Abstract
Fas ligand (FasL) expression induces apoptosis of activated T cells and has been suggested as a strategy to inhibit graft rejection. Unfortunately, the use of FasL to confer ‘immune privilege’ in this setting has been hampered by the finding that it may also provoke a destructive granulocytic response. While the Fas/FasL-mediated apoptotic pathways are well defined, the pro-inflammatory effects of FasL are poorly understood. Our aim in this study was to define in vitro the biological effects of FasL on neutrophil recruitment and activation. DAP-3 cells expressing human FasL on the cell membrane (mFasL) potently induced apoptosis in human neutrophils and in activated T lymphocytes. Recombinant human soluble FasL (sFasL), by contrast, was a very weak inducer of apoptosis, even at high concentrations. This latter observation suggests that cleavage of mFasL by naturally occurring matrix metalloproteinases may serve to down-regulate FasL activity in vivo. However, in the presence of a cross-linking antibody, the efficiency of apoptosis-induction by sFasL was greatly increased, suggesting that the lesser pro-apoptotic potency of sFasL reflects an inability to induce trimerization of the Fas receptor. With regard to pro-inflammatory effects, we found that sFasL is a potent neutrophil chemoattractant and, given that it induces little apoptosis, the dominance of sFasL over mFasL may mean that graft-infiltrating neutrophils will survive to mediate inflammation. Neither sFasL nor mFasL produced neutrophil activation as assessed by chemiluminescence assay. This suggests that neutrophils recruited to an inflammatory site by FasL will be activated by mechanisms other than Fas–FasL signalling.
Keywords: apoptosis, Fas ligand, immune privilege, neutrophil, transplantation
Introduction
Since the 19th century, it has been recognized that sites such as the anterior chamber of the eye and the testis permit prolonged, and sometimes permanent, survival of foreign tissue and tumour grafts.1–5 For those with an interest in transplantation, this naturally occurring ‘immune privilege’ provides a tempting model to try to mimic in order to down-regulate allogeneic and xenogeneic immune responses.
Fas ligand (FasL), a member of the tumour necrosis factor family, is thought to play a key role in conferring immune privileged status at such sites,6,7 presumably by inhibiting immune responses through the induction of apoptosis in infiltrating lymphocytes. This observation led to the hope that the artificial expression of FasL on transplanted tissues would act as a local immunosuppressant. Several investigators have sought to mimic this natural immune privilege using expression of FasL as a strategy to protect engrafted tissue. Selawry and Cameron showed that rat pancreatic islet grafts enjoyed prolonged survival when co-transplanted with (FasL-expressing) Sertoli cells.8 Similar results have been achieved by co-transplanting allogeneic islets with allogeneic testicular cell aggregates9 or FasL-expressing myoblasts.10 Other studies have shown prolonged survival of rat liver allografts transfected with a FasL-expressing plasmid.11
However, other data appear to be in conflict with these observations, suggesting that FasL expression may in fact be pro-inflammatory and, rather than conferring immune privilege, targets grafts for destruction.12 Allison and others have shown that islets transplanted from FasL-transgenic mice into allogeneic recipients may be rapidly destroyed.13,14 It is now clear that the mechanism of graft loss in these animals is very different to classical rejection. Islets taken from FasL-transgenic donor animals showed a marked granulocytic destruction even when transplanted into a syngeneic recipient. Indeed, lymphocytes were not involved in this process because similar results were obtained in rag-deficient13 or severe combined immunodeficiency12 mice. Seino and colleagues reported similar neutrophil-mediated destruction of FasL-expressing tumour cells injected subcutaneously in T-cell-deficient nude mice.14,15
These experiments have served to highlight a deficiency in our understanding of FasL biology, namely that while the pro-apoptotic effects of FasL have been well delineated, its pro-inflammatory effects remain poorly characterized. Yet if deployment of FasL is ever to be used successfully to protect grafts, the potential downsides of enforced graft FasL expression, namely induction of neutrophil responses,12,16–18 will need to be addressed. A clearer understanding of the roles of the membrane and soluble forms of the molecule is also needed. Cleavage of the 40 000 molecular weight (MW) membrane FasL (mFasL) by a cell-associated metalloprotease (MMP-7/matrilysin) yields a 26 000 MW soluble form (sFasL).19 It has been suggested that transmembrane FasL is a more efficient ligand and that cleavage to yield sFasL serves to generate an antagonist of mFasL as well as to reduce the amount of the active FasL molecule present.19,20
Our aim in this study was to define in vitro the biological effects of FasL on neutrophil recruitment and activation and to examine the relative contributions of the membrane and soluble forms of the molecule.
