Abstract
Water oxidation in photosystem II (PSII) is still insufficiently understood and is assumed to involve HCO3−. A Chlamydomonas mutant lacking a carbonic anhydrase associated with the PSII donor side shows impaired O2 evolution in the absence of HCO3−. The O2 evolution for saturating, continuous illumination (RO2) was slower than in the wild type, but was elevated by HCO3− and increased further by Cah3. The RO2 limitation in the absence of Cah3/HCO3− was amplified by H2O/D2O exchange, but relieved by an amphiphilic proton carrier, suggesting a role of Cah3/HCO3− in proton translocation. Chlorophyll fluorescence indicates a Cah3/HCO3− effect at the donor side of PSII. Time-resolved delayed fluorescence and O2-release measurements suggest specific effects on proton-release steps but not on electron transfer. We propose that Cah3 promotes proton removal from the Mn complex by locally providing HCO3−, which may function as proton carrier. Without Cah3, proton removal could become rate limiting during O2 formation and thus, limit water oxidation under high light. Our results underlie the general importance of proton release at the donor side of PSII during water oxidation.
Keywords: carbonic anhydrase, Chlamydomonas reinhardtii, photosystem II, proton removal, water oxidation
Introduction
Carbonic anhydrases (CAs) serve different functions in the metabolism of algae and plants. Their role is in carboxylation/decarboxylation reactions, and therefore CA functions in algal carbon-concentrating mechanisms, ion transport, pH homeostasis and in the production of carbon skeletons by mitochondria (for reviews, see Raven, 1995; Moroney et al, 2001; Giordano et al, 2005). The first intracellular algal α-CA (Cah3) was identified in the green alga Chlamydomonas reinhardtii (Karlsson et al, 1998). Immunolocalization showed that Cah3 is located on the lumenal side of thylakoid membranes, including those that penetrate the pyrenoid (Mitra et al, 2005). Moreover, the Cah3 protein was enriched in a core complex fraction compared with photosystem II (PSII) membrane fragments (Villarejo et al, 2002). Cah3 was further found to be functionally associated with the electron donor side of photosystem II (PSII), which is the site of proton release and O2 production. Intact cells, thylakoids and PSII-enriched membranes fragments of a mutant lacking the Cah3 protein (cia3) showed impaired water-splitting ability (Villarejo et al, 2002).
Although O2 and protons are both produced at the electron donor side of PSII, their release patterns differ greatly. O2 is released only during the terminal transition of a four-electron cycle completed by a metal centre, when this centre returns from its highest (S4) to its lowest (S0) oxidation state. Proton production, on the other hand, is more evenly distributed over several of these state transitions. It is a further complication that release of protons at the catalytic centre is superimposed on transient proton release and uptake caused by electrostatic interactions of peripheral acidic and basic groups with this centre. It is now accepted that proton release at the catalytic centre proper follows a 1:0:1:2 pattern during the transitions sequence S0 → S1 → S2 → S3 → S4- - -S0; (Fowler, 1977; Schlodder and Witt, 1999; Junge et al, 2002; Dau and Haumann, 2007). The sequence and relation of the transfer of electrons (to YZ, a redox-active tyrosine residue) and protons (to the aqueous bulk) have recently been described by a nine-step reaction cycle (I-cycle; Dau and Haumann, 2006). Evidence for a crucial role of proton release in the S3 → S4- - -S0 transition has been presented (Haumann et al, 2005; Dau and Haumann, 2008). At low pH, this proton-release step likely limits the yield of dioxygen (Bernat et al, 2002). The release of protons during the terminal transition is the slowest step, with the reduction of the metal centre and the release of O2 occurring simultaneously with a typical half-rise time of about 1.3 ms (Joliot, 1968; Kok et al, 1970; Haumann and Junge, 1994; Junge et al, 2002; Clausen et al, 2004). The kinetic H/D-isotope effect of proton transfer (2.4) is larger than that of electron transfer (1.4) (Haumann et al, 1997). Thus, if O2 production was rate-limited by proton release, then the limitation would be expected to occur during this terminal step (the I0 → I1 transition; Dau and Haumann, 2006).
Evidence that HCO3− might be a structural component of the PSII acceptor side between QA and QB, and that it binds to a non-heme iron, were published (Blubaugh and Govindjee, 1988; Diner and Petrouleas, 1990). Possible roles for HCO3− on the donor side of PSII (Stemler et al, 1974; Villarejo et al, 2002; van Rensen and Klimov, 2005) remain controversial, although the proposed functions of HCO3− as an electron donor (Warburg and Krippahl, 1958) and a catalyst (Metzner et al, 1979) have been ruled out by recent experiment (Clausen et al, 2005a; Hillier et al, 2006). Furthermore, evidence that HCO3− has a structural role (Dasgupta et al, 2004; Ferreira et al, 2004) remains contradictory (Loll et al, 2005; Siegbahn and Lundberg, 2006) and it was this possibility that prompted us to test the effects of HCO3− and Cah3 supplementation on the rate of O2 evolution in a Cah3-less mutant. We report here that isolated PSII-enriched membrane fragments derived from the Cah3-less mutant showed a lower rate of O2 evolution than membrane fragments from wild-type (wt) Chlamydomonas under exposure to both continuous light and flash light. Our experimental findings support a proposition that the rate limitation of O2 evolution results from a kinetic role of HCO3− in the removal of protons produced by the oxidation of water.
Results
The experimental system consisted of isolated PSII membrane fragments from either wt C. reinhardtii cells containing the Cah3 protein, wt PSII, or from the Cah3-less mutant (cia3), PSII(Cah3−). Both wt and PSII(Cah3−) PSII membrane fragments were suspended in an MES–KOH buffer initially free of inorganic carbon (Ci) at pH 5.5. This value is close to (Hager and Holocher, 1994) or slightly below (Kramer et al, 1999) the pH of the thylakoid lumen in the light and lower than the pK2 of carbonic acid, favouring conversion of HCO3− to CO2. PSII activity was measured before and after addition of HCO3− and/or overexpressed Cah3. The rate of O2 evolution was first determined under continuous illumination.
The HCO3−-induced increase in O2 evolution in cia3-mutant PSII membrane fragments is twofold greater in the presence of the Cah3 protein
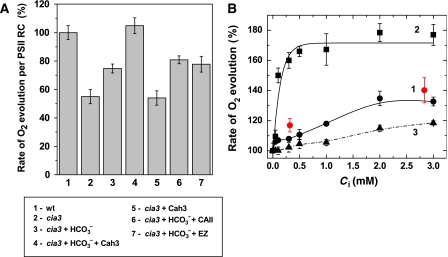
Expressed per reaction centre (RC), the O2 evolution rate of PSII(Cah3−) fragments in Ci-free medium was lower than that of the wt PSII fragments (Figure 1A). This particular study was a logical continuation of our earlier observation (Villarejo et al, 2002) that addition of HCO3− to PSII(Cah3−) fragments in the O2 electrode chamber led to enhanced O2 release. Two millimolar HCO3− stimulated the rate of O2 evolution, on average, by 40%. When overexpressed and purified CA, Cah3, enzyme was added simultaneously with HCO3−, the stimulation increased twofold and reached the wt PSII rate if calculated per RC (Figure 1A). HCO3− alone, at all concentrations and experimental conditions tested (pH, temperature, buffer system etc.), was unable to restore O2 evolution in PSII(Cah3−) to the full wt PSII rate. From Figure 1B and thereafter, 100% corresponds to the O2 evolution rate of PSII(Cah3−) in Ci-depleted media (the typical rate was 180–200 μmol O2 (mg Chl)−1 h−1).
Figure 1.
