Abstract
A sensitive and fast method was developed to quantitate the carcinogenic polycyclic aromatic hydrocarbon benzo(a)pyrene (BaP) and eight of its oxidized metabolites by ultra-performance liquid chromatography (UPLC) coupling with mass spectrometry (MS). The UPLC method, using an acetonitrile:water gradient as a mobile phase, provided baseline separation of the BaP metabolites including three BaP diones. Linearity of detection was in the range of 0.2 to 5.0 ng/μL, and limits of detection (LODs) were lower than 0.01 ng/μL for BaP and all of the metabolites except BaP tetrol. In order to test this method in environmentally relevant samples, we exposed the small fish Fundulus heteroclitus to BaP and quantitated biliary BaP metabolites. Extraction recovery of all compounds varied from 65.4 ± 21.3% to 92.4 ± 3.0%. In exposed fish bile, the BaP diones, BaP-7,8-dihydrodiol, and 3-hydroxy BaP metabolites predominated, existing mainly as glucuronic acid conjugates. This UPLC-MS method will be useful for further defining the roles of cytochrome P450s with both in vivo and in vitro models in the understanding of the mechanisms of metabolic activation and detoxification of BaP.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a group of compounds, which share the same core structure: two or more fused rings consisting of only carbon and hydrogen[1,2]. PAHs are formed during incomplete combustion of organic substances, such as coal, oil, wood, and tobacco. Some PAH mixtures and individual PAHs are considered as carcinogens by the International Agency for Research on Cancer (IARC). Due to their ubiquitous presence and their toxic potency, PAHs are one of the most important environmental pollutants[3].
Benzo[a]pyrene (BaP), a carcinogenic model PAH, has been extensively studied[4]. Bio-activation is needed for BaP to exert its toxic, mutagenic, and carcinogenic effects[5,6]. Hepatobiliary excretion is the primary route for BaP metabolic elimination[7], so the analysis of bile metabolites is a reasonable and convenient way to understand BaP metabolism in animal models. The major metabolites of BaP include phenols, diones and dihydrodiols, most of which can be conjugated to glucuronic acid, sulfate and glutathione to become more water soluble facilitating excretion[8]. Unfortunately one dihydrodiol, BaP-7,8- dihydrodiol, can be further oxidized to BaP-7,8-diol-9,10-epoxide (BPDE). BPDE can react with proteins, lipids and DNA, hence it is considered the ultimate carcinogenic metabolite of BaP[5]. Some studies have found BaP quinones can also form DNA adducts when they became semi-quinones by one electron reduction[9,10]. Therefore, an important research question is how is BaP metabolized in susceptible tissues or species, and ultimately, what enzymes are involved in bioactivation vs. detoxification. BaP is a potent inducer of CYP1 enzymes including CYP1A, CYP1B and CYP1C[11–14]. In turn, CYP1A and CYP1B also play an important role in BaP metabolism[15–17] and genotoxicity[18,19]. Recently CYP1C has been identified in fish[20–22], and the induction of this enzyme by BaP exposure was observed in Fundulus heteroclitus[11], however its metabolic role is currently unknown. Furthermore, PAH exposure causes liver cancer in this organism[23,24], so our goal was to develop a sensitive method to quantitate BaP metabolism in this small fish species.
High performance liquid chromatography (HPLC) has been widely used for the analysis of drug or environmental contaminant metabolites, including BaP metabolites[25–32], for the last several decades. In the 1970’s, HPLC was used for separating BaP metabolites[25–27] combined with UV or scintillation counter detection. Although HPLC certainly provided better separation than TLC, phenols were barely separated, while dihydrodiols and BaP diones were not resolved[25–27]. In 1984, Krahn et al developed a method of biliary PAH quantitation by measuring fluorescence at 380/430 nm excitation/emission to detect “BaP-like” metabolites[28]. This method has been widely accepted as a sensitive and predictable method to detect and monitor PAH contamination[29–32]. However BaP diones, do not exhibit fluorescence, and the method does not quantitate individual metabolites prohibiting the application of fluorescence detection for a detailed study of BaP metabolism. During the 1990’s, on-line or off-line radioactivity detection played a major role in the study of BaP metabolism[6,17,33–36]. The disadvantages of the radioactive method include cost, radiolabeled individual metabolites are not available, and it cannot be used for the analysis of environmental samples. The improvement of chromatographic separation methods have allowed for significantly better separation of BaP metabolites during this decade[17,34,37], although a long gradient elution protocol had to be used, and BaP diones were still unresolved. From 2000, HPLC coupled with mass spectrometry was introduced in the study of BaP metabolism though there were still problems associated with high detection limits for the diones[32]. Willett and colleagues[38] successfully compared the metabolism and excretion of BaP in two species of Ictalurid catfish by using HPLC-MS. However, the run time was still more than 60 minutes per sample. So, the analytical challenge associated with developing a rapid, efficient and convenient method by traditional techniques to determine BaP metabolites is still considerable.
Recently, ultra-performance liquid chromatography (UPLC) has been introduced with improved performance over traditional HPLC[39]. UPLC takes advantage of smaller (sub-2 μm) column-filling particles and is dimensioned to allow for higher pressures (up to 14,000 psi). These column characteristics allow for better resolution, more rapid analysis, and improved detection sensitivity compared to traditional HPLC[40,41]. In this manuscript we report on the development and validation of a new UPLC-MS method to separate and analyze BaP and eight of its metabolites from F. heteroclitus bile, which showed overall improvement compared to currently used methods, and provided the methodological support to study the role of CYP1s in BaP’s metabolism.
