Abstract
Thiazolidinediones (TZDs) have been used for the treatment of hyperglycaemia in type 2 diabetes for the past 10 years. They may delay the development of type 2 diabetes in individuals at high risk of developing the condition, and have been shown to have potentially beneficial effects on cardiovascular risk factors. TZDs act as agonists of peroxisome proliferator-activated receptor-γ (PPAR-γ) primarily in adipose tissue. PPAR-γ receptor activation by TZDs improves insulin sensitivity by promoting fatty acid uptake into adipose tissue, increasing production of adiponectin and reducing levels of inflammatory mediators such as tumour necrosis factor-alpha (TNF-α), plasminogen activator inhibitor-1(PAI-1) and interleukin-6 (IL-6). Clinically, TZDs have been shown to reduce measures of atherosclerosis such as carotid intima-media thickness (CIMT). However, in spite of beneficial effects on markers of cardiovascular risk, TZDs have not been definitively shown to reduce cardiovascular events in patients, and the safety of rosiglitazone in this respect has recently been called into question. Dual PPAR-α/γ agonists may offer superior treatment of insulin resistance and cardioprotection, but their safety has not yet been assured.
Keywords: thiazolidinedione, peroxisome proliferator-activated receptor-γ, insulin resistance, glucose, adipose, adiponectin, cardiovascular, inflammation, atherosclerosis
Introduction
Type II diabetes is an increasing problem in the developed world. Most costs associated with the condition relate to hospital-based care to treat microvascular and macrovascular complications. Patients with diabetes are known to be at increased risk of coronary artery disease, myocardial infarction and stroke (Huang, 2005), that is increased two- to four-fold compared with non-diabetic subjects (Dormandy et al., 2005). Intensive glucose control is the accepted standard for management of patients with type II diabetes (Kendall, 2006). The UK Prospective Diabetes Study showed that intensive glycaemic control can significantly reduce the complications of diabetes, principally microvascular complications especially retinopathy (UKPDS, 1998). To significantly reduce macrovascular complications, we require comprehensive risk factor management that includes treatment of hypertension, hyperlipidaemia and hypercoagulability in addition to optimizing blood glucose control.
Thiazolidinediones (TZDs) have been used for the treatment of hyperglycaemia in type II diabetes since 1997. Troglitazone was the first of this class of drugs to be introduced into clinical practice, but was withdrawn due to liver toxicity. Currently, pioglitazone and rosiglitazone are the only compounds licensed for patients with type II diabetes. TZDs can be used as monotherapy or in combination with other glucose-lowering agents. Pioglitazone is licensed for use in combination with insulin in patients for whom metformin is inappropriate due to contraindications or intolerance. The recent PROspective PioglitAzone Clinical Trial in macroVascular Events (PROactive) study provided tentative evidence that pioglitazone may have a beneficial effect on cardiovascular risk in type II diabetics, an effect independent of its glucose-lowering properties (Dormandy et al., 2005). TZDs may also have a role retarding the development of type II diabetes in individuals at high risk of developing the condition. They have been shown to have potentially beneficial effects on traditional and some novel cardiovascular risk factors (The DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators, 2006). TZDs act as agonists of peroxisome proliferator-activated receptor-γ (PPAR-γ). This review explores the potential value of PPAR-γ agonists in medicine with particular emphasis on their effects on insulin resistance and the cardiovascular system.
Pathogenesis of insulin resistance and adipokines
The insulin receptor is a hetero-tetrameric transmembrane glycoprotein composed of two extracellular α-subunits and two transmembrane β-subunits (Becker and Roth, 1990). Insulin binds to the α-subunit and the receptor undergoes autophosphorylation which is catalysed by the tyrosine-specific protein kinase in the β-subunit (Denton et al., 1981). Phosphorylation of tyrosine residues occurs and leads to phosphorylation of insulin receptor substrates (IRSs), which are released to a new site and interact with phosphatidylinositol 3-kinase (Lam et al., 1994). This connects with glucose transporter-4 (GLUT-4), which has a direct effect on peripheral tissue glucose uptake (Watson and Pessin, 2001).
IRS phosphorylation, phosphatidylinositol 3-kinase activity and GLUT-4 activity have been shown to be impaired in patients with insulin resistance (Krook et al., 2004). Elevated free fatty acids (FFAs) are associated with this condition and it has been postulated that there is a competition between FFA and glucose for mitochondrial oxidation as part of the glucose–fatty acid cycle. Increased free fatty acid oxidation causes elevation of the intramitochondrial acetyl-CoA/CoA and NADH/NAD+ ratios with subsequent inactivation of pyruvate dehydrogenase. Citrate concentrations increase leading to inhibition of phosphofructokinase and accumulation of glucose-6-phosphate which in turn inhibits hexokinase II and results in reduced glucose uptake (Randle et al., 1963). However, an alternative theory states that FFAs inhibit glucose transport/phosphorylation, leading to a reduction in glucose oxidation and muscle glycogen synthesis (Roden et al., 1996). Metabolites of FFA such as diacylglycerol, fatty acyl Co-A and ceramide may also cause defects in the insulin signalling pathway (Shulman, 2000), which leads to impairment of IRS-1 tyrosine phosphorylation and a decrease in glucose transport.
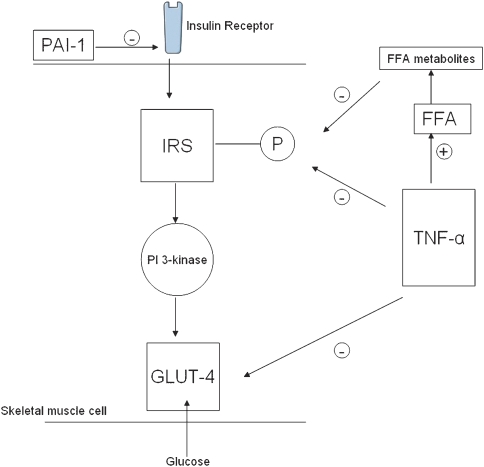
In obese patients, white adipose tissue displays low-grade inflammation characterized by infiltration of macrophages, which produce proinflammatory cytokines (Weisberg et al., 2003). There is overproduction of cytokines such as tumour necrosis factor-α (TNF-α) and interleukin-6, which are thought to have a detrimental effect on the insulin signalling pathway and therefore alter insulin sensitivity (Bastard et al., 2006). TNF-α exerts its effects on insulin signalling in three ways: firstly, it reduces tyrosine phosphorylation by increasing serine or threonine phosphorylation of the insulin receptor (Hotamisligil et al., 1994). Secondly, it downregulates GLUT-4 and the enzyme responsible for insulin signal transduction (Stephens et al., 1997). It has also been shown that TNF-α increases ceramide, a lipid which downregulates GLUT-4 gene transcription in adipocytes (Long and Pekala, 1996). Thirdly, TNF-α increases release of free fatty acids by stimulation of lipolysis. This process is dependent on the downregulation of the lipid droplet-associated protein perilipin. Perilipin is thought to prevent the accession of hormone-sensitive lipase to the surface of the fat droplet where lipid degradation takes place. The elevated level of FFA leads to insulin resistance as described above (Guo and Tabrizchi, 2006). Other adipocyte-derived proteins such as resistin and leptin may also affect the pathway, as does plasminogen activator inhibitor-1 (PAI-1) which interrupts insulin signalling by preventing the interaction of integrins with the insulin receptor (Lopez-Alemany et al., 2003). In addition, high plasma PAI-1 levels have been considered a risk factor for coronary heart disease in diabetic patients and are associated with morbidity and mortality in diabetes (Alessi and Juhan-Vague, 2004; Guo and Tabrizchi, 2006; Figure 1).
