Abstract
Osteoclasts are large multinucleated, bone-resorbing cells derived from hematopoietic precursors in response to receptor activator of nuclear factor-κB ligand (RANKL). RANKL activates a number of signal transduction pathways, which stimulate, in turn, a series of specific transcription factors that initiate the process of osteoclastogenesis. Perhaps the most important of these is nuclear factor of activated T cells cytoplasmic 1 (NFATc1), a DNA-binding protein that upon activation translocates to the nucleus where it stimulates transcription. The objective of this study was to explore the process whereby RANKL induces NFATc1 and to assess the role of this factor in the activation of an additional key osteoclast target gene. We found that whereas several NFAT members are expressed in RAW264.7 cells, soluble RANKL-induced up-regulation is limited to NFATc1 through a mechanism that is largely autoregulatory. Thus, although we observed the presence of resident NFAT members at the inducible Nfatc1 P1 promoter at very early times after RANKL treatment, a selective and time-dependent increase in the binding of up-regulated NFATc1 to Nfatc1 was observed beginning at 12 h. Several additional factors that are activated by soluble RANKL and also participate in NFATc1 up-regulation include c-Fos and RNA polymerase II. Chromatin immunoprecipitation analysis also revealed a similar, time-dependent accumulation of NFATc1 at multiple sites on the Acp5 promoter, thereby highlighting a central contributing role for NFATc1 in the activation of this gene as well. Our studies provide additional molecular detail regarding the mechanisms through which RANKL induces NFATc1 in osteoclast precursors and into mechanisms by which NFATc1 induces the expression of at least one gene responsible for the osteoclast phenotype.
NORMAL TURNOVER IN adult bone is maintained through the coordinated activities of bone-resorbing osteoclasts and matrix-secreting osteoblasts. Osteoclasts are giant, multinucleated cells that form on bone surfaces, express tartrate-resistant acid phosphatase 5 (TRAP), and function both to degrade osteoid matrix and to release mineral from bone. Osteoclasts are derived from monocytic/macrophagic hematopoietic precursors, which are prompted to differentiate into multinucleated cells in response to receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL), a cell surface cytokine produced by a variety of support cells including stroma and osteoblasts (1). The binding of RANKL to receptor activator of NF-κB (RANK), a receptor located on the surface of monocyte precursors, triggers the stimulation of a number of signaling cascades the integrated actions of which not only initiate osteoclast differentiation, but induce activation and survival as well (2).
Nuclear factor of activated T cells cytoplasmic 1 (NFATc1) represents a key transcription factor target that is up-regulated by RANKL stimulation (3,4). The up-regulation of this transcription factor has been shown to be a necessary precursor to the formation of multinucleated polykaryons capable of actively resorbing bone. The essentiality of NFATc1 in the differentiation process was confirmed though the use of knockout and rescue experiments (5,6), although the direct targets of NFATc1 action and the mechanism by which this transcription factor functions to induce osteoclastogenesis is not entirely clear. The NFAT family of transcription factors comprises the prototypical NFATs (NFATc1–4) as well as NFAT5 (7). These proteins possess a conserved Rel DNA-binding motif reminiscent of that found in NF-κB proteins (7). Activation of NFATs occurs in the cytoplasm through an interaction with calcineurin, a phosphatase which functions to dephosphorylate specific serine residues within the NFAT protein that results in the unmasking of a sequence that facilitates rapid nuclear localization (7). The NFAT proteins bind to a conserved WGGARAA consensus sequence. This consensus can be considerably degenerate, however, due to interactions with heterodimer partners shown in previous studies to include such proteins as p300, CCAAT enhancer-binding protein, mouse embryo fibroblast 2, activator protein 1 (AP-1), and GATA binding proteins (8). Simultaneous activation of the partner protein through additional signaling pathways may be, in some cases, a requirement for NFATc1-DNA binding or full NFATc1 activity.
In this paper, we investigate the mechanism by which RANKL induces and maintains the expression of the key osteoclast transcription factor NFATc1. We also explore the mechanism whereby NFATc1 induces, in turn, the expression of the classic osteoclast target gene TRAP (Acp5). We find that although several NFATs are expressed in the undifferentiated monocyte precursor and may play an initial role in NFATc1 regulation, transcriptional induction of NFATc1 by soluble RANKL (sRANKL) leads to an NFATc1 autoregulatory process that is likely to be critical for sustained osteoclast differentiation. Induced NFATc1 also binds to multiple sites on the Acp5 promoter, thereby inducing this gene’s expression as well. Our results provide additional molecular insight into the mechanism by which RANKL induces osteoclastogenesis from hematopoietic cell precursors.
RESULTS
Induction of Nfatc1 mRNA and NFATc1 Protein by RANKL
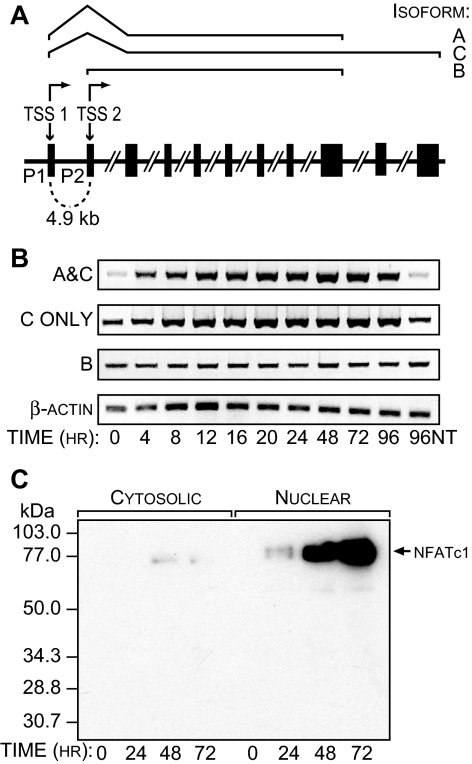
The induction of NFATc1 by RANKL is believed to be central to the maturation and differentiation of mononuclear osteoclast precursors into functional multinucleated osteoclasts (3,4). NFATc1, however, comprises three isoforms (A–C) derived from two separate NFATc1 promoters (P1 and P2), as depicted in Fig. 1A (9). To explore the induction of NFATc1 in osteoclasts, we used the murine cell line RAW264.7. This monocyte/macrophage-like line has been shown previously to be capable of differentiating into functional bone-resorbing osteoclasts in the absence of other supporting cell types (10). These cells were treated with a truncated and soluble form of RANKL that lacks the transmembrane domain (sRANKL) for periods up to 96 h, after which the appearance of NFATc1 mRNA isoforms B, C, and A/C were measured. Isoform A is not independently distinguishable using the primer sets we employed. The results in Fig. 1B reveal that the B isoform is constitutively present in RAW264.7 cells and is not induced by sRANKL. In contrast, the C isoform, and possibly A as well, are induced by sRANKL. We also investigated the kinetics of the production of NFATc1 by subjecting RAW264.7 cells to maximal levels of sRANKL, isolating both the cytoplasmic and nuclear fractions, and assessing the abundance of NFATc1 in both cellular compartments by Western blot analysis. As shown in Fig. 1C, whereas NFATc1 levels are extremely low in the uninduced state in both the cytoplasmic and nuclear fractions, they increase dramatically in the nuclear compartment after treatment with sRANKL. Interestingly, the increase in protein levels within the cell appears substantially delayed relative to the increase in mRNA levels seen within 4 h. Whether this difference reflects a true lag period in protein production or reflects a difference in analytical detection is unclear. All NFATc1 mRNA levels in the studies that follow are determined using a primer set that is specific for the A/C isoforms.
Figure 1.
RANKL Induces the Synthesis and Expression of Nuclear NFATc1 in RAW264.7 Cells
A, Expression of the murine NFATc1 gene is controlled by two independent promoters P1 and P2. The P1 promoter regulates the synthesis of isoforms A and C, which arise exclusively through the use of alternative polyadenylation sites in the 3′-untranslated region. B, RANKL induces NFATc1 transcripts in RAW264.7 cells. Cells were treated with soluble RANKL (300 ng/ml) for the indicated time periods, and isolated RNA was examined for the presence of β-actin as well as NFATc1 isoforms C, B, and A plus C. C, Expression of NFATc1 in RAW264.7 cells. Cells were treated with sRANKL as above, and the nuclear and cytosolic fractions were subjected to Western blot analysis using antibodies to NFATc1. These data are representative of three independent studies. NT, No treatment.
