Abstract
Tacrolimus is a widely used immunosuppressive agent. Although T cells are the main targets of these pharmacological drugs, antigen presentation may also be affected. Among antigen-presenting cells, plasmacytoid dendritic cells (PDCs) are the main source of type I interferons upon microbial challenge, and are involved in several diseases and autoimmune disorders. The aim of this study was to evaluate whether tacrolimus can modulate the function of PDCs in vitro. Maturation and function of PDCs was determined using flow cytometry, enzyme-linked immunosorbent assay and cytometry bead arrays. The effect of tacrolimus on PDCs was observed mainly when the cells were pretreated with the immunosuppressive agent before activation. Upon dinucleotide–oligodeoxynucleotide (CpG–ODN) activation, tacrolimus pretreated PDCs showed a significant reduction in the surface expression of co-stimulatory molecules and human leucocyte antigen D-related (HLA-DR) and secreted reduced levels of tumour necrosis factor (TNF)-α. These results show that tacrolimus treatment of PDCs impairs CpG-induced activation, which could affect the outcome of the immune response.
Keywords: autoimmunity, immunosupression, plasmacytoid dendritic cells, tacrolimus, transplantation
Introduction
Immunosuppressive agents (IAs) are used currently to reduce allograft rejection following organ transplantation1,2 and also in the treatment of several autoimmune disorders and inflammatory diseases, such as rheumatoid arthritis and atopic dermatitis.3,4 One of the most widely used IAs is tacrolimus, a macrolide lactone antibiotic obtained from a Japanese fungus (Streptomyces tsukuabensis). Similar to cyclosporin A, the main effect of tacrolimus in T lymphocytes is the inhibition of the calcineurin activity, although it binds to FKBP12, whereas cyclosporin binds to cyclophilin A.5 These intracellular proteins form a complex with calcium, calmodulin and calcineurin, inhibiting the phosphatase activity of calcineurin. One of the final consequences is the sequestration of phosphorylated-NF-AT in the cytoplasm and the inhibition of interleukin (IL)-2 secretion by T cells. Several authors have pointed out that IAs, including tacrolimus, may be also affecting the immune response at the level of antigen presentation,6,7 especially in dendritic cells (DCs).8–13 In particular, CD34+-derived DCs cultured in the presence of tacrolimus showed a poor capacity to stimulate allogeneic T cell responses and secreted low levels of IL-12.14 Also, monocyte-derived DCs (MDDCs) cultured in the presence of tacrolimus display a reduced allostimulatory capacity and impaired cytokine production, including IL-12, IL-6 and tumour necrosis factor (TNF)-α,15,16 possibly affecting the nuclear factor (NF)-κB translocation to the nucleus as shown in other cell types.17
The effect of different IAs has been studied mainly on myeloid DCs. However, DCs form a heterogeneous family represented by two main populations, the myeloid and the plasmacytoid subsets.18,19 Plasmacytoid DCs (PDCs) are the main producers of type I interferons (IFN) upon microbial infection, linking innate and adaptative immune responses.20 In vitro, PDC may be activated by CD40 ligation and/or dinucleotide (CpG) oligodeoxynucleotide (CpG–ODN),21 inducing different types of responses, including peripheral tolerance.22,23 CpG oligodeoxynucleotides are often used to induce PDC activation via recognition of Toll-like receptor 9 (TLR9). It has been shown that diverse CpG–ODNs may induce a different type of maturation/activation on PDCs, especially with regard to IFN-α secretion.21 PDCs may migrate constitutively to lymph nodes in steady-state conditions.24 However, PDCs have been also found in different tissues upon inflammation and in some autoimmune disorders, such as rheumatoid and psoriatic arthritis.25 Importantly, autoimmune disorders such as systemic lupus erythematosus (SLE)26,27 and primary Sjögren's syndrome28 are also related to PDC function. This relation is based mainly in the capacity of PDCs to secrete type I IFN, the presence of factors inducing this function − such as anti-dsDNA antibodies29 in the serum of autoimmune patients30– and the consistent up-regulation of IFN-regulated genes observed in SLE patients.31 Also, the induction of SLE-like syndromes in patients after type I IFN treatment further confirmed the role of the cytokines and PDCs in the etiology of the disorder. Often, patients affected by lupus and other autoimmune diseases are treated with corticosteroids, which have been shown to reduce both the number of circulating cells and the ability to produce IFN per PDC.32 In this regard, a recent study exposed the role of dexamethasone in PDC-enhanced apoptosis and suppression of differentiation from haematopoietic precursors.33
Due to the implication of PDCs in several autoimmune disorders and their essential role in innate and adaptative immunity, our aim was to investigate whether tacrolimus could modulate PDC function. Our results show that tacrolimus may affect the immune response induced by PDCs through co-stimulatory blockade and impairment in cytokine secretion.
Materials and methods
Cells
Human peripheral blood mononuclear cells (PBMC) were isolated from apheresis products of healthy blood donors from the Blood and Tissue Bank (BTB) by Ficoll-Paque density gradient centrifugation (Lymphoprep, Axis Shield, Oslo, Norway). Blood PDCs were enriched from PBMC using the BDCA-4 cell isolation kit and the Automacs device (Miltenyi Biotec, Bergisch Gladbach, Germany). In some experiments PDCs were obtained by cell sorting, as reported previously.34 In all samples, the purity and viability of the enriched PDCs was over 90%, as assessed by expression of specific markers and trypan blue exclusion or annexin V + 7-actinomycin D labelling (BD Biosciences, Oxford, UK), respectively. Naive cord blood T cells (CbT) were obtained from umbilical cord blood units (supplied by the Cord Bank of Barcelona) rejected for banking owing to their low volume. Cord blood mononuclear cells were obtained as in PBMCs and CD3+ T lymphocytes were purified by negative selection (Miltenyi Biotec). The purity of the CbT cells was 90–95% in all experiments.