Materials and methods
Cells
To study the membrane form of FasL (mFasL), we used cells expressing wild-type human FasL. DAP-3 murine fibroblasts (a kind gift from Dr Nicola Rogers, Imperial College London, UK) were transfected using an electroporation method with cDNA for wild-type human FasL (a kind gift from Professor Jurg Tschopp, University of Lausanne, Switzerland) cloned into the plasmid expression vector, pcDNA 3.1(–) (Invitrogen Corporation, Renfrew, UK).
Briefly, cells to be transfected were harvested, washed in phosphate-buffered saline (PBS) and resuspended in Dulbecco's modified Eagle medium (DMEM) (Invitrogen Corporation) at a concentration of 1·4 × 106/ml. Aliquots of 350 μl containing 5 × 105 cells were transferred to pre-chilled cuvettes and the DNA was added in amounts ranging from 1 μg to 20 μg (typically 5 μg). All samples were made up to a total of 500 μl with medium and incubated on ice for 5 min. Following incubation, the cells were pulsed with 960 mF at 300 V using a Bio-Rad Gene Pulser (Bio-Rad, Hemel Hempstead, UK) and then rapidly transferred to pre-warmed T25 cell culture flasks containing 5 ml DMEM with 10% fetal calf serum (FCS) (MB Meldrum Ltd, Bourne End, UK). Geneticin (Sigma, Poole, UK) was added the following day as a selection agent, initially at a concentration of 1 mg/ml tapering to a typical maintenance level of 200 μg/ml by day 7.
sFasL
The recombinant human sFasL (Sigma) used had a 6× histidine tag to allow cross-linking of the molecules by addition of a murine polyclonal anti-histidine antibody (Sigma).
Antibodies and flow cytometry
To confirm cell-surface expression of FasL, the transfected cells were stained using an indirect immunofluorescence technique. To prevent cleavage of mFasL by MMP, the cells were incubated overnight with the MMP inhibitor KB8301 (10 μm; BD Pharmingen, Oxford, UK) before testing. A murine monoclonal anti-human FasL (NOK-1) antibody (Becton Dickinson, Oxford, UK) was used as the primary antibody and a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin (Sigma) was used as the secondary antibody. In other experiments, T cells were identified by direct immunofluorescence using an FITC-labelled anti-human CD3 antibody (Dako, Glostrup, Denmark). Cells were analysed by flow cytometry performed on a FACScalibur instrument, using cellquest software (both Becton Dickinson). Successfully transfected cells were cloned by a standard limiting dilution technique.
T-lymphocyte isolation
Peripheral blood mononuclear cells (PBMCs) were obtained by a standard Ficoll density gradient separation technique. From this preparation, fresh unactivated T cells were obtained by negative depletion using Dynabeads (Dynal Biotech, Bromborough, UK). Briefly, the prepared PBMCs were resuspended at 107 cells in 100–200 μl PBS/0·1% bovine serum albumin (BSA) (Sigma, Poole, UK). Next, 20 μl heat-inactivated FCS (MB Meldrum Ltd) was added, together with 20 μl per 107 PBMC of proprietary antibody mix (mouse monoclonal antibodies for CD14, CD16, CD56 and HLA class II DR/DP, all Dynal Biotech, Bromborough, UK). The cells were then incubated at 4° for 10 min. Following incubation, the cells were washed with PBS/0·1% BSA and resuspended in 0·9 ml PBS/0·1% BSA per 107 PBMC.
Washed Dynabeads were added to the cells and incubated at room temperature with gentle tilting and rotation for 20 min, 1–2 ml PBS/0·1% BSA was then added to the cells and the tube was placed in a Dynal magnetic particle concentrator for 2 min. Supernatant containing the negatively isolated T cells was pipetted to a fresh tube. Isolates were consistently 90–95% pure and viability was greater than 98% as assessed by trypan blue exclusion.