Figure 1.
Fatty acid and cytokine-induced insulin resistance in skeletal muscle. Fatty acid metabolites inhibit phosphorylation of IRS, which leads to reduced uptake of glucose via GLUT-4. TNF-α also interrupts insulin signalling by inhibiting this phosphorylation, along with downregulating GLUT-4 and increasing FFA release. PAI-1 inhibits the interaction of integrins with the insulin receptor. IRS, insulin receptor substrate; GLUT-4, glucose transporter-4; PI3K, phosphatidylinositol 3-kinase; TNF-α, tumour necrosis factor; PAI-1, plasminogen activator inhibitor-1; FFA, free fatty acid.
In contrast to other adipocyte-derived proteins, adiponectin (also known as Acrp30) is an adipokine, which is thought to have antidiabetic and antiatherogenic properties, although further study of this molecule in humans is required to confirm this theory. Adiponectin is secreted by both white and brown adipose tissue and is structurally similar to complement with a C-terminal globular domain and an N-terminal collagen domain. It exists in a wide range of multimer complexes in plasma and combines via its collagen domain to create three major oligomeric forms: a low-molecular-weight trimer, a middle-molecular-weight hexamer and a high-molecular-weight 12- to 18-mer adiponectin (Kadowaki et al., 2006). High-molecular-weight adiponectin is the most active form of the protein and is most relevant to insulin sensitivity. In contrast to PAI-1 and TNF-α, levels of adiponectin have been found to be reduced in obesity and insulin resistance. Low levels are also independently associated with coronary artery disease and may predict development of atherosclerosis in both diabetic and non-diabetic patients (Kumada et al., 2003). It is thought that adiponectin enhances hepatic insulin action and promotes fatty acid oxidation in skeletal muscle, thus reducing glucose levels, although much of the evidence for this is derived from animal studies (Berg et al., 2001; Tomas et al., 2002). However, one group has investigated the effect of the globular head of adiponectin (gAcrp30) on human skeletal muscle and found evidence that gAcrp30 plays a role in regulating fatty acid and glucose metabolism in this tissue. Treatment of lean muscle with this protein increased fatty acid oxidation by 70%, and in obese muscle by 30% (Bruce et al., 2005). gAcrp30 increased glucose uptake by 37% in lean muscle and 33% in obese muscle. Combined exposure of insulin and gAcrp30 demonstrated an additive effect on glucose uptake in lean and obese individuals, but this effect was reduced by 50% in obese muscle. These effects are thought to be due to an increase in AMP-activated protein kinase-α1 and AMP-activated protein kinase-α2 activity via specific receptor signalling. However, in obese muscle, the activation of AMP-activated protein kinase-α2 by gAcrp30 was blunted indicating the possible development of adiponectin resistance. Adiponectin may also counteract the proinflammatory effects of TNF-α on the arterial wall and hence protect against atherosclerosis (Bastard et al., 2006). Adiponectin stimulates nitric oxide (NO) production in endothelial cells and protects endothelial cells from apoptosis which may further contribute to its antiatherogenic effects (Chen et al., 2003). In view of these actions, a therapeutic strategy for the treatment of insulin resistance, type II diabetes, the metabolic syndrome and cardiovascular disease may include the upregulation of adiponectin levels, the upregulation of adiponectin receptors or the development of adiponectin receptor agonists (Kadowaki et al., 2006).
Atherosclerosis
Inflammation has a pathogenic role in both insulin resistance and atherosclerosis and a significant inverse relationship between insulin sensitivity and coronary heart disease has been described (Rewers et al., 2004). This relationship is independent of insulin levels and other risk factors for cardiovascular disease. Development of atheroma is thought to be due to inflammatory mechanisms (Ross, 1999). Atherogenesis begins with endothelial cell activation and accumulation of low-density lipoprotein (LDL) cholesterol in the subendothelium (Berliner et al., 1995). Oxidation of LDL cholesterol promotes release of monocyte chemotactic protein-1 that attracts and activates circulating monocytes and T cells. Vascular cell adhesion molecule-1 and intracellular adhesion molecule-1 facilitate the adhesion of the inflammatory cells to the vascular wall (Ross, 1999). Monocytes differentiate into macrophages and ingest cholesterol from the oxidised LDL to form foam cells, initiating the process of atheromatous plaque formation and growth (Rios-Vazquez et al., 2006). Vascular smooth muscle cells migrate to this region and produce growth factors. Collagen and elastin form a fibrous cap over the lipid core (Weissberg, 2000). Metalloproteinases secreted by macrophages can degrade the collagen of the fibrous cap, predisposing to plaque rupture with the clinical outcome of a myocardial infarction or unstable angina (Fuster et al., 1992).
Reduced levels of NO and subsequent endothelial dysfunction are involved in the earliest stages of this process of atheroma formation. NO is produced by platelets and endothelial cells and reduces smooth muscle proliferation, leukocyte activation and platelet aggregation and adhesion, making it antiatherogenic (Radomski et al., 1991; Bath, 1993; Mooradian et al., 1995).
Insulin resistance is an obvious therapeutic target given its association with the development of type II diabetes and vascular complications. The advent of TZDs provided the first therapeutic agents whose predominant action is to enhance insulin sensitivity.
PPARs
PPARs are a subfamily of the superfamily of nuclear receptors that are closely related to the thyroid hormone and retinoid receptors (Rios-Vazquez et al., 2006). PPARs are ligand-activated transcription factors that regulate target gene expression. The PPAR heterodimerizes with retinoid X receptor and agonist binding leads to altered conformation of the PPAR. Recruitment of transcriptional co-activators occurs and the result is an increase in gene transcription (Berger and Moller, 2002). PPARs regulate the expression of many genes involved in lipid metabolism and play a key role in adipocyte differentiation (Tontonoz et al., 1994; Nedergaard et al., 2005). Three PPARs have been identified—PPAR-α, PPAR-β (or δ) and PPAR-γ. PPAR-γ is found most abundantly in adipose tissue, but also in pancreatic β-cells, vascular endothelium and macrophages (Dubois et al., 2000; Willson et al., 2001). It is also present in skeletal muscle (Norris et al., 2003). PPAR-γ receptor activation has an important role in the modulation of glucose metabolism.
