Abstract
Mitogen-activated protein/ERK kinase kinase 3 (MEKK3) is a Ser/Thr protein kinase belonging to the MEKK/STE11 subgroup of the MAP3K family. Recently, we found that MEKK3 plays a critical role in interleukin-1 (IL-1) receptor and Toll-like receptor 4 signalling using established primary mouse embryonic fibroblast (MEF) cell lines. However, the function of MEKK3 in immune cells has not been studied because germ-line MEKK3 knockout mice are embryonically lethal between embryonic days 10 and 11. In this study, we used small interference RNA to the mouse Mekk3 gene to specifically knock down MEKK3 expression in the macrophage line Raw264.7. We found that the lipopolysaccharide-induced IL-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF) production was dramatically decreased in MEKK3 knockdown cells whereas the tumour necrosis factor-α and IL-1β production were not affected. We also observed that the ERK1/2, p38 and JNK MAPK induction in MEKK3 knockdown cells were moderately inhibited within the first 60 min of stimulation, while the ERK and p38 were more severely inhibited after 2–4 hr of stimulation. Degradation of IκBα was also partially blocked in MEKK3 knockdown cells. Notably, the impairment in IL-6 and GM-CSF production in the MEKK3 knockdown cells was restored by reintroducing a human Mekk3 cDNA that could not be targeted by mouse Mekk3-siRNAs. In conclusion, this study showed that MEKK3 is a crucial and specific regulator of the proinflammatory cytokines IL-6 and GM-CSF in macrophages and provided a novel method for investigating MEKK3 function in other immune cells.
Keywords: Cytokine, MAPK, MEKK3, Macrophages, TLR signalling
Introduction
Lipopolysaccharide (LPS) is a potent inducer of inflammation by stimulating immune cells to produce proinflammatory cytokines, proteases and reactive oxygen and nitrogen species.1 LPS acts by binding to its cognate receptor, toll-like receptor 4 (TLR4), a member of the evolutionarily conserved interleukin-1 receptor (IL-1R)-TLR supergene family of proteins that are essential for mounting a host defence against infectious pathogens.2–5 Upon stimulation, IL-1R-TLRs activate four intracellular protein kinase cascades, the IκBα kinase (IKK) nuclear factor-κB (NF-κB), extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK) and p38 cascades, which are essential for the induction of genes encoding proinflammatory cytokines.5–7 The IL-1R-TLR signals are mediated by a conserved Toll/IL-1 receptor (TIR) domain that recruits and assembles intracellular signalling molecules, including MyD88, IL-1R-associated protein kinase, TIR domain-containing adaptor protein/MyD88-adaptor-like and tumour necrosis factor (TNF) receptor-associated factor 6 (TRAF6), into complexes.5,8,9 Biochemical and genetic studies have confirmed the essential roles of these signalling molecules in the activation of downstream kinase cascades and in IL-1R- and TLR-mediated cytokine gene expression.
IL-1R-TLR-mediated signal transduction to the downstream protein kinase cascades requires members of the mitogen-activated protein kinase (MAPK) kinase kinase (MAP3K) family. Studies from many laboratories have shown that the following members of the MAP3K family are involved in this process: transforming growth factor-β-activating kinase 1 (TAK1),10,11 mitogen-activated protein/ERK kinase kinase 1 (MEKK1),12–14 MEKK2,15 MEKK3,15,16 apoprosis signal-regulating kinase 1 (ASK1), NF-κB-inducing kinase17 and tumour progression locus 2.18 However, the underlying molecular mechanism responsible for their regulation, specificity and function in each distinctive IL-1R-TLR signalling pathway remains largely unknown.
Of particular interest is MEKK3, a Ser/Thr protein kinase belonging to the MEKK/STE11 subgroup of the MAP3K family19,20 that is ubiquitously expressed in multiple tissues and is a potent activator of the NF-κB and MAPK pathways.19–21In vitro, MEKK3 activates the MAPKs JNK, ERK1/2, p38 and ERK5/big MAPK.19,22–25 It was recently demonstrated that MEKK3 plays a critical role in IL-1R and TLR4 signalling in studies performed in established primary mouse embryonic fibroblast (MEF) cell lines. In particular, it was found that MEKK3 is essential for IL-1-induced and LPS-induced IL-6 production via the IKK-NF-κB and JNK-p38 MAPK pathways. However, because the germ-line MEKK3 knockout in mice is embryonically lethal between embryonic days 10 and 11, its role has not be studied in immune cells such as macrophages, which are critical for promoting inflammation and innate immunity.
