Abstract
Spatiotemporal variability in floral resources can have ecological and evolutionary consequences for both plants and the pollinators on which they depend. Seldom, however, can patterns of flower abundance and visitation in the field be linked with the behavioral mechanisms that allow floral visitors to persist when a preferred resource is scarce. To explore these mechanisms better, we examined factors controlling floral preference in the hawkmoth Manduca sexta in the semiarid grassland of Arizona. Here, hawkmoths forage primarily on flowers of the bat-adapted agave, Agave palmeri, but shift to the moth-adapted flowers of their larval host plant, Datura wrightii, when these become abundant. Both plants emit similar concentrations of floral odor, but scent composition, nectar, and flower reflectance are distinct between the two species, and A. palmeri flowers provide six times as much chemical energy as flowers of D. wrightii. Behavioral experiments with both naïve and experienced moths revealed that hawkmoths learn to feed from agave flowers through olfactory conditioning but readily switch to D. wrightii flowers, for which they are the primary pollinator, based on an innate odor preference. Behavioral flexibility and the olfactory contrast between flowers permit the hawkmoths to persist within a dynamic environment, while at the same time to function as the major pollinator of one plant species.
Keywords: flower visitation, foraging behavior, moth, pollination
Abundance and composition of flower species are fundamental aspects of pollination biology, and both can change over a pollinator's lifetime. For any nectar or pollen forager, the ability to discriminate, learn, and switch among flowers in the face of an ever-changing environment is critically important. For example, availability of floral resources at a landscape scale can constrain the size (1) and behavior (2) of a population of floral visitors (3). Unfortunately, aside from research on social bees (e.g., Apis mellifera, Bombus spp.), our understanding of mechanisms controlling nectar foraging by pollinators is limited. Whereas observational studies of flower visitation (reviewed in ref. 4) and proportionally fewer studies of the effects of changing flower composition on foraging (5) have been conducted (6), the causal mechanisms controlling floral visitation remain unclear and seldom can be demonstrated from the observed correlations. Thus, there remains a fundamental gap between the processes that occur in the field and the underlying behavioral mechanisms mediating those interactions.
Nectar foraging by insects involves a suite of behaviors, both innate and learned. Much of our understanding of flower choice and cognition comes from work with generalist honey bees (Apis mellifera) and bumble bees (Bombus spp.), for which learning is a critical component. For instance, honey bee workers exhibit innate color preferences, but when trained on flowers of alternative colors, the preference is extinguished (7). Learning permits individual bees the flexibility to forage on different flowers without being restricted to one floral phenotype over the course of their lifetime (7). In contrast to foraging behavior mediated almost exclusively by learning, innate preferences are believed to account for biases that aid in recognition, and possibly learning, of critical floral resources. Indeed, studies of nonsocial insects that are relatively specialized to a given floral phenotype provide intriguing insights (reviewed in ref. 8). The swallowtail butterfly, Battus philenor, and the diurnal hawkmoth, Macroglossum stellatarum, exhibit innate floral color and shape preferences. When exposed to an alternate stimulus with a nectar reward, however, both species can switch hosts, although the instinctive preference is never lost (9–12). The ability to learn novel floral resources while retaining innate preferences could provide a mechanism by which a specialist forager could maintain an association with a specific floral phenotype despite changes in the floral landscape.
Despite the potential importance of innate preference and learning for floral visitation, little is known about how behaviors by nonsocial insects mediate plant–pollinator interactions in the field. A pollinator species that exhibits such behavioral flexibility is the hawkmoth, Manduca sexta (Sphingidae). M. sexta has a wide geographic range and typically feeds from a narrow range of moth-adapted flowers (13–15). In the semiarid grassland of Arizona, M. sexta is a frequent visitor to Datura wrightii (Solanaceae) (16). D. wrightii possesses the typical phenotype of hawkmoth-pollinated flowers: nocturnal anthesis, intense and sweet fragrance, and reflective coloration (16, 17). Flowers of D. wrightii are rare in these habitats during early (May–June) and late (October–November) summer months but peak in abundance during a portion of the summer rainy (monsoon) season (July–September) when M. sexta is most active, presumably forcing the hawkmoths to use other floral resources during part of their adult stage. This system thus offers investigators a unique opportunity to explore the nature of flower–forager interactions both in the field and in the laboratory and hence to ask how behavior may shape pollination associations.
In this article, we examine the behavioral mechanisms controlling floral preference. We present findings from (i) field studies of floral-foraging shift by M. sexta, (ii) analyses of floral characteristics that influence foraging by M. sexta, and (iii) experiments on the proximate causes controlling floral switching using naïve and conditioned, freely flying moths. Using this integrative approach, we demonstrate that this hawkmoth has an innate preference for certain floral traits but can exploit “non-hawkmoth” flowers through its learning capabilities and sensory flexibility.
Results
Pollen Load and Flower Phenology.