Overexpressed and purified Cah3 protein fully restores O2 evolution in PSII membrane fragments derived from the Cah3-less cia3 mutant. (A) Means±s.e. of O2 evolution rates expressed as a percentage of the wt rate and per PSII RC in Ci-free buffer: (1) PSII from wt cells in Ci-free SMS buffer without additions, (2) PSII(Cah3−) without additions, (3) in the presence of 2 mM KHCO3, (4) in the presence of 2 mM KHCO3 and 0.1 μM recombinant Cah3 protein, (5) in the presence of 0.1 μM recombinant Cah3 protein, (6) in the presence of 2 mM KHCO3 and 0.1 μM bovine CAII protein (EC 4.2.1.1) and (7) in the presence of 2 mM KHCO3 and both 0.1 μM recombinant Cah3 protein and 0.2 μM EZ. (B) HCO3− stimulation of O2 evolution rates in (1) PSII(Cah3−), (2) PSII(Cah3−) reconstituted with 0.1 μM recombinant Cah3 protein and (3) wt PSII. Red circles show O2 evolution rates in the presence of the indicated KHCO3 concentration and 0.1 μM bovine CAII (EC 4.2.1.1). The control (100%) rate of O2 evolution corresponds to the PSII(Cah3−) O2 evolution rate in Ci-depleted media and was 180 μmol O2 (mg Chl)−1 h−1. The light intensity was 1200 μmol m−2 s−1 PAR. Chl concentration was 15 μg ml−1. All measurements were conducted at 25°C in the presence of 1 mM DCBQ and 0.5 mM FeCN. Values are means±s.e. (n=3). A full-colour version of this figure is available at The EMBO Journal Online.
Addition of Cah3 to PSII(Cah3−) membrane fragments in the presence of HCO3− reactivated O2 evolution but this reactivation could be entirely prevented by the addition of either 0.2 μM of the CA-specific inhibitor ethoxyzolamide (EZ) or by removing the substrate HCO3− (Figure 1A). In contrast to the addition of Cah3, addition of bovine CA, CAII (EC 4.2.1.1), under similar conditions did not stimulate O2 evolution above that obtained with HCO3− alone (Figure 1A and B), despite its high enzymatic activity in solution.
The O2 evolution rate of PSII(Cah3−) saturated at 0.25 mM HCO3− in the presence of added Cah3 enzyme, but in the absence of Cah3 supplementation, saturation was reached first at 2 mM HCO3− (Figure 1B). Thus, Cah3 had a twofold greater stimulatory effect at an almost 10-fold lower HCO3− concentration compared with HCO3− alone. A stimulatory effect on the O2 evolution rate was observed also in wt PSII upon addition of HCO3− alone or together with Cah3, but in this case the stimulation never exceeded 15% (Figure 1B). Thus, under continuous illumination, when neither the light intensity nor the electron acceptor side of PSII(Cah3−) were rate limiting, Cah3 in the presence of its substrate, HCO3−, stimulated the O2 evolution rate twofold (Figure 1A and B; Supplementary Figure S1A and B).
Cah3/HCO3− accelerates O2-release kinetics
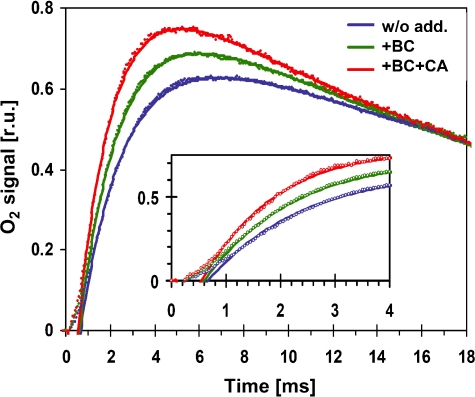
After dark adaptation of PSII, all Mn4CaTyr clusters of the water oxidizing complex (WOC) are synchronized mainly in the first oxidized state, which is designated S1. Excitation with a train of short light flashes stepwise oxidizes the Mn4CaTyr centres until after the third flash O2 is released. O2 is produced and released only after the centre has reached its fourth oxidation state, S4. Figure 2 shows polarographic recordings of O2 release of dark-adapted PSII(Cah3−) upon release of the third flash. A typical half-rise time of O2 release is 1.3 ms (Clausen et al, 2004). In agreement with steady-state O2 evolution, O2 rise in PSII(Cah3) membranes suspended in Ci-free buffer and exposed to flash light was accelerated by the addition of Cah3 or just HCO3− (Figure 2). In the wt PSII, on the other hand, there was no noticeable effect of these additions on the rise of O2 (data not shown). The half-rise time of wt PSII was 1.3 ms, whereas in PSII(Cah3−) it was 1.9 ms. In the presence of HCO3−, the apparent half-rise time decreased by about 20% and even further when HCO3− and Cah3 were added simultaneously. The amplitude of flash-induced polarographic recordings of O2 release was not influenced significantly by Cah3/HCO3−, implying that the number of active centres remained unaffected.
Figure 2.
Flash-induced raw polarographic transients recorded with a Clark-type O2 electrode in Ci-free SMS buffer with PSII(Cah3−) membranes, blue symbols; PSII(Cah3−) membranes in the presence of 1 mM KHCO3, green symbols and PSII(Cah3−) membranes reconstituted with 0.7 μM recombinant Cah3 protein and 1 mM KHCO3, red symbols. The lines result from simulations involving a delayed onset of the exponential rise, an either mono- or bi-exponential rise and an exponential decay. The following parameters were used in the simulations shown (delay//first rise time and amplitude//second rise time and amplitude//decay time): without additions (blue)—670 μs//1.8 ms, 65%//6 ms, 35%//24 ms; with HCO3− (green)—600 μs//1.8 ms, 84%//4 ms, 16%//24 ms; with HCO3− and Cah3 (red)—550 μs//1.6 ms, 100%//not required//24 ms. Unless HCO3− and Cah3 are simultaneously supplemented, a bi-exponential simulation of the O2 rise is required (see Supplementary data). However, due to extensive parameter correlations, various combinations of amplitudes and time constants can result in simulations of similar quality. A full-colour version of this figure is available at The EMBO Journal Online.
The above description of the O2-release kinetics by a single half-rise time is oversimplified. The O2 electrode signal indeed comprises (i) an initial delay (lag phase, t<600 μs) reflecting mostly a delayed O2 release by PSII (Clausen et al, 2004), (ii) a rise in O2 concentration reflecting O2 formation by PSII and (iii) a decay due to O2 diffusion within the electrode cell. For PSII(Cah3−) supplemented with HCO3− and Cah3, the rise phase is well described by a single exponential. However, without HCO3−/Cah3, a bi-exponential simulation of the rise phase of O2 release is required for good agreement, where the slower phase contributes by 10–30% to the total amplitude of the rise (Supplementary Figure S2, data simulation). The simulations inter alia show that the time constants of the major rise phase are unaffected by HCO3− addition and are essentially identical to the values determined by the delayed fluorescence measurements described below. Therefore, the acceleration of O2 release by HCO3− and Cah3 supplementation is mostly explainable by a decrease in the contribution of a minor slower component to the exponential rise of O2 release; a minor contribution presumably stems from a reduction in the duration of the initial lag phase.
Cah3/HCO3− affects the PSII donor side
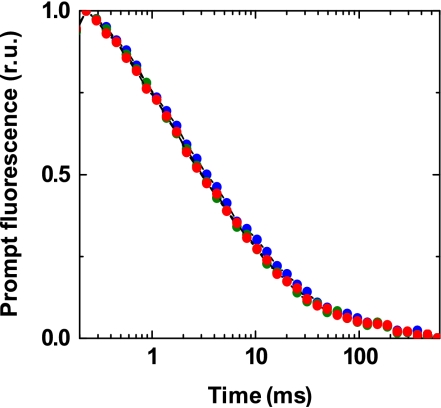
HCO3− binds to the non-heme iron at the acceptor side of PSII (Ferreira et al, 2004; Loll et al, 2005). After formate treatment, HCO3− addition is required to restore QA → QB electron transfer (Blubaugh and Govindjee, 1988; Diner and Petrouleas, 1990). It was therefore important to clarify whether or not the stimulatory effect of HCO3− in PSII(Cah3−) was caused by an acceptor-side effect. To address this question, we measured flash-induced changes in the yield of prompt chlorophyll (Chl) fluorescence, which reflect the re-oxidation of reduced QA by electron transfer to QB (Dau, 1994; Steffen et al, 2001). As is evident, there was no difference in the decay kinetics between various samples (Figure 3). This observation strongly suggests that the Cah3/HCO3− effect is not associated with changes at the acceptor side of PSII(Cah3−) (no formate treatment). The increase in Chl fluorescence yield (ΔF) under continuous light also supports a limitation at the donor side (Supplementary Figure S2).
