Abstract
Hyper-immunoglobulin M (IgM) syndrome (HIGM) is a rare primary immunodeficiency characterized by elevated or normal IgM and absent or markedly decreased amounts of IgG, IgA and IgE. The X-linked form (HIGM1) is the most common type and is caused by mutations in the gene for CD40L, a T-cell surface molecule required for T-cell driven immunoglobulin class switching by B cells. In the present study we have identified a patient with X-linked hyper-IgM who failed to express CD40L on the cell surface of CD4+ T lymphocytes. Sequence analysis of CD40L genomic DNA showed no mutations. CD40L mRNA was absent and sequence analysis of the CD40L promotor revealed a mutation at position −123 from the transcription start site. The mutation in the promotor region likely contributed to the decreased transcription as demonstrated by a luciferase reporter assay.
Keywords: hyper-IgM, CD40L, immunodeficiency
Introduction
Hyper-immunoglobulin M (HIGM) syndrome is a rare primary immunodeficiency characterized by elevated or normal immunoglobulin M (IgM) and absent or decreased amounts of IgG, IgA and IgE. Several molecular defects have been described that result in the HIGM syndrome. The X-linked form is the most common type and is caused by mutations in the gene encoding the CD40 ligand (CD40L or CD154) (XHIGM; HIGM1).1–5 CD40L is expressed on activated T cells, and its interaction with CD40, which is constitutively expressed on B cells and antigen-presenting cells, is required for class switch recombination. Patients with mutation in CD40L are susceptible to recurrent sino-pulmonary and opportunistic infections, neutropenia, autoimmune complications such as sclerosing cholangitis, and malignancies.6,7
In this report we describe a patient with X-linked HIGM caused by a mutation in the promotor for CD40L. Mutations in the promoter of CD40L causing HIGM1 have not been reported previously8 (CD40Lbase, a registry for mutations in CD40L http://bioinf.uta.fi/CD40Lbase/index.html).
Materials and methods
Sequence analysis of CD40L
Genomic DNA was isolated from peripheral blood using the QIAamp DNA blood mini kit (Qiagen, Hilden, Germany). Polymerase chain reaction (PCR) amplification of the five exons of the CD40L gene was performed in 50 µl reaction mixture containing 100 ng genomic DNA, 750 nm (exon 1–4) or 250 nm (exon 5) primers, 1× Gene Amp PCR buffer (Applied Biosystems, Foster City, CA), 1·5 mm MgCl2, 200 µm of each dinucleotide triphosphate (dNTP) and 1 U AmpliTaq Gold. Oligonucleotides used as primers were as described by Shimadzu et al.16 Forty cycles of amplification were performed in a 9700 thermocycler (Applied Biosystems). Each cycle consisted of 30 s denaturation at 94°, a 30 s annealing step at 55°, and a 60 s extension step at 72°. The PCR products were sequenced on the ABI310 using the Big Dye Terminator Cycle sequencing kit (Applied Biosystems).
RNA isolation, reverse transcription (RT) and PCR amplification
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood on Lymphoprep density gradient (Nycomed Pharma). Cells were resuspended in phosphate-buffered saline. Total RNA was isolated from PBMC after stimulation with phorbol 12-myristate 13-acetate (PMA; 25 ng/ml/6 hr) and ionomycin using the RNeasy mini kit (Qiagen), and reverse transcribed into cDNA using murine moloney leukaemia virus reverse transcriptase (Invitrogen, San Diego, CA) and a random hexamer primer (Amersham Biosciences, Amersham, UK) in a 40 µl final volume. First-strand cDNA was used directly for PCR amplification. PCR amplification was performed in 50 µl reaction mixture containing 3 µl cDNA, 1× Gene Amp PCR buffer (Applied Biosystems), 1·5 mm MgCl2, 200 µm of each dNTP, 1 U AmpliTaq Gold, 5% dimethyl sulphoxide, and 250 nm primers. Oligonucleotides used as primers are described in Table 1. Forty cycles of amplification were performed in a 9700 thermocycler (Applied Biosystems). Each cycle consisted of 30 s denaturation at 94°, a 30 s annealing step at 55°, and a 60 s extension step at 72°. The PCR products were visualized on a 2% agarose gel.