RANKL Induction of NFATc1 Expression Is Due to Transcriptional Activation and Not mRNA Stabilization
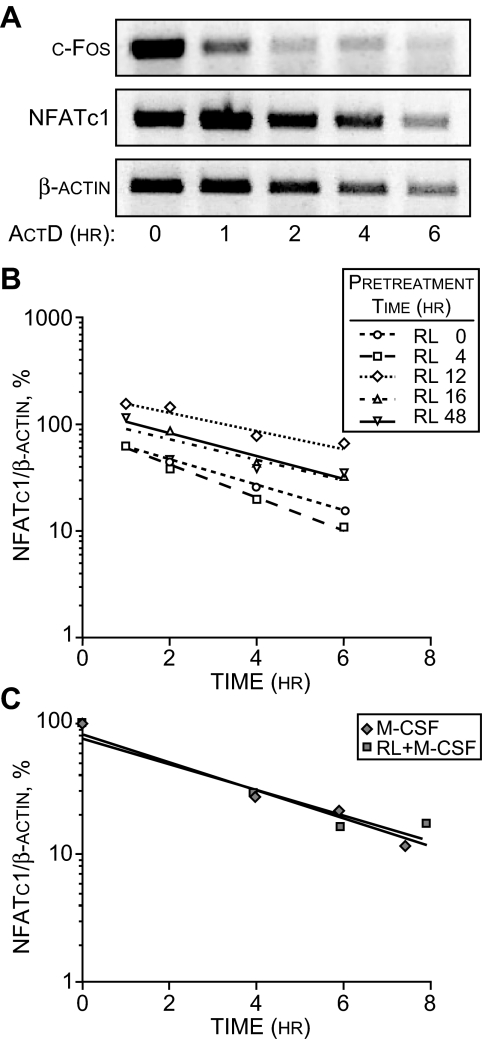
An increase in mRNA levels can arise as a result of either increased production or decreased degradation. Whereas previous studies have indicated a largely transcriptional mechanism of NFATc1 up-regulation (5,6), we first explored the possibility that the up-regulation of NFATc1 by RANKL might also involve an increase in NFATc1 mRNA stability. We therefore performed a standard actinomycin D (ActD) experiment wherein we treated RAW264.7 cells with sRANKL for 12 h (to induce NFATc1 levels), added a maximal dose of ActD, and then measured the levels of NFATc1 mRNA from 1–6 h later. We contrasted the decay rate of mRNA for NFATc1 with that of c-Fos, an mRNA that is known to be rapidly degraded and displays a half-life of less than 30 min. As can be seen in Fig. 2A, although NFATc1 mRNA decreases as a function of time in the presence of ActD as expected, its decay rate of more than 3 h was much slower than that for c-Fos. Thus, the NFATc1 mRNA is more stable than that of c-Fos and similar to that of β-actin. Figure 2B reveals, however, that regardless of the length of time of sRANKL induction, the decay rate of NFATc1 mRNA remained unchanged and independent of this parameter. NFATc1 is also induced by sRANKL in splenic monocytes (5,6). To test whether RANKL influenced the stability of NFATc1 mRNA in these primary osteoclast precursors, we first established an NFATc1 mRNA induction profile in monocytes in response to sRANKL and macrophage colony-stimulating factor (M-CSF) (compared with M-CSF alone). sRANKL was shown to induce NFATc1 in splenic monocytes within 2 d of treatment (data not shown). Based upon this finding, we then treated the monocytes with sRANKL and M-CSF or M-CSF alone for 2.5 d, added ActD at an effective concentration of 3 μg/ml, and measured the decrease in NFATc1 mRNA levels over the ensuing 8-h period. As can be seen in Fig. 2C, although the absolute cellular levels of NFATc1 mRNA differed substantially after treatment with either M-CSF or M-CSF and RANKL, inhibition of NFATc1 mRNA by ActD resulted in a similar degradation rate between the two treatments. This half-life of approximately 2 h was very similar to that seen in the RAW264.7 cell line. We conclude that the increase in NFATc1 mRNA abundance in osteoclast precursors after RANKL treatment is not due to an increase in mRNA stability.
Figure 2.
The Presence of RANKL Does Not Alter NFATc1 mRNA Stability
A, NFATc1 transcripts are more stable than c-Fos mRNA. RAW264.7 were treated with sRANKL (sRL) (300 ng/ml) for 12 h to up-regulate NFATc1 mRNA and then exposed to ActD (3 μg/ml) for the periods indicated. RNA was then collected and subjected to RT-PCR analysis for β-actin (20 cycles), NFATc1 (21 cycles), or c-Fos (25 cycles) using the primers indicated in Materials and Methods. B, The stability of NFATc1 mRNA is independent of RANKL induction time in RAW264.7 cells. Cells were treated with sRANKL for increasing periods of time and exposed to ActD (3 μg/ml), and isolated RNA was subjected to qRT-PCR analysis. The amount of NFATc1 mRNA present at each time point after ActD treatment was contrasted to that before the administration of ActD, and all concentrations were normalized to the amount of β-actin mRNA present. Each determination is the average of three independent measurements. C, RANKL does not alter the stability of NFATc1 mRNA derived from primary splenic monocytes. Monocytes were isolated from C57Bl/6 mice and cultured for 2.5 d in the presence of M-CSF (10 ng/ml) alone or M-CSF (10 ng/ml) plus sRANKL (300 ng/ml). Cells were then treated with ActD (3 μg/ml), and total RNA was harvested at the times indicated in the figure. The amount of NFATc1 mRNA present at each time was determined using qRT-PCR analysis and normalized to that present before the addition of ActD. Each determination represents the average of three independent measurements.
NFATc1 Is Rapidly Degraded
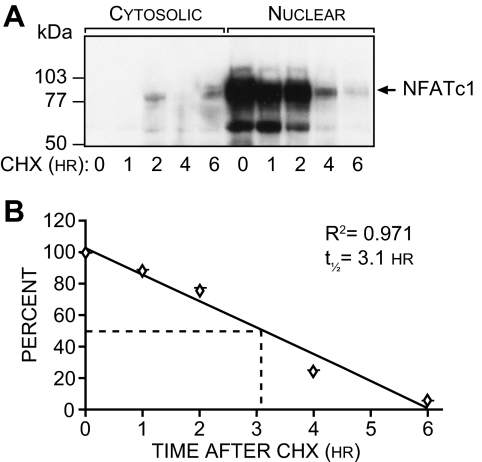
Most transcription factors are rapidly degraded in cells and manifest a relatively short half-life. In view of the relative stability of the NFATc1 mRNA, we next measured the turnover rate of NFATc1. RAW264.7 cells were treated with sRANKL for a period of 12 h and then exposed to cycloheximide (CHX). Cells were harvested at 0, 1, 2, 4, and 6 h post-CHX treatment, and the level of nuclear NFATc1 was assessed by Western blot analysis as seen in Fig. 3A. Quantitation of the decay rate of NFATc1 protein is depicted in Fig. 3B, revealing an NFATc1 half-life of approximately 3.1 h. These results suggest that whereas NFATc1 mRNA is remarkably stable, the half-life of the protein itself is short and similar to that of other rapidly turned over transcription factors. Thus, it seems likely that a positive mechanism would be required to maintain the sustained NFATc1 up-regulation during the lengthy process of osteoclast differentiation.
Figure 3.
NFATc1 Is a Rapidly Degraded Nuclear Protein
A, Western analysis of NFATc1 expression. RAW264.7 cells were treated with sRANKL (300 ng/ml) for 24 h and then subjected to an additional treatment with CHX (10 μg/ml). Cells were isolated at the times indicated and fractionated to obtain cytosolic and nuclear components, and the components were then subjected to Western blot analysis using antibodies to NFATc1. The arrow indicates the presence of NFATc1 protein at approximately 90 kDa. B, Determination of NFATc1 half-life. The signals generated in panel A were quantitated densitometrically and normalized to the levels present before CHX administration. NFATc1, t1/2 = 3.1 h; r2 = 0.97. These data are representative of three independent experiments.