Neutrophil isolation
Neutrophil isolation was performed using Polymorphprep (Dextran 500 8%; sodium diatrizoate 13·8%– Axis-Shield, Oslo, Norway), a proprietary solution for isolation of polymorphonuclear granulocytes (PMN) from whole blood. The method is based on a modification of a one-step centrifugal technique first described by Boyum.21 Cell purity was typically 90–95% using Giemsa staining with viability > 98% as assessed by trypan blue exclusion.
Apoptosis assays
Induction of apoptosis by Fas–FasL interactions was assessed using human T cells or human neutrophils as target cells. To render the T cells more susceptible to Fas-mediated apoptosis, they were first cultured for 48 hr in the presence of phytohaemagglutinin 1 μg/ml (Sigma) and recombinant human interleukin-2 (IL-2) 10 μg/ml (Leinco Technologies, St Louis, MO).
To assess apoptosis induction by sFasL, target cells were plated out in a 24-well plate at 106 cells/ml suspended in RPMI-1640/10% FCS. sFasL was added at concentrations ranging from 10 to 2000 ng/ml and the cells were incubated for 4 hr at 37° with 5% CO2. Induction of apoptosis was determined by double-staining with propidium iodide and annexin V-FITC (both Becton Dickinson) followed by flow cytometric analysis. Apoptotic cells were propidium iodide negative and annexin V positive.
To assess apoptosis induction by mFasL, DAP-3 murine fibroblasts, either untransfected or transfected to express human FasL, were seeded onto 24-well tissue culture plates so as to create an adherent monolayer of cells. Being cognisant that some degree of cleavage of membrane FasL might occur in the course of this assay (resulting in the generation of low levels of sFasL in the medium), we quantified the amount of sFasL in conditioned medium using a commercially available enzyme-linked immunosorbent assay (Oncogene Research Products, San Diego, CA). This confirmed that sFasL was present at a level unlikely to produce apoptosis in target cells (typically less than 10 ng/ml). We also washed the DAP-3 cells and instilled fresh medium just before incubation with the relevant (Fas-expressing) target cells so as to ensure that no significant amount of sFasL remained. The cells were then incubated for 4 hr at 37° with 5% CO2. As before, induction of apoptosis was determined by double-staining with propidium iodide and annexin V-FITC followed by flow cytometric analysis.
Neutrophil chemotaxis assay
Freshly isolated neutrophils (106 cells) were placed in the upper chamber of a transwell system (Becton Dickinson) and sFasL 0·01–10 nm dissolved in PBS was placed in the lower chamber, separated by a semi-permeable membrane with 3-μm pore size. We assessed neutrophil migration by counting the number of cells appearing in the lower chamber after 2 hr using a Neubauer haemocytometer (Brand, Wertheim, Germany). The chemoattractant peptide, formyl-met-leu-phe (fMLP) 10−9 m (Sigma), was used as a positive control and PBS was used as a negative control.
Neutrophil activation assay
Neutrophil activation produces a characteristic respiratory burst that can be detected by means of a chemiluminescence assay. Neutrophils (105 cells) were added to the wells of a 96-well plate suspended in 200 μl RPMI-1640/10% FCS together with luminol 5 mm (Sigma). The intensity of luminescence produced correlates with the degree of neutrophil activation. The assay was read using an Anthos Lucy-1 chemiluminometer (Labtech International, Ringmer, UK). The phorbol ester, phorbol 12-myristate 13-acetate (Sigma) was used as a positive control (50 ng/ml).