Oligodeoxynucleotides
Endotoxin-free phosphorothioate-modified stimulatory CpG–ODNs (CpG-2216 and CpG-2006) (Invitrogen Corporation, Paisley, UK) have been described previously.21 CpG–ODNs were resuspended in TE buffer, diluted in phosphate-buffered saline (PBS) and used as indicated.
Monoclonal antibodies
The following murine monoclonal antibodies (mAbs) and isotype controls were used: peridinin chlorophyll protein (PerCP)-labelled CD3 and human leucocyte antigen D-related (HLA-DR); fluorescein isothiocyanate (FITC)-labelled CD4; phycoerythrin (PE)-labelled CD11c, CD80, CD83, CD86 and CD123 (BD Biosciences); allophycocyanin (APC)-labelled IFN-γ (Caltag Laboratories, Hamburg, Germany); FITC-labelled anti-BDCA2 (Miltenyi Biotec); and RPE-Cy5-labelled CD14, CD19 (Serotec Ltd, Kidlington, UK).
Immunostaining and flow cytometry
Cells were washed, resuspended in 50 µl of buffer [PBS + 0·1% bovine serum albumin (BSA)] and incubated with mAbs for 20 min at 4°. Cells were then washed, resuspended in PBS and analysed in a FACSCalibur flow cytometer using the standard CellQuest™ acquisition software (BD Biosciences, Oxford, UK). Samples were gated using forward (FSC) and side (SSC) scatter to exclude dead cells and debris.
Cell culture media and reagents
A minimum of 50 000 PDCs were cultured in 200 µl in flat-bottomed 96-well plates (Nunc, Roskilde, Denmark) containing RPMI-1640 (Gibco, Invitrogen Corporation, Paisley, UK) supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS) (Gibco), 2 mm l-glutamine (Sigma-Aldrich, St Louis, MO), 100 U/ml penicillin (Cepa SL, Madrid, Spain), 100 µg/ml streptomycin (Laboratorios Normon SA, Madrid, Spain), 250 ng/ml Fungizone® (Sigma-Aldrich) and recombinant human IL-3 (Peprotech, London, UK) used at 25 ng/ml. Tacrolimus (PROGRAF® Fujisawa Ireland Limited, Killorglin, Ireland) was used mainly at 5 µm, based on published observations.11 Other doses are indicated in each experiment. CpG–ODNs were used at 50 µg/ml to yield maximal activity and were added at the indicated time-points. After the incubation, supernatants were collected and cells counted using perfect count beads (BD Biosciences). Dead cells were excluded by size and annexin V detection.
Cytokine analyses
Cytokines were determined on supernatants using the cytometric bead array (CBA) assay (BD Biosciences). PDCs secreted IFN-α was determined on supernatants using an enzyme-linked immunosorbent assay (ELISA) kit (Bender Medisystems, Vienna, Austria) following the manufacturer's instructions. The presence of tacrolimus in supernatants was analysed using a specific immunoassay (IMX system, tacrolimus II; Abbott Diagnostics, Madrid, Spain).
Relative mRNA expression by real-time polymerase chain reaction (RT–PCR)
Total RNA was extracted from cells using TRIzol reagent (Sigma-Aldrich) according the manufacturer's protocol. Aliquots of 1 μg total RNA, 0·5 μg of oligo-(dT) (Sigma-Aldrich) as primers and 2 μl dNTPs (mix, 5 mm each; Sigma-Aldrich) were dissolved with sterile RNAse-free water until 12 μl, heated at 65° for 5 min and cooled at 4°. Then, 4 μl of first-strand buffer 5× (250 mm Tris-HCl, 375 mm KCl, 15 mm MgCl, pH 8·3; Invitrogen), 2 μl of 100 mm dithiothreitol (Invitrogen) and 1 μl of RNAse inhibitor (RNasa OUT; Invitrogen) were added and incubated at 42° for 2 min. The reaction was started adding 1 μl (200 units) of SuperScript II RT (Invitrogen), incubated at 42° for 50 min and finished at 70° for 15 min. The cDNAs were amplified using a commercially available kit (LightCycler FastStart DNA MasterPLUS SYBR Green I; Roche). Each reaction was carried out with 2 μl of cDNA, 4 μl of 5× Master Mix (FastStart DNA MasterPLUS SYBR Green I), 0·5 μm of each primer and sterile RNAse-free water at final volume of 20 μl. RT–PCR was carried out for 35 cycles using the LightCycler instrument (Roche). Specific primers were selected according to the GenBank database resource. Primers and PCR conditions were as follows: TNF-α: amplimer 266 base pairs (bp), forward: 5′-CTT CTC CTT CCT GAT CGT GC-3′, reverse: 5′-GCT GGT TAT CTC TCA GCT CCA-3′ 5 seconds at 95°, 5 s annealing at 60°, 11 seconds at 72°, and acquire for 4 seconds at 84°); β2-mycroglobulin: amplimer 107 bp, forward: 5′-ACA CAA CTG TGT TCA CTA GC-3′, reverse: 5′-CAA CTT CAT CCA CGT TCA CC-3′ 5 seconds at 95°, 10 s annealing at 58°, 4 seconds at 72°, and acquire for 4 seconds at 85°). β2-mycroglobulin mRNA levels were used as an endogenous control to normalize mRNA quantities. The relative mRNA levels of TNF-α were calculated as 2−(Ct TNF-α − Ct β2-mycroglobulin).
