Abstract
CD8+ regulatory (suppressor) T cells are induced by complex cellular pathways in the spleens of mice that have received an injection of antigen into the anterior chamber (AC) of an eye, an immune-privileged site. Although these CD8+ regulatory T cells perform an antigen-specific regulatory function for an immune response to self and non-self antigens, the mechanisms of the activation or function of these regulatory cells are not clear. Here, we describe a novel mechanism for the activation of splenic CD8+ regulatory T cells induced by injection of antigen into the AC. Immunization of mice with trinitrophenyl and bovine serum albumin (TNP-BSA) amplified AC-induced splenic CD8+ regulatory T cells that suppressed the initiation of contact sensitivity when transferred to immunized, challenged mice. These CD8+ regulatory T cells were produced independently of perforin, indicating that they are not canonical cytotoxic T cells. Fas ligand (FasL)-deficient CD8+ regulatory T-cell function was rescued by inclusion of exogenous interferon-γ (IFN-γ), demonstrating that the expression of FasL by CD8+ regulatory T cells was dispensable, but IFN-γ was not. Ultimately, we demonstrated that the generation of these CD8+ regulatory T cells occurred independently of IFN-γ, but their suppressor function required IFN-γ receptor stimulation.
Keywords: CD8+ regulatory T cells, DTH, immunosuppression, interferon-γ
Introduction
The powerful effector mechanisms of cell-mediated immunity may cause pathology to healthy tissue or induce autoimmunity. Accordingly, cell-mediated immune responses are subject to strong regulatory influences by antigen (anergy) and/or regulatory T cells, which comprise at least three distinct peripheral regulatory T-cell populations: NKT cells;1–4 CD4+ CD25+ T cells;5,6 and CD8+ T cells.7–10 The latter population was first described more than 30 years ago as ‘suppressor T cells’ (reviewed in in refs 7 and 8) and has gained renewed attention in the past few years. CD8+ regulatory (suppressor) T cells are instrumental in regulating autoimmune diseases, such as experimental autoimmune encephalomyelitis, and function in a highly antigen (Ag)-specific manner (reviewed in ref. 8). Moreover, the numbers of Ag-specific, CD8+ regulatory T cells are increased during an immune response, but require certain signals to be activated.11
So-called ‘immune privileged’ sites, where damaging immune reactions are less likely, may utilize mechanisms that increase and/or activate regulatory T cells as a means to inhibit and/or pre-empt a cell-mediated immune response.12 For example, the production and/or activation of splenic CD8+ regulatory T cells by the injection of Ag into the anterior chamber (AC) of an eye requires: (i) that circulating F4/80+ cells have probably been exposed to the anterior chamber environment;12–14 (ii) splenic B cells;15 (iii) recent NKT thymic emigrants;4,11,16 and (iv) peripheral NKT cells.17–19 All of these cells participate in the production of CD8+ regulatory T cells by presenting Ag, by recruitment of regulatory cells and by providing regulatory cytokines.
We have observed that immunization regimens biased towards the induction of delayed-type hypersensitivity (DTH) are associated with the production of splenic CD8+ regulatory T cells activated by injection of the immunizing Ag into the AC.11 Although antigen-presenting cells (APC) pulsed with Ag and transforming growth factor-β (TGF-β) can reprogrammme activated T cells to a suppressive phenotype,20 an increase in antigen-specific CD8+ regulatory T cells would require immunization with the same Ag as that injected into the anterior chamber.11 In addition, the suppressive activity of CD8+ regulatory T cells in vitro is activated by interferon-γ (IFN-γ).21 These observations suggest that the generation and/or activity of CD8+ regulatory T cells occurs during a T helper (Th)-1-biased immune response.
Because the requirements for the increase and activation of CD8+ regulatory T cells are not clear, we investigated a role for IFN-γ in the activation of splenic regulatory CD8+ T cells induced in immunized mice that received an injection of Ag into the AC. We found that the suppression of DTH by perforin-independent, splenic CD8+ regulatory T cells, induced by the injection of Ag into the AC of immunized mice, requires IFN-γ. Moreover, IFN-γ restored suppressive activity to regulatory CD8+ T cells recovered from Fas ligand (FasL)–/– mice that received an injection of Ag into the AC, demonstrating that suppressive activity by CD8+ regulatory T cells does not necessarily depend upon Fas-bearing suppressor cells.
