Abstract
Human papillomaviruses (HPVs) are obligate epithelial pathogens and typically cause localized mucosal infections. We therefore hypothesized that T-cell responses to HPV antigens would be greater at sites of pathology than in the blood. Focusing on HPV-16 because of its association with cervical cancer, the magnitude of HPV-specific T-cell responses at the cervix was compared with those in the peripheral blood by intracellular cytokine staining following direct ex vivo stimulation with both virus-like particles assembled from the major capsid protein L1, and the major HPV oncoprotein, E7. We show that both CD4+ and CD8+ T cells from the cervix responded to the HPV-16 antigens and that interferon-γ (IFN-γ) production was HPV type-specific. Comparing HPV-specific T-cell IFN-γ responses at the cervix with those in the blood, we found that while CD4+ and CD8+ T-cell responses to L1 were significantly correlated between compartments (P = 0·02 and P = 0·05, respectively), IFN-γ responses in both T-cell subsets were significantly greater in magnitude at the cervix than in peripheral blood (P = 0·02 and P = 0·003, respectively). In contrast, both CD4+ and CD8+ T-cell IFN-γ responses to E7 were of similar magnitude in both compartments and CD8+ responses were significantly correlated between these distinct immunological compartments (P = 0·04). We therefore show that inflammatory T-cell responses against L1 (but not E7) demonstrate clear compartmental bias and the magnitude of these responses do reflect local viral replication but that correlation of HPV-specific responses between compartments indicates their linkage.
Keywords: mucosal, human papillomavirus, cervical, T-cell immunity
Introduction
Cervical cancer is one of the most common causes of cancer-related deaths in women and is associated with persistent infections with a high-risk subset of human papillomaviruses (HPVs). It is the first cancer recognized by the World Health Organization to be 100% attributable to infection.1 HPV type 16 (HPV-16) is the most prevalent HPV type associated with cervical intraepithelial neoplasia (CIN) and cervical cancer both in South Africa and worldwide, with 50% of women with cervical disease being infected with HPV-16.2,3
HPV infection of the cervix is relatively common in young sexually active women. The majority of these infections are transient and are not clinically evident, with 70–90% of infected women resolving their infections within 12–30 months.4–6 This suggests that host immunity is generally able to clear HPV infection. Women who do not clear their infections are at much higher risk of developing CIN and cervical cancer.6 Patients diagnosed with CIN 1 (mild cervical dysplasia) carry a 1% probability of developing invasive cancer.7 The transition from CIN 1 to CIN 2/3 (moderate/severe cervical dysplasia) occurs quickly, with about 5% of patients diagnosed with CIN 2 progressing to invasive cervical cancer.7 Both cell-mediated immunity (CMI) and antibody responses have been implicated in influencing the persistence or clearance of genital HPV infection.8
HPV does not stimulate an inflammatory response because it uses deliberate strategies to avoid detection.9 HPV causes cell proliferation instead of lysis and does not cause systemic infection.9 As a result, both HPV-specific CMI responses and antibody responses are much more difficult to detect than those elicited by many other viral pathogens.9,10
The mucosal immune system therefore serves a major role in protection against HPV. There are, however, very few studies of mucosal immune responses to HPV infection. Studies on the rate of genital wart clearance have shown that persisting warts are characterized by the absence of immune infiltration.11,12 Wart regression is associated with both CD4+ T-cell and, to a lesser extent, CD8+ T-cell infiltration into the wart stroma and epithelium. Wart infiltrating T cells express both markers of activation and antigenic memory, and express predominantly T helper (Th) 1 type (or proinflammatory) cytokines (interferon (IFN)-γ, tumour necrosis factor-α, and interleukin-12).11
Because HPVs do not have a systemic phase in their infection cycles and are known to cause only localized mucosal infections that are not associated with significant inflammation, we wanted to investigate whether inflammatory (IFN-γ) T-cell responses at the primary site of pathology, the uterine cervix, to HPV antigens L1 and E7 were more prevalent and/or of greater magnitude than those detected in peripheral blood T cells in a cohort of women either with varying grades of CIN (CIN 1 to CIN 2/3) or who had recently cleared their cervical disease (CIN 0). This study focused on HPV-16 infection in women with cervical disease since they have been shown to have a high frequency of high risk HPV infection with HPV-16 being the most prevalent type detected.2,3
Materials and methods
Description of cohort
Thirty-three women were recruited from amongst first time attendees of the Colposcopy Clinic at Groote Schuur Hospital, Cape Town, South Africa. The Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town approved all aspects of the investigation, and written informed consent was obtained from all women before initiation of the study. Samples were not collected from women if: (i) they were menstruating on the day of enrolment; (ii) blood was visible in the cervical area; (iii) if the epithelium appeared disrupted; (iv) if they had known or macroscopically visible vaginal inflammation or sexually transmitted infections; or (v) if they were menopausal.
