Abstract
Particularly potent cellular or humoral immune responses are needed to confer protection in animal models against such pathogens as HIV/SIV, Mycobacterium tuberculosis, and malarial parasites. Persistent, high-level vaccine Ag expression may be required for eliciting such potent and durable immune responses. Although plasmid DNA immunogens are being explored as potential vaccines for protection against these pathogens, little is known about host factors that restrict long-term plasmid DNA vaccine Ag expression in vivo. We observed rapid damping of transgene expression from a plasmid DNA immunogen in wild-type, but not in T cell-deficient mice. This damping of Ag expression was temporally associated with the emergence of Ag-specific cellular immune responses. A requirement for Fas and the appearance of apoptotic nuclei at the site of vaccine inoculation suggest that T cells induce Fas-mediated apoptosis of plasmid DNA vaccine Ag-expressing cells. These studies demonstrate that high levels of in vivo Ag expression are associated with high-frequency cellular immune responses that in turn rapidly down-regulate vaccine Ag expression in vivo. These findings argue that it may not be possible to maintain persistent, high-level production of vaccine Ag in vivo to drive persistent immune responses as long as vaccine Ag production can be limited by host immune responses.
Plasmid DNA holds promise as a vaccine modality for immunization against diseases such as AIDS, malaria, and tuberculosis. However, the utility of DNA immunogens has been limited by their failure to elicit sufficiently potent immune responses. One potential explanation for the limited immunogenicity of plasmid DNA is that it is associated with only a transient or low magnitude of Ag expression. There is substantial evidence suggesting that insufficient or suboptimal Ag expression by any vector can lead to brief or weak immune responses (1–6). Efforts have therefore been focused on developing novel vaccine vectors that can provide a more persistent source of Ag. These vectors either integrate into the host genome, such as adeno-associated virus (7), or replicate persistently in vivo, such as mycobacteria (8). Yet little is known about the relative contributions of host factors in restricting long-term expression of plasmid DNA vaccine Ags in vivo. It is therefore unclear whether the transient expression of plasmid DNA Ags is a result of inherent properties of the vector or a result of host factors that might act on any vaccine vector.
A number of mechanisms have been shown to limit the expression of vaccine vectors in vivo. In particular, vector- and transgene-specific CTLs have been shown to damp expression from adenoviral vectors (9–12). In contrast, it is not clear whether cellular immune responses regulate the expression of plasmid DNA vaccine Ags. Although immune-mediated destruction of Ag-producing muscle fibers has been reported following immunization with plasmid DNA expressing a surface-expressed hepatitis B virus Ag (HBsAg),4 this destruction was not seen with a plasmid DNA expressing the intracellular protein luciferase (13). Furthermore, attenuation of Ag expression was temporally associated with the appearance of anti-HBsAg Abs, and MHC class I and perforin molecules were not required for the damping of Ag expression (13,14). These findings argue against a role for CTL-mediated apoptosis in eliminating plasmid DNA immunogens. Rather, these data are consistent with an Ab-dependent cell-mediated cytotoxicity or a complement-mediated lysis (15) mechanism for the in vivo clearance of HBsAg-expressing plasmid DNA. However, the HBsAg system may be unusually skewed toward a strong humoral immune response (16), leading to an underestimation of the role of CTL or other mechanisms in limiting the expression of plasmid DNA vaccine Ags. In addition to Ag-specific immune responses, host factors may limit plasmid DNA expression, including non-Ag-specific inflammation (17), vector redistribution (18), death of transfected cells (19), or circulating DNases (20). An ability to monitor precisely the kinetics of Ag expression and immune responses is needed to clarify the role of vaccine-elicited T cells in eliminating plasmid DNA immunogens.
In this study, we show that vaccine Ag-elicited T lymphocytes damp plasmid DNA vaccine Ag expression in vivo through Fasmediated apoptosis. Because this damping of expression also occurs with nonreplicating viral vectors, and can be mediated by transgene product-specific rather than vector-specific immune cells, it follows that autologous T cells may limit the persistence of Ag expression by any vaccine vector. These findings argue that strategies for maintaining persistent, high-level production of vaccine Ag in vivo must circumvent immune-mediated Ag clearance.
Materials and Methods
Animals and immunizations
Six- to 8-wk-old female BALB/c, BALB/c nude, C57BL/6, Rag1−/−, perforin−/− (Pfptm1Sdz), Fas−/− (Tnfrf6lpr), and IFN-γ−/− (Ifngtm1Ts) mice were obtained from The Jackson Laboratory. Mice were maintained under pathogen-free conditions, and experimental protocols were approved by the Harvard Institutional Animal Care and Use Committee.
For immunizations, 50 µg of plasmid DNA or 107–1010 particles of recombinant adenovirus serotype 5 (rAd) in 100 µl of sterile saline were divided between quadriceps muscles.
Vectors
E1/E3-deleted rAd containing GL2 luciferase (Promega) was provided by G. Nabel (Vaccine Research Center, National Institute of Allergy and Infectious Diseases, Bethesda, MD). pGWiz-Luc, referred to in this study as plasmid Luc0, was purchased from Genlantis.
For constructing plasmid Luc, the GL4.10 luciferase sequence (Promega) was PCR amplified and inserted into the pVRC2000 plasmid vector, as described previously (21). For AL11-tagged plasmid Luc, a forward PCR primer encoding the immunodominant H-2Db-restricted SIV Gag AL11 epitope (AAVKNWMTQTL) inside triple-alanine spacers was used (5′-TTATCTGCAGGCCACCATGGCCGCAGCCGCAGCCGTGAAGAACTGGATGACTCAAACGCTCGCCGCTGCAATGGAAGATGCCAAAAACATTAAGAAGGGC-3′)
Plasmid DNA was prepared using an endotoxin-free Qiagen Giga-prep kit.
In vivo bioluminescence measurement
Animals were injected i.p. with 100 µl of a 30 mg/ml solution of firefly luciferin in PBS (Xenogen), and 100 µl of a 20 mg/ml ketamine and 1.72 µg/ml xylazine mixture. After 20 min, imaging was performed using the in vivo imaging system (IVIS) Series 100 (Xenogen) with an integration time of 1 min. Overlay images and luminescence measurements were made using Living Image software (version 2.50.1; Xenogen).
Immunological assays
ELISPOT assays were performed, as previously described (22). A luciferase peptide pool of 67 18-mers, overlapping by 10, was synthesized by Quality Control Biochemicals.