Allostimulatory assays
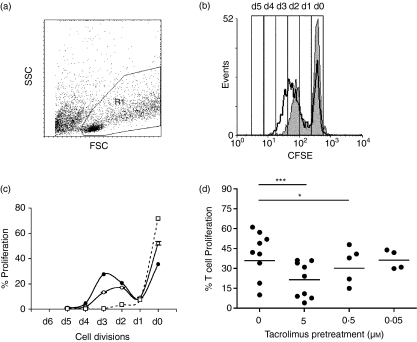
Allostimulatory conditions were set up following previous results.34 Briefly, allogeneic 5 × 104 CbT cells were stimulated in vitro with the indicated PDCs at a 10:1 ratio during 4 days. Previously, PDCs were washed three times with an excess of PBS + 0·1% BSA to exclude dead cells and debris and to minimize the direct effect of tacrolimus on CbT cells. Cell proliferation was assessed using the carboxyfluorescein diacetate succinimidyl ester (CFSE)-based (Molecular Probes Europe, Leiden, the Netherlands) flow cytometry assay. Briefly, 5 × 106 isolated CbT cells were resuspended in 1 ml PBS + 0·1% BSA and incubated with an equal volume of 1·25 µm CFSE. After 10 min, unbound dye was quenched with an equal volume of RPMI-1640 + 10% FCS at 37°. Labelled cells were washed twice in RPMI-1640 + 10% FCS before placing in culture. Alloproliferative CbT cells were determined as the CFSE-low/negative population by FACS analyses (BD Biosciences). The number of cell divisions was estimated by gating the cells in areas based on the non-proliferating T cells area (as shown in Fig. 4b).
Figure 4.
Allostimulatory capacity of dinucleotide (CpG)-activated tacrolimus-pretreated plasmacytoid dendritic cells (Tp-PDCs). PDCs were incubated during 48 hr in the presence of interleukin (IL)-3 alone or IL-3 plus tacrolimus. After this pretreatment period, dinucleotide–oligodeoxynucleotide (CpG–ODNs) were added to induce cell activation. After 48 hr, cells were recovered, washed and counted before allostimulatory experiments were set up, using carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled cord blood T cell (CbT) lymphocytes as responders. After 4 days, cells contained in the living gate (R1 in panel a) were analysed for CFSE labelling. (b) The loss of CFSE in proliferating T cells (gated in R1) in a single representative experiment. The thick line represents the proliferation of T cells responding to activated PDCs (control), whereas the grey area represents the proliferation of T cells responding to Tp-activated PDCs. (c) Number of cell divisions was estimated based on the area of CFSE-labelled non-proliferating cells. The dotted line shows the non-stimulated control. Circles (black CpG2006 stimulated-PDCs, white CpG2006 stimulated Tp-PDCS) show the number of cell divisions and the proportion of proliferating T cells. Each symbol represents the mean of at least three independent determinations. A representative experiment of nine is shown. (d) Summary of results of the alloproliferation experiments induced by CpG2216 activated PDCs pretreated or not with tacrolimus at the concentrations indicated. Proliferation is represented as the percentage of CFSE-low T cells. Each symbol represents an individual experiment (mean of at least two replicates). Significant differences are indicated (*P < 0·05, paired t-test).
Intracellular cytokines
Day 4 alloproliferative CbT cells were washed and restimulated for 5 hr with phorbol-12-myristate 13-acetate (PMA) (50 ng/ml) and ionomycin (500 ng/ml) in the presence of monensin (2·5 µg/ml). Then cells were washed, fixed and permeabilized using the IntraStain kit (Dako Cytomation, Glostrup, Denmark). Finally, cells were labelled with anti-IFN-γ allophycocyanin (APC) mAbs (Caltag Laboratories) or the corresponding isotype control.
Statistical analysis
Results are expressed as the mean ± standard deviation (SD). Comparison between groups was conducted using the Wilcoxon test for paired nonparametric data and the paired t-test for parametric data after confirming normal distribution by the Kolmogorov–Smirnov test, as indicated. Unpaired observations were analysed by Mann–Whitney test (nonparametric data) and unpaired t-test (parametric data), respectively. Analyses were performed using SigmaStat version 3·0 software (SPSS, Ekrath, Germany). A value of P < 0·05 was considered significant.
Results
Tacrolimus does not modify the viability and phenotype of unactivated cultured PDCs and does not block CpG activation of PDCs
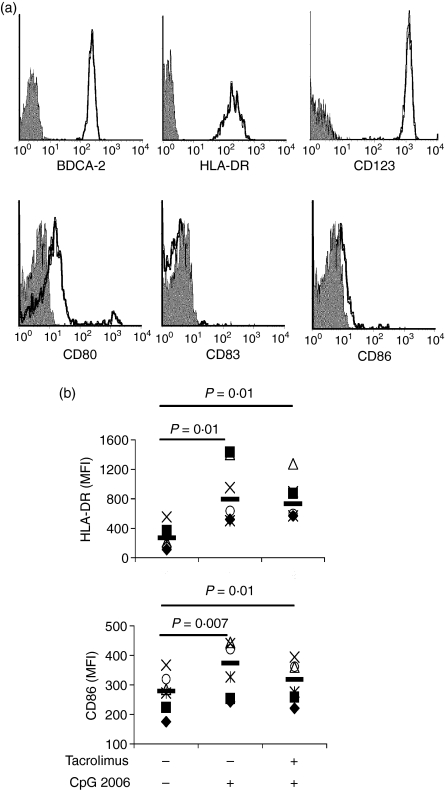
Freshly isolated PDCs were defined as CD4+ HLADR+ CD14–CD19–CD11c– and confirmed by the expression of the PDC-specific marker BDCA-2. Immaturity of cells was demonstrated by the low expression of co-stimulatory molecules (CD80 and CD86) and the absence of CD83 (Fig. 1a). Cells were cultured in complete medium in the presence or absence of tacrolimus. After 48 hr, non-activated PDCs showed a similar phenotype (data not shown) and survival (46 ± 12% versus 41 ± 16%, n = 6, ns) in both conditions, indicating that tacrolimus did not affect the viability of the cells. As expected, the addition of CpG–ODN at the onset of the culture period increased the survival of PDCs and induced the activation of PDCs, as revealed by the up-regulation of HLA-DR and co-stimulatory molecules (Fig. 1b). Activation was not blocked by the presence of tacrolimus, although CD86 expression did not reach the levels of the CpG-activated controls. An unexpected observation was a reduced survival of PDCs in the presence of tacrolimus only upon CpG2216 activation (49 ± 7% versus 26 ± 1%, n = 3, P = 0·006, paired t-test). In contrast, this reduction was less evident when CpG 2006 was used in the same conditions (62 ± 10% versus 55 ± 13%, n = 4, ns).
