Abstract
The potency of CD8+ cytotoxic T lymphocyte (CTL) responses toward core antigen has been shown to affect the outcomes of hepatitis B virus (HBV) infection. Since single-chain trimers (SCT) composed of peptide epitope β2-microglobulin (β2m) and major histocompatiblity complex (MHC) class I heavy chain covalently linked together in a single molecule have been shown to stimulate efficient CTL responses, we investigated the properties of human leucocyte antigen (HLA)-A2 SCTs encoding the HBV core antigen (HBcAg) epitopes C18−27 and C107−115. Transfection of NIH-3T3 cells with pcDNA3.0-SCT-C18−27 and SCT-C107−115 leads to stable presentation of HBcAg epitopes at the cell surface. HLA-A2.1/Kb transgenic mice vaccinated with the SCT constructs, either as a DNA vaccine alone or followed by a boost with recombinant vaccinia virus, were shown to generate HBcAg-specific CTL responses by enzyme-linked immunospot assay (ELISPOT) and in vitro interferon-γ release experiments. HBcAg-specific CTLs from vaccinated HLA-A2.1/Kb transgenic mice were able to inhibit HBV surface and e antigen expression as indicated by HepG2.2.15 cells. Our data indicate that a DNA vaccine encoding a human HLA-A2 SCT with HBV epitopes can lead to stable, enhanced HBV core antigen presentation, and may be useful for the control of HBV infection in HLA-A2-positive HBV carriers.
Keywords: cytotoxic T lymphocytes, hepatitis B virus, human leucocyte antigen A2 single-chain trimer, human leucocyte antigen A2 transgenic mice
Introduction
Hepatitis B virus (HBV) infection represents a significant unmet medical problem worldwide. An estimated 350 million people are chronically infected with the virus; they can develop chronic hepatitis, cirrhosis and in some cases ultimately hepatocellular carcinoma. Despite an effective prophylactic vaccine that has been broadly used, vaccination is not an effective treatment for established infection. Attempts at treatment of chronic infection by interferon-α (IFN-α) and nucleotide analogues have had limited success in elimination of the virus.1 A combinational strategy of first down-regulating HBV replication with antiviral chemicals and then stimulating cellular immunity of the host showed promising results.2 Patients who successfully clear the virus usually have efficient HBV core antigen (HBcAg)-specific cytotoxic T-lymphocyte (CTL) responses; however, these responses are reduced or below detection levels in patients with chronic infection.3–5 Efficient stimulation of HBcAg-specific CTLs may provide a possibility for virus elimination from chronic HBV carriers.
To date, different strategies have been tried in enhancing HBV-specific CTL responses, including CpG DNA, heat-shock proteins, DNA vaccines and various epitope or protein vaccines.2,6–9 DNA vaccines were shown to elicit both humoral and cellular immune responses in small animal models; however, this method has achieved limited success in large non-human primates.6,10 Antigens are mainly expressed on muscle cells following routine DNA vaccination by intramuscular injection, so the CTL-priming efficiency was relatively low because of the lack of expression of the costimulatory molecules B7.1 and B7.2 on these cells. Transfer of DNA-coated gold beads via a gene gun has been shown to efficiently deliver the DNA to professional antigen-presenting cells (Langerhans cells), which can greatly enhance the CTL-priming efficiency.11,12 Despite the high efficiency of CTL priming by the gene-gun method, this application has been limited because of the low availability of the equipment. Recombinant viral vectors that contain sufficient adjuvant elements, such as CpG motifs, have been used for a number of years in laboratory animals and in human trials, and have been shown to induce strong humoral and cellular immune responses for the possible prevention and treatment of various diseases. DNA vaccines have been used in combination with attenuated vaccinia virus Ankara (MVA), NYVAC (derived from the Copenhagen strain of vaccinia virus), avipoxvirus FP9 and ALVAC (derived from canarypox virus), which have been modified to express antigens from bacteria, parasites, viruses and tumour cells.13–18 Vaccinia TianTan (VTT) was used as a vaccine against smallpox in China for millions of people before 1980 and proved to be significantly less virulent than most other vaccines.19 A study of the CTL-priming ability of VTT encoding an HBV antigen would be of interest.