H-2Db/AL11 tetramers were prepared and used to stain epitope-specific CD8+ T lymphocytes, as previously described (23). Samples were analyzed on a FACS array (BD Pharmingen), and CD8+ T lymphocytes were examined for staining with the Db/AL11 tetramer. CD8+ T lymphocytes from control mice immunized with untagged plasmid Luc exhibited <0.1% tetramer staining.
Intracellular cytokine staining was performed, as previously described (24). For CD8+ T cell stimulation, cells were incubated with AL11 peptide at 1 µg/ml.
Histopathology
Muscles were isolated 17 days following inoculation, fixed in formalin, and then paraffin embedded. Serial H&E sections were used to identify regions with significant inflammatory infiltrates. For CD3 staining, slides were then deparaffinized and unmasked. Endogenous peroxidase activity, avidin, biotin, and nonspecific binding were blocked using hydrogen peroxide, avidin-biotin blocking kit (SP-2001; Vector Laboratories), and normal goat serum, respectively. Rabbit anti-CD3 mAb (ab5690; Abcam) was diluted (1/250) and incubated on slides overnight. Biotinylated goat anti-rabbit secondary Ab was used at a dilution of 1/200. Slides were developed using the avidin-biotin complex/diaminobenzidine system. Sections were counterstained with hematoxylin, and then dehydrated and coverslipped with permount/xylene solution.
The TUNEL assay was used to detect apoptotic nuclei. Slides were deparaffinized, and treated with proteinase K and then 3% hydrogen peroxide. Slides were then incubated in a TdT reaction for 60 min at 37°C. Slides were incubated in antidigoxigenin and then developed using diaminobenzidine. Sections were counterstained with hematoxylin, dehydrated using xylene, and coverslipped.
Statistics
The statistical significance of differences between groups was determined as described in figure legends using the program GraphPad Prism (version 4.03). A value of p < 0.05 was considered statistically significant. Error bars represent the SEM.
Results
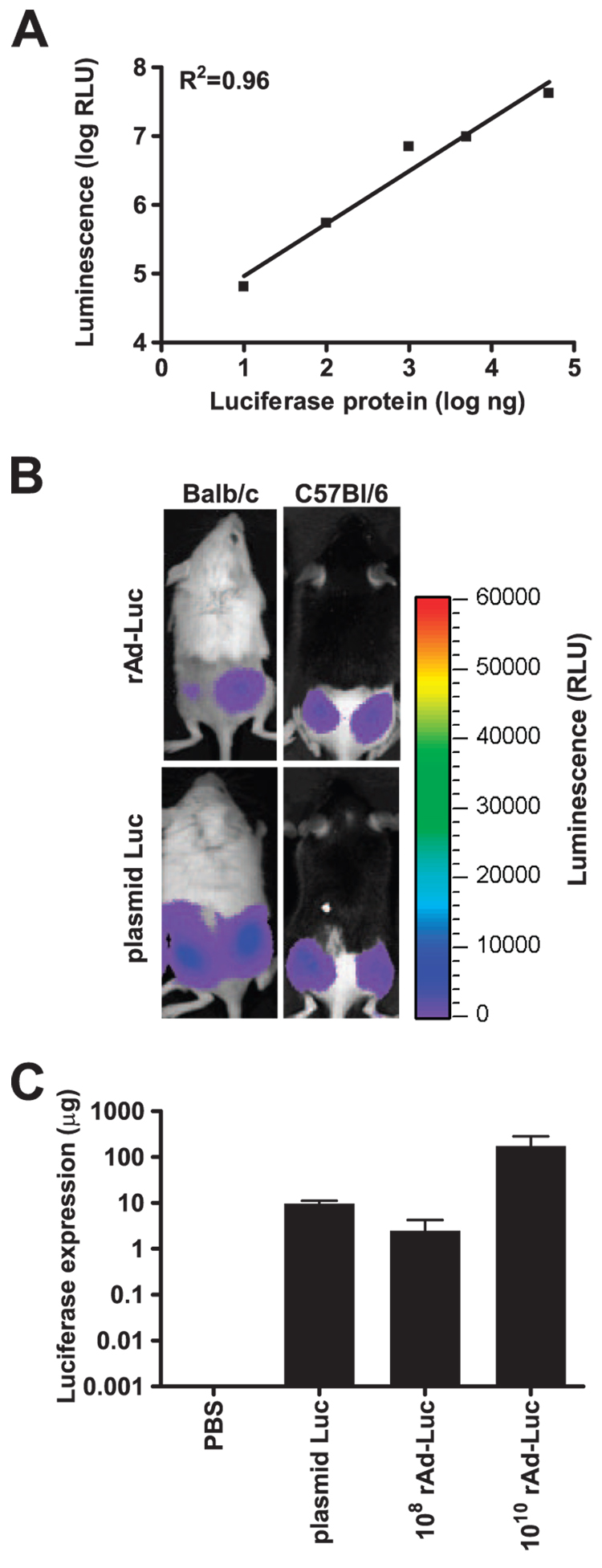
To explore the relationship between the kinetics of in vivo vaccine Ag expression and immunogenicity, we used nonreplicating vaccine vectors as model immunogens and an IVIS to measure the expression of luciferase by these vectors. This imaging strategy exploits the ability of luciferase protein to catalyze the light-producing oxidation of the small molecule luciferin. Luciferin is inoculated into mice that have received luciferase-expressing immunogens, and the quantity of light emitted by this reaction is monitored in living mice (25). Inoculating mice with increasing quantities of luciferase protein by the i.m. route, we first showed that the imaging system could detect the expression of luciferase in vivo with quantitative precision over a 4-log range (Fig. 1A). Moreover, we demonstrated that luciferase protein had an in vivo t1/2 of 13 h (data not shown), indicating that periodic measurements of luciferase expression reflected the kinetics of the expression of this protein. Using this system, we were readily able to quantitate luciferase expressed in mice by two prototypic nonreplicating immunogens, plasmid DNA and gene-deleted rAd (Fig. 1, B and C).
Figure 1.
Detection of luciferase immunogen expression in vivo by imaging. A, Luciferase protein was injected i.m., and in vivo luminescence was immediately measured using an IVIS. The correlation (R² = 0.96) between degree of luminescence and quantity of injected protein is shown. B, Peak luciferase expression is shown in BALB/c and C57BL/6 mice injected i.m. with 108 particles of rAd-Luc or 50 µg of luciferase-expressing plasmid DNA (plasmid Luc). C, Peak luciferase expression levels are shown for BALB/c mice injected with 50 µg of plasmid Luc, or with 108 or 1010 particles of rAd-Luc.
