Abstract
We have developed a generic transient transfection process at 100 L scale, using HEK293-EBNA cells and PEI as the transfection reagent for the production of recombinant IgG. The process, including large-scale plasmid preparation, expression at bioreactor scale, capture, purification and, if necessary, endotoxin removal allows reproducible production of more than 0.5 g IgG for in vitro and in vivo studies. We compared the performance of two HEK cell lines, investigated the effect of conditioned medium, optimized the DNA:PEI ratio and implemented a feed strategy to prolong the culture time to increase product yield. The transient transfection protocol developed enables a closed process from seeding culture to protein capture. The challenge of performing a medium exchange before transfection at large scale is solved by applying a continuous centrifugation step between the seeding bioreactor and the production bioreactor. After 7–8 days the harvest and capture is performed in a one-step operation using a Streamline expanded bed chromatography system. Following a polishing step the purified antibody is transferred to the final formulation buffer. The method has shown to be reproducible at 10, 50, and 100 L scale expressing between 5 and 8 mg L−1 IgG.
Keywords: Bioreactor, HEK293, Large scale, PEI, Transient transfection
Introduction
Transient expression in animal or human cells is an important tool for producing recombinant proteins for research or pharmaceutical use. During the last decade this technology has been developed at larger scale, providing a mean to enable fast supply of milligram to gram amounts of protein in early development stages (Wurm and Bernard 1999).
Today the majority of therapeutic proteins are produced in stably transfected Chinese hamster ovary (CHO) cells (Birch and Onakunle 2005). Although scalable transient expression using CHO cells is frequently reported (Derouazi et al. 2004; Tait et al. 2004; Kunaparaju et al. 2005; Galbraith et al. 2006) the human embryonic kidney 293 cell line (HEK293) is the most widely used cell line for transient protein expression. HEK293 cells readily take up DNA using various gene transfer vehicles such as calcium phosphate (Jordan et al. 1998; Meissner et al. 1999; Girard et al. 2001; Meissner et al. 2001; Durocher et al. 2002; Baldi et al. 2005), XtremeGENE Ro1539 (Schlaeger et al. 1998; Schlaeger et al. 2003) and PEI (Schlaeger and Christensen 1999; Durocher et al. 2002; Pham et al. 2003; Schlaeger et al. 2003; Baldi et al. 2005). Transient gene expression with PEI in a high-cell density perfusion culture was reported recently (Sun et al. 2008). The HEK293 cell line is easily grown in suspension culture and can be adapted to serum free medium (Schlaeger et al. 2003; Geisse et al. 2003; Geisse and Henke 2005). Two genetic variants, the HEK293-EBNA (Cachianes et al. 1993) and the HEK293-T (Lebkowski et al. 1985) cell lines have been developed. The HEK-EBNA cell line expresses the Epstein-Barr virus (EBV) nuclear antigen 1 (EBNA1) and the HEK293-T cell line expresses the SV40 large T antigen. Both elements allow episomal amplification of plasmids containing the viral EBV (HEK293-EBNA) or SV40 (HEK293-T) origins of replication.
A great deal of effort has been invested in the development of specific vectors suitable for transient expression. The vector must sustain a high plasmid copy number that is maintained through the production phase. A high level of DNA transcription is achieved by using promotors that are highly active in the host cell line, such as the human cytomegalovirus (CMV) promotor (Durocher et al. 2002; Geisse et al. 2003; Geisse and Henke 2005).
For the transfer of plasmid DNA into mammalian cells a transport vehicle is needed. Although many highly effective transfection reagents are commercially available, only a few are cost effective when considering application at large scale. Additional features to consider are ease of use, efficiency in combination with cells growing in suspension and possible cytotoxic effects. The well established calcium phosphate precipitation technique (Graham and van der Eb 1973) and the more recently described cationic polymer polyethyleneimine (PEI) (Boussif et al. 1995) meet these criteria. A major breakthrough was achieved when PEI technology demonstrated its ability to produce milligram amounts of recombinant protein within a few days in large-scale suspension cultures (Jordan et al. 1998). Calcium phosphate has been reported to support transient gene expression for 2–100 L scale (Jordan et al. 1998; Girard et al. 2002) and PEI has been used successfully up to 15 L scale (Durocher et al. 2002; Pham et al. 2003; Geisse and Henke 2005).
The last critical aspect in transient transfection is related to the cell culture medium. The medium should support good cell growth and transfection efficiencies above 80%. Calcium phosphate mediated transfection in HEK-EBNA cells, as described by Meissner et al. (1999), was successful only if the cells were transferred into DMEM/F12 medium supplemented with serum prior to transfection, although the cells could be grown in various serum-free culture media (Meissner et al. 1999). PEI-mediated transfection, as described by Geisse and Henke (2005) is insensitive to the presence of serum (Geisse and Henke 2005). However, most commercially available serum free culture media with proprietary composition preclude successful transfection with PEI for unknown reasons (Geisse et al. 2003). Another important aspect to consider is the presence of cellular products in conditioned medium. These products have been found to exert a strong inhibitory effect on transfection efficiency and subsequent protein expression (Schlaeger and Christensen 1999), suggesting that a medium exchange before transfection could be beneficial.
Here we describe the development of a generic protocol for PEI mediated transient transfection in a 100 L bioreactor using HEK293 cells as the host cell line. A process for the production and purification of the expression plasmid at quantities needed at large scale was also established. We compared the performance of two HEK cell lines, investigated the effect of conditioned medium, optimized the DNA:PEI ratio and implemented a feeding strategy to prolong the culture time to increase product yield. The resulting process was scaled up to 10 L Wave bioreactor and then to 50 and 100 L production scale stirred tank bioreactors.
Material and methods
Plasmid production
The backbone of the antibody-expressing plasmid (proprietary to AstraZeneca) carries a neomycin resistance marker for selection of stable transfectants and encodes for both the heavy and light antibody chains. The pCEP4-AmCyan plasmid expressing the fluorescence protein AmCyan was described elsewhere (Davies et al. 2005).