Studies on antigen presentation have revealed that different major histocompatibility complex (MHC) class I alleles compete with each other in binding epitopes that may alter CTL responses.20,21 In addition, different viruses have acquired the ability to down-regulate the MHC class I antigen presentation pathway, leading to increased viral persistence and immune escape. However, single-chain trimers (SCT), composed of antigenic epitope, β2-microglobulin (β2m) and MHC class I heavy chain all covalently joined together via flexible linker sequences, bypass the requirement for antigen processing and lead to stable, enhanced presentation of the epitope. A mouse Kb SCT with an epitope from human papillomavirus type 16 E6 protein was able to protect DNA-vaccinated mice from lethal E6-expressing tumour challenge, whereas a DNA vaccine encoding full-length antigen was much less effective,11 suggesting that SCTs may improve DNA vaccine efficacy. Similarly, two different human leucocyte antigen (HLA)-A2 SCTs have been shown to be effective at inducing CD8+ T-cell responses.22,23 Because HBcAg-specific CTLs are thought to play a vital role in virus resolution, we investigated the efficiency of responses against HLA-A2 SCTs with HBcAg epitopes C18−27 and C107−115 either as a DNA vaccine alone or in a DNA-rVTT prime-boost vaccine strategy in HLA-A2·1/Kb transgenic mice. The effector function of generated CTLs was studied using HepG2.2.15 cells, which express HLA-A2 and contain a 1·3-fold HBV genome (U95551, ayw subtype).
Materials and methods
Cell lines and mice
NIH-3T3 cells and Vero cells cultured in Dulbecco's modified Eagle's minimum essential medium (DMEM) supplemented with 10% fetal calf serum (FCS) and antibiotics were used to study the cell surface expression of SCTs and for recombinant selection and expansion of vaccinia viruses. HepG2.2.15 cells, which have a 1·3-fold length of HBV genome (U95551, subtype ayw), were cultured in DMEM supplemented with 10% FCS, antibiotics and 200 μg/ml G418. Human immunodeficiency virus (HIV) gag epitope (SLYNTVATL), HBcAg epitopes C18−27 (FLPSDFFPSV) and C107−115 (CLTFGRETV) were synthesized by GL Biochem (Shanghai, China) and their purity (> 95%) was confirmed by fast protein liquid chromatography and mass spectrometry. HLA-A2.1/Kb transgenic mice were intramuscularly injected with 100 μg pcDNA3.0-SCT plasmids at 2-week intervals three times or were boosted with the appropriate recombinant vaccinia virus for the third vaccination. Mice were killed 14 days after the last vaccination and splenocytes were dispersed for ELISPOT and in vitro IFN-γ staining analysis directly.
Construction of pcDNA3.0-SCTs
HBcAg-specific SCTs were derived by overlapping polymerase chain reaction (PCR) mutation from a pKG5-A2SCT-HIVgag construct (K. G., unpublished) using the upstream and downstream primers 5′-CAGCTGTGGAATGTGTGTCAGTTAG-3′ and 5′-AATCAGCAAGCTTGGTACCGA-3′ with the following mutagenic primers: SCT-C18 mutagenic primers (epitope HBcAg 18–27): 5′-AACAGAA GGAAAGAAGTCAGAAGGCAAAAAAGCCTCCAGGCCAGAAA-3′ and 5′-TTTTTGCCTTCTGACTTCTTTCCTTCTGTTGGAGGTGGGGGAGGCGGA-3′; SCT-C107 mutagenic primers (epitope HBcAg107–115): 5′-AACAGTTTCTCTTCCAAAAGTAAGACAAGCCTCCAGGCCAGAAAG-3′ 5′-TGTCTTACTTTTGGAAGAGAAACTGTTGGAGGTGGGGGAGGCGGA-3′. PCR fragments of SCT-C18 and SCT-C107 were digested with XhoI/HindIII and cloned into pcDNA3.0 digested vector. The pcDNA3-SCTs were sequenced and then subcloned with the following primers for correct inserting directions in pcDNA3.0 vector: SCT-5′-BamHI primer TGAGGATCCGATATGTCTCGCTCCGTG and SCT-3′-XhoI primer GAGCTCGAGTCACACTTTACAAGCTGTGAGAGAC.
Construction of recombinant vaccinia virus transfer vector and selection of recombinant vaccinia viruses
Recombinant vaccinia virus (rVTT) was constructed by homologous recombination of wild-type VTT with a recombinant transfer vector that contains HA flanking sequences. Transfer vector AE was constructed by inserting an enhanced green fluorescent protein gene (EGFP) gene to the vector A × 2d (provided by Dr Zhiwei Chen, Wuhan University). EGFP was PCR amplified from pEGFP-N1 vector (BD Biosciences, San Jose, CA) using a 5′ primer: 5′-AACAGATCTGGAATGGTGAGCAAGGGCGA-3′ and a 3′ primer: 5′-GCAGGATCCTTACTTGTACAGCTCGTC-3′ and inserted under the A × 2d promoter pH 5. Recombinant AE-SCT-C18−27 and AE-SCT-C107−115 were constructed by inserting the corresponding SCTs under the promoter pSyn of the AE vector separately.