Figure 3.
Flash-induced changes in the yield of prompt Chl fluorescence measured in a pump-probe experiment as described under Materials and methods. The time course was detected after the third flash was applied to dark-adapted PSII membrane fragments isolated from the cia3 mutant in Ci-free SMS buffer. PSII(Cah3−) membranes, blue; PSII(Cah3−) membranes in the presence of 1 mM KHCO3, green and PSII(Cah3−) membranes reconstituted with 0.07 μM recombinant Cah3 protein in the presence of 1 mM KHCO3, red. Chl concentration was 10 μg ml−1. See Buchta et al (2007) for more details on the prompt fluorescence measurements. A full-colour version of this figure is available at The EMBO Journal Online.
Cah3 stimulates O2 evolution through its substrate/product bicarbonate as a carrier facilitating proton transfer
Cah3, an α-type CA with a rate of about one million turnovers per second (Lindskog, 1997), speeds up the reaction CO2+H2O↔HCO3−+H+. It is conceivable that Cah3 facilitates the removal of H+ at the donor side of PSII and, by doing so, facilitates H2O oxidation. This would be consistent with the data in Figures 1 and 2; Supplementary Figure S2 showing that Cah3 enzymatic activity was required to obtain full reactivation of water oxidation. In agreement with earlier reports (Clausen et al, 2005a; Hillier et al, 2006), we disregard the possibility that HCO3− is directly involved in the electron donation in PSII. Instead, our hypothesis is that HCO3− accelerates proton removal from the vicinity of the WOC, which is essential for the energetics of water oxidation (Krishtalik, 1986; Haumann and Junge, 1994; Dau and Haumann, 2007; Renger, 2007).
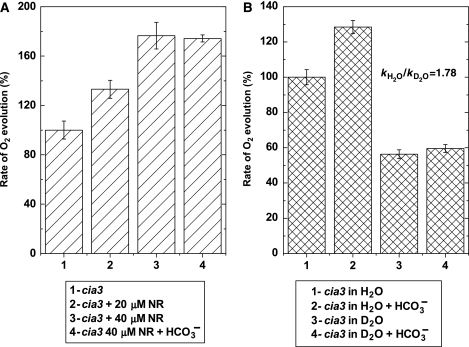
This hypothesis was tested using the amphiphilic pH indicator/buffer neutral red (NR), which has been shown to be an efficient H+ donor/acceptor through bimolecular interactions at the thylakoid membrane surface (Auslander and Junge, 1975; Junge et al, 2002). A clear stimulation of O2 evolution by this dye was obtained. In the presence of 20 μM NR, the rate of O2 evolution of PSII(Cah3−) increased to the same extent as with 2 mM HCO3− (Figure 4A). At higher concentrations of NR (40 μM), the stimulation was comparable to that of HCO3− and Cah3 added simultaneously. At optimal NR concentrations, there was no further stimulation by 2 mM HCO3−. A decrease of the O2 evolution rate was observed at NR concentrations exceeding 50 μM (data not shown). In wt PSII, no significant increase in the O2 evolution rate was found by the addition of NR. The fact that NR mimics the effect of HCO3− and Cah3 in the water oxidation reaction supports the conclusion that Cah3 accelerates O2 evolution by facilitating the removal of protons from the WOC.
Figure 4.
Bicarbonate stimulates O2 evolution in PSII(Cah3−) by facilitating proton transfer away from WOC. (A) Stimulatory effect of the amphiphilic pH indicator NR on the O2 evolution rate of membrane fragments from cia3. PSII(Cah3−), 1; PSII(Cah3−) in the presence of 20 μM NR, 2; in the presence of 40 μM NR, 3 and in the presence of 40 μM NR and 2 mM KHCO3, 4. The initial rate of O2 evolution (indicated as 100% level in the figure) was 183 μmol O2 (mg Chl)−1 h−1. The assay medium was depleted of endogenous Ci and the Chl concentration was 15 μg ml−1. All measurements were conducted at 25°C in the presence of 1 mM DCBQ and 0.5 mM FeCN. Values are means±s.e. (n=4). (B) Rate of O2 evolution in PSII membrane fragments from the cia3 mutant measured in H2O and D2O media. PSII(Cah3−) in SMS buffer, 1; PSII(Cah3−) in SMS buffer in the presence of 1 mM KHCO3, 2; PSII(Cah3−) in D2O-SMS buffer, 3 and PSII(Cah3−) in D2O–SMS buffer in the presence of 1 mM KHCO3, 4. The kH2O/kD2O in the PSII(Cah3−) was 1.78 and 1.15 in wt PSII. Values are means±s.e. (n=6). The initial rate of O2 evolution (indicated as 100% level) was 190 μmol O2 (mg Chl)−1 h−1.
Additional independent support for a proton limitation in PSII(Cah3−) comes from isotope-exchange experiments (Figure 4B). The kinetic isotope effect kH2O/kD2O in the mutant was 1.78, whereas in it was only 1.15 the wt PSII. Importantly, stimulation of O2 release by HCO3− in PSII(Cah3−) in an H2O-based buffer was 37%, whereas no statistically significant stimulation could be detected in a D2O-based buffer.
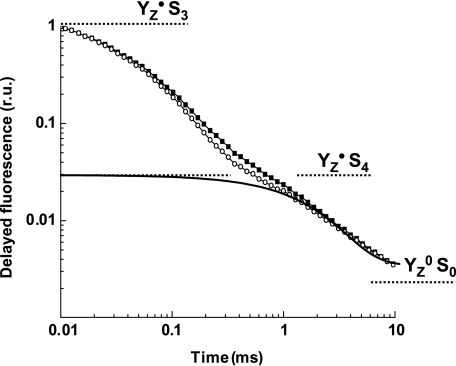
To investigate more directly the influence of HCO3− on the rate of proton transfer from the Mn complex to the lumenal bulk phase, measurements of delayed Chl fluorescence (DF) were conducted. Results obtained previously by time-resolved DF and X-ray measurements (Haumann et al, 2005; Buchta et al, 2007) and other methods (Rappaport et al, 1994) suggest that in the O2 evolution transition (S3 → S4 → S0), an S4 intermediate is formed by transfer of a proton from the Mn complex to the lumenal bulk phase (for a detailed discussion, see Dau and Haumann, 2007). This step of removing a proton from the Mn complex precedes electron transfer and dioxygen formation itself. It is likely that this proton-removal step is an essential prerequisite for dioxygen formation (Dau and Haumann, 2005, 2008).
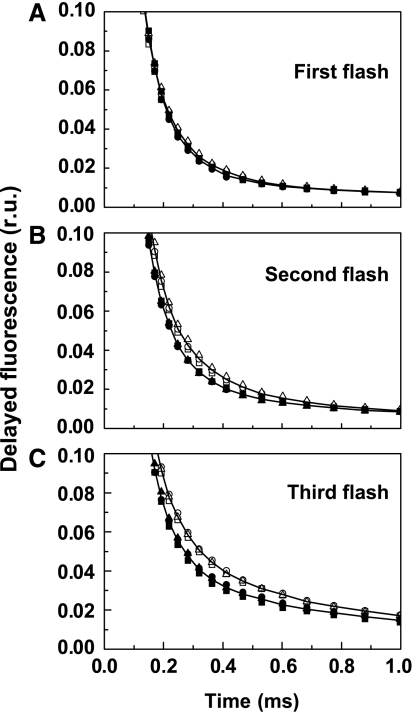
The third nanosecond-laser flash applied to dark-adapted PSII membrane fragments induces S3 → S4 → S0 transition. In the time course of DF excited by the third flash (Figure 5), the S3 → S4 transition (t<1 ms) is reflected in the decay from the initial value at 10 μs to the plateau reached at about 1 ms. The subsequent DF decay in the millisecond time domain reflects the reduction of the YZ•+ radical and the simultaneous dioxygen formation step itself (Zankel, 1971; Grabolle and Dau, 2005; Clausen et al, 2005b; Buchta et al, 2007). In PSII(Cah3−) fragments suspended in Ci-depleted media, the rate of proton removal in the S4 formation step was reproducibly found to be accelerated by about 25% by the addition of HCO3− (visual inspection of DF; Figure 6) but not so in the wt PSII (not shown). A tri-exponential simulation of the DF time courses (Buchta et al, 2007) revealed that the corresponding time constant changed from 200 to 150 μs upon HCO3− addition, whereas in the wt it was close to 150 μs and was unaffected by HCO3−. However, the time constant assignable to YZ•+ reduction and O2 formation is apparently unaffected by HCO3− addition (time constant of 1.9±0.1 ms, with and without HCO3−).