PPAR-γ
Seven PPAR-γ mRNAs have been identified (Zhou et al., 2002). PPAR-γ-1 and -2 are expressed mostly in adipose tissue and large intestine. Lower levels are found in kidney, liver and small intestine (Fajas et al., 1997). PPAR-γ-3 is found in adipose tissue and large intestine, PPAR-γ-4 and -5 are expressed only in macrophages, whereas PPAR-γ-6 and -7 have been detected in adipose tissue (Fajas et al., 1998). The existence of these isoforms and the wide distribution of these receptors suggests a diversity of ligands and tissue-specific transcriptional responses (Desvergne and Wahli, 1999). The ligand-binding pocket of PPAR-γ is larger than that of other nuclear receptors, permitting affinity for a variety of ligands. In addition to the TZDs, which are potent exogenous agonists of PPAR-γ (Lehmann et al., 1995), these ligands include peroxisome proliferators such as nafenopin, clofibric acid and warfarin; mono- and poly-unsaturated fatty acids and arachidonic acid metabolites, which implies a role for the receptor in lipid metabolism (Kliewer et al., 1997); and certain non-steroidal anti-inflammatory drugs, for example ibuprofen (Lehmann et al., 1997). The angiotensin type 1 receptor antagonist telmisartan also acts as a partial agonist of PPAR-γ and may have a role in the treatment of insulin resistance and diabetes in the future (Kurtz, 2005).
Consequences of PPAR-γ receptor activation byTZDs
TZDs decrease insulin levels which suggests they act as insulin sensitisers and enhance glucose uptake by insulin-sensitive tissues. They are thought to exert all these effects by acting as selective ligands of the PPAR-γ receptors primarily in adipose tissue (Berger et al., 2005). The activated receptors work in a number of ways to achieve these effects. They alter the expression of genes involved in lipid metabolism and promote fatty acid uptake and storage in adipose tissue. Elevated FFA levels have been found to be associated with insulin resistance and, as described previously, there have been a number of theories as to how FFA accumulation can influence insulin resistance. PPAR-γ activation by TZDs has been shown to reduce the amount of circulating FFA in the body via adipocyte differentiation and apoptosis. The number of small adipocytes, which are able to accumulate FFA, increases at the expense of hypertrophied adipocytes that release FFA. The distribution of adipose tissue is changed from visceral sites to subcutaneous parts of the body. PPAR-γ activation has the effect of containing the fatty acids subcutaneously (Okuno et al., 1998). Fatty acid translocase, the enzyme which moves circulating FFA into adipocytes, is upregulated by TZDs which further facilitates this ‘fatty acid steal' (Teboul et al., 2001). As lipolysis and levels of circulating FFA are reduced by PPAR-γ activation adipose tissue mass is increased, so other insulin-sensitive tissues such as liver, skeletal muscle and possibly pancreatic β-cells are spared the harmful metabolic effects of high concentrations of free fatty acids that induce insulin resistance (Yki-Jarvinen, 2004). Glucose metabolism by liver and muscle is therefore improved. The ‘lipotoxicity' in pancreatic β-cells from elevated fat content is also reduced which leads to decreased β-cell apoptosis, improved β-cell mass and therefore insulin secretion in type II diabetic subjects (Bays et al., 2004).
The insulin-sensitizing effects of TZDs on muscle have been shown to be indirect. A study using mice with a muscle-specific deletion of PPAR-γ found development of excess adiposity and whole-body insulin resistance. Treatment with TZDs ameliorated these effects and altered expression of several lipid metabolism genes in the muscle of these mice. Therefore, muscle PPAR-γ is not required for the antidiabetic effects of TZDs but is needed for maintenance of normal adiposity, whole-body insulin sensitivity and hepatic insulin action, perhaps via altered lipid metabolism in muscle (Norris et al., 2003).
PPAR-γ activation by TZDs also occurs in macrophages and is thought to reduce macrophage numbers in adipose tissue. The expression of proinflammatory genes is inhibited, and production of the cytokines TNF-α, interleukin-6 and PAI-1 is reduced (Sharma and Staels, 2007). Expression of IRS-2, a protein with a facilitatory role in the insulin signalling pathway, is increased in adipose tissue cultured with PPAR-γ agonists (Smith et al., 2001) with subsequent enhancement of insulin sensitivity.
PPAR-γ activation also increases adiponectin production from adipose tissue which may be due to a direct effect of PPAR-γ on adiponectin transcription (Iwaki et al., 2003). Treatment with PPAR-γ agonists has resulted in significantly improved glycaemia in diabetic mice and was associated with an increase in circulating adiponectin levels (Berg et al., 2001; Bruce et al., 2005). As mentioned previously, adiponectin has been shown to increase fatty acid oxidation in human skeletal muscle via activation of AMP-activated protein kinase, with subsequent increased glucose uptake (Bruce et al., 2005). Animal and in vitro studies indicate that adiponectin may also protect against atherosclerosis by decreasing adhesion molecule expression on endothelial cells to inhibit foam cell formation and vascular smooth muscle cell proliferation. It inhibits TNF-α-induced adhesion molecule expression on endothelial cells, including vascular cell adhesion molecule-1, intracellular adhesion molecule-1 and E-selectin (Blaschke et al., 2006). In addition, adiponectin directly stimulates NO production in endothelial cells and protects these cells from apoptosis (Chen et al., 2003). Adiponectin may also be involved in plaque stability through increasing tissue inhibitors of metalloproteinases expression and secretion in human monocyte-derived macrophages (Kumada et al., 2004).
PPAR-γ agonists also influence the signalling pathways that promote atherosclerosis and cardiovascular events (Rios-Vazquez et al., 2006). PPAR-γ agonists inhibit the activation of nuclear factor-κB, a transcription factor that controls the expression of many genes involved in immune and inflammatory responses (Barnes and Karin, 1997). This has the effect of downregulating proinflammatory genes involved in the formation of the atheromatous plaque (Castrillo et al., 2000). Other nuclear and transcription factors involved in atherogenesis are also suppressed by PPAR-γ activation, such as CCAAT enhancer-binding protein-δ and activator protein 1 (Delerive et al., 1999; Takata et al., 2002). Vascular cell adhesion molecule-1 and intracellular adhesion molecule-1 expression is inhibited which reduces macrophage migration to atherosclerotic plaque, and PPAR-γ activation by troglitazone has been shown to inhibit vascular smooth muscle cell proliferation and migration (Law et al., 1996).