Results
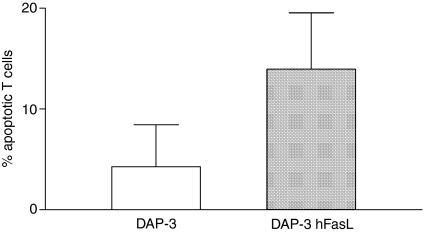
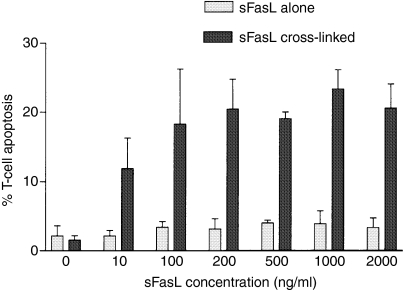
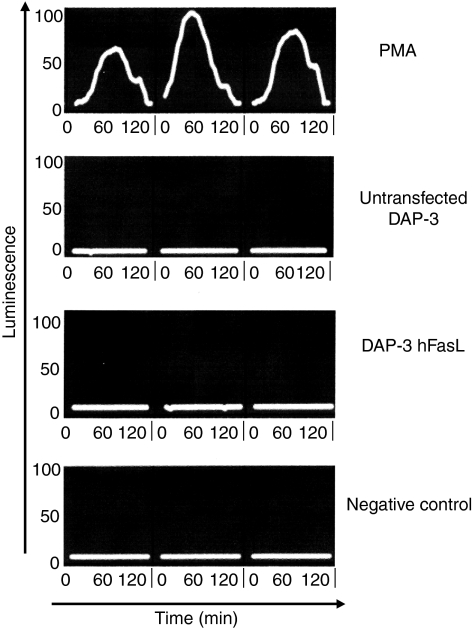
We began by examining the differential effects of mFasL and sFasL using activated T lymphocytes, and in subsequent experiments neutrophils, as targets. Exposure to cell-surface human FasL expressed on DAP-3 murine fibroblasts potently induced apoptosis in activated human T lymphocytes in vitro(Fig.1). By contrast, sFasL was a very weak inducer of apoptosis, even when deployed at high concentrations. However, if the molecule was cross-linked (using a murine polyclonal anti-6× histidine antibody directed against the 6× His tag present on the recombinant FasL), the efficiency of cell killing was greatly increased (Fig.2).
Figure 1.
DAP-3 cells expressing human FasL induce apoptosis in activated human T cells. DAP-3 murine fibroblasts, either untransfected (DAP-3) or transfected to express wild-type human FasL (DAP-3 hFasL) were seeded at 104 cells/well in 24-well tissue culture plates and incubated overnight at 37° with 5% CO2 to create an adherent monolayer of cells. Human CD3 cells, activated by stimulation with phytohaemagglutinin 1 μg/ml and recombinant human interleukin-2 10 μg/ml for 48 hr, were used as Fas-expressing target cells; 2 × 105 CD3 cells were added to the DAP-3 monolayers and incubated for 4 hr. Apoptosis was assessed by staining the T cells with propidium iodide and annexin V-FITC and then analysing by flow cytometry. Apoptotic cells were defined as propidium iodide negative/annexin V positive. A representative example of three experiments is shown.
Figure 2.
Soluble FasL (sFasL) requires cross-linking to deliver an apoptotic signal. T lymphocytes (CD3) were isolated as described above and plated out in a 24-well plate at 106 cells/ml suspended in RPMI-1640/10% FCS. To render the T cells more susceptible to Fas-mediated apoptosis, they were first cultured for 48 hr in the presence of phytohemagglutinin 1 μg/ml and IL-2 10 μg/ml to induce activation and up-regulate Fas expression. For the assay, 2 × 105 cells were incubated for 4 hr with either recombinant human sFasL alone (0–2000 ng/ml) or sFasL cross-linked by a murine polyclonal anti-6× histidine antibody directed against the 6× His tag present on this recombinant molecule. T-cell apoptosis was detected by double-staining with propidium iodide and annexin V-FITC followed by flow cytometry. Apoptotic cells were defined as being propidium iodide negative/annexin V positive. The typical dose–response pattern is shown above. A representative example of three experiments is shown.