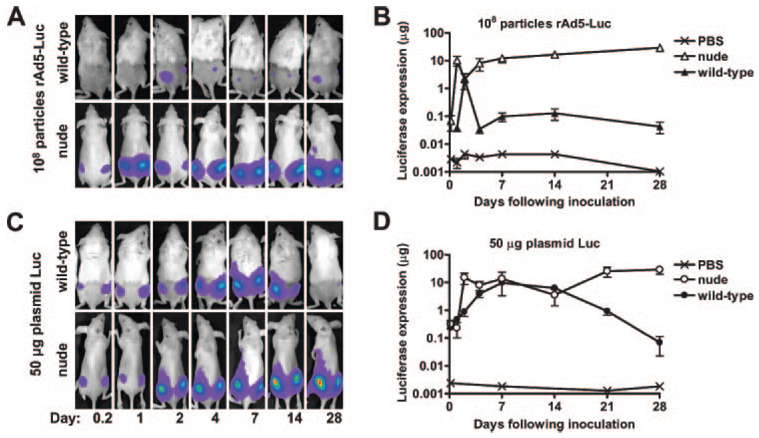
We then used this detection system to monitor the kinetics of luciferase expression in mice following vaccination with luciferase-producing rAd and plasmid DNA immunogens. Luciferase expression in mice immunized with rAd-Luc was maximal 48 h following immunization and declined dramatically over subsequent days (Fig. 2, A and B). Similarly, luciferase expression in plasmid Luc-immunized mice reached a maximum at 14 days and declined significantly thereafter (Fig. 2, C and D). By contrast, athymic nude mice evidenced high-level expression of luciferase by days 2 and 4 following rAd-Luc and plasmid Luc immunization, respectively, but showed no damping of expression over the entire course of the experiment (Fig. 2). Although nude mice have multiple immune deficiencies (26), the dramatic difference in the kinetics of luciferase expression following vaccination of wild-type and athymic nude mice is consistent with a role for T lymphocytes in attenuating immunogen expression in vivo.
Figure 2.
In vivo elimination of luciferase transgene expression from plasmid DNA and adenovirus vectors in wild-type, but not in athymic nude mice. BALB/c or athymic BALB/c nude mice (n = 4) were immunized i.m. with 108 particles of rAd5-Luc (A and B) or 50 µg of plasmid Luc DNA (C and D), and in vivo transgene expression was monitored over 28 days. Representative images are shown in A and C, whereas mean values (±SEM) for each group of mice are shown in B and D. Expression was significantly higher in nude than in wild-type mice at days 21 (p = 0.03) and 28 (p = 0.01) following plasmid Luc immunization, and at all time points after day 4 (p = 0.01) in rAd-Luc-immunized mice, as determined by Student’s t test.
Loss of vaccine Ag expression in the wild-type mice became apparent at approximately the same time that Ag-specific T cell immune responses to these immunogens are reported to be first detectable (27, 28). To assess the kinetics of the emergence of immune responses elicited by plasmid DNA and recombinant adenovirus, we immunized mice with vectors encoding gp120 and prospectively measured p18 tetramer responses (data not shown). p18-specific CD8+ T lymphocyte responses were detected earlier in the mice immunized with the recombinant adenovirus construct than in the mice immunized with the plasmid DNA construct. Immune responses to the adenovirus construct were detectable at least 7 days earlier than responses to plasmid DNA. The differences in kinetics of these immune responses may explain the differing kinetics of Ag expression in these two systems.
Previous work has suggested that the destruction of myocytes expressing HBsAg was temporally associated with the emergence of Ab responses (13, 14). We analyzed humoral immune responses in serum from mice inoculated with plasmid DNA and rAd immunogens at several time points following inoculation. However, we were unable to detect luciferase-specific Ab titers greater than 1:10 until at least 2 mo following immunization with either vector.
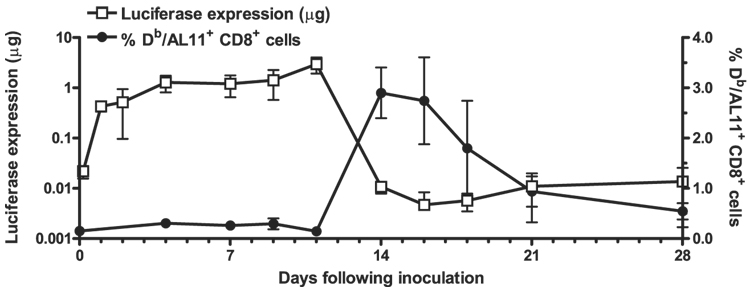
To determine whether the disappearance of vaccine Ag expression was temporally associated with the emergence of a cytotoxic T cell response in this system, we created a plasmid DNA construct that would allow for the measurement of T cell responses and Ag expression prospectively in the same individual animal. We inserted the H-2Db-restricted dominant epitope of SIV gag, AL11, into the N terminus of a luciferase-expressing plasmid DNA. Mice were immunized i.m. with 50 µg of this construct, and we assessed Ag expression by in vivo imaging and monitored Agspecific immune responses by PBL staining with a Db/AL11 MHC tetramer. We observed high-level expression of luciferase and undetectable tetramer responses through day 11 following immunization. Over the next 3 days, however, we observed a nearly 300-fold decline in luciferase activity and a 20-fold increase in the frequency of AL11-specific CD8+ T lymphocytes in the peripheral blood. Over the subsequent weeks, luciferase expression remained persistently low, whereas the frequency of AL11-specific lymphocytes declined (Fig. 3). The temporal association between the appearance of detectable Ag-specific T cells and the damping of vaccine Ag expression is consistent with a role for T cells in limiting vaccine Ag expression.
Figure 3.
Damping of expression is coincident with the emergence of cellular immune responses. C57BL/6 mice (n = 4) were immunized with a plasmid containing AL11-tagged luciferase. Expression was measured by in vivo imaging (left axis, □), whereas AL11-specific cellular immune responses (right axis,●) were monitored by Dd/AL11 tetramer staining of CD8+ PBLs at the indicated time points.