NFATc1 Autoregulates Its Own Transcription from the Inducible Nfatc1 Promoter P1
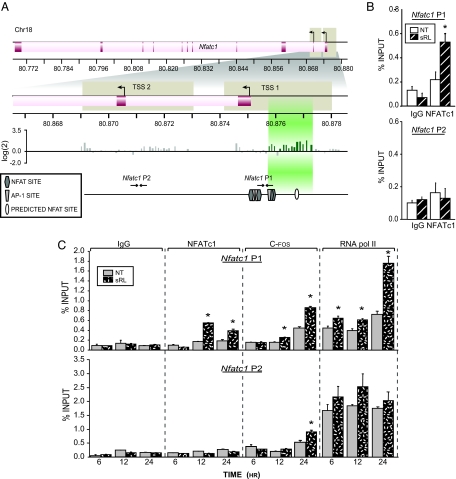
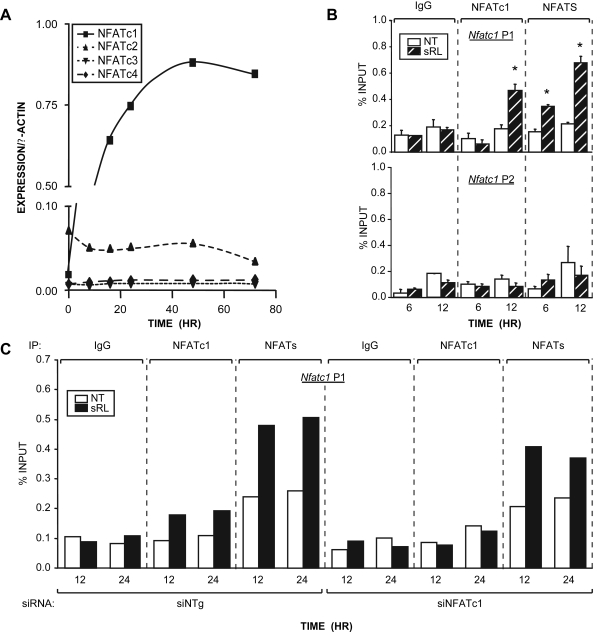
The expression of Nfatc1 is known to be regulated in T cells by both NFATc1 and NFATc2 (9,11). Indeed, binding sites have been identified at the Nfatc1 gene promoter that interact directly with NFATc1 and NFATc2 (9,11). We therefore explored the possibility that NFATc1, and perhaps NFATc2, might regulate the activity of Nfatc1 in osteoclast precursors as well. NFATc1 expression is mediated by two promoters: P1, which is responsible for the expression of the NFATc1 A and C isoforms, and P2, which is responsible for the expression of the additional NFATc1 isoform. Because several functional NFAT binding sites have been identified in the P1 promoter (9,11), we used an initial chromatin immunoprecipitation-DNA chip (ChIP-chip) approach to address whether RANKL could induce localization of NFATc1 to the Nfatc1 locus. Cells were treated with sRANKL for a period of 48 h and subjected to chromatin immunoprecipitation (ChIP) using antibodies to NFATc1, and the coprecipitated DNA was then used to scan the extended region comprising both P1 and P2 on a DNA microarray containing oligonucleotides that spanned the promoter regions at approximately 100-bp resolution. Figure 4A reveals the presence of a significant NFATc1 binding peak located between −650 and −2099. This peak of NFATc1 activity appears to partially overlap a previously defined NFATc1 binding site located immediately adjacent to an AP-1 site and extends also to an upstream region, which contains a new and well-defined consensus site for NFATc1 at −2148 bp (TGGAAGT) (see Fig. 4C). The region containing this upstream element confers RANKL responsiveness to a reporter plasmid and mutation of the NFAT consensus site reduces this activation (see supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals online web site at http://mend.endojournals.org). No NFATc1 binding was observed at the Nfatc1 P2 promoter located downstream.
Figure 4.
NFATc1 Autoregulates Its Expression in Developing Osteoclasts
A, ChIP-chip analysis of the mouse Nfatc1 gene. RAW264.7 cells were cultured in the presence or absence of sRANKL (sRL) for 48 h and then subjected to a ChIP analysis using antibodies to NFATc1. The DNAs from sRL and control treated cells were separately amplified and labeled as outlined in Materials and Methods. The regions probed in the DNA array on chromosome 18 (Chr18) are delineated by tan boxes. The TSS and translational start sites (ATG) of Nfatc1 are shown. Signal intensity as log(cy3/cy5) ratios are shown for each probe. Peaks of NFAT binding are highlighted in green. Confirmed NFATc1 (hexagon) and AP-1 (trapezoid) binding sites as well as a predicted NFAT site (oval) are indicated for the P1 and P2 regions. The locations of the primers designed to amplify these regions are depicted with arrows and numbered according to their location relative to the P1 TSS. B, Confirmation of ChIP-chip results at promoters of Nfatc1. RAW264.7 cells were cultured in the presence or absence of sRL for 48 h and then subjected to ChIP analysis using antibodies to NFATc1 and an IgG control. The resulting coprecipitated DNA was evaluated by qRT-PCR analysis using primers capable of detecting the Nfatc1 P1 or P2. Signal intensities were normalized to their corresponding input signals. C, NFATc1 occupation of the Nfatc1 P1 region is delayed relative to the appearance of RNA pol II. RAW264.7 cells were treated with sRL for either 6, 12, or 24 h and then subjected to ChIP analysis using antibodies to NFATc1, c-Fos, RNA pol II, or IgG. The precipitated DNA was then evaluated for each of the time points as in panel B above. The data represent the average of triplicate determinations ± sem. *, P ≤ 0.05 compared with time-matched NT (no treatment) control (one-way ANOVA). These data are representative of more than 10 independent experiments.
To confirm these findings, we performed a direct ChIP analysis using primer sets capable of detecting NFATc1 at sites located within either the P1 or P2 promoters (see Fig. 4A). The results assessed 48 h after sRANKL treatment and documented in Fig. 4B confirm the presence of NFATc1 at the P1 but not the P2 promoter. This accumulation of NFATc1, as documented in Fig. 4C, was evident as early as 12 h after treatment. Importantly, this increase in NFATc1 localization at the P1 promoter was also associated temporally with an increase in c-Fos, as measured by ChIP analysis and documented in Fig. 4C. c-Fos represents a transcription factor that is also known to be activated by RANKL and to participate in NFATc1 activation. Modest c-Fos binding was also noted at the P2 promoter, suggesting a role for this factor in P2-mediated NFATc1 isoform expression. Finally, the recruitment of both c-Fos and NFATc1 were also correlated with an increase in the recruitment of RNA polymerase II (RNA pol II), a potential marker of enhanced mRNA output. Whereas RNA pol II was also present at Nfatc1 P2 and perhaps responsible for the low levels of constitutively expressed NFATc1 isoforms, it did not appear to be up-regulated in response to sRANKL. These findings suggest that NFATc1 may indeed autoregulate its own expression via a direct interaction with cis elements located within the vicinity of P1.
Nfatc1 autoregulation does not provide a mechanism for the initial induction of NFATc1 mRNA, however, because the protein levels of this factor are quite low for the first 12 h. c-Fos protein is also not detected at this early time (data not shown). We therefore explored the possibility that other members of the NFATc family might contribute to this initial induction by RANKL. To observe whether other NFATc family members were expressed in our model we first treated RAW264.7 cells with sRANKL and performed RT-PCR analysis to measure the amounts of NFATc2, -c3, and -c4 mRNA at sequential time points after the addition of sRANKL. Figure 5A reveals that NFATc2 mRNA is indeed present in RAW264.7 cells, although it is not induced by sRANKL. Western analysis of NFATc2 levels in RAW264.7 supports the relatively low expression seen at the mRNA levels (data not shown). NFATc3 and NFATc4 mRNAs were also present, although at very low levels and likewise not induced by sRANKL treatment.
Figure 5.
NFATc2 as well as Other NFAT Proteins Participate in the Initial Upregulation of Nfatc1 expression
A, NFAT expression in RAW264.7 cells is limited to NFATc1 and NFATc2. Cells were treated with sRANKL (sRL) (300 ng/ml) for the times indicated. Total RNA was collected and subjected to RT-PCR analysis using primers designed to amplify NFATc1 (21 cycles), NFATc2 (25 cycles), NFATc3 (35 cycles), NFATc4 (35 cycles), and β-actin (21 cycles). Densitometry was performed, and the relative expression levels of the test mRNAs were normalized to β-actin levels. B, Additional NFATc members activate Nfatc1 at early times. RAW264.7 cells were treated with sRL for either 6 or 12 h and then subjected to ChIP analysis using antibodies to NFATc1, IgG, or a pan antibody to NFATc1–4. The resulting coprecipitated DNA was evaluated by qRT-PCR analysis using primers capable of detecting the Nfatc1 P1 or P2. Signal intensities were normalized to their corresponding input signals. C, Binding of other NFATc members to the Nfatc1 promoter is supported by NFATc1 knockdown. RAW264.7 cells were transfected with siRNA to knockdown expression of NFATc1 (siNFATc1) or a genome-selected nontargeted negative control siRNA (siNTg). These cells were treated with sRL for either 12 or 24 h and then subjected to ChIP analysis and evaluated as described in panel B above. The data represent the average of triplicate determinations ± sem. *, P ≤ 0.05 compared with time-matched NT control (one-way ANOVA). These data are representative of more than three independent experiments. IP, Immunoprecipitation; NT, no treatment.