The host E. coli strain LibraryEfficiency™ DH5 a (Invitrogen, Stockholm, Sweden) was used for plasmid production. The pCEP4-AmCyan plasmid was produced and purified in small-scale using a commercial kit according to the manufacturer instructions (Macherey-Nagel, Duren, Germany). The antibody-encoding plasmid was produced in a 100 L bioreactor (Sartorius BBI Systems GmbH, Melsungen, Germany) with TB medium (24 g L−1 Tryptone-Peptone, 12 g L−1 Bacto™ yeast extract, 17 mM KH2PO4, 72 mM K2HPO4, 5 g L−1 glucose). Upon reaching OD 20, the culture broth was harvested by semi-continuous centrifuge (Cepa Z41G, Carl Padberg Zentrifugenbau, Lahr, Germany). The cell paste (2,600 g) was stored at −70 °C until plasmid purification. Tryptone-Peptone and Bacto™ yeast extract were from Becton Dickinson (Sparks, MD, USA), other chemicals from Sigma-Aldrich (Stockholm, Sweden). The temperature in all cultures was controlled to 37 °C and pH was monitored, but not controlled.
The alkaline lysis procedure used here was based on methods described elsewhere (Varley and Birch 1999; Chamsart et al. 2001). Briefly, 450 grams of cell paste was resuspended in buffer (50 mM Tris–HCl, 10 mM EDTA, 100 mg L−1 RnaseA, pH 8.0) to yield a cell suspension of 100 g L−1, lysed for 3 min by addition of two volumes of 150 mM NaOH 1% SDS solution and neutralized by one volume of a solution containing 3 M potassium acetate and 10 mM EDTA (titrated to pH 5.5 by glacial acetic acid). One hour after neutralization the solution was passed through a bag filter (500 μm, Plastok, Merseyside, UK), a depth filter (1.2 μm Sartopure PP2, Sartorius AG, Göttingen, Germany) and a membrane filter (0.2 μm Acropak 200, Pall, Lund, Sweden).
The cleared lysate was diluted 1:1 with two-fold concentrated C1 buffer (50 mM triethylamine acetate, 25 mM Na2HPO4, pH 7.0) and applied onto a pre-equilibrated XK50 column (GE Healthcare, Uppsala, Sweden) containing 350 mL of Perfluorosorb S resin S (Prometic Life Sciences, Mont-Royal, Canada). The column was then washed with three column volumes of C1 buffer and with five column volumes of C2 buffer (20 mM Tris, 1 mM EDTA, 5 mM NaCl, 2% ethanol pH 8.0). The plasmid was eluted with two column volumes of C3 buffer (100 mM sodium acetate, 8% ethanol, pH 8.5). The chromatography was run at a constant flow rate of 23 mL min−1 and monitored by UV absorbance at 260 and 280 nm. The eluate was concentrated by ultrafiltration (Pellicon XL50 Ultracel 30 kDa cut-off, Millipore, Bedford, MA, USA) to a plasmid concentration of about 1 mg L−1 and exchanged with 5 volumes of TE buffer. The resulting plasmid solution contained too high endotoxin content (400 EU mg L−1), which necessitated additional purification. Endotoxin removal was performed by four cycles of two-phase extraction with Triton X-114 (Petsch and Anspach 2000). The aqueous fraction recovered after two-phase extraction contained residual soluble Triton X-114, which was removed by buffer exchange with 10 volumes of TE buffer. The plasmid solution was then sterile-filtered, aliquoted and stored at −20 °C until use. The final plasmid product (112 mg) had an endotoxin content of below 4 EU mg−1, was free from RNA (judged by agarose electrophoresis stained by ethidium bromide) and was predominantly in the supercoiled form. Plasmid DNA was quantified by optical density measurements at 260 nm assuming an extinction coefficient of 50 mg L−1 cm−1.
Cell lines and culture medium
In this work the original adherent cell line HEK293-EBNA (Invitrogen, Stockholm, Sweden) stably expressing the Epstein Barr virus Nuclear Antigen-1 gene and the original adherent cell line HEK293-T (GeneHunter Corporation, Brookline, MA) stably expressing the SV40 large T antigen were used. Both cell lines were grown in adherent culture using DMEM medium (Invitrogen, Stockholm, Sweden). They were adapted to suspension growth before being transferred into DHI medium (custom version made by SAFC Biosciences, Andover, UK) by stepwise medium replacement (Davies et al. 2005).
After adaptation, a working cell bank was made and both cell lines were grown routinely in DHI medium supplemented with 4 mM Glutamine, 2% v/v ultra-low IgG fetal bovine serum, 250 μg mL−1 G418 (all from Invitrogen, Stockholm, Sweden) and 0.1% w/v Pluronic F68 (Sigma-Aldrich, Stockholm, Sweden) to a maximum of 20 passages.
The HEK293-EBNA cells were also stepwise adapted to two serum-free media, ExCell™ 293 and ExCell™ V-Pro (SAFC Biosciences, Andover, UK).
Transfection procedure
The 1 mg mL−1 stock solution of linear 25 kDa polyethylenimin (Polysciences Europe, Eppenheim, Germany) was prepared in water, pH adjusted to 7.0, sterile filtered and stored in small aliquots at −80 °C until use.
The transfection cocktail was prepared shortly before transfection in non-supplemented DHI medium in a volume equivalent to one-tenth of the transfection volume. For preparing the transfection cocktail the DHI media was divided into two halves. 0,8 μg DNA per mL transfection volume was added to one half of the DHI medium and into the other half 2 μg PEI per mL transfection volume was added. After shaking the two solutions briefly and incubating them for 5 min, the DNA solution was slowly added to the PEI solution. The transfection cocktail was incubated for 20–30 min at room temperature before addition to the bioreactor.
Four hours post transfection the culture was fed to the final production volume with supplemented DHI medium and HypPep1510 (Kerry Bio-Sciences, Almere, the Netherlands) to a final concentration of 0.3% (w/v).
Seeding cultures
For expansion of the seeding culture the cells were grown in plastic shake bottles at 37 °C in 5% CO2 atmosphere placed in an orbital shaker incubator (Infors AG, Bottmingen, Switzerland). The cells were routinely passaged twice weekly, reaching approximately 2 × 106 cells mL−1 before splitting. Cell density and viability were assessed using a Cedex automatic cell counter (Innovatis AG, Bielefeld, Germany).
For small-scale shaker experiments and Wave cultures the cells were split to 1 × 106 cells mL−1 1 day before transfection to ensure that they were in logarithmic growth phase at the start of the experiment. This could not be done for seeding cultures grown in bioreactors.
Small-scale shaker experiments and Wave cultures were inoculated directly from shakers whereas for the 50 and 100 L cultures, the inocula were pre-cultured for 3 days in a 20 L stirred tank bioreactor (Sartorius BBI Systems GmbH, Melsungen, Germany). All seeding cultures were concentrated by centrifugation and resuspended in fresh culture medium before addition to the production bioreactors.