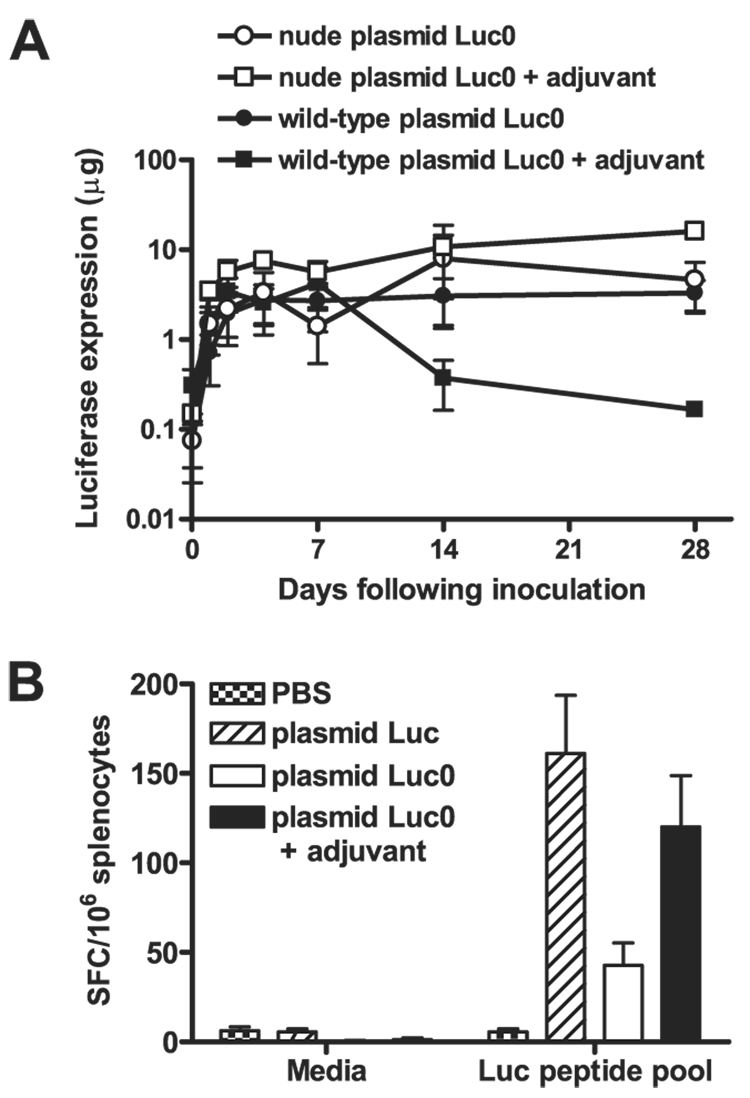
However, these findings contradicted earlier reports that luciferase expression from plasmid DNA immunogens is not limited by immune responses (13, 29). In assessing why the results of the present study differed from previously reported studies, we noted that the expression of the plasmid Luc immunogen was not persistent in the present experiments. We speculated that some luciferase-expressing plasmid DNA constructs may express persistently as a result of their inability to induce a strong T lymphocyte response. Indeed, we observed persistent expression in vivo by the immunogen plasmid Luc0, a vaccine construct that was not codon optimized and lacked two of the CpG motifs present in plasmid Luc (Fig. 4A). We evaluated luciferase-specific cellular immune responses by splenocytes of mice immunized with the Luc and Luc0 vaccine constructs, using an IFN-γ ELISPOT assay and lymphocyte exposure to a luciferase peptide pool, and observed only low-frequency responses by cells of animals immunized with the suboptimal construct (Fig. 4B). These findings suggested that persistent vaccine Ag expression was associated with poor vaccine immunogenicity.
Figure 4.
Damping of transgene expression in vivo is associated with strong cellular immune responses. A, BALB/c or athymic BALB/c nude mice (n = 4) were immunized with 50 µg of plasmid Luc0, with or without polymer adjuvant, and luciferase expression was monitored in vivo over 28 days. Ag expression was significantly lower in wild-type mice immunized with polymer-adjuvanted plasmid Luc0 as compared with wild-type animals receiving unadjuvanted plasmid Luc0 at day 28 (p < 0.05), as determined by Student’s t test. B, Immune responses of mice immunized with plasmid Luc or the suboptimal plasmid Luc0 with or without polymer adjuvant were measured 2 mo following immunization by IFN-γ ELISPOT assay using pooled luciferase peptides. A one-way ANOVA showed the differences between the three experimental groups to be statistically significant (p < 0.01), and a Dunnett’s multiple comparison test with a cutoff of p = 0.05 showed immune responses to be significantly lower in animals receiving unadjuvanted plasmid Luc0.
We have shown previously that poly(β-amino ester) polymers can significantly enhance CTL responses to plasmid DNA immunogens (22). To determine whether this adjuvant could enhance the T cell immunogenicity and damping of luciferase expression by this suboptimal construct, we vaccinated mice with plasmid Luc0, either alone or with a polymer adjuvant. We observed significantly lower levels of long-term luciferase expression in mice immunized with adjuvanted plasmid Luc0 as compared with mice receiving unadjuvanted plasmid Luc0 in wild-type animals (p < 0.05), whereas no damping of adjuvanted or unadjuvanted immunogen expression was observed in athymic nude animals (Fig. 4A). This damping of luciferase expression in the mice receiving adjuvanted plasmid Luc0 was associated with potent cellular immune responses, comparable in magnitude to those elicited by plasmid Luc alone (Fig. 4B).
A role for T lymphocytes in the regulation of immunogen expression was further supported by the observation that the magnitude of the cellular immune response elicited by a vaccine immunogen correlated with the kinetics of vaccine Ag damping in vivo. There was a statistically significant correlation between the magnitude of the immune response as measured by ELISPOT assay on day 28 and the log decrease in luciferase expression between weeks 2 and 4 (p < 0.001, R² = 0.6, n = 16).