2. Experimental
2.1. Chemicals
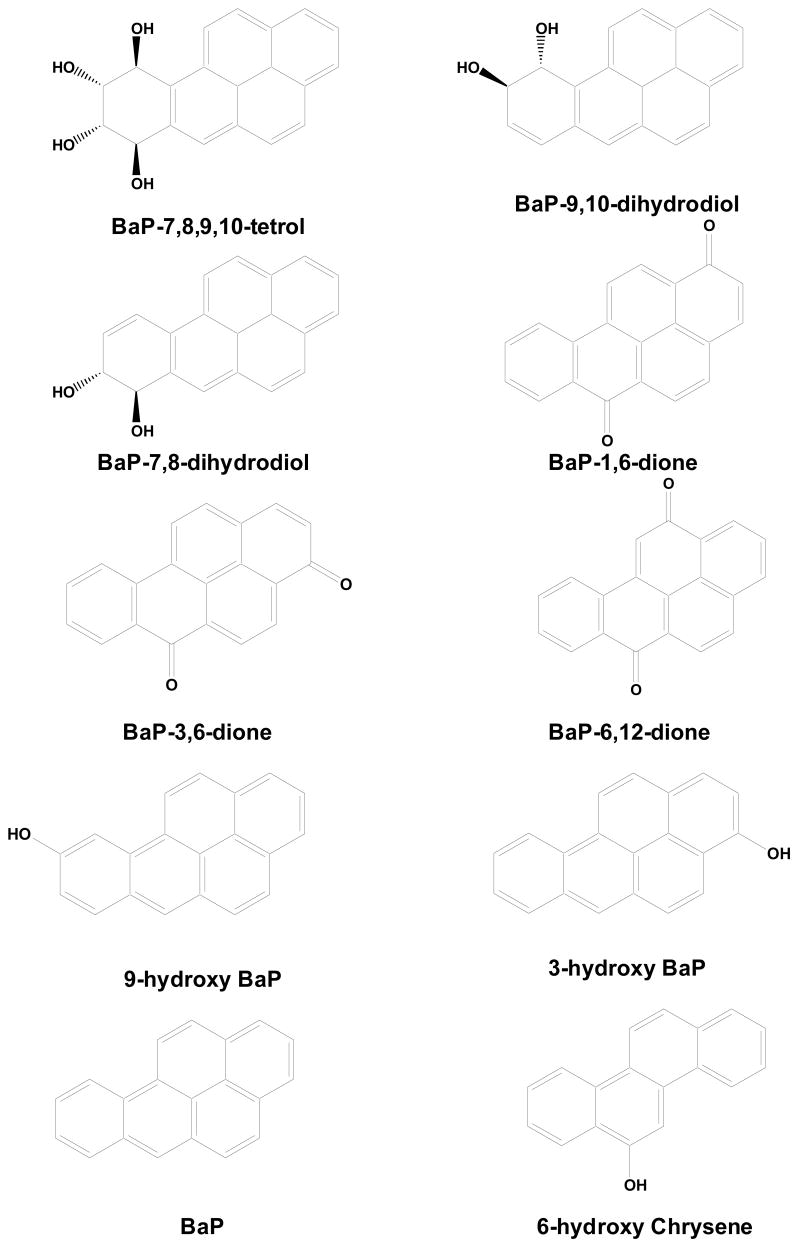
BaP, benzo[a]pyrene solution (100 μg/ml in dichloromethane, Cat. No.: P-650, Lot Number: CB-1109, 1 ml) was purchased from Ultra Scientific Analytical Solutions (North Kingstown, RI). 6-hydroxychrysene (100% purity (GC/FID), R-095N, CAS No. 37515-51-8, 2 ×10 mg) was purchased from AccuStandard® (New Haven, CT). BaP1,6-dione, BaP-3,6-dione, BaP-6,12-dione, 3-hydroxy BaP, 9-hydroxy BaP, BaP-7,8-dihydrodiol, BaP-9,10-dihydrodiol, and BaP-7,8,9,10-tetrahydrotetrol BaP were purchased from National Cancer Institute Chemical Carcinogen Reference Standard Repository (Kansas City, MO). The structures of BaP, its metabolites and 6-hydroxy chrysene (the internal standard) are shown in Fig. 1. Acetone (optima*), acetonitrile (HPLC grade), ethyl acetate (HPLC grade), and water (HPLC grade) were purchased from Fisher (Fairlawn, NJ); formic acid and sodium acetate trihydrate (95% acetic acid sodium salt, CAS number: 6131-90-4) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Fig. 1.
Chemical structures of BaP, eight BaP metabolites and 6-hydroxy chrysene (the internal standard).
2.2. UPLC instrument and chromatographic conditions
Waters Acquity UPLC system was used (Milford, MA, USA). Chromatographic separation was performed on a Waters Acquity UPLC™ BEH C18 column (1.7 μm, 2.1 × 50 mm) at 28°C. A 0.2 μm precolumn filter (Acquity UPLC™ stainless steel in-line filter) was used to protect the analytical column. One micro liter sample, dissolved in acetonitrile, was injected for UPLC analysis. The flow rate was 0.25 ml/min. Mobile phase A was acetonitrile and mobile phase B was water containing 0.3% formic acid. The time program for the multi-step gradient was: 0–6 min: 35/65 (A/B, v/v) to 60/40 (A/B, v/v), 6–9 min: 60/40 (A/B, v/v) to 100/0 (A/B, v/v), 9–10 min: 100/0 (A/B, v/v) to 35/65 (A/B, v/v). Total run time was 10 min per sample.