In this study, we developed an alternative strategy for verifying these results by using a lentiviral vector to deliver small interference RNA (siRNA) to knock down Mekk3 gene expression in the macrophage line Raw264.7. We found that this knocked-down Mekk3 expression in Raw264.7 cells specifically impaired IL-6 production induced by LPS, which was consistent with our previous findings in MEKK3-deficient MEFs.26 Importantly, we also show that MEKK3 is required for the production of another important cytokine, granulocyte–macrophage colony-stimulating factor (GM-CSF), but not for TNF-α and IL-1β production, in the Raw264.7 cells. Together, our findings showed that MEKK3 is a crucial and specific regulator of the production of the proinflammatory cytokines IL-6 and GM-CSF but not of TNF-α and IL-1β in macrophages. This study also provides a novel method for investigating MEKK3 function in other immune cells.
Materials and methods
Reagents
LPS was purchased from Sigma (St. Louis, MO), anti-ERK2 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and the anti-p38 antibody used for immunoblotting was purchased from Cell Signaling (Boston, MA). Anti-MEKK3 was produced by immunizing rabbits with a mouse MEKK3 peptide (amino acids 22–41 of the N terminus) and was affinity purified. Anti-β-actin was purchased from Sigma. The IL-6, GM-CSF, IL-1β, and TNF-α enzyme-linked immunosorbent assay kits were purchased from R and D Systems (Minneapolis, MN).
Vector construction
Construction of the pBS-SKII-huU6 vector has been described previously.27 Briefly, a BbsI site was introduced at the 3′ end, which allowed insertion of the siRNA sequences at the + 1 position of the U6 transcript. To construct the hairpin siRNA expression cassette, two complementary DNA oligonucleotide sequences (see description below) were synthesized, annealed, and inserted between the BbsI and XhoI sites immediately downstream from the U6 promoter: 5′-accg(n)19ttcaagaga(n)19ctttttc-3′; 3′-(n)19aagttctct(n)19-gaaaaagagct-5′. The (n)19 in the oligonucleotide sequence stands for the 19-nucleotide sense and reverse complementary targeting sequences as described below. The siRNA cassette also features a TTCAAGAGA loop situated between the sense and reverse complementary targeting sequences and a TTTTT terminator at the 3′ end. The Mekk3-siRNA15 contains the sense targeting sequence of ggatcctatacgagcatca, corresponding to the 894–912 nucleotide positions whereas the Mekk3-siRNA35 has the targeting sequence of ggcgagaacatgggtgtag, corresponding to the 1272–1290 nucleotide positions of the mouse Mekk3 coding sequence (GenBank accession no. Q61084). The targeting sequence for luciferase-siRNA (Luci-siRNA) is ggcgagaacatgggtgtag, which is directed to the 153–172 region of the firefly gene. The FG12 lentiviral vector derived from the vector FUGW has been described previously.28 To construct the siRNA-expressing lentiviral vectors, the siRNA expression cassette was digested out from the pBS-SKII-huU6-(siRNA) and subcloned into FG12 between the XbaI and XhoI sites. Restriction enzyme digestion and DNA sequencing confirmed the resulting plasmid.
Cell culture and lentiviral vector transduction
Macrophage Raw264.7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) and 10% fetal calf serum containing antimycotic antibiotics. The expression of green fluorescent protein (GFP) in the Raw264.7 cells was confirmed in a fluorescence-activated cell sorter (FACS) analysis. The cells were transduced with concentrated lentiviral vector stocks at a multiplicity of infection (MOI) of 10–25 (note that Raw264.7 cells have a considerably lower transducing efficiency) in the presence of 8 μg/ml Polybrene (hexadimethrine bromide; Sigma). The transduced cells were harvested 7 days later and analysed by FACS. Briefly, 1 × 103 cells were centrifuged with various lentiviral vectors at an MOI of 10 for 1 hr 30 min at 1000 g in the presence of 8 μg/ml Polybrene. After centrifugation, virus supernatants were removed and replaced with 1·5 ml fresh DMEM plus 10% fetal calf serum containing antibiotics. The cells were incubated for 24 hr and then transduced again with lentiviral vectors, as described above. After the cells were incubated for an additional 40–48 hr, the rate of infection was determined by FACS analysis and the data were analysed using cellquest (FACSCaliber, BD Biosciences, Sparks, MD).
The lentiviral vector stocks were produced by calcium phosphate-mediated transient transfection with appropriate amounts of vector plasmid, the HIV-1 lentiviral packaging constructs pRSVREV and pMDLg/pRRE,29 and the VSV-G expression plasmid pHCMVG of HEK-293 T cells, as described previously.30 The viruses were collected from the culture supernatant on days 2 and 3 after transfection.