Based upon these findings, we next asked whether transcriptional regulation of the Nfatc1 gene was restricted to NFATc1 in RAW264.7 cells. Cells were treated with sRANKL for 6 or 12 h and subjected to a ChIP analysis using an antibody to NFATc1 as well as a pan antibody capable of detecting the presence of NFATc1, -c2, -c3, and -c4. As can be seen in Fig. 5B, whereas NFATc1 is not observed at the Nfatc1 P1 or P2 promoters at 6 h, other members of the NFATc family are clearly present at P1. This observed recruitment of other NFAT proteins was reinforced by retention of the pan-specific signal after knockdown of NFATc1 using short interfering RNA (siRNA) and shown in Fig. 5C (The efficacy of these siRNAs to suppress NFATc1 expression and osteoclast formation are supplied as supplemental Fig. 2). These results suggest that NFATc2, which is expressed in RAW264.7 cells, and known to have similar DNA binding characteristics and transactivating properties, may contribute to the initial induction of the NFATc1 gene.
Inhibition of NFATc1 Function Results in Reduced Osteoclast Formation and a Decrease in NFATc1 mRNA Expression
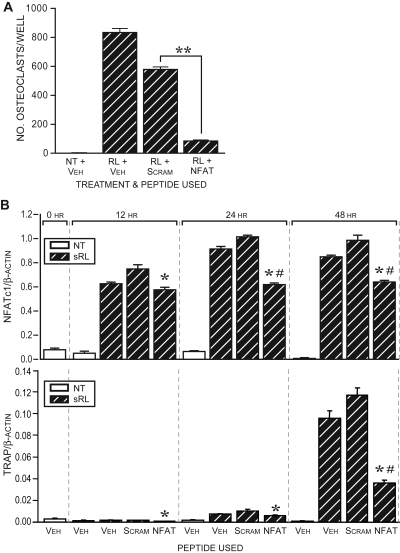
Previous studies have demonstrated that the process of osteoclast formation is fully dependent upon the induction of NFATc1 (6). To confirm this dependency and to test whether a reduction in NFAT activity results in reduced NFATc1 expression, we used a peptide inhibitor of the NFAT family of proteins and explored its effects on both osteoclast formation and NFATc1 expression (12). Calcineurin is an upstream activator of NFAT that functions to dephosphorylate multiple serine residues in the NFAT-regulatory domain located N-terminal to the DNA-binding domain (13). The actions of this phosphatase allow for nuclear accumulation of NFATc1 and an increase in the binding affinity of NFAT proteins for their response elements. In fact, the suppressive actions of the calcineurin inhibitors, FK506 and cyclosporine A on osteoclast formation have been known for several years (14,15). In contrast to these broad chemical inhibitors of calcineurin, the peptide inhibitor we used is specific for calcineurin-mediated activation of NFAT proteins (12). This peptide binds to all NFATs, prevents calcineurin docking, and consequently prevents translocation to the nucleus. Accordingly, RAW264.7 cells were treated with sRANKL in the presence of either the inhibitor peptide or a scrambled control peptide (peptide sequences are provided in Materials and Methods) and the number of TRAP-positive, multinucleated osteoclast-like cells was quantitated 5 d later. As can be seen in Fig. 6A, whereas the control peptide modestly reduced the number of osteoclasts produced in response to sRANKL, the presence of the NFAT inhibitor peptide resulted in a dramatic and statistically significant reduction in the number of osteoclasts formed after 5 d. To assess the effect of the peptide inhibitor on NFATc1 expression, we quantitated NFATc1 mRNA levels 12, 24, and 48 h after sRANKL treatment in the presence of vehicle, scrambled peptide, or peptide inhibitor. The results depicted in Fig. 6B reveal that the inhibitor is capable of reducing NFATc1 expression in a statistically significant manner, although this reduction was somewhat limited. Inhibition of NFATc1 up-regulation and function also resulted in a decrease in the expression of the NFATc1 target TRAP. These results suggest that NFATc1 activity is necessary for osteoclast formation, although NFATc1 autoregulation may represent only one of several mechanisms whereby RANKL induces and maintains NFATc1 gene expression over time.
Figure 6.
NFATc1 Activity Is Essential for Osteoclast Formation but Only Contributory to NFATc1 Gene Activation
A, A peptide inhibitor of NFATc1 activity prevents osteoclast formation in RAW264.7. Cells were cultured in the presence of sRANKL (sRL) (300 ng/ml) with either vehicle, palmitic-linked NFATc1 inhibitor peptide (50 μm), or scrambled control peptide (50 μm). TRAP-positive multinucleated osteoclasts were quantitated after 5 d in culture. The data represent the average of triplicate determinations ± sem. **, P ≤ 0.05 compared with scrambled control (one-way ANOVA). B, NFATc1 inhibitor peptide partially blocks RANKL-induced NFATc1 mRNA and the up-regulation of the NFATc1 target mRNA TRAP in RAW264.7 cells. Cells were treated for the indicated times without or with sRL (300 ng/ml) and dimethylsulfoxide vehicle, scrambled peptide, or NFATc1-inhibitory peptide. RNA was collected at the times indicated and evaluated for the presence of either β-actin, NFATc1 (upper panel), or TRAP (lower panel). Real-time PCR was performed on reverse transcribed products and expressed as the ratio of NFATc1/β-actin or TRAP/β-actin. The data represent the average of triplicate determinations ± sem. *, P ≤ 0.05 compared with time-matched scrambled control; #, P ≤ 0.05 compared with the time-matched vehicle control (one-way ANOVA). These data are representative of two independent experiments. NT, No treatment; Scram, scrambled; Veh, vehicle.
NFATc1 Modulates the Expression of Acp5 (TRAP)
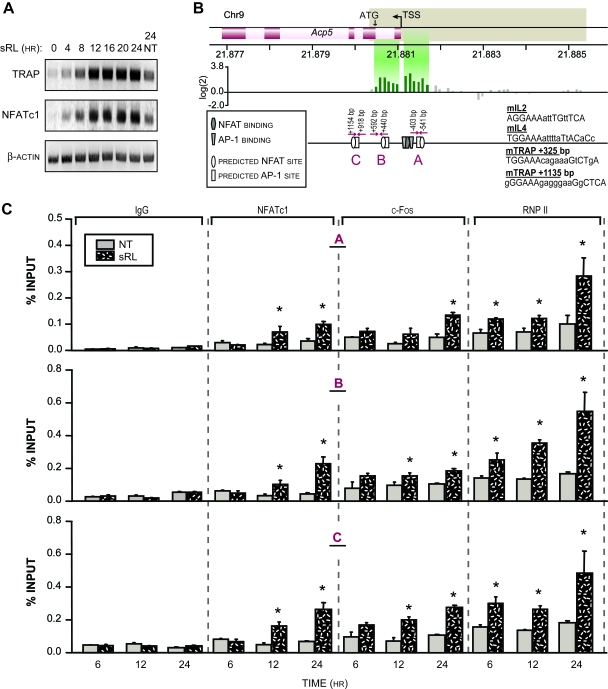
The up-regulation of NFATc1 in response to RANKL and its essentiality in the process of osteoclast differentiation suggests that this factor may be key to the induction of osteoclastic genes such as Acp5 (TRAP) and others (5,16,17,18,19,20). Indeed, considerable evidence suggests that many osteoclastic genes are regulated by NFATc1 and contain defined NFATc1-binding sites (5,16,17,18,19,20). We therefore explored the kinetics of activation of Acp5. We treated RAW264.7 cells for increasing periods of time, harvested total RNA as indicated, and assessed the abundance of TRAP and NFATc1 transcripts using RT-PCR. As can be seen in Fig. 7A, both TRAP and NFATc1 are induced by sRANKL in RAW264.7 cells with a similar kinetic pattern that begins approximately 4 h after treatment.
Figure 7.