Specimen collection
From each woman enrolled in the study: (i) a Digene cervical sampler was used to obtain cervical cells from the endocervix for direct ex vivo analysis; (ii) a second Digene cervical sampler was taken for detection of HPV infection and typing; (iii) heparinized peripheral blood specimens were taken for isolation of mononuclear cells for direct ex vivo analysis; and (iv) clotted peripheral blood specimens were taken for measurement of serum antibody responses to HPV-16 virus-like particles (VLPs).
Detection of cervical HPV infection by Digene HC2
Infection at the cervix with ‘high risk’ HPV types was evaluated using Digene HC2 (Digene Corporation, Gaithersburg, MD) as previously described.2 Digene HC2 detects (but does not differentiate) 13 high risk HPV types including HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, and -68.
Typing of HPV cervical infection by Roche linear array HPV genotyping assay
HPV typing was performed using a Roche linear array HPV genotyping assay (kindly supplied by Dr Janet Kornegay, Roche) according to the manufacturers' instructions. The Roche linear array HPV genotyping assay has the capacity to detect 37 different HPV genotypes (high risk types: HPV-16, -18, -26, -31, -33, -35, -39, -45, -51, -52, -53, -56, -58, -59, -66, -67, -68, -69, -70, -73, -82, -IS39 (n = 22); low risk types: HPV-6, -11, -40, -42, -54, -55, -61, -62, -64, -71, -72, -81, -83, -84, -CP6108 (n = 15)).
Detection of HPV-16 L1-specific serum antibody responses by enzyme-linked immunosorbent assay (ELISA)
Serum was collected from each participant for evaluation of seropositivity to HPV-16 virus-like particles (VLPs) and stored at −20° until processing. HPV-16 serum antibodies were detected according to the method described by Marais et al.13 Cut-off levels for HPV-16 VLP sero-positivity were calculated using the mean OD492nm value of children controls (minus outliers) plus 2 standard deviations.13
Collection and processing of cervical specimens
Cervical cells were collected using a Digene cervical cytobrush as described previously.14 The cytobrush was inserted within the cervical os and rotated 360°. The cytobrush was immediately placed in a 10-ml transport tube containing 3 ml ice-cold 10% fetal calf serum (FCS) RPMI-1640, 50 U/ml penicillin, 50 mg/ml streptomycin and 2·5 µg/ml amphotericin B. Specimens were stored on ice and processed within 4 hr of sampling. Cervical cells were isolated from the cytobrush by vigorously rotating it against the sides of the transport tube after incubating the sample with 5 mm dl-dithiothreitol (Sigma, St Louis, MO) at 37° for 15 min (to reduce the mucus component of the sample). The cell suspension was washed twice in RPMI supplemented with 10% heat-inactivated FCS. Cervical specimens visibly contaminated with blood were excluded from further analysis. Red blood cell contamination of cervical specimens was further assessed using CD235 staining for glycophorin A (PharMingen, San Diego, CA) and fluorescence-activated cell sorting (FACS) analysis. Only cytobrush specimens with less than ∼0·0005% peripheral blood mononuclear cells (PBMC) contamination were included in the study (data not shown). To assess the suitability of cervical samples for further in vitro analysis by intracellular cytokine staining, the number of CD3+ T cells present in the total cervical sample was investigated by FACS analysis. Samples with 30 000 CD3+ T cells per cytobrush were processed further. This cut-off was established to ensure statistical validity of FACS rare-event analyses. Cervical samples were adjusted to between 1·5 × 105 and 1 × 106 CD3+ cells/ml (depending on the CD3+ yields per cytobrush) for direct ex vivo stimulation.
Isolation PBMC
Heparinized peripheral blood specimens (for isolation of PBMC) were obtained from all women by venepuncture. PBMC were isolated by Ficoll gradient density centrifugation and T-cell responses were evaluated on the day of isolation.