Figure 1.
Assessment of plasmacytoid dendritic cells (PDCs) phenotype upon isolation and after dinucleotide–oligodeoxynucleotide (CpG–ODN) activation. (a) The phenotype of PDCs upon isolation. Isotype controls are shown in grey. (b) PDCs were cultured for 48 hr in the presence of interleukin (IL)-3, tacrolimus and CpG–ODN as indicated. After the incubation period, the expression of human leucocyte antigen D-related (HLA-DR) and CD86 was analysed by flow cytometry. Each symbol represents an independent sample. Significant differences are indicated in the figure (n ≥ 5, paired t-test).
Tacrolimus-pretreated PDCs (Tp-PDCs) show lower activation and secretion of inflammatory cytokines
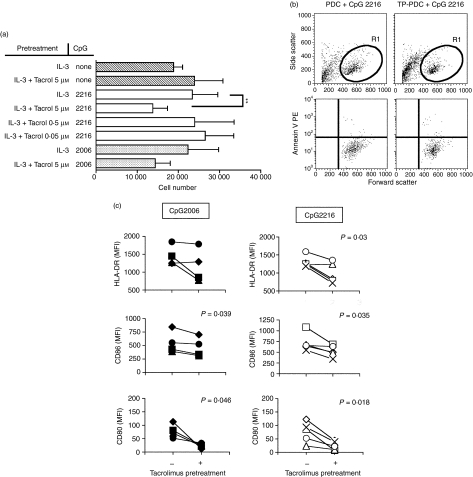
As tacrolimus pretreatment could affect PDCs survival upon activation, dose-viability experiments were performed. Isolated PDCs were cultured in the absence or presence of tacrolimus (tacrolimus-pretreatment, Tp). After 48 hr, CpG–ODNs were added to the cultures to induce activation of PDCs and the cells were incubated further for 48 hr (Tp-CpG PDCs). In these experiments, the recovered cells were counted using perfect count beads (Fig. 2a). A significant reduction of cell numbers was detected in CpG2216 activated Tp-PDCs at the higher dose of tacrolimus used (5 µm) (23416 ± 6149 versus 13805 ± 3560, n = 7, P < 0·002, paired t-test). Also, a slightly reduced cell survival was detected in CpG2006 activated Tp-PDCs, although this was not found significant. At lower doses, the survival of activated Tp-PDCs was not different compared to activated controls, suggesting a direct implication of the drug in the activation-induced cell death. However, in the absence of CpG activation, the higher dose of tacrolimus used did not affect PDCs survival (Fig. 2a). Importantly, over 90% of PDCs contained in the living gate in all situations were annexin V negative, suggesting that they were not prone to cell death (Fig. 2b). These cells were used later as stimulators in alloresponse experiments.
Figure 2.
Tacrolimus pretreatment impairs plasmacytoid dendritic cells (PDC) survival and maturation upon dinucleotide (CpG) activation. PDCs were cultured during 48 hr in the presence of interleukin (IL)-3 and tacrolimus at the concentrations indicated. After this pretreatment, dinucleotide–oligodeoxynucleotide (CpG–ODNs) were added and cells further incubated during 48 hr. (a) The number of viable cells in each condition (mean ± SD of at least four independent experiments) recovered at the end of this period as determined by perfect count. The cells contained in the R1 gate were mainly annexin V negative (a single representive experiment is shown in panel b) (c) The expression of the indicated markers was analysed after the activation period. Each symbol represents an individual sample. Black symbols represent cells activated by CpG–ODN 2006 and white symbols represent cells activated by CpG–ODN 2216. Significant differences are shown by the indicated P-value (n ≥ 4, paired t-test).
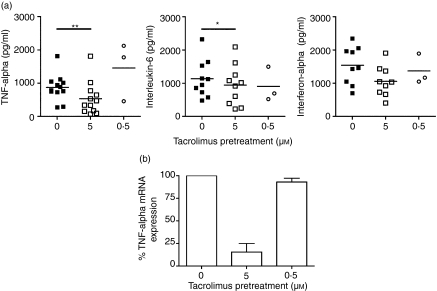
In all experiments, the majority of cells were positive for the analysed markers upon activation. However, Tp-CpG PDCs showed reduced levels of HLA-DR, CD80 and CD86 compared to their fully activated controls (Fig. 2c). This effect was independent of the CpG–ODN used, and was marked specially on the expression of CD80. Finally, supernatants of the cultured Tp-CpG PDCs and controls were analysed to evaluate the presence of proinflammatory cytokines. The results, summarized in Table 1, showed a consistent (14 of 16 experiments) and important reduction (up to 85%) in the secreted levels of TNF-α by Tp-PDCs stimulated by CpG. In CpG2216-stimulated cultures secretion of IL-6 was also reduced (Fig. 3a). However, overall this reduction was quantitatively less important, and only four of 13 experiments showed values below 50% compared to controls. Finally, the presence of tacrolimus did not significantly affect the levels of secreted IFN-α (Fig. 3a).