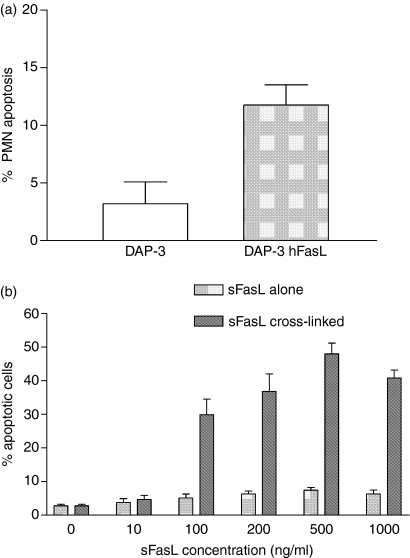
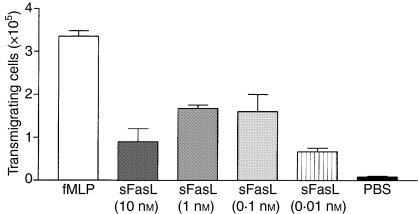
Similarly, when neutrophils were used as the target cell, sFasL remained a very weak inducer of apoptosis. Again, this contrasts with the effect of cross-linked sFasL and with mFasL (Fig.3), both of which effectively induced apoptosis. However, although sFasL lacked efficacy in apoptosis induction, it was a potent chemotactic factor for neutrophils, with peak activity at nanomolar concentrations (Fig.4), suggesting that this is a phenomenon that may have relevance in vivo.
Figure 3.
Membrane FasL (mFasL) and cross-linked soluble FasL (sFasL), but not uncross-linked sFasL, induce apoptosis in neutrophils. To assess apoptosis induction by mFasL (a), 2 × 105 PMN were added to monolayers of DAP-3 or DAP-3 hFasL and incubated for 4 hr. Neutrophil apoptosis was detected by double-staining with propidium iodide and annexin V-FITC followed by flow cytometric analysis. To assess apoptosis induction by sFasL (b), PMN were added to a 96-well plate at 2 × 105 cells/well suspended in 200 μl RPMI-1640/10% FCS and incubated for 4 hr with sFasL alone (0–1000 ng/ml) or sFasL cross-linked by a murine polyclonal anti-6× histidine antibody. Again, apoptosis was detected by double-staining with propidium iodide and annexin V-FITC. Representative examples of three experiments are shown.
Figure 4.
Soluble FasL (sFasL) is chemotactic for neutrophils: 106 freshly isolated neutrophils were placed in the upper chamber of a transwell and varying concentrations of sFasL in 500 μl PBS were placed in the lower chamber separated by a semi-permeable membrane (3-μm pore size). We assessed neutrophil migration by counting the number of cells appearing in the lower chamber after 2 hr. fMLP 10−9 m in the lower chamber was used as a positive control. PBS in the lower chamber was used as a negative control. A representative example of three experiments is shown.
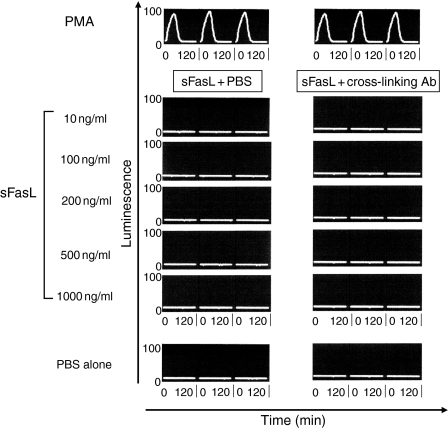
In light of these findings that FasL might mediate neutrophil recruitment, we next examined its ability to activate neutrophils. To address this question, we used a chemiluminescence assay to detect the characteristic oxidative respiratory burst which represents the final phase of neutrophil activation. We saw no evidence of neutrophil activation in this assay, even at high concentrations of sFasL or in the presence of cross-linking antibody (Fig.5). Similarly, mFasL, expressed on the surface of DAP-3 murine fibroblasts, did not produce any detectable neutrophil activation (Fig.6).
Figure 5.
Soluble FasL (sFasL) does not produce neutrophil activation: 105 freshly isolated neutrophils suspended in 200 μl RPMI-1640/10% FCS were added to the wells of a 96-well plate then stimulated with varying concentrations of sFasL in the presence or absence of cross-linking antibody. Neutrophil activation was assessed using a standard chemiluminescence assay read with an Anthos Lucy-1 chemiluminometer. Luminol 5 mm was used as the indicator. Readings were taken at 3-min intervals over 120 min. The intensity of luminescence (arbitrary units) produced correlates with the degree of neutrophil activation. Triplicates are shown for each condition. Figure shown is a representative example of three experiments. Time is shown on the x-axis (0–120 min for each well). Luminesence is shown on the y-axis measured in arbitrary ‘light units’ (0–100).