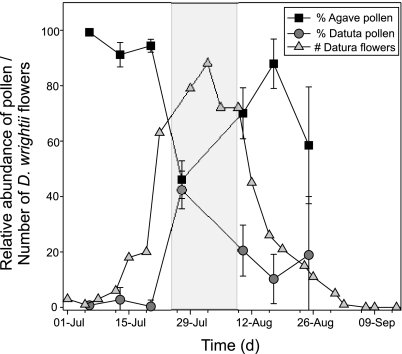
In 2004, 109 hawkmoths were captured at mercury-vapor lamps and blacklights at the base of the Santa Rita Mountains, near Tucson, AZ (31°78′ N, 110°82′ W, 1320 m). Pollen was removed from M. sexta moths and identified, in most cases to the level of plant species. Nearly all (n = 101) individuals carried measurable quantities of pollen, the bulk (≥90%) of which was from D. wrightii and/or Agave palmeri (Agavaceae). Less than 10% of the pollen came from plants of other species (54). The relative abundance of D. wrightii and A. palmeri pollen carried by M. sexta, however, varied through the summer (Fig. 1). Individual adult M. sexta tended to carry predominantly A. palmeri pollen early (91–99% of pollen load) and late (58–88%) in the summer. During this time, D. wrightii flowers were relatively rare, while A. palmeri flowers remained abundant (flowering umbels found on >60% of A. palmeri inflorescences). However, when the abundance of D. wrightii flower peaked at the end of July, a corresponding peak in the proportion of the pollen load came from D. wrightii (Fig. 1), although A. palmeri pollen was still present on the hawkmoths. When flowers of both species were present, D. wrightii plants had 1–29 flowers per plant (mean = 11.38 per plant, ±2.01 SEM, n = 16), and A. palmeri had 1–12 flowering umbels per plant (mean = 3.52, ±0.25 SEM, n = 51) with each umbel comprised of 7 and 12 open flowers. There was a positive correlation between the mean proportion of D. wrightii pollen carried by M. sexta that was D. wrightii and the number of D. wrightii flowers available in a given week in an adjacent census plot [see supporting information (SI) Methods] (Spearman's ρ = 0.679, P = 0.047, n = 7 weeks). In contrast, there was a negative relationship between the mean A. palmeri pollen load and the abundance of D. wrightii flowers (Spearman's ρ = −0.643, P = 0.06, n = 7 weeks). Although flowering of D. wrightii peaked ≈1 month later in 2005 than in 2004, the 2005 pollen-load data and flowering phenology of D. wrightii were similar to those observed in 2004: there was a positive association between the abundance of D. wrightii flowers in a given week, and the mean proportion of pollen carried by M. sexta that was from D. wrightii (Spearman's ρ = 1.0, P < 0.01, n = 4 weeks), and a negative but nonsignificant relationship between abundance of D. wrightii flowers and the mean proportion of pollen that was from A. palmeri (Spearman's ρ = −0.80, P = 0.10, n = 4 weeks).
Fig. 1.
Hawkmoth pollen load in relation to flower phenology. Flowering phenology of D. wrightii, and mean (±SEM) relative abundance of D. wrightii and A. palmeri pollen on the probosces of M. sexta hawkmoths observed during the summer of 2004. During the entirety of this period, A. palmeri remains in bloom (>60% of inflorescences having flowering umbels). Shaded area denotes the time period when behavioral experiments were conducted on wild moths (during peak of both A. palmeri and D. wrightii flower abundance).
Flower Characteristics.
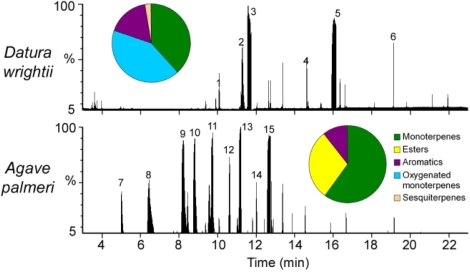
Flowers of D. wrightii and A. palmeri were examined to characterize the floral traits that might be responsible for M. sexta's foraging preferences. First, analysis of headspace volatiles by gas chromatography with mass-spectrometric detection (GC-MS) revealed distinct differences between the two flowers (Fig. 2). Although both plants have similar floral odor emission rates (SI Table 2; one-way ANOVA: F1,18 = 0.28, P = 0.59), D. wrightii scent is composed of terpenoids (mono- and sesquiterpenoids) (79%) and benzenoids (18%) that produce a strong sweet smell, whereas the A. palmeri floral bouquet is dominated by esters (30%) and monoterpenoids (59%) that generate a scent of rotten fruit (SI Table 3). Both flowers share a handful of monoterpenoids (sabinene, β-myrcene, limonene) and an aromatic (benzaldehyde) in their headspace, but D. wrightii completely lacks the pungent esters of A. palmeri (SI Table 3). The flowers differ visually as well. The corollas of D. wrightii flowers are highly reflective (52%) at wavelengths of 400–700 nm, whereas corollas of A. palmeri flowers are dull (reflectance <25%) (SI Fig. 6A), but both flowers cause excitation in M. sexta's blue and green receptors (SI Fig. 6 B and C). Finally, nectar differs dramatically between the two species, in standing crop, sugar composition, and content (Table 1). A D. wrightii flower produces an average of 56 μl of sucrose-rich nectar in an evening (Table 1). By contrast, an A. palmeri flower produces a 10-fold greater nightly standing crop (616 μl) of hexose (glucose and fructose)-rich nectar. Although the D. wrightii flower provides a higher sugar concentration (22%) than A. palmeri (12%), A. palmeri's abundant standing crop provides a 6-fold higher total energy content than D. wrightii that can sustain hawkmoth hovering times for much longer durations (Table 1). Principal-component analysis of the floral characteristics (odor, nectar sugar chemistry, and physical features) revealed a tight, but distinct, clustering of D. wrightii and A. palmeri flowers (SI Fig. 7; SI Tables 2 and 4). Thus, in nearly every floral characteristic that we measured (odor, visual, morphological, gustatory, and nutritional), these two plant species contrast substantially.
Fig. 2.
GC-MS analysis of floral headspace volatiles. (A) Major constituents of D. wrightii floral headspace scent shown in the total ion chromatogram are monoterpenoids β-myrcene (1), E-β-ocimene (3), and geraniol (5), aromatics including benzyl alcohol (2) and methyl salicylate (4), and sesquiterpenoids such as α-farnasene (6). (B) Major constituents of A. palmeri floral odor shown in the total ion chromatogram are monoterpenoids α-pinene (9), camphene (10), β-pinene (11), and limonene (13), aromatics including xylene (8), and esters such as ethyl isovalerate and analogs (7, 12) and ethyl sorbate isomers (14 and 15).