PPAR-γ activation results in improved endothelial-dependent vasodilatation via increased NO production from endothelial cells which has antithrombotic and antiatherogenic effects. Treatment with ciglitazone has been shown to significantly increase release of NO from pulmonary artery endothelial cells and human umbilical vein endothelial cells. This was thought to be due to a transcriptional mechanism independent of endothelial NO synthase expression (Calnek et al., 2003). PPAR-γ activation also inhibits endothelin-1, which is a vasoconstrictor peptide involved in vascular smooth muscle cell proliferation and is associated with coronary artery disease (Salomone et al., 1996), and inhibits platelet aggregation and adhesion by decreasing production of thromboxane A2 (Hamberg et al., 1975). These influences contribute further to improved endothelial function.
Urinary albumin creatinine ratio, another marker of cardiovascular risk, is reduced by treatment with rosiglitazone (Lebovitz et al., 2001). Mesangial cells in the kidney have been found to express PPAR-γ (Asano et al., 2000) and diabetic nephropathy is thought to be due to the alteration of mesangial cells from a quiescent phenotype to a proliferative myofibroblast-like phenotype, characterized by increased α-smooth muscle actin and proinflammatory cytokines, as well as enhanced production of extracellular matrix proteins. PPAR-γ ligands have been shown to suppress production of these substances and inhibit proliferation of mesangial cells (Guan and Breyer, 2001; Figure 2).
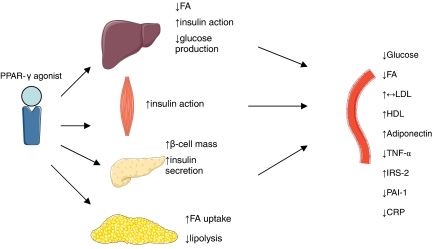
Figure 2.
Mechanism of action of TZDs. PPAR-γ, peroxisome proliferator-activated receptor-γ; FA, fatty acids; LDL, low density lipoprotein; HDL, high-density lipoprotein; TNF-α, tumour necrosis factor-α; IRS-2, insulin receptor substrate-2; PAI-1, plasminogen activator inhibitor-1; CRP, C-reactive protein; TZD, thiazolidinedione.
Clinical evaluation of TZDs
Clinical trials have shown that TZDs lower fasting and postprandial glucose and it has been demonstrated that pioglitazone and rosiglitazone at maximal doses can lower glycosylated haemoglobin by on average 1–1.5% (Yki-Jarvinen, 2004). A Diabetes Outcome Progression Trial (ADOPT) study evaluated the durability of glycaemic control in patients receiving monotherapy with rosiglitazone, metformin or glyburide over 4 years. It showed that treatment with rosiglitazone slowed progression to monotherapy failure more effectively than metformin or glyburide. Rosiglitazone was also shown to slow the rate of loss of β-cell function and improve insulin sensitivity to a greater extent than the other two drugs. However, rosiglitazone was associated with increased levels of LDL cholesterol and weight gain (Kahn et al., 2006).
The recent Diabetes REducation Assessment with ramipril and rosiglitazone Medication (DREAM) study shows rosiglitazone can reduce the incidence of diabetes in individuals with impaired glucose tolerance or impaired fasting glucose (The DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators, 2006). However, this trial showed no clear benefit on cardiovascular outcomes at 3 years—the rate of all cardiovascular events was higher in the rosiglitazone group, albeit nonsignificantly (P=0.08), and there was a significant increase in heart failure in the rosiglitazone group compared with placebo (Heneghan et al., 2006). The safety of rosiglitazone was questioned further in a recent meta-analysis which found that rosiglitazone was associated with a significant increase in myocardial infarction and an increased risk of death from cardiovascular causes that approached statistical significance (Nissen and Wolski, 2007). Forty-two studies of rosiglitazone vs placebo or other antihyperglycaemic agents of at least 24 weeks duration were included and, overall, rosiglitazone was associated with a statistically significant 43% increase in risk for myocardial infarction (odds ratio 1.43; 95% confidence interval 1.03–1.98; P=0.03) and a nonstatistically significant increased risk of death from cardiovascular causes (odds ratio 1.64; 95% confidence interval 0.98–2.74; P=0.06). The study was limited by the small size and short-term nature of some of the trials included, and the lack of availability of original source data that prevented the use of more statistically powerful time-to-event analysis. However, despite the shortcomings of this meta-analysis, concerns have been raised regarding the use of rosiglitazone in the treatment of type II diabetes, particularly as alternative agents are available. The ongoing Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of glycaemia in Diabetes (RECORD) study should help to answer these questions regarding the safety of rosiglitazone when results are available in 2009. Results of a recent unplanned interim analysis of this trial were inconclusive with respect to the effect of rosiglitazone on overall risk of hospitalization or death from cardiovascular causes (Home et al., 2007).
In contrast, the PROactive study, which monitored cardiovascular outcomes in type II diabetic subjects at high risk for cardiovascular events treated with pioglitazone, showed more encouraging results. In the primary composite end point of time to first occurrence of all-cause mortality, myocardial infarction, acute coronary syndrome, stroke, leg or coronary artery revascularization and amputation above the ankle, there was a nonsignificant 10% reduction in the subjects treated with pioglitazone. For the secondary composite end point of time to first occurrence of all-cause mortality, myocardial infarction or stroke, there was a statistically significant reduction of 16% in the pioglitazone group compared to placebo. There was a 3% increase in the incidence of heart failure in the pioglitazone group but no difference in fatal heart failure between the two groups (Dormandy et al., 2005).
The results of these cardiovascular outcome studies are somewhat disappointing considering that TZDs have a favourable effect on markers of cardiovascular risk. They have been shown to reduce blood pressure in both diabetic and non-diabetic patients (Nolan et al., 1994; Ogihara et al., 1995) and decrease free fatty acid concentrations (Suter et al., 1992). They have a beneficial effect on lipid profile with both pioglitazone and rosiglitazone raising high-density lipoprotein cholesterol levels, reducing triglyceride levels and increasing concentration of large as opposed to more atherogenic small LDL particles. Pioglitazone compared with rosiglitazone is associated with significant improvements in triglycerides, high-density lipoprotein cholesterol, LDL particle concentration and LDL particle size (Goldberg et al., 2005).
TZDs have also been shown to reduce clinical measures of atherosclerosis. Pioglitazone reduces neointimal tissue proliferation after coronary stent implantation in type II diabetic patients and hence produces a lower rate of restenosis (Takagi et al., 2003; Choi et al., 2004). Troglitazone has been shown to reduce the progression of carotid artery intima-media thickness (CIMT), a known marker for future cardiovascular events, by 31% compared with placebo in young women at high risk for type II diabetes (Xiang et al., 2005; Mazzone et al., 2006). CIMT is a marker of coronary atherosclerosis, and this parameter was also used in the recent Carotid Intima-Media Thickness in Atherosclerosis Using Pioglitazone (CHICAGO) study, which compared the effects of pioglitazone and glimepiride on CIMT in a racially diverse population. The investigators found that progression of CIMT after 18 months was slowed with pioglitazone compared with glimepiride in patients stratified by age, gender, systolic blood pressure, duration of diabetes, body mass index, haemoglobin A1C value and statin use (Mazzone et al., 2006). Another recent study has shown that rosiglitazone may improve plaque stability in symptomatic patients with carotid disease awaiting carotid endarterectomy (Marfella et al., 2006). This is thought to be due to a reduction of ubiquitin-proteasome and nuclear factor-κB activity, which are involved in the inflammation, proliferation and apoptosis, which leads to plaque instability.