Table 1.
Cytokine secretion by tacrolimus-pretreated plasmacytoid dendritic cells (Tp-PDCs). The indicated cytokines (pg/ml) were determined in supernatants after culture. The number of independent experiments is indicated (n). Statistical differences are indicated by the P-value (paired t-test)
| Cytokine secretion (pg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|
| CpG 2006 | CpG2216 | |||||||
| Control | Tacrolimus (5 μm) | P | n | Control | Tacrolimus (5 μm) | P | n | |
| TNF-α | 126 ± 46 | 69 ± 47 | 0·02 | 4 | 871 ± 400 | 538 ± 496 | 0·002 | 12 |
| IL-6 | 752 ± 504 | 716 ± 491 | ns | 3 | 1138 ± 562 | 947 ± 605 | 0·02 | 10 |
| IFN-α | 212 ± 186 | 198 ± 249 | ns | 3 | 1540 ± 571 | 1059 ± 457 | ns | 9 |
CpG: dinucleotide; IFN: interferon; IL: interleukin; TNF: tumour necrosis factor.
Figure 3.
Tumour necrosis factor (TNF)-α secretion by activated plasmacytoid dendritic cells (PDCs) is impaired by tacrolimus pretreatment. (a) PDCs were incubated during 48 hr in the presence of interleukin (IL)-3 and tacrolimus at the dose indicated. After this pretreatment, dinucleotide–oligodeoxynucleotide (CpG–ODNs) were added. After the additional 48 hr of incubation, supernatants were recovered. Indicated cytokines were determined either by enzyme-linked immunosorbent assay or cytometric bead array (CBA). Each symbol represents an individual sample. Significant differences are indicated (*P < 0·05, paired t-test). (b) Cells were pretreated with the indicated dose of tacrolimus for 12 hr. Then CpG–ODN were added and cells incubated further during 6–8 hr before mRNA was extracted. The expression of TNF-α mRNA is shown as the relative proportion (TNF-α/β2 m) normalized to the maximum level of stimulation (CpG-activated non-pretreated PDCs). The mean ± SD of two independent experiments is shown.
As the impaired survival observed in 5 µm Tp-PDCs could account for the reduced secretion of some cytokines, i.e. TNF-α, and to investigate further the involvement of tacrolimus in the reduced cytokine secretion, PDCs were pretreated with two different doses of the drug for 12 hr. Then CpG2216 was added to cultures and both mRNA and protein levels were analysed in short-term cultures (up to 8 hr upon activation). Under these experimental conditions, in which cell viability was not affected, 5 µm Tp-PDCs showed an important reduction in the TNF-α mRNA levels. When used at lower doses, this reduction was no longer detected (Fig. 3b). Accordingly, protein levels were also reduced in these conditions (about 60%, data not shown), indicating that tacrolimus was indeed affecting TNF secretion by PDCs at the transcriptional level.
Tp-PDCs are poor inducers of allostimulation
We next studied whether the defect on maturation observed in Tp-PDCs was affecting their allogeneic stimulatory capacity. As before, Tp-PDCS were activated with CpG2216 for 48 hr. Cells were then recovered and washed extensively. PDCs included in the living gate were counted and added to fresh U-bottomed wells in which CFSE-labelled naive CbT cells had been dispensed. As shown before, cells included in the living gate were not prone to cell death (Fig. 2b). After 96 hr, cells contained in the living gate (R1) were analysed and the proportion of proliferating T cells was significantly lower in allogeneic cultures induced by activated Tp-PDCs than in activated control PDCs (39 ± 18% versus 20 ± 14%, n = 8, P = 0·0003, paired t-test) (Fig. 4). Similar results were obtained with CpG2006 (data not shown). PDCs pretreated with a lower dose of tacrolimus (0·5 µm) still showed a reduced capacity to induce allogeneic T cell proliferation compared to untreatred controls (28 ± 5%, n = 4, P = 0·02, paired t-test). In these experiments, the higher proportion of proliferating T cells in control cultures had suffered three divisions, whereas Tp-PDCs-stimulated T cells reached only two divisions in the same period (Fig. 4). Additionally, the presence of tacrolimus in 24-hr and 48-hr mixed lymphocyte culture (MLC) supernatants was determined. Although all measured values were below the negative control (data not shown), technical limitations (lower detection limit was 1·8 nm) do not permit to completely rule out the possibility of a carryover effect.
T cells responding to Tp-PDCS show impaired cytokine production
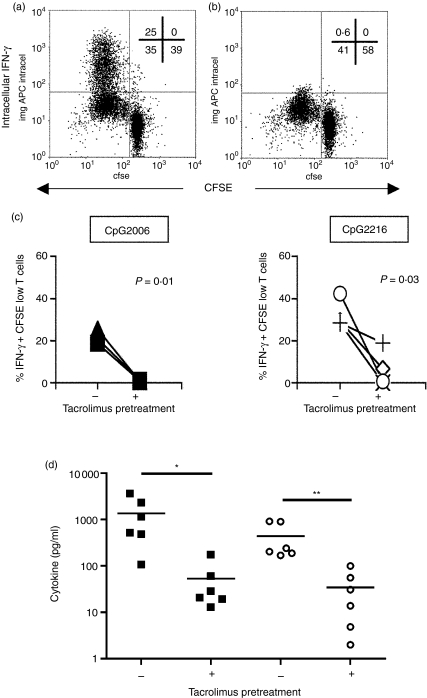
To gain some knowledge of the cytokines secreted by these responding cells, CFSE-low alloproliferative T lymphocytes were re-stimulated with PMA + ionomycin and the production of IFN-γ was measured by intracellular staining. The capacity of responder T cells to produce IFN-γ was almost completely abrogated in cultures where activated Tp-PDCs were used as stimulators compared to controls (Fig. 5). As before, the effect was equally observed when both CpG 2216 or 2006 were used to activate PDCs. Also, CBA analyses of allogeneic culture supernatants stimulated with CpG2216 confirmed these results (Fig. 5d). In general, pretreatment of PDC with tacrolimus reduced the production of IFN-γ and IL-2 to less than 10% by allogeneic T cells.