Table 1.
Sugar composition and energy of floral nectar
| Species | Nectar volume per flower, ml | Glucose, mg/flower | Fructose, mg/flower | Sucrose, mg/flower | Sucrose:hexose ratio | Sugar/flower, mg | Cal/flower | Hovering time provided by flower nectar, min |
|---|---|---|---|---|---|---|---|---|
| D. wrightii | 0.056 (0.008)* | 1.89 (0.13)* | 1.55 (0.11)* | 8.90 (0.56)* | 2.66 (0.08)* | 12.33 (0.63)* | 49.32 (2.5)* | 8.58 (0.43)* |
| A. palmeri | 0.616 (0.140) | 40.12 (5.5) | 33.73 (0.53) | 0.12 (0.03) | 0.001 (0.0002) | 73.94 (5.45) | 315.76 (22.1) | 54.95 (3.85) |
Values are mean ± SEM (N = 10/species).
*, P < 0.0001; asterisk denotes a significant difference between flower species (one-way ANOVA: F-1,18 >32.31, all comparisons).
Feeding Choices of Wild Moths.
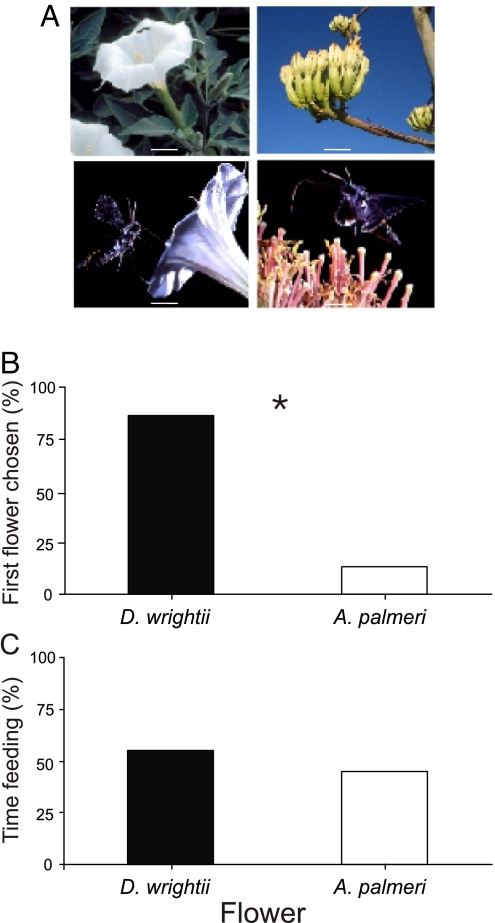
During the summer of 2005 and 2006, field-caught M. sexta showed a strong preference for D. wrightii flowers. Of 28 male moths caught and tested the following evening, 32% (9/28) visited flowers within the 10-min period in which they were allowed to forage. Only one of the nine moths chose the A. palmeri umbel first, and that individual quickly shifted to feeding from the D. wrightii flower (Fig. 3 A and B). In contrast, eight of nine first chose to feed from the D. wrightii flower (G1,9 = 10.31, P < 0.05; Fig. 3B). They fed by “diving” into the flower's corolla for several seconds before emerging, hovering for a few seconds, and then diving into the flower again (Fig. 3A; SI Movie 1). Typically, moths entered the D. wrightii flower several times (and spent up to 20 s feeding) before flying again. An unvisited D. wrightii flower contained 56 μl of nectar (Table 1); after one hawkmoth had fed from the flower, only 7 ± 4 μl (± SEM, n = 9) remained. Once nectar was depleted from the D. wrightii flower, all of the moths (eight of eight) then switched to feeding from the A. palmeri flowers (SI Movie 2) until they either stopped flying or the experimental period ended. Although wild moths had a significant preference for D. wrightii flowers, the total time from which moths fed from flowers of the two species did not differ significantly (G1,9 = 2.13, P > 0.20; Fig. 3C).
Fig. 3.
Wild hawkmoth floral preference. (A) (Upper) Morphology of D. wrightii flower (Left) and an A. palmeri (Right) umbel. (Lower) Wild M. sexta hawkmoths feeding on the flowers. (Scale bars: Upper, 2 cm; Lower, 3 cm.) (B) Data reporting the first choices of wild M. sexta for nectar-feeding from either the D. wrightii or A. palmeri flowers. Bars indicate the percentage of individuals that first chose either flower. (C) Percentage of time spent feeding from either of the flowers. An asterisk (*) denotes a significant deviation from a random distribution (G test, P < 0.05).
Feeding Choices of Naïve Moths.
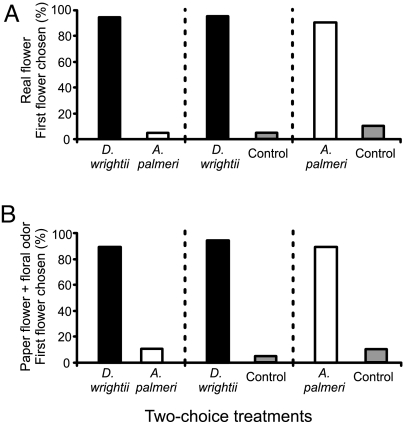
The behavioral mechanism that accounts for floral preference in M. sexta was first examined by using naïve laboratory-reared moths. When given a choice between D. wrightii and A. palmeri, 95% (19/20) of naïve hawkmoths first chose the D. wrightii flower and spent most of the observation time feeding from it (G1,20 = 19.73, P < 0.001; Fig. 4A; SI Table 5). In a separate experiment, when given a choice either between an A. palmeri umbel and a control paper flower emitting no odor or between a D. wrightii flower and a control paper flower, 90% (18/20) and 95% (19/20), respectively, of the moths tested first fed from the real flower (both comparisons: G1,20 > 14.72, P < 0.001; Fig. 4A), suggesting that moths perceive natural floral cues. An additional control experiment using two paper flowers with no odor suggested that scent may be the critical factor dictating moth behavior: only 5/41 of the moths introduced into a flight arena fed at the odorless paper flowers (data not shown).