Table 1.
Oligonucleotide primers for PCR amplification
| Oligonucleotide sequence | |
|---|---|
| Oligonucleotide primers for PCR amplification of CD40L cDNA | |
| Sense | 5′-gccagaagataccatttcaa |
| Antisense | 5′-cttatgacatgtgccgcaatt |
| Sense | 5′-aaggtgatcagaatcctca |
| Antisense | 5′-ctgcaaggtgacactgttc |
| Oligonucleotide primers for PCR amplification of CD40L promotor | |
| Sense | 5′-actggggagagcattcagg |
| Antisense | 5′-tctgccaagtaggtggcttc |
| Sense | 5′-ccaaagcctctgacttgactg |
| Antisense | 5′-tgcacctgtcttgcttatcct |
| Sense | 5′-gtgtcaaatttcttccatgcac |
| Antisense | 5′-tgactggtgtcccatcaact |
| Sense | 5′-gccaggctttcattgagtttag |
| Antisense | 5′-gcagaggcagcatgagaag |
| Oligonucleotides used as primers for amplification of CD40L 3′ untranslated region and poly A site | |
| Sense | 5′-gcaaatacccacagttccgc |
| Antisense | 5′-gggacagggtggaaagaagag |
| Sense | 5′-acacagagtcaggccgttgc |
| Antisense | 5′-tgtcacagacttccagaaagtgtg |
| Sense | 5′-aagcagcaaccccactgat |
| Antisense | 5′-tctttccccaacctggctg |
Sequence analysis of CD40L and CD40L promotor
The CD40L gene was sequenced using oligonucleotide primers flanking the five exons as described by Shimadzu et al.9 PCR amplification of the promotor of the CD40L gene was performed using four overlapping primer sets. Oligonucleotides used as primers are described in Table 1. Oligonucleotides used as primers for PCR amplification of the CD40L untranslated region are described in Table 1. PCR amplification was performed using the Expand High Fidelity PCR System (Roche Diagnostics, Indianapolis, IN), according to the manufacturer's instructions. Briefly, 50 µl reaction mixture contained 100 ng DNA, 300 nm primers, 1× Gene Amp PCR buffer (Applied Biosystems), 1·5 mm MgCl2, 200 µm of each dNTP and 2·6 U Expand High Fidelity PCR Systemenzyme mix. Amplification was performed in a 9700 thermocycler (Applied Biosystems). The PCR protocol was as follows: 2 min denaturation at 94°; 10 cycles consisting of 15 s denaturation at 94°, a 30 s annealing step at 54° and a 1 min elongation step at 72°; 15 cycles consisting of 15 s denaturation at 94°, a 30 s annealing step at 54° and a 1 min elongation step at 72° plus cycle elongation of 5 s for each cycle; and a prolonged elongation time of 45 s. PCR products were sequenced using the Big Dye Terminator Cycle sequencing kit (Applied Biosystems).
Reporter plasmid construction, transient transfection and luciferase reporter gene assay
The CD40L promoter region of the patient and a control subject from (−1227 to +67) relative to the start of transcription was isolated by PCR and subcloned into the luciferase reporter plasmid pGL3 (Promega, Madison, WI) as previously described.10 Sequence analysis was performed to document the presence of the mutation in the patient's CD40L promoter and the absence of other mutations in the patient and control CD40L promoter. A plasmid expressing renilla luciferase under the control of the β-actin promoter that was subcloned in the multiple cloning site of the pRL-null vector (Promega) was used to control for transfection efficiency. Transient transfection of the patient or control plasmid with 1 µg of the β-actin-renilla plasmid into Jurkat T cells (American Type Culture Collection, Bethesda, MD) was performed as previously described.10 Transfected cells were stimulated with PMA (20 ng/ml) and ionomycin (1·5 µm) for 16 hr and luciferase assays performed using the Dual-Glo Luciferase Assay System (Promega) following the manufacturer's instructions. The firefly results were corrected for transfection efficiency using the unstimulated results for renilla luciferase. Significance was determined using the paired Student's t-test on the mean of three different experiments.