Materials and methods
Mice
Female C57Bl/6-IFN-γtm1Ts (IFN-γ–/–), B6Smn.C3-Tnfsf6gld (FasL–/–), B6.12957-lfn-6γr4mlngt[IFN-γ receptor–/– (IFN-γR–/–)], were purchased from Jackson Laboratories (Bar Harbor, ME). Female C57Bl/6 or BALB/c mice, 6–8 weeks old, were purchased from Jackson Laboratories or Charles River/NCI (Frederick, MD). The mice were maintained in the Center for Laboratory Animal Care of the University of Connecticut Health Center. All work with animals was approved previously by the University of Connecticut Health Center Animal Care Committee (ACC2004-098). All animals were treated according to the Association for Research in Vision and Ophthamology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Antigens/immunization
2,4,6-Trinitrobenzene sulphonic acid [trinitrophenyl (TNP)] and bovine serum albumin (BSA) were purchased from Sigma Chemical Co. (St Louis, MO). TNP-BSA was prepared as described previously.11 Picryl chloride (PCl), 2-chloro-1,3,5-trinitorobenzene (the antigenic equivalent of TNP used to elicit contact sensitivity) was purchased as 2-chloro-5-trypthtane from Chemical Alta Ltd (Edmonton, AB, Canada). Mice were immunized by a single subcutaneous injection of 200 µg of TNP-BSA in 50 µl of Freund's complete adjuvant (Sigma Chemical Co.). In general, the mice were challenged to induce contact sensitivity (CS) to TNP 7–10 days after immunization.
Induction of DTH
CS to TNP in TNP-BSA-sensitized or naïve mice was induced by the epicutaneous application of 15 µl of 1% PCl in acetone/olive oil (4 :1, v/v) to a footpad. The CS response was usually measured approximately 24 hr after the application of PCl by measuring footpad thickness with an engineer's digital micrometer (Mitatoyo, Tokyo, Japan). Mice were anesthetized with ketamine/xylazine (described in greater detail below), and the thickness of each footpad was measured. One footpad was then challenged with PCl and the other with vehicle only. Twenty-four hours later, the mice were anesthetized and the thickness of each footpad measured. The thickness of each footpad, before the application of PCl, was subtracted from that of the thickness of each footpad 24 hr after the application of PCl. Swelling was computed as the difference between the 24-hr thickness of the challenged footpad minus that of the unchallenged footpad.
Injection of antigen into the AC
Mice were immunized with TNP-BSA 7 days before the injection of Ag into the anterior chamber, as described previously.4 Mice were anesthetized with ketamine (75 mg/kg)/xylazine (15 mg/kg) and, under a dissecting microscope, a 30-gauge needle was inserted into the anterior chamber. A 32-gauge needle attached to a cannula, which was attached to a Hamilton syringe (Stoelting Co., Woodale, IL), was inserted into the opening to the anterior chamber made by the 30-gauge needle. Approximately 3 µl of phosphate-buffered saline (PBS), pH 7·2, containing 4 µg of TNP-BSA, was injected into the AC with the manually controlled Hamilton syringe. The mice recovered approximately 30 min after the injection and exhibited no distress, eating and drinking normally.
Induction of splenic regulatory T cells
Mice were immunized 7 days before receiving an injection of Ag into an AC (intracameral injection). In one set of experiments, naïve mice also received an intracameral injection of TNP-BSA. Seven days after receiving an intracameral injection of TNP-BSA, spleens were removed from the mice, diced and expressed through a 40-mm nylon mesh into PBS. The cells were washed twice and suspended (at a concentration of 1 × 108/ml) in PBS.
Immunomagnetic cell separation
Spleen cells from immunized, AC-injected mice were separated, based on the expression of CD8 or CD4, by incubation of the spleen cells with biotinylated monoclonal anti-CD8 or anti-CD4 (Pharmingen, San Jose, CA) at their optimum titre.11 The cells were washed twice with 20× volume labeling buffer and resuspended in 90 µl of labeling buffer per 107 cells. Ten microliters/107 cells of magnetic antibody cell sorting (MACS) streptavidin microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were added and the mixture was incubated for 15 min at 6–12°. The cells were washed with separation buffer (PBS containing 0·5% BSA and 2 mm EDTA) and suspended in 500 µl of separation buffer/1 × 108 cells. The cells were then loaded onto a positive selection column (LS+ Miltenyi Biotec) or a depletion column (BS, Vario, MACS; Miltenyi Biotec) to separate the cells. The cells were washed twice in PBS and resuspended in PBS for injection.