Vero cells were cultured until they reached 40–80% confluence in six-well plates. Sufficient wild-type virus to give a multiplicity of infection (MOI) of 0·1 was diluted in 400 μl DMEM and used to infect the Vero cells for 1·5 hr. Then, 1 μg transfer plasmid AE or AE-SCTs was transfected into the infected Vero cells using Effectene transfection reagent (Qiagen, Valencia, CA) according to the manufacturer's protocol. The cultures were left to grow for 24 hr and split into six 60-mm plates. EGFP-positive plaques were selected and plaque purified for six rounds; finally, the purified rVTT was harvested by sucrose-gradient centrifugation before being injected into transgenic mice. The titre of rVTT was determined by EGFP expression and defined as EFU (EGFP-forming units per ml virus stock). Vaccinia virus rVTT-AE (EGFP only), rVTT-AE-SCTC18−27 (EGFP and SCT-C18−27), rVTT-AE-SCTC107−115 (EGFP and SCT-C107−115) were injected into HLA-A2.1/Kb transgenic mice intramuscularly at a dose of 2 × 107 EFU/mouse in some experiments to boost the immune effects following DNA priming.
Transfection and cell surface detection of SCTs
NIH-3T3 cells were seeded in six-well plates and incubated at 37° in 5% CO2 for 24 hr to 40–80% confluence. Transfection was performed using Effectene transfection reagent (Qiagen) according to the manufacturer's protocol. The cells were cultured for 24 hr and cell surface expression of SCTs was analysed both by flow cytometry and confocal microscopy using fluorescein isothiocyanate (FITC)-labelled anti-HLA-A2 antibody clone BB7.2.
Elispot
Interferon-γ ELISPOT analysis was performed according to the protocols supplied by the manufacturer (U-Cytech). Briefly, 96-well polyvinylidene difluoride (PVDF) plates were preincubated with the coating antibody at 4° overnight and blocked for 1 hr at 37°. Splenocytes (1 × 105 to 5 × 105 cells/well pulsed with 10 μg/ml peptides separately) were added to wells in triplicate and incubated at 37° for 24 hr. Spot-forming cells were counted and analysed with an ELISPOT plate reader (Biosys, Karben, Germany). Statistical analysis was performed using Student's t-test with Excel.
In vitro IFN-γ staining
Splenocytes were incubated with the corresponding epitope peptides for 6 hr in the presence of Brefeldin A (5 μg/ml). Cells were stained according to the protocol of the IFN-γ staining kit (BD Pharmingen, San Diego, CA). Briefly, splenocytes were surfaced stained with anti-CD8α-FITC (BD Pharmingen) for 30 min on ice then fixed and rendered permeable on ice for 20 min. Cells were then stained with anti-IFN-γ-APC at room temperature for 30 min and washed. Cells were dispersed in 1% paraformaldehyde and analysed by flow cytometry using BD FACS software (BD Biosciences).
Functional analysis of CTLs
Splenocytes were cocultured with HepG2.2.15 cells (1 × 105 cells) at ratios of 20 : 1 and 40 : 1 in a total volume of 600 μl RPMI-1640 + 10% FCS in 24-well plates and incubated at 37° for 2 days. Culture media were collected and centrifuged at 500 g for 5 min. HBsAg and HBeAg expression was tested in triplicate using a commercial HBV S and e antigen detection kit (Shanghai Siic Kehua Biotech, Shanghai, China) by enzyme-linked immunosorbent assay (ELISA). Expression differences were calculated using the following equation: average S or e down-expression = [(HepG2.2.15 average OD450 − test group average OD450)/(HepG2.2.15 average OD450)] × 100%, where OD450 is the optical density at 450 nm.
Results
Construction of DNA vaccines and vaccinia viruses encoding HLA-A2-SCTs
HLA-A2-restricted HBcAg-specific epitopes HBcAg18−27 (FLPSDFFPSV) and HBcAg107−115 (CLTFGRETV) were constructed by PCR mutation from pKG5-SCT-HIVgag (SLYNTVATL) as described in the Materials and methods and verified by DNA sequencing. Vectors pcDNA3.0 and pcDNA3.0-SCT-HIVgag were used as controls for DNA vaccination. Recombinant vaccinia viruses were constructed by homologous recombination of transfer vector AE and AE-SCTs with wild-type virus in Vero cells and were designated rVTT-AE, rVTT-AE-SCT-C18−27 and rVTT-AE-SCT-C107−115. The DNA and vaccinia virus vaccines used in this study are shown schematically in Fig. 1.
Figure 1.