Figure 6.
Membrane FasL does not produce neutrophil activation: 105 freshly isolated neutrophils suspended in 200 μl RPMI-1640/10% fetal calf serum were added to the wells of a six-well plate coated with a monolayer of DAP-3 fibroblasts either untransfected (DAP-3) or transfected to express human FasL (DAP-3 hFasL). Neutrophil activation was assessed using a standard chemiluminescence assay read with an Anthos Lucy-1 chemiluminometer. Luminol 5 mm was used as the indicator. The intensity of luminescence produced correlates with the degree of neutrophil activation. A representative example of three experiments is shown.
Discussion
If we are to use enforced expression of FasL to protect grafts from rejection, we will need to separate its beneficial pro-apoptotic effects from its pro-inflammatory effects. Our in vitro data suggest that membrane-expressed FasL is efficient at eliminating activated T lymphocytes. However, it is clear that the membrane and soluble forms of FasL have very different functional activities in respect of apoptosis induction, although the mechanism(s) underlying this difference remain unclear. Sieg and colleagues suggested that accessory molecules, specifically intercellular adhesion molecule-1 (ICAM-1), may play a role in modulating FasL activity, postulating that the absence of such accessory molecular interactions might explain the lesser activity of the soluble form.22 However, the finding that cross-linking of sFasL in the absence of interacting with any other surface molecules produces efficient apoptosis-induction, suggests that involvement of ICAM-1 is not an absolute requirement. An alternative explanation is that the lesser potency of sFasL reflects an inability to induce trimerization of the Fas receptor, resulting in defective transduction of ‘death signals’.23 Cross-linking may act to allow trimerization of the Fas–FasL signalling complex to an extent that overcomes the need for additional signals. The lesser activity of sFasL suggests that cleavage of mFasL by naturally occurring matrix metalloproteinases provides a means of down-regulating FasL activity in vivo, not only by reducing levels of the active form of the molecule but also by generating an antagonist. With regard to the pro-inflammatory effects of FasL, the soluble form of FasL is clearly directly chemotactic for neutrophils, in vitro at least. Since sFasL produces little apoptosis one would expect that neutrophils recruited to the graft via sFasL gradients would remain viable for a considerable period.
The exact mechanism by which sFasL exerts its chemotactic effect is unknown. It appears to be mediated via Fas because others have shown that ‘blocking’ anti-Fas antibodies can inhibit FasL-mediated neutrophil chemoattraction.24 Furthermore, neutrophils from lymphoproliferative (lpr) mice, which lack functional Fas receptor expression, do not respond to sFasL.24 By contrast, neutrophils from lpr(cg) mice, which express Fas molecules with a mutated death domain, respond normally to sFasL chemotaxis.24 These data suggest a novel signalling function of Fas, independent of death-domain-mediated apoptosis.
Details of these Fas-mediated pro-inflammatory pathways have yet to be elucidated. It may be that pathways producing neutrophil activation can be triggered when Fas-mediated apoptosis is prevented, for example through up-regulation of anti-apoptotic proteins. This possibility was explored in another context by Bouchet who described differential susceptibility among endothelial cells to apoptosis mediated via up-regulation of FLICE-inhibitory protein (FLIP).25 Bouchet speculated that such cells might prove relatively insusceptible to apoptosis while pro-inflammatory pathways could be initiated by Fas-mediated nuclear factor-κB and extracellular signal-regulated kinase activation.25 Further delineation of such pathways may provide future targets for selective blocking of the FasL-initiated inflammatory response while sparing its apoptotic properties.