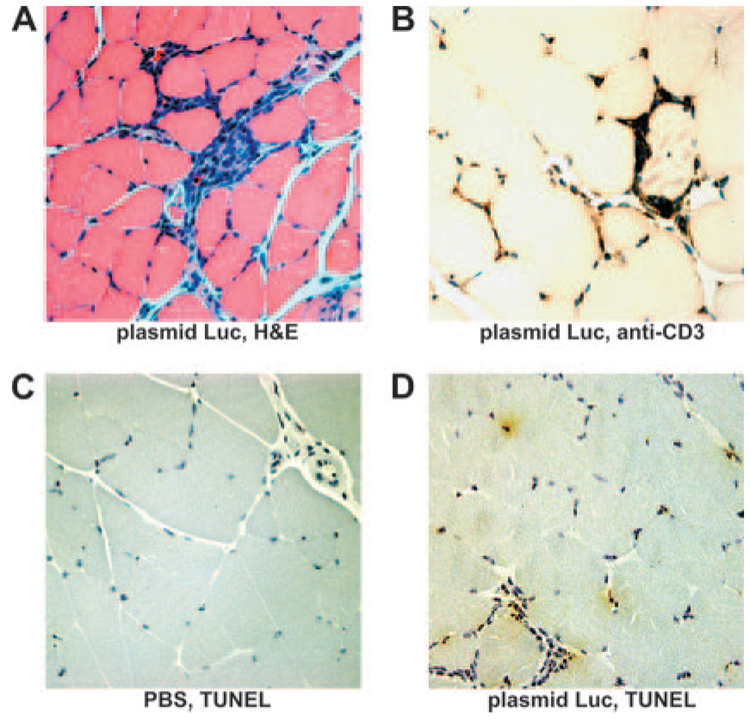
To explore the mechanism underlying this T lymphocyte-mediated damping of vaccine Ag expression, we performed a histological evaluation of plasmid Luc-inoculated muscles at the time of the damping of Ag expression. H&E-stained muscle sections demonstrated a local inflammatory cell infiltrate surrounding myocytes 17 days following plasmid DNA inoculation (Fig. 5A). Infiltrating cells stained positively with an anti-CD3 mAb, suggesting that this infiltrate was composed predominantly of T cells (Fig. 5B). Positive TUNEL staining (30) for the fragmented chromatin that is characteristic of apoptotic nuclei was observed in plasmid Luc inoculated, but not in control saline-inoculated muscles (Fig. 5, C and D). Positive TUNEL staining was observed in the nuclei of both myocytes and infiltrating cells. These observations therefore indicate that there was apoptosis of cells at the site of inoculation associated with an inflammatory infiltrate that included T lymphocytes.
Figure 5.
Histologic examination of vaccine-inoculated muscles demonstrates a CD3+ lymphocytic infiltrate and evidence of apoptosis. Quadriceps muscles were isolated and fixed 17 days following immunization with PBS or 50 µg of plasmid Luc. Paraffin sections from plasmid-inoculated muscles were stained with H&E (A) or an anti-CD3 Ab (B). TUNEL staining was performed on sections from muscles 17 days following inoculation either with saline (C) or with plasmid Luc (D). TUNEL-reactive apoptotic nuclei and CD3-positive cells are stained brown. Slides were counterstained with hematoxylin and are shown at ×40 magnification. No staining was observed either with an anti-CD3 Ab isotype control or following TUNEL staining in the absence of TdT.
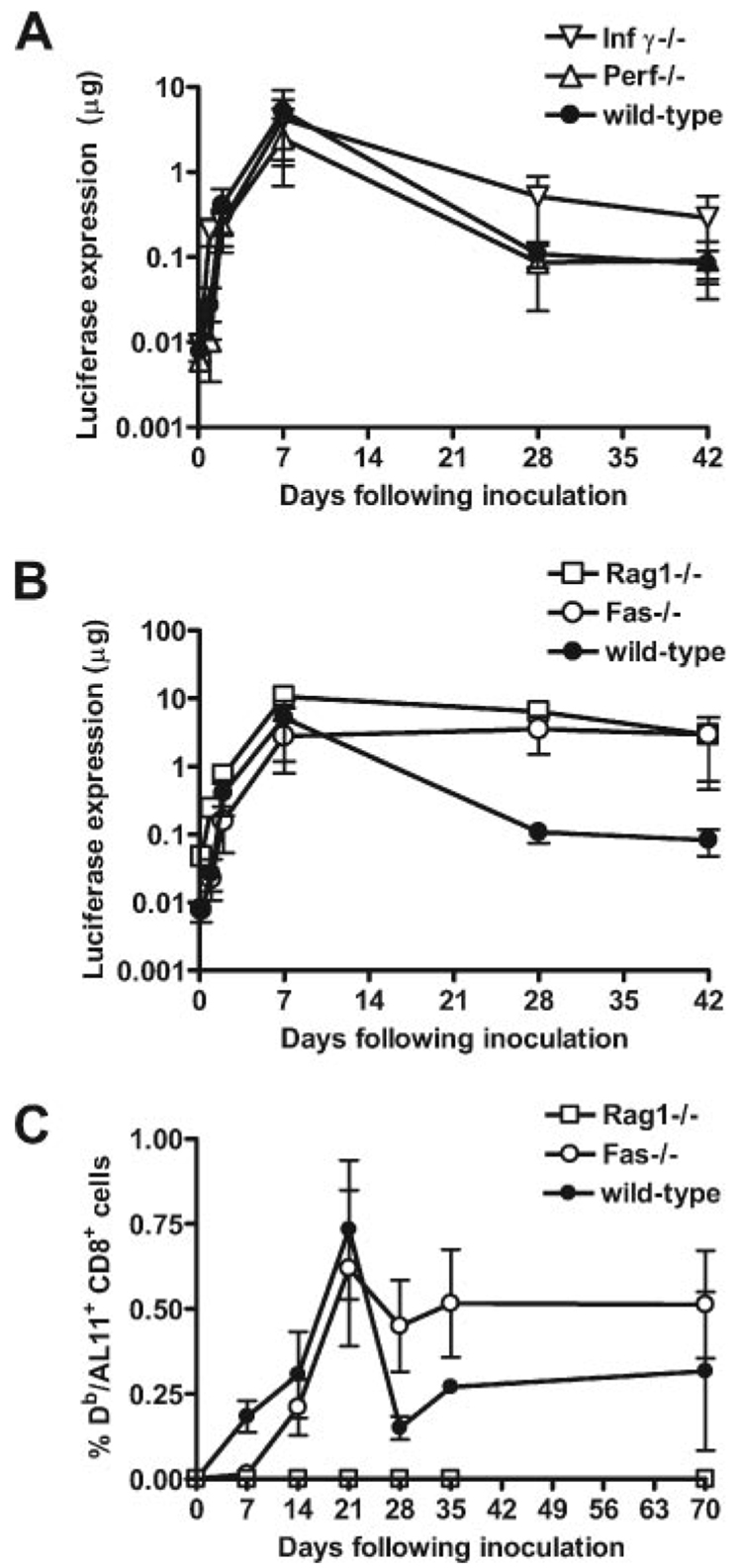
To investigate the mechanisms underlying this apoptosis of muscles, we evaluated the contribution of selected molecules associated with T lymphocyte effector functions (31–33). Mice with homozygous deletions in perforin and IFN-γ genes were immunized with plasmid Luc and prospectively monitored for luciferase expression. The damping of luciferase expression in these animals was comparable to that seen in wild-type mice (Fig. 6A), suggesting that these T lymphocyte mediators are not associated with this functional activity. However, Rag1-deficient mice did not demonstrate damping of luciferase expression following vaccination with plasmid Luc, indicating that the cells mediating this effect were Ag specific (Fig. 6B). Moreover, we also observed persistent, high-level luciferase expression in Fas-deficient mice (Fig. 6B). This finding suggests that the regulation of plasmid DNA expression in immunized mice is dependent on Fas/Fas ligand interactions.
