Abstract
5-lipoxygenase (5-LOX) is the key enzyme responsible for the synthesis of the biologically active leukotrienes. Its presence has been reported in cells of the myeloid lineage and B lymphocytes but has not been formally defined in T lymphocytes. In this study, we provide evidence for 5-LOX expression on both transcriptional and translational levels in highly purified peripheral blood T cells as well as in human T lymphoblastoid cell lines (MOLT4 and Jurkat). Messenger RNA (mRNA) of 5-LOX was amplified by conventional reverse transcription–polymerase chain reaction (RT-PCR; MOLT4 and Jurkat cells) and by in situ RT-PCR (T lymphocytes). 5-LOX protein expression was confirmed by Western blot and immunofluorescence studies. 5-LOX was present primarily in the cytoplasm with some nuclear localization and was translocated to the nuclear periphery after culture in a mitosis-supporting medium. Fluorescence-activated cell sorter analysis of different T-lymphocyte populations, including CD4, CD8, CD45RO, CD45RA, T helper type 2, and T-cell receptor-αβ and -γδ expressing cells, did not identify a differential distribution of the enzyme. Purified peripheral blood T lymphocytes were incapable of synthesizing leukotrienes in the absence of exogenous arachidonic acid. Jurkat cells produced leukotriene C4 and a small amount of leukotriene B4 in response to CD3–CD28 cross-linking. This synthesis was abolished by two inhibitors of leukotriene synthesis, MK-886 and AA-861. The presence of 5-LOX in T lymphocytes but the absence of endogenous lipoxygenase metabolite production compared to Jurkat cells may constitute a fundamental difference between resting peripheral lymphocytes and leukaemic cells.
Keywords: in situ reverse transcription–polymerase chain reaction, Jurkat, leukotrienes, MOLT4, T lymphocytes
Introduction
Several families of molecules, called eicosanoids, derive from arachidonic acid (AA) (including leukotrienes, prostaglandins, prostacyclins, thromboxane, isoprostanes and cytochrome 450 oxidative products) and exert a wide variety of biological actions in inflammation, immunity, oxidative stress and neoangiogenesis.1,2 The best known are prostaglandins, which are synthesized in most cell types by the cyclo-oxygenases and participate in inflammatory reactions by promoting vasodilation and fever. Prostaglandin E2 (PGE2), for instance, exerts strong immunosuppressive effects on T-cell proliferation and responses.3 Another prominent group of eicosanoids, obtained after the action of 5-lipoxygenase (5-LOX, arachidonate:oxygen 5-oxidoreductase, EC 1.13.11.34) is the leukotriene (LT) family, which mediates key inflammatory reactions including bronchoconstriction, vasodilatation and increased mucus secretion.4 Leukotrienes arise by the action of 5-LOX on arachidonic acid, which is enzymatically liberated from membrane phospholipids following cellular activation by bacteria, immune complexes, cytokines and other stimuli. Free arachidonic acid is presented by 5-lipoxygenase-activating protein (FLAP) to 5-LOX, which has translocated to the nuclear envelope.5,6 A two-step reaction successively forms 5-hydroxy-6,8,11,14-eicosatetraenoic acid (5-HETE) and LTA4, which is then further converted into LTB4 or the cysteinyl leukotrienes LTC4, LTD4 and LTE4. Certain cysteinyl LTs are implicated in immunopathological processes such as asthma, allergy, inflammatory bowel disease and psoriasis.7 Indeed, elevated levels of LTs have been demonstrated in bronchoalveolar lavage of asthmatic patients and are increased during asthma attacks.8,9