It has also been proposed that TZDs reduce cardiac hypertrophy. This is mediated directly by activation of PPAR-γ, and subsequent inhibition of factors such as activator protein-1, nuclear factor-κB, endothelin-1, TNF-α and NO synthase, which are implicated in cardiac hypertrophy (Frey and Olson, 2002). Pioglitazone has been observed to improve left ventricular diastolic function and decrease collagen accumulation in the hearts of prediabetic rats (Tsuji et al., 2001). Other studies have shown that TZDs reduce tissue injury caused by regional myocardial ischaemia and reperfusion, and can reduce myocardial infarct size and improve cardiac contractile function (Zhu et al., 2000; Guo and Tabrizchi, 2006). This is thought to be due to a reduction in the formation of proapoptotic molecules such as peroxynitrite and inactivation of proapoptotic signalling pathways (such as p38 mitogen-activated protein kinase; Liu et al., 2004). In contrast to these results, Frantz et al. (2004) showed PPAR-γ receptor activation did not improve survival or left ventricular remodelling in mice with chronic myocardial infarction. Moreover, this study also showed no changes in metabolic parameters, inflammation, collagen deposition or endothelial function in the aorta with PPAR-γ activation. Another study found PPAR-γ activation does not provide myocardial protection in ischaemia and reperfusion in pigs (Xu et al., 2005). Both troglitazone and pioglitazone were used in pigs subjected to myocardial ischaemia and reperfusion. Troglitazone, but not pioglitazone, had a beneficial effect on myocardial contractile function and reduced proinflammatory cytokine expression. These protective effects are thought to be due to the α-tocopherol moiety of troglitazone, which is not present in pioglitazone. Clinical end point data will be required to convince clinicians that the theoretical benefits of TZDs do indeed translate into improved patient outcomes.
Beyond their actions on the cardiovascular system, TZDs have been employed to treat other conditions characterised by insulin resistance (Yki-Jarvinen, 2004). In the polycystic ovary syndrome, symptoms of hyperandrogenism such as hirsutism and acne are present along with ovulatory dysfunction (Knochenhauer et al., 1998). Weight loss and metformin which both reduce insulin levels have been shown to improve these symptoms (Iuorno and Nestler, 2001) as have troglitazone and rosiglitazone (Azziz et al., 2001; Ghazeeri et al., 2003). However, TZDs have not been licensed for this indication.
Non-alcoholic steatohepatitis, which is associated with type II diabetes, has also been successfully treated with TZDs. It is the most common cause of elevated liver enzymes and can result in potentially fatal cirrhosis (Clark et al., 2002). Non-alcoholic steatohepatitis is associated with an insulin-resistant state, which causes increased lipolysis and delivery of free fatty acids to the liver. This sensitizes the liver to metabolic injury leading to necrosis, inflammation and fibrosis (Promrat et al., 2004). Improvements in liver aminotransferase levels and histological findings such as steatosis, ballooning necrosis and inflammation were observed in subjects with impaired glucose tolerance or type II diabetes and non-alcoholic steatohepatitis treated with pioglitazone (Belfort et al., 2006). By improving the insulin sensitivity in adipose tissue, TZDs reduce excessive rates of lipolysis and substrate supply to the liver. Lower plasma insulin and glucose levels lead to reduced hepatic lipid synthesis by decreasing activity of sterol regulatory element-binding protein 1c and carbohydrate response element-binding protein (Browning and Horton, 2004). TZDs and metformin also stimulate AMP-activated protein kinase which inhibits hepatic lipogenic enzymes (Belfort et al., 2006).
Lipodystrophy is a condition that is seen with antiretroviral therapy in patients with human immunodeficiency virus and is accompanied by insulin resistance. However, the effects of TZDs as a treatment are minimal (Arioglu et al., 2000), despite improving insulin sensitivity and increasing the subcutaneous fat depot.
Adverse effects of TZDs
A number of side effects are well recognised with the use of TZDs in clinical practice. Treatment with these agents leads to weight gain due to expansion of the subcutaneous fat depot, whereas visceral fat mass remains unchanged or is reduced (Nesto et al., 2004). Average weight gain is 3–6 kg over the first year which is comparable to that seen with initiation of insulin (Kendall, 2006).
Fluid retention can also occur and is reported in around 4–6% of patients (Yki-Jarvinen, 2004). At least in part, this may explain the higher incidence of heart failure reported with TZD therapy compared with placebo. Ejection fraction and ventricular end-diastolic volume do not appear to change with TZD treatment (St John et al., 2002). Oedema formation is especially common in insulin-treated patients due to sodium and water retention. TZDs may enhance fluid retention by increasing sensitivity to insulin (Kalambokis et al., 2004). As described earlier, they also precipitate sodium and water retention by upregulating renal tubule sodium transport proteins and decreasing glomerular filtration rate. This reduction in glomerular filtration rate may be due to vasodilatation as a result of endothelial NO production (Song et al., 2004). PPAR-γ is present in the collecting duct, so activation by TZDs may also stimulate the epithelial sodium channel directly thereby increasing sodium and water absorption (Guan et al., 2005). In addition to peripheral oedema, both pioglitazone and rosiglitazone have also been reported to cause macular oedema, with resolution and improvement in vision occurring after drug cessation (Ryan et al., 2006).
Hepatotoxicity has been observed in studies using troglitazone, which led to its withdrawal from clinical use. This was thought to be mediated by the troglitazone–quinone metabolite and its metabolism via CYP 3A4 enzyme system (Guo and Tabrizchi, 2006). Hepatotoxicity does not appear to be a class effect—alanine aminotransferase levels more than 10 times the upper limit of normal were observed in 0.68% of patients taking troglitazone in a total of 13 studies, compared with none taking rosiglitazone and pioglitazone (Yki-Jarvinen, 2004).
TZDs appear to have detrimental effects on bone. The ADOPT study, monitored glycaemic control over 4 years in type II diabetic patients receiving rosiglitazone, metformin or glyburide, identified a higher rate of fractures in the group receiving rosiglitazone (Kahn et al., 2006). More women in the rosiglitazone group had upper limb fractures involving the humerus and hand, but the number of men with fractures did not differ according to the treatment group. This was an unexpected event that was not part of the prespecified analysis plan. These findings were supported by a more recent study which examined biochemical markers of bone formation and bone resorption, and bone mineral density in healthy postmenopausal women prescribed rosiglitazone or placebo. Osteoblast markers procollagen type I N-terminal propeptide and osteocalcin decreased significantly but there was no change in the bone resorption marker β-C-terminal telopeptide of type I collagen. Total hip bone density decreased significantly in the rosiglitazone group. Lumbar spine density was not significantly different between the groups. The mechanism by which rosiglitazone alters bone remodelling likely involves direct effects on osteoblast development and function (Grey et al., 2007).