With regard to inhibiting neutrophil recruitment, use of non-cleavable forms of FasL (which produce no sFasL) has been mooted as one potential approach. However, the limited data available suggest that the absence of sFasL may not be sufficient to avoid the induction of a neutrophil response. Kang etal. reported that a non-cleavable mutant of FasL, although more active in inducing apoptosis in Fas-expressing targets, did not prevent neutrophilic destruction of islet transplants.26 This has led some to postulate that the initial event leading to an inflammatory response in vivo is in fact the apoptotic demise of a Fas-expressing target cell rather than the generation of sFasL gradients.27 It has been suggested that FasL-mediated apoptosis may not be a passive process, but rather that dying cells can release functionally significant levels of the cytokines IL-1 and IL-10.28 FasL-induced production of IL-1β by target cells has also been suggested as a further mechanism that might incite neutrophil infiltration.29 Our in vitro data argue against such a secondary mechanism, or at least suggest that sFasL may be important on its own. If the chemotactic substance were only released after sFasL had interacted with target cells, there would be no chemotactic gradient across the semi-permeable membrane in our split chamber assays. Accordingly there should be no transmigration. Subsequent release of chemokine molecules from the transmigrated PMNs might amplify the chemotactic response, but only if there had been a direct chemotactic effect of the sFasL initially.
Although several mechanisms have been proposed to explain how FasL might induce neutrophil recruitment, there has been relatively little investigation into the means by which graft FasL expression promotes neutrophil activation. To date the only significant insight has been provided by Chen and colleagues,30 who showed that FasL induced activation of neutrophil mitogen-activated protein kinase, although it remains unclear whether this is sufficient to produce full cellular activation. We could not detect any neutrophil activation in response to exposure to FasL, in either membrane or soluble forms (the latter in either cross-linked or uncross-linked state). It seems likely that neutrophils recruited to the graft by sFasL gradients, or by the release of other chemotactic factors elaborated by apoptotic cells, require mechanisms other than (or in addition to) Fas–FasL signalling to induce subsequent activation. The question of whether FasL facilitates activation by an effect on neutrophil priming or pro-inflammatory cytokine production is one which warrants further study.
Acknowledgments
P.D. was supported by a Clinical Research Fellowship from the Medical Research Council, UK.
Glossary
Abbreviations:
- BSA
bovine serum albumin
- cDNA
complementary DNA
- DMEM
Dulbecco's modified Eagle medium
- FasL
fas ligand
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- FLICE
fas ligand interleukin-1β converting enzyme
- FLIP
FLICE inhibitory protein
- fMLP
N-formyl-methionine-leucine-phenylalanine
- His
histidine
- ICAM-1
intercellular adhesion molecule-1
- IL
interleukin
- lpr
lymphoproliferative
- MMP
matrix metalloproteinase
- mFasL
membrane fas ligand
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PMN
polymorphonuclear granulocytes
- sFasL
soluble fas ligand
References
- 1.van Dooremal J. AkbrecgVab Graefes Arch Ophthalomol. 1873;19:358–73. [Google Scholar]
- 2.Niederkorn JY. The immune privilege of corneal allografts. Transplantation. 1999;67:1503–8. doi: 10.1097/00007890-199906270-00001. [DOI] [PubMed] [Google Scholar]
- 3.Niederkorn JY, Streilein JW, Kripke ML. Promotion of syngeneic intraocular tumor growth in mice by anterior chamber-associated immune deviation. J Natl Cancer Inst. 1983;71:193–9. [PubMed] [Google Scholar]
- 4.Jiang LQ, Streilein JW. Immune privilege extended to allogeneic tumor cells in the vitreous cavity. Invest Ophthalmol Vis Sci. 1991;32:224–8. [PubMed] [Google Scholar]
- 5.Ksander BR, Streilein JW. Immune privilege to MHC-disparate tumor grafts in the anterior chamber of the eye. I. Quantitative analysis of intraocular tumor growth and the corresponding delayed hypersensitivity response. Transplantation. 1989;47:661–7. doi: 10.1097/00007890-198904000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–2. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 7.Filippini A, Riccioli A, Padula F, et al. Control and impairment of immune privilege in the testis and in semen. Hum Reprod Update. 2001;7:444–9. doi: 10.1093/humupd/7.5.444. [DOI] [PubMed] [Google Scholar]