HPV-16 L1 and E7 antigen preparation
HPV-16 virus-like particles (VLPs) was kindly provided by MedImmune Inc. (Gaithersburg, MD). The quality of the VLP preparation was confirmed by polyacrylamide gel electrophoresis (PAGE) and Western blot, electron microscopy and ELISA. Western blots and ELISA tests used V5 and J4, mouse monoclonal antibodies directed against conformational and linear epitopes, respectively (kindly provided by Dr Neil Christenson, The Jake Gitlen Cancer Research Institute, PA). HPV-16 E7 gene (Rb–) was obtained from John Schiller (Laboratory of Cellular Oncology, National Cancer Institute, Bethesda, MD), cloned into pProEx™ HTa Prokaryotic Expression Vector (Life Technologies, GibcoBRL, Grand Island, NY) and transformed into competent Escherichia coli (strain DH5α). Histidine-tagged E7 protein was induced with 0·6 mm isopropyl-beta-D-thiogalactopyranoside (IPTG) for 3 hr at 37° once the cultures reached an A590 of 0·5–1·0. The cells were harvested by centrifugation of 3800 g for 10 min (GSA rotor) and the cells were resuspended in 4 volumes lysis buffer (50 mm Tris pH 8·5, 5 mmβ-mercaptoethanol) with addition of 800 µg lysozyme and 10 µl of 40 mm phenylmethylsulphonyl fluoride (PMSF) per gram of cells pelleted. Pellets were resuspended and were incubated 20 min on ice, and then Triton-X-100 was added to final concentration of 1%. Cells were kept at 37° until solution became viscous and this was followed by DNA and RNA degradation with 5 µg DNAse I and 50 µg RNAse A per ml of lysis mix. Samples were incubated at room temperature for 30 min, followed by centrifugation for 15 min at 4° in a microfuge. E7 was purified from this supernatant using a batch wise purification protocol with Ni-NTA resin (QIAGEN GmBH, Hilden, Germany) according to the manufacturer's instructions. Purified E7 was dialysed against PBS at 4° overnight. To confirm the identity of the protein, E7 was Western blotted and detected with anti-E7 antibody H16E7 (provided by Neil Christensen).
Detection of HPV-16 L1- and E7-specific intracellular cytokine responses
Cervical cytobrush-derived cells and PBMC were stimulated with HPV-16 antigens and then stained for intracellular IFN-γ production essentially as described by Passmore et al.14 For intracellular cytokine staining, PBMC (1 × 106 cell/ml) or cervical cells (approximately 0·15–1 × 106 lymphocytes/ml) were stimulated in 100 µl cultures in a 96-well plate format with either PMA/ionomycin (25 ng/ml; 1 µg/ml, 4 hr; Sigma-Aldrich), HPV-16 VLPs (15 µg/ml, 20 hr), HPV-16 E7 (10 µg/ml, 20 hr) or untreated (20 hr) at 37°, 5%CO2. Brefeldin A (10 µg/ml; Sigma) was added after the first hour of incubation for PMA/ionomycin stimulation and for the last 5 hr of stimulation for unstimulated, HPV-16 L1 and E7 stimulation. Following stimulation, cells were washed once in 10% FCS phosphate-buffered saline (PBS; 0·01% NaN3), fixed and permeabilized using BD CytoFix/CytoPerm for 10 min at room temperature, washed once with 0·1% Saponin (Fluka; Sigma-Aldrich (Pty) Ltd., Astor Manor, South Africa) PBS (containing 5% FCS and 0·01% NaN3) and then stained with allophycocyanin (APC)-labelled anti-CD3 (Becton-Dickinson, San Jose, CA), fluoroscein isothiocyanate (FITC)-labelled anti-CD8 (Becton-Dickenson), and phycoerythrin (PE)-conjugated anti-IFN-γ (PharMingen) for 30 min on ice. Cells were finally washed once with 0·1% Saponin PBS buffer, once with PBS and fixed with 1% paraformaldehyde PBS or BD Cell Fix. Cell fluorescence was measured using a FACSCalibar flow cytometer and data were analysed using either CellQuest (Becton Dickinson) or FlowJo (Tree Star, Inc., Ashland, OR) software.
Statistical analysis
Statistical analyses were performed using the commercial statistical software package Statistica®. As indicated in the results, Mann–Whitney U-test was applied for independent sample comparisons, The Wilcoxon rank test was applied for matched paired comparisons, Spearman's rank correlation was applied for correlation comparisons and Chi-squared test was applied for data in binary format. P-values ≤0·05 were considered significant.