Wave cultures
Initial scale-up studies were performed in Wave bioreactors (Wave Biotech AG, Tagelswangen, Switzerland) at a working volume of 10 L. The wave bioreactors were seeded to 1 × 106 cells mL−1 in 4.5 L supplemented DHI medium. After a 2 h adaptation phase the culture was transfected with 0.5 L transfection cocktail.
Four hours post transfection the culture was fed to 10 L total volume with supplemented DHI medium and HyPep1510 to a final concentration of 0.3% (w/v). Samples were taken daily to determine cell density, viability and protein concentration.
50 and 100 L bioreactor cultures
50 and 100 L working volume stirred tank bioreactors (both Sartorius BBI Systems GmbH, Melsungen, Germany) were used. Both bioreactors were equipped with micro spargers and operated with the following set points: the dissolved oxygen concentration was controlled to 50% and the pH to 7.20. The head-space aeration was set to 0.1–0.2 vvm and the pH was down regulated by addition of CO2 into the head-space on demand. The dissolved oxygen concentration was regulated by addition of O2 on demand using a micro sparger. The temperature was set at 37 °C. The stirrer of the 50 L bioreactor was set to 50 rpm (tip-speed 0.38 m/s) and the stirrer of the 100 L bioreactor was set to 40 rpm (tip-speed 0.42 m s−1). A Centritech Lab II continuous centrifuge (Thermo-Kendro, Langenselbold, Germany) was used for direct transfer of cell concentrate from the 20 L inoculum reactor to the production bioreactor allowing a 95% medium exchange. The production bioreactors were seeded to 1 × 106 cells mL−1 in 22.5 L for the 50 L productions and in 45 L for the 100 L productions. After 2 h, 2.5 or 5 L transfection cocktail was added to the respective bioreactor resulting in a transfection volume equivalent to half the final production volume. Four hours post transfection the culture was fed to the final production volume with supplemented DHI medium and HyPep1510 to a final concentration of 0.3% (w/v). Samples were taken daily to determine cell density, viability and protein concentration. Glucose, lactate and glutamine concentrations were monitored only during the 100 L cultures using an YSI analyser (YSI Incorporated, Ohio, USA).
Antibody purification
For the 50 or 100 L process, expanded bed chromatography was used for antibody capture. A Streamline100 column (GE Healthcare, Uppsala, Sweden) was directly coupled to the fermentor via a membrane pump (Quatro flow, Hardegsen-Hevensen, Germany). The broth containing cells was pumped directly over the column containing 900 mL rProtA Streamline resin (GE Healthcare, Uppsala, Sweden), at a flow rate that allowed the bed to expand about three times. Subsequently the column was washed with PBS in expanded mode until all cells and debris were removed. The resin was transferred to a XK50 column by siphoning it out of the Streamline100 column and a packed bed column was prepared according to standard procedure. This was then connected to an Äkta purification system (GE Healthcare, Uppsala, Sweden). The matrix was washed at a flow rate of 76 cm h−1 using 20 mM phosphate-buffer pH 7.3 until a stable baseline was obtained. The antibody was eluted at the same flow rate using 100 mM citrate buffer pH 3.2. The pool containing the eluted antibody was neutralized to pH 5.5 using 1 M Tris–HCl pH 9.
A polishing step using ion exchange was performed. The protein was adsorbed to SP Sepharose FF (GE Healthcare, Uppsala, Sweden) in batch overnight at 4 °C under continuous stirring. The column was packed and washed with 20 mM sodium acetate pH 5.0 at a flow rate of 31 cm h−1 until a stable baseline was obtained. The antibody was eluted with a gradient to 100% 20 mM sodium acetate pH 5.0, 1 M sodium chloride over 10 column volumes and the antibody containing fractions were pooled.
The purified antibody was transferred to the final formulation buffer (PBS, 0.05% Tween-20) using a desalting column with G-25 medium Sephadex (GE Healthcare, Uppsala, Sweden) according to manufacturers instructions. IgG concentrations were determined by IgG-ELISA.
Fluorescence assay
A signal-to-noise assay was used to monitor the success of the pCEP4-AmCyan transfection experiments (Davies et al. 2005). A sample was taken at the earliest 24 h post transfection and cell numbers determined with the CEDEX cell counter. For the background measurement a sample of non-transfected cells was taken and measured with the CEDEX cell counter. Both samples were diluted with supplemented DHI medium to 0.2 × 106 cells mL-1 and 200 μL well-1 were plated in different lanes in a 96-well plate. The plates were read at 453 nm (excitation), 495 nm (emission) and 475 nm (cut-off) using a Spectra Max Gemini plate reader (Molecular Devices, Sunnyvale, CA, USA). The signal-to-noise-ratio was measured and calculated in relative fluorescence units (RFU).
IgG-ELISA
Coating of NUNC MaxiSorp plates (GTF #45-439454, Fisher Scientific, Stockholm, Sweden) was performed with anti-kappa (#A0191, Dako, Stockholm, Sweden) antibody. The coating antibody was diluted in PBS to 10 μg mL−1 and 50 μL was pipetted into each well. The plate was incubated at room temperature with shaking for 1 h and then transferred to +4 °C over night without shaking. After removing the coating solution, the plate was washed three times with 300 μL PBS. Before adding the samples, 50 μL PBS/Gelatin (0.33%) solution was added to each well. Thereafter, 50 μL of standard (3 μg mL−1 L in PBS/Gelatin) or sample was added to the wells of column 1. Kappa standard used was ATM027 (internal AstraZeneca antibody).The samples and standard were then subjected to a series of 1:1 dilution in columns 1–11. Column 12 was left as blank. The plate was incubated as above. Samples and standards were removed and the plate washed as above. The HRP-detection antibody (P0214, Dako, Stockholm, Sweden) was diluted 1:1,000 in PBS/Gelatin solution and 50 μL of this solution was added to each well of the plate. The plate was placed on a shaker at room temperature for 1–6 h. Finally, the detection antibody was removed and the plate was washed as above. Detection was preformed by removing all PBS from the wells and adding 100 μL OPD substrate to each well (P9187, Sigma-Aldrich, Stockholm, Sweden). The samples were incubated for approximately 20 min in the dark at room temperature before absorbance at 450 nm was read in a micro plate reader, Spectramax 340PC.