The future—dual PPAR-α/γ agonists
PPAR-γ agonists have beneficial effects on markers for cardiovascular disease in addition to their glucose-lowering actions. PPAR-α agonists, such as fenofibrate, have more marked lipid-lowering effects than PPAR-γ agonists. Animal studies have suggested they may also provide protection against weight gain and enhance insulin-mediated muscle glucose metabolism (Ljung et al., 2002). The combination of PPAR-α and -γ effects could therefore offer superior treatment of insulin resistance and cardioprotection than the individual agonists (Willson et al., 2000; Chinetti et al., 2001). Dual PPAR-α/γ agonists are in development at present. Muraglitazar, one such novel agent, was found to provide greater improvements in haemoglobin A1C and lipid parameters than pioglitazone in patients with type II diabetes (Kendall et al., 2006). Plasma insulin levels and circulating free fatty acids were reduced to a greater extent in the muraglitazar group. However, despite these beneficial effects on cardiovascular risk factors, an analysis of muraglitazar trials in 2005 led to approval being withdrawn by the Food and Drug Administration due to an excess incidence in the composite end point of death, major adverse cardiovascular events (myocardial infarction, stroke and transient ischaemic attack) and congestive heart failure, giving a relative risk 2.23 compared to placebo or pioglitazone (Nissen et al., 2005).
Conclusion
TZDs are useful agents in the treatment of hyperglycaemia, acting as insulin sensitisers and enhancing glucose uptake via their action on PPAR-γ receptors. They have been shown to have additional beneficial effects on surrogate markers of cardiovascular risk. However, this has not been definitively shown to translate into improved cardiovascular outcomes for patients, and the recent meta-analysis by Nissen and Wolski (2007) raises significant concerns of adverse cardiovascular effects of treatment with rosiglitazone. It is also important to remember that much of the data on these agents is derived from animal and in vitro studies using supraphysiological concentrations which may produce different effects to those observed in humans. There are many actions of genes activated by PPAR-γ agonists, only some of which are currently known, and further research is required to determine the net clinical benefit of these agents.
Abbreviations
- CIMT
carotid artery intima-media thickness
- CRP
C-reactive protein
- FFA
free fatty acid
- GLUT-4
glucose transporter-4
- IRS
insulin receptor substrate
- LDL
low-density lipoprotein
- NO
nitric oxide
- PAI-1
plasminogen activator inhibitor-1
- PPAR
peroxisome proliferator-activated receptor
- TNF
tumour necrosis factor
- TZD
thiazolidinedione
Conflict of interest
The authors state no conflict of interest.
References
- Alessi MC, Juhan-Vague I. Contribution of PAI-1 in cardiovascular pathology. Arch Mal Coeur Vaiss. 2004;97:673–678. [PubMed] [Google Scholar]
- Arioglu E, Duncan-Morin J, Sebring N, Rother KI, Gottlieb N, Lieberman J, et al. Efficacy and safety of troglitazone in the treatment of lipodystrophy syndromes. Ann Intern Med. 2000;133:263–274. doi: 10.7326/0003-4819-133-4-200008150-00009. [DOI] [PubMed] [Google Scholar]
- Asano T, Wakisaka M, Yoshinari M, Iino K, Sonoki K, Iwase M, et al. Peroxisome proliferator-activated receptor gamma1 (PPARgamma1) expresses in rat mesangial cells and PPARgamma agonists modulate its differentiation. Biochim Biophys Acta. 2000;1497:148–154. doi: 10.1016/s0167-4889(00)00054-9. [DOI] [PubMed] [Google Scholar]
- Azziz R, Ehrmann D, Legro RS, Whitcomb RW, Hanley R, Fereshetian AG, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2001;86:1626–1632. doi: 10.1210/jcem.86.4.7375. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- Bath PM. The effect of nitric oxide-donating vasodilators on monocyte chemotaxis and intracellular cGMP concentrations in vitro. Eur J Clin Pharmacol. 1993;45:53–58. doi: 10.1007/BF00315350. [DOI] [PubMed] [Google Scholar]
- Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- Becker AB, Roth RA. Insulin receptor structure and function in normal and pathological conditions. Annu Rev Med. 1990;41:99–115. doi: 10.1146/annurev.me.41.020190.000531. [DOI] [PubMed] [Google Scholar]
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- Blaschke F, Spanheimer R, Khan M, Law RE. Vascular effects of TZDs: new implications. Vascul Pharmacol. 2006;45:3–18. doi: 10.1016/j.vph.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CR, Mertz VA, Heigenhauser GJ, Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes. 2005;54:3154–3160. doi: 10.2337/diabetes.54.11.3154. [DOI] [PubMed] [Google Scholar]
- Calnek DS, Mazzella L, Roser S, Roman J, Hart CM. Peroxisome proliferator-activated receptor gamma ligands increase release of nitric oxide from endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:52–57. doi: 10.1161/01.atv.0000044461.01844.c9. [DOI] [PubMed] [Google Scholar]
- Castrillo A, Diaz-Guerra MJ, Hortelano S, Martin-Sanz P, Bosca L. Inhibition of IkappaB kinase and IkappaB phosphorylation by 15-deoxy-delta(12,14)-prostaglandin J(2) in activated murine macrophages. Mol Cell Biol. 2000;20:1692–1698. doi: 10.1128/mcb.20.5.1692-1698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- Choi D, Kim SK, Choi SH, Ko YG, Ahn CW, Jang Y, et al. Preventative effects of rosiglitazone on restenosis after coronary stent implantation in patients with type 2 diabetes. Diabetes Care. 2004;27:2654–2660. doi: 10.2337/diacare.27.11.2654. [DOI] [PubMed] [Google Scholar]
- Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- Delerive P, Martin-Nizard F, Chinetti G, Trottein F, Fruchart JC, Najib J, et al. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res. 1999;85:394–402. doi: 10.1161/01.res.85.5.394. [DOI] [PubMed] [Google Scholar]
- Denton RM, Brownsey RW, Belsham GJ. A partial view of the mechanism of insulin action. Diabetologia. 1981;21:347–362. doi: 10.1007/BF00252681. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- Dubois M, Pattou F, Kerr-Conte J, Gmyr V, Vandewalle B, Desreumaux P, et al. Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in normal human pancreatic islet cells. Diabetologia. 2000;43:1165–1169. doi: 10.1007/s001250051508. [DOI] [PubMed] [Google Scholar]
- Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- Fajas L, Fruchart JC, Auwerx J. PPARgamma3 mRNA: a distinct PPARgamma mRNA subtype transcribed from an independent promoter. FEBS Lett. 1998;438:55–60. doi: 10.1016/s0014-5793(98)01273-3. [DOI] [PubMed] [Google Scholar]