Regulation of Acp5 by NFATc1
A, RANKL induces TRAP mRNA. RAW264.7 cells were treated with sRANKL (sRL) (300 ng/ml), and total RNA was isolated at the specific time points indicated. An RT-PCR analysis was performed using primers capable of detecting TRAP (25 cycles), NFATc1 (21 cycles), and β-actin (20 cycles). NT, No treatment. B, ChIP-chip analysis of the mouse Acp5 gene. RAW264.7 cells were cultured in the presence or absence of sRL for 48 h and then subjected to a ChIP analysis using antibodies to NFATc1. The DNA from sRL and control-treated cells were separately amplified and labeled as outlined in Materials and Methods. The regions probed in the DNA array on chromosome 9 (Chr9) are delineated by tan boxes. The TSS and translational start sites (ATG) are shown. The location of the primers designed to detect DNA fragments derived from these regions are indicated with arrows. The locations of known NFAT (hexagon) and AP-1 (trapezoid) binding sites are marked schematically. Putative NFATc1/AP-1 composite response elements are also indicated downstream of the TSS. The sequences for these putative sites are shown at the left together with two reference composite elements for the IL-2 and IL-4 genes that are known to bind these transcription factors. C, RANKL induces the localization of NFATc1, c-fos, and RNA pol II to the Acp5 promoter in RAW264.7 cells. Cells were treated with sRL for 6, 12, or 24 h and then subjected to ChIP analysis using antibodies to NFATc1, c-fos, RNA pol II, and IgG. Coprecipitated DNA was evaluated using qPCR for the presence of DNA fragments A, B, and C as described by the primer sets indicated in panel B above. Signal intensities were normalized to their corresponding input signals. The data represent the average of triplicate determinations ± sem. *, P ≤ 0.05 compared with time-matched NT control (one-way ANOVA). These results are representative of more than 10 independent experiments. RNP II, RNA polymerase II.
Based on this finding, we next examined whether NFATc1 could be detected at the Acp5 promoter. As with the NFATc1 promoter, several NFATc1- and AP-1-binding sites have been previously identified in the Acp5 gene as well (5,21). These sites for NFATc1 are located upstream of the transcription start site (TSS), although sites for other factors important to TRAP up-regulation have been located downstream within the first intron (22). We therefore used ChIP-chip analysis to scan this extended region as well using the methods described more extensively for the NFATc1 locus. Cells were treated with sRANKL for 48 h and subjected to the ChIP procedure using antibodies to NFATc1, after which the coprecipitated DNA was used to scan the region beginning −3250 bp upstream and ending 750 bp downstream of the Acp5 TSS. The locations of the known NFAT-binding sites are identified by the darkened symbols in Fig. 7. Two peaks of NFATc1-binding activity were observed, one located upstream of the TSS from −150 to −600 and the second within the first intron between +50 and +550. The results of this limited ChIP-chip scan support the functionality of previously identified NFAT-binding sites located upstream of the Acp5 TSS at −120 and also revealed the possibility that an additional NFAT site(s) might exist downstream within intron 1. Surprisingly, close examination of this general region of the Acp5 gene using COMPEL revealed at least two additional putative NFAT-regulatory regions, one located at +325 within intron 1 that corresponded to the peak detected in the ChIP-chip scan as well as one at +1135, which was not included within the scan. The results documented in supplemental Fig. 3 demonstrate that each of these novel regions was fully capable of mediating RANKL-induced transcription when fused to a thymidine kinase promoter-luciferase reporter plasmid and transfected into RAW264.7 cells. Indeed, mutagenesis of each of the downstream putative NFAT consensus elements significantly compromised inducibility by RANKL and, in the context of a DNA fragment containing both, provided evidence of a potential synergistic interaction. Thus, multiple regulatory elements for NFAT appear responsible for Acp5 gene induction after RANKL treatment.
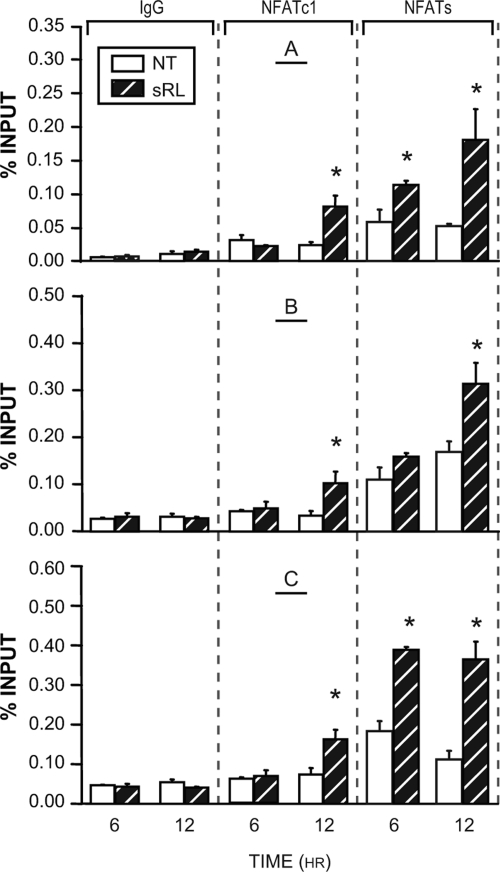
Figure 7C documents the time course of appearance of NFATc1 at all three of these sites by direct ChIP analysis using the primer sets identified in Fig. 7B. As can be seen in Fig. 7C, NFATc1 localization was confirmed at these sites and was detected as early as 12 h after sRANKL treatment. Both c-Fos and RNA pol II are also detected at these sites as well. Finally, as observed in Fig. 8A, whereas the NFATc1-specific antibody failed to detect NFATc1 at the TRAP promoter within 6 h, NFATc2, or perhaps other NFATs, are detected using the pan antibody employed earlier. Thus, it appears likely that as with the NFATc1 gene, early control of TRAP expression may be mediated by NFATc2 and/or other members of the NFAT family of transcription factors. These data confirm a role for NFAT in Acp5 regulation in osteoclasts and suggest that the time course of response is temporally related to the up-regulation of NFATc1.
Figure 8.
Additional NFATc Members Activate Acp5 at Early Times
RAW264.7 cells were treated with sRL for either 6 or 12 h and then subjected to ChIP analysis using antibodies to NFATc1, IgG, or a pan antibody to NFATc1–4. The resulting coprecipitated DNA was evaluated by qRT-PCR analysis using primers capable of detecting regions of the Acp5 locus as indicated in Fig. 7. Signal intensities were normalized to their corresponding input signals. The data represent the average of triplicate determinations ± sem. *, P ≤ 0.05 compared with time-matched NT (no treatment) controls (one-way ANOVA). These results are representative of more than four independent experiments. sRL, sRANKL.
DISCUSSION
The induction of NFATc1 by RANKL represents a seminal event in the process of osteoclastogenesis (3,4). Whereas the proximal RANKL-stimulated signaling pathways that induce the expression of this gene and that activate the transcriptional capabilities of the protein product remain to be fully characterized, the role of NFATc1 in the expression of osteoclast target genes is rapidly emerging. In this report, we demonstrate clearly that the induction of NFATc1 mRNA is controlled almost exclusively through an increase in the transcriptional output of the NFATc1 gene and not via an increase in mRNA stability. Whereas the mRNA appears to be a relatively stable transcript in the context of mRNA, the protein itself manifests a relatively short half-life similar to that of other transcription factors. The transcriptional up-regulation of NFATc1 gene is mediated by the P1 promoter and is achieved initially in part via the presence and activity of existing members of the NFATc family. Within 12 h of sRANKL treatment, however, NFATc1 is found predominantly at the NFATc1 promoter and, via autoregulation, appears to play a dominant role in NFATc1 mRNA expression. The relationship between NFATc1 mRNA and protein concentration is not entirely clear, because at early times the mRNA levels do not appear to be associated with corresponding levels of NFATc1 protein. This suggests the possibility of a posttranscriptional mechanism essential to the production of NFATc1 itself. Indeed, posttranscriptional regulation of protein production is also observed with c-Fos, a well-known osteoclastogenic factor (23,24). Thus, there is a dramatic increase in c-Fos protein level observed in RAW264.7 after sRANKL treatment, but no observable change in mRNA expression (our unpublished data).