Results
Selection of host cell line and determination of the DNA:PEI ratio
The recombinant IgG plasmid was intended for stable cell line generation using HEK293-T as the host cell line. Although this vector is not specifically designed for transient transfection, a PEI-mediated transient transfection approach in HEK293-T and HEK293-EBNA cells was undertaken. Ten different DNA:PEI ratios for both HEK293-EBNA and HEK293-T cells were evaluated in shaker flasks and the antibody concentration was measured 5 days post transfection. It was repeatedly shown that a PEI concentration over 3 μg mL−1 was toxic to the cells and consequently had a negative effect on the transfection efficiency. For both cell lines the best IgG expression was obtained with a DNA:PEI ratio of 1:2.5 (0.8 μg DNA:2 μg PEI/mL cell suspension). HEK293-EBNA was chosen as the production cell line because the IgG expression levels were significantly higher compared to HEK293-T cells.
Influence of cell culture medium and conditioned medium on the transfection efficiency
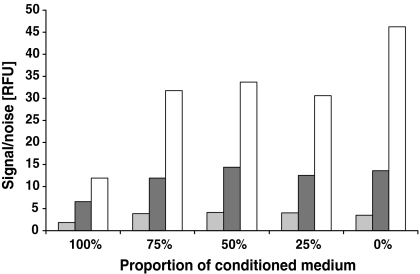
The HEK293-EBNA cell line could easily be adapted to two commercially available serum-free culture media, ExCell™ 293 and ExCell™ V-Pro. Unfortunately both media prevented transient transfection and the DHI medium supplemented with 2% FBS was selected as culture and transfection medium. It was observed that transfection of the cells a relatively short time after seeding into fresh medium resulted in higher protein expression than transfections at the same cell density 24 h later. We noticed that the transfected cells, which were simply diluted into fresh medium showed lower protein expression than transfected cells that were centrifuged and resuspended in 100% fresh medium. Therefore we investigated how much conditioned medium could be tolerated at the time of transfection. The experiment was done in shake flasks and several concentrations between 0% and 100% of a 72-h-old conditioned medium was added to the culture prior to transfection. The cells were transfected with a reporter plasmid (pCEP4-AmCyan) and the protein expression was measured daily for 3 days post transfection using the fluorescence assay. The use of more than 25% conditioned medium had no significant effect on protein expression up to 48 h post transfection but at 72 h the protein concentration was significantly reduced (Fig. 1). Surprisingly this effect was not observed when the conditioned medium was kept at 4 °C for 2–3 days before the initiation of a similar experiment. This strongly suggests that transfer of conditioned medium from the seeding culture to cultures intended for transient protein expression should be minimized.
Fig. 1.
Influence of conditioned medium on transient protein expression. Cells were seeded in culture medium at a density of 0.5 × 106 cells mL−1 with five different ratios of 72-h-old conditioned medium. The experiment was performed in 30 mL shaked cultures with pCEP4-AmCyan as expression vector. AmCyan expression was measured 24, 48 and 72 h post transfection using the signal-to-noise assay. Data (RFU) of one representative experiment are presented (░24 h, ▒48 h, □72 h)
Influence of the cell density and the time of transfection on expression levels
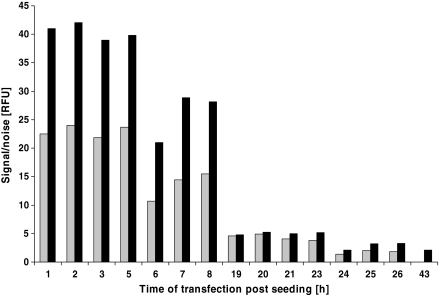
Different cell densities during transfection were tested and the best results were obtained at 0.5–1 × 106 cells mL−1. Transfection at cell densities over 1.5 × 106 cells mL−1 and below 0.5 × 106 cells mL−1 L showed reduction in protein yield. In order to define a time window for transfection, two Wave cultures were started at a cell density of 0.5 × 106 cells mL−1. Cells were taken from the Wave bags once every hour between 0 and 8 h and 19 and 26 h post inoculation. The cells were transferred into shaker flasks and transfected immediately with the pCEP4-AmCyan plasmid. The protein expression in the shake flasks was assayed 48 and 72 h post transfection using the fluorescence assay. The best time for the transfection of the cells was seen to be between 1 and 5 h post seeding. At 6–8 h the protein expression started to decline and transfection at 19 h and later showed an 80% drop in the signal-to-noise ratio. The data are presented in Fig. 2. Based on this result we routinely transfected the cells 2–4 h post seeding at a cell density of either 0.5 or 1 × 106 cells mL−1.
Fig. 2.
Influence of the time of transfection on transient protein expression. Cells were seeded in Wave bags at a density of 0,5 × 106 cells mL−1. Samples of 30 mL were taken almost every hour, transferred to a shaker or spinner flask and immediately transfected with a freshly prepared transfection complex using pCEP4-AmCyan. AmCyan expression was followed with the signal-to-noise assay for 3 days. Data of one representative experiment (Wave—shake flasks) are presented (░48 h, ▒72 h)
Implementation of a feeding strategy
When transfecting at a cell density of 1 × 106 cells mL−1 the culture started to lose viability after 4 days post transfection. The question of how to extend the production time was raised, as this would increase the final recombinant IgG yield. A bolus medium addition, doubling the culture volume post transfection and diluting the cells to 0.5 × 106 cell mL−1 was implemented. We have observed that the transfection process is completed approximately 2 h after adding the transfection complex. Results obtained demonstrated that any feeding time between 2 and 8 h post transfection leads to higher protein expression. However, feeding 4 h post transfection resulted in the highest protein expression. A feeding time 4 h post transfection was chosen for all further experiments. The effects of medium supplement on transient protein expression were addressed with a factorial experimental design approach. This study showed that different plant hydrolysates had a positive effect on protein expression (data not shown). Several of the tested hydrolysates showed a positive effect on cell growth, viability and protein expression and HyPep1510 was selected for further evaluation. The effect was found to be concentration dependent. When feeding the bioreactor to the final culture volume HyPep1510 was added as an individual feed. We conclude that feeding with HyPep1510 delays the decrease in cell viability after transfection.
Transient transfection in Wave bioreactor
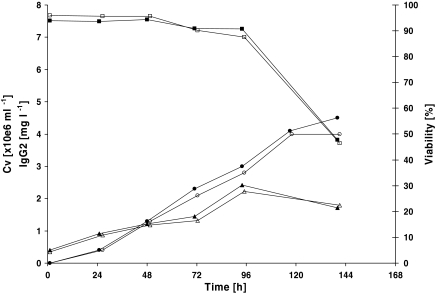
A first scale up was done in two 10 L (working volume) Wave bioreactors. Growth and productivity patterns were similar between the cultures. After 4 days of growth the viable cell density reached 2.4 × 106 cells mL−1 in both cultures and these were harvested 6 days post transfection. The IgG level in both cultures was 4 and 4.5 mg L−1 respectively (Fig. 3).