Results
Description of the cohort
The 33 women included into this study were referred to the Colposcopy Clinic based on cytological diagnoses of cervical disease (CIN 1 to CIN 3). Upon follow-up at the clinic, 9 of the 33 women were confirmed negative for cervical disease (CIN 0) by both colposcopy and histology and had either cleared their cervical disease by follow-up or the primary cytological diagnosis was not supported by histology. Twenty-four of the 33 women had histologically confirmed CIN 1 (10/33) or CIN 2/3 (14/33). Table 1 provides the details of these patient groups. Women recruited into each group did not differ significantly by age, current cervical infection with high-risk HPV (HPV DNA positive determined by Digene HC2), relative HPV viral load (relative light units as determined by Digene HC2), infection with HPV-16 at the cervix (determined by Roche linear array HPV genotyping assay), or previous exposure to HPV-16 (determined by HPV-16 L1-specific serum immunoglobulin G (IgG) responses).
Table 1.
Clinical details of patients included in the study
| Cervical HPV-16 infection status | CIN status | Patient ID | Age | Cervical HPV types detected (Roche Linear Array Assay) | HPV-16 VLP serum IgG response1 | HPV-16 VLP IgG level of recognition (OD492nm) |
|---|---|---|---|---|---|---|
| HPV-16 DNA Positive | CIN 0 | 47 | 45 | 16, 69, 83 | − | 0·085 |
| 88 | 22 | 11, 16, 18, 35, 42, 55 | − | 0·328 | ||
| 58 | 30 | 16 | + | 1·357 | ||
| CIN 2/3 | 49 | 35 | 16, 18, 35, 45, 52, 70 | − | 0·251 | |
| 55 | 42 | 16 | − | 0·203 | ||
| 11 | 28 | 16 | + | 0·860 | ||
| 17 | 36 | 16 | + | 1·623 | ||
| 43 | 38 | 16, 33 | + | 0·804 | ||
| 48 | 38 | 16, 18, 31, 53, 56 | + | 0·714 | ||
| HPV-16 DNA Negative | CIN 0 | 34 | 28 | 51 | − | 0·474 |
| 35 | 25 | 26, 73 | − | 0·515 | ||
| 1 | 50 | 33 | + | 1·620 | ||
| 10 | 25 | 51 | + | 2·062 | ||
| 18 | 24 | 6, 33 | + | 1·432 | ||
| 28 | 34 | 45 | + | 1·040 | ||
| 41 | 41 | 35 | + | 1·570 | ||
| 45 | 29 | None2 | + | 0·918 | ||
| 61 | 28 | 33, 52, 68 | + | 1·204 | ||
| CIN 1 | 7 | 37 | None | − | 0·274 | |
| 89 | 35 | 35, 62 | − | 0·530 | ||
| 13 | 23 | 53, 51, 39 | + | 1·700 | ||
| 33 | 38 | 59 | + | 1·526 | ||
| 84 | 40 | 56 | + | 0·846 | ||
| 91 | 36 | 51, 53, 54, 59, 68 | + | 1·056 | ||
| CIN 2/3 | 8 | 42 | 68 | − | 0·131 | |
| 60 | 31 | 35 | − | 0·356 | ||
| 62 | 35 | 35 | − | 0·394 | ||
| 5 | 42 | 52 | − | 0·151 | ||
| 29 | 31 | 18, 35, 39 | − | 0·234 | ||
| 14 | 52 | 35 | − | 0·754 | ||
| 2 | 49 | 73 | + | 0·690 | ||
| 66 | 34 | 31, 33 | + | 1·472 | ||
| 68 | 34 | None | + | 0·974 |
Cut-off values for serum IgG positivity = 0·54. Cut-off levels for HPV-16 VLP sero-positivity were calculated using the mean OD492nm value of children controls (minus outliers) plus 2 standard deviations13.
None of the 37 types probed using the Roche Assay were detected.
Prevalence of HPV types in cervical specimens selected for the study
The overall prevalence of high-risk HPV types in this cohort was found to be 66·67% (22/33; Digene HC2; data not shown). HPV-16 was the most prevalent HPV type infecting the cervices of these women (27·3%, 9/33) with HPV-35 (21·2%, 7/33), HPV-33 (15·2%, 5/33) and HPV-51 (12·1%, 4/33) being the next most prevalent (Table 1). Thirteen of the 33 women (39·4%) were infected with multiple (more than two) HPV types.
HPV-16 L1-specific serum IgG responses as evidence of previous infection
As an indicator of previous infection with HPV-16, serum IgG levels against HPV-16 L1 were determined (Table 1). HPV-16 L1 seropositivity was detected in 20/33 (60·6%) of the women. Of the patients who were found to be antibody positive to HPV-16, only 25·0% (5/20) were currently infected with HPV-16 at the cervix indicating that seropositivity to HPV is a better indicator of infection history than current exposure. Conversely, of the patients that were currently infected with HPV-16, only 55·6% (5/9) also had clearly detectable levels of antibodies to HPV-16 L1.