Diagrams depicting structure of HBV SCTs and chimeric DNA and vaccinia virus constructs. (a) SCT which includes a signal peptide, epitope, β2m and human MHC-I molecule HLA-A2 was linked by a 15 amino acid GS linker. (b) SCTs containing HBcAg epitope C18−27 (pcDNA3.0-SCT-C18−27, FLPSDFFPSV), epitope C107−115 (pcDNA3-SCT-C107−115, CLTFGRETV) and a control epitope from HIV gag (SLYNTVATL) were cloned into pCDNA3.0 vector separately to make the DNA vaccine in the study. (c) Diagram of transfer vector AE-SCT-C18−27, which includes an EGFP gene and SCT-C18−27 gene. (d) Transfer vectors AE (control vaccinia vector with EGFP), AE-SCT-C18−27 and AE-SCT-C107−115 were constructed and recombined with wild-type vaccinia virus TianTan strain (VTT) to make recombinant vaccinia virus (rVTT) in this study.
HBV core antigen HLA-A2 SCTs are efficiently expressed on the cell surface
To test if HBcAg-epitope-encoding SCTs were able to fold correctly and be expressed at the cell surface, the DNA vaccines were used to transfect NIH-3T3 cells. Cell surface expression of SCT-HBcAg18−27 and SCT-HBcAg107−115 was tested by flow cytometry and confocal microscopy 24 hr after transient transfection. Cell surface staining with conformation-dependent HLA-A2-FITC antibody (clone BB7.2) showed that HBcAg epitope-encoding SCTs could be expressed efficiently on the cell surface (Fig. 2).
Figure 2.
Cell surface expressions of SCTs. (a) Flow cytometry analysis of cell surface expression of SCTs. The pcDNA3.0-SCT constructs were transiently transfected to NIH-3T3 cells. Cell surface expression was determined by FITC-labelled anti-HLA-A2 antibody (clone BB7.2). (b) Confocal microscopic detection of the cell surface expression of SCTs on NIH-3T3 cells. After transient transfection, NIH-3T3 cells were stained with FITC-labelled HLA-A2 antibody (clone BB7.2) and analysed using confocal microscopy. NIH-3T3 (negative control), NIH-3T3-pcDNA-SCT-HIVgag (NIH-3T3 cells transfected with pcDNA3.0-SCT-HIVgag).
Selection of recombinant vaccinia viruses encoding HLA-A2 SCTs
Recombinant vaccinia viruses were constructed by homologous recombination of transfer vector AE and AE-SCTs with wild-type VTT strain in Vero cells and were selected from plaques that were positive for EGFP expression. After six rounds of purification, recombinant virus was expanded in Vero cells seeded in 10-cm plates. Figure 3(a) shows a representative recombinant vaccinia virus plaque and the recombinant vaccinia virus-infected Vero cells. Figure 3(b) shows a transmission electron micrograph of sucrose-purified recombinant vaccinia virus.
Figure 3.
Construction and detection of recombinant vaccinia virus. (a) Example of recombinant vaccinia virus rVTT-AESCT-C18−27 selection. Vero cells were transfected with 0·1 MOI wild-type VTT for 90 min in six-well plates, followed by transfection of 1 μg transfer vector AESCT-C18−27 plasmids by Effectene transfection reagent. Cells were left to grow for 24 hr and then frozen and thawed three times. Supernatants were planted on six 60-mm plates and cultured for 24 hr. EGFP-expressing cells were selected using a fluorescent microscopy and further purified for six rounds. (b) Purification of rVTT-AESCT-C18−27 was performed using 20–60% sucrose-gradient centrifugation at 18 000 g and diluted in phosphate-buffered saline. Purified recombinant virus was subjected to transmission electron microscopy.
DNA vaccines encoding HBV core antigen epitope HLA-A2 SCTs prime HBV core antigen-specific CD8+ T cells in HLA-A2.1/Kb transgenic mice
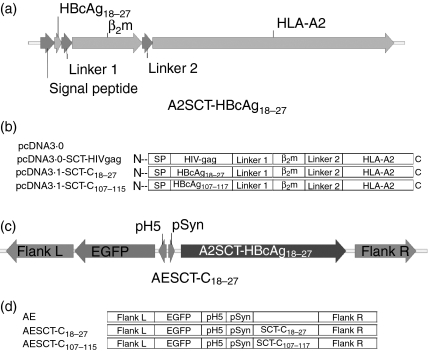
HLA-A2.1/Kb transgenic mice were vaccinated with three injections of pcDNA3.0-SCTs at 2-week intervals by intramuscular injection. Two weeks after the last vaccination, spleens were removed and dispersed in RPMI-1640 medium supplemented with 10% FCS as described in the Materials and methods. Splenocytes were stimulated in 24-well plates with the corresponding peptides for 24 hr and then subjected to ELISPOT analysis for a further 24 hr. In vitro IFN-γ production was tested after 2 days of peptide stimulation followed by a 6-hr stimulation with the appropriate peptide in the presence of Brefeldin A. ELISPOT experiments indicated that the DNA vaccines encoding HBcAg and HIV gag epitope-specific HLA-A2 SCTs were able to induce epitope-specific CD8+ T-cell responses in HLA-A2.1/Kb transgenic mice (with P =0·002, P = 0·009 and P = 0·007 for SCT-C18−27, SCT-C107−115 and SCT-HIV gag, respectively, compared to control pcDNA3·0 vaccinated group) (Fig. 4a). Similar results were also obtained using in vitro IFN-γ staining experiments (Fig. 4b).
