholar]
- 13.Cottard, V., C. Valvason, G. Falgarone, D. Lutomski, M. C. Boissier, and N. Bessis. 2004. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J. Clin. Immunol. 24162-169. [DOI] [PubMed] [Google Scholar]
- 14.Driscoll, K. E. 2000. TNFalpha and MIP-2: role in particle-induced inflammation and regulation by oxidative stress. Toxicol. Lett. 112-113177-183. [DOI] [PubMed] [Google Scholar]
- 15.During, M. J., C. W. Symes, P. A. Lawlor, J. Lin, J. Dunning, H. L. Fitzsimons, D. Poulsen, P. Leone, R. Xu, B. L. Dicker, J. Lipski, and D. Young. 2000. An oral vaccine against NMDAR1 with efficacy in experimental stroke and epilepsy. Science 2871453-1460. [DOI] [PubMed] [Google Scholar]
- 16.Ehlers, M. R. 2000. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2289-294. [DOI] [PubMed] [Google Scholar]
- 17.Erles, K., P. Sebokova, and J. R. Schlehofer. 1999. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J. Med. Virol. 59406-411. [DOI] [PubMed] [Google Scholar]
- 18.Fagiolo, U., F. Kricek, C. Ruf, A. Peserico, A. Amadori, and M. Cancian. 2000. Effects of complement inactivation and IgG depletion on skin reactivity to autologous serum in chronic idiopathic urticaria. J. Allergy Clin. Immunol. 106567-572. [DOI] [PubMed] [Google Scholar]
- 19.Fang, Y., C. Xu, Y. X. Fu, V. M. Holers, and H. Molina. 1998. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J. Immunol. 1605273-5279. [PubMed] [Google Scholar]
- 20.Favoreel, H. W., G. R. Van de Walle, H. J. Nauwynck, and M. B. Pensaert. 2003. Virus complement evasion strategies. J. Gen. Virol. 841-15. [DOI] [PubMed] [Google Scholar]
- 21.Favre, D., V. Blouin, N. Provost, R. Spisek, F. Porrot, D. Bohl, F. Marme, Y. Cherel, A. Salvetti, B. Hurtrel, J. M. Heard, Y. Riviere, and P. Moullier. 2002. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J. Virol. 7611605-11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fields, P. A., D. W. Kowalczyk, V. R. Arruda, E. Armstrong, M. L. McCleland, J. N. Hagstrom, K. J. Pasi, H. C. Ertl, R. W. Herzog, and K. A. High. 2000. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol. Ther. 1225-235. [DOI] [PubMed] [Google Scholar]
- 23.Fischer, M. B., S. Goerg, L. Shen, A. P. Prodeus, C. C. Goodnow, G. Kelsoe, and M. C. Carroll. 1998. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science 280582-585. [DOI] [PubMed] [Google Scholar]
- 24.Frank, M. M., and L. F. Fries. 1991. The role of complement in inflammation and phagocytosis. Immunol. Today 12322-326. [DOI] [PubMed] [Google Scholar]
- 25.Giannakis, E., T. S. Jokiranta, R. J. Ormsby, T. G. Duthy, D. A. Male, D. Christiansen, V. A. Fischetti, C. Bagley, B. E. Loveland, and D. L. Gordon. 2002. Identification of the streptococcal M protein binding site on membrane cofactor protein (CD46). J. Immunol. 1684585-4592. [DOI] [PubMed] [Google Scholar]
- 26.Hansen, J., K. Qing, H. J. Kwon, C. Mah, and A. Srivastava. 2000. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J. Virol. 74992-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helmy, K. Y., K. J. Katschke, Jr., N. N. Gorgani, N. M. Kljavin, J. M. Elliott, L. Diehl, S. J. Scales, N. Ghilardi, and M. van Lookeren Campagne. 2006. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124915-927. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez, Y. J., J. Wang, W. G. Kearns, S. Loiler, A. Poirier, and T. R. Flotte. 1999. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J. Virol. 738549-8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herzog, R. W., E. Y. Yang, L. B. Couto, J. N. Hagstrom, D. Elwell, P. A. Fields, M. Burton, D. A. Bellinger, M. S. Read, K. M. Brinkhous, G. M. Podsakoff, T. C. Nichols, G. J. Kurtzman, and K. A. High. 1999. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat. Med. 556-63. [DOI] [PubMed] [Google Scholar]
- 30.Horstmann, R. D., H. J. Sievertsen, J. Knobloch, and V. A. Fischetti. 1988. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. USA 851657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang, H., Z. Wang, D. Serra, M. M. Frank, and A. Amalfitano. 2004. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol. Ther. 101140-1142. [DOI] [PubMed] [Google Scholar]
- 32.Jooss, K., Y. Yang, K. J. Fisher, and J. M. Wilson. 1998. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J. Virol. 724212-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemper, C., and J. P. Atkinson. 2007. T-cell regulation: with complements from innate immunity. Nat. Rev. Immunol. 79-18. [DOI] [PubMed] [Google Scholar]