An immunomodulatory role has been postulated for 5-LOX metabolites, especially LTB4. For example, LTB4 is a powerful chemoattractant agent for inflammatory cells and induces degranulation, superoxide anion production and adherence of neutrophils to vascular endothelial cells. LTB4 also stimulates the production of proinflammatory cytokines, including interleukin-1β (IL-1β),10,11 IL-2,12,13 IL-614 and interferon-γ (IFN-γ),15 anti-inflammatory cytokines, such as IL-416 and IL-10,17 and activates c-fos, c-jun18 and nuclear factor-κB gene transcription.10 It also increases the expression of the IL-2 receptor β-chain in natural killer cells and to a lesser extent in CD8+ lymphocytes, resulting in a cytotoxic response.19 LTB4 promotes DNA synthesis, activation and proliferation of B lymphocytes.20 In T cells, LTB4 has been reported to induce suppressor T cells21 although another study showed that LTB4 was immunostimulatory22,23 by partially substituting for the CD28 costimulatory signal that is necessary for T-cell activation.12 The biological activity of different 5-LOX metabolites and, especially the fact that 5-LOX-deficient mice have altered ovalbumin-dependent cellular and humoral immune responses, implies that 5-LOX may play an important role in adaptive immunity.24
Many cell types, including blood monocytes, neutrophils, eosinophils, basophils, alveolar and peritoneal macrophages, mast cells and B lymphocytes, are well known to constitutively express 5-LOX. However, the ability of T lymphocytes to synthesize 5-LOX products has been a matter of controversy for over a decade. Early studies claimed that human T lymphocytes synthesized 5-HETE and LTC4,25–27 which would require 5-LOX expression, while other groups could not find evidence for this.28,29 T lymphocytes were considered to express messenger RNA (mRNA) for FLAP but not for 5-LOX.30,31 More recent studies, using cell-sorting methods, reported synthesis and release of LTB4 and cysteinyl LTs in purified T lymphocytes and T-cell lines. For example, T-cell receptor (TCR)/CD3 cross-linking was accompanied by LTE4 but not LTB4 production in Jurkat and MOLT4 cells and Western blot of Jurkat cells detected a 78 000 molecular weight (MW) band corresponding to the molecular weight of 5-LOX.32 Triggering of CD28 led to LTB4 release by primary T lymphocytes, which could be inhibited by lipoxygenase inhibitors.12 Furthermore, the LTB4 receptor is expressed by T cells and an LTB4-specific antagonist abolished anti-CD3 induced T-lymphocyte proliferation33 as well as significantly inhibiting IL-2, IL-4 and IFN-γ synthesis by these cells. Experiments using 5-LOX inhibitors suggest that 5-LOX is an essential signal for NF-κΒ and c-jun activation and for cell proliferation in T cells.33–36
These results led us to reconsider the question of 5-LOX expression in T cells and to initiate studies on whether 5-LOX was expressed in highly purified resting human T lymphocytes from peripheral blood as well as to confirm its presence in the human T-cell lines Jurkat and MOLT4 cells. We then sought to identify different T-cell subpopulations to determine if there was a differential distribution of 5-LOX which would be a first step in understanding its physiological role, keeping in mind that 5-LOX could be a potential target for molecular inhibition leading to future therapeutic applications.
Materials and methods
Cells
Mycoplasma-free human T-cell lines, Jurkat and MOLT4 (ECACC, Salisbury, UK), were grown in RPMI-1640 medium supplemented with 10% fetal calf serum and 2 mm glutamine and maintained at 37° in a humidified atmosphere containing 5% CO2. T lymphocytes from healthy donors were isolated by density centrifugation over Ficoll–Hypaque (Sigma-Aldrich, St Quentin Fallavier, France) and then were negatively sorted by magnetic separation using the Pan T-Cell Isolation Kit II (Miltenyi Biotec, Paris, France). Fluorescence-activated cell sorting analysis of the resulting fraction routinely showed > 99% purity. For certain experiments, enriched T cells were stimulated for 3 days with Chang medium MF (Irvine Scientific, Santa Ana, CA).