As with all genes, transcriptional activation by NFATc1 is facilitated by additional transcription factors as well. At least one of these transcription factors is c-Fos, the appearance of which at Nfatc1 P1 is also induced by sRANKL. Its central role in the formation of osteoclasts has been extensively described (5,23,25,26). Given the diverse signaling pathways activated by RANKL, it is almost certain that other transcription factors are also likely to play a role in NFATc1 regulation. The timely appearance of RNA pol II is indicative of mRNA induction. Interestingly, recent studies demonstrate that RNA pol II can be found associated with not only promoter regions, but with enhancer regions and at sites within genes as well (27). Thus, further studies using phospho-specific antibodies will be necessary to establish a direct correlation between NFATc1 and c-Fos DNA binding and gene activation.
A typical feature of transcription factors is their ability to autoregulate the expression of their own genes (28,29,30). Our results suggest that this is the case for NFATc1 as well. Thus, NFATc1 binds directly to and participates in the expression of the Nfatc1 gene. Interestingly, this activity appears in osteoclasts to be focused exclusively at the Nfatc1 P1 promoter, a promoter responsible for the expression of both the A and C isoforms. Numerous examples of autoregulation are evident in cells, most of them associated with the production of proteins with relatively short half-lives such as NFATc1. Of the many that have been identified, c-Fos may be the most striking example (31). Others, however, include members of the NF-κB activation pathway (32,33) and several nuclear receptors such as the vitamin D receptor (34). Interestingly, some transcription factors autoregulate their expression in a negative fashion (35,36). Thus, although the need for the up-regulation of a transcription factor appears to be intuitively logical, the underlying principles whereby regulatory activity is controlled are likely to be diverse and require additional exploration.
RANKL activates multiple signaling cascades, which converge on a diverse set of transcription factors that, in addition to NFATc1, include the c-Jun/c-Fos heterodimer, ELK1/2, the NF-κB activation pathway, microphthalmia-associated transcription factor, and likely a number of others (26). Thus, our finding that additional transcription factors, such as c-Fos, localize to the NFATc1 promoter is not surprising. Indeed, it seems likely that activation of c-Fos may mediate a primary RANKL signal at the NFATc1 promoter to initiate osteoclast formation (25). This view is supported by the observation in vivo that ectopic overexpression of NFATc1 is able to rescue the apparent block in osteoclastogenesis in the c-Fos null mouse (5). Furthermore, our observation that inhibition of NFAT activation using competitive peptides results in a modest effect on NFATc1 mRNA induction during the first 12 h of RANKL treatment, but a more exaggerated effect at later times, once NFATc1 has accumulated in the cells, suggests that other transcriptional regulators are dominant during these early times. A more precise time course will be necessary, however, to determine the earliest time point for which c-Fos can be detected at nfatc1. Interestingly, NFATc1 functions almost exclusively on regulatory elements as heterodimers (8). Thus, it is known to interact at target genes via a rather diverse set of regulatory factors that include c-Fos, c-Jun, cAMP response element-binding protein, and many others (8). Whether NFATc1 forms heterodimers with factors other than c-Fos on these promoters remains to be determined.
NFATc1 is essential for osteoclast formation (6). A full understanding of NFATc1 induction, synthesis, and activation is currently unresolved, however, due to the complexity of the pathways that appear to be integrally involved. Indeed, the recent identification of coreceptors, the ITAM pathways, and their involvement in establishing critical calcium oscillations in the osteoclast precursor supports this view (37,38). The direct gene targets of NFATc1 during the process of osteoclast formation also remain to be identified. These targets, however, cannot be identified by simply inhibiting or reducing NFATc1 activity, because this maneuver prevents osteoclastogenesis as well. Thus, additional studies using ChIP and/or ChIP-chip analysis, as conducted here for the Nfatc1 and Acp5 promoters, will be necessary to achieve this goal.
In conclusion, we show in this report that the induction of NFATc1 is critical to the production of osteoclast-specific genes and to the formation of TRAP-positive, multinucleated osteoclasts. Through at least two independent regulatory sites in the P1 promoter, NFATc1 induction is initiated by existing members of the NFAT family of transcription factors and potentiated in an autoregulatory manner by the increasing production of new NFATc1 protein. NFATc1 also modulates directly the expression of TRAP, likely via at least three independent regulatory sties. Whereas the recruitment of NFATc1 and other NFATs to the promoters of these two targets are temporally related, it appears that other transcription factors may be required also for initial up-regulation of the NFATc1 gene. TRAP, in contrast, appears to be highly dependent upon the recruitment of NFAT proteins.
MATERIALS AND METHODS
Reagents
General biochemical reagents were obtained from Fisher Scientific (Pittsburgh, PA) and Sigma Chemical Co. (St. Louis, MO). Oligonucleotide primers were purchased from Integrated DNA Technologies, Inc. (Coralville, IA). Anti-NFATc1 (sc-13033), anti-pan NFAT (sc-7294), and anti c-Fos (sc-125) antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-RNA polymerase II antibody (8WG16) was obtained from Covance, Inc. (Berkley, CA). Trans-IT siQuest reagent was purchased from Mirus Bio Corporation (Madison, WI). CHX and Histopaque-1077 were obtained from Sigma, and ActD was purchased from Fisher Scientific (Pittsburgh, PA).
Generation of sRANKL
A human sRANKL (residues 137–316) cDNA was expressed as a glutathione S-transferase fusion protein in BL21 cells and purified on glutathione affinity columns as previously described (39). We used 300 ng/ml sRANKL to achieve a maximal effect on osteoclast formation and gene activation in vitro.
Cell Culture
RAW264.7 cells were maintained in DMEM supplemented with 10% heat-inactivated defined fetal bovine serum from Hyclone Laboratories, Inc. (Logan, UT). Osteoclast generation media for all cell types consisted of phenol red-free α-MEM (Invitrogen, Carlsbad, CA) supplemented with 10% preselected charcoal-stripped fetal bovine serum (Hyclone).
Animal Studies
C57BL/6 wild-type mice were maintained at our facilities. Experimental protocols were reviewed and approved by the Research Animal Resources Center (University of Wisconsin-Madison, Madison, WI). Mice (4–8 wk of age) were killed through CO2 asphyxiation, and spleens were collected. The mononuclear cell fraction was enriched by Ficoll density gradient centrifugation and then cultured in osteoclast generation media as previously described (40).
Characterization and Quantification of Osteoclast-Like Cells
Osteoclast formation was induced in RAW264.7 cells (2 × 103 cells per well) or primary spleen cells (1 × 105 cells per well) in 48-well dishes using sRANKL (300 ng/ml). Osteoclast formation was assessed by counting the total number of multinucleated (> three nuclei), TRAP 5-positive cells present per well after 5 d in culture (40,41).
Chromatin Immunoprecipitation (ChIP) Assay
Chromatin Immunoprecipitation assays were performed as previously described (42). Primer sets used for amplifying the mouse NFATc1 regions of interest included mNfatc1 P1 (forward, 5′-GGAAGCCTGCGATTTTACAT; reverse, 5′-ACGAAACGGGAAGGAAAG), mNfatc1 P2 (forward, 5′-CACCTCCTTCGGCTGTGG; reverse, 5′-GGGCTTCGCTCTCCCTTA), mAcp5 A (forward, 5′-AGTCCCATTCTGGCCCAGCTTTA; reverse, 5′-TCTTCCGCACACTGCAGGTCTTT); mAcp5 B (forward, 5′-GGTGGTGAGTGTTCAAGGAAGGAGT; reverse, 5′-TCATCTAAGTGTGGGACTGTCTCCG); mAcp5 C (forward, 5′-TGACTGTGCTGGCTTCGAGAAAC; reverse, 5′-AGGCAGTTAAGCTCCTGGACCAA).