Modified local adoptive transfer assay for regulatory suppressor (effector) cells
Splenic CD8+ regulatory effector T cells were assayed by a modification11 of the local adoptive transfer (LAT) assay.22 Spleen cells (2·5 × 104 at 5 × 106/ml were injected subcutaneously into the footpad of immunized mice immediately following epicutaneous challenge with PCl. Swelling was measured 24 hr later. In experiments investigating the effects of IFN-γ on CD8+ T-cell-mediated suppression, AC spleen (AC-SPL) cells in PBS were mixed with sufficient recombinant IFN-γ (Genzyme, Boston, MA) so that 10 µg was injected into the footpad with the spleen cells.
Statistics
Statistical significance was calculated by one-way analysis of variance (anova). P-values were determined by the Student Neuman–Keuls test, and P-values of < 0·05 were considered significant.
Results
CD8+ regulatory T cells suppress CS
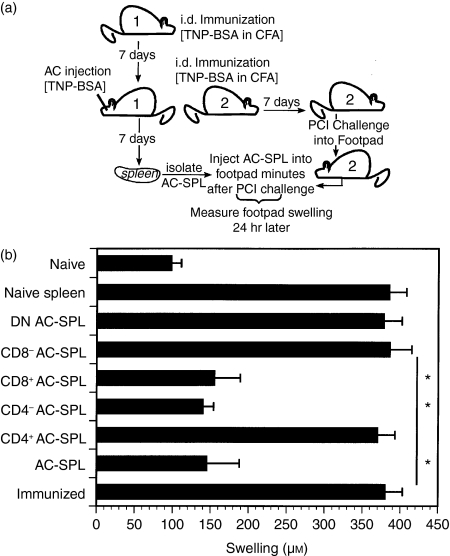
To confirm the nature of the splenic suppressor cells induced by immunization and an injection of Ag into the AC, spleen cells from at least three TNP-BSA-immunized BALB/c or C57Bl/6 mice that received an injection of TNP-BSA into the AC were pooled (respectively) and the pooled cells were separated by immunomagnetic beads into CD8+ or CD4+ cells.11 These cells were injected into the footpads of TNP-BSA-immunized mice immediately after an epicutaneous challenge with PCl to a footpad (Fig.1a). Negatively selected cells were < 1·5% CD4+ or CD8+. Positively selected cells were 94–95% CD4+ or CD8+. The swelling of the challenged footpads of mice receiving CD8+- or CD4+-depleted spleen cells from donors that received an injection of TNP-BSA into the AC was similar to that of naïve mice (Fig.1b). The footpad swelling of mice that received naïve spleen cells was indistinguishable from the swelling of immunized, challenged mice that did not receive spleen cells. The initiation of CS was not suppressed when CD4+ splenic T cells from AC-injected donors were injected into the footpads of immunized mice immediately after challenge (data not shown). Although the results shown are those of AC-SPL cells from BALB/c mice, similar data were obtained with C57Bl/6 AC-SPL cells.11 These results are consistent with observations that the splenic regulatory effector T cells produced after the injection of Ag into the AC are CD8+ T cells.11,22
Figure 1.
CD8+, CD4– anterior chamber spleen (AC-SPL) cells effect the suppression of delayed-type hypersensitivity (DTH) in the modified local adoptive transfer (LAT) assay of suppression.(a)Scheme of modified LAT.(b)One week after trinitrophenyl/bovine serum albumin (TNP-BSA)-immunized BALB/c mice received an injection of TNP-BSA into the AC, spleen cell suspensions were prepared and separated with magnetic antibody cell sorting (MACS) beads, based on the expression of CD4 and CD8. Separated or unseparated spleen cells from AC-injected donors (AC-SPL), or naïve spleen cells, were injected subcutaneously into the challenged footpad minutes after the footpad of a TNP-BSA-immunized mouse received epicutaneous picryl chloride (PCl). Footpad swelling was measured 24 hr later. The data are representative of two experiments, one with BALB/c mice (shown) and one with C57Bl/6 mice, and show the mean swelling ± standard error of the mean (SEM) of four mice per group (*P < 0·01). CFA, Freund's complete adjuvant; DN, double negative; i.d., intradermal.