Figure 6.
T lymphocyte regulation of plasmid DNA expression in vivo is Fas ligand dependent.A and B, Animals (n = 4–8) were immunized with 50 µg of plasmid Luc, and expression was monitored over 42 days in wild-type C57BL/6 mice, IFN-γ−/− mice, and perforin−/− mice (A), or in Fas−/− and Rag1−/− mice (B). A one-way ANOVA showed the differences between groups at day 28 to be statistically significant (p < 0.0001), and a Dunnett’s multiple comparison test with a cutoff of p = 0.05 showed expression levels to be higher in Fas−/− and Rag1−/− mice, but not in IFN-γ−/− or perforin−/− mice as compared with wild-type control mice. C, Wild-type, Fas−/−, and Rag1−/− mice were immunized with a plasmid containing AL11-tagged luciferase. Immune responses were measured by Dd/AL11 tetramer staining of CD8+ PBLs at the indicated time points.
One possible explanation for the persistence of plasmid DNA vaccine Ag expression in Fas-deficient mice is that these mice were incapable of mounting an immune response to plasmid DNA vaccines. To examine the kinetics of immune responses in the absence of Fas-mediated Ag clearance, we immunized wild-type, Rag1−/−, and Fas−/− mice with an AL11-tagged luciferase expression plasmid and monitored Ag expression and immune responses. As before, we observed persistent luciferase expression by in vivo imaging following immunization of Fas−/− and Rag1−/− mice, whereas luciferase expression decayed in wildtype mice (data not shown). As expected, we detected an AL11-specific immune response following immunization in wild-type, but not in Rag1−/− animals. Interestingly, we observed a persistent and potent immune response in Fas−/− animals in association with the persistent Ag expression (Fig. 6C). These data suggest that persistent immune responses can be generated if Fas-mediated vaccine Ag clearance is avoided.
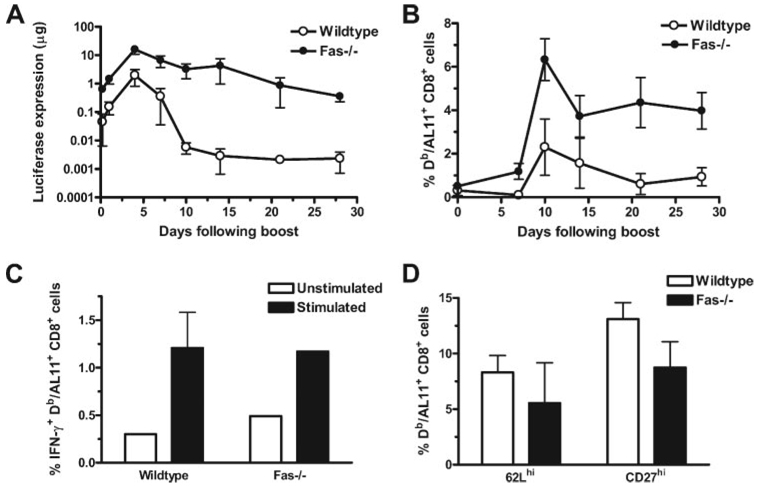
We then sought to determine how persistent Ag expression could occur in the setting of a high-frequency Ag-specific CD8+ T cell response. To examine whether Ag-specific cells in Fas−/− animals are functionally defective and could therefore not eliminate cells expressing vaccine Ag, we compared the proliferative capacity, effector function, and phenotype between Ag-specific T cells generated in Fas−/− animals and those generated in wild-type animals. We inoculated Fas−/− and wild-type mice with priming and boosting inoculations of plasmid AL11-Luc. Responses to the priming immunizations were comparable to those illustrated in Fig. 6, B and C. Following the boosting immunizations, we observed luciferase expression in both strains of mice (Fig. 7A). Interestingly, vaccine Ag expression was attenuated significantly more rapidly following the boosting immunization than following the priming immunization. Peak expression levels in wild-type animals were observed at 11 days following a priming immunization and 4 days following a boost immunization. A more rapid damping of expression following a boosting inoculation is consistent with the central role of the cellular immune response in vaccine Ag clearance. Consistent with what was observed following a priming immunization, vaccine Ag expression following boosting was more persistent in the Fas−/− than in the wild-type control mice. However, in contrast to what was seen following a single immunization, some attenuation of luciferase expression was observed following boosting of the Fas−/− animals. These data suggest that other mechanisms in addition to Fas-mediated apoptosis may be capable of eliminating Ag-producing muscle cells in the setting of the potent immune response elicited by a prime-boost immunization.
Figure 7.
Immune responses in Fas−/− mice are larger in magnitude and comparable in both function and phenotype when compared with those in wild-type mice following a boosting immunization. Seventy days following a priming immunization with 50 µg of plasmid AL11-Luc, mice (n = 4–8) were inoculated again with the same immunogen. A, Expression was monitored over the ensuing 30 days. Statistically significant differences in expression levels between wild-type and Fas−/− mice were observed at all time points beginning 7 days following the boost. Significance was calculated using Student’s t test with a cutoff of p = 0.05. B, Immune responses were monitored by Db/AL11 tetramer and anti-CD8 staining. Differences in frequencies of tetramer+ CD8+ lymphocytes were statistically significant between wild-type and Fas−/− animals at 10, 14, 21, and 28 days following the boost using Student’s t test with a cutoff of p = 0.05. C, Intracellular IFN-γ staining was performed on lymphocytes from wild-type and Fas−/− mice 14 days following boosting. Lymphocytes were cultivated for 6 h without (□) or with (■) AL11 peptide, and the percentage of total CD8+ lymphocytes producing IFN-γ is shown. Differences between lymphocytes of the wild-type and Fas−/− groups were not statistically significant. D, Phenotypic analysis of Db/AL11+ CD8+ lymphocytes was performed 14 days following boosting. The percentage of 62Lhigh and CD27high Db/AL11+ CD8+ T cells is shown for wild-type (□) or Fas−/− mice (■). Phenotypic differences between lymphocytes of the wild-type and Fas−/− groups were not statistically significant.
We also examined the immune responses induced in these mice by plasmid DNA inoculation (Fig. 7B). In both strains of mice, we observed more rapid and higher magnitude responses than were seen following the priming immunizations. We also observed significantly higher immune responses in the Fas−/− than in the wild-type control mice following the boost immunizations.