5-LOX reverse transcription–polymerase chain reaction (RT-PCR)
Total cellular mRNA (1·5 μg) from MOLT4, Jurkat and peripheral T cells was reverse transcribed into complementary DNA (cDNA; Superscript Preamplification System, Life Technologies, Cergy Pontoise, France) and amplified. PCR was performed as previously described31 using the following primers: 5′-AGTCCTCAGGCTTCCCCAAGT-3′ and 5′-CATGCCCAGGAACAGCTCGTT-3′. Samples without reverse transcriptase were used to confirm that genomic 5-LOX DNA was not amplified. Conditions were as follows: 95° for 2 min and then 35 cycles at 94° for 45 seconds, 62° for 45 seconds and 72° for 1 min. The reaction was terminated with a run at 72° for 5 min. Aliquots of the PCR product were analysed by electrophoresis on 1% agarose gels to detect a specific 295-base-pair (bp) fragment; PCR products were verified by restriction enzyme digestion with BamHI and were thereafter sequenced (Genome Express, Meylan, France).
In situ amplification of 5-LOX mRNA by RT-PCR
Magnetically sorted peripheral blood T lymphocytes were applied to slides by cytospin centrifugation and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) followed by methanol for 5 min at −20° and stored at −80° until use. Cells were brought to room temperature in acetone–methanol (vol : vol) for 10 min and mRNA was reversed transcribed using an Omniscript Reverse Transcriptase Kit (Qiagen, Courtaboeuf, France). Briefly, 20 μl reaction mixture containing 1 mm oligo-dT, 2 mm dNTP, 10 U RNase OUT recombinant ribonuclease inhibitor (Invitrogen, Cergy Pontoise, France) was added to each zone. Coverslips were sealed to slides with rubber cement and slides were placed in a thermocycler (Omnigene Hybaid, Scheicher and Schuell, Ecquivelly, France) for 60 min at 37°. The reaction was terminated by heating at 93° for 5 min followed by cooling on ice. Coverslips were removed and slides were immediately used for PCR.
The 5-LOX cDNA was amplified using a PCR DIG (digoxygenin) Probe synthesis kit (Roche, Mannheim, Germany). Cells received 50 μl reaction mixture (2·6 U Expand High Fidelity; 200 μm each dATP, dCTP, dGTP; 130 μm dTTP; 70 μm DIG-11-dUTP and 20 μm sense and antisense 5-LOX primers. Coverslips were sealed again to the slides. The amplification conditions were identical to those for liquid-phase PCR. Controls included: (1) T cells submitted to reverse transcription without the enzyme reverse transcriptase, (2) T cells submitted to reverse transcription and cDNA amplification with non-LOX-specific primers (primers for chloramphenicol acetyl transferase), and (3) macrophages submitted to both reverse transcription and cDNA amplification as a positive control.
Amplified DIG-labelled cDNA was detected using an anti-DIG-labelled antibody conjugated to alkaline phosphatase (Roche). Slides were washed twice for 15 min each at 42° in 2 × saline sodium citrate (SSC), 0·2 × SSC and 0·1 × SSC followed by one wash for 5 min in 100 mm maleic acid, 150 mm NaCl, pH 7·5 at 25° (buffer 1). After 30 min saturation at 25° in blocking solution (buffer 1, 0·3% Triton X-100, 1% blocking agent; Roche), slides were incubated for 30 min with 1 : 100 anti-DIG antibody. Excess antibody was eliminated by 15-min washes in buffer 1. The 5-LOX cDNA was localized by an enzyme-catalysed colour reaction with 5-bromo-4-chloro-3-indoyl phosphate (X-phosphate) and nitroblue tetrazolium salt in 100 mm Tris–HCl, 100 mm NaCl, 50 mm MgCl2, pH 9·5. The reaction was stopped by immersion in Tris–EDTA (TE) buffer. Slides were then rinsed in PBS and saturated in PBS−3% bovine serum albumin for 30 min. Cells were incubated with anti-CD3 antibody for 30 min, washed and 1 : 500 Alexa-Fluor-488-conjugated goat anti-mouse immunoglobulin G (IgG; Molecular Probes, Eugene, OR) was added for an additional 30 min. Nuclei were stained with propidium iodide and slides were mounted and visualized by light and fluorescence microscopy.