- Frantz S, Hu K, Widder J, Bayer B, Witzel CC, Schmidt I, et al. Peroxisome proliferator activated-receptor agonism and left ventricular remodeling in mice with chronic myocardial infarction. Br J Pharmacol. 2004;141:9–14. doi: 10.1038/sj.bjp.0705585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey N, Olson EN. Modulating cardiac hypertrophy by manipulating myocardial lipid metabolism. Circulation. 2002;105:1152–1154. [PubMed] [Google Scholar]
- Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1) N Engl J Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- Ghazeeri G, Kutteh WH, Bryer-Ash M, Haas D, Ke RW. Effect of rosiglitazone on spontaneous and clomiphene citrate-induced ovulation in women with polycystic ovary syndrome. Fertil Steril. 2003;79:562–566. doi: 10.1016/s0015-0282(02)04843-4. [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, et al. The peroxisome proliferator-activated receptor-{gamma} agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305–1310. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- Guan Y, Breyer MD. Peroxisome proliferator-activated receptors (PPARs): novel therapeutic targets in renal disease. Kidney Int. 2001;60:14–30. doi: 10.1046/j.1523-1755.2001.00766.x. [DOI] [PubMed] [Google Scholar]
- Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, et al. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- Guo L, Tabrizchi R. Peroxisome proliferator-activated receptor gamma as a drug target in the pathogenesis of insulin resistance. Pharmacol Ther. 2006;111:145–173. doi: 10.1016/j.pharmthera.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Hamberg M, Svensson J, Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci USA. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneghan C, Thompson M, Perera R. Prevention of diabetes. BMJ. 2006;333:764–765. doi: 10.1136/bmj.38996.709340.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielson H, Gomis R, Hanefeld M, Jones NP, et al. Rosiglitazone evaluated for cardiovascular outcomes—an interim analysis. N Engl J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci USA. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PL. Unraveling the links between diabetes, obesity, and cardiovascular disease. Circ Res. 2005;96:1129–1131. doi: 10.1161/01.RES.0000170705.56583.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuorno MJ, Nestler JE. Insulin-lowering drugs in polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28:153–164. doi: 10.1016/s0889-8545(05)70191-1. [DOI] [PubMed] [Google Scholar]
- Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- Kalambokis GN, Tsatsoulis AA, Tsianos EV. The edematogenic properties of insulin. Am J Kidney Dis. 2004;44:575–590. [PubMed] [Google Scholar]
- Kendall DM. Thiazolidinediones: the case for early use. Diabetes Care. 2006;29:154–157. doi: 10.2337/diacare.29.1.154. [DOI] [PubMed] [Google Scholar]
- Kendall DM, Rubin CJ, Mohideen O, Ledeine JM, Belder R, Gross J, et al. Improvement of glycemic control, triglycerides, and HDL cholesterol levels with muraglitazar, a dual (alpha/gamma) peroxisome proliferator-activated receptor activator, in patients with type 2 diabetes inadequately controlled with metformin monotherapy: A double-blind, randomized, pioglitazone-comparative study. Diabetes Care. 2006;29:1016–1023. doi: 10.2337/diacare.2951016. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- Krook A, Wallberg-Henriksson H, Zierath JR. Sending the signal: molecular mechanisms regulating glucose uptake. Med Sci Sports Exerc. 2004;36:1212–1217. doi: 10.1249/01.mss.0000132387.25853.3b. [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- Kurtz TW. Treating the metabolic syndrome: telmisartan as a peroxisome proliferator-activated receptor-gamma activator. Acta Diabetol. 2005;42 Suppl 1:S9–S16. doi: 10.1007/s00592-005-0176-0. [DOI] [PubMed] [Google Scholar]
- Lam K, Carpenter CL, Ruderman NB, Friel JC, Kelly KL. The phosphatidylinositol 3-kinase serine kinase phosphorylates IRS-1. Stimulation by insulin and inhibition by Wortmannin. J Biol Chem. 1994;269:20648–20652. [PubMed] [Google Scholar]
- Law RE, Meehan WP, Xi XP, Graf K, Wuthrich DA, Coats W, et al. Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia. J Clin Invest. 1996;98:1897–1905. doi: 10.1172/JCI118991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz HE, Dole JF, Patwardhan R, Rappaport EB, Freed MI. Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab. 2001;86:280–288. doi: 10.1210/jcem.86.1.7157. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Liu HR, Tao L, Gao E, Lopez BL, Christopher TA, Willette RN, et al. Anti-apoptotic effects of rosiglitazone in hypercholesterolemic rabbits subjected to myocardial ischemia and reperfusion. Cardiovasc Res. 2004;62:135–144. doi: 10.1016/j.cardiores.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Ljung B, Bamberg K, Dahllof B, Kjellstedt A, Oakes ND, Ostling J, et al. AZ 242, a novel PPARalpha/gamma agonist with beneficial effects on insulin resistance and carbohydrate and lipid metabolism in ob/ob mice and obese Zucker rats. J Lipid Res. 2002;43:1855–1863. doi: 10.1194/jlr.m200127-jlr200. [DOI] [PubMed] [Google Scholar]
- Long SD, Pekala PH. Lipid mediators of insulin resistance: ceramide signalling down-regulates GLUT4 gene transcription in 3T3-L1 adipocytes. Biochem J. 1996;319:179–184. doi: 10.1042/bj3190179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Alemany R, Redondo JM, Nagamine Y, Munoz-Canoves P. Plasminogen activator inhibitor type-1 inhibits insulin signaling by competing with alphavbeta3 integrin for vitronectin binding. Eur J Biochem. 2003;270:814–821. doi: 10.1046/j.1432-1033.2003.03453.x. [DOI] [PubMed] [Google Scholar]
- Marfella R, D'Amico M, Di Filippo C, Baldi A, Siniscalchi M, Sasso FC, et al. Increased activity of the ubiquitin-proteasome system in patients with symptomatic carotid disease is associated with enhanced inflammation and may destabilize the atherosclerotic plaque: effects of rosiglitazone treatment. J Am Coll Cardiol. 2006;47:2444–2455. doi: 10.1016/j.jacc.2006.01.073. [DOI] [PubMed] [Google Scholar]
- Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, D'Agostino RB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomised trial. JAMA. 2006;296:2572–2581. doi: 10.1001/jama.296.21.joc60158. [DOI] [PubMed] [Google Scholar]
- Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol. 1995;25:674–678. [PubMed] [Google Scholar]
- Nedergaard J, Petrovic N, Lindgren EM, Jacobsson A, Cannon B. PPARgamma in the control of brown adipocyte differentiation. Biochim Biophys Acta. 2005;1740:293–304. doi: 10.1016/j.bbadis.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2004;27:256–263. doi: 10.2337/diacare.27.1.256. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581–2586. doi: 10.1001/jama.294.20.joc50147. [DOI] [PubMed] [Google Scholar]
- Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, et al. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003;112:608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogihara T, Rakugi H, Ikegami H, Mikami H, Masuo K. Enhancement of insulin sensitivity by troglitazone lowers blood pressure in diabetic hypertensives. Am J Hypertens. 1995;8:316–320. doi: 10.1016/0895-7061(95)96214-5. [DOI] [PubMed] [Google Scholar]
- Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998;101:1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- Radomski MW, Palmer RM, Moncada S. Modulation of platelet aggregation by an L-arginine–nitric oxide pathway. Trends Pharmacol Sci. 1991;12:87–88. doi: 10.1016/0165-6147(91)90510-y. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Rewers M, Zaccaro D, D'Agostino R, Haffner S, Saad MF, Selby JV, et al. Insulin sensitivity, insulinemia, and coronary artery disease: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27:781–787. doi: 10.2337/diacare.27.3.781. [DOI] [PubMed] [Google Scholar]
- Rios-Vazquez R, Marzoa-Rivas R, Gil-Ortega I, Kaski JC. Peroxisome proliferator-activated receptor-gamma agonists for management and prevention of vascular disease in patients with and without diabetes mellitus. Am J Cardiovasc Drugs. 2006;6:231–242. doi: 10.2165/00129784-200606040-00003. [DOI] [PubMed] [Google Scholar]
- Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Ryan EH, Jr, Han DP, Ramsay RC, Cantrill HL, Bennett SR, Dev S, et al. Diabetic macular edema associated with glitazone use. Retina. 2006;26:562–570. doi: 10.1097/00006982-200605000-00011. [DOI] [PubMed] [Google Scholar]
- Salomone OA, Elliott PM, Calvino R, Holt D, Kaski JC. Plasma immunoreactive endothelin concentration correlates with severity of coronary artery disease in patients with stable angina pectoris and normal ventricular function. J Am Coll Cardiol. 1996;28:14–19. doi: 10.1016/0735-1097(96)00110-6. [DOI] [PubMed] [Google Scholar]
- Sharma AM, Staels B. Review: Peroxisome proliferator-activated receptor gamma and adipose tissue—understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab. 2007;92:386–395. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith U, Gogg S, Johansson A, Olausson T, Rotter V, Svalstedt B. Thiazolidinediones (PPARgamma agonists) but not PPARalpha agonists increase IRS-2 gene expression in 3T3-L1 and human adipocytes. FASEB J. 2001;15:215–220. doi: 10.1096/fj.00-0020com. [DOI] [PubMed] [Google Scholar]
- Song J, Knepper MA, Hu X, Verbalis JG, Ecelbarger CA. Rosiglitazone activates renal sodium- and water-reabsorptive pathways and lowers blood pressure in normal rats. J Pharmacol Exp Ther. 2004;308:426–433. doi: 10.1124/jpet.103.058008. [DOI] [PubMed] [Google Scholar]
- St John SM, Rendell M, Dandona P, Dole JF, Murphy K, Patwardhan R, et al. A comparison of the effects of rosiglitazone and glyburide on cardiovascular function and glycemic control in patients with type 2 diabetes. Diabetes Care. 2002;25:2058–2064. doi: 10.2337/diacare.25.11.2058. [DOI] [PubMed] [Google Scholar]
- Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem. 1997;272:971–976. doi: 10.1074/jbc.272.2.971. [DOI] [PubMed] [Google Scholar]
- Suter SL, Nolan JJ, Wallace P, Gumbiner B, Olefsky JM. Metabolic effects of new oral hypoglycemic agent CS-045 in NIDDM subjects. Diabetes Care. 1992;15:193–203. doi: 10.2337/diacare.15.2.193. [DOI] [PubMed] [Google Scholar]
- Takagi T, Yamamuro A, Tamita K, Yamabe K, Katayama M, Mizoguchi S, et al. Pioglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with type 2 diabetes mellitus: an intravascular ultrasound scanning study. Am Heart J. 2003;146:E5. doi: 10.1016/S0002-8703(03)00146-7. [DOI] [PubMed] [Google Scholar]
- Takata Y, Kitami Y, Yang ZH, Nakamura M, Okura T, Hiwada K. Vascular inflammation is negatively autoregulated by interaction between CCAAT/enhancer-binding protein-delta and peroxisome proliferator-activated receptor-gamma. Circ Res. 2002;91:427–433. doi: 10.1161/01.res.0000031271.20771.4f. [DOI] [PubMed] [Google Scholar]
- Teboul L, Febbraio M, Gaillard D, Amri EZ, Silverstein R, Grimaldi PA. Structural and functional characterization of the mouse fatty acid translocase promoter: activation during adipose differentiation. Biochem J. 2001;360:305–312. doi: 10.1042/0264-6021:3600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;363:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CC, Itani SI, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Mizushige K, Noma T, Murakami K, Ohmori K, Miyatake A, et al. Pioglitazone improves left ventricular diastolic function and decreases collagen accumulation in prediabetic stage of a type II diabetic rat. J Cardiovasc Pharmacol. 2001;38:868–874. doi: 10.1097/00005344-200112000-00008. [DOI] [PubMed] [Google Scholar]
- UKPDS Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- Watson RT, Pessin JE. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog Horm Res. 2001;56:175–193. doi: 10.1210/rp.56.1.175. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberg PL. Atherogenesis: current understanding of the causes of atheroma. Indian Heart J. 2000;52:467–472. [PubMed] [Google Scholar]
- Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem. 2001;70:341–367. doi: 10.1146/annurev.biochem.70.1.341. [DOI] [PubMed] [Google Scholar]
- Xiang AH, Peters RK, Kjos SL, Ochoa C, Marroquin A, Goico J, et al. Effect of thiazolidinedione treatment on progression of subclinical atherosclerosis in premenopausal women at high risk for type 2 diabetes. J Clin Endocrinol Metab. 2005;90:1986–1991. doi: 10.1210/jc.2004-1685. [DOI] [PubMed] [Google Scholar]
- Xu Y, Gen M, Lu L, Fox J, Weiss SO, Brown RD, et al. PPAR-gamma activation fails to provide myocardial protection in ischemia and reperfusion in pigs. Am J Physiol Heart Circ Physiol. 2005;288:H1314–H1323. doi: 10.1152/ajpheart.00618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wilson KM, Medh JD. Genetic analysis of four novel peroxisome proliferator activated receptor-gamma splice variants in monkey macrophages. Biochem Biophys Res Commun. 2002;293:274–283. doi: 10.1016/S0006-291X(02)00138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Lu L, Xu Y, Schwartz GG. Troglitazone improves recovery of left ventricular function after regional ischemia in pigs. Circulation. 2000;101:1165–1171. doi: 10.1161/01.cir.101.10.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]