Fig. 3.
Transfection of a 10 L HEK293-EBNA cells culture in a Wave Bioreactor. In two experiments (open symbols experiment 1, closed symbols experiment 2) cells were seeded in Wave bags at a density of 1 × 106 cells mL−1. Two hours later the cultures were transfected and after an additional 4 h incubation, the cultures were fed to the final culture volume. IgG concentration (○●) was determined by ELISA and the viable cell concentration (△▲) and viability (□■) were assessed using a Cedex automatic cell counter
Production of IgG in 50 and 100 L bioreactors
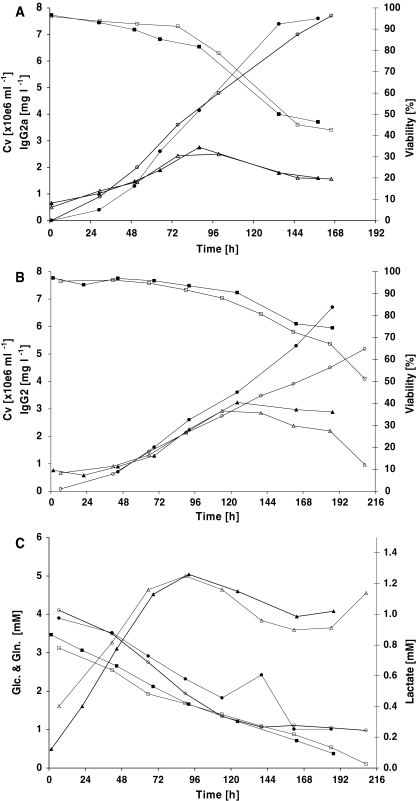
Further scale up to two 50 L production and two 100 L production bioreactors was carried out. Growth and productivity showed high reproducibility between duplicate runs in the same scale and were comparable between the two scales (Fig. 4a and b). A maximum cell density of approximately 3 × 106 cells mL−1 was reached in all cultures. The viability decreased faster for the 50 L scale productions and dropped below 50% after 6 days when the culture was harvested. The 100 L productions were terminated after 8–9 days although the viability still exceeded 50%. Glucose, lactate and glutamine concentrations were monitored only for the 100 L cultivations (Fig. 4c). The 50 L cultures reached slightly more than 7.5 mg L−1 IgG on the day of harvest. The first 100 L production reached 5.2 mg L−1 whereas the second reached as high as 6.7 mg L−1 on the day of harvest. The lower cell density can explain the reason for the lower production in the first run.
Fig. 4.
Transfection of 50 L HEK293-EBNA cells cultured in a 50 L (a) and 100 L (b) stirred tank bioreactor. In two experiments (open symbols experiment 1, closed symbols experiment 2) cells were seeded from a seeding bioreactor at a density of 1 × 106 cells mL−1. Two hours later the cultures were transfected and after an additional 4 h the cultures were fed to the final culture volume. IgG concentrations (○●) were determined by ELISA and the viable cell concentration (△▲) and viability (□■) were assessed using a Cedex automatic cell counter. (c) Shows the glucose (□■), lactate (△▲) and glutamine (○●) concentration monitored for the two 100 L cultures
It can be seen that for all scales, including the Wave experiments, the IgG was accumulating although the culture was degenerating. Due to logistical reasons it was decided to terminate the productions after a maximum of 9 days, although extending the culture for some time could have increased the final product yield.
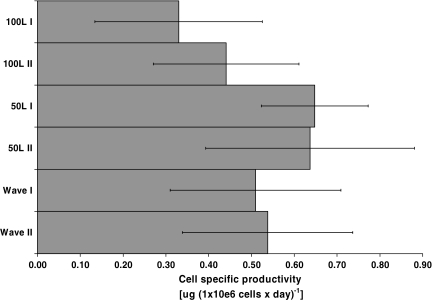
In Fig. 5 the average cell specific productivities are compared. The highest specific productivity was achieved in the 50 L scale followed by the Wave experiments. The 100 L cultures showed the lowest cell specific productivity indicating possible lower transfection efficiency at this scale.
Fig. 5.
Comparison of the average cell specific productivities at 10, 50 and 100 L production scale. The average cell specific productivities were calculated over the whole production process as ((ΔIgG Δtime−1 ΔCv−1) × 24 h). The error bars show the standard deviation
Purification of IgG
The purification of the IgG consisted of rProteinA affinity chromatography, cation exchange chromatography and buffer exchange over a size exclusion column as described under material and methods. The antibody was purified reproducibly to greater than 99% purity with a yield of 86% from cell broth to formulation buffer. For material intended for in vivo studies an endotoxin reduction step was added when necessary (Ritzen et al. 2007).
Process time lines
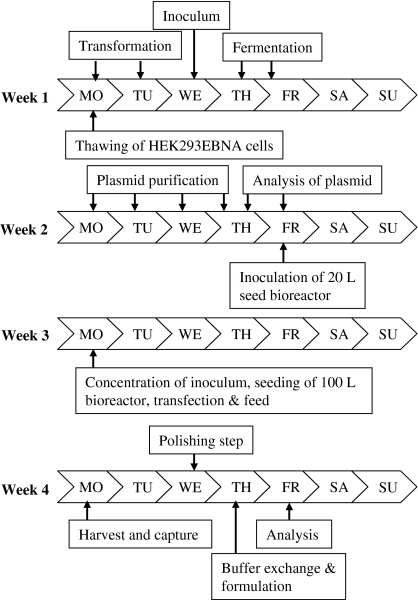
The transient transfection process for a 50 and 100 L transient production starting from large-scale plasmid production to final product formulation is summarised in Fig. 6. The process time lines are based on a 7-day production time and take a total of 4 weeks. This working scheme has been applied several times in different projects allowing flexibility without changing the total time needed.
Fig. 6.