- 34.Kessler, P. D., G. M. Podsakoff, X. Chen, S. A. McQuiston, P. C. Colosi, L. A. Matelis, G. J. Kurtzman, and B. J. Byrne. 1996. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl. Acad. Sci. USA 9314082-14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiang, A., Z. C. Hartman, R. S. Everett, D. Serra, H. Jiang, M. M. Frank, and A. Amalfitano. 2006. Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol. Ther. 14588-598. [DOI] [PubMed] [Google Scholar]
- 36.Kopf, M., B. Abel, A. Gallimore, M. Carroll, and M. F. Bachmann. 2002. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 8373-378. [DOI] [PubMed] [Google Scholar]
- 37.Kotwal, G. J., S. N. Isaacs, R. McKenzie, M. M. Frank, and B. Moss. 1990. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250827-830. [DOI] [PubMed] [Google Scholar]
- 38.Krych-Goldberg, M., and J. P. Atkinson. 2001. Structure-function relationships of complement receptor type 1. Immunol. Rev. 180112-122. [DOI] [PubMed] [Google Scholar]
- 39.Lee, S. H., J. U. Jung, and R. E. Means. 2003. ‘Complementing’ viral infection: mechanisms for evading innate immunity. Trends Microbiol. 11449-452. [DOI] [PubMed] [Google Scholar]
- 40.Lindahl, G., U. Sjobring, and E. Johnsson. 2000. Human complement regulators: a major target for pathogenic microorganisms. Curr. Opin. Immunol. 1244-51. [DOI] [PubMed] [Google Scholar]
- 41.Liu, D. W., Y. P. Tsao, J. T. Kung, Y. A. Ding, H. K. Sytwu, X. Xiao, and S. L. Chen. 2000. Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 peptide DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer. J. Virol. 742888-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutz, H. U., and E. Jelezarova. 2006. Complement amplification revisited. Mol. Immunol. 432-12. [DOI] [PubMed] [Google Scholar]
- 43.Manning, W. C., X. Paliard, S. Zhou, M. P. Bland, A. Y. Lee, K. Hong, C. M. Walker, J. A. Escobedo, and V. Dwarki. 1997. Genetic immunization with adeno-associated virus vectors expressing herpes simplex virus type 2 glycoproteins B and D. J. Virol. 717960-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manno, C. S., G. F. Pierce, V. R. Arruda, B. Glader, M. Ragni, J. J. Rasko, M. C. Ozelo, K. Hoots, P. Blatt, B. Konkle, M. Dake, R. Kaye, M. Razavi, A. Zajko, J. Zehnder, P. K. Rustagi, H. Nakai, A. Chew, D. Leonard, J. F. Wright, R. R. Lessard, J. M. Sommer, M. Tigges, D. Sabatino, A. Luk, H. Jiang, F. Mingozzi, L. Couto, H. C. Ertl, K. A. High, and M. A. Kay. 2006. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 12342-347. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto, A. K., J. Kopicky-Burd, R. H. Carter, D. A. Tuveson, T. F. Tedder, and D. T. Fearon. 1991. Intersection of the complement and immune systems: a signal transduction complex of the B lymphocyte-containing complement receptor type 2 and CD19. J. Exp. Med. 17355-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKenzie, R., G. J. Kotwal, B. Moss, C. H. Hammer, and M. M. Frank. 1992. Regulation of complement activity by vaccinia virus complement-control protein. J. Infect. Dis. 1661245-1250. [DOI] [PubMed] [Google Scholar]
- 47.McNearney, T. A., C. Odell, V. M. Holers, P. G. Spear, and J. P. Atkinson. 1987. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J. Exp. Med. 1661525-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehlhop, E., K. Whitby, T. Oliphant, A. Marri, M. Engle, and M. S. Diamond. 2005. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J. Virol. 797466-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mingozzi, F., M. V. Maus, D. J. Hui, D. E. Sabatino, S. L. Murphy, J. E. Rasko, M. V. Ragni, C. S. Manno, J. Sommer, H. Jiang, G. F. Pierce, H. C. Ertl, and K. A. High. 2007. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 13419-422. [DOI] [PubMed] [Google Scholar]
- 50.Molina, H., V. M. Holers, B. Li, Y. Fung, S. Mariathasan, J. Goellner, J. Strauss-Schoenberger, R. W. Karr, and D. D. Chaplin. 1996. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc. Natl. Acad. Sci. USA 933357-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molina, H., T. Kinoshita, C. B. Webster, and V. M. Holers. 1994. Analysis of C3b/C3d binding sites and factor I cofactor regions within mouse complement receptors 1 and 2. J. Immunol. 153789-795. [PubMed] [Google Scholar]
- 52.Moskalenko, M., L. Chen, M. van Roey, B. A. Donahue, R. O. Snyder, J. G. McArthur, and S. D. Patel. 2000. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J. Virol. 741761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pangburn, M. K. 2000. Host recognition and target differentiation by factor H, a regulator of the alternative pathway of complement. Immunopharmacology 49149-157. [DOI] [PubMed] [Google Scholar]
- 54.Pangburn, M. K., K. L. Pangburn, V. Koistinen, S. Meri, and A. K. Sharma. 2000. Molecular mechanisms of target recognition in an innate immune system: interactions among factor H, C3b, and target in the alternative pathway of human complement. J. Immunol. 1644742-4751. [DOI] [PubMed] [Google Scholar]