2.3. Mass spectrometry
The MS equipment consisted of a Waters Micromass Quattro Micro™ triple-quadrupole system (Manchester, UK). The MS system was controlled by MassLynx software, version 4.0. The APCI+ (positive Atmospheric Pressure Chemical Ionization) interface consisted of a heated nebulizer probe and a standard atmospheric pressure source equipped with a corona discharge pin. The source and probe temperatures were set to 100°C and 550°C, respectively. The corona current was 6.0 μA; the cone voltage was 80 V; the extractor voltage was 4 V, and the RF lens voltage was set to 0.1 V. The desolvation and cone gases were 400 and 50 L/hr, respectively. Analysis was performed in selected ion recording (SIR).
2.4. Standard and working solution
Individual stock solutions in acetonitrile of BaP metabolites (100 ng/μL), and internal standard (30 ng/μL) were prepared. For BaP tetrol, water and DMSO were added to acetonitrile to improve solubility. Stock solutions of BaP and its metabolites were mixed and further diluted. The final concentrations of BaP and each metabolite in the standard solutions ranged from 0.1 to 6 ng/μL (0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0 ng/μL), while the concentration of the internal standard was fixed at 3 ng/μL.
Working solutions were prepared by adding each standard solution (100 μL) in a borosilicate glass disposable culture tube (13×100 mm). After evaporation by a stream of nitrogen, the residue was dissolved in blank bile from two gallbladders and then extracted as described in Section 2.5. Quality control (QC) samples at 3 different concentrations (0.4, 2, 4 ng/μL) were prepared in the same way, and were used to assess accuracy and precision.
2.5. Fish exposure and bile sample preparation
Adult F. heteroclitus were divided to three groups: 1) control group, treated with ethanol (vehicle) at 100 μl/L; 2) low BaP group, exposed with 10 μg/L BaP; 3) high BaP group, exposed with 100 μg/L BaP for 15 days with ~90% static renewal daily. Two waterborne BaP exposures were done with 12 fish per group for experiment 1, and 16 fish per group for experiment 2. The water concentration of BaP was confirmed by GC/MS to be: 9.0 ± 1.3 μg/L and 101 ± 6.6 μg/L for experiment 1; 9.2 ± 0.6 μg/L and 101 ± 15.4 μg/L for experiment 2, respectively for the low and high concentration groups. The gallbladders were carefully dissected from each fish and stored at −80°C.
Two gallbladders (about 3 μL bile each) and 10 μL of internal stock solution (30 ng/μL of 6-hydroxy chrysene) were placed in a borosilicate glass disposable culture tube (13×100 mm). After adding 0.5 ml sodium acetate buffer (200 mM, pH 5.0) to each tube, extractions were done with 1 ml ethyl acetate:acetone (2:1) first, then followed by 1 ml ethyl acetate two more times. The organic fractions were combined and dried down under nitrogen. The residue was dissolved in 100 μL acetonitrile, and filtered with 0.45 μm filter (Millex®-HN, 4mm). This extract contained the “free” unconjugated BaP metabolites.
The aqueous phase from the extract described above was blown down to remove any trace of the organic phase. Glucurase (200 μl, 1000 units, β-D-Glucuronide glucuronosohydrolase, Sigma) was added, and tubes were capped, vortexed, and incubated for 6 hr at 37°C. The reaction was stopped by addition of a mixture of ethyl acetate:acetone (2:1) and the internal standard was added. Two additional extractions were done: one with ethyl acetate:acetone (2:1) and a second with ethyl acetate alone. Once concentrated, resuspended in acetonitrile, and filtered, this extract contained the BaP metabolites that had been conjugated with glucuronic acid. To isolate metabolites that had been sulfate conjugated, aqueous samples were then brought up to 1 mL with 0.2 M sodium acetate, pH 5.0. Nineteen units of aryl sulfatase (Type V from limpets, Sigma) and 20 μmol/mL saccharic acid lactone were added to the tubes and incubated again for 6 hr at 37°C. After incubation, the samples were again extracted as previously described [38]. All samples were protected from oxidation by a layer of argon gas.
2.6. Method validation
The validation of this UPLC-MS method included linearity, inter- and intra-day precision, intra-day accuracy, and recovery.
2.6.1. Linearity, limits of detection (LOD), and limits of quantitation (LOQ)
Calibration curves were obtained by plotting the peak area ratio of BaP or its metabolites to internal standard against the theoretical concentration of each compound added, using a weighted (1/x) least-squares regression. The calibration was processed with the MassLynx 4.0 QuanLynx software. The limit of detection (LOD) was determined as the concentration corresponding to a peak height that was three times of the baseline noise. A 10:1 ratio of peak height to baseline noise was used to determine the limit of quantification (LOQ).
2.6.2. Precision and accuracy
To test the reproducibility of this analytical method, quality control samples were analyzed. The intra-day precision was measured as the relative standard deviation (RSD) of the peak area ratio of BaP or its metabolites to the internal standard from five consecutive injections of the same sample. The inter-day precision was measured as the relative standard deviation (RSD) of the peak area ratio of BaP or its metabolites to internal standard from five injections of the same sample, one injection per day. Accuracy was assessed by comparison of the calculated mean concentration (n=3) to nominal concentration for each compound.
2.6.3. Extraction recovery
The recoveries of BaP and its metabolites were determined in triplicate using samples spiked at 2 ng/μL for each compound individually. Stock solution (2 μL) of BaP or its metabolites (100 ng/μL) was placed in a borosilicate glass disposable culture tube (13 × 100 mm) with 5 μl blank bile, and then it was extracted as described in section 2.5. The extraction recoveries were calculated by comparing peak areas from spiked samples to the same amounts of standard solution directly diluted from 2 μL to 100 μL with acetonitrile. The internal standard at the concentration of 3 ng/μL was determined by the same way.