Figure 4.
DNA vaccine with SCT constructs elicits epitope-specific CTL responses in HLA-A2.1/Kb transgenic mice. (a) Four groups of HLA-A2.1/Kb transgenic mice, each contain four to eight mice, were vaccinated with 100 μg pcDNA-3.0-SCTs intramuscularly at 2-week intervals for three injections. Splenocytes were dissected 2 weeks after the last vaccination. Epitope-specific IFN-γ-releasing cells were counted by ELISPOT experiments. pcDNA3.0-SCT vaccines were able to prime CD8+ T-cell responses with statistical significance (P = 0·002, P = 0·009 and P = 0·007 for C18−27, C107−115 and HIV gag, respectively, compared to pcDNA3.0 control group vaccination; SFC, spot-forming cells). (b) Splenocytes were prestimulated with 10 μg/ml epitopes for 2 days and then tested by flow cytometry using FITC-CD8a and APC-IFN-γ antibodies. CD8+ IFN-γ-expressing cells were gated against CD8+ T cells.
Splenocytes from vaccinated mice inhibit HBV antigen production by HepG2·2.15 cells
The HepG2.2.15 cell line, which is HLA-A2 positive and stably transfected with a hepatitis B virus genome (U95551, ayw subtype) and produces all the viral RNAs and proteins, was used to study the effector function of splenocytes derived from vaccinated mice as described in the Materials and methods. Splenocytes from vaccinated mice were isolated and mixed with 1 × 105 HepG2.2.15 cells at ratios of 20 : 1 and 40 : 1. HBV S and e antigen expression in culture supernatant was tested by ELISA after 2 days of cocultivation. The results indicated that HBV antigen expression was down-regulated when HepG2.2.15 cells were cocultured with splenocytes from mice vaccinated with SCTs, and that this down-regulation was antigen specific because splenocytes from mice vaccinated with plasmid vector alone or HIV gag SCT were less effective at down-regulating the expression of S and e antigen (Fig. 5).
Figure 5.
Epitope-specific CTLs from HLA-A2.1/Kb transgenic mice inhibit HBV surface and e antigen expression from HepG2.2.15 cells. Splenocytes were immediately cocultured with HepG2.2.15 cells at ratios of 20 : 1 and 40 : 1 for 2 days. S and e antigen expression in the culture media was tested with HBV S and e antigen detection kits by ELISA. (a) Splenocytes from pcDNA3.0-SCT-C18−27-vaccinated and pcDNA3.0-SCT-C107−115-vaccinated mice were shown to inhibit HBV S antigen expression of HepG2.2.15 cells with statistical significance (P < 0·05 compared to mice from control pcDNA3.0- and pcDNA3.0-SCT-HIVgag-vaccinated groups). (b) HBV e antigen expression was inhibited by CTLs from core-antigen-specific SCT-vaccinated mice with statistical significance compared to control groups.
Boosting with recombinant vaccinia virus greatly enhances the frequency of specific CD8+ T cells elicited by SCT DNA vaccine
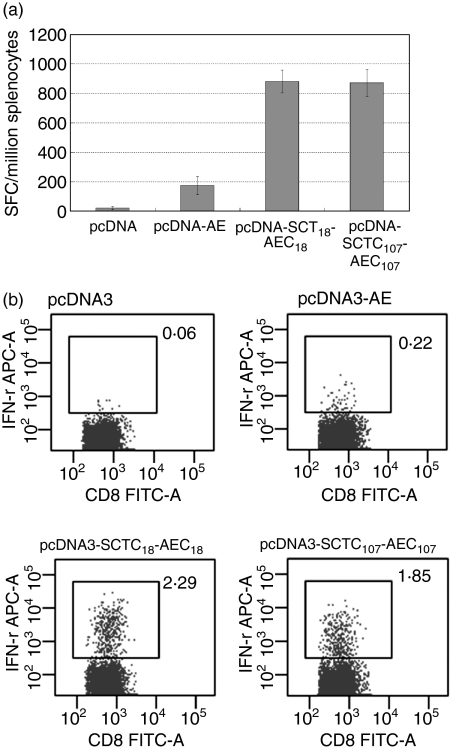
Our results indicated that HBcAg-specific responses were primed in HLA-A2 transgenic mice using HLA-A2 SCT pcDNA3.0 constructs. However, the magnitude of CD8+ T-cell responses generated did not seem to show an obvious advantage compared to other DNA vaccine strategies published, and was not at a comparable level to the responses reported for a mouse SCT DNA vaccine,11 although there are many possible explanations for this difference in efficiency. To test whether boosting with recombinant vaccinia virus would enhance the generation of specific T cells, 2 × 107 EFU recombinant vaccinia viruses rVTT-AE, rVTT-AESCT-C18−27 and rVTT-AESCT-C107−115 were injected into mice 2 weeks after priming with the corresponding DNA vaccine. ELISPOT and in vitro IFN-γ production were used to determine the efficiency of specific CD8+ T-cell responses. Our results confirmed that boosting with VTT strain expressing an HLA-A2 SCT could greatly enhance the CD8+ T-cell responses primed by a DNA vaccine encoding the same SCT molecule (four-fold to seven-fold compared to DNA vaccine alone) (Fig. 6).