Indirect immunofluorescence and flow cytometry
For fluorescence microscopy observations, cells were fixed in 2% paraformaldehyde–PBS for 15 min, followed by methanol for 5 min at − 20°, and applied to slides which were stored at −80° until use. Cells were permeabilized, incubated with PBS containing 10% goat serum for 1 hr at 25° and then with 1 : 100 anti-human 5-LOX monoclonal antibody (BD Transduction Laboratories, Verdelburg, Germany), a negative isotypic control or PBS alone for 1 hr at room temperature. After washing in PBS, 1 : 200 Alexa Fluor 488 conjugated F(ab)′2 goat anti-mouse IgG (Molecular Probes) was added for 1 hr followed by additional washes. Blood T lymphocytes were labelled with anti-CD3-PE-PC5 antibody after 5-LOX detection to avoid binding of secondary antibody to anti-CD3. Nuclei were stained with diaminidophenylindol (DAPI). The fluorescent signal was observed with a Nikon Eclipse E800 microscope equipped with a Photonic Science camera (Nikon France, Champigny sur Marne, France).
For flow cytometry experiments 2 × 106 purified T cells were fixed and permeabilized using the Intraprep Permeabilization Reagent kit (Immunotech, Marseille, France). Again, to avoid non-specific binding of the secondary antibody, lymphocytes were first incubated with the anti 5-LOX monoclonal antibody or with PBS alone for 40 min at 4°, abundantly washed three times and then submitted to a supplemental permeabilization step before the addition of fluorescein isothiocyanate-labelled F(ab)′2 goat anti-mouse IgG antibody (1 : 100) for 25 min at 25°. After five washes in PBS, cells were selected for analysis with anti-CD19-Cychrome 5 (Cy5; Immunotech) [or anti-CD19-phycoerythrin (PE) according to the labels of the other antibodies used for analysis] and anti-CD45-Cy7 (Immunotech) antibodies. In addition, to determine whether or not 5-LOX was restricted to a specific subpopulation, cells were stained with anti-CD3-Cy5, CD1a-PE and CD2-PE (Cytostat Coulter Clone; Coulter Corporation, Miami, FL), anti-CD4-RPE-Cy5, CD8-RPE-Cy5 and CD10-PE (DAKO; Dako Cytomation, Trappes, France), anti-CD45RO-PE, CD45RA-PE, CD7-PE and CRTH2-PE (Immunotech), anti-TCR-αβ-PE and anti-TCR-γδ-PE (BD Biosciences, San José, CA) antibodies and analysed using a Beckman Coulter XL cell sorter. All gates were set with appropriate isotypic controls. Cells incubated with anti 5-LOX antibody and secondary antibody had the same fluorescence intensity for membrane markers while secondary antibody alone did not bind.
Western blot analysis
Cells were washed four times in PBS and the resulting cell pellet was resuspended in RIPA lysis buffer [50 mm HEPES (pH 7·5), 150 mm NaCl, 1% deoxycholate, 1% Nonidet-P40, 1% sodium dodecyl sulphate (SDS), 20 μg/ml aprotinine] supplemented with a cocktail of protease inhibitors (Complete, Roche). Cells were sonicated three times for 15 seconds each (Vibra-cell, Bioblock Scientific, Illkirch, France). Sonicated samples were then ultracentrifuged at 10 000 g for 30 min at 4°. Supernatants were collected and total protein concentrations were determined by the Lowry method.37 Samples (25 μg) were loaded on 4–15% SDS–polyacrylamide gel electophoresis (PAGE) gradient gels and electroblotted on Immobilon-P membranes (Roche) for 1 hr at 0·2 A. After blocking in 25 mm Tris–HCl, 140 mm NaCl, 27 mm KCl, pH 8 (TBS) containing 5% skim milk, membranes were incubated overnight at 4° with 1 : 250 mouse anti-5-LOX antibody in TBS. Following washing, horseradish peroxidase-coupled rabbit anti-mouse immunoglobulin antibody (DAKO) was added at a 1 : 5000 dilution for 1 hr. Immunodetection was performed by chemoluminescence (ECL Detection System, Amersham Biosciences, Buckinghamshire, UK). To better assess 5-LOX protein amounts, β-actin was estimated by incubating the same membrane overnight with a 1 : 5000 dilution of mouse anti-β-actin monoclonal antibody (Sigma) in 5% skim milk. After washing, the membrane was developed as described above. The 5-LOX : β-actin ratio was calculated using Bio-Vision image acquisition software (Vilber Loumat, Marne-la-Vallée, France).