3. Results and discussion
3.1. Chromatography
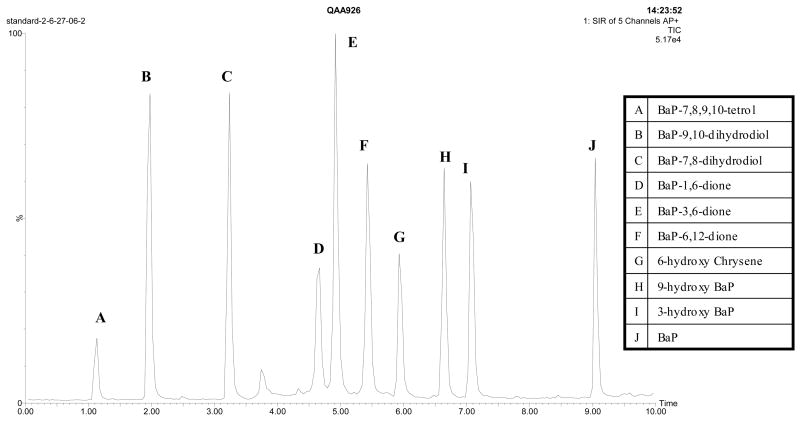
Although several methods described in the literature used acetonitrile/water as mobile phase in HPLC for the separation of polycyclic aromatic hydrocarbons, including BaP[42–44], most BaP HPLC methods [6,29,31,32,38,45–47] used methanol/water as the mobile phase with a gradient program. We tried methanol/water first, however, even after optimization, we did not achieve ideal separation of the three BaP diones with UPLC, and some bile samples showed a peak of unknown endogenous compound that overlaid with the internal standard. When we changed the organic phase to acetonitrile, with the gradient program described in section 2.2, the three BaP diones were almost base-line-separated, along with perfect separation of other metabolites and the internal standard (Fig. 2). The peak of the endogenous compound was shifted to 2.7 min without overlaying any metabolites. In addition, the labile compounds, such as the BaP diones, showed better stability in acetonitrile than in methanol (data not shown).
Fig. 2.
Chromatogram of BaP, eight metabolites and the internal standard under the gradient elution of acetonitrile/acid water system as described in section 2.2.
The peak name, retention time and selected ion for each compound are shown in Table 1. The separation was done in just 10 minutes with better resolution than those with 45–80 minutes protocols[6,48]. This rapid method saves time and solvent. Furthermore, the mass spectrometry detector with selected ion recording provided better specificity for determination of BaP metabolites, compared to common UV or fluorescent detectors, and radiolabeled parent compounds were unnecessary. In addition, the higher peak capacity of UPLC resulted in a net reduction in ion suppression for MS detector, which gave an improvement in the detection sensitivity, compared to traditional HPLC.
Table 1.
Compound name, retention time, selected ion and the extraction recoveries (n=3, average ± SE) from fish bile at 2 ng/μL spiked concentration.
| Peak name | Compound | Retention time (min) | Selected ion | Recovery (%) |
|---|---|---|---|---|
| A | BaP-7,8,9,10-tetrol | 1.13 | 226 | 65.4±21.3 |
| B | BaP-9,10-dihydrodiol | 1.98 | 239 | 75.2±4.3 |
| C | BaP-7,8-dihydrodiol | 3.24 | 239 | 82.2±8.0 |
| D | BaP-1,6-dione | 4.66 | 226 | 71.1±2.8 |
| E | BaP-3,6-dione | 4.92 | 226 | 86.2±5.0 |
| F | BaP-6,12-dione | 5.42 | 226 | 74.1±11.8 |
| G | 6-hydroxy Chrysene | 5.97 | 202 | 90.6±2.1 |
| H | 9-hydroxy BaP | 6.64 | 239 | 86.4±7.7 |
| I | 3-hydroxy BaP | 7.06 | 239 | 82.7±6.7 |
| J | BaP | 9.04 | 252 | 92.4±3.0 |
3.2. Optimization of extraction procedure
Ethyl ether was tried for extraction, and it resulted in better recovery than ethyl acetate:acetone for most metabolites with the exception of BaP tetrol. However, because the improvement was not particularly dramatic and taking safety into account, the previously published[38] ethyl acetate:acetone extraction was ultimately used. Oxygen and trace amounts of acid in the ethyl acetate, and the pH value of the buffer had significant impacts on the extraction efficiency for the different metabolites. When ACS grade ethyl acetate was used, the recovery of 6-OH chrysene (IS) and 3-OH BaP was unacceptably low. Accordingly, HPLC grade ethyl acetate was required; and an argon bubbling degassing procedure (10 minutes) was helpful to get better recoveries. Sample extraction methods presented in section 2.5 represent optimized methods and resulted in acceptable recovery for each of the compounds (Table 1). The extraction recovery of all compounds varied from 65.4 ± 21.3% to 92.4 ± 3.0%.
3.3. Method validation
Good linearities (regression coefficient between 0.969 to 0.998) were obtained between the peak-area ratio to the internal standard and the corresponding concentration of BaP and its metabolites. The linear range was 0.6 – 5.0 ng/μL for BaP tetrol, while for all other metabolites and BaP, the range was 0.2 – 5.0 ng/μL. The equations for the regression lines and the regression coefficients are shown in Table 2, with all LOD and LOQ values. Generally, except for BaP tetrol (0.08 ng/μL), LOD of BaP and other metabolites were lower than 0.01 ng/μL. LOQs ranged from 0.6 ng/μL for BaP tetrol to lower than 0.01 ng/μL for BaP. The LODs of BaP metabolites in our previous study[38], which also used APCI-MS as detector, were in the range of 1–5 ng (25 ng for BaP tetrol), considering 1 μL injection volume in our method, the LODs were 100 times lower (40 times lower for BaP tetrol), which shows the greater sensitivity of this UPLC method compared traditional HPLC-MS.