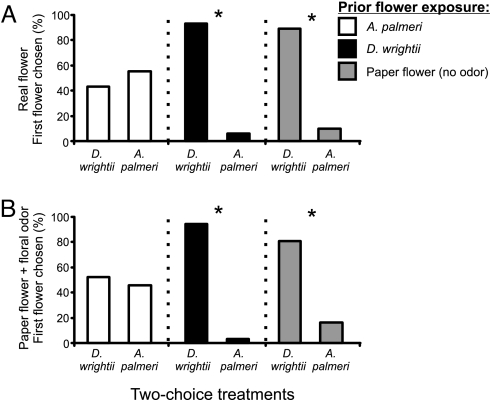
Fig. 4.
Two-choice experiments examining the floral preferences of naïve male M. sexta moths. (A) With real flowers, the percentages of moths that chose D. wrightii (black bars) or A. palmeri (white bars) (Left), D. wrightii or control (gray bars) (Center), and A. palmeri or control (Right). Two-choice experiments using two D. wrightii flowers or two A. palmeri flowers showed no significant difference in the first flower chosen (G test: G < 0.32, P > 0.50, n = 40) and thus revealing no positional effects on moths' preferences. (B) With artificial flowers, the percentages of moths that chose paper flowers emitting D. wrightii or A. palmeri scent (Left), D. wrightii scent or no scent (control) (Center), and A. palmeri scent or no scent (control) paper flowers (Right).
Although these experiments suggest that floral characteristics may play a role in feeding, they do not control for the effects that visual traits may have on moth behavior. To normalize the visual characteristics of the flowers, two identical paper flowers emitting scents of A. palmeri and D. wrightii were set up in the flight arena. Consistent with the results described above, 90% of the naïve hawkmoths chose paper flowers emitting the scent of D. wrightii over those emitting A. palmeri scent (Fig. 4B). When given a choice between a scented and an unscented (control) paper flower, however, hawkmoths almost always (92%; 37/40) chose the flower emitting the floral odor, whether derived from D. wrightii or A. palmeri (Fig. 4B; SI Table 4). These findings reveal the preference of M. sexta for D. wrightii odor but also demonstrate that hawkmoths have the olfactory flexibility to feed on novel floral resources when offered no other alternative.
Feeding Choices by Experienced Moths.
Learning the association between nectar reward and floral odor provides a mechanism by which hawkmoths can shift their feeding preferences. When offered a choice between D. wrightii and A. palmeri flowers, moths previously exposed to D. wrightii flowers chose D. wrightii flowers more frequently and spent most of the observation period feeding from them (G1,30 = 24.12, P < 0.001; Fig. 5A; SI Table 6). In contrast, individuals previously exposed to A. palmeri, when offered a choice between the two flowers, fed from A. palmeri and D. wrightii at equal frequencies and durations (G1,30 < 0.53, P > 0.50; Fig. 5A; SI Table 6). Hawkmoths previously exposed to odorless paper flowers, thereby normalizing the ability to learn the physical-visual display while keeping the moths “odor naïve,” significantly preferred the D. wrightii flower (G1,30 = 15.49, P < 0.001; Fig. 5A; SI Table 3).
Fig. 5.
Two-choice experiments examining the floral preferences of experienced male M. sexta moths. Twenty-four hours before testing, moths were assigned to one of three treatment groups: moths exposed to D. wrightii flowers (black bars), A. palmeri flowers (white bars), or controls (odorless paper flower, gray bars). Moths were removed from the flowers in photophase and tested the next evening. (A) Using real flowers, the percentages of moths that chose D. wrightii or A. palmeri flowers after having prior experience with D. wrightii (Left), A. palmeri (Center), and control flowers (Right). (B) With paper flowers that emit real floral scent, the percentages of moths that chose D. wrightii scented flowers or A. palmeri scented flowers after prior experience with D. wrightii (Left), A. palmeri (Center), or control flowers (Right). An asterisk (*) denotes a significant deviation from a random distribution (G test: P < 0.05). n = 30 for each two-choice experiment.
Hawkmoths may use multiple sensory modalities to learn to associate flowers and nectar rewards (14, 15). To determine the strength of the association between the olfactory stimulus and the nectar resource, experiments were conducted by using paper flowers emitting D. wrightii and A. palmeri scents. As in experiments with real flowers, moths previously exposed to paper flowers emitting D. wrightii odor significantly preferred the one emitting the D. wrightii odor over one emitting A. palmeri odor (G1,30 = 32.82, P < 0.001; Fig. 5B; SI Table 6). Moreover, moths with no prior exposure to either floral odor that were offered a choice between paper flowers with D. wrightii and A. palmeri odors significantly chose those emitting D. wrightii odor (G1,30 = 6.93, P < 0.05; Fig. 5B; SI Table 6). In contrast, moths that had been exposed to paper flowers emitting A. palmeri odor did not show a significant preference (G1,30 = 0.36, P > 0.50; Fig. 5B; SI Table 6). Thus, results from the two sets of experiments suggest that learning the association between nectar reward and flower type is primarily olfactory-mediated.