ChIP-chip Analysis
ChIP-chip analysis was carried out as described by others (43). In brief, DNA was isolated by specific immunoprecipitation using the ChIP methodology outlined above and then subjected to ligation-mediated PCR (43). DNA samples immunoprecipitated during the ChIP analysis using antibodies to NFATc1 were blunt ended by incubating with T4 DNA polymerase from New England Biolabs (Ipswich, MA) for 1 h and then purified with the Qiaquick PCR purification kit from QIAGEN (Valencia, CA). Double-stranded unidirectional linkers (forward, GCGGTGACCCGGGAGATCTGAATTC; reverse, GAATTCAGATC) were ligated to the blunt-ended DNA overnight at 16 C using T4 DNA Ligase (New England Biolabs). Samples were purified using the Qiaquick PCR purification kit and then PCR amplified to create amplicon stocks. Each 50-μl PCR was performed in the presence of 1 μl of Taq polymerase and 5 μl of 10× Taq polymerase buffer from Promega Corp. (Madison, WI), 0.3 mm deoxynucleotide triphosphates, 1.5 mm MgCl2, 6.5 μl betane (Promega), and 1 μm forward linker primer. PCR was performed by heating to 55 C for 2 min, 72 C for 5 min, and 95 C for 2 min. This was followed by 15 cycles at 95 C for 0.5 min, 55 C for 0.5 min, and 72 C for 1 min, and finished at 72 C for 4 min and then holding at 4 C. After each PCR, the samples were purified with the Qiaquick PCR purification kit. Amplification was repeated until 1–10 μg of each sample was generated. The resulting amplicons (∼500 bp) were then end labeled with oligos containing either Cy3 or Cy5 dyes. Cy3- and Cy5-labeled DNA samples were then mixed, denatured, and cohybridized to MM8 promoter dual-chip oligonucleotide microarrays (Nimblegen Systems, Inc., Madison, WI). The microarrays were then washed extensively and scanned using an Axon 4000B scanner at the appropriate wavelengths. A peak-finding algorithm was used to score the relative level of binding within the promoter regions (44).
RNA Isolation and Analysis
Total RNA was isolated from cells using TRIzol reagent obtained from MRC (Cincinnati, OH). The isolated RNA was reverse transcribed using the Superscript III RNase H reverse transcriptase kit from Invitrogen and then subjected to PCR analysis using quantitative (q) PCR (qPCR) or standard PCR methods. qPCR reactions were performed using Power SYBR Green master mix (no. 4367660) from Applied Biosciences, Inc. (Foster City, CA); semiquantitative PCR analysis was performed using a master mix purchased from Promega (Madison, WI). Primers used included those for mβ-actin (forward, 5′-TGTTTGAGACCTTCAACACCC; reverse, 5′-CGTTGCCAATAGTGATGACCT), mNFATc1 A and C (forward, 5′-CCCTTCCAAGTTTCCACTC; reverse, 5′-CCCAGGTAGGAGGTGATCT), mNFATc1 B (forward, 5′-GACCCGGAGTTCGACTTCGATTT; reverse, 5′-CGCTTGCAGCTAGGAAGTACGTCTT), mNFATc1 C (forward, 5′-GTGTGGTGCAGGCCATTCT; reverse, 5′-AGGGCATTTCTAGGGGTTTCC), c-Fos (forward, 5′-GCCGGGGACAGCCTTTCCT; reverse, 5′-CCACTGCAGGTCTGGGCTGG), mNFATc2 (forward, 5′-ATCGCACATAAGGCCATCAGCTC; reverse, 5′-TTGAAACTGGGGACACAGGTCGT), mNFATc3 (forward, 5′-CACCATCGTTTCAGCTCCAAAGTC; reverse, 5′-AAGAGTGAATCGTGCAGCAGCTTC), mNFATc4 (forward, 5′-CTGGGGGTGGTCGTGTTC; reverse, 5′-GCCTTCTGGTGGAGGGTAA), and mTRAP (forward, 5′-TTCATGGGTGGTGCTGCTGG; reverse, 5′-GAGCGCCCATCGTCTGCA).
Western Blot Analysis
RAW264.7 cells were treated with sRANKL (300 ng/ml) for the indicated periods of time. Cells were washed twice with PBS and suspended in a low-salt buffer containing 0.1% Nonidet P-40. Nuclei were collected by centrifugation, and the supernatant was used as cytosolic protein. The nuclear pellets were resuspended in 50 mm HEPES-KOH (pH 7.8), 420 mm KCl, 0.1 mm EDTA, 5 mm MgCl2, 1 mm dithiothreitol, and 0.5 mm phenylmethylsulfonylfluoride for 30 min at 4 C, and the insoluble fraction was collected and discarded. The resulting supernatant was retained as nuclear protein. Lysates were evaluated for protein content, and 60 and 30 μg of cytosolic and nuclear protein extract, respectively, were subjected to SDS-PAGE. Proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA) and subjected to Western blot analysis using the anti-NFATc1 antibody 7A6 (Santa Cruz Biotechnology, Santa Cruz, CA).
siRNA Knockdown
RAW264.7 (3.5 × 106 cells per well) were plated in 10-cm culture dishes and left overnight. The cells were transfected with siRNA duplexes (40 ng/ml) directed against NFATc1 (M-054700–00) or a nontargeted control (D-001206–13-20). All siRNA pools were purchased from Dharmacon, Inc. (Lafayette, CO). After transfection, the cells were cultured for 72 h before they were trypsinized, counted, and replated for osteoclast formation analysis, RNA collection, or ChIP assay as described above.
Palmityl-Linked Peptide Inhibitors
Palmityl-linked peptides were synthesized as previously described (12). The sequence of the NFATc1-inhibitory peptide was palmitic-linked MAGPHPVIVITGPHEE. The sequence of the scrambled control peptide was palmitic-linked MGAPGSTPIVHVPEGE. RAW264.7 were plated in 48-well plates for osteoclast formation assay and in 10-cm culture dishes for RNA isolation studies and treated with 50 μm of either peptide or dimethylsulfoxide vehicle in the presence of sRANKL.
Plasmids
Generation of the pCH110-β-galactosidase reporter plasmid was previously described (45). pTK-mNfatc1 P1-luc and pTK-mAcp5-luc plasmids were prepared by cloning the appropriate mouse DNA fragments obtained through DNA amplification of RAW264.7 genomic DNA into the pTK-luc vector using the BamHI and HindIII restriction sites. Mutagenesis of the NFAT consensus elements located in the constructs was performed using the QuikChange Site-Directed Mutagenesis Kit from Stratagene (San Diego, CA). The sequences altered in mutagenesis were as follows: NFATc1 −1330 mutant (from tTTTCAT to taTTgAc), Acp5 +330 mutant (from gTTTCCAtt to gaTTggAtc), and Acp5 +1140 (from cTTTCCct to caTTggct).
Transfection Analysis
RAW264.7 cells were seeded into 24-well plates (1.5 × 104 cells per well) and treated with sRANKL at the indicated concentrations. Cells were transfected 24 h later with TransIT-Neural reagent from Mirus Bio, Inc. (Madison, WI) according to the manufacturer’s protocol. Individual wells were transfected with 500 ng of a luciferase-reporter vector and 100 ng of pCH110-βgal. After transfection, the cells were cultured in complete media. Cells were harvested 24 h after stimulation, and the lysates were assayed for luciferase and β-galactosidase activities as previously described (45). Luciferase activity was normalized to β-galactosidase activity in all cases.
Statistical Analysis
All values are expressed as mean ± sem. We evaluated differences between groups through ANOVA with significance indicated when P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank members of the Pike laboratory for their helpful discussions and additional contributions to this work.
Footnotes
This work was supported by National Institutes of Health Grant DK-56059 (to J.W.P.).
Disclosure Statement: The authors have nothing to declare.
First Published Online December 6, 2007
Abbreviations: ActD, Actinomycin D; AP-1, activator protein 1; ChIP, chromatin immunoprecipitation; ChIP-chip, chromatin immunoprecipitation-DNA chip; CHX, cycloheximide; M-CSF, macrophage colony-stimulating factor; NFATc1, nuclear factor of activated T cells cytoplasmic 1; NF-κB, nuclear factor-κB; qPCR, quantitative PCR; RANK, receptor activator of NF-κB; RANKL, RANK ligand; RNA pol II, RNA polymerase II; siRNA, short interfering RNA; sRANKL, soluble RANKL; TRAP, tartrate-resistant acid phosphatase; TSS, transcriptional start site.