Figure 6.
A vaccinia virus boost greatly enhanced the magnitude of HBV core-antigen-specific CTL responses primed by DNA vaccine. HLA-A2.1/Kb transgenic mice were vaccinated with pcDNA-SCTs twice and then boosted with 2 × 107 EFU recombinant vaccinia virus rVTT-AE and rVTT-AE-SCTs. Splenocytes were dissected 2 weeks after the last vaccination. (a) ELISPOT analysis of HBcAg18−27-specific and HBcAg107−115-specific CTLs was performed immediately after the splenocyte dissection. Vaccinia virus boost greatly enhanced the CTL responses in an epitope-specific manner (with P = 0·01, P =0·002 and P = 0·005 for pcDNA-AE, pcDNA-AE-SCTC18 and pcDNA-AE-SCTC107, respectively, compared to the pcDNA3.0-vaccinated group; P = 0·0003 and P = 0·02 for pcDNA-AE-SCTC18 and pcDNA-AE-SCTC107, respectively, compared to the pcDNA-AE-vaccinated group). (b) Splenocytes were stimulated with corresponding epitopes for 2 days and were then subjected to in vitro IFN-γ staining analysis.
Discussion
HBV infection is a major public-health concern worldwide because of its association with chronic infection and the disease progression to liver cirrhosis and hepatocellular carcinoma. DNA vaccines encoding HBV surface and core antigens have been shown to elicit both humoral and cellular responses; however, the application of DNA vaccination with HBV structural antigens for HBV infection has its limitations. Kidney and liver damage caused by circulating immune complexes as a result of the long-term persistence of antigen and antibody was reported after DNA vaccination in an animal model.24 In the context of HBV infection, surface antigen and core antibodies are usually present during acute and chronic HBV infection; a vaccination strategy for clinical application should consider the existing over-expressed antigen and persistent antibodies.25 More importantly, HBV recurrence following dominant epitope mutation after medical treatment often occurs, and low-affinity (non-dominant) epitopes that are less likely to be subject to immune selection and mutation may play even more vital roles in therapeutic vaccination.26 In addition, vaccination with full-length antigen does not necessarily induce a polyclonal CTL response in vivo, as indicated by a phase I clinical study on melanoma patients who were vaccinated with DNA and vaccinia virus encoding intact antigen, and in which CTL responses were only generated against one of the reported epitopes.17
Using human MHC class I SCTs encoding HBV core antigen epitopes, we report here that core antigen-specific CD8+ T-cell responses were primed by DNA vaccination and that the magnitude of these responses was greatly enhanced by boosting with a recombinant vaccinia virus encoding the same construct. This approach has the potential advantage that efficient responses may be made against non-immunodominant, low-affinity epitopes, because the peptide epitope is covalently linked to the MHC class I molecule, and the normal requirements for antigen processing are bypassed. Two other studies have also successfully used different HLA-A2 SCTs in the form of DNA vaccines22,23 but the properties of a recombinant vaccinia virus encoding an SCT have not yet been reported. In this study, a DNA vaccine encoding the SCT delivered by intramuscular injection was shown to elicit specific CD8+ T-cell responses less than a mouse SCT construct delivered by gold particles.11 There are several possible reasons for this. First, antigens encoded by plasmid DNA given by intramuscular injection are mainly expressed in muscle cells, which lack the costimulatory molecules B7.1 and B7.2. Second, the transgenic mice used in the experiments express heterodimeric HLA-A2.1/Kb molecules in the context of a background of H-2 class I molecules, and these transgenic mice were shown to have a relatively lower efficiency in CTL priming.26–29 However, boosting with recombinant VTT strain encoding the same SCT constructs was shown to significantly enhance the CD8+ T-cell responses primed by DNA vaccines carrying the SCT constructs.