Enzyme-linked immunosorbent assay for IL-6, GM-CSF, IL-1β and TNF-α
Cells were plated at a density of 2 × 104 cells in a 48-well culture plate and incubated for 5 hr. The siRNA-transduced Raw264.7 cells were stimulated with various concentrations of LPS (0, 0·1, 0·5 μg/ml) in 300 μl DMEM containing 5% fetal bovine serum for 24 hr. The medium was collected, and the levels of IL-6, GM-CSF, IL-1β and TNF-α released into the medium from siRNA-infected Raw264.7 cells were measured by an enzyme-linked immunoassay kit (ELISA; R and D Systems) specific for each protein, according to the manufacturer's instructions. Each sample was tested in triplicate.
Immunoblotting analysis
Raw264.7 cells cultured in 60-mm tissue culture dishes were stimulated, and cell lysates were prepared and analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and in immunoblot studies. Raw264.7 cell lysates (10 μg cell lysate protein per lane) were electrophoresed on a 10% SDS–PAGE gel and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). Non-specific binding was blocked by a 2-hr incubation of membranes at room temperature with 5% non-fat dried milk and 0·05% Tween-20 in triethanolamine-buffered saline (pH 7·6). The membranes were then probed with a 1/1000 dilution of anti-phospho-JNK, anti-JNK, anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-p38, anti-p38 MAPK antibodies (Cell Signaling), anti-MEKK3 (BD Biosciences), and anti-IκBα antibody (Santa Cruz Biotechnology). As a loading control, β-actin was detected by immunoblotting analysis in the same samples using an anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO) at a 1/5000 dilution. Peroxidase-conjugated anti-rabbit immunoglobulin G was purchased from Sigma-Aldrich. Immunoreactive proteins were detected with a chemiluminescent system (Pierce, Rockford, IL).
FACS analysis
Lentivirus-siRNA-transduced cells were analysed and sorted by FACSArial. Briefly, 1 × 105 cells were sorted at 4° in DMEM containing 0·5% fetal bovine serum. GFP-positive cells were then analysed using FACScalibur and cellquest software (BD Biosciences). Sorted cells were cultured for 3–4 days more and then used for experiments.
Results
Construction of Mekk3-siRNA expression cassettes
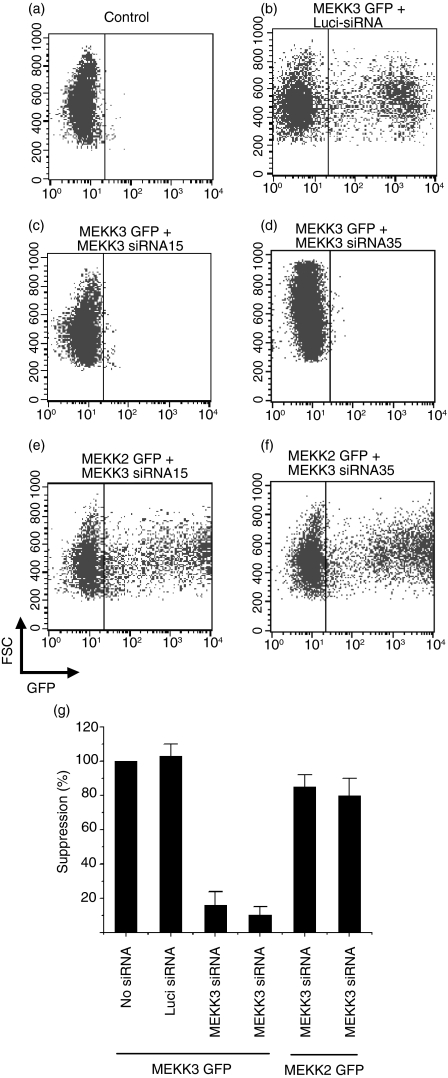
We designed four Mekk3 hairpin siRNA expression cassettes by subcloning the annealed oligonucleotide sequences into the BbsI and XhoI sites in the pBS-SKII-huU6 expression vector, as described in Materials and methods. We tested the specificity of these siRNA cassettes by cotransfecting the siRNA expression vectors with a MEKK3-GFP fusion protein expression vector.26 As shown in Fig. 1, while the control non-specific luciferase-siRNA (Luci-siRNA) had no effect on MEKK3-GFP expression (Fig. 1b), two Mekk3-siRNA expression plasmids, Mekk3-siRNA15 and Mekk3-siRNA35 (Fig. 1c,d), were found to specifically suppress MEKK3-GPF expression. To confirm that the knockdown of MEKK3-GFP expression by the Mekk3-siRNAs was specific, we examined their effect on a similar control expression vector for MEKK2-GFP expression and showed that they had no effect on MEKK2-GFP expression (Fig. 1e,f). Expression of the Mekk3-siRNAs was capable of inhibiting more than 90% of the expression of the cotransfected MEKK3-GFP as compared with the expression of the cotransfected Luci-siRNA, which had no effect on MEKK3-GFP expression (Fig. 1g). These results thus demonstrated that the expression of either Mekk3-siRNA15 or Mekk3-siRNA35 specifically knocked down Mekk3 gene expression.