We examined the function and phenotype of the vaccine-elicited CD8+ T cells 14 days following boosting in both strains of mice. AL11-specific CD8+ T lymphocytes from these mice produced higher levels of IFN-γ following exposure to AL11 peptide than did unexposed cells (Fig. 7C). Importantly, no differences in the percentages of IFN-γ+; AL11-specific CD8+ T cells were observed between Fas−/− and wild-type mice, even though the total number of Ag-specific T cells was higher in Fas−/− animals. These data suggest that Fas−/− T cells and wild-type T cells have similar effector function.
Because it has been suggested that persistent Ag expression can prevent the transition of Ag-specific T lymphocytes to a central memory subpopulation (34), we sought to determine whether vaccinated Fas−/− animals were deficient in central memory cells. Expression of both the CD27 and CD62L molecules have been used to distinguish between central and effector T cells, central memory T cells being both CD27high and CD62Lhigh (34, 35). We observed no statistically significant differences between wild-type and Fas−/− AL11-specific CD8+ T cells in their CD27 and CD62L expression (Fig. 7D). These data suggest that differences in central and effector phenotype between Fas−/− and wild-type T cells animals do not account for the increased cellular immune responses and persistent Ag expression observed in Fas−/− animals.
Discussion
The present studies show that vaccine-elicited T lymphocytes play a central role in modulating vaccine Ag expression in vivo. Using in vivo imaging of luciferase expression, we demonstrate that Ag expression from plasmid DNA or rAd immunogens is significantly reduced in mice with functional cellular immune systems, and that this reduction is temporally coincident with the emergence of T cell immune responses to these immunogens. Persistent transgene expression is observed with a vector that does not induce significant cellular immune responses, but augmentation of these immune responses with an adjuvant leads to the elimination of transgene expression.
Because Rag is needed for the TCR rearrangements that allow Ag recognition (36), the Rag requirement for the damping of Ag expression suggests that this phenomenon is mediated by Ag-specific lymphocytes. Similarly, the absence of a detectable Ab response in animals in which Ag expression is damped, the close temporal association between the elimination of Ag expression and the emergence of a T lymphocyte response, the absence of damping in athymic nude mice, and the more rapid attenuation of luciferase expression following boosting immunization all suggest that T lymphocytes mediate this phenomenon. Finally, the observation of apoptosis in muscle cells following plasmid DNA inoculation and the requirement for Fas for terminating vaccine immunogen expression suggest that Ag-specific T lymphocytes may induce apoptosis of cells expressing vaccine immunogens in vivo.
Previous work suggested that inoculation of plasmid DNA encoding a surface-expressed immunogen can lead to Ab-mediated destruction of Ag-expressing cells, but immunogens such as luciferase that are expressed intracellularly can express persistently (13, 14, 29). These earlier findings suggested that cellular immune responses do not play a role in limiting expression from plasmid DNA immunogens, and that unadjuvanted plasmid DNA could serve as a persistent source of Ag for cellular immune responses, as long as it does not evoke a humoral immune response. Consistent with those reports, we observed that certain luciferase-expressing plasmid DNA constructs were not eliminated by cellular immune responses; however, these same constructs were incapable of eliciting potent cellular immune responses. High-frequency cellular immune responses with an associated clearance of Ag-expressing cells in vivo were both observed either when the suboptimal vaccine construct was formulated with an adjuvant or when an immunogenically optimized vaccine construct was used.
Because potent immune responses prevent persistent Ag expression by vaccine immunogens, persistent expression will only possible if these immune mechanisms can be evaded. Immune evasion may prove possible if plasmid DNA is released in vivo from an immunologically silent depot (37), or if vaccine Ag-producing cells can avoid elimination. Although it is theoretically possible that the persistence of high-level Ag expression in a vaccinee may lead to tolerization (38) or prevent the transition of Ag-specific lymphocytes to a central memory subpopulation (34), prolongation of Ag expression in vivo may also substantially potentiate cellular immune responses. Indeed, we did not observe defective anamnestic responses, effector function, or transition to central memory cells in Fas−/− animals that persistently expressed luciferase Ag.
The present findings suggest a flaw in current vaccine strategies designed to achieve persistent Ag expression. Because vectors that induce potent immune responses cause the elimination of Ag-expressing cells, avoidance of this immune response will be required to achieve high-level, persistent expression of vaccine Ag to drive immune responses. The observations in the present study will therefore inform the expectations and strategies that will be used by investigators attempting to vaccinate against pathogens such HIV, M. tuberculosis, and malaria.
Acknowledgments
We acknowledge Richard Mulligan, Laising Yen, Jinyan Liu, Diana Lynch, Dan Barouch, Michelle Lifton, Dilani Dombagoda, and Gary Nabel for advice, support, and reagents.
Footnotes
This work was supported by the National Institute of Allergy and Infectious Diseases Center for HIV/AIDS Vaccine Immunology, Grant AI067854.
Abbreviations used in this paper: HBsAg, surface-expressed hepatitis B virus Ag; IVIS, in vivo imaging system; rAd, recombinant adenovirus serotype 5.
Disclosures The authors have no financial conflict of interest.