References
- Tsukii K, Shima N, Mochizuki S, Yamaguchi K, Kinosaki M, Yano K, Shibata O, Udagawa N, Yasuda H, Suda T, Higashio K 1998 Osteoclast differentiation factor mediates an essential signal for bone resorption induced by 1 α,25-dihydroxyvitamin D3, prostaglandin E2, or parathyroid hormone in the microenvironment of bone. Biochem Biophys Res Commun 246:337–341 [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T 1998 Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N, Hayashi K, Hoshijima M, Ogawa T, Koga S, Miyatake Y, Kumegawa M, Kimura T, Takeya T 2002 Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem 277:41147–41156 [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T 2002 Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901 [DOI] [PubMed] [Google Scholar]
- Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H, Wagner EF 2004 Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem 279:26475–26480 [DOI] [PubMed] [Google Scholar]
- Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H 2005 Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202:1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfling E, Berberich-Siebelt F, Chuvpilo S, Jankevics E, Klein-Hessling S, Twardzik T, Avots A 2000 The role of NF-AT transcription factors in T cell activation and differentiation. Biochim Biophys Acta 1498:1–18 [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A 2003 Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17:2205–2232 [DOI] [PubMed] [Google Scholar]
- Chuvpilo S, Jankevics E, Tyrsin D, Akimzhanov A, Moroz D, Jha MK, Schulze-Luehrmann J, Santner-Nanan B, Feoktistova E, König T, Avots A, Schmitt E, Berberich-Siebelt F, Schimpl A, Serfling E 2002 Autoregulation of NFATc1/A expression facilitates effector T cells to escape from rapid apoptosis. Immunity 16:881–895 [DOI] [PubMed] [Google Scholar]
- Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ 1999 Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 96:3540–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Cron RQ, Wu B, Genin A, Wang Z, Liu S, Robson P, Baldwin HS 2002 Regulation of the murine Nfatc1 gene by NFATc2. J Biol Chem 277:10704–10711 [DOI] [PubMed] [Google Scholar]
- Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A 1999 Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285:2129–2133 [DOI] [PubMed] [Google Scholar]
- Beals CR, Clipstone NA, Ho SN, Crabtree GR 1997 Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev 11:824–834 [DOI] [PubMed] [Google Scholar]
- Sun L, Moonga BS, Lu M, Zaidi N, Iqbal J, Blair HC, Epstein S, Abe E, Troen BR, Huang CL, Zaidi M 2003 Molecular cloning, expression, and function of osteoclastic calcineurin Aα. Am J Physiol Renal Physiol 284:F575–583 [DOI] [PubMed] [Google Scholar]
- Hirotani H, Tuohy NA, Woo JT, Stern PH, Clipstone NA 2004 The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J Biol Chem 279:13984–13992 [DOI] [PubMed] [Google Scholar]
- Kim Y, Sato K, Asagiri M, Morita I, Soma K, Takayanagi H 2005 Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J Biol Chem 280:32905–32913 [DOI] [PubMed] [Google Scholar]
- Anusaksathien O, Laplace C, Li X, Ren Y, Peng L, Goldring SR, Galson DL 2001 Tissue-specific and ubiquitous promoters direct the expression of alternatively spliced transcripts from the calcitonin receptor gene. J Biol Chem 276:22663–22674 [DOI] [PubMed] [Google Scholar]
- Crotti TN, Flannery M, Walsh NC, Fleming JD, Goldring SR, McHugh KP 2006 NFATc1 regulation of the human β3 integrin promoter in osteoclast differentiation. Gene 372:92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram K, Nishimura R, Senn J, Youssef RF, London SD, Reddy SV 2007 RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp Cell Res 313:168–178 [DOI] [PubMed] [Google Scholar]
- Troen BR 2006 The regulation of cathepsin K gene expression. Ann NY Acad Sci 1068:165–172 [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hisatake K, Nogi Y, Tsujimoto M 2001 Regulation of receptor activator of NF-κB ligand-induced tartrate-resistant acid phosphatase gene expression by PU. 1-interacting protein/interferon regulatory factor-4. Synergism with microphthalmia transcription factor. J Biol Chem 276:33086–33092 [DOI] [PubMed] [Google Scholar]
- Reddy SV, Hundley JE, Windle JJ, Alcantara O, Linn R, Leach RJ, Boldt DH, Roodman GD 1995 Characterization of the mouse tartrate-resistant acid phosphatase (TRAP) gene promoter. J Bone Miner Res 10:601–606 [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Liang J, Schellander K, Wagner EF, Grigoriadis AE 1995 c-fos-Induced osteosarcoma formation in transgenic mice: cooperativity with c-jun and the role of endogenous c-fos. Cancer Res 55:6244–6251 [PubMed] [Google Scholar]
- Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF 1994 c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266:443–448 [DOI] [PubMed] [Google Scholar]
- Wagner EF 2002 Functions of AP1 (Fos/Jun) in bone development. Ann Rheum Dis 61(Suppl 2):40–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL 2003 Osteoclast differentiation and activation. Nature 423:337–342 [DOI] [PubMed] [Google Scholar]
- Chernukhin I, Shamsuddin S, Kang SY, Bergström R, Kwon YW, Yu W, Whitehead J, Mukhopadhyay R, Docquier F, Farrar D, Morrison I, Vigneron M, Wu SY, Chiang CM, Loukinov D, Lobanenkov V, Ohlsson R, Klenova E 2007 CTCF interacts with and recruits the largest subunit of RNA polymerase II to CTCF target sites genome-wide. Mol Cell Biol 27:1631–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi U, Espinoza HM, Ganga M, Martin DM, Liu X, Engelhardt JF, Amendt BA 2005 PITX2, β-catenin and LEF-1 interact to synergistically regulate the LEF-1 promoter. J Cell Sci 118:1129–1137 [DOI] [PubMed] [Google Scholar]
- Zella LA, Kim S, Shevde NK, Pike JW 2006 Enhancers located within two introns of the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3. Mol Endocrinol 20:1231–1247 [DOI] [PubMed] [Google Scholar]
- Delany I, Spohn G, Pacheco AB, Ieva R, Alaimo C, Rappuoli R, Scarlato V 2002 Autoregulation of Helicobacter pylori Fur revealed by functional analysis of the iron-binding site. Mol Microbiol 46:1107–1122 [DOI] [PubMed] [Google Scholar]
- Acquaviva C, Salvat C, Brockly F, Bossis G, Ferrara P, Piechaczyk M, Jariel-Encontre I 2001 Cellular and viral Fos proteins are degraded by different proteolytic systems. Oncogene 20:942–950 [DOI] [PubMed] [Google Scholar]
- Hohmann HP, Remy R, Scheidereit C, van Loon AP 1991 Maintenance of NF-κ B activity is dependent on protein synthesis and the continuous presence of external stimuli. Mol Cell Biol 11:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice NR, Ernst MK 1993 In vivo control of NF-κ B activation by I κ B α. EMBO J 12:4685–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob F, Gieseler F, Tresch A, Hammer S, Seufert J, Schneider D 1992 Kinetics of nuclear translocation and turnover of the vitamin D receptor in human HL60 leukemia cells and peripheral blood lymphocytes—coincident rise of DNA-relaxing activity in nuclear extracts. J Steroid Biochem Mol Biol 42:11–16 [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Levchenko A, Scott ML, Baltimore D 2002 The IκB-NF-κB signaling module: temporal control and selective gene activation. Science 298:1241–1245 [DOI] [PubMed] [Google Scholar]
- Gyling M, Leclercq G, Heuson JC 1990 Estrogenic and antiestrogenic down-regulation of estrogen receptor levels: evidence for two different mechanisms. J Recept Res 10:217–234 [DOI] [PubMed] [Google Scholar]
- Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T 2004 Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428:758–763 [DOI] [PubMed] [Google Scholar]
- Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC 2004 The immunomodulatory adapter proteins DAP12 and Fc receptor γ-chain (FcRγ) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA 101:6158–6163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevde NK, Bendixen AC, Dienger KM, Pike JW 2000 Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA 97:7829–7834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevde NK, Pike JW 1996 Estrogen modulates the recruitment of myelopoietic cell progenitors in rat through a stromal cell-independent mechanism involving apoptosis. Blood 87:2683–2692 [PubMed] [Google Scholar]
- Roodman GD 1999 Cell biology of the osteoclast. Exp Hematol 27:1229–1241 [DOI] [PubMed] [Google Scholar]
- Kim S, Shevde NK, Pike JW 2005 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J Bone Miner Res 20:305–317 [DOI] [PubMed] [Google Scholar]
- Oberley MJ, Farnham PJ 2003 Probing chromatin immunoprecipitates with CpG-island microarrays to identify genomic sites occupied by DNA-binding proteins. Methods Enzymol 371:577–596 [DOI] [PubMed] [Google Scholar]
- Scacheri PC, Crawford GE, Davis S 2006 Statistics for ChIP-chip and DNase hypersensitivity experiments on NimbleGen arrays. Methods Enzymol 411:270–282 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Shevde NK, Warrier A, Plum LA, DeLuca HF, Pike JW 2003 2-Methylene-19-nor-(20S)-1,25-dihydroxyvitamin D3 potently stimulates gene-specific DNA binding of the vitamin D receptor in osteoblasts. J Biol Chem 278:31756–31765 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.