- 55.Peden, C. S., C. Burger, N. Muzyczka, and R. J. Mandel. 2004. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J. Virol. 786344-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pramoonjago, P., J. Takeda, Y. U. Kim, K. Inoue, and T. Kinoshita. 1993. Ligand specificities of mouse complement receptor types 1 (CR1) and 2 (CR2) purified from spleen cells. Int. Immunol. 5337-343. [DOI] [PubMed] [Google Scholar]
- 57.Qing, K., J. Hansen, K. A. Weigel-Kelley, M. Tan, S. Zhou, and A. Srivastava. 2001. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J. Virol. 758968-8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ram, S., D. P. McQuillen, S. Gulati, C. Elkins, M. K. Pangburn, and P. A. Rice. 1998. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J. Exp. Med. 188671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross, G. D. 2000. Regulation of the adhesion versus cytotoxic functions of the Mac-1/CR3/alphaMbeta2-integrin glycoprotein. Crit. Rev. Immunol. 20197-222. [PubMed] [Google Scholar]
- 60.Sahu, A., S. N. Isaacs, A. M. Soulika, and J. D. Lambris. 1998. Interaction of vaccinia virus complement control protein with human complement proteins: factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway. J. Immunol. 1605596-5604. [PubMed] [Google Scholar]
- 61.Sahu, A., and J. D. Lambris. 2001. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol. Rev. 18035-48. [DOI] [PubMed] [Google Scholar]
- 62.Schneider, M. C., R. M. Exley, H. Chan, I. Feavers, Y. H. Kang, R. B. Sim, and C. M. Tang. 2006. Functional significance of factor H binding to Neisseria meningitidis. J. Immunol. 1767566-7575. [DOI] [PubMed] [Google Scholar]
- 63.Schwende, H., E. Fitzke, P. Ambs, and P. Dieter. 1996. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 59555-561. [PubMed] [Google Scholar]
- 64.Shayakhmetov, D. M., A. Gaggar, S. Ni, Z. Y. Li, and A. Lieber. 2005. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 797478-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szomolanyi-Tsuda, E., M. O. Seedhom, M. C. Carroll, and R. L. Garcea. 2006. T cell-independent and T cell-dependent immunoglobulin G responses to polyomavirus infection are impaired in complement receptor 2-deficient mice. Virology 35252-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tal-Singer, R., C. Seidel-Dugan, L. Fries, H. P. Huemer, R. J. Eisenberg, G. H. Cohen, and H. M. Friedman. 1991. Herpes simplex virus glycoprotein C is a receptor for complement component iC3b. J. Infect. Dis. 164750-753. [DOI] [PubMed] [Google Scholar]
- 67.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26171-176. [DOI] [PubMed] [Google Scholar]
- 68.van Lookeren Campagne, M., C. Wiesmann, and E. J. Brown. 2007. Macrophage complement receptors and pathogen clearance. Cell. Microbiol. 92095-2102. [DOI] [PubMed] [Google Scholar]
- 69.Verschoor, A., M. A. Brockman, M. Gadjeva, D. M. Knipe, and M. C. Carroll. 2003. Myeloid C3 determines induction of humoral responses to peripheral herpes simplex virus infection. J. Immunol. 1715363-5371. [DOI] [PubMed] [Google Scholar]
- 70.Wang, L., K. Takabe, S. M. Bidlingmaier, C. R. Ill, and I. M. Verma. 1999. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc. Natl. Acad. Sci. USA 963906-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuasa, K., M. Sakamoto, Y. Miyagoe-Suzuki, A. Tanouchi, H. Yamamoto, J. Li, J. S. Chamberlain, X. Xiao, and S. Takeda. 2002. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Ther. 91576-1588. [DOI] [PubMed] [Google Scholar]
- 72.Zaiss, A. K., Q. Liu, G. P. Bowen, N. C. Wong, J. S. Bartlett, and D. A. Muruve. 2002. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J. Virol. 764580-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zaiss, A. K., and D. A. Muruve. 2005. Immune responses to adeno-associated virus vectors. Curr. Gene Ther. 5323-331. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, Y., N. Chirmule, G. Gao, and J. Wilson. 2000. CD40 ligand-dependent activation of cytotoxic T lymphocytes by adeno-associated virus vectors in vivo: role of immature dendritic cells. J. Virol. 748003-8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhong, L., W. Li, Z. Yang, K. Qing, M. Tan, J. Hansen, Y. Li, L. Chen, R. J. Chan, D. Bischof, N. Maina, K. A. Weigel-Kelley, W. Zhao, S. H. Larsen, M. C. Yoder, W. Shou, and A. Srivastava. 2004. Impaired nuclear transport and uncoating limit recombinant adeno-associated virus 2 vector-mediated transduction of primary murine hematopoietic cells. Hum. Gene Ther. 151207-1218. [DOI] [PubMed] [Google Scholar]