Metabolite production
LTB4 and LTC4 production was assayed in Jurkat and purified T-cell supernatants. To verify that the CD3 signalling pathway was intact, Jurkat and purified T lymphocytes were stimulated using the T-cell Activation/Expansion kit (Miltenyi Biotec), which mimics antigen-presenting cells and activates the resting T cells. For this 10 × 106 cells were incubated for 5, 10, 15, 30, 60, 90, 120 and 240 min at 37° with beads loaded with biotinylated anti-CD2, anti-CD3 and anti-CD28 monoclonal antibodies in serum-free RPMI. Supernatants were stored at −80° until quantification by enzyme immunoassay (EIA; Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions.
For pharmacological stimulation, MOLT4, Jurkat and purified T lymphocytes were incubated with 2 mm CaCl2 and 0·5 mm MgCl2 for 5 min. Calcium ionophore A23187 (0·5 μg) and exogenous arachidonic acid (100 μm final concentration, Sigma) were added in an ethanolic solution that never exceeded 0·4% for 10 min at 37°. Arachidonic acid was tested to verify the absence of autooxidation before use. Control cells received an equivalent amount of ethanol that did not affect AA metabolism. When 5-LOX inhibitors were used, cells were preincubated for 1 hr at 37° alone or in the presence of 10−6m or 10−5m MK-886 (Biomol, Plymouth Meeting, PA) or AA-861 (Sigma) before stimulation.
Results
Detection of 5-LOX mRNA in lymphoblastoid T-cell lines
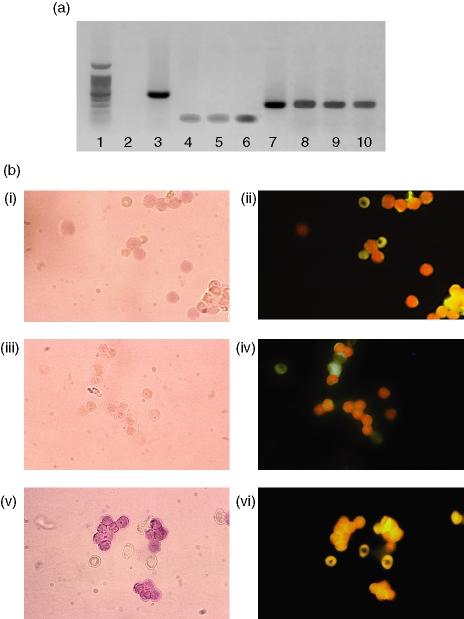
RT-PCR analysis of mRNA from MOLT4 and Jurkat cell lines as well as purified peripheral T lymphocytes showed the expected 295-bp band corresponding in size to that obtained when 5-LOX cDNA (kindly provided by Dr J.F. Evans, Merck-Frosst, Rahway, NJ) was amplified as a positive control under the same conditions (Fig. 1a). Restriction enzyme digestion by BamHI gave two fragments of 169 and 126 bp, which matched the fragments obtained when the 5-LOX cDNA-positive control was digested. The sequence of the PCR products was identical to the published 5-LOX sequence (nucleotides 1609–1904),38 confirming the presence of 5-LOX mRNA in both cell lines.
Figure 1.