Figure 5.
Decay of DF following the third flash, with correction for the influence of QA re-oxidation. PSII(Cah3−) in Ci-free SMS buffer without additions, open symbols; PSII(Cah3−) in Ci-free SMS buffer in the presence of 1 mM KHCO3, filled symbols. Chl concentration was 10 μg ml−1. For details of the DF measurement and its analysis, see Buchta et al (2007).
Figure 6.
Time course of DF for the first three flashes (A–C) applied to dark-adapted PSII(Cah3−) isolated from the cia3 mutant in Ci-free SMS buffer. Open symbols, without additions; filled symbols, in the presence of 1 mM KHCO3. The figure shows three independent replicates for each treatment and the lines are the best-fit curves for each group of three replicates within a treatment. All curves are normalized at 10 μs.
As in the S3 → S4 → S0 transition, in the S2 → S3 transition electron transfer likely is also preceded by an essential step of removing a proton from the Mn complex, as proposed by Dau and Haumann (2006), and reviewed elsewhere (Dau and Haumann, 2007). The S1 → S2 transition does not involve any proton removal from the Mn complex. As shown in Figure 8, in the S2 → S3 transition (but not in S1 → S2 transition), the DF decay was also accelerated by the addition of HCO3− (by ∼20%). Thus, the DF analysis supports a role of HCO3− in the removal of protons from the Mn complex.
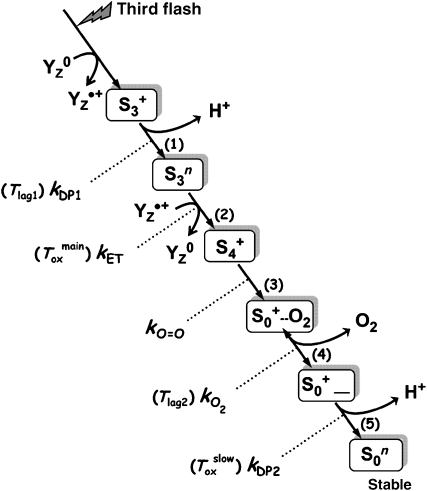
Figure 8.
Proposed sequence of events induced by the third flash of saturating light applied to dark-adapted PSII. The initial oxidation of YZ, a specific redox-active tyrosine residue, is followed by the removal of a proton from the Mn complex (step 1) and only subsequently by electron transfer from the Mn complex to YZ•+ (step 2). The latter ET step is kinetically indistinguishable from O2 formation and the associated reduction of the Mn complex (step 3). The subsequent dioxygen release (step 4) is assumed to be reversible. Finally, a second proton is removed from the Mn complex (step 5), a process that may involve a significant rearrangement of the Mn ligand environment. Steps (1) and (5) are assumed to be accelerated by the Cah3/HCO3− system. The given time constants refer to possible contributions to the O2-release signal, which accordingly may reflect not only Toxmain but also Tlag1 and Tlag2 as well as Toxslow (the latter only in PSII(Cah3−) in the absence of HCO3− or Cah3). In the nomenclature used here, the S-states refer to states of the Mn complex. The states S3, S4 and S4′ of Haumann et al (2005) correspond to states S3+, S3n and S4+, respectively. The subscripts indicate the number of oxidizing equivalents accumulated by the Mn complex, and the superscripts refer to the relative charge of Mn complex, which is assumed to be either neutral (n) or single positive charge (+).
Purified Cah3 protein stoichiometrically rebinds to mutant PSII membrane fragments and restores their activity
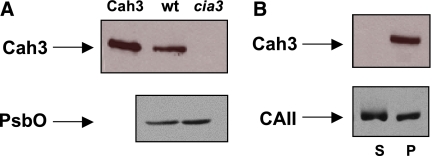
According to the functional analysis presented above, lumenal Cah3 is a protein with the molecular architecture required to bind to a specific site of PSII, where its function is accomplished. To test its rebinding ability, we conducted western blot analysis using Cah3-specific antibodies. The single 29-kDa band corresponding to Cah3 is clearly visible in the lane containing wt PSII, while no cross-reaction occurred with PSII(Cah3−). The same samples were examined using PsbO-specific antibodies (Figure 7A). Following reactivation of the O2-evolving activity of PSII(Cah3−) in the presence of overexpressed and purified Cah3, the samples were centrifuged to separate the membrane-bound protein from the soluble fraction (Figure 7B). Both pellet and supernatant were subjected to western blot analysis using Cah3-specific antibodies. All Cah3 protein detected was in the pellet (lane P). It is therefore concluded that the Cah3 protein binds to PSII(Cah3−). The KD for binding of Cah3 was about 0.1–0.2 μM, indicating specific binding. KD was calculated at ratios from 0.1 to 5 Cah3 per PSII RC. Increasing the ratio further caused unspecific binding. The reactivation of O2-evolving activity was also tested with bovine CAII (see Figure 1A and B), and the sample was separated into membrane-bound and soluble fractions after the O2 assay. Importantly, CAII also had a different distribution than Cah3 between pellet and supernatant (Figure 7B). Much less CAII was bound to the membrane phase compared with Cah3. It was not possible to determine the KD for bovine CAII because CAII was evenly distributed between pellet and soluble fraction at all concentrations. In agreement with the rebinding experiments, the greatest stimulation of mutant O2 evolution by Cah3 was observed at a 1:1 protein to PSII RC ratio (Supplementary Figure S3).
Figure 7.
Stimulation of O2 evolution of PSII membrane fragments caused by binding of Cah3. (A) Immunoblot analysis of PSII membrane fragments from the wt and the cia3 mutant (cia3). The recombinant Cah3 protein was loaded as a positive control (Cah3). (B) Immunoblot analysis of PSII(Cah3−) after reconstitution with recombinant Cah3 protein, followed by centrifugation to obtain pellet (P) and soluble (S) fractions. The antibodies used were against Cah3, PsbO and CAII. Lanes cia3, wt and P were loaded with 7 μg of Chl and Cah3 along with 1.2 μg Cah3 protein.
Discussion
Our starting point was the striking observation that in the Cah3-deficient mutant assayed in Ci-free media, the rate of O2 evolution per PSII under continuous light was approximately half of that of the wt, and that complete restoration to the wt rate in the mutant was only achieved after addition of overexpressed Cah3 together with HCO3− (Figure 1A and B). This observation raises the question of what important function could Cah3 have within the PSII complex to so strongly enhance O2 release?
One possibility that we could exclude is that the Cah3 protein itself fulfils a structural function within the PSII multi-protein complex. Such a function would remain after addition of low concentrations of the CA-specific inhibitor EZ, a rather small molecule that binds to the active site of Cah3 and with no influence on the structure of the enzyme (Huang et al, 1998). As is demonstrated in Figure 1A, the stimulatory effect obtained by Cah3 disappeared after the addition of this inhibitor. Even in the presence of 1 mM HCO3−, no stimulatory effect was found for EZ-inhibited Cah3. The lack of stimulation by the Cah3 enzyme in the absence of its substrate, HCO3 (Figure 1A), is further corroborating evidence that there is no structural effect of this protein on the O2-evolving activity of PSII. The specific association of Cah3 with PSII is proven by the low KD of about 0.1 μM and a maximal stimulation of O2 evolution at a 1:1 ratio of Cah3 to PSII. In contrast, bovine CAII did not show any specific binding over a broad concentration range and resulted in no stimulation of O2 evolution despite its high activity (Figure 1A and B; Supplementary Figure S3). Thus, on the basis of the results presented here and earlier results (Karlsson et al, 1998; Villarejo et al, 2002), we feel confident to propose that lumenal Cah3 is a PSII-specific CA and that it restores O2 evolution by exerting a locally directed, rather than a general, CA activity in the lumen bulk at low HCO3− concentration.