Results and discussion
F was born at term as the first child of non-consanguineous parents of Italian descent. At the age of 5 months he started a fever with cough and respiratory distress that progressed to severe hypoxaemia after a few days. Chest X-ray revealed interstitial type pneumonia. F was intubated and ventilated. Pneumocystis carinii was detected in the bronchoalveolar lavage fluid. The boy was treated with high-dose trimethoprim-sulfamethoxazol intravenously. Several pneumothoraces occurred. F was eventually weaned from the ventilator after 3 weeks. In the mean time immunological evaluation revealed near absent serum IgG and IgA, but normal serum IgM of 1·85 g/l Peripheral blood cell counts were normal as well as B and T lymphocyte counts and in vitro proliferation to mitogens and antigens. Monthly intravenous substitution therapy with immunoglobulin was started and the clinical evolution was favourable. No infectious episodes occurred and the boy thrived well: weight on the 25th centile, height on the 3rd centile. Trough IgG level was kept above 6 g/l. The diagnosis of X-linked HIGM syndrome was only made at age 5 years by demonstrating absent CD40L on T lymphocytes. Immunoglobulin substitution and trimethoprim sulfamethoxazol prophylaxis were continued. Linkage analysis revealed that the CD40L gene was inherited via the mother from a healthy grandfather and thus represents a de novo mutation. At age 9·5 years, liver enzyme tests were found to be abnormal for the first time: AST 119 U/l (normal < 30), ALT 151 U/l (normal < 40), gamma-glutamyltransferase (γGT) 289 U/l (normal < 50), alkaline phosphatase 1317 U/l (normal < 720). At age 10 a firm hard liver was palpated. Ultrasound revealed an enlarged nodular liver consistent with cirrhosis. Liver histology was compatible with sclerosing cholangitis. No infectious cause such as hepatitis B virus, hepatitis C virus, cytomegalovirus or Cryptosporidium could be determined. At present F is 12 years old. He continues to thrive normally. He has never had neutropenia, ulcers, significant infections nor chronic diarrhoea. He has a normal chest radiograph and normal lung function. Liver enzymes remain abnormal but so far there has been no progression to liver failure.
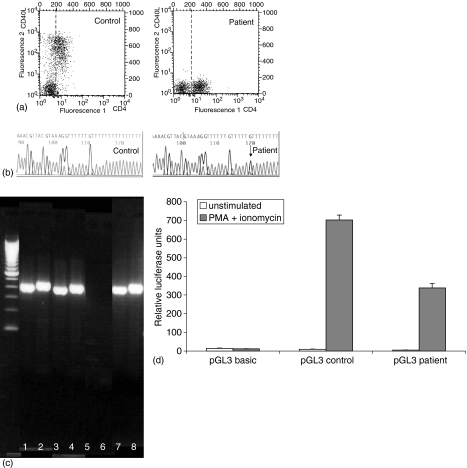
The following investigations were done in order to establish the diagnosis of X-linked HIGM. First, expression of CD40L on CD4+ T lymphocytes was evaluated by flow cytometry. In contrast to control T lymphocytes, T lymphocytes from the patient did not express CD40L upon stimulation with PMA and ionomycin (Fig. 1a).
Figure 1.