Discussion
In semiarid grassland habitats of southern Arizona, adult M. sexta hawkmoths feed primarily on nectar from flowers of two unrelated plants: A. palmeri and D. wrightii. These flowers contrast greatly in their physical, visual, and chemical characteristics, making this a unique system in which to examine the cognitive and behavioral mechanisms controlling floral choice. At some locations, A. palmeri flowers are abundant throughout the summer months when adult M. sexta are present. During this time, M. sexta feeds almost exclusively on A. palmeri flowers, which exhibit a characteristic set of “bat-adapted” traits: they are pale, dull-colored, produce a high volume of hexose-dominant nectar, and emit a scent comprising sulfur-containing carboxy-ester and terpenoid volatiles known to be attractive to bats (18). When flowers of their preferred larval host plant, D. wrightii, become abundant, M. sexta switches to incorporate D. wrightii nectar into its diet. D. wrightii flowers exhibit typical “moth-adapted” traits that commonly attract and reward hawkmoths, including reflective coloration, sucrose-dominant nectar, and strong, sweet scent (16, 17). In fact, M. sexta is the primary visitor to D. wrightii at our study site (54), and in the course of its nectar-feeding activities acts as a highly effective pollinator (54).
The shift in flower preference by M. sexta in the field is mediated by a combination of innate preference and olfactory learning. Both A. palmeri and D. wrightii plants emit the same concentration of floral scent, but naïve hawkmoths have an innate affinity for D. wrightii. If D. wrightii is not abundant, however, moths have the behavioral flexibility to investigate, and feed from, A. palmeri flowers. The abundant nectar resource of A. palmeri, along with its distinctive odor, appear to favor hawkmoths that can learn quickly to associate its odor and nectar reward. Hawkmoth preference for D. wrightii flowers, however, is not completely extinguished by learning to feed from A. palmeri flowers.
Cognitive Processes and Olfactory Control of Flower Choice.
The behavioral and cognitive mechanisms governing flower choice are better understood for the generalist honey bee and bumble bees than for any other nectar forager. This information provides a foundation for understanding the mechanisms controlling floral preference in other pollinator systems. Floral preference in honey bees is controlled by a suite of factors including floral abundance, odor, and morphology (19, 20), but an important sensory modality influencing floral choice is vision (7, 21). Flowers are chosen based on their similarity of color, odor, and shape to flowers previously experienced, thereby maintaining “constancy” in floral choice (22–24). Experience-based flexibility provides bees with a mechanism to use flowers that are encountered infrequently (25).
Despite an overwhelming focus on the processes governing bee behavior, the relatively fewer studies of other nectar foragers provide interesting contrasts. A combination of olfactory and visual cues are necessary for successful foraging behavior in many nocturnal and diurnal insects, including moths (10, 14, 15), solitary bees (26), beetles (27), and flies (28, 29). Many of these nectarivores have innate preferences for a combination of odor and visible characteristics of plants for which they are pollinators (10, 15, 16) and can learn to associate new floral features with nectar rewards (10, 30, 31). This combination of innate preference and learning may represent ubiquitous cognitive processes that control foraging behavior in pollinators and possibly all insects.
The affinity of M. sexta for D. wrightii scent may be due to a strong neural representation of the floral blend in the moth's olfactory system. D. wrightii emits many odorants that are common in other nocturnal moth-pollinated flowers, including benzenoids (e.g., benzaldehyde, benzyl alcohol, and methyl salicylate) and oxygenated monoterpenenes (e.g., linalool, nerol, and geraniol) (16). In contrast, A. palmeri's odor is composed of esters, in particular ethyl isovalerate and the structural isomers of ethyl sorbate (32). The contrast between floral scents enables the hawkmoth to distinguish and locate both types of flowers. Moreover, associating nectar reward with a unique type of odor may enable M. sexta to process information about the flowers through different cognitive pathways. In the regions of the brain where olfactory information is first processed (the antennal lobe), the pattern of neural activity in response to an odor has been demonstrated to change as an animal learns (33). Conversely, for odors of innate significance such as pheromones, unique neural patterns encode the critical odor information (34). Neural-ensemble recordings in the antennal lobe of M. sexta have shown that this is indeed the case for D. wrightii odor, with neural responses reflecting a unique spatiotemporal pattern (J.R.A. and J.G.H., unpublished data). The use of two distinct neural patterns that control floral nectar feeding may enable the moth to sustain a preference for both flowers without one being lost or extinguished.
Hawkmoth–Plant Interactions in the Southwest.
The innate affinity of M. sexta for D. wrightii flowers may be a result of selective pressure operating on both sexes. D. wrightii plants, patchily distributed in southwestern Arizona, are the primary larval host plant for M. sexta, and both male and female M. sexta hawkmoths innately prefer D. wrightii flowers for nectar-feeding. In a preliminary experiment with virgin females, we found that 93% (15/16) of the females first fed from the D. wrightii flower rather than A. palmeri (G test: G1,16 = 14.6, P < 0.05). Attraction to the floral odor of D. wrightii may be related to the fact that the flower is a signal not only of a nectar resource but also of an appropriate oviposition site. Floral signals acting in such a manner have been found in females of the host-specialist senita (Upiga virescens) (35) and yucca moths (Tegeticula and Parategeticula spp.) (36, 37) and the facultative specialist sunflower moth (Homoeosoma electellum) (38). For M. sexta females, field observations and laboratory experiments suggest that host-plant flowers may play a similar role (39, 40). Why, however, do males also have an innate preference for D. wrightii, given that they do not need to locate the host plant, and A. palmeri is energetically a much greater nectar source than D. wrightii? For male M. sexta, the D. wrightii floral odor may be an attractive chemical signal acting in parallel with the sex pheromone released by females. Plant-derived odors are known to enhance male behavioral attraction to (41, 42), and increase detection of (43), female pheromones. Because hawkmoths disperse widely, with few being recaptured in mark-release studies (44), the D. wrightii floral odor may serve as a beacon for males to locations where the probability of both food and sex is high. Observations of M. sexta hawkmoths visiting an unlit D. wrightii patch during the summer of 2006 revealed a somewhat, but not significantly, higher percentage of males than females (59%, 13 males to 9 females; G test: G1,22 = 0.73, P > 0.50). A floral odor used to attract pollinators thus may have been coopted by both sexes to facilitate other life-history functions.