Figure 1.
MEKK3-GFP expression knocked down by Mekk3-siRNAs. (a–f) 293T cells were transfected with an empty vector (a) or with siRNA expression vectors against luciferase (Luci-siRNA) (b) or against Mekk3 (c, d) alone (a) or together with expression vectors for MEKK3-GFP (b–d) or MEKK2-GFP (e, f). Transfected cells were analysed 36 hr later by FACS. (g) Quantification of knock down of MEKK3-GFP and MEKK2-GFP expression by FACS. Data shown are the percentage of GFP-positive cells transfected with different siRNAs compared with GFP-positive cells that were transfected with an empty vector. One representative FACS plot is shown for each condition.
Knockdown of MEKK3 expression in Raw264.7 cells by Mekk3-siRNA lentivirus
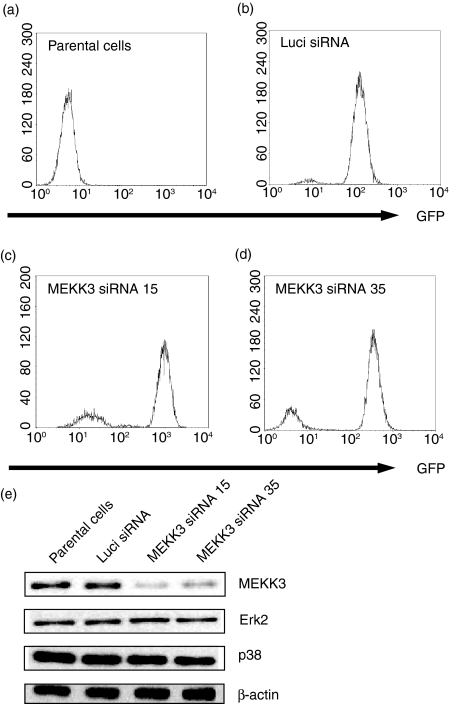
We next subcloned the Mekk3-siRNA expression cassettes into the lentiviral vector FG12 that had been originally designed for germ-line gene transduction.28 This vector also expresses a human UbiC-driven GFP gene that serves as a marker and enables transduced cells to be tracked.28 To understand the function of MEKK3 in macrophages, we used the lentiviruses that express either Luci-siRNA or one of the two Mekk3-siRNAs to transduce Raw264.7 cells. We enriched the numbers of transduced Raw264.7 cells by performing FACS sorting of the GFP-positive cells. As shown in Fig. 2(a), mock-transduced Raw264.7 cells expressed no GFP. However, more than 95% of the cells transduced by Luci-siRNA or by Mekk3-siRNA (Mekk3-siRNA15 or Mekk3-siRNA35) were GFP-expressing cells (i.e. positively transduced cells) (Fig. 2c,d).
Figure 2.
Knocked down MEKK3 expression in the macrophage line Raw264.7. (a–d) Raw264.7 cells (a) and lentivirus-infected Raw264.7 cells that express Luci-siRNA (b), Mekk3-siRNA15 (c), or Mekk3-siRNA35 (d) after sorting for GFP-positive cells followed by FACS analysis. (e) Equal amounts of cell lysates prepared from the cells described above in (a–d) were analysed by immunoblotting using antibodies against MEKK3, ERK2, p38 and β-actin, as indicated. One representative experiment of three is shown.
Next, the levels of the MEKK3 protein and several control proteins in Raw264.7 cells transduced with different siRNAs were measured by performing an immunoblot analysis. As shown in Fig. 2(e), while the endogenous levels of the control proteins ERK2, p38 and β-actin were constant, the endogenous MEKK3 protein level decreased dramatically in cells transduced with either Mekk3-siRNA15 or Mekk3-siRNA35 but not in control cells transduced with Luci-siRNA. These results demonstrated that expressing either the Mekk3-siRNA15 or Mekk3-siRNA35 by means of a lentiviral vector effectively and specifically suppressed endogenous MEKK3 expression in the macrophage line Raw264.7.