(a) CD4 (+) T-cells of the patient do not express CD40L upon stimulation. Peripheral blood obtained from the patient and from a control child was incubated with ionomycin (1 µg/ml) and PMA (25 ng/ml) for 3 hr at 37°. Thereafter, red blood cells were lysed and stained with CD4 (fluoroscein isothiocyanate, FITC) and CD40L (phycoerythrin, PE) (BD biosciences). The figure represents the dot blots of the flow cytometric analysis (FACScan instrument, BD Biosciences). The right-hand side panel shows the data from the patient and the left-hand side panel from the control child. The x-axis (fluorescence 1) represents CD4 fluorescence, whereas the y-axis (fluorescence 2) represents CD40L fluorescence. (b) Nucleotide sequence (reverse strand) of the normal to the patient's CD40L promotor. An A(T)to C (G) transversion (indicated by an arrow) was found in the patient's promoter at base −123 from the transcription start site. The right-hand side panel shows the sequence from the patient and the left-hand side panel from the control child. (c) RT–PCR for CD40L on total RNA isolated from peripheral mononuclear cells from patient and control. Two overlapping primer sets (see Data supplement) were used in order to cover the complete sequence. The PCR products were visualized on a 2% agarose gel. Lanes 1 and 2: father of patient, lanes 3 and 4: mother of patient, lanes 5 and 6: patient, lanes 7 and 8: control person. The lane on the left-hand side (with no number) is the 100 bp DNA ladder with 15 fragments between 100 and 1500 bp in multiples of 100 bp. (d) Transcriptional activity of the patient's CD40L promoter is decreased compared to the transcriptional activity of the CD40L promoter from a healthy control. Relative luciferase units (y-axis) of PMA and ionomycin stimulated Jurkat cells transfected with patient or control pGL3 plasmids were corrected for transfection efficiency and demonstrate a markedly decreased activity from the patient's CD40L promoter (P = 0·005). The results are the average of three different experiments.
In a first step to unravel the molecular defect in the patient we sequenced the CD40L gene (coding region and associated splice sites). No mutations were found (data not shown). No mutations were found in the 3′ untranslated region and the polyA site (a short sequence from base 12749 to base 12835 was not covered).
In a next step, we performed RT–PCR on total RNA isolated from PBMC after a stimulation of 3 hr with PMA and ionomycin in order to obtain a full-length cDNA probe of CD40L. This was done for the patient, the parents of the patient and a control individual. cDNA products were obtained for both parents and the control individual but not for the patient (Fig. 1c). This indicated that the absence of CD40L mRNA was responsible for the lack of CD40L protein in the patient.
Based on these results, we considered the possibility that the absence of demonstrable mRNA was the result of a mutation in the CD40L promotor. Therefore, the promotor was sequenced using four primer pairs covering the whole promotor region. Within the sequence covered by the fourth primer pair an A to C transversion was found at base −123 from the transcription start site in the patient's promotor but not in the control's promotor. The relevant sequence is shown in Fig. 1(b). Sequencing of the CD40L promotor was also performed on DNA from the parents and from 10 male controls. None of them were found to have the same mutation as the patient, which indicated that the mutation was a de novo mutation in the patient. Moreover, other authors that have looked for promoter polymorphisms have identified single nucleotide polymorphisms in the promoter region but not in the sequence of poly A's.11,12
In order to determine the involvement of the mutation identified in the promoter region in the transcription process, a luciferase reporter gene construct was generated. After transient transfection with the reporter gene constructs, Jurkat T-cell lines were stimulated with PMA and ionomycin for 16 hr. The results are shown in Fig. 1(d). The inducible activity of the luciferase reporter gene construct containing the promoter of the patient was compared with the inducible activity of the construct containing the promoter of a control individual, which was identical to patient construct except for the alteration at −123. The construct containing the promoter sequence of the patient generated lower activities than the construct containing the promoter sequence of the control, which suggested that the mutation identified in the patient is responsible for a lack of transcription of the CD40L gene and the absence of the CD40L protein on the cell surface of activated T lymphocytes. There was, however, a discrepancy between the reduction in promoter activity in transfected Jurkat cells, which was not complete, and the lack of detectable CD40L mRNA, which precluded cDNA sequencing. This raises the possibility that another unidentified factor may be involved in the pathology described in the patient. On the other hand, the presence of a promoter fragment in a reporter plasmid transfected into a T-cell line is a very artificial situation, and it is possible that the mutation identified has a more significant effect on gene transcription in vivo.