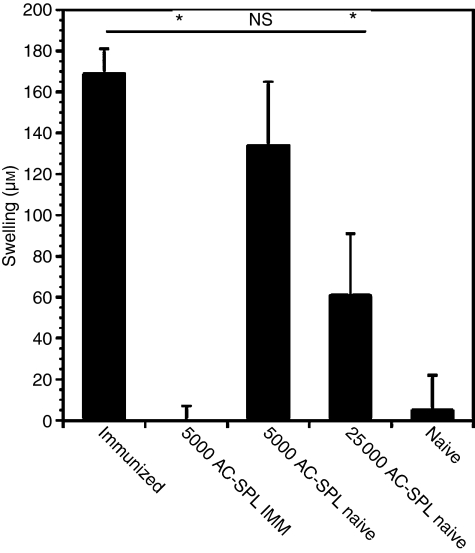
Previously we demonstrated that CD8+ splenic regulatory T cells were detected in mice receiving Th1-biased systemic immunization.11 Accordingly, we investigated whether immunization amplifies the splenic CD8+ regulatory T cells induced by the injection of antigen into the AC. TNP-BSA-immunized or naïve mice received an injection of TNP-BSA into the AC. One week later, spleens were recovered from these two groups, and 25 000 or 5000 AC-SPL cells were injected into the footpads of mice immunized 1 week previously with TNP-BSA. Immediately after transfer the mice received an epicutaneous challenge of PCl and swelling was measured 24 hr later. The DTH response of mice receiving 5000 AC-SPL cells from immunized mice was profoundly suppressed (Fig.2), but AC-SPL cells from non-immunized donors did not suppress DTH unless 25 000 cells were injected.
Figure 2.
Immunization of anterior chamber spleen (AC-SPL) cell donors amplifies splenic CD8+ regulatory T cells. AC-SPL cell donors immunized with trinitrophenyl/bovine serum albumin (TNP-BSA), emulsified in Freund's complete adjuvant, 1 week previously, and naïve mice, received an injection of TNP-BSA into the AC. One week after the injection of TNP-BSA into the AC, spleens were removed and spleen cells from immunized (AC-SPL IMM) and naive AC-SPL donors were recovered. Either 5000 or 25 000 AC-SPL cells were injected into a footpad of TNP-BSA-immunized recipients immediately after the recipient was challenged with epicutaneous picryl chloride (PCl). Swelling was measured 24 hr later. The data represents the mean ± standard error of the mean (SEM) swelling of three mice per group. The data are representative of three experiments, with the same result obtained on each occasion. *P < 0·01; NS, not significant.
The suppression of DTH by CD8+ AC-SPL cells is perforin independent, but requires IFN-γ
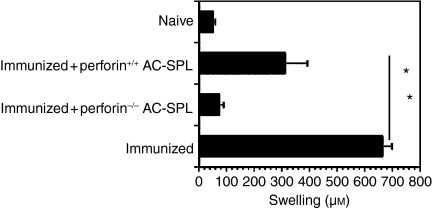
To determine if the suppression of DTH by CD8+ AC-SPL cells was the result of perforin-dependent cytoxicity, perforin–/– and perforin+/+ C57Bl/6 mice were immunized with TNP-BSA and then received an AC injection of TNP-BSA. One week later, spleen cells from these mice were injected into the footpads of TNP-BSA-immunized mice immediately after the immunized mice were challenged epicutaneously with PCl. CS to TNP in the recipients of spleen cells from AC-injected, TNP-BSA immunized mice from perforin–/– donors was similar to that of TNP-BSA-immunized mice challenged with PCl only (Fig.3).
Figure 3.
The suppression of delayed-type hypersensitivity (DTH) by anterior chamber spleen (AC-SPL) cells is perforin independent. Seven days after C57Bl/6 mice were immunized with trinitrophenyl/bovine serum albumin (TNP-BSA), a footpad of C57Bl/6-immunized or naïve recipients was challenged with epicutaneous picryl chloride (PCl). Immediately after challenge, some mice received an intradermal injection of AC-SPL cells from TNP-BSA-immunized C57Bl/6 perforin+/+ or perforin–/– donors. Swelling was measured 24 hr after challenge. The data represent the mean ± standard error of the mean (SEM) swelling of four mice per group. *P < 0·01.
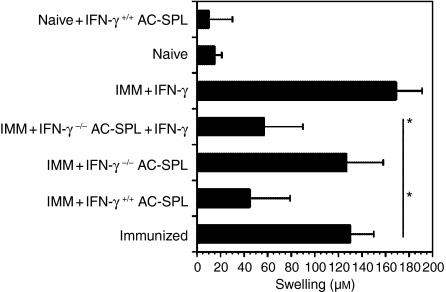
A major product of stimulated CD8+ T cells is the effector cytokine, IFN-γ. Because Th1-biased immunization amplified the generation of splenic CD8+ regulatory T cells induced by the injection of antigen into the AC, we reasoned that IFN-γ may influence the development or activity of CD8+ regulatory T cells. To test this notion, C57Bl/6 wild-type and IFN-γ–/– mice were immunized with TNP-BSA as before and then TNP-BSA was injected into the AC. One week after AC injection, spleen cell suspensions were prepared and injected into a footpad of TNP-BSA-immunized mice immediately after an epicutaneous application of PCl. Swelling of the footpads was measured 24 hr later. The Ag-induced swelling of the footpad was significantly reduced in mice receiving spleen cells from AC-injected wild-type mice (Fig.4). In contrast, the swelling of footpads of the recipients of spleen cells from IFN-γ–/– mice was not suppressed unless IFN-γ was included in the inoculum with the AC-SPL cells. Importantly, injection of IFN-γ alone slightly increased swelling, but did not suppress DTH.