The oxidation of YZ is very fast (rise time <1 μs) and the detected rise time of O2 evolution is independent of the rate constants at the acceptor side of PSII. That we observed an influence of Cah3 and HCO3− on this rise (Figure 2) but not on the prompt fluorescence decay kinetics (Figure 3) are strong arguments for the localization of the HCO3− effect at the electron donor side. Whereas rise was accelerated by HCO3−, the magnitude of the laser flash-induced O2 pulse was largely unaffected, implying a constant number of active PSIIs. In conclusion, five different experimental approaches: (i) O2 evolution and (ii) variable fluorescence (ΔF) for continuous illumination (Figure 1; Supplementary Figure S2), (iii) O2 liberation, (iv) prompt and (v) delayed fluorescence kinetics detected after flash excitation (Figures 2, 3, 5 and 6) provide consistent coherent results and support a direct function of HCO3− and Cah3 at the PSII donor side.
Warburg and Krippahl (1958) proposed that CO2 is the source of O2 in photosynthesis. Later, this hypothesis was modified by Metzner et al (1979), who suggested that HCO3− acted as the H2O supply to PSII. In two recent papers on O2 evolution in cyanobacteria and green plants (Clausen et al, 2005a; Hillier et al, 2006), it was corroborated that H2O, and not HCO3−, is the donor of electrons and the sole source of O2. However, a structural role of HCO3− at the donor side of PSII, for example, in reconstitution and stabilization of the Mn4CaTyr cluster, has remained a possibility (Dasgupta et al, 2004; van Rensen and Klimov, 2005). In the results wt PSII exhibited no measurable stimulation when HCO3− was added to the Ci-depleted sample buffer, while PSII(Cah3−) was stimulated. This suggests that the wt binds HCO3− with a much higher affinity than PSII(Cah3−). Under our experimental conditions, HCO3− could not be removed from wt PSII and therefore we cannot exclude that there is a tight HCO3−-binding site at the PSII donor side (Dasgupta et al, 2004; van Rensen and Klimov, 2005). This site could be arginine 357 of the CP43 protein, which was suggested to be an HCO3− ligand (Ananyev et al, 2005; Hwang et al, 2007). However, it appears likely that HCO3− either leaves the Mn cluster after photo-assembly or is tightly bound and cannot be removed by bubbling with CO2-free gas in spinach thylakoids (Shevela et al, 2007). Because it is now excluded that HCO3− is the primary electron donor to PSII, and because Cah3 or HCO3− does not likely exerts its effects only through a structural function, the possibility of HCO3− acting as a local proton acceptor/carrier came into focus.
To test this hypothesis, we used NR, an amphiphilic buffer known to bind protons on the surface of thylakoid membranes (Auslander and Junge, 1975; Junge et al, 2002). The stimulatory effect of NR on the rate of O2 evolution was as great that as observed for the joint addition of HCO3− and Cah3 to PSII(Cah3−), whereas there was no NR effect observed in wt PSII (Figure 4A). We note that NR effectively restores H2O oxidation in PSII(Cah3−), whereas other buffers with suitable pKa do not (data not shown). As opposed to these buffers, NR is amphiphilic and aligns along the membrane surface, where it accepts protons directly from the donor(s) instead of accepting protons from the bulk phase of the lumen.
The rate of O2 evolution in PSII(Cah3−) was also modified when exchangeable protons were replaced by deuterons (Figure 4B). The significant kinetic isotope effect (kH2O/kD2O of 1.78) suggests that in Ci-depleted media and in the absence of Cah3 activity, the water oxidation is rate limited by a process involving proton translocation. In contrast, in wt PSII the replacement of H2O by D2O causes only minor changes (⩽15%) in the O2 evolution rate, similar to the report by Sinclair and Arnason (1974) for Chlorella cells (kH2O/kD2O of 1.29). In the D2O-based buffer solution, only an insignificant HCO3−-induced increase of the O2 evolution rate was found in PSII(Cah3−). There are several conceivable explanations for the latter finding, for example, slow or inefficient deuteronation of HCO3− or changed pK-values in D2O (upshift by 0.2–0.7 units; Glasoe and Long, 1960) could impair the role of HCO3− as a proton carrier in the Cah3-less PSII. The kinetics of electron transfer is virtually the same in H2O and D2O (Haumann et al, 1997; Christen and Renger, 1999). In marked contrast, proton release from the WOC, especially in the terminal step of the water oxidation reaction, exhibits greater isotope effects (Haumann et al, 1997; Karge et al, 1997). The result obtained for PSII(Cah3−) in a D2O-based solution at pH 5.5 revealed a kH2O/kD2O value consistent with proton transfer being the rate-limiting step of the water oxidation reaction in the absence of Cah3 activity and HCO3−.
Further support for a role of the Cah3/HCO3− system in proton removal from the Mn complex comes from our time-resolved measurements of DF after laser-flash excitation (Figures 5 and 6). In the S2 → S3 and S3 → S4 → S0 transitions of the WOC, an essential proton-release step has been suggested to precede electron transfer (Haumann et al, 2005; Dau and Haumann, 2007). The corresponding proton movement towards the lumenal bulk is directly reflected in the DF time courses (Buchta et al, 2007). The DF decays indeed suggest that in PSII(Cah3−), HCO3− addition is required for restoration of the proton-removal rate to the wt level. These time-domain experiments demonstrate acceleration of proton removal at the PSII donor side, and thus support the suggested role of HCO3− as a proton acceptor and/or carrier.
A stimulating effect on O2 evolution was obtained by adding HCO3−, Cah3 or both of them together under continuous light and excitation with short light flashes (Figures 1, 2, 5 and 6; Supplementary Figure S2). The lower stimulation detected for flash illumination, in comparison with continuous illumination, can be explained in several ways. We favour the explanation that under continuous light the protons produced in PSII(Cah3−) will saturate the proton acceptors. When subjected to a small number of short flashes, saturation of acceptors will not take place and therefore the stimulatory effect by HCO3− and Cah3 is less pronounced.
In each water oxidation cycle, four protons are transferred from the Mn complex to the lumenal bulk phase; the sequence and distinct properties of the four steps of removal of ‘intrinsic' protons from the Mn complex have been discussed elsewhere (Junge et al, 2002; Dau and Haumann, 2006, 2008). The results reported here consistently suggest that HCO3− may promote more efficient proton removal from the Mn complex (but not the proton production at the catalytic site itself), and we propose that it does so in each of the four proton-removal steps. However, the various experiments reported here relate differently to each of the four distinct proton-removal steps. In the S2 → S3 and S3 → S4 → S0 transitions, proton removal from the Mn complex has been proposed to precede electron transfer to the YZ radical, and a specific Cah3/HCO3− influence on these proton-removal steps is suggested by the DF transients (Figure 6). In the S3 → S4 transition, the preceding proton transfer delays Mn reduction and O2 formation (Haumann et al, 2005). Thus, the Cah3/HCO3− influence on the time course of O2 release (Figure 2) could result from an effect on this specific proton movement step. However, the deceleration of this proton removal step in the absence of Cah3 and HCO3− is clearly too small to explain the severe limitation in the O2 evolution rate detected for continuous illumination. Earlier time-resolved proton-release studies (Haumann et al, 1997) suggest that in the S4 → S0 transition a second proton is removed from the Mn complex concomitantly with (in H2O at pH 7.4) or after (in D2O or at pH⩽6.3) the kinetically indistinguishable events of electron transfer to YZ, Mn reduction and O2 release. Consequently, at lowered pH and in D2O, this delayed proton removal may be the slowest step in the water oxidation cycle and this could limit the O2 evolution rate under continuous, saturating illumination. Evidently, in the absence of Cah3 and HCO3−, this proton release is delayed with respect to dioxygen formation and thus affects the rate of O2 evolution under continuous light. However, this delay remains undetectable in flash-induced DF and in the major rise phase detected in the O2-release experiments.