Transcriptional activity of CD40L is highly regulated and requires the transcription factor of activated T cells (NF-AT) and AP 1 protein, which act in a co-ordinated manner. An NF-AT binding motif (TTTTCC) has been identified at position −761 to −765, −258 to −265 and −62 to −69 of the CD40L promoter.9,10,13 The mutation we identified was located at position −123 from the CD40L transcription start site and is not part of a NF-AT binding motif. It has been suggested previously that enhancing elements other than NF-AT may be present in the sequence from −100 to −240.10 Future studies should address the question whether any factors bind to that portion of the promotor and whether it is part of a regulatory element. Alternatively, as the mutation was situated in a poly A region, it could be that the mutation results in conformational changes that affect transcription.
In conclusion, we identified a patient with HIGM syndrome in whom a mutation in the promoter was identified.
Acknowledgments
Xavier Bossuyt is a Senior Clinical Investigator of the FWO Vlaanderen. Ramsay Fuleihan is supported by a grant from the Jeffrey Modell Foundation.
We thank C. Massomet and F. Houtmeyers for expert technical assistance.
Glossary
Abbreviations:
- CD40L
CD40 ligand
- HIGM
hyper-IgM
- PCR
polymerase chain reaction
References
- 1.Fuleihan RS, Ramesh N, Loh R, et al. Defective expression of the CD40 ligand in X chromosome-linked immunoglobulin deficiency with normal or elevated IgM. Proc Natl Acad Sci USA. 1993;90:2170–3. doi: 10.1073/pnas.90.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aruffo A, Farrington M, Hollenbaugh D, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- 3.Allen RC, Armitage RJ, Conley ME, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259:990–3. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 4.DiSanto JP, Bonnefoy JY, Gauchat JF, Fisher A, de Saint Basile G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993;361:541–3. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 5.Korthauer U, Graf D, Mages HW, et al. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper IgM. Nature. 1993;361:539–41. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- 6.Winkelstein JA, Marino MC, Ochs H, et al. The X-linked hyper-Igm syndrome. Clinical and immunologic features of 79 patients. Medicine. 2003;82:373–84. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]
- 7.Levy J, Espanol-Boren L, Thomas C, et al. LD. Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr. 1997;131:47–54. doi: 10.1016/s0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 8.Notarangelo LD, Peitsch MC. CD40Lbase: a database of CD40L gene mutations causing X-linked hyper-IgM syndrome. Immunol Today. 1996;17:511–6. doi: 10.1016/0167-5699(96)30059-5. [DOI] [PubMed] [Google Scholar]
- 9.Shimadzu M, Nunoi H, Terasaki H, et al. Structural organization of the gene for CD40 ligand: molecular analysis for diagnosis of X-linked hyper-IgM syndrome. Biochim Biophys Acta. 1995;1260:67–72. doi: 10.1016/0167-4781(94)00179-7. [DOI] [PubMed] [Google Scholar]
- 10.Lobo FM, Xu S, Lee C, Fuleihan R. Transcriptional activity of the distal CD40 ligand promoter. Biochem Biophys Res Commun. 2000;279:245–50. doi: 10.1006/bbrc.2000.3914. [DOI] [PubMed] [Google Scholar]
- 11.Campbell SJ, Sabeti P, Fielding K, et al. Variants of the CD40 ligand gene are not associated with increased susceptibility to tuberculosis in West Africa. Immunogenetics. 2003;55:502–7. doi: 10.1007/s00251-003-0602-9. [DOI] [PubMed] [Google Scholar]
- 12.Sabeti P, Usen S, Farhadian S, et al. CD40L association with protection from severe malaria. Genes Immunity. 2002;3:286–91. doi: 10.1038/sj.gene.6363877. [DOI] [PubMed] [Google Scholar]
- 13.Schubert LA, King G, Cron RQ, Lewis DB, Aruffo A, Holenbaugh D. The human gp39 promotor. J Biol Chem. 1995;270:29624–7. doi: 10.1074/jbc.270.50.29624. [DOI] [PubMed] [Google Scholar]