Direct ex vivo stimulation with HPV-16 L1 and E7 antigens
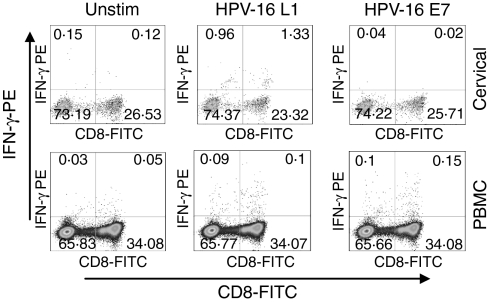
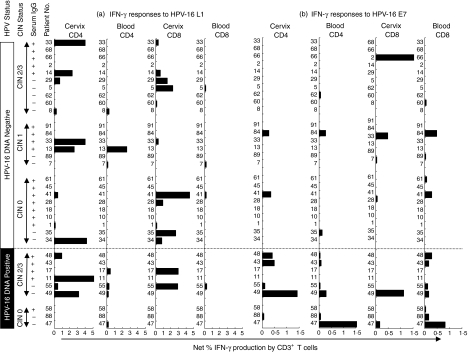
In order to investigate the comparative magnitude and HPV type specificity of T-cell immune responses in cervical and blood samples from this cohort, paired cervical and PBMC T cells were stimulated directly ex vivo with HPV-16 specific antigens (major capsid protein L1 within virus-like particles (VLPs) and major oncoprotein E7) and stained for intracellular IFN-γ cytokine expression. Representative plots from patient 55 (CIN 2/3; infected with HPV-16) are shown in Fig. 1. This donor generated clearly detectable HPV-16-specific responses to HPV-16 L1 in the cervical sample and to both HPV antigens in blood. Figure 2 shows the net frequency of IFN-γ production by CD4 and CD8 T cells at the cervix and in blood for each of the 33 women. Net IFN-γ production for each donor is defined as the frequency of IFN-γ producing cells above the unstimulated negative control (Fig. 1 left panels). Protein antigens such as the recombinant HPV L1 and E7 used in this study are recognized to be effective at stimulating CD4 T-cell responses. There is published evidence, however, to suggest that L1 assembled into virus-like particles is a potent inducer of CD8 cytotoxic T lymphocyte (CTL) responses and that cross-presentation account for this.15,16 In support of this, using HPV-16 L1 and E7-expanded CTL lines, we have confirmed that both L1 and E7 antigens used in this study show comparable efficacy at activating CD8 and CD4 T cells to express both CD69 and IFN-γ (data not shown).
Figure 1.
Representative plots of cervical and blood mononuclear cells from a patient with CIN 2/3 stimulated directly ex vivo with HPV-16 L1 and E7 and stained for intracellular IFN-γ production. IFN-γ production by cervical (upper panel) and blood CD3+ cells (lower panel) either unstimulated or following stimulation with HPV-16 antigens L1 (VLPs) and E7. Each plot was gated on CD3+ cells and then analysed for CD8+ and IFN-γ production.
Figure 2.
HPV-16 (a) L1- and (b) E7-specific IFN-γ production by CD4+ and CD8+ T cells from all donors following direct ex vivo stimulation. Individual donors have been grouped according to whether they were (i) actively infected with HPV-16 (DNA Positive; bottom panel) or (ii) were negative for HPV-16 DNA (top panel). Within each of these groups, individual donors have been grouped according to CIN status and serum IgG status. Each bar represents an individual's net percentage IFN-γ production by either CD4+ or CD8+ CD3+ T cells. Net percentage IFN-γ responses were calculated by subtracting background percentage IFN-γ production by unstimulated cells.