Table 2.
Calibration data of the examined compounds, 9 concentrations (0.2, 0.4, 0.6, 0.8, 1.0, 2.0, 3.0, 4.0, 5.0 ng/μL), n = 3
| Compound | Linearity | r | LOD (ng/μL) | LOQ (ng/μL) |
|---|---|---|---|---|
| BaP-7,8,9,10-tetrol | y=0.450975x-0.10325* | 0.977 | 0.08 | 0.6 |
| BaP-9,10-dihydrodiol | y=2.45477x-0.353215 | 0.988 | <0.01 | 0.04 |
| BaP-7,8-dihydrodiol | y=2.35149x-0.371544 | 0.991 | <0.01 | 0.04 |
| BaP-1,6-dione | y=1.95707x-0.0972766 | 0.995 | <0.01 | 0.04 |
| BaP-3,6-dione | y=6.344325x-1.52853 | 0.969 | <0.01 | 0.02 |
| BaP-6,12-dione | y=4.23934x-0.822734 | 0.982 | <0.01 | 0.02 |
| 9-hydroxy BaP | y=1.49807x-0.218547 | 0.993 | <0.01 | 0.02 |
| 3-hydroxy BaP | y=1.45174x-0.142785 | 0.998 | <0.01 | 0.04 |
| BaP | y=1.79128x-0.298198 | 0.991 | <0.01 | <0.01 |
: The linear range of BaP tetrol was 0.6–5.0 ng/μL
Intra-day precision values of BaP and all of its metabolites were within 9.8% RSD, and inter-day precision values were within 13.9% RSD at all three concentrations: 0.4, 2.0 and 4.0 ng/μL (Table 3). The intra-day accuracy varied from 78.8% to 108.8% for all compounds at these three concentrations (Table 4).
Table 3.
Intra- and inter- precision data (n = 5 injections) of BaP and its eight metabolites at three different concentrations
| Compound | Intra-day precision (RSD %) | Inter-day precision (RSD %) | ||||
|---|---|---|---|---|---|---|
| 0.4 ng/μL | 2 ng/μL | 4 ng/μL | 0.4 ng/μL | 2 ng/μL | 4 ng/μL | |
| BaP-7,8,9,10-tetrol | 9.4 | 3.8 | 7.6 | 11.4 | 8.3 | 8.6 |
| BaP-9,10-dihydrodiol | 4.0 | 3.9 | 8.7 | 11.0 | 6.3 | 5.5 |
| BaP-7,8-dihydrodiol | 8.2 | 2.0 | 8.0 | 4.5 | 7.3 | 4.0 |
| BaP-1,6-dione | 3.8 | 4.5 | 6.8 | 7.9 | 11.0 | 8.3 |
| BaP-3,6-dione | 6.3 | 4.5 | 7.3 | 5.9 | 5.6 | 7.1 |
| BaP-6,12-dione | 8.9 | 3.4 | 5.1 | 9.7 | 7.5 | 2.7 |
| 9-hydroxy BaP | 6.4 | 3.3 | 7.4 | 10.5 | 5.4 | 3.0 |
| 3-hydroxy BaP | 7.7 | 3.3 | 6.5 | 6.7 | 7.3 | 3.7 |
| BaP | 5.8 | 9.8 | 8.1 | 12.9 | 10.3 | 13.9 |
Table 4.
Accuracy data (n = 3, average ± SE) of BaP and its eight metabolites at three different concentrations
| Compound | Accuracy (%) | ||
|---|---|---|---|
| 0.4 ng/μL | 2 ng/μL | 4 ng/μL | |
| BaP-7,8,9,10-tetrol | 108.8±2.8 | 83.7±5.7 | 108.5±9.2 |
| BaP-9,10-dihydrodiol | 106.3±1.7 | 85.6±3.7 | 104.4±5.3 |
| BaP-7,8-dihydrodiol | 108.8±1.6 | 88.5±4.4 | 100.7±2.2 |
| BaP-1,6-dione | 108.1±5.8 | 92.8±2.1 | 97.3±1.7 |
| BaP-3,6-dione | 108.7±2.5 | 78.8±3.8 | 101.9±8.3 |
| BaP-6,12-dione | 105.9±3.9 | 82.5±3.6 | 103.7±6.3 |
| 9-hydroxy BaP | 105.0±4.4 | 90.8±1.4 | 104.2±2.5 |
| 3-hydroxy BaP | 100.3±5.4 | 99.6±1.2 | 104.1±4.2 |
| BaP | 103.9±3.4 | 92.0±7.7 | 102.3±4.2 |
3.4. Application for fish bile
The method has been satisfactorily applied for detection of biliary BaP metabolites from BaP exposed fish. The sensitivity of the UPLC method described has made it possible to study BaP metabolism in F. heteroclitus, a small fish with minimal amounts of bile. The average bile volume was only about 3 μL per gallbladder, and it was impossible to drain this small volume from each gallbladder. In these experiments, we pooled two gallbladders prior to extraction representing approximately 6 μL bile. With less sensitive methods, bile from more fish would be need, or the studies would have to be limited to bigger fish with more bile which are more difficult to maintain in the laboratory or expose to BaP[30,38].