Transient transfection working scheme for transfections in 50 and 100 L bioreactors. The key-events from cell thawing to the final product formulation are indicated
Discussion
The increasing demand for recombinant proteins expressed in mammalian cells emphasizes the need for alternative scalable expression methods other than stable clones. Here we provide data showing that large-scale transient transfection can speed up recombinant protein supply of moderate amounts of pure protein (1–2 g) for research. In comparison to stable expression, the transient system is less time consuming. The described process takes four weeks from large-scale plasmid preparation to purified IgG protein. In this study we have expressed 4–7.5 mg L−1 depending on the bioreactor system and scale using the same plasmid as is used for stable cell line generation. The plasmid was constructed for stable integration into HEK293-T cells and does not contain the EBNA-1 sequence supporting episomal replication. Our results showed that the HEK293-EBNA cell line expressed significantly higher IgG titres than the HEK293-T cells. The most successful cell line currently employed in transient expression is HEK293-EBNA (Durocher et al. 2002; Girard et al. 2002; Schlaeger et al. 2003; Pham et al. 2003; Geisse et al. 2005). One genetic feature is the presence of the Adenovirus-derived E1a protein in the cells driving and enhancing transcription from a CMV promotor preceding the gene of choice. Additionally these cells are easily transfectable and easy to cultivate adherently and in suspension. The cell line used can be maintained easily in culture medium containing 1–2% (v/v) foetal bovine serum and was adapted successfully to serum free suspension culture by sequential weaning from the original DMEM medium plus 10% FBS to two commercially available serum-free media. Unfortunately these media were not compatible with our transfection protocol. This problem was solved by Geisse and Henke (2005), which resulted in the development of a proprietary culture medium for HEK293-EBNA cells supporting growth and transfection. Pham et al. (2003) took a different approach and stably transfected the 293SF cell line with an expression vector encoding the EBNA protein creating the 293SFE cell line that can be cultured and transfected in a serum free medium.
In another project, a similar IgG gene was cloned into the pCEP4 vector. The pCEP4 vector is, contrary to the vector used in this work, specially designed for transient expression and contains the viral EBV origin of replication and the EBNA-1 sequence in addition to the CMV promotor. When using this more suitable construct, IgG concentrations of up to 20 mg L−1 were reached at large-scale (data not presented). This result emphasises the importance of choosing an optimal vector designed for episomal transcription. Even though we used a sub optimal vector, we achieved expression levels up to 8 mg mL−1, demonstrating that, in some cases, re-cloning of the gene of interest is not necessary. Previously reported yields for secreted proteins in stirred bioreactors are 2.5–28 mg L−1 (Durocher et al. 2002; Girard et al. 2002; Baldi et al. 2005). Durocher et al. (2002) have expressed various secretory proteins in either a 3 L bioreactor or a 14 L bioreactor. Although they used the same plasmid and transfection protocol, protein expression levels varied between 1 and 20 mg L−1 at time of harvest (9 days), showing that the expression level is very much protein dependent. The variation in expression level was even higher for recombinant intracellular proteins (3–50 mg L−1). These experiments were performed in shaker flasks (Durocher et al. 2002).
Polyethyleneimine is commercially available as branched and linear molecules of different molecular weights. The linear 25 kDa PEI reagent has been shown to be most effective in transient transfection trials (Durocher et al. 2002). Following an optimization and evaluation process at small scale (Davies et al. 2005), it became our transfection reagent of choice at large scale. The ratio of plasmid DNA to PEI reagent is one of the most critical parameters to be assessed. The results of several studies (Durocher et al. 2002) showed that (Schlaeger and Christensen 1999; Schlaeger et al. 2003; Geisse and Henke 2005) a two-fold excess of PEI reagent over DNA appears to be crucial, whereas the absolute quantities of plasmid and PEI seems to be dependent on the plasmid used.
Pham et al. (2003) and Baldi et al. (2005) described a transfection protocol where the cells are seeded in fresh culture medium at cell densities between 0.25 × 106 and 1 × 106 cells 24–48 h prior to transfection. When a similar protocol was used we noticed a significant reduction in protein expression. Our results confirm previous data by Schlaeger and Christensen (1999) showing that PEI-mediated transient transfection is influenced negatively by conditioned medium. It is well known that cells produce autocrine factors that can have an influence on cell growth (Ozturk and Palsson 1990). These molecules accumulate in the medium and it is believed that they can interfere with the transfection process. Glycosaminoglycans (GAG’s) are known to be critical in intercellular communication. GAG’s are linear, highly charged, acidic polysaccharides commonly found linked to core proteins, so called proteoglycans (PG’s) present on the surface of all adherent animal cells and in the extra cellular matrix. These PGs appear to perform a vital role in cell signalling and cell-cell communication (Linhardt and Toida 2004). Cationic lipids (CL) or polymers are efficient carriers for DNA in vitro. Belting and Petersson (1999) could demonstrate that CL-mediated gene transfer is inhibited by secreted cellular proteins, in particular PG’s (Belting and Petersson 1999). PG’s form complexes with CL’s, leading to the release of DNA and intracellular accumulation of CL-PG complexes. This results in inhibition of DNA uptake and a significant reduction in reporter-gene expression (Belting and Petersson 1999; Belting et al. 2005; Gardner et al. 2007). These findings support our observation that fresh conditioned medium has a negative effect on protein expression. Surprisingly, this negative effect of conditioned medium disappeared when the medium was stored for at least 48 h at 4 °C. One possible explanation could be the adsorption of the inhibiting substances to the wall of the storage vessel when stored at 4 °C. Another possible explanation could be that GAG’s secreted into the culture medium become enzymatically or chemically degraded during storage. It is interesting that Durocher et al. (2002) did not observe a significant effect of conditioned medium on protein expression (Durocher et al. 2002). This could be related to the genetic variant of the HEK host cell line (suspension cell line) and the cell culture medium used. Our host cell lines (HEK293-EBNA and HEK293-T) were both adherent cell lines adapted to growth in suspension. Both suspension adapted cell lines adhere to tissue culture dishes almost immediately in static cultures indicating that the cells maintained the capability of adhesion related to the composition of the extracellular matrix. In order to circumvent any influence of conditioned medium a cell concentration step was included when seeding the bioreactor. The cell density at the time of transfection was optimized. It seems to be important that the seeding culture is in the logarithmic growth phase and that the cell density at the beginning of the production phase reaches approximately 0.5 × 106 cells mL−1. At the time of transfection a cell density between 0.5 and 1 × 106 cells mL−1 is recommended. These results are in agreement with previous reports (Durocher et al. 2002; Geisse and Henke 2005; Baldi et al. 2005). The supplementation of medium with the plant hydrolysate HyPep1510 has a beneficial effect on the final protein concentration due to the fact that the HEK293-EBNA cells have a prolonged time at high viability post transfection. The addition of low Primatone concentrations in the feed mixture at maximal cell number was found by Schlaeger et al. to support a significant increase in protein yield per transfected cell (Schlaeger 1996; Schlaeger et al. 2003). Pham et al. (2003) evaluated different peptones and showed that many were effective in promoting cell growth and two of them had an additional positive effect on transfection efficiency allowing transfection to be performed in serum-free medium with an expression level close to that obtained with 1% serum-supplemented medium (Pham et al. 2003).