Figure 8 illustrates the possible sequence of events in the S3 → S0 transition initiated by the third flash of saturating light (Dau and Haumann, 2007, 2008), which in the following sections is discussed with regard to the HCO3− influence on O2 formation in the Cah3-less mutant. The formation of YZ•+ is followed by a proton-removal step ((1) in Figure 8), which is accelerated by HCO3− addition by about 50 μs, as suggested by the time-resolved DF and O2-release measurements (contribution to the lag preceding the O2 rise, Tlag1). However, the electron transfer to YZ•+ (2) remains unaffected by HCO3− (Toxmain≈1.9±0.1 ms in the DF and O2 signal). The subsequent O2 formation step (3) is faster than the preceding ET step (kET≪kO=O) and thus is not directly detectable in any kinetic study. O2 formation is followed by an O2-release step (4); here this step is assumed to be reversible (Clausen and Junge, 2004) such that a sizable fraction of O2 remains bound. O2 release could also contribute to the lag in the O2 signal (Tlag2) (Clausen et al, 2004). The final step in Figure 8 involves the release of a proton (5), which, for PSII(Cah3−) in the absence of HCO3−, is slower than the YZ•+ reduction and O2 formation (kDP2<kET). This explains the biphasic O2 release suggested by the time-resolved polarographic measurements, because the slow proton release in conjunction with the reversible O2-release step implies a contribution of the slow phase to the O2 signal (Toxslow). Upon addition of HCO3− and Cah3 to the mutant PSII, the final proton-release step accelerates (kDP2<kET) and becomes faster than YZ•+ reduction/O2 formation, and thus invisible in the O2 signal. We conclude that, assuming a specific HCO3− influence on the two proton-release steps, the reaction sequence of Figure 8 provides a consistent and plausible explanation of the time-resolved DF and O2 data on S3 → S0 transition.
The negative consequences, if protons were not removed fast enough, should be more pronounced for saturating continuous illumination than for excitation by a small number of light flashes. In the absence of Cah3 and added HCO3−, photodamage occurred fast with a half time of 17 s even though the light intensity was as low as 200 μmol m−2 s1, photosynthetic active radiation (PAR) (Supplementary Figure S4). Photo-inhibition becomes more pronounced at acidic pH, as shown elsewhere (Spetea et al, 1997). A lack of HCO3− would, according to our hypothesis, lead to an increased concentration of protons in PSII that could increase the risk of photodamage at the donor side. To compensate for the slowed donor-side reactions, which result from less efficient proton removal at the donor side, and the impaired stability of PSII, the cia3-mutant cells accumulate more PSII at standard growth conditions than does the wt (Villarejo et al, 2002). As a consequence, electron supply from PSII is not rate limiting when whole-cell photosynthesis is measured (Hanson et al, 2003). The difference between wt and cia3-mutant cells in vivo was revealed under high-light conditions, where an increase in the accompanied protons release is predicted (Villarejo et al, 2002; Hanson et al, 2003). On the other hand, the cia3 thylakoids were impaired in ATP synthesis due to an altered proton gradient (van Hunnik et al, 2002), probably reflecting that Cah3 also have an important function in maintaining an optimal H+/HCO3−/CO2 balance. In comparison with higher plants (Kanervo et al, 2005), the light reactions of the photosynthetic apparatus in green algae are less well studied (Wilson et al, 2006). Several photosynthetic mutants of C. reinhardtii have been valuable tools in gaining an understanding of light adaptation in this model organism (Rochaix, 2002). Non-photochemical quenching is composed of two components, state transition and ΔpH-driven quenching. In C. reinhardtii, the state transition component is smaller than in higher plants (Finazzi et al, 2006). Thus, a reduced ΔpH feedback on the PSII activity in algae may require an additional fine-tuning of the lumenal pH through regulation of CA activity. For C. reinhardtii we propose that Cah3 is one component out of several, involved in regulating activity at the donor side of PSII.
So far C. reinhardtii is, to our knowledge, the only organism with a characterized CA associated with PSII. CA activity has also been found in PSII preparations from higher plants (Moubarak-Milad and Stemler, 1994; Clausen et al, 2005a; Hillier et al, 2006; McConnell et al, 2007), although no PSII-associated enzyme has yet been characterized. An in-depth analysis of the thylakoid membrane proteome of Arabidopsis thaliana chloroplasts (Friso et al, 2004) revealed an α-CA homologue At4g20990. However, a database search of higher plant genomes did not identify any close homologue to Cah3. Nevertheless, 35 candidates putatively located in the chloroplast can be proposed from the data (Supplementary Table T1; Supplementary data). The reactions at the PSII donor side have been inferred to be almost invariant to the evolution from cyanobacteria to higher plants (Karge et al, 1997). However, although all photosynthetic organisms share the primary mechanism for water oxidation, differences may occur between how this is facilitated. In the cyanobacteria studied, no CA activity associated to PSII has been detected (Hillier et al, 2006). While HCO3− is also clearly required at the donor side of cyanobacterial PSII (Carrieri et al, 2007), the HCO3− concentration around PSII is kept high by active HCO3− transport in these cells. Different strategies to keep the HCO3− concentration high around PSII therefore seem to have evolved. We suggest based on our and others results (Hillier et al, 2006; Carrieri et al, 2007), that PSII-associated CA first appeared in eukaryotic unicellular algae. However, the data currently available do not facilitate a clear-cut conclusion of the role of CA in water oxidation in higher plants.
Irrespective of the still open question whether in higher plants a Cah3 homologue is associated with PSII, the results reported here relate to questions that are of general importance for photosynthetic water oxidation. Dioxygen and protons are reaction products of water oxidation at the donor side of PSII. Evidence has been reported that the dioxygen-release step may be critical for the energetics, and thus the efficiency, of photosynthetic water oxidation (Clausen and Junge, 2004). Our findings suggest that also proton removal from the catalytic Mn complex of PSII is a process that can potentially limit photosynthetic dioxygen formation seriously, at least for continuous, saturating illumination at the lumenal ‘working pH' of about 5.5. The PSII-associated Cah3/HCO3− system likely does not eliminate completely, but presumably reduces this limitation in some or even all organisms of oxygenic photosynthesis. We also note that the findings reported here provide circumstantial, but relevant, support to recent considerations (Dau and Haumann, 2007, 2008) on the crucial role of distinct proton-removal steps for energetics and mechanism of photosynthetic water oxidation (Krishtalik, 1986), thereby supporting a shift of focus in PSII research from the electron transfer reactions towards the role of the associated proton movements.
Materials and methods
Isolation of PSII membrane fragments
PSII membrane fragments were usually prepared simultaneously from 20 l algal cultures of strains cw92 and cia3 of C. reinhardtii using a 1:20 Chl-to-Triton ratio (w/v) (Berthold et al, 1981). The typical O2 evolution rate of PSII membrane fragments was 170–190 μmol (mg Chl)−1 h−1. For more experimental details, see Supplementary data.
Expression and purification of recombinant Cah3
The plasmid pET32-Xa-cah3 encodes a 394-amino-acid long fusion protein in which the mature Cah3 is linked to a 156-amino-acid N-terminal extension containing His6-tag thioredoxin and a Factor Xa cleavage site. The construct was verified by DNA restriction digestion analysis and DNA sequencing. The recombinant fusion protein was purified using a fast-performance liquid chromatography system (Pharmacia Biotech, Uppsala, Sweden) by affinity chromatography on a HisTrap Chelating HP column with 5 ml bed volume (Amersham Biosciences AB, Uppsala, Sweden). For more experimental details, see Supplementary data.
PSII activity measurements
Steady-state O2 evolution was measured with a Hansatech Clark-type O2 electrode (CB1D; Hansatech, Norfolk, UK) in media containing 50 mM MES–KOH (pH 5.5), 400 mM sucrose and 35 mM NaCl (SMS buffer). The media was depleted of endogenous CO2/HCO3− by bubbling for 1 h with CO2-free air. CO2-free air was generated by passing air through a solution of 50% KOH and a 20-cm layer of ascarite (5–20 mesh particle size; Sigma-Aldrich, Seelze, Germany). Chl concentration during the measurements was 10 μg ml−1. All measurements were conducted at 25°C in the presence of 1 mM 2.b-dichloro-p-benzoquinone (DCBQ) and 0.5 mM FeCN as electron acceptors.