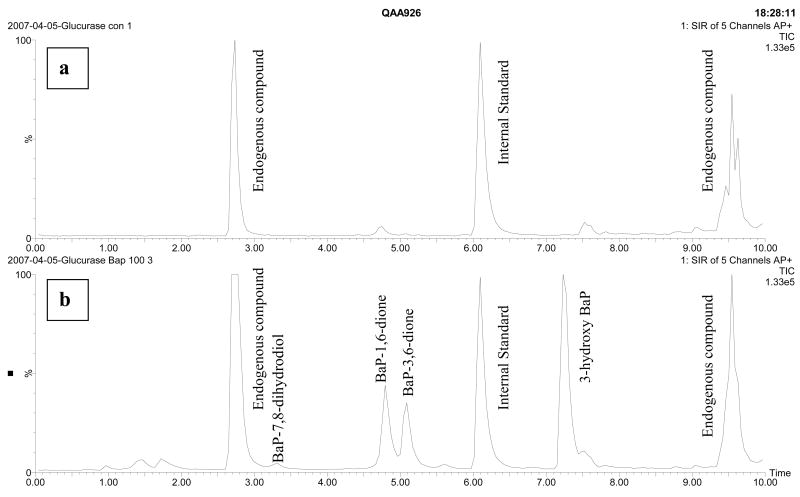
As with any biological sample, potential matrix interference by endogenous compounds present in bile must be considered. Several blank bile samples from F. heteroclitus of different genders and ages were tested. Most of them had two peaks of unknown endogenous compound at retention time 2.7 and 9.4 min, which did not overlay with any peaks of the examined compounds (Fig 3a). However, a matrix effect compromised our ability to accurately quantitate the most polar metabolite, BaP tetrol in some of the free metabolites samples. At around 1 min, other polar endogenous compounds sometimes also eluted and the ratio of the quantitation ion (226) to the confirmation ion (252) was not indicative of BaP tetrol at this retention time. Therefore, BaP tetrol was not quantitated in any of the free metabolites samples.
Fig. 3.
Chromatogram of: (a) a control F. heteroclitus bile sample after glucurase digestion; (b) a BaP (100 μg/L) exposed F. heteroclitus bile sample after glucurase digestion.
The results indicated that most BaP metabolites were conjugated (Table 5 columns F vs. G or S; Fig 3b). The ratio of conjugated metabolites to free metabolites varied with each compound. For example, BaP-7,8-dihydrodiol was mainly in the free form: 53–100% of total free metabolites at the high BaP dose, but the BaP diones and the 3-OH BaP were mostly or exclusively detected in the glucurase-digested extraction. The average total ng/gallbladder of glucuronic acid conjugates were 59 and 89 for the low (10 μg/L) and high (100 μg/L) BaP exposure group, respectively in the first experiment and 412 and 602 ng/gallbladder in the second experiment. While the amount of BaP metabolites was higher in the first experiment relative to the second, we believe this is for a biological reason rather than an analytical method/extraction reason. Nevertheless, the percentage that each metabolite contributed to the total glucuronic acid conjugates was consistent between experiment and doses. For example, the 3-OH BaP contributed between 58 – 66 percent and BaP-3,6 dione was 14 – 17 percent of the total glucuronic acid metabolites regardless of dose or experiment.
Table 5.
BaP metabolite concentrations detected following two waterborne experiments (average ± SE ng/gallbladder). Values are not corrected for extraction recoveries.
| Metabolites | BaP Doseμg/L | Experiment 1 | Experiment 2 | |||
|---|---|---|---|---|---|---|
| F | G | S | F | G | ||
| BaP-7,8-dihydrodiol | 10 | nd | nd | nd | * | 12.4±6.8 |
| 100 | 7.0±1.7 | * | nd | 13.6±3.5 | 13.1±3.2 | |
| BaP-1,6-dione | 10 | nd | 14.4±4.8 | nd | nd | 64.5±19.8 |
| 100 | nd | 20.2±2.8 | * | nd | 103±15.0 | |
| BaP-3,6-dione | 10 | * | 10.1±3.0 | nd | 9.2±3.7 | 62.4±21.9 |
| 100 | * | 13.2±1.5 | nd | 4.7±2.5 | 84.8±12.5 | |
| BaP-6,12-dione | 10 | nd | nd | nd | nd | 5.4±2.7 |
| 100 | nd | nd | nd | nd | 3.8±0.5 | |
| 3-hydroxy BaP | 10 | * | 34.1±19.2 | nd | 5.6±0.9 | 267±75.7 |
| 100 | * | 55.8±15.0 | 6.2±3.3 | 7.1±1.1 | 397±61.3 | |
There was no BaP-9,10-dihydrodiol and 9-hydroxy BaP detected in any sample. F= free metabolites; G= Glucurase digestion released metabolites; S= Sulfatase digestion released metabolites.
nd: Not detected.
: Detectable, but lower than the linear range.
In experiment 1, there were 4 samples in low BaP group (10 μg/L), and 6 samples in high BaP group (100 μg/L); in experiment 2, there are 6 samples in low BaP group and 7 samples in high BaP group.
In the first experiment, there was a predominance of the glucuronic acid conjugated metabolites. Sulfate conjugated metabolites were barely detectable and hence were not extracted in the second experiment. The preferential glucuronic acid conjugation of BaP metabolites was consistent with results observed in studies using hamster embryo fibroblasts[49], English sole and starry flounder[47], and mirror carp[37]. Higher amounts of BaP-7,8-dihydrodiol existing as free metabolites may contribute to the susceptibility of F. heteroclitus to BaP-associated genotoxicity. The percentage of glucuronic acid conjugated BaP-7,8-dihydrodiol was comparable with channel catfish[38], but much lower than carp[50], English sole and starry flounder[47]. However the species, dose, routes, and time of BaP exposure were different between these experiments so an absolute correspondence between the fish BaP metabolism studies is not expected.