Figure 4.
Suppression by regulatory anterior chamber spleen (AC-SPL) cells requires interferon-γ (IFN-γ). Twenty-five thousand AC-SPL cells from trinitrophenyl/bovine serum albumin (TNP-BSA)-immunized (IMM) C57Bl/6 IFN-γ+/+ and IFN-γ–/– donors and 25 000 AC-SPL cells from the IFN-γ–/– donors + 10 µg of IFN-γ, or IFN-γ only, were injected into the footpad of TNP-BSA-immunized mice minutes after epicutaneous challenge with picryl chloride (PCl). Swelling was measured 24 hr later and the data represent the mean swelling ± standard error of the mean (SEM) of four mice per group. The data are representative of two experiments, with three to four mice per group. *P < 0·05.
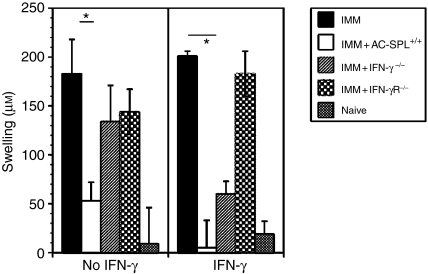
To investigate further the role of IFN-γ in the regulation of CD8+ regulatory T cells, spleen cells from TNP-BSA-immunized, AC-injected C57Bl/6 IFN-γR–/– mice were recovered and injected into the footpads of TNP-BSA-immunized C57Bl/6 mice minutes after the footpads were challenged with epicutaneous PCl. Spleen cells from IFN-γ–/– or IFN-γR–/– mice did not suppress DTH, although DTH was suppressed by spleen cells from AC-injected wild-type mice (Fig.5, left panel). In contrast, IFN-γ restored the suppressive ability to spleen cells from IFN-γ–/– mice, but did not restore suppression to spleen cells from IFN-γR–/– mice (Fig.5, right panel).
Figure 5.
CD8+ anterior chamber spleen (AC-SPL) cells require an interferon-γ receptor (IFN-γR). C57Bl/6 IFN-γ–/–, IFN-γR–/– and wild-type mice were immunized (IMM) with trinitrophenyl/bovine serum albumin (TNP-BSA) and received an injection of TNP-BSA into the AC. AC-SPL cells were recovered 1 week after injection of TNP-BSA into the AC and were injected into a footpad without (left panel) or with (right panel) IFN-γ minutes after epicutaneous challenge with picryl chloride (PCl). Swelling was measured 24 hr later. Data represent the mean swelling ± standard error of the mean (SEM) of two separate experiments, with a total of six to eight mice per group. *P < 0·01.
IFN-γ restores CD8+-mediated suppression by FasL–/– AC-SPL cells
Although the suppression of DTH by AC-SPL cells is not perforin dependent, we investigated whether these cells may be cytotoxic through the expression of FasL. To test this hypothesis, TNP-BSA-immunized C57Bl/6 FasL–/– and wild-type mice received an injection of TNP-BSA into the AC. One week later, spleen cell suspensions from these donors were prepared and the spleen cells were injected into a footpad of TNP-BSA-immunized C57Bl/6 wild-type mice immediately after the footpad was challenged with PCl. Swelling was measured 24 hr later. The swelling of a footpad of TNP-BSA-immunized mice challenged with PCl was suppressed in the recipients of spleen cells from AC-injected FasL+/+ donors (Fig.6). In contrast, the swelling of a footpad of recipients of spleen cells from AC-injected FasL–/– donors was no different from that of TNP-BSA-immunized challenged mice that did not receive spleen cells (Fig.6). To determine if IFN-γ could rescue suppression in the FasL–/– AC-SPL cell population, we also injected AC-SPL cells and IFN-γ into the footpads of PCl-challenged, TNP-BSA-immunized recipients. As observed previously (Fig.4), the injection of IFN-γ only enhanced the swelling of the footpad. In contrast, footpad swelling was significantly reduced in the recipients of IFN-γ and AC-SPL cells from FasL–/– mice (Fig.6).