With its copious standing nectar crop, A. palmeri presents a unique floral resource for hawkmoths to exploit. A. palmeri and many other agaves in southwestern United States and Mexico attract a diverse and large assemblage of pollinators, including mammals (45), insects (including hawkmoths), and birds (46, 47). Agaves, therefore, are thought to be “keystone” species in these arid and semiarid environments, because they provide nectar and pollen resources to a large pollinator community. Given the wide distribution of agaves, Datura spp., and M. sexta in the southwestern United States and Mexico, similar flower–hawkmoth interactions may occur in other locations (13, 14, 48). That hawkmoths are commonly seen foraging from agave flowers (49, 50) suggests our results in the Santa Rita mountains may not be uncommon, although this needs to be verified.
Although M. sexta and D. wrightii overlap in parts of their range, M. sexta remains a plastic opportunistic forager that is not physically or behaviorally excluded from nectaring on “non-hawkmoth” flowers. The ability to feed from diverse floral phenotypes over an individual's lifetime should be advantageous, because it would permit hawkmoths to persist at times or locations where preferred resources are scarce or absent. In this case, A. palmerii might support the local M. sexta population before and after the period in which D. wrightii is in bloom and thus facilitate the M. sexta–D. wrightii interaction by maintaining the pollinator pool for D. wrightii (51, 52).
Materials and Methods
Procedures for collection and phenotypic analyses of flowers (headspace collection and analysis, nectar compensation, flower reflectance, and morphology) and collection, rearing, maintenance of moths, and behavioral experiments with paper flowers are described in SI Methods.
Surveys of Hawkmoths and Pollen.
In 2004 and 2005, we collected moths in the field by blacklighting as follows. Two mercury vapor lamps (175 W) were placed on both sides of a reflective white sheet (2 × 3 m), with one 15-W UV lamp hanging on each side of the sheet, for 2 hours starting at sunset once per week from mid-June through mid-September. When a hawkmoth landed on the sheet, we recorded the time of its arrival, sex, and species identity. To determine which plant species M. sexta moths visit for nectar-feeding, we removed the pollen carried by captured moths by slowly unrolling the proboscis and rubbing a small cube (≈2 mm3) of glycerin jelly containing basic fuchsin stain (53) along the dorsal and ventral lengths of the proboscis. We then melted the glycerin cube onto a slide to make a permanent record. With the help of a reference pollen collection made in 2005, we classified pollen into one of three groups, D. wrightii, A. palmerii, and “Other,” and then counted the pollen grains in each category at ×40–100 magnification. We calculated the mean proportion of D. wrightii and A. palmerii pollen found on the bodies of individual moths (number of pollen grains of X/total number of pollen grains per individual) for dates on which we collected multiple (≥4) M. sexta moths were attracted to collection lights (R.A. et al., unpublished work).
Behavioral Flower-Preference Experiments.
Two-choice experiment with field-caught moths.
Details about the collection and maintenance of wild moths can be found in SI Methods. Experiments were conducted by releasing single moths into a flight arena (1.8 × 1.8 × 1.8 m) containing both a D. wrightii flower and an A. palmeri umbel (a cluster of 7–12 flowers on a single stem). The plants were positioned randomly in the arena and spaced 1 m apart. The flower at which the first proboscis extension and active feeding took place, number of proboscis extensions into the floral corollas, and the time spent feeding on the flowers were recorded. Each trial was 10 min in duration or lasted until the moth stopped flying for >3 min. The moth was then removed from the cage, and after an interval of at least 5 min, another moth was released. D. wrightii flowers were replaced after every trial, and A. palmeri umbels were replaced after every two moths had been tested (because of a limitation in the number of available umbels).
Two-choice experiments with real flowers and naïve moths.
To establish whether M. sexta might have an innate preference for the two flower species in question, we performed behavioral tests with laboratory-reared male moths that had eclosed 3 d before testing. At no time before experimentation were moths exposed to plant odor. Each moth was used only once, released alone into the cage used for the two-choice tests. Twenty moths were used for each two-choice treatment. To test whether M. sexta moths have an innate preference for D. wrightii or A. palmeri flowers, moths were exposed to one of six treatments: (i) D. wrightii flower vs. A. palmeri umbel; (ii) D. wrightii flower vs. paper flower (no odor) control; (iii) A. palmeri umbel vs. paper flower (no odor) control; (iv) paper flower (no odor) control vs. paper flower (no odor) control; (v) D. wrightii flower vs. D. wrightii flower; or (vi) A. palmeri umbel vs. A. palmeri umbel. Flowers were randomly positioned in the flight arena and spaced 1 m apart. Paper flowers were white paper cones with an opening 8 cm in diameter and a length of 18 cm and served as a neutral visual display (15). Experiment i tested whether the moths have a preference between the two flowers. Experiments ii and iii tested whether the moths would feed on a flower when given no other odor choice. Experiment iv examined the importance of odor stimuli and examined whether odor contamination might occur. Experiments v and vi controlled for effects of the position of a flower on behavior of a moth. Floral preference was determined by means of the same criteria used for field-caught moths.