Since an SCT construct can efficiently present the epitope of interest, vaccination with DNA vectors encoding multiple SCTs presenting different epitopes may show an advantage in inducing efficient polyclonal CTL responses. Boosting with VTT strain can greatly enhance the efficiency of DNA priming. This prime-boost strategy of vaccination with HLA-A2 SCTs encoding HBV epitopes may be useful for HBV virus infection control.
Acknowledgments
We are very grateful for the advice of Prof. Charles Bangham and Dr Mala Maini during different stages of the experiments. We thank Dr Zhiwei Chen for help with the vaccinia virus system, Mrs Fulian Liao for technical assistance and Weihua Zhuang for graphical assistance. This work was supported by a grant from the National Frontier Research Program (Project 973) of the Ministry of Science and Technology of the People's Republic of China (Grant nos 2005CB523001 and 2005CB522901). K.G. thanks the Medical Research Council (UK) for support.
Abbreviations
- CTL
cytotoxic T lymphocytes
- DMEM
Dulbecco's modified Eagle's minimum essential medium
- EFU
EGFP-forming unit
- ELISA
enzyme-linked immunosorbent assay
- ELISPOT
enzyme-linked immunospot assay
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- HBV
hepatitis B virus
- HBcAg
HBV core antigen
- HIV
human immunodeficiency virus
- HLA
human leucocyte antigen
- IFN
interferon
- MHC
major histocompatibility complex
- MOI
multiplicity of infection
- PCR
polymerase chain reaction
- SCT
single-chain trimers
- SFC
spot-forming cell
- VTT
vaccinia TianTan
References
- 1.Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang SH, Lee CG, Park SH, et al. Correlation of antiviral T-cell responses with suppression of viral rebound in chronic hepatitis B carriers: a proof-of-concept study. Gene Ther. 2006;13:1110–17. doi: 10.1038/sj.gt.3302751. [DOI] [PubMed] [Google Scholar]
- 3.Penna A, Chisari FV, Bertoletti A, Missale G, Fowler P, Giuberti T, Fiaccadori F, Ferrari C. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J Exp Med. 1991;174:1565–70. doi: 10.1084/jem.174.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohr HF, Gerken G, Schlicht HJ, Meryer zum Buschenfelde KH, Fleischer B. Low frequency of cytotoxic liver-infiltrating T lymphocytes specific for endogenous processed surface and core proteins in chronic hepatitis B. J Infect Dis. 1993;168:1133–9. doi: 10.1093/infdis/168.5.1133. [DOI] [PubMed] [Google Scholar]
- 5.Lohr HF, Krug S, Herr W, Weyer S, Schlaak J, Wolfel T, Gerken G, Meyer zum Buschenfelde KH. Quantitative and functional analysis of core-specific T-helper cell and CTL activities in acute and chronic hepatitis B. Liver. 1998;18:405–13. doi: 10.1111/j.1600-0676.1998.tb00825.x. [DOI] [PubMed] [Google Scholar]
- 6.Payette PJ, Ma X, Weeratna RD, McCluskie MJ, Shapiro M, Engle RE, Davis HL, Purcell RH. Testing of CpG-optimized protein and DNA vaccines against the hepatitis B virus in chimpanzees for immunogenicity and protection from challenge. Intervirology. 2006;49:144–51. doi: 10.1159/000089375. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Yang X, Li L, Liu H, Liu J. A truncated C-terminal fragment of Mycobacterium tuberculosis HSP70 gene enhanced potency of HBV DNA vaccine. Vaccine. 2006;24:3321–31. doi: 10.1016/j.vaccine.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Li N, Fan XG, Chen ZH, Zhu C, Liu HB, Huang Y. Inhibition of the hepatitis B virus replication in vitro by an oligodeoxynucleotide containing cytidine-guanosine motifs. Immunol Lett. 2006;102:60–6. doi: 10.1016/j.imlet.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Zhou M, Han J, Zhu X, Dong T, Gao GF, Tien P. Generation of murine CTL by a hepatitis B virus-specific peptide and evaluation of the adjuvant effect of heat shock protein glycoprotein 96 and its terminal fragments. J Immunol. 2005;174:195–204. doi: 10.4049/jimmunol.174.1.195. [DOI] [PubMed] [Google Scholar]
- 10.Zhao YG, Peng B, Deng H, et al. Anti-HBV immune responses in rhesus macaques elicited by electroporation mediated DNA vaccination. Vaccine. 2006;24:897–903. doi: 10.1016/j.vaccine.2005.08.093. [DOI] [PubMed] [Google Scholar]