Knockdown MEKK3 expression impairs LPS-induced IL-6 and GM-CSF but not TNF-α and IL-1β production in Raw264.7 cells
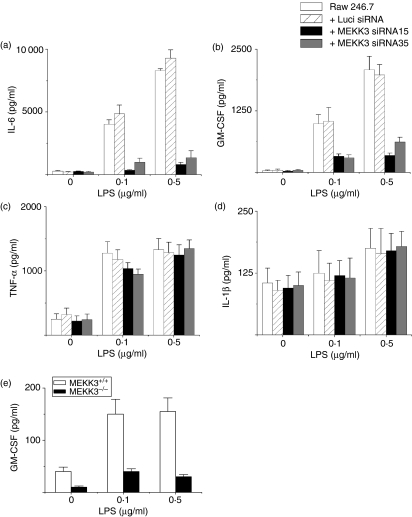
To determine whether MEKK3 must be present for macrophages to produce proinflammatory cytokines in response to LPS, we determined the effect of knocked-down MEKK3 expression by Mekk3-siRNA-expressing lentiviral vectors on the production of various cytokines before and after LPS stimulation in Raw264.7 cells. While the parental and Luci-siRNA-transduced Raw264.7 cells produced high levels of IL-6, the Mekk3-siRNA15- and Mekk3-siRNA35-transduced cells produced significantly reduced levels of IL-6 (Fig. 3a). In addition, the production of GM-CSF was severely impaired in Mekk3-siRNA-transduced cells but not in parental or Luci-siRNA-transduced cells (Fig. 3b). Surprisingly, the LPS-induced TNF-α (Fig. 3c) and IL-1β (Fig. 3d) production were not affected in Mekk3-siRNA-transduced cells. These results suggest that MEKK3 plays a pivotal role in LPS-stimulated IL-6 and GM-CSF production in macrophage Raw264.7 cells. In contrast, TNF-α and IL-1β production may not require MEKK3. Alternatively, the loss of MEKK3 function may be compensated for by different pathways.
Figure 3.
MEKK3 is required for LPS-induced IL-6 and GM-CSF production but not TNF-α and IL-1β production. (a–d) Parental Raw264.7 cells or lentiviral siRNA-transduced cells were stimulated with the indicated amounts of LPS for 24 hr. The culture supernatants were collected for determination of IL-6, GM-CSF, TNF-α and IL-1β production by ELISA. (e) Wild-type and MEKK3–/– MEFs were stimulated with LPS for 24 hr, and analysed for GM-CSF production by ELISA. One representative experiment of three is shown.
To confirm the critical role of MEKK3 in GM-CSF production, we stimulated wild-type and MEKK3–/– MEFs and then determined their GM-CSF production. The MEKK3–/– MEFs produced significantly less GM-CSF than did the wild-type cells, consistent with the results obtained in the MEKK3 knockdown Raw264.7 cells (Fig. 3E). Together, these results demonstrated that MEKK3 is critical for both IL-6 and GM-CSF production induced by LPS.
Effects of MEKK3 knockdown on JNK, p38 and ERK activation and IκBα degradation in Raw264.7 cells
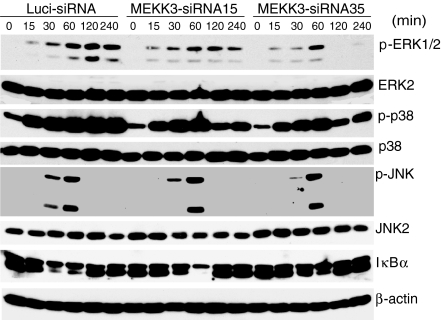
The JNK, ERK and p38 MAPKs are crucial downstream molecules for TLR signalling. To determine whether MEKK3 is required for the LPS-mediated activation of the JNK, ERK and p38 pathways in macrophages, we stimulated the control and Mekk3-siRNA-transduced Raw264.7 cells with LPS for different time-points. Cell extracts were prepared for the determination of ERK1/2, p38 and JNK MAPK activation by immunoblotting analyses. Interestingly, although the ERK1/2, p38 and JNK MAPKs were still induced in the Mekk3 knockdown cells, their levels were consistently lower than those in the control cells with similar kinetics at early time-points (at 15, 30 and 60 min stimulation) (Fig. 4). However, at later time-points (120 and 240 min post-stimulation), we found that both ERK1/2 and p38 activation were significantly reduced. These results suggest that knockdown MEKK3 may be crucial for the ERK1/2 and p38 MAPK activation at later time-points. We also determined the NF-κB activation in MEKK3 knockdown cells and found a partial inhibition of IκBα degradation compared to that in wild-type cells. Thus, these results indicate that the blockade of MAPK activation at later time-points, together with a partial inhibition of the NF-κB pathway caused the inhibition of cytokine gene expression. Interestingly, in our previous study, we did not observe any defect in ERK activation by LPS in fibroblasts. In the Mekk3 knockdown macrophages, ERK1/2 activation was markedly inhibited at later time-points, especially in the Mekk3-siRNA35-transduced cells. These results suggest that MEKK3 participates in the ERK1/2 activation in macrophages but not in fibroblasts. These results also indicate that the MAPK pathways still function in the MEKK3 knockdown Raw264.7 cells and that initial TLR4 signalling to MAPKs is not completely blocked.
Figure 4.