Two-choice experiments with real flowers and experienced moths.
We conducted experiments to determine how learning might change the floral preference of M. sexta moths. Twenty-four hours before testing, male moths 2 days posteclosion were transferred to a partially covered Plexiglas cage (1 m3) and subjected to one of three treatments: (i) moths were exposed to an array of four cut D. wrightii flowers; (ii) moths were exposed to an A. palmeri umbel (7–12 flowers); or (iii) two groups of moths, in separate cages, were exposed to an array of six odorless paper flowers (each containing 100 μl of 25% sucrose solution). Experiments with moths that had been exposed to the paper flowers but without prior exposure to the D. wrightii or A. palmeri flowers allowed comparisons with the other treatment groups to examine the effects of flower conditioning, while also controlling for cage effects. For each treatment, data were collected from 30 moths that actively fed on the flowers. On a given experimental evening, eight moths were assigned to each treatment group (32 moths total). Once placed in the cage, moths were observed for 0.5–1.5 h at anthesis to determine whether moths fed from the flowers. From our observations, ≈40% (±8%) of the moths fed on the A. palmeri flowers, and 52% (±17%) fed on the D. wrightii flowers, with the proportion of moths that fed between the two treatment groups not differing significantly (χ2 test: P = 0.70).
Six hours before testing (although still in photophase), moths were removed from the cages containing the flowers and placed into fiberglass-screen cages (31 × 31 × 32 cm) separated according to treatment group. Behavioral testing of the experienced moths began in the evening once moths had entered scotophase (19:30 Pacific Standard time) and continued for up to 3 h. In contrast to the two-choice experiments described above, these experiments had only one treatment: the choice between a D. wrightii flower and an A. palmeri umbel. Floral preference was determined on the basis of the same criteria used for experiments with naïve moth and field-caught moths.
Supplementary Material
ACKNOWLEDGMENTS.
We thank J. Barker, G. Barron-Gafford, E. Chen, B. Collins, I. Davidowitz, S. Diamond, J. Graber, B. Helm, B. Horvath, A. Levine, J. Lin, K. Mackay, C. Meyers, H. Miller, J. Pearson, V. Pham, K. Potter, B. Pri-Tal, R. Ruppel, V. Rychka, E. Saperstein, R. Sananmuang, K. Scott, D. Sung, and M. Williams, who provided invaluable assistance in blacklighting. A. Acosta, J. Graber, F. Santa Maria, and A. Urquidez helped analyze and identify pollens. C. Hedgcock (Division of Neurobiology, University of Arizona, Tucson) kindly provided hawkmoth foraging images. R. Bennett, R. White, and J. Sprayberry provided insight into the M. sexta visual system. C. Reisenman, H. Lei, and R. Raguso provided helpful guidance during all phases of this project, and their assistance improved this manuscript. This work was supported by National Institutes of Health Grant DC-02751 (to J.G.H.), National Science Foundation Grants DEB-0316205 (to J.L.B. and G.D.) and CHE-0216226 (to L.A.), and by a seed grant from University of Arizona's Center for Insect Science. J.A.R. and R.A. were supported by National Institutes of Health Postdoctoral Training Grant 2 K12 GM000708-06.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709811105/DC1.
References
- 1.Herrmann SM, Westphal CATR, Moritz RFA, Steffan-Dewenter I. Mol Ecol. 2007;16:1167–1178. doi: 10.1111/j.1365-294X.2007.03226.x. [DOI] [PubMed] [Google Scholar]
- 2.Kunin WE. Ecology. 1993;74:2145–2160. [Google Scholar]
- 3.Kunin WE. J Ecol. 1997;85:225–234. [Google Scholar]
- 4.Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. Ecology. 1996;77:1043–1060. [Google Scholar]
- 5.Fleming TH, Sahley CT, Holland JN, Nason JD, Hamrick JL. Ecol Monogr. 2001;71:511–530. [Google Scholar]
- 6.Bosch J, Retana J, Cerda X. Oecologia. 1997;109:583–591. doi: 10.1007/s004420050120. [DOI] [PubMed] [Google Scholar]
- 7.Giurfa M, Nunez J, Chittka L, Menzel R. J Comp Physiol A. 1995;177:247–259. [Google Scholar]
- 8.Weiss MR. In: Cognitive Ecology of Pollination; Animal Behavior and Floral Evolution. Chittka L, Thomson JD, editors. Cambridge, UK: Cambridge Univ Press; 2001. pp. 171–190. [Google Scholar]
- 9.Weiss MR. Anim Behav. 1997;53:1043–1052. [Google Scholar]
- 10.Kelber A. Proc R Soc London Ser B. 2002;269:2573–2577. doi: 10.1098/rspb.2002.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss MR, Papaj DR. Anim Behav. 2003;65:425–434. [Google Scholar]
- 12.Balkenius A, Kelber A. Naturwissenschaften. 2006;93:255–258. doi: 10.1007/s00114-006-0099-9. [DOI] [PubMed] [Google Scholar]