Impact of current HPV-16 infection at the cervix on cervical responses
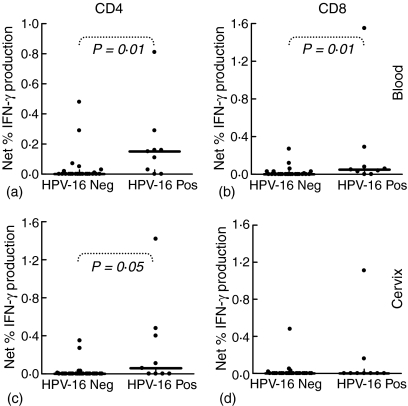
To determine whether the presence of HPV-16 DNA at the cervix influences the frequency of T-cell responses detected in these patients, the ability of T cells to produce IFN-γ in response to HPV-16 antigens were compared in women with current HPV-16 cervical infections (HPV-16 DNA positive) and in those previously infected with HPV-16 (HPV-16 serum IgG positive) (Figs 2 and 3). Both in the blood and at the cervix, CD4+/IFN-γ responses to HPV-16 E7 were significantly increased in HPV-16 DNA positive women compared with HPV-16 DNA negative but antibody positive women (P = 0·01 in the blood and P = 0·05 at the cervix; Fig. 3(a) and (c)). No significant difference was observed in the frequency of CD4+ T cells producing IFN-γ in response to HPV-16 L1 protein if the patients were stratified according to current HPV-16 infection. Similarly, CD8+/IFN-γ T cell responses to HPV-16 E7 in blood but not at the cervix were significantly higher in HPV-16 DNA positive women compared with women previously infected with HPV-16 (HPV-16 DNA negative but antibody positive; P = 0·01; Fig. 3b). In response to HPV-16 L1, CD8+/IFN-γ T-cell responses in blood and at the cervix were not significantly higher in HPV-16 DNA positive women than in women previously infected with HPV-16 (HPV-16 DNA negative, antibody positive; Fig. 2). At the cervix, active HPV-16 infection was found to be associated with a significant increase in CD4+ IFN-γ responses to E7 but not to L1, supporting a stimulatory role for current HPV-16 infection on HPV-16-specific T-cell immunity. The absence of significantly increased inflammatory responses to L1 in either T-cell subset, however, does suggest that current mucosal HPV replication does not necessarily drive a stronger mucosal T-cell response.
Figure 3.
Impact of current HPV-16 infection at the cervix on inflammatory T cell responses to HPV-16 E7. Blood (a, b) and cervical (c, d) CD4+ and CD8+ T cell IFN-γ production following stimulation with HPV-16 E7 in women either having no HPV-16 DNA at the cervix (HPV-16 Neg) or with active HPV-16 infection (HPV-16 DNA Pos). Each (?) represents an individual's net percentage IFN-γ production by either CD4+ or CD8+ T cells. Net percentage IFN-γ responses were calculated by subtracting background percentage IFN-γ production by unstimulated cells. The solid lines indicate median response indices for each group. P-values were calculated using the Mann–Whitney U-test (Statistica®) and P-values = 0·05 were considered significant.
Comparison of T-cell response magnitudes between the cervix and blood
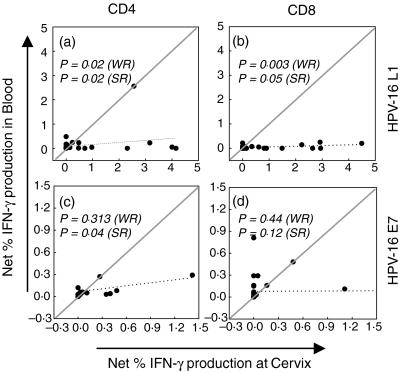
To compare the magnitude of HPV-specific T-cell responses present at the cervix with those detected in the blood, CD4+ and CD8+ T-cell responses in these two separate immunological compartments were directly compared (Fig. 4). The magnitude of net IFN-γ responses by both CD4 and CD8 T cell to L1 were significantly higher at the cervix than in peripheral blood (Fig. 4a; P = 0·02 for CD4 and P = 0·003 for CD8, Wilcoxon rank tests) but there was still a significant correlation between responses to L1 in the blood and at the cervix (P = 0·02 for CD4 and P = 0·05 for CD8; Spearman rank correlations). The magnitude of responses to E7 antigen in blood and at the cervix, however, were comparable between compartments (Fig. 4c, d) and significantly correlated for CD4 T cells only (P = 0·04 for CD4; Spearman rank correlation).
Figure 4.
Correlation of cervical and blood CD4+ (a and c) and CD8+ (b and d) HPV-16 L1 and E7 specific T-cell response magnitudes. Each (•) represents an individual's net percentage IFN-γ production by either CD4+ or CD8+ T cells. Net percentage IFN-γ responses were calculated by subtracting background percentage IFN-γ production by unstimulated cells. The dotted line indicates the linear regression. The solid line has a slope = 1 and y-intercept = 0. To test for differences in response magnitudes between compartments, Wilcoxon rank (WR) tests for paired non-parametric data was used and these P-values have been suffixed with (WR). To test for correlation between compartments, the Spearman rank (SR) test was used and significant P-values have been suffixed with (SR). P-values = 0·05 were considered significant and appear in bold text.