Expression of 5-LOX mRNA by MOLT4 and Jurkat T cell lines as well as peripheral blood T cells. (a) Amplification of 5-LOX mRNA by RT-PCR (35 cycles). Lane 1, molecular weight ladder; lane 2, negative control – cDNA from chloramphenicol acetyl transferase mRNA with 5-LOX primers; lane 3, positive control – same cDNA with corresponding primers; lane 4, Jurkat mRNA run without reverse transcriptase; lane 5, MOLT4 mRNA run without reverse transcriptase; lane 6, T-lymphocyte mRNA run without reverse transcriptase; lane 7, 5-LOX cDNA-positive control; lane 8, Jurkat cells; lane 9, MOLT4 cells; lane 10, purified peripheral T lymphocytes. (b) Amplification of 5-LOX mRNA by RT-PCR in situ. Panel i, negative control: peripheral blood T lymphocytes submitted to RT-PCR in situ without reverse transcriptase; panel ii, same field visualized by fluorescence microscopy after incubation with anti-CD3 antibodies; panel iii, negative control: peripheral blood T lymphocytes submitted to RT-PCR in situ with primers specific for chloramphenicol acetyl transferase; panel iv, same field visualized by fluorescence microscopy after incubation with anti-CD3 antibodies, panel v, peripheral blood T lymphocytes submitted to RT-PCR in situ with specific 5-LOX primers; panel vi, same field visualized by fluorescence microscopy after incubation with anti-CD3 antibodies. Magnification 65×.
Detection of 5-LOX mRNA in peripheral blood T lymphocytes by in situ RT-PCR
After in situ amplification of 5-LOX mRNA in highly purified peripheral blood T lymphocytes, cells were stained with an anti-CD3 antibody to ensure that positive cells were actually T lymphocytes. Cells amplified with specific 5-LOX primers showed a lavender to intense violet coloration because of the formation of an insoluble precipitate which was localized in the nucleus (Fig. 1b). Immunofluorescence examination of the same field confirmed that these were indeed T cells. Staining was somewhat attenuated in 5-LOX-positive cells because of quenching by precipitate formation. Negative controls (amplification without reverse transcriptase and amplification with primers for chloramphenicol acetyl transferase) showed no nuclear labelling.
Indirect immunofluorescence of 5-LOX in MOLT4, Jurkat and peripheral blood T cells
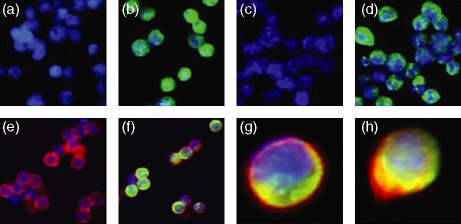
5-LOX was detected in both T-cell lines by indirect immunofluorescence using a monoclonal anti-5-LOX antibody. As shown in Fig. 2, 5-LOX was predominantly located in the cytosol with a lesser amount present in the nuclear compartment. This was confirmed by confocal microscopy (data not shown). Incubation with F(ab)′2 secondary antibody alone or with negative isotypic controls did not show any staining, confirming the specificity of the technique (Fig. 2a,c).
Figure 2.
5-LOX expression in Jurkat and MOLT4 cell lines and peripheral blood T lymphocytes by indirect immunofluorescence. (a and c) Jurkat and MOLT4 cells, respectively, incubated with a negative isotypic control serum; (b and d) Jurkat and MOLT4 cells, respectively, incubated with anti-5-LOX monoclonal antibody; (e and f) peripheral blood T lymphocytes incubated with either a negative isotypic control serum (e) or anti-5-LOX monoclonal antibody (f) followed by staining with anti-CD3 monoclonal antibodies (magnification × 40); (g) peripheral blood T lymphocytes stained with anti-5-LOX and anti-CD3 monoclonal antibodies and (h) after 3 days incubation in Chang MF medium and stained with anti-5-LOX and anti-CD3 monoclonal antibodies (arrow indicates localization of 5-LOX at nuclear periphery).
For peripheral T cells, double-labelling immunostaining [anti-CD3 (red) and anti-5-LOX (green)] was used to demonstrate unequivocally that 5-LOX is present in normal T lymphocytes. As in MOLT4 and Jurkat cells, 5-LOX was located in the cytosol and nucleus. Cells stimulated in Chang medium MF showed a shift in staining from the cytoplasm to the nuclear periphery indicating that 5-LOX translocation had occurred (Fig. 2h).
FACS sorting and immunostaining in MOLT4, Jurkat and peripheral blood T cells