- 13.Hodges RW. The Moths of North America North of Mexico. Fascicle 21:Sphingidae. London: EW Classey and RBD Publications; 1972. [Google Scholar]
- 14.Raguso RA, Willis MA. Anim Behav. 2002;64:685–695. [Google Scholar]
- 15.Raguso RA, Willis MA. Anim Behav. 2005;69:407–418. [Google Scholar]
- 16.Raguso RA, Henzel C, Buchman SL, Nabhan GP. Int J Plant Sci. 2003;164:877–892. [Google Scholar]
- 17.Grant V, Grant KA. Bot Gaz. 1983;144:280–284. [Google Scholar]
- 18.von Helversen O, Winkler L, Bestmann HJ. J Comp Physiol A. 2000;186:143–153. doi: 10.1007/s003590050014. [DOI] [PubMed] [Google Scholar]
- 19.Chittka L, Thomson JD, Waser NM. Naturwissenschaften. 1999;86:361–377. [Google Scholar]
- 20.Ashman TL, Cole DH, Bradburn M, Blaney B, Raguso RA. Ecology. 2005;86:2099–2105. [Google Scholar]
- 21.Chittka L, Menzel R. J Comp Physiol A. 1992;171:171–181. doi: 10.1007/BF00199332. [DOI] [PubMed] [Google Scholar]
- 22.Waser NM. Am Nat. 1986;127:593–603. [Google Scholar]
- 23.Gegear RJ, Laverty TM. In: Cognitive Ecology of Pollination; Animal Behavior and Evolution. Chittka L, Thomson JD, editors. Cambridge, UK: Cambridge Univ Press; 2001. pp. 1–21. [Google Scholar]
- 24.Menzel R. In: Cognitive Ecology of Pollination; Animal Behavior and Floral Evolution. Chittka L, Thomson JD, editors. Cambridge: Cambridge Univ Press; 2001. pp. 21–40. [Google Scholar]
- 25.Giurfa M, Lehrer M. In: Cognitive Ecology of Pollination; Animal Behavior and Floral Evolution. Chittka L, Thomson JD, editors. Cambridge, UK: Cambridge Univ Press; 2001. pp. 61–82. [Google Scholar]
- 26.Howell AD, Alarcon R. Anim Behav. 2007;74:199–205. [Google Scholar]
- 27.Bernhardt P. Plant Syst Evol. 2000;222:293–320. [Google Scholar]
- 28.Faegri K, van der Pijl L. The Principles of Pollination Ecology. Oxford, UK: Pergamon Press; 1979. [Google Scholar]
- 29.Erhardt A. Bio J Linn Soc. 1993;111:229–240. [Google Scholar]
- 30.Azuma H, Thien LB, Kawano S. Plant Species Biol. 1999;14:121–127. [Google Scholar]
- 31.Cunningham J, Moore C, Zalucki M, Cribb B. Proc R Soc London Ser B. 2006;273:2035–2040. doi: 10.1098/rspb.2006.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raguso RA. Ecology. 2004;85:1486–1494. [Google Scholar]
- 33.Daly KC, Christensen TA, Lei H, Smith BH, Hildebrand JG. Proc Natl Acad Sci USA. 2004;101:10476–10481. doi: 10.1073/pnas.0401902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei H, Christensen TA, Hildebrand JG. Nat Neurosci. 2002;5:557–565. doi: 10.1038/nn0602-859. [DOI] [PubMed] [Google Scholar]
- 35.Holland JN, Fleming TH. Ecology. 1999;80:2074–2084. [Google Scholar]
- 36.Pellmyr O. Ann Mo Bot Gard. 2003;90:35–55. [Google Scholar]
- 37.Svensson GP, Hickman MO, Jr, Bartram S, Boland W, Pellmyr O, Raguso RA. Am J Bot. 2005;92:1624–1631. doi: 10.3732/ajb.92.10.1624. [DOI] [PubMed] [Google Scholar]
- 38.Delisle J, McNeil JN, Underhill EW, Barton D. Ent Exp Appl. 1989;50:53–60. [Google Scholar]
- 39.Madden AH, Chamberlin FS. US Department of Agriculture Technical Bulletin. 1945;86:2–51. [Google Scholar]
- 40.Adler LS, Bronstein JL. Ecology. 2004;85:1519–1526. [Google Scholar]
- 41.Dickens JC, Jang EB, Light DM, Alford AR. Naturwissenschaften. 1990;77:29–31. [Google Scholar]
- 42.Deng JY, Wei HY, Huang YP, Du JW. J Chem Ecol. 2004;30:2037–2045. doi: 10.1023/b:joec.0000045593.62422.73. [DOI] [PubMed] [Google Scholar]
- 43.Ochieng S, Park, Park K, Baker, Baker T. J Comp Physiol A. 2002;188:325–333. doi: 10.1007/s00359-002-0308-8. [DOI] [PubMed] [Google Scholar]
- 44.Powell JA, Brown JW. Biotropica. 1990;22:316–319. [Google Scholar]
- 45.Scott PE. Southwestern Nat. 2004;49:425–434. [Google Scholar]
- 46.del Rio CM, Eguiarte LE. Condor. 1987;89:357–363. [Google Scholar]
- 47.Slauson LA. Am J Bot. 2000;87:825–836. [PubMed] [Google Scholar]
- 48.Good-Avila SV, Souza V, Gaut BS, Eguiarte LE. Proc Natl Acad Sci USA. 2006;103:9124–9129. doi: 10.1073/pnas.0603312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molina-Freaner F, Eguiarte LE. Am J Bot. 2003;90:1016–1024. doi: 10.3732/ajb.90.7.1016. [DOI] [PubMed] [Google Scholar]
- 50.Silva-Montellano A, Eguiarte LE. Am J Bot. 2003;90:377–387. doi: 10.3732/ajb.90.3.377. [DOI] [PubMed] [Google Scholar]
- 51.Waser NM, Real LA. Nature. 1979;281:670–672. [Google Scholar]
- 52.Moeller DA. Ecology. 2004;85:3289–3301. [Google Scholar]
- 53.Kearns CA, Inouye DW. Techniques for Population Biologists. Boulder, CO: Univ Press of Colorado; 1993. [Google Scholar]
- 54.Alarcón R, Bronstein JL, Davidowitz G. Ecol Entomol. 2008 in press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.