Figure 5.
The production and secretion of cytokines by T lymphocytes stimulated with tacrolimus-pretreated plasmacytoid dendritic cells (PDCs) is altered. Proliferating T lymphocytes were obtained from allostimulatory experiments. The production of interferon (IFN)-γ was measured by intracellular staining upon restimulation of the cells in the presence of monensin. Cells were gated as in Figure 4(a). (a, b) The IFN-γ production of T cells responding to control CpG2216-activated PDCs (a) or 5 µm Tp-pretreated PDCs (b) in a representative experiment. The numbers inside each plot indicate the percentage of cells in each quadrant. (c) Summary of the results of the intracellular IFN-γ production in both CpG2006 (n = 3) and 2216 (n = 4) activated cultures, respectively. Each symbol represents an individual sample. Significant differences are shown by the indicated P-value (paired t-test). (d) IFN-γ (black squares) and interleukin (IL)-2 (white circles) were measured by cytometric bead array (CBA) in supernatants of T cell alloproliferation experiments in which CpG2216-activated PDCs or 5 µm Tp-PDCs were used. Each symbol represents an individual experiment. The line shows the mean of all experiments. Statistical differences are indicated (*P < 0·05, paired t-test).
Discussion
The results of this study provide evidence that tacrolimus affects the adaptative immune response induced by activated PDCs. PDCs have been broadly shown to induce T cell responses only upon activation. In particular, some CpG-ODNs are well known to promote TNF-α secretion by PDCs and to induce IFN-γ production by allogenic T cells.21,35 When tacrolimus and the activating factor (CpG) were added simultaneously, PDCs acquired an activated phenotype similar to controls. However, when PDCs were pretreated with tacrolimus, CpG-activated Tp-PDCs showed a reduced level of co-stimulatory molecules upon activation, an effect already described in PDCs treated with cyclosporin A.9 These observations could indicate that in the presence of calcineurin inhibitors PDCs may reach a state of ‘partial’ maturation only upon activation. Also, a reduced capacity to secrete IFN-α was reported.9 Our results showed only a slightly reduced capacity of Tp-CpG PDCs to secrete IFN-α upon activation. This minor difference may be due to the timing at which interferon was measured (24 hr in the cyclosporine study versus 48 hr in our experiments). Although it has been described that the maximum level of IFN-α production by activated PDCs may be around 24 hr,20,21 it is possible that incubation with the immunosuppressive agent may delay the production and secretion of the cytokine, which may accumulate and be detected at 48 hr. Also, tacrolimus pretreatment could modulate TLR9 levels on PDCs. In this sense, no differences were found in intracellular staining experiments using monoclonal antibodies to TLR-9 between tacrolimus-treated PDCs and controls (data not shown). This observation is in tune with those reported for other immunosuppressive agents, such as dexamethasone.33
Secretion of TNF-α and IL-6, both negatively regulated by calcineurin inhibitors in myeloid DCs,15,16 was also studied. TNF-α secretion showed a consistent and significant reduction in Tp-CpG PDCs. The secretion of IL-6 was also reduced significantly, although to a much lesser extent. Tacrolimus, as other calcineurin inhibitors, blocks the binding of nuclear factor (NF)-κB to their regulatory domains.8 The promoter regions of TNF-α and IL-636,37 contain NF-κB regulatory domains. Interestingly, the NF-κB domain in the IL-6 promoter functions as a potent IL-1/TNF-α-responsive element in myeloid cells.38 In addition, NF-κB activation is crucial for TNF production.39 Recently, in NF-κB(–/–) mice, it has been shown that murine pDCs produced type I interferon but not IL-6.40 In our system, IL-6 secretion by PDCs was impaired importantly only when TNF-α levels were reduced drastically (> 80% reduction). These results further suggest TNF-α as one of the potential regulators of IL-6 secretion by PDCs. Also, a cross-regulation between TNF-α and IFN-α has been suggested in autoimmune diseases.41 It is considered that TNF-α may block IFN-α secretion, a capacity owned by immature PDCs, by inducing PDC maturation. Consequently, TNF blockade is thought to impair PDC maturation, thus indirectly promoting IFN-α secretion. Our results show that tacrolimus treatment impaired TNF-α secretion by PDCs and affected PDC maturation, but did not increase IFN-α secretion upon activation. This is due most probably to the multiple factors affected by tacrolimus on PDCs that can modulate IFN-α secretion, rendering basal or even lower levels of type I IFN upon stimulation.
Of note, tacrolimus-pretreated PDCs showed a dose-dependent impaired survival exclusively upon activation. Glucocorticoids33 but not cyclosporin A9 have been shown to induce apoptosis in PDCs. As NF-κB translocation is related to PDC survival,40 it is possible that blockade of this factor may be involved in the induction of apoptosis on PDCs upon activation.