Effect of MEKK3 knockdown on NF-κB and MAPK pathway activation. Raw264.7 cells transduced with lentiviruses expressing Luci-siRNA, Mekk3-siRNA15, or Mekk3-siRNA35 were stimulated with LPS (0·5 μg/ml) for 0, 15, 30, 60, 120 and 240 min, as indicated. Cell lysates were prepared and analysed by immunoblotting using anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-p38, anti-p38, anti-phospho-JNK, anti-JNK2, anti-IκBα and anti-β-actin antibodies, as indicated.
Restoration of LPS-induced IL-6 and GM-CSF expression in MEKK3 knockdown macrophages with human Mekk3 cDNA
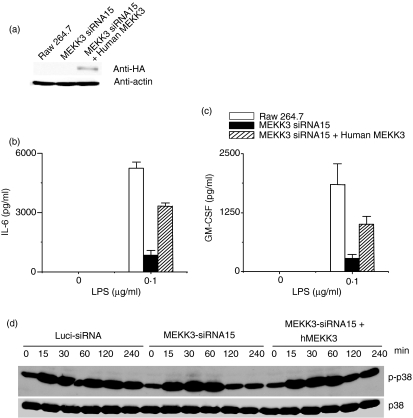
Because siRNA-mediated gene knockdown sometimes introduces artefacts that are not associated with the targeted genes, we decided to confirm our results by examining the restoration of MEKK3 expression in the knockdown cells. Human and mouse MEKK3 is 99% identical at the protein level but only 85% identical at the DNA level. Because three nucleotides in Mekk3-siRNA15 differ from those in the corresponding human Mekk3 DNA sequence, we expected that it would not target the human Mekk3 cDNA. Therefore, we transfected the Mekk3-siRNA15-transduced Raw264.7 cells with an expression vector for a haemagglutinin-tagged human MEKK3 (HA-hMEKK3). Expression of the HA-hMEKK3 was determined by immunoblotting with an anti-HA-antibody (Fig. 5a). To determine if the expression of human MEKK3 in the knockdown Raw264.7 cells restored LPS-induced IL-6 and GM-CSF production, we stimulated the parental, Mekk3-siRNA15-transduced or Mekk3-siRNA15-transduced Raw264.7 cells restored with human MEKK3 with LPS for 24 hr and then measured IL-6 and GM-CSF production in the culture supernatants by ELISA. As shown in Fig. 5(b,c), expression of the human Mekk3 cDNA restored almost 50–60% of IL-6 and GM-CSF production in the Mekk3-siRNA15-transduced cells. To determine whether the expression of human MEKK3 in the Mekk3-siRNA15-transduced Raw264.7 cells restored LPS-induced signalling cascade, we stimulated the parental (Luciferase-siRNA), Mekk3-siRNA15-transduced, or Mekk3-siRNA15-Raw264.7 cells that were restored with human MEKK3 with LPS. As shown in Fig. 5(d), expression of the human Mekk3 cDNA restored about 40–60% of p38 activation in the hMEKK3-transfected Mekk3-siRNA15-transduced cells. This confirmed that the defect in IL-6 and GM-CSF production in Raw264.7 cells transduced with Mekk3-specific siRNAs was indeed the result of the knockdown of MEKK3 expression.
Figure 5.
Restoration of LPS-induced IL-6 and GM-CSF expression in MEKK3 knockdown macrophages by a human Mekk3 cDNA (hMEKK3). Raw264.7 cells infected with lentiviral vector for Mekk3-siRNA15 were transfected with an empty vector or HA-hMEKK3-expressing vector. HA-hMEKK3 expression in the transfected cells was determined by immunoblotting using an anti-HA antibody (a) (b) Control or (c) HA-hMEKK3-transfected cells were stimulated with LPS for 24 hr. The culture supernatants were collected and analysed for IL-6 and GM-CSF production by ELISA as described in Fig. 3. One representative experiment of three is shown. (d) Raw264.7 cells transduced with lentiviruses expressing Luci-siRNA, Mekk3-siRNA15 or HA-hMEKK3-transfected siRNA15 were stimulated with LPS (0·5 μg/ml) for 0, 15, 30, 60, 120 and 240 min, as indicated. Cell lysates were prepared and analysed by immunoblotting using anti-phospho-p38 and total p38 as indicated.
Discussion
In this study, we observed that MEKK3 plays a pivotal role in LPS-induced IL-6 and GM-CSF production but not in the production of two other cytokines, TNF-α and IL-1β. Thus, we conclude that MEKK3 is a crucial and specific regulator of the proinflammatory cytokines IL-6 and GM-CSF in macrophages. We found that the ERK1/2 and p38 MAPK induction by LPS was also inhibited in the MEKK3 knockdown cells. The JNK activation and the IκBα degradation appear to be moderately affected. This study also provided an alternative method for investigating MEKK3 function in other immune cells.