The correlation between inflammatory T-cell responses to HPV antigens (CD4+ responses to L1 and E7 and CD8+ responses to L1 only) at the cervix and in blood indicates that these responses in the different compartments may be codependent. The significantly higher magnitude of IFN-γ responses to L1 at the cervix relative to response in the blood does, however, indicate an important compartmental bias in the HPV antigens being recognized. With respect to certain HPV antigens, localized mucosal (cervical) viral replication does appear to be associated with significantly increased T-cell inflammatory responses.
Discussion
Studies on individuals infected with human immunodeficiency virus (HIV) have demonstrated that identical HIV-specific CD8+ T cells (based on T-cell receptor usage and antigen epitope specificity) are found in the cervical and blood compartments.17,18 In HIV, however, mucosal infections rapidly establish productive systemic infections during the early phases of disease19 and there is convincing evidence that the mucosal and systemic compartments are linked with memory T cells trafficking between these sites during the course of infection.17–21 In contrast, HPVs do not have a systemic phase in their infection cycles and are known to cause only localized mucosal infections. We hypothesized that cervical T-cell responses to HPV antigens would be more prevalent and/or of greater magnitude than responses in the blood.
We show that both CD4+ and CD8+ T cells responded to HPV-16 antigens11,12,16,22,23 and that the frequency of IFN-γ producing T cells to E7 antigen was generally higher in women with current HPV-16 infection (DNA+) than those previously infected with HPV-16 (DNA– but IgG+). In comparison to E7 responses, the net frequency of both CD4 and CD8 T-cell responses to L1 antigen was comparable between patient groups and did not seem to be associated with active HPV-16 infection (DNA positive).
We clearly demonstrate here that inflammatory responses to the major capsid protein L1 (IFN-γ production) in both T-cell subsets were significantly greater in magnitude at the cervix than they were in the blood, suggesting that localized replication and expression of HPV capsid proteins is associated with increased local T-cell recognition. For the major oncoprotein E7, however, both CD4+ and CD8+ responses were of similar magnitude at the cervix and blood. This comparative magnitude of responses to E7 at the cervix and in the blood occurs despite the highly localized mucosal expression of this oncoprotein.
This study is the first to have investigated cervical HPV-specific T-cell responses by direct ex vivo stimulation of cytobrush-derived cervical lymphocytes. Despite the obvious role of mucosal surfaces in the sexual transmission of high-risk HPV types, the majority of published HPV vaccine trials have not included assessment of mucosal immune responses. We have found our methodology to be well tolerated (data not shown) and relatively non-invasive. This, together with the ease of the HPV-specific mucosal immune assays described, should make such an approach a particularly relevant addition to HPV vaccine trial protocols.
In summary, this study has compared the frequency of HPV-16-specific T-cell responses to L1 and E7 at the cervix and in peripheral blood. In support of our hypothesis, we found that the frequencies of T-cell responses to L1 were significantly elevated at the cervix compared with in the blood but that responses in these two distinct immunological compartments were significantly correlated.
Acknowledgments
We thank Roche Molecular Systems for providing the Roche linear array HPV genotyping assay reagents. We are grateful to Dr Darren Martin and Dr Wendy Burgers for critically reading this manuscript and useful suggestions. This work was supported by grants from the (MRC) SA, UCT FRC, CANSA and PRF SA.