Flash-induced release of O2 was measured polarographically with a bare Pt electrode as described earlier (Clausen and Junge, 2004; Clausen et al, 2004). Both prompt and delayed time-resolved measurements of Chl fluorescence were conducted according to the procedure by Buchta et al (2007). For the DF measurements, dark-adapted samples were exited by a train of saturating nanosecond-laser flashes (FWHM ∼5 ns, λ=532 mn) with a 0.7- s interval between each flash.
Prompt Chl fluorescence was measured as follows. The sample was excited with nanosecond-laser pulses as described above. The time course of the prompt fluorescence was measured with a commercially available instrument (FL 300; Photon Systems Instruments, Brno, Czech Republic) using a pump-probe technique with logarithmically spaced probe pulses (six pulses per decade, 100 μs to 690 ms, λmax=615 nm). The temperature of the sample was 24.5°C.
Supplementary Material
Supplementary Information
Supplementary Figures, Table and Information
Acknowledgments
This work was supported by grants to GS from VR, SSF and the Foundation for International Cooperation in Research and Higher Education (STINT). We are grateful to Professor B Martin and Professor V Hurry for fruitful discussions on the manuscript.
References
- Ananyev G, Nguyen T, Putnam-Evans C, Dismukes GC (2005) Mutagenesis of CP43-arginine-357 to serine reveals new evidence for bicarbonate functioning in the water oxidizing complex of photosystem II. Photochem Photobiol Sci 4: 991–998 [DOI] [PubMed] [Google Scholar]
- Auslander W, Junge W (1975) Neutral red, a rapid indicator for pH-changes in the inner phase of thylakoids. FEBS Lett 59: 310–315 [Google Scholar]
- Bernat G, Morvaridi F, Feyziyev Y, Styring S (2002) pH-dependence of the four individual transitions in the catalytic S-cycle during photosynthetic oxygen evolution. Biochemistry 41: 5830–5843 [DOI] [PubMed] [Google Scholar]
- Berthold DA, Babcock GT, Yocum CF (1981) A highly resolved, oxygen-evolving photosystem II preparations from spinach thylakoid membranes. EPR and electron-transport properties. FEBS Lett 134: 231–234 [Google Scholar]
- Blubaugh DJ, Govindjee (1988) The molecular mechanism of the bicarbonate effect at the plastoquinone reductase site of photosynthesis. Photosyn Res 19: 85–128 [DOI] [PubMed] [Google Scholar]
- Buchta J, Grabolle M, Dau H (2007) Photosynthetic dioxygen formation studied by time-resolved delayed fluorescence measurements—method, rationale, and results on the activation energy of dioxygen formation. Biochim Biophys Acta 1767: 565–574 [DOI] [PubMed] [Google Scholar]
- Carrieri D, Ananyev G, Brown T, Dismukes GC (2007) In vivo bicarbonate requirement for water oxidation by photosystem II in the hypercarbonate-requiring cyanobacterium Arthrospira maxima. J Inorg Biochem 101: 1865–1874 [DOI] [PubMed] [Google Scholar]
- Christen G, Renger G (1999) The role of hydrogen bonds for the multiphasic P680+* reduction by YZ in photosystem II with intact oxygen evolution capacity. Analysis of kinetic H/D isotope exchange effects. Biochemistry 38: 2068–2077 [DOI] [PubMed] [Google Scholar]
- Clausen J, Beckmann K, Junge W, Messinger J (2005a) Evidence that bicarbonate is not the substrate in photosynthetic oxygen evolution. Plant Physiol 139: 1444–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen J, Debus RJ, Junge W (2004) Time-resolved oxygen production by PSII: chasing chemical intermediates. Biochim Biophys Acta 1655: 184–194 [DOI] [PubMed] [Google Scholar]
- Clausen J, Junge W (2004) Detection of an intermediate of photosynthetic water oxidation. Nature 430: 480–483 [DOI] [PubMed] [Google Scholar]
- Clausen J, Junge W, Dau H, Haumann M (2005b) Photosynthetic water oxidation at high O2 backpressure monitored by delayed chlorophyll fluorescence. Biochemistry 44: 1275–1279 [DOI] [PubMed] [Google Scholar]
- Dasgupta J, van Willigen RT, Dismukes GC (2004) Consequences of structural and biophysical studies for the molecular mechanism of photosynthetic oxygen evolution: functional roles for calcium and bicarbonate. Phys Chem Chem Phys 6: 4793–4802 [Google Scholar]
- Dau H (1994) Molecular mechanisms and quantitative models of variable photosystem-II fluorescence. Photochem Photobiol 60: 1–23 [Google Scholar]
- Dau H, Haumann M (2005) Considerations on the mechanism of photosynthetic water oxidation—dual role of oxo-bridges between Mn ions in (i) redox-potential maintenance and (ii) proton abstraction from substrate water. Photosynth Res 84: 325–331 [DOI] [PubMed] [Google Scholar]
- Dau H, Haumann M (2006) Reaction cycle of photosynthetic water oxidation in plants and cyanobacteria. Science 312: 1471–1472 [Google Scholar]
- Dau H, Haumann M (2007) Eight steps preceding O–O bond formation in oxygenic photosynthesis—a basic reaction cycle of the photosystem II manganese complex. Biochim Biophys Acta 1767: 472–483 [DOI] [PubMed] [Google Scholar]
- Dau H, Haumann M (2008) The manganese complex of photosystem II in its reaction cycle—basic framework and possible realization at the atomic level. Coord Chem Rev 252: 273–293 [Google Scholar]
- Diner BA, Petrouleas V (1990) Formation by NO of nitrosyl adducts of redox components of the photosystem-II reaction center. 2. Evidence that HCO3−/CO2 binds to the acceptor-side non-heme iron. Biochim Biophys Acta 1015: 141–149 [Google Scholar]
- Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303: 1831–1838 [DOI] [PubMed] [Google Scholar]
- Finazzi G, Johnson GN, Dall'Osto L, Zito F, Bonente G, Bassi R, Wollman FA (2006) Nonphotochemical quenching of chlorophyll fluorescence in Chlamydomonas reinhardtii. Biochemistry 45: 1490–1498 [DOI] [PubMed] [Google Scholar]
- Fowler CF (1977) Proton evolution from photosystem II. Stoichiometry and mechanistic considerations. Biochim Biophys Acta 462: 414–421 [DOI] [PubMed] [Google Scholar]
- Friso G, Giacomelli L, Ytterberg AJ, Peltier JB, Rudella A, Sun Q, van Wijk KJ (2004) In-depth analysis of the thylakoid membrane proteome of Arabidopsis thaliana chloroplasts: new proteins, new functions, and a plastid proteome database. Plant Cell 16: 478–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56: 99–131 [DOI] [PubMed] [Google Scholar]
- Glasoe PK, Long FA (1960) Use of glass electrodes to measure acidities in deuterium oxide. J Phys Chem 64: 188–190 [Google Scholar]
- Grabolle M, Dau H (2005) Energetics of primary and secondary electron transfer in photosystem II membrane particles of spinach revisited on basis of recombination-fluorescence measurements. Biochim Biophys Acta 1708: 209–218 [DOI] [PubMed] [Google Scholar]
- Hager A, Holocher K (1994) Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a light-dependent pH decrease. Planta 192: 581–589 [Google Scholar]
- Hanson DT, Franklin LA, Samuelsson G, Badger MR (2003) The Chlamydomonas reinhardtii cia3 mutant lacking a thylakoid lumen-localized carbonic anhydrase is limited by CO2 supply to rubisco and not photosystem II function in vivo. Plant Physiol 132: 2267–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haumann M, Bogershausen O, Cherepanov D, Ahlbrink R, Junge W (1997) Photosynthetic oxygen evolution: H/D isotope effects and the coupling between electron and proton transfer during the redox reactions at the oxidizing side of photosystem II. Photosyn Res 51: 193–208 [Google Scholar]