In our previous study, we demonstrated that MEKK3 plays a critical role in IL-1- and LPS-induced IL-6 production in embryonic fibroblasts.26 However, because of the early embryonic lethality of the MEKK3 germ-line knockout in mice, we have been prevented from investigating the role of MEKK3 in proinflammatory cytokine production in immune cells such as macrophages and dendritic cells, which are the key types of cells controlling the innate immune responses and inflammation. To overcome this problem, we developed a strategy to knock down Mekk3 gene expression in the macrophage line Raw264.7 through the use of a lentiviral vector-mediated delivery of siRNA expression vectors. This strategy inhibited the expression of the cotransfected Mekk3 gene and also the endogenous Mekk3 gene by up to 80%. We showed in this study that MEKK3 knockdown in macrophages caused a severe impairment in IL-6 and GM-CSF expression.
Only two of the four siRNAs, Mekk3-siRNA15 and Mekk3-siRNA35 that we tested against the mouse Mekk3 gene worked in this study. However, these two siRNAs were specific because they had no effect on the expression of Mekk2, a close homologue of Mekk3. We also tested these two siRNAs on another unrelated gene, Mip1 and again there was no effect (data not shown). In addition, we designed an anti-human Mekk3-siRNA, hMekk3-siRNA9, with only one nucleotide change from the mouse sequence. While this siRNA appeared able to knock down human Mekk3 expression, it had no effect on mouse Mekk3 expression (data not shown). These results strongly suggest that Mekk3-siRNA15 and Mekk3-siRNA35 were specific to the Mekk3 gene and the impairment in cytokine expression was probably the result of the knock down of MEKK3.
To confirm that the observed impairment in cytokine production was indeed the result of the defective MEKK3 expression, we restored the cytokine expression by reconstitution of MEKK3 expression in the knockdown cells using a human Mekk3 cDNA that could not be targeted by the mouse Mekk3 siRNAs. Although this strategy did not completely restore IL-6 and GM-CSF production, the production of both cytokines was significantly increased compared with production in cells transfected with an empty vector. This incomplete restoration may have been partially the result of transfection inefficiency because not all cells were transfected.
Although the activation of the ERK1/2, p38 and JNK MAPKs in MEKK3 knockdown cells was only moderately affected at the early time-points, we found strong inhibition of the ERK1/2 and p38 activation later, at 2–4 hr after stimulation. We also observed a partial inhibition of IκBα degradation in MEKK3 knockdown cells suggesting that the NF-κB pathway may also be affected in the MEKK3 knockdown cells. These results suggest that the defect in cytokine gene expression in MEKK3 knockdown cells was the result of the inhibition of MAPK at later time-points, and may be partially the result of NF-κB inhibition. Whether other pathways besides the ERK1/2, JNK and p38, or NF-κB pathways, are also impaired in MEKK3 knockdown macrophages is currently unknown. The ERK5/BMPK activation is defective in MEKK2-deficient cells.31 Interestingly, while we found that ERK1/2 activation was defective in MEKK3 knockdown macrophages, we did not find such a defect in our previous study involving embryonic fibroblasts.26 This finding in the present study suggests that MEKK3 signalling may be cell-type-specific.
In addition to the defect in IL-6 production in the MEKK3 knockdown cells, this study also revealed that MEKK3 is required for the expression of another cytokine, GM-CSF, which was confirmed in MEKK3-deficient fibroblasts (Fig. 4e). Interestingly, MEKK3 does not seem to be required for TNF-α and IL-1β production because both cytokines were expressed at a similar level in both the knockdown and control cells. Together, these results suggest that distinct cytokine genes may specifically use the MEKK3 signalling pathway.
In summary, we used siRNAs to knock down MEKK3 expression in a macrophage line and provided evidence that MEKK3 plays an important and specific role in mediating proinflammatory cytokine gene expression in immune cells. While other experimental systems such as conditional MEKK3 knockout mice are important and may be central to investigating the function and regulation of MEKK3 in different immune cells, the siRNA strategy developed in this study promises to serve as a valuable alternative system in the study of other immune cells, especially in the light of the potential improvement in the delivery efficiency of lentiviruses expected in the future. Finally, this strategy is crucial for the study of human immune cells, because mouse genetic manipulation does not work for studies of the human system.
Acknowledgments
This work was supported partially by National Institutes of Health grants AI063348 and HL070225 and a Texas Higher Education grant ARP to B.S. and a Cancer Center Core grant CA16672 (M. D. Anderson Cancer Center). We thank B. Wang for technical assistance, Z. Dong for Raw264.7 cells and B. Notzon for editing the manuscript.