References
- 1.Bosch FX, Munoz N. The viral etiology of cervical cancer. Virus Res. 2002;89:183–9. doi: 10.1016/s0168-1702(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 2.Kay P, Soeters R, Nevin J, Denny L, Dehaeck CM, Williamson A. High prevalence of HPV 16 in South African women with cancer of the cervix and cervical intraepithelial neoplasia. J Med Virol. 2003;71:265–73. doi: 10.1002/jmv.10479. [DOI] [PubMed] [Google Scholar]
- 3.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 4.Evander M, Edlund K, Gustafsson A, Jonsson M, Karlsson R, Rylander E, Wadell G. Human papillomavirus infection is transient in young women: a population-based cohort study. J Infect Dis. 1995;171:1026–30. doi: 10.1093/infdis/171.4.1026. [DOI] [PubMed] [Google Scholar]
- 5.Herrero R, Hildesheim A, Bratti C, et al. Population based study of HPV infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2002;92:464–74. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- 6.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. New Engl J Med. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 7.Pinto AP, Crum CP. Natural history of cervical neoplasia. defining progression and its consequence. Clin Obstet Gynecol. 2000;43:352–62. doi: 10.1097/00003081-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Bontkes HJ, de Gruijl TD, Walboomers JMM, et al. Immune responses against HPV type 16 VLPs in a cohort study of women with CIN, II. Systemic but not local IgA responses correlate with clearance of HPV-16. J Gen Virol. 1999;80:409–17. doi: 10.1099/0022-1317-80-2-409. [DOI] [PubMed] [Google Scholar]
- 9.Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Nat Rev Immunol. 2004;4:46–54. doi: 10.1038/nri1260. [DOI] [PubMed] [Google Scholar]
- 10.Tindle RW. Immune evasion in HPV-associated cervical cancer. Nat Rev Cancer. 2002;2:59–65. doi: 10.1038/nrc700. [DOI] [PubMed] [Google Scholar]
- 11.Nicholls PK, Moore PF, Anderson DM, Moore RA, Parry NR, Gough GW, Stanley MA. Regression of canine oral papillomavirus is associated with infiltration of CD4 and CD8 lymphocytes. Virology. 2001;283:31–9. doi: 10.1006/viro.2000.0789. [DOI] [PubMed] [Google Scholar]
- 12.Stanley M. Immunobiology of papillomavirus infections. J Reprod Immunol. 2001;52:45–59. doi: 10.1016/s0165-0378(01)00113-9. [DOI] [PubMed] [Google Scholar]
- 13.Marais DJ, Rose RC, Williamson A. Age distribution of antibodies to human papillomavirus in children, women with cervical intraepithelial neoplasia and blood donors from South Africa. J Med Virol. 1997;51:126–31. doi: 10.1002/(sici)1096-9071(199702)51:2<126::aid-jmv7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Passmore JS, Burch V, Shephard EV, Marais DJ, Allan B, Kay P, Rose R, Williamson A. Single cell cytokine analysis allows detection of cervical T cell responses against human papillomavirus (HPV) type 16, L1 in women infected with genital HPV. J Med Virol. 2002;67:234–40. doi: 10.1002/jmv.2212. [DOI] [PubMed] [Google Scholar]
- 15.Yan M, Peng J, Jabbar IA, Liu X, Filgueira L, Frazer IH, Thomas R. Despite differences between dendritic cells and Langerhans cells in the mechanism of papillomavirus-like particle antigen uptake, both cells cross-prime T cells 2004. Virology. 2004;324:297–310. doi: 10.1016/j.virol.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 16.Rudolf MP, Nieland JD, DaSilva DM, Velders MP, Muller M, Greenstone HL, Schiller JT, Kast WM. Induction of HPV16 capsid protein-specific human T cell responses by virus-like particles. Biol Chem. 1999;380:335–40. doi: 10.1515/BC.1999.045. [DOI] [PubMed] [Google Scholar]
- 17.Musey L, Ding Y, Cao J, Lee J, Galloway C, Yuen A, Jerome KR, McElrath MJ. Ontogeny and specificities of mucosal and blood human immunodeficiency virus type 1-specific CD8+ cytotoxic T lymphocytes. J Virol. 2003;77:291–300. doi: 10.1128/JVI.77.1.291-300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibarrondo FJ, Anton PA, Fuerst M, et al. Parallel HIV-specific CD8+ T-lymphocyte responses in blood and mucosa during chronic infection. J Virol. 2005;79:4289–97. doi: 10.1128/JVI.79.7.4289-4297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller CJ, Li Q, Abel K, et al. Propagation and dissemination of infection after vaginal transmission of SIV. J Virol. 2005;79:9217–27. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandtzaeg P, Farstad IN, Haraldsen G. Regional specialization in the mucosal immune system: primed cells do not always home along the same track. Immunol Today. 1999;20:267–77. doi: 10.1016/s0167-5699(99)01468-1. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds MR, Rakasz E, Skinner PJ, et al. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol. 2005;79:9228–35. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bontkes HJ, de Gruijl TD, Walboomers JMM, van den Muysenberg AJ, Gunther AW, Scheper RJ, Meijer CJ, Kummer JA. Assessment of cytotoxic T-lymphocyte phenotype using the specific markers granzyme B and TIA-1 in cervical neoplastic lesions. Br J Cancer. 1997;76:1353–60. doi: 10.1038/bjc.1997.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi A, Greenblatt RM, Anastos K, et al. Functional attributes of mucosal immunity in CIN and effects of HIV infection. Cancer Res. 2004;64:6766–74. doi: 10.1158/0008-5472.CAN-04-1091. [DOI] [PubMed] [Google Scholar]