4. Conclusion
This method represents the first development and validation of a UPLC-MS method for the determination of BaP metabolism. The new UPLC-MS-based method allows for separation and analysis of BaP metabolites with increased resolution, rapid analysis time, and high detection sensitivity compared to conventional HPLC methods. With some modification, this method could be adapted to quantitate other PAH metabolites such as hydroxypyrenes and hydroxyphenanthrenes. Also it can be used for environmental monitoring of PAH contamination. Furthermore, the application of this method is definitely not limited to marine samples or biliary metabolites, but other matrices could be extracted to detect, for example, human urinary BaP metabolites, or in vitro BaP metabolism from cell culture or recombinant protein assays. In summary, this method will be useful as we continue to try and understand the mechanisms of BaP and the role of its metabolites in toxicity.
Acknowledgments
This project was supported by Grant Number R01ES012710 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institute of Health. In addition, we would like to gratefully acknowledge the Center of Disease Control (Grant Number 5 U01 CI000211-03) for providing the instrument, and the help of all our fish care technicians.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shoeny R, Muller P, Mumford JL, Douben PET, editors. Pollution Risk Assessment and Management. Chichester: John Wiley; 1998. [Google Scholar]
- 2.International Programme on Chemical Safety (IPCS) Environmental Health Criteria 202. Selected Non-Heterocyclic Polycyclic Aromatic Hydrocarbons. 1998 Appendix I. [Google Scholar]
- 3.Flowers L, Rieth SH, Cogliano VJ, Foureman GL, Hertzberg R, Hofmann EL, Murphy DL, Nesnow S, Schoeny RS. Polycyclic Aromatic Compounds. 2002;22:811. [Google Scholar]
- 4.IARC. Lyon, France: 2004. List of IARC evaluations. International Agency for Research on Cancer. Available from http://monographs.iarc.fr/monoeval/grlist.html. [Google Scholar]
- 5.Miller KP, Ramos KS. Drug Metab Rev. 2001;33:1. doi: 10.1081/dmr-100000138. [DOI] [PubMed] [Google Scholar]
- 6.Shou M, Korzekwa KR, Crespi CL, Gonzalez FJ, Gelboin HV. Mol Carcinog. 1994;10:159. doi: 10.1002/mc.2940100307. [DOI] [PubMed] [Google Scholar]
- 7.United States Environmental Protection Agency (EPA) Drinking Water Criteria for Polycyclic Aromatic Hydrocarbons (PAHs) ECAO-CIN-D010. 1991. [Google Scholar]
- 8.Faust RA. Toxicity summary for benzo[a]pyrene, Prepared for: Oak Ridge Reservation Environmental Restoration Program. 1994 available from http://rais.ornl.gov/tox/profiles/bap.doc.
- 9.Chesis PL, Levin DE, Smith MT, Ernster L, Ames BN. Proc Natl Acad Sci USA. 1984;81:1696. doi: 10.1073/pnas.81.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph P, Jaiswal AK. Proc Natl Acad Sci USA. 1994;91:8413. doi: 10.1073/pnas.91.18.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Scheffler BE, Willett KL. Toxicol Sci. 2006;93:331. doi: 10.1093/toxsci/kfl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonsson EM, Abrahamson A, Brunstrom B, Brandt I. Aquat Toxicol. 2006;79:226. doi: 10.1016/j.aquatox.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Willett KL, Ganesan S, Patel M, Metzger C, Quiniou S, Waldbieser G, Scheffler B. Mar Environ Res. 2006;62(Suppl):S332. doi: 10.1016/j.marenvres.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Harrigan JA, McGarrigle BP, Sutter TR, Olson JR. Toxicol In Vitro. 2006;20:426. doi: 10.1016/j.tiv.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Miranda CL, Chung WG, Wang-Buhler JL, Musafia-Jeknic T, Baird WM, Buhler DR. Aquat Toxicol. 2006;80:101. doi: 10.1016/j.aquatox.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Galvan N, Teske DE, Zhou G, Moorthy B, MacWilliams PS, Czuprynski CJ, Jefcoate CR. Toxicol Appl Pharmacol. 2005;202:244. doi: 10.1016/j.taap.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Stansbury KH, Walker NJ, Trush MA, Strickland PT, Sutter TR. Carcinogenesis. 1998;19:1847. doi: 10.1093/carcin/19.10.1847. [DOI] [PubMed] [Google Scholar]
- 18.Harrigan JA, Vezina CM, McGarrigle BP, Ersing N, Box HC, Maccubbin AE, Olson JR. Toxicol Sci. 2004;77:307. doi: 10.1093/toxsci/kfh030. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson ME, Brunstrom B, Ingebrigtsen K, Brandt I. Environ Toxicol Chem. 2004;23:874. doi: 10.1897/03-211. [DOI] [PubMed] [Google Scholar]