Our results show that the IgG concentration was lower at the 100 L scale than at the 50 L scale (Fig. 4a and b). This was also the case for the average cell specific productivity (Fig. 5) and for the daily cell specific productivity at the corresponding cell densities during the culture period (data not shown). One possible explanation for the lower cell specific productivity could be a difference in transfection efficiency or uptake of the DNA into the nucleus. Carpentier et al. (2007) investigated limiting factors governing protein expression following polyethylenimine-mediated gene transfer in HEK293-EBNA1 cells. This study described that transfection efficiencies can vary significantly from one experiment to another even though the same transfection parameters and culture conditions were rigorously applied at all times. Additionally it is discussed that gene expression depends on certain cellular characteristics of physiological traits that allow the DNA-PEI polyplexes to be efficiently translocated in the nucleus and transcribed.
The reduced growth rates at 100 L scale could be an effect of different mixing characteristics between the 50 and the 100 L scale. The scale-up from 50 to 100 L was done based on geometry by keeping the relative bioreactor dimensions and the impeller positions constant at both scales. But we noted that the relative impeller size was not kept constant. The impeller diameter to vessel diameter ratio was 10% larger for the 50 L bioreactor than for the 100 L bioreactor (0.55 compared to 0.5). The agitation was scaled-up keeping the tip speed constant. As shown by Yang et al. (2007), scaling up agitation based on constant tip speed gives a constant shear rate but leads to longer mixing time at larger scale. This difference in mixing characteristics can possibly explain the reduced growth rates at the 100 L scale.
The purification method followed a simple and efficient purification cascade using only three chromatography steps that is standard in large-scale downstream processing for monoclonal antibodies and Fc fusion proteins (mAbs) (Kelley 2007; Abhinav et al. 2007). Protein A chromatography served as the key volume reduction and capture step. This step has proven to be highly selective for mAbs and usually yields >99% purity starting from the cell culture supernatant. As polishing step we used ion exchange chromatography to remove host cell proteins. Following the completion of downstream purification, the protein is buffer exchanged into the formulation buffer. This is usually best accomplished by the use of an ultrafiltration/diafiltration setup at large scale (Abhinav et al. 2007). However, we decided to us a standard desalting column with G-25 medium Sephadex because it could be adapted very easily to our relative small volumes.
The time lines for the cell culture part from thawing of the cells to the final harvest (two weeks) and for the purification of the antibody (one week) were consistent, whereas process time for the production of 50 to 100 mg of plasmid may vary because of various copy numbers. Low copy number plasmids necessitate the processing of larger amounts of biomass with two weeks required at most.
In the present work we described the robustness of our transient transfection process by demonstrating reproducibility at three different scales (10, 50 and 100 L) and in two different bioreactor systems (Wave bioreactor and stirred tank bioreactors). All experiments were performed as individual experiments at different times. As a result of this work, a large-scale plasmid production, expression and purification process for IgG is established. Although this process has demonstrated robustness, improvements can still be made, such as the development of a specially designed plasmid for transient transfection as described by Durocher et al. (2002) and Geisse and Henke (2005) and the implementation of a serum-free culture medium allowing successful transfection with PEI to facilitate recombinant protein purification (Geisse and Henke 2005).
Acknowledgements
We thank our colleagues at process R&D Anna Bogstedt, Ingrid Fagervall for ELISA support and Maud Lennmark for endotoxin measurements. We thank or colleagues Sebastian Baumann, Thomas Falkman, Ulrika Ritzén and Stefan Schmidt for their contribution and support to this work and Catherine Heddle and Ian W. Taylor for critical reading of the manuscript.
References
- Abhinav A, Shukla A, Hubbard B, Tressel T et al (2007) Downstream processing of monoclonal antibodies—Application of platform approaches. J Chromatogr 848:28–39 [DOI] [PubMed]
- Baldi L, Muller N, Picasso S et al (2005) Transient gene expression in suspension HEK-293 cells: application to large-scale protein production. Biotechnol Prog 21:148–153 [DOI] [PubMed]
- Belting M, Petersson P (1999) Intracellular accumulation of secreted proteoglycans inhibits cationic lipid-mediated gene transfer. Co-transfer of glycosaminoglycans to the nucleus. J Biol Chem 274(27):19375–19382 [DOI] [PubMed]
- Belting M, Sandgren S, Wittrup A (2005) Nuclear delivery of macromolecules: barriers and carriers. Adv Drug Deliv Rev 57(4):505–527 [DOI] [PubMed]
- Birch J, Onakunle Y (2005) Biopharmaceutical proteins: opportunities and challenges. In: Smales CM, James DC (eds) Therapeutic proteins: methods and protocols. Totowa, Humana Press, pp 1–16 [DOI] [PubMed]
- Boussif O, Lezoualch F, Zanta MA et al (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethyleneimine. Proc Natl Acad Sci USA 92:7297–7301 [DOI] [PMC free article] [PubMed]
- Cachianes G, Ho C, Weber RF et al (1993) Epstein-Barr virus-derived vectors for transient and stable expression of recombinant proteins. Biotechniques 15:255–259 [PubMed]
- Carpentier E, Paris S, Kamen AA et al (2007) Limiting factors governing protein expression following polyethylenimine-mediated gene transfer in HEK293-EBNA1 cells. J Biotechnol 128(2):268–280 [DOI] [PubMed]
- Chamsart S, Patel H, Hanak JA et al (2001) The impact of fluid-dynamic-generated stresses on chDNA and pDNA stability during alkaline cell lysis for gene therapy products. Biotechnol Bioeng 75:387–389 [DOI] [PubMed]