Figure 6.
Interferon-γ (IFN-γ) restores the suppression of delayed-type hypersensitivity (DTH) by Fas ligand–/– (FasL–/–) anterior chamber spleen (AC-SPL) cells. Twenty-five thousand AC-SPL cells from trinitrophenyl/bovine serum albumin (TNP-BSA)-immunized C57Bl/6 FasL+/+ or FasL–/– donors were injected, with or without IFN-γ, into the footpads of TNP-BSA-immunized C57Bl/6 mice immediately after the recipients were challenged with epicutaneous picryl chloride (PCl). Swelling of the footpads was measured 24 hr later. The data represents the mean swelling ± standard error of the mean (SEM) of three mice per group. The data are representative of three experiments, with similar results obtained on each occasion. *P < 0·01.
Discussion
CD8+ regulatory T cells produced after the injection of Ag into the AC may be induced in at least two ways. Antigen and TGF-β in the aqueous humor confer a suppressive phenotype on F4/80+ iris cells that, in turn, probably confer a suppressive phenotype on T cells in the anterior chamber.20 Although it has been suggested that these F4/80+ iris cells migrate into the circulation and home to the spleen, thereby delivering intracameral antigen,13,23 we have suggested that the resident iris APC may confer a suppressive phenotype on circulating F4/80+ cells that entered the AC after the intracameral injection of antigen, and that these circulating F4/80+ cells re-enter the circulation and home to the thymus and spleen.14 Antigen introduced into the anterior chamber does enter the circulation.11,24,25 Some soluble ocular-derived Ag could also be in a form that induces regulatory T cells.26 Antigen injected into the anterior chamber is found in the thymus, but cell-free intravenous Ag does not penetrate the thymus.11 Thus, available evidence suggests that F4/80+ cells provide a critical signal that induces and/or activates splenic and thymic regulatory T cells. The observation that AC-SPL cells from immunized mice were more effective in suppressing DTH than AC-SPL from naïve mice suggests that immunization may increase the number and/or the efficiency of antigen-specific CD8+ regulatory T cells induced by the injection of Ag into the AC. Accordingly, the splenic CD8+ suppressor T cells generated are activated by the presentation of intracameral antigen13,14,19 and/or are converted from CD8+ effector T cells to a suppressive phenotype.20 Preliminary data generated by the injection of three to four dilutions of AC-SPL cells into the footpads of immunized mice after challenge suggest that immunization does increase the number of CD8+ AC-SPL cells. However, spleen cells from immunized (only) mice do not suppress DTH when used in the modified LAT assay.11 Although we have not investigated variations in the suppressive activity of CD8+ AC-SPL cells from individual mice, our experience has been that the suppressive activity of pooled AC-SPL cells is very consistent (> 75% suppression by 5000–25 000 AC-SPL cells).
The requirement for IFN-γ to activate the CD8+ regulatory T cells is consistent with our observation that CD8+ regulatory T cells are detected in mice immunized by regimens that induce a Th1 response, but not regimens biased towards a Th2 response.11 Although CD8+ regulatory T cells are produced in the absence of IFN-γ, the suppressive activity of these cells requires IFN-γ. The necessity for IFN-γ in CD8+ AC-SPL cell-mediated suppression is buttressed by the demonstration that CD8+ regulatory T cells are not activated by IFN-γ in IFN-γR–/– mice (Fig.5). Moreover, the suppressive activity of AC-SPL cells is removed by the removal of spleen cells expressing IFN-γR from AC-SPL cells (X. Li, R. E. Cone, unpublished). Additionally, preliminary evidence suggests that the APC required to activate the CD8+ AC-SPL cells are not dependent on IFN-γ (R. E. Cone, unpublished). Although IFN-γ plays a central role in a Th1 response, the immunosuppressive characteristics of this cytokine have been demonstrated in vivo as protective from autoimmune disease or asthma.27–30
AC-SPL cells from perforin–/– mice suppressed DTH in the modified LAT, indicating that the CD8+ AC-SPL suppressor T cells are not canonical cytotoxic T cells. The higher DTH response observed in these experiments (Fig.3) is probably a result of these experiments being performed 5 months before the other experiments described herein and the use of a different batch of TNP-BSA. Moreover, the suppressive activity of CD8+ regulatory T cells in the modified LAT assay is independent of FasL, because in the presence of IFN-γ, FasL–/– T cells from AC-injected donors suppressed DTH. This observation was based on a previous finding, demonstrating that CD8+ T-cell-mediated suppression in certain models depends upon IFN-γ production in order to elaborate TGF-β activity.21 In addition, CD8+ AC-SPL cells do produce TGF-β but do not produce IL-10.31 Nevertheless, Kezuka & Streilen reported that CD8+ suppressor T cells are produced in FasL–/– mice.32 The modified LAT we use to assay for CD8+ regulatory T cells uses a limiting (25 000) number of spleen cells, while the assay used by Kezuka & Streilein uses at least 40-fold more spleen cells to inhibit large numbers of DTH-inducing T cells from immunized mice. Therefore, it is possible that the use of larger numbers of AC-SPL cells from FasL–/– mice may provide sufficient levels of IFN-γ to facilitate suppression by the regulatory effector T cells. We have not tested this idea, but it is the subject of another investigation.