With regard to allogeneic T cell responses, both proliferation and cytokine secretion were clearly impaired when Tp-CpG PDCs were used as stimulators. A putative carryover effect of tacrolimus, suggested in MDDC cultures,15 could also be affecting these MLCs. In our titration experiments 1 nm tacrolimus affected only 1% of PMA + ionomycin-induced T cell proliferation (data not shown). However, it has been reported that murine T cell proliferation and IFN-γ secretion were reduced by approximately 50% using 1 nm tacrolimus.12 Using a commercial immunoassay we could not detect tacrolimus in supernatants from 24-hr and 48-hr MLCs, although due to technical limitations we cannot completely exclude that minute amounts of tacrolimus would still be present in those cultures and may affect, at least partially, the outcome of the alloresponse. However, other factors, such as the lower expression of HLA-DR and co-stimulatory molecules on Tp-activated PDCs and the reduced secretion of cytokines such as TNF-α, may also account for the functional impairment exhibited by these cells. Also, in the context of an immune response, the reduced secretion of proinflammatory cytokines such as TNF-α may have an autoparacrine activity to direct the PDCs final differentiation and also affect the function of other cells, such as monocytes, neutrophils or endothelial cells.
In conclusion, tacrolimus pretreatment importantly affected the secretion of TNF-α by PDCs upon activation. TNF blockade using monoclonal antibodies is an alternative approach to control autoimmune disorders, but it may induce the secretion of IFN-α by PBMCs, and particularly PDCs.41 In fact, 0·2% of rheumatoid arthritis patients on TNF-blockade treatment have developed symptoms of SLE, including increased anti-dsDNA antibodies.42 Regulation of IFN-α secretion may be crucial in some autoimmune disorders such as SLE, where increased levels of IFN-α are found routinely. Use of tacrolimus, providing reduced levels of TNF-α and a moderate decrease in IFN-α secretion, may be an alternative choice in these situations. Studies on samples from patients under continuous immunosuppressive therapy would further clarify the role of IAs in PDCs function.
Acknowledgments
The authors would like to thank Dr Carlos Margarit (Unitat de Cirurgia Hepato-Bilio-Pancreàtica i Trasplantament Hepàtic, Hospital Universitari Vall d'Hebron) for kindly providing tacrolimus; also Dr Alejandro Olivé (Rheumatology Unit, Hospital U. Germans Trias i Pujol) for critically revising the manuscript, and Ms Laura Ocaña (Centre de Teixits i Teràpia Cellular, BST) for helpful discussions. We also thank Drs Cruz Pastor and Susana Montes (Hospital U. Germans Trias i Pujol) for tacrolimus determinations. This work was supported partially by grants from the ‘Fondo de Investigaciones Sanitarias’ FIS 01/3120 and FIS 03/0142 to F.E.B. and ‘Red Temática de Investigación en SIDA (Red de grupos 173)’ to M.B. M.N.G is supported by grant FIS 03/0142. F.E.B. is supported by contract FIS 01/3120 from the BST in collaboration with the Spanish Health Department.
References
- 1.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States 1988–96. N Engl J Med. 2000;342:605–12. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 2.Fung JJ. Tacrolimus and transplantation: a decade in review. Transplantation. 2004;77(9 Suppl.):S41–3. doi: 10.1097/01.tp.0000126926.61434.a5. [DOI] [PubMed] [Google Scholar]
- 3.Tugwell P, Bombardier C, Gent M, et al. Low-dose cyclosporin versus placebo in patients with rheumatoid arthritis. Lancet. 1990;335:1051–5. doi: 10.1016/0140-6736(90)92630-z. [DOI] [PubMed] [Google Scholar]
- 4.Cheer SM, Plosker GL. Tacrolimus ointment. A review of its therapeutic potential as a topical therapy in atopic dermatitis. Am J Clin Dermatol. 2001;2:389–406. doi: 10.2165/00128071-200102060-00005. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin–cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 6.Jirapongsananuruk O, Leung DY. The modulation of B7.2 and B7.1 on B cells by immunosuppressive agents. Clin Exp Immunol. 1999;118:1–8. doi: 10.1046/j.1365-2249.1999.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasowska BA, Zheng XX, Strom TB, Kupieck-Weglinski JW. Adjunctive rapamycin and CsA treatment inhibits monocyte/macrophage associated cytokines/chemokines in sensitized cardiac graft recipients. Transplantation. 2001;71:1179–83. doi: 10.1097/00007890-200104270-00029. [DOI] [PubMed] [Google Scholar]
- 8.Lee JI, Ganster RW, Geller DA, Burckart GJ, Thomson AW, Lu L. Cyclosporine A inhibits the expression of costimulatory molecules on in vitro-generated dendritic cells: association with reduced nuclear translocation of nuclear factor kappa B. Transplantation. 1999;68:1255–63. doi: 10.1097/00007890-199911150-00007. [DOI] [PubMed] [Google Scholar]
- 9.Tajima K, Amakawa R, Ito T, Miyaji M, Takebayashi M, Fukuhara S. Immunomodulatory effects of cyclosporin A on human peripheral blood dendritic cell subsets. Immunology. 2003;108:321–8. doi: 10.1046/j.1365-2567.2003.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vieira PL, Kaliñski P, Wierenga EA, Kapsenberg ML, de Jong EC. Glucocorticoids inhibit bioactive IL-12p70 production by in vitro-generated human dendritic cells without affecting their T cell stimulatory potential. J Immunol. 1998;161:5245–51. [PubMed] [Google Scholar]
- 11.Cos J, Villalba T, Parra R, et al. FK506 in the maturation of dendritic cells. Haematologica. 2002;87:679–87. discussion 687. [PubMed] [Google Scholar]
- 12.Matsue H, Yang C, Matsue K, Edelbaum D, Mummert M, Takashima A. Contrasting impacts of immunosuppressive agents (rapamycin, FK506, cyclosporin A, and dexamethasone) on bidirectional dendritic cell–T cell interaction during antigen presentation. J Immunol. 2002;169:3555–64. doi: 10.4049/jimmunol.169.7.3555. [DOI] [PubMed] [Google Scholar]