- Davies A, Greene A, Lullau E et al (2005) Optimisation and evaluation of a high-throughput mammalian protein expression system. Protein Expr Purif 42(1):111–121 [DOI] [PubMed]
- Derouazi M, Girard P, Van Tilborgh F et al (2004) Serum-free large-scale transient transfection of CHO cells. Biotechnol Bioeng 87:537–545 [DOI] [PubMed]
- Durocher Y, Perret S, Kamen A (2002) High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acid Res 30: E9 [DOI] [PMC free article] [PubMed]
- Galbraith DJ, Tait AS, Racher AJ et al (2006) Control of culture environment for improved polyethylenimine-mediated transient production of recombinant monoclonal antibodies by CHO cells. Biotechnol Prog 22:753–762 [DOI] [PubMed]
- Gardner RA, Belting M, Svensson K et al (2007) Synthesis and transfection efficiencies of new lipophilic polyamines. J Med Chem 50(2):308–318 [DOI] [PubMed]
- Geisse S, Henke M (2005) Large-scale transient transfection of mammalian cells: a newly emerging attractive option for recombinant protein production. J Struct Funct Genomics 6:165–170 [DOI] [PubMed]
- Geisse S, Di Maiuta N, Ten Buren B, Henke M (2003) The secrets of transfection in serum-free suspension culture. In: Godia F, Fussenegger M (eds) Proceedings of the 18th ESACT meeting, Animal cell technology meets genomics. Springer, Dordrecht, pp 373–376
- Geisse S, Jordan M, Wurm FM (2005) Large-scale transient expression of therapeutic proteins in mammalian cells. Meth Mol Biol 308:87–98 [DOI] [PubMed]
- Girard P, Derouazi M, Baumgartner G et al (2002) 100-Liter transient transfection. Cytotechnology 38:15–21 [DOI] [PMC free article] [PubMed]
- Girard P, Porte L, Berta T et al (2001) Calcium phosphate transfection optimization for serum-free suspension culture. Cytotechnology 35:175–180 [DOI] [PMC free article] [PubMed]
- Graham FL, van der Eb AJ (1973) A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52:456–467 [DOI] [PubMed]
- Jordan M, Kohne C, Wurm FM (1998) Caclium-phosphate mediated DNA transfer into HEK-293 cells in suspension—controle of physicochemeical parameters allows transfection in stirred media—Transfection and protein expression in mammalian cells. Cytotechnology 26:39–47 [DOI] [PMC free article] [PubMed]
- Kelley B (2007) Very large scale monoclonal antibody purification: the case for conventional unit operations. Biotechnol Prog 23:995–1008 [DOI] [PubMed]
- Kunaparaju R, Liao M, Sunstrom NA (2005) Epi-CHO, an episomal expression system for recombinant protein production in CHO cells. Biotechnol Bioeng 91:670–677 [DOI] [PubMed]
- Lebkowski JS, Clancy S, Calos MP (1985) Simian virus 40 replication in adenovirus-transformed human cells antagonizes gene expression. Nature 317:169–171 [DOI] [PubMed]
- Linhardt RJ, Toida T (2004) Role of glycosaminoglycans in cellular communication. Acc Chem Res 37:431–438 [DOI] [PubMed]
- Meissner P, Girard P, Kulangara A, Tasao MC, Jordan M, Wurm FM (1999) Process development for transient gene expression in mammalian cells at the 3 L scale: 10-50 mg of r-protein in days. In: Bernard A, Griffiths B, Noé W, Wurm FM (eds) Proceedings of the 16th ESACT meeting, Animal Cell Technology: Products from cells, cells as product. Kluwer Academic Publisher, Dordrecht, pp 351-357
- Meissner P, Pick H, Kulangara A et al (2001) Transient gene expression: Recombinant protein production with suspension-adapted HEK293-EBNA cells. Biotechnol Bioeng 75:197–203 [DOI] [PubMed]
- Ozturk SS, Palsson BO (1990) Effect of initial cell density on hybridoma growth, metabolism, and monoclonal antibody production. J Biotechnol 16(3–4):259–278 [DOI] [PubMed]
- Petsch D, Anspach FB (2000) Endotoxin removal from protein solutions. J Biotechnol 76(2–3):97–119 [DOI] [PubMed]
- Pham PL, Perret S, Doan HC et al (2003) Large-scale transient transfection of serum-free suspension-growing HEK293 EBNA1 cells: peptone additives improve cell growth and transfection efficiency. Biotechnol Bioeng 84:332–342 [DOI] [PubMed]
- Ritzen U, Rotticci-Mulder J, Stromberg P et al (2007) Endotoxin reduction in monoclonal antibody preparations using arginine. J Chrom B: Anal Technol Biomed Life Sci 856(1–2):343–347 [DOI] [PubMed]
- Schlaeger EJ (1996) The protein hydrolysate, Primatone RL, is a cost-effective multiple growth promoter of mammalian cell culture in serum-containing and serum-free media and displays anti-apoptosis properties. J Immunol Methods 194(2):191–199 [DOI] [PubMed]
- Schlaeger E-J, Christensen K (1999) Transient gene expression in mammalian cells grown in serum-free suspension culture. Cytotechnology 30:71–83 [DOI] [PMC free article] [PubMed]
- Schlaeger E-J, Legendre JY, Trzeciak A et al (1998) Transient transfection in mammalian cells: a basic study for an efficient and cost-effective scale up process. In: Meren O-W, Perrin P, Griffith R (eds) Proceedings of the 15th ESACT meeting, New developments and new applications in animal cell technology. Kluver Academic Publisher, Dordrecht, pp 105–111
- Schlaeger E-J, Kitas EA, Dorn A (2003) SEAP expression in transiently transfected mammalian cells grown in serum-free suspension culture. Cytotechnology 42:47–55 [DOI] [PMC free article] [PubMed]
- Sun X, Hia HC, Goh PE et al (2008) High-density transient gene expression in suspension-adapted 293 EBNA1 cells. Biotechnol Bioeng 99:108–116 [DOI] [PubMed]
- Tait AS, Brown CJ, Galbraith DJ et al (2004) Transient production of recombinant proteins by Chinese hamster ovary cells using polyethyleneimine/DNA complexes in combination with microtubule disrupting anti-mitotic agents. Biotechnol Bioeng 88:707–721 [DOI] [PubMed]
- Varley J, Birch J (1999) Reactor design for large scale suspension animal cell culture. Cytotechnology 29(3):177–205 [DOI] [PMC free article] [PubMed]
- Wurm F, Bernard A (1999) Large-scale transient expression in mammalian cells for recombinant protein production. Curr Opin Biotechnol 10:156–159 [DOI] [PubMed]
- Yang JD, Lu C, Stasny B et al (2007) Fed-batch bioreactor process scale-up from 3-L to 2,500-L scale for monoclonal antibody production from cell culture. Biotechnol Bioeng 98:141–154 [DOI] [PubMed]