In summary, our results buttress the proposal8 that CD8+ regulatory T cells are produced during an immune response. That these cells require the products of activated T cells indicates that the therapeutic generation and maintenance of these cells requires a strategy that utilizes an ongoing immune response.
Acknowledgments
This work was supported by NIH grants EY013243, EY017289 (REC), NIH grants AI052108 and AI142858 (ATV), the University of Connecticut Health Center Research Advisory Committee and the Connecticut Lions Eye Research Foundation.
Glossary
Abbreviations:
- AC
anterior chamber
- Ag
antigen
- BSA
bovine serum albumin
- CS
contact sensitivity
- DTH
delayed-type hypersensitivity
- IFN-γ
interferon-γ
- IFN-γR
interferon-γ receptor
- LAT
local adoptive transfer
- MACS
magnetic antibody cell sorting
- PBS
phosphate-buffered saline
- PCl
picryl chloride
- SEM
standard error of the mean
- TCR
T-cell receptor
- Th
T helper
- TGF
transforming growth factor
- TNP
trinitrophenyl
References
- 1.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;14:535–62. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 2.Laloux V, Beaudoin L, Jeske D, Carnaud C, Lehuen A. NK T cell-induced protection against diabetes in Va 14-Ja281 transgenic nonobese diabetic mice is associated with a Th2 shift circumscribed regionally to the islets and functionally to islet autoantigen. J Immunol. 2001;166:3749–56. doi: 10.4049/jimmunol.166.6.3749. [DOI] [PubMed] [Google Scholar]
- 3.Sonoda K-H, Exley M, Snapper S, Balk ST, Stein-Streilein J. CD1-reactive natural killer T cells are required for the development of systemic tolerance through an immune privileged site. J Exp Med. 1999;190:1215–26. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Goldschneider I, O'Rourke J, Cone RE. Blood mononuclear cells induce regulatory NK thymocytes in anterior chamber-associated immune deviation. J Leukoc Biol. 2001;69:741–6. [PubMed] [Google Scholar]
- 5.Sakaguchi S, Sakaguchi NM, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 6.Shevach EM. CD4+, CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 7.Green DR, Flood PM, Gershon RK. Immunoregulatory T cell pathways. Annu Rev Immunol. 1983;1:439–61. doi: 10.1146/annurev.iy.01.040183.002255. [DOI] [PubMed] [Google Scholar]
- 8.Jiang H, Chess L. An integrated model of immunoregulation mediated by regulatory T cell subsets. Adv Immunol. 2004;83:253–88. doi: 10.1016/S0065-2776(04)83008-6. [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, Braunstein NS, Winchester B, Yu R, Chess L. CD8+ T cells control the TH phenotype of MBP-reactive CD4+ T cells in EAE mice. Proc Natl Acad Sci USA. 2001;98:6301–6. doi: 10.1073/pnas.101123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–23. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Wang Y, Urso D, O'Rourke J, Cone RE. Thymocytes induced by antigen injection into the anterior chamber activate splenic CD8+ suppressor cells and enhance the antigen-induced production of immunoglobulin G1 antibodies. Immunology. 2004;113:44–56. doi: 10.1111/j.1365-2567.2004.01928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niederkorn JY. Immune privilege in the anterior chamber of the eye. Crit Rev Immunol. 2002;11:13–46. [PubMed] [Google Scholar]
- 13.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID) II. Eye-derived cells participate in generating blood-borne signals that induce ACAID. J Immunol. 1991;146:3018–24. [PubMed] [Google Scholar]
- 14.Li X, Shen S, Urso D, Kalique S, Park SH, Sharafieh R, O'Rourke J, Cone RE. Phenotypic and immunoregulatory characteristics of monocytic iris cells. Immunology. 2006;117:566–75. doi: 10.1111/j.1365-2567.2006.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Orazio TG, Niederkorn JY. Splenic B cells are required for tolerogenic antigen presentation in the production of anterior chamber-associated immune deviation. Immunology. 1998;95:47–55. doi: 10.1046/j.1365-2567.1998.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Goldschneider I, Foss D, Wu D, O'Rourke J, Cone RE. Direct thymic involvement in anterior chamber-associated immune deviation: evidence for a nondeletional mechanism of centrally induced tolerance to extrathymic antigens in adult mice. J Immunol. 1997;158:2150–5. [PubMed] [Google Scholar]