- 20.Kaminishi Y, El-Kady MA, Mitsuo R, Itakura T. Environ Sci. 2007;14:23. [PubMed] [Google Scholar]
- 21.Godard CA, Goldstone JV, Said MR, Dickerson RL, Woodin BR, Stegeman JJ. Biochem Biophys Res Commun. 2005;331:1016. doi: 10.1016/j.bbrc.2005.03.231. [DOI] [PubMed] [Google Scholar]
- 22.Jonsson ME, Orrego R, Woodin BR, Goldstone JV, Stegeman JJ. Toxicol Appl Pharmacol. 2007;221:29. doi: 10.1016/j.taap.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stine CB, Smith DL, Vogelbein WK, Harshbarger JC, Gudla PR, Lipsky MM, Kane AS. Toxicol Pathol. 2004;32:375. doi: 10.1080/01926230490440899. [DOI] [PubMed] [Google Scholar]
- 24.Vogelbein WK, Fournie JW, Van Veld PA, Huggett RJ. Cancer Res. 1990;50:5978. [PubMed] [Google Scholar]
- 25.Selkirk JK, Croy RG, Gelboin HV. Science. 1974;184:169. doi: 10.1126/science.184.4133.169. [DOI] [PubMed] [Google Scholar]
- 26.Selkirk JK, Croy RG, Roller PP, Gelboin HV. Cancer Res. 1974;34:3474. [PubMed] [Google Scholar]
- 27.Nemoto N, Takayama S. Cancer Res. 1977;37:4125. [PubMed] [Google Scholar]
- 28.Krahn MM, Myers MS, Burrows DG, Malins DC. Xenobiotica. 1984;14:633. doi: 10.3109/00498258409151461. [DOI] [PubMed] [Google Scholar]
- 29.Bentsen-Farmen RK, Botnen IV, Notø H, Jacob J, Ovrebø S. Int Arch Occup Environ Health. 1999;72:161. doi: 10.1007/s004200050355. [DOI] [PubMed] [Google Scholar]
- 30.Ruddock PJ, Bird DJ, McCalley DV. Ecotoxicol Environ Saf. 2002;51:97. doi: 10.1006/eesa.2001.2131. [DOI] [PubMed] [Google Scholar]
- 31.Ruddock PJ, Bird DJ, McEvoy J, Peters LD. Sci Total Environ. 2003;301:105. doi: 10.1016/s0048-9697(02)00292-9. [DOI] [PubMed] [Google Scholar]
- 32.van Schanke A, Holtz F, van der Meer JP, Boon JP, Ariese F, Stroomberg G, van den Berg M, Everaarts JM. Environ Toxicol Chem. 2001;20:1641. [PubMed] [Google Scholar]
- 33.Sikka HC, Rutkowski JP, Kandaswami C. Aquat Toxicol. 1990;16:101. [Google Scholar]
- 34.James MO, Altman AH, Li CL, Schell JD. Chemico-biological interactions. 1995;95:141. doi: 10.1016/0009-2797(94)03355-2. [DOI] [PubMed] [Google Scholar]
- 35.James MO, Altman AH, Morris K, Kleinow KM, Tong Z. Drug Metab Dispos. 1997;25:346. [PubMed] [Google Scholar]
- 36.Steward AR, Zaleski J, Sikka HC. Chem Biol Interact. 1990;74:119. doi: 10.1016/0009-2797(90)90063-s. [DOI] [PubMed] [Google Scholar]
- 37.Zaleski J, Steward AR, Sikka HC. Carcinogenesis. 1991;12:167. doi: 10.1093/carcin/12.2.167. [DOI] [PubMed] [Google Scholar]
- 38.Willett KL, Gardinali PR, Lienesch LA, Di Giulio RT. Toxicol Sci. 2000;58:68. doi: 10.1093/toxsci/58.1.68. [DOI] [PubMed] [Google Scholar]
- 39.Sherma J. J AOAC Int. 2005;88:63A. [PubMed] [Google Scholar]
- 40.Castro-Perez J, Plumb R, Granger JH, Beattie I, Joncour K, Wright A. Rapid Commun Mass Spectrom. 2005;19:843. doi: 10.1002/rcm.1859. [DOI] [PubMed] [Google Scholar]
- 41.Plumb RS, Granger JH, Stumpf CL, Johnson KA, Smith BW, Gaulitz S, Wilson ID, Castro-Perez J. Analyst. 2005;130:844. doi: 10.1039/b501767j. [DOI] [PubMed] [Google Scholar]
- 42.Deshpande AD. Arch Environ Contam Toxicol. 1989;18:900. doi: 10.1007/BF01160307. [DOI] [PubMed] [Google Scholar]
- 43.van der Wielen JCA, Jansen JTA, Martena MJ, De Groot HN, In’t Veld PH. Food Addit Contam. 2006;23:709. doi: 10.1080/02652030600631869. [DOI] [PubMed] [Google Scholar]
- 44.Wang JJ, Frazer DG, Stone S, Goldsmith T, Law B, Moseley A, Simpson J, Afshari A, Lewis DM. Anal Chem. 2003;75:5953. doi: 10.1021/ac030017a. [DOI] [PubMed] [Google Scholar]
- 45.James MO, Kleinow KM, Zhang Y, Zheng R, Wang L, Faux LR. Mar Environ Res. 2004;58:343. doi: 10.1016/j.marenvres.2004.03.079. [DOI] [PubMed] [Google Scholar]
- 46.Li CL, James MO. Toxicol Sci. 2000;57:75. doi: 10.1093/toxsci/57.1.75. [DOI] [PubMed] [Google Scholar]
- 47.Varanasi U, Nishimoto M, Reichert WL, Le Eberhart BT. Cancer Res. 1986;46:3817. [PubMed] [Google Scholar]
- 48.Steward AR, Zaleski J, Sikka HC. Chem Biol Interact. 1990;74:119. doi: 10.1016/0009-2797(90)90063-s. [DOI] [PubMed] [Google Scholar]
- 49.Merrick BA, Selkirk JK. Carcinogenesis. 1985;6:1303. doi: 10.1093/carcin/6.9.1303. [DOI] [PubMed] [Google Scholar]
- 50.Sikka HC, Rutkowski JP, Kandaswami C. Aquat Toxicol. 1990;16:101. [Google Scholar]