- 13.Lee YR, Yang IH, Lee YH, Im SA, Song S, et al. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005;105:3951–5. doi: 10.1182/blood-2004-10-3927. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu K, Fujii S, Fujimoto K, Kawa K, Yamada A, Kawano F. Tacrolimus (FK506) treatment of CD34+ hematopoietic progenitor cells promote the development of dendritic cells that drive CD4+ T cells toward Th2 responses. J Leukoc Biol. 2000;68:633–40. [PubMed] [Google Scholar]
- 15.Woltman AM, de Fijter JW, Kamerling SW, Paul LC, Daha MR, van Kooten C. The effect of calcineurin inhibitors and corticosteroids on the differentiation of human dendritic cells. Eur J Immunol. 2000;3:1807–12. doi: 10.1002/1521-4141(200007)30:7<1807::AID-IMMU1807>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Szabo G, Gavala C, Mandrekar P. Tacrolimus and cyclosporine A inhibit allostimulatory capacity and cytokine production of human myeloid dendritic cells. J Invest Med. 2001;49:442–9. doi: 10.2310/6650.2001.33789. [DOI] [PubMed] [Google Scholar]
- 17.Tuñón MJ, Sánchez-Campos S, Gutiérrez B, Culebras JM, González-Gallego J. Effects of FK506 and rapamycin on generation of reactive oxygen species, nitric oxide production and nuclear factor kappa B activation in rat hepatocytes. Biochem Pharmacol. 2003;66:439–45. doi: 10.1016/s0006-2952(03)00288-0. [DOI] [PubMed] [Google Scholar]
- 18.Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–78. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.O'Doherty U, Peng M, Gezelter S, et al. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–93. [PMC free article] [PubMed] [Google Scholar]
- 20.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 21.Krug A, Rothenfusser S, Hornung V, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;3:2154–63. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–11. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 24.Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 25.Jongbloed SL, Lebre MC, Fraser AR, et al. Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis. Arthritis Res Ther. 2006;8:R15. doi: 10.1186/ar1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–43. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lövgren T, Eloranta ML, Båve U, Alm GV, Rönnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–72. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 28.Båve U, Nordmark G, Lövgren T, et al. Activation of the type I interferon system in primary Sjögren's syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005;52:1185–95. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- 29.Vallin H, Perers A, Alm GV, Rönnblom L. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J Immunol. 1999;163:6306–13. [PubMed] [Google Scholar]
- 30.Vallin H, Blomberg S, Alm GV, Cederblad B, Rönnblom L. Patients with systemic lupus erythematosus (SLE) have a circulating inducer of interferon-alpha (IFN-alpha) production acting on leucocytes resembling immature dendritic cells. Clin Exp Immunol. 1999;115:196–202. doi: 10.1046/j.1365-2249.1999.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qing X, Putterman C. Gene expression profiling in the study of the pathogenesis of systemic lupus erythematosus. Autoimmun Rev. 2004;3:505–9. doi: 10.1016/j.autrev.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Shodell M, Shah K, Siegal FP. Circulating human plasmacytoid dendritic cells are highly sensitive to corticosteroid administration. Lupus. 2003;12:222–30. doi: 10.1191/0961203303lu362xx. [DOI] [PubMed] [Google Scholar]
- 33.Abe M, Thomson AW. Dexamethasone preferentially suppresses plasmacytoid dendritic cell differentiation and enhances their apoptotic death. Clin Immunol. 2005;118:300–6. doi: 10.1016/j.clim.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Naranjo-Gómez M, Fernández MA, Bofill M, et al. Primary alloproliferative TH1 response induced by immature plasmacytoid dendritic cells in collaboration with myeloid DCs. Am J Transplant. 2005;5:2838–48. doi: 10.1111/j.1600-6143.2005.01097.x. [DOI] [PubMed] [Google Scholar]
- 35.Krug A, Veeraswamy R, Pekosz A, et al. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J Exp Med. 2003;197:899–906. doi: 10.1084/jem.20021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drouet C, Shakhov AN, Jongeneel CV. Enhancers and transcription factors controlling the inducibility of the tumor necrosis factor-alpha promoter in primary macrophages. J Immunol. 1991;147:1694–700. [PubMed] [Google Scholar]
- 37.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10:2327–34. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakayama K, Shimizu H, Mitomo K, Watanabe T, Okamoto S, Yamamoto K. A lymphoid cell-specific nuclear factor containing c-Rel-like proteins preferentially interacts with interleukin-6 kappa B-related motifs whose activities are repressed in lymphoid cells. Mol Cell Biol. 1992;12:1736–46. doi: 10.1128/mcb.12.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foxwell B, Browne K, Bondeson J, et al. Efficient adenoviral infection with IkappaB alpha reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-kappaB dependent. Proc Natl Acad Sci USA. 1998;95:8211–15. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Keeffe M, Grumont RJ, Hochrein H, et al. Distinct roles for the NF-kappaB1 and c-Rel transcription factors in the differentiation and survival of plasmacytoid and conventional dendritic cells activated by TLR-9 signals. Blood. 2005;106:3457–64. doi: 10.1182/blood-2004-12-4965. [DOI] [PubMed] [Google Scholar]
- 41.Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci USA. 2005;102:3372–7. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aringer M, Graninger WB, Steiner G, Smolen JS. Safety and efficacy of tumor necrosis factor alpha blockade in systemic lupus erythematosus: an open-label study. Arthritis Rheum. 2004;50:3161–9. doi: 10.1002/art.20576. [DOI] [PubMed] [Google Scholar]