- 17.Sonoda K-H, Faunce DE, Taniguchi M, Exley M, Balk S, Stein-Streilein J. NK T cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells in systemic tolerance. J Immunol. 2001;166:42–50. doi: 10.4049/jimmunol.166.1.42. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Sonoda K-H, Faunce DE, Gumperz J, Yamamura T, Mikake S, Stein-Streilein J. CD4+ NKT cells, but not conventional CD4+ T cells are required to generate efferent CD8+ regulatory T cells following antigen inoculation in an immune privileged site. J Immunol. 2003;171:1266–71. doi: 10.4049/jimmunol.171.3.1266. [DOI] [PubMed] [Google Scholar]
- 19.Faunce DE, Sonoda K-H, Stein-Streilein J. MIP-2 mediated recruitment of NKT cells to the spleen during tolerance induction. J Immunol. 2001;166:313–21. doi: 10.4049/jimmunol.166.1.313. [DOI] [PubMed] [Google Scholar]
- 20.Kezuka T, Streilein JW. In vitro generation of regulatory CD8+ T cells similar to those in mice with anterior chamber-associated immune deviation. Inv Opthalmol Vis Sci. 2000;41:1803–11. [PubMed] [Google Scholar]
- 21.Myers L, Croft M, Kwon BS, Mittler RS, Vella AT. Peptide-specific CD8 regulatory T cells use IFN-γ to elaborate TGF-β- based suppression. J Immunol. 2005;174:7625–32. doi: 10.4049/jimmunol.174.12.7625. [DOI] [PubMed] [Google Scholar]
- 22.Niederkorn JY, Streilein JW. Characterization of the suppressor cell(s) responsible for anterior chamber-associated immune deviation (ACAID) induced in BALB/c mice by P815 cells. J Immunol. 1985;134:1381–7. [PubMed] [Google Scholar]
- 23.Wilbanks GA, Streilein JW. Macrophages capable of inducing anterior chamber associated immune deviation demonstrate spleen-seeking migratory properties. Reg Immunol. 1992;4:130–7. [PubMed] [Google Scholar]
- 24.Dulforce PA, Gareman KL, Seitz GW, Fleischman RJ, Crespo SM, Planck SR, Parker DC, Rosenbaum JT. APCs in the anterior uveal tract do not migrate to draining lymph nodes. J Immunol. 2004;172:6701–8. doi: 10.4049/jimmunol.172.11.6701. [DOI] [PubMed] [Google Scholar]
- 25.Camelo S, Kezic J, Shanley A, Rigby P, McMenamin P. Antigen from the anterior chamber travels in soluble form to secondary lymphoid organs via lymphatic and vascular routes. Invest Ophthamol Vis Sci. 2005;47:1039–46. doi: 10.1167/iovs.05-1041. [DOI] [PubMed] [Google Scholar]
- 26.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 27.Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-γ production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–35. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hachem P, Lisbonne M, Michel M, et al. a-galactosylceramide-induced iNKT cells suppress experimental allergic asthma in sensitized mice: role of IFN-γ. Eur J Immunol. 2005;35:2793–802. doi: 10.1002/eji.200535268. [DOI] [PubMed] [Google Scholar]
- 29.Krakowsi M, Owens T. Interferon γ confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–6. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 30.Ferber IAS, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 31.Wang Y, Ghali WE, Pingle P, Traboulsi A, Dalal T, O'Rourke J, Cone RE. Splenic T cells from mice receiving intracameral antigen suppress in-vitro antigen-induced proliferation and interferon-gamma production by sensitized lymph node cells. Ocular Immunol Inflamm. 2003;11:39–52. doi: 10.1076/ocii.11.1.39.15578. [DOI] [PubMed] [Google Scholar]
- 32.Kezuka T, Streilein JW. Evidence for multiple CD95–CD95 ligand interactions in anterior chamber-associated immune deviation induced by soluble protein antigen. Immunology. 2000;99:451–7. doi: 10.1046/j.1365-2567.2000.00983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]