References
- 1.Zinkernagel RM, Hengartner H. Regulation of the immune response by antigen. Science. 2001;293:251–253. doi: 10.1126/science.1063005. [DOI] [PubMed] [Google Scholar]
- 2.Kundig TM, Bachmann MF, Oehen S, Hoffmann UW, Simard JJ, Kalberer CP, Pircher H, Ohashi PS, Hengartner H, Zinkernagel RM. On the role of antigen in maintaining cytotoxic T-cell memory. Proc. Natl. Acad. Sci. USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Faassen H, Dudani R, Krishnan L, Sad S. Prolonged antigen presentation, APC−, and CD8+ T cell turnover during mycobacterial infection: comparison with Listeria monocytogenes. J. Immunol. 2004;172:3491–3500. doi: 10.4049/jimmunol.172.6.3491. [DOI] [PubMed] [Google Scholar]
- 4.Gray D, Matzinger P. T cell memory is short-lived in the absence of antigen. J. Exp. Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann MF, Beerli RR, Agnellini P, Wolint P, Schwarz K, Oxenius A. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur. J. Immunol. 2006;36:842–854. doi: 10.1002/eji.200535730. [DOI] [PubMed] [Google Scholar]
- 6.Oehen S, Waldner H, Kundig TM, Hengartner H, Zinkernagel RM. Antivirally protective cytotoxic T cell memory to lymphocytic choriomeningitis virus is governed by persisting antigen. J. Exp. Med. 1992;176:1273–1281. doi: 10.1084/jem.176.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson PR, Schnepp BC, Connell MJ, Rohne D, Robinson S, Krivulka GR, Lord CI, Zinn R, Montefiori DC, Letvin NL, Clark KR. Novel adeno-associated virus vector vaccine restricts replication of simian immunodeficiency virus in macaques. J. Virol. 2005;79:955–965. doi: 10.1128/JVI.79.2.955-965.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, Bansal GP, Young JF, Lee MH, Hatfull GF, et al. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Ertl HC, Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 10.Zoltick PW, Chirmule N, Schnell MA, Gao GP, Hughes JV, Wilson JM. Biology of E1-deleted adenovirus vectors in nonhuman primate muscle. J. Virol. 2001;75:5222–5229. doi: 10.1128/JVI.75.11.5222-5229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathy SK, Black HB, Goldwasser E, Leiden JM. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat. Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 13.Davis HL, Millan CL, Watkins SC. Immune-mediated destruction of transfected muscle fibers after direct gene transfer with antigen-expressing plasmid DNA. Gene Ther. 1997;4:181–188. doi: 10.1038/sj.gt.3300380. [DOI] [PubMed] [Google Scholar]
- 14.Payette PJ, Weeratna RD, McCluskie MJ, Davis HL. Immunemediated destruction of transfected myocytes following DNA vaccination occurs via multiple mechanisms. Gene Ther. 2001;8:1395–1400. doi: 10.1038/sj.gt.3301534. [DOI] [PubMed] [Google Scholar]
- 15.Hezareh M, Hessell AJ, Jensen RC, van de Winkel JG, Parren PW. Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J. Virol. 2001;75:12161–12168. doi: 10.1128/JVI.75.24.12161-12168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao-Yuan Zi, Y Y-C, Zhu H-Y, Xiong J, Wu X-J, Zhang N, Ba Y, Li W-L, Wang X-M, Li J-X, Yu H-Y, et al. Long-term persistence of hepatitis B surface antigen and antibody induced by DNA-mediated immunization results in liver and kidney lesions in mice. Eur. J. Immunol. 2006;36:875–886. doi: 10.1002/eji.200535468. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama M, Hassett DE, Zhang J, Whitton JL. DNA immunization can stimulate florid local inflammation, and the antiviral immunity induced varies depending on injection site. Vaccine. 1997;15:553–560. doi: 10.1016/s0264-410x(97)00213-2. [DOI] [PubMed] [Google Scholar]
- 18.Lecocq M, Andrianaivo F, Warnier MT, Wattiaux-De Coninck S, Wattiaux R, Jadot M. Uptake by mouse liver and intracellular fate of plasmid DNA after a rapid tail vein injection of a small or a large volume. J. Gene Med. 2003;5:142–156. doi: 10.1002/jgm.328. [DOI] [PubMed] [Google Scholar]
- 19.Sabbatini P, Chiou S, Rao L, White E. Modulation of p53-mediated transcriptional repression and apoptosis by the adenovirus E1B 19K protein. Mol. Cell. Biol. 1995;15:1060–1070. doi: 10.1128/mcb.15.2.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napirei M, Wulf S, Eulitz D, Mannherz HG, Kloeckl T. Comparative characterization of rat deoxyribonuclease 1 (Dnase1) and murine deoxyribonuclease 1-like 3 (Dnase113) Biochem. J. 2005;389:355–364. doi: 10.1042/BJ20042124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barouch DH, Santra S, Tenner-Racz K, Racz P, Kuroda MJ, Schmitz JE, Jackson SS, Lifton MA, Freed DC, Perry HC, et al. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J. Immunol. 2002;168:562–568. doi: 10.4049/jimmunol.168.2.562. [DOI] [PubMed] [Google Scholar]
- 22.Greenland JR, Liu H, Berry D, Anderson DG, Kim WK, Irvine DJ, Langer R, Letvin NL. β-Amino ester polymers facilitate in vivo DNA transfection and adjuvant plasmid DNA immunization. Mol. Ther. 2005;12:164–170. doi: 10.1016/j.ymthe.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 24.Hovav AH, Cayabyab MJ, Panas MW, Santra S, Greenland J, Geiben R, Haynes BF, Jacobs WR, Jr, Letvin NL. Rapid memory CD8+ T-lymphocyte induction through priming with recombinant Mycobacterium smegmatis. J. Virol. 2007;81:74–83. doi: 10.1128/JVI.01269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook SH, Griffin DE. Luciferase imaging of a neurotropic viral infection in intact animals. J. Virol. 2003;77:5333–5338. doi: 10.1128/JVI.77.9.5333-5338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelleitier M, Montplaisir S. The nude mouse: a model of deficient T-cell function. Methods Achiev. Exp. Pathol. 1975;7:149–166. [PubMed] [Google Scholar]
- 27.McKay PF, Barouch DH, Santra S, Sumida SM, Jackson SS, Gorgone DA, Lifton MA, Letvin NL. Recruitment of different subsets of antigen-presenting cells selectively modulates DNA vaccine-elicited CD4+ and CD8+ T lymphocyte responses. Eur. J. Immunol. 2004;34:1011–1020. doi: 10.1002/eji.200324840. [DOI] [PubMed] [Google Scholar]
- 28.Sumida SM, Truitt DM, Kishko MG, Arthur JC, Jackson SS, Gorgone DA, Lifton MA, Koudstaal W, Pau MG, Kostense S, et al. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J. Virol. 2004;78:2666–2673. doi: 10.1128/JVI.78.6.2666-2673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum. Mol. Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 30.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 32.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol. Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 33.Dobbs ME, Strasser JE, Chu CF, Chalk C, Milligan GN. Clearance of herpes simplex virus type 2 by CD8+ T cells requires γ interferon and either perforin- or Fas-mediated cytolytic mechanisms. J. Virol. 2005;79:14546–14554. doi: 10.1128/JVI.79.23.14546-14554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 35.Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, Wu BB, Cheung KP, Ding Z, Goldrath AW. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat. Immunol. 2006;7:1317–1325. doi: 10.1038/ni1403. [DOI] [PubMed] [Google Scholar]
- 36.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 37.Huang YC, Riddle K, Rice KG, Mooney DJ. Long-term in vivo gene expression via delivery of PEI-DNA condensates from porous polymer scaffolds. Hum. Gene Ther. 2005;16:609–617. doi: 10.1089/hum.2005.16.609. [DOI] [PubMed] [Google Scholar]
- 38.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]