- 11.Huang CH, Peng S, He L, Tsai YC, Boyd DA, Hansen TH, Wu TC, Hung CF. Cancer immunotherapy using a DNA vaccine encoding a single-chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Ther. 2005;12:1180–6. doi: 10.1038/sj.gt.3302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188:1075–82. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster DP, Dunachie S, McConkey S, et al. Safety of recombinant fowlpox strain FP9 and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine. 2006;24:3026–34. doi: 10.1016/j.vaccine.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 14.Ranasinghe C, Medveczky JC, Woltring D, et al. Evaluation of fowlpox-vaccinia virus prime-boost vaccine strategies for high-level mucosal and systemic immunity against HIV-1. Vaccine. 2006;24:5881–95. doi: 10.1016/j.vaccine.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Jimenez E, Kochan G, Gherardi MM, Esteban M. MVA-LACK as a safe and efficient vector for vaccination against leishmaniasis. Microbes Infect. 2006;8:810–22. doi: 10.1016/j.micinf.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert SC, Moorthy VS, Andrews L, et al. Synergistic DNA-MVA prime-boost vaccination regimes for malaria and tuberculosis. Vaccine. 2006;24:4554–61. doi: 10.1016/j.vaccine.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 17.Smith CL, Dunbar PR, Mirza F, et al. Recombinant modified vaccinia Ankara primes functionally activated CTL specific for a melanoma tumor antigen epitope in melanoma patients with a high risk of disease recurrence. Int J Cancer. 2005;113:259–66. doi: 10.1002/ijc.20569. [DOI] [PubMed] [Google Scholar]
- 18.Hutchings CL, Gilbert SC, Hill AV, Moore AC. Novel protein and poxvirus-based vaccine combinations for simultaneous induction of humoral and cell-mediated immunity. J Immunol. 2005;175:599–606. doi: 10.4049/jimmunol.175.1.599. [DOI] [PubMed] [Google Scholar]
- 19.Fang Q, Yang L, Zhu W, et al. Host range, growth property, and virulence of the smallpox vaccine: vaccinia virus Tian Tan strain. Virology. 2005;335:242–51. doi: 10.1016/j.virol.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Tourdot S, Nejmeddine M, Powis SJ, Gould KG. Different MHC class I heavy chains compete with each other for folding independently of beta 2-microglobulin and peptide. J Immunol. 2005;174:925–33. doi: 10.4049/jimmunol.174.2.925. [DOI] [PubMed] [Google Scholar]
- 21.Tourdot S, Gould KG. Competition between MHC class I alleles for cell surface expression alters CTL responses to influenza A virus. J Immunol. 2002;169:5615–21. doi: 10.4049/jimmunol.169.10.5615. [DOI] [PubMed] [Google Scholar]
- 22.Hung CF, Calizo R, Tsai YC, He L, Wu TC. A DNA vaccine encoding a single-chain trimer of HLA-A2 linked to human mesothelin peptide generates anti-tumor effects against human mesothelin-expressing tumors. Vaccine. 2006;25:127–35. doi: 10.1016/j.vaccine.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 23.Jaramillo A, Narayanan K, Campbell LG, et al. Recognition of HLA-A2-restricted mammaglobin-A-derived epitopes by CD8+ cytotoxic T lymphocytes from breast cancer patients. Breast Cancer Res Treat. 2004;88:29–41. doi: 10.1007/s10549-004-8918-1. [DOI] [PubMed] [Google Scholar]
- 24.Zi XY, Yao YC, Zhu HY, et al. Long-term persistence of hepatitis B surface antigen and antibody induced by DNA-mediated immunization results in liver and kidney lesions in mice. Eur J Immunol. 2006;36:875–86. doi: 10.1002/eji.200535468. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson FK, Rice J. Optimizing cancer immunotherapy trials: back to basics. Eur J Immunol. 2006;36:1070–3. doi: 10.1002/eji.200636085. [DOI] [PubMed] [Google Scholar]
- 26.Ramage JM, Metheringham R, Moss R, Spendlove I, Rees R, Durrant LG. Comparison of the immune response to a self antigen after DNA immunisation of HLA*A201/H-2Kb and HHD transgenic mice. Vaccine. 2004;22:1728–31. doi: 10.1016/j.vaccine.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 27.Firat H, Cochet M, Rohrlich PS, Garcia-Pons F, Darche S, Danos O, Lemonnier FA, Langlade-Demoyen P. Comparative analysis of the CD8(+) T cell repertoires of H-2 class I wild-type/HLA-A2.1 and H-2 class I knockout/HLA-A2.1 transgenic mice. Int Immunol. 2002;14:925–34. doi: 10.1093/intimm/dxf056. [DOI] [PubMed] [Google Scholar]
- 28.Street MD, Doan T, Herd KA, Tindle RW. Limitations of HLA-transgenic mice in presentation of HLA-restricted cytotoxic T-cell epitopes from endogenously processed human papillomavirus type 16, E7 protein. Immunology. 2002;106:526–36. doi: 10.1046/j.1365-2567.2002.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med. 1997;185:2043–51. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]