- Haumann M, Junge W (1994) Extent and rate of proton release by photosynthetic water oxidation in thylakoids—electrostatic relaxation versus chemical production. Biochemistry 33: 864–872 [DOI] [PubMed] [Google Scholar]
- Haumann M, Liebisch P, Muller C, Barra M, Grabolle M, Dau H (2005) Photosynthetic O2 formation tracked by time-resolved X-ray experiments. Science 310: 1019–1021 [DOI] [PubMed] [Google Scholar]
- Hillier W, McConnell I, Badger MR, Boussac A, Klimov VV, Dismukes GC, Wydrzynski T (2006) Quantitative assessment of intrinsic carbonic anhydrase activity and the capacity for bicarbonate oxidation in photosystem II. Biochemistry 45: 2094–2102 [DOI] [PubMed] [Google Scholar]
- Huang S, Xue Y, Sauer-Eriksson E, Chirica L, Lindskog S, Jonsson BH (1998) Crystal structure of carbonic anhydrase from Neisseria gonorrhoeae and its complex with the inhibitor acetazolamide. J Mol Biol 283: 301–310 [DOI] [PubMed] [Google Scholar]
- Hwang HJ, Dilbeck P, Debus R, Burnap RL (2007) Mutation of arginine 357 of the CP43 protein of photosystem II severely impairs the catalytic S-state cycle of the H2O oxidation complex. Biochemistry 46: 11987–11997 [DOI] [PubMed] [Google Scholar]
- Joliot P (1968) Kinetic studies of photosystem II in photosynthesis. Photochem Photobiol 8: 451–463 [DOI] [PubMed] [Google Scholar]
- Junge W, Haumann M, Ahlbrink R, Mulkidjanian A, Clausen J (2002) Electrostatics and proton transfer in photosynthetic water oxidation. Philos Trans R Soc Lond B Biol Sci 357: 1407–1417, discussion 1417–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanervo E, Suorsa M, Aro EM (2005) Functional flexibility and acclimation of the thylakoid membrane. Photochem Photobiol Sci 4: 1072–1080 [DOI] [PubMed] [Google Scholar]
- Karge M, Irrgang KD, Renger G (1997) Analysis of the reaction coordinate of photosynthetic water oxidation by kinetic measurements of 355 nm absorption changes at different temperatures in photosystem II preparations suspended in either H2O or D2O. Biochemistry 36: 8904–8913 [DOI] [PubMed] [Google Scholar]
- Karlsson J, Clarke AK, Chen ZY, Hugghins SY, Park YI, Husic HD, Moroney JV, Samuelsson G (1998) A novel alpha-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J 17: 1208–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B, Forbush B, McGloin M (1970) Cooperation of charges in photosynthetic O2 evolution—I. A linear four-step mechanism. Photochem Photobiol 11: 457–475 [DOI] [PubMed] [Google Scholar]
- Kramer DM, Sacksteder CA, Cruz JA (1999) How acidic is the lumen? Photosyn Res 60: 151–163 [Google Scholar]
- Krishtalik LI (1986) Energetics of multielectron reactions. Photosynthetic oxygen evolution. Biochim Biophys Acta 849: 162–171 [Google Scholar]
- Lindskog S (1997) Structure and mechanism of carbonic anhydrase. Pharmacol Ther 74: 1–20 [DOI] [PubMed] [Google Scholar]
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J (2005) Towards complete cofactor arrangement in the 3.0 Angstrom resolution structure of photosystem II. Nature 438: 1040–1044 [DOI] [PubMed] [Google Scholar]
- McConnell IL, Badger MR, Wydrzynski T, Hillier W (2007) A quantitative assessment of the carbonic anhydrase activity in photosystem II. Biochim Biophys Acta 1767: 639–647 [DOI] [PubMed] [Google Scholar]
- Metzner H, Fischer K, Bazlen O (1979) Isotope ratios in photosynthetic oxygen. Biochim Biophys Acta 548: 287–295 [DOI] [PubMed] [Google Scholar]
- Mitra M, Mason CB, Xiao Y, Ynalvez RA, Lato SM, Moroney JV (2005) The carbonic anhydrase gene families of Chlamydomonas reinhardtii. Can J Bot 83: 780–795 [Google Scholar]
- Moroney JV, Bartlett SG, Samuelsson G (2001) Carbonic anhydrases in plants and algae. Plant Cell Environ 24: 141–153 [Google Scholar]
- Moubarak-Milad M, Stemler A (1994) Oxidation-reduction potential dependence of photosystem II carbonic anhydrase in maize thylakoids. Biochemistry 33: 4432–4438 [DOI] [PubMed] [Google Scholar]
- Rappaport F, Blanchard-Desce M, Lavergne J (1994) Kinetics of electron transfer and electrochromic change during the redox transition of the photosynthetic oxygen-evolving complex. Biochim Biophys Acta 1184: 178–192 [Google Scholar]
- Raven JA (1995) Phycological reviews. Photosynthetic and nonphotosynthetic roles of carbonic-anhydrase in algae and cyanobacteria. Phycologia 34: 93–101 [Google Scholar]
- Renger G (2007) Oxidative photosynthetic water splitting: energetics, kinetics and mechanism. Photosyn Res 92: 407–425 [DOI] [PubMed] [Google Scholar]
- Rochaix JD (2002) Chlamydomonas, a model system for studying the assembly and dynamics of photosynthetic complexes. FEBS Lett 529: 34–38 [DOI] [PubMed] [Google Scholar]
- Schlodder E, Witt HT (1999) Stoichiometry of proton release from the catalytic center in photosynthetic water oxidation. Reexamination by a glass electrode study at ph 5.5–7.2. J Biol Chem 274: 30387–30392 [DOI] [PubMed] [Google Scholar]
- Shevela D, Klimov V, Messinger J (2007) Interaction of photosystem II with bicarbonate, formate and acetate. Photosyn Res 94: 247–264 [DOI] [PubMed] [Google Scholar]
- Siegbahn PE, Lundberg M (2006) Hydroxide instead of bicarbonate in the structure of the oxygen evolving complex. J Inorg Biochem 100: 1035–1040 [DOI] [PubMed] [Google Scholar]
- Sinclair J, Arnason T (1974) Studies on a thermal reaction associated with photosynthetic oxygen evolution. Biochim Biophys Acta 368: 393–400 [DOI] [PubMed] [Google Scholar]
- Spetea C, Hideg E, Vass I (1997) Low pH accelerates light-induced damage of photosystem II by enhancing the probability of the donor-side mechanism of photoinhibition. Biochim Biophys Acta 1318: 275–283 [Google Scholar]
- Steffen R, Christen H, Renger G (2001) Time-resolved monitoring of flash-induced changes of fluorescence quantum yield and decay of delayed light emission in oxygen-evolving photosynthetic organisms. Biochemistry 40: 173–180 [DOI] [PubMed] [Google Scholar]
- Stemler A, Babcock GT, Govindjee (1974) The effect of bicarbonate on photosynthetic oxygen evolution in flashing light in chloroplast fragments. Proc Natl Acad Sci USA 71: 4679–4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hunnik E, Amoroso G, Sultemeyer D (2002) Uptake of CO2 and bicarbonate by intact cells and chloroplasts of Tetraedron minimum and Chlamydomonas noctigama. Planta 215: 763–769 [DOI] [PubMed] [Google Scholar]
- van Rensen JJS, Klimov VV (2005) Bicarbonate interactions. In Photosystem II: the Light-Driven Water:Plastoquinone Oxidoreductase, Wydrzynski T, Satoh K (eds), pp 329–345. The Netherlands: Springer [Google Scholar]
- Villarejo A, Shutova T, Moskvin O, Forssen M, Klimov VV, Samuelsson G (2002) A photosystem II-associated carbonic anhydrase regulates the efficiency of photosynthetic oxygen evolution. EMBO J 21: 1930–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O, Krippahl G (1958) Hill-Reaktionen. Z Naturforsch 13B: 509–514 [PubMed] [Google Scholar]
- Wilson KE, Ivanov AG, Oquist G, Grodzinski B, Sarhan F, Huner NPA (2006) Energy balance, organellar redox status, and acclimation to environmental stress. Can J Bot 84: 1355–1370 [Google Scholar]
- Zankel K (1971) Rapid delayed luminescence from chloroplasts: kinetic analysis of components; the relationship to the O2 evolving system. Biochim Biophys Acta 245: 373–385 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Figures, Table and Information