References
- 1.West MA, Heagy W. Endotoxin tolerance. A review. Crit Care Med. 2002;30:S64–S73. [PubMed] [Google Scholar]
- 2.Dunne A, O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- 3.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625–35. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi O, Akira S. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr Top Microbiol Immunol. 2002;270:155–67. doi: 10.1007/978-3-642-59430-4_10. [DOI] [PubMed] [Google Scholar]
- 9.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 10.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–6. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–51. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 12.Kopp E, Medzhitov R, Carothers J, Xiao C, Douglas I, Janeway CA, Ghosh S. ECSIT is an evolutionarily conserved intermediate in the Toll/IL-1 signal transduction pathway. Genes Dev. 1999;13:2059–71. doi: 10.1101/gad.13.16.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee FS, Hagler J, Chen ZJ, Maniatis T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–22. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 14.Baud V, Liu ZG, Bennett B, Suzuki N, Xia Y, Karin M. Signaling by proinflammatory cytokines. Oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999;13:1297–308. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Q, Lee FS. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activate nuclear factor-kappaB through IkappaB kinase-alpha and IkappaB kinase-beta. J Biol Chem. 1999;274:8355–8. doi: 10.1074/jbc.274.13.8355. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Lin Y, Guo Z, et al. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol. 2001;2:620–4. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- 17.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–4. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 18.Dumitru CD, Ceci JD, Tsatsanis C, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–83. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 19.Blank JL, Gerwins P, Elliott EM, Sather S, Johnson GL. Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. Regulation of sequential phosphorylation pathways involving mitogen-activated protein kinase and c-Jun kinase. J Biol Chem. 1996;271:5361–8. doi: 10.1074/jbc.271.10.5361. [DOI] [PubMed] [Google Scholar]
- 20.Su B, Cheng J, Yang J, Guo Z. MEKK2 is required for T-cell receptor signals in JNK activation and interleukin-2 gene expression. J Biol Chem. 2001;276:14784–90. doi: 10.1074/jbc.M010134200. [DOI] [PubMed] [Google Scholar]
- 21.Ellinger-Ziegelbauer H, Brown K, Kelly K, Siebenlist U. Direct activation of the stress-activated protein kinase (SAPK) and extracellular signal-regulated protein kinase (ERK) pathways by an inducible mitogen-activated protein kinase/ERK kinase kinase 3 (MEKK) derivative. J Biol Chem. 1997;272:2668–74. doi: 10.1074/jbc.272.5.2668. [DOI] [PubMed] [Google Scholar]
- 22.Deacon K, Blank JL. Characterization of the mitogen-activated protein kinase kinase 4 (MKK4) /c-Jun NH2-terminal kinase 1 and MKK3/p38 pathways regulated by MEK kinases 2 and 3. MEK kinase 3 activates MKK3 but does not cause activation of p38 kinase in vivo. J Biol Chem. 1997;272:14489–96. doi: 10.1074/jbc.272.22.14489. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer BC, Ware MF, Marrack P, Fanger GR, Kappler JW, Johnson GL, Monks CR. Live cell fluorescence imaging of T cell MEKK2: redistribution and activation in response to antigen stimulation of the T cell receptor. Immunity. 1999;11:411–21. doi: 10.1016/s1074-7613(00)80116-8. [DOI] [PubMed] [Google Scholar]
- 24.Cheng J, Yang J, Xia Y, Karin M, Su B. Synergistic interaction of MEK kinase 2, c-Jun N-terminal kinase (JNK) kinase 2, and JNK1 results in efficient and specific JNK1 activation. Mol Cell Biol. 2000;20:2334–42. doi: 10.1128/mcb.20.7.2334-2342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrington TP, Ishizuka T, Papst PJ, et al. MEKK2 gene disruption causes loss of cytokine production in response to IgE and c-Kit ligand stimulation of ES cell-derived mast cells [In Process Citation] Embo J. 2000;19:5387–95. doi: 10.1093/emboj/19.20.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Q, Yang J, Lin Y, Walker C, Cheng J, Liu ZG, Su B. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat Immunol. 2004;5:98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- 27.Myslinski E, Ame JC, Krol A, Carbon P. An unusually compact external promoter for RNA polymerase III transcription of the human H1RNA gene. Nucl Acids Res. 2001;29:2502–9. doi: 10.1093/nar/29.12.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin XF, An DS, Chen IS, Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA. 2003;100:183–8. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–80. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yee JK, Friedmann T, Burns JC. Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol. 1994;43 Part A:99–112. doi: 10.1016/s0091-679x(08)60600-7. [DOI] [PubMed] [Google Scholar]
- 31.Chayama K, Papst PJ, Garrington TP, et al. Role of MEKK2-MEK5 in the regulation of TNF-alpha gene expression and MEKK2-MKK7 in the activation of c-Jun N-terminal kinase in mast cells. Proc Natl Acad Sci USA. 2001;98:4599–604. doi: 10.1073/pnas.081021898. [DOI] [PMC free article] [PubMed] [Google Scholar]