- 8.Selawry HP, Cameron DF. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant. 1993;2:123–9. doi: 10.1177/096368979300200206. [DOI] [PubMed] [Google Scholar]
- 9.Korbutt GS, Elliott JF, Rajotte RV. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes. 1997;46:317–22. doi: 10.2337/diab.46.2.317. [DOI] [PubMed] [Google Scholar]
- 10.Lau HT, Yu M, Fontana A, Stoeckert CJ., Jr Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996;273:109–12. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 11.Li XK, Okuyama T, Tamura A, et al. Prolonged survival of rat liver allografts transfected with Fas ligand-expressing plasmid. Transplantation. 1998;66:1416–23. doi: 10.1097/00007890-199812150-00003. [DOI] [PubMed] [Google Scholar]
- 12.Kang SM, Schneider DB, Lin Z, Hanahan D, Dichek DA, Stock PG, Baekkeskov S. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat Med. 1997;3:738–43. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- 13.Allison J, Georgiou HM, Strasser A, Vaux DL. Transgenic expression of CD95 ligand on islet beta cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts. Proc Natl Acad Sci USA. 1997;94:3943–7. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yagita H, Seino K, Kayagaki N, Okumura K. CD95 ligand in graft rejection. Nature. 1996;379:682. doi: 10.1038/379682a0. [DOI] [PubMed] [Google Scholar]
- 15.Seino K, Kayagaki N, Fukao K, Okumura K, Yagita H. Rejection of Fas ligand-expressing grafts. Transplant Proc. 1997;29:1092–3. doi: 10.1016/s0041-1345(96)00421-6. [DOI] [PubMed] [Google Scholar]
- 16.Ottonello L, Tortolina G, Amelotti M, Dallegri F. Soluble Fas ligand is chemotactic for human neutrophilic polymorphonuclear leukocytes. J Immunol. 1999;162:3601–6. [PubMed] [Google Scholar]
- 17.Kang SM, Lin Z, Ascher NL, Stock PG. Fas ligand expression on islets as well as multiple cell lines results in accelerated neutrophilic rejection. Transplant Proc. 1998;30:538. doi: 10.1016/s0041-1345(97)01396-1. [DOI] [PubMed] [Google Scholar]
- 18.O'Connell J. Immune privilege or inflammation? The paradoxical effects of Fas ligand. Arch Immunol Ther Exp (Warsz) 2000;48:73–9. [PubMed] [Google Scholar]
- 19.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–13. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–6. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 21.Boyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1g gravity field. Scand J Clin Lab Invest Suppl. 1968;97:51–76. [PubMed] [Google Scholar]
- 22.Sieg S, Smith D, Kaplan D. Differential activity of soluble versus cellular Fas ligand: regulation by an accessory molecule. Cell Immunol. 1999;195:89–95. doi: 10.1006/cimm.1999.1530. [DOI] [PubMed] [Google Scholar]
- 23.Holler N, Tardivel A, Kovacsovics-Bankowski M, et al. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23:1428–40. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seino K, Iwabuchi K, Kayagaki N, et al. Chemotactic activity of soluble Fas ligand against phagocytes. J Immunol. 1998;161:4484–8. [PubMed] [Google Scholar]
- 25.Bouchet D, Tesson L, Menoret S, Charreau B, Mathieu P, Yagita H, Duisit G, Anegon I. Differential sensitivity of endothelial cells of various species to apoptosis induced by gene transfer of fas ligand: role of flip levels. Mol Med. 2002;8:612–23. [PMC free article] [PubMed] [Google Scholar]
- 26.Kang SM, Braat D, Schneider DB, et al. A non-cleavable mutant of Fas ligand does not prevent neutrophilic destruction of islet transplants. Transplantation. 2000;69:1813–7. doi: 10.1097/00007890-200005150-00014. [DOI] [PubMed] [Google Scholar]
- 27.Hohlbaum AM, Gregory MS, Ju ST, Marshak-Rothstein A. Fas ligand engagement of resident peritoneal macrophages in vivo induces apoptosis and the production of neutrophil chemotactic factors. J Immunol. 2001;167:6217–24. doi: 10.4049/jimmunol.167.11.6217. [DOI] [PubMed] [Google Scholar]
- 28.Lloyd AR, Oppenheim JJ. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992;13:169–72. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- 29.Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med. 1998;4:1287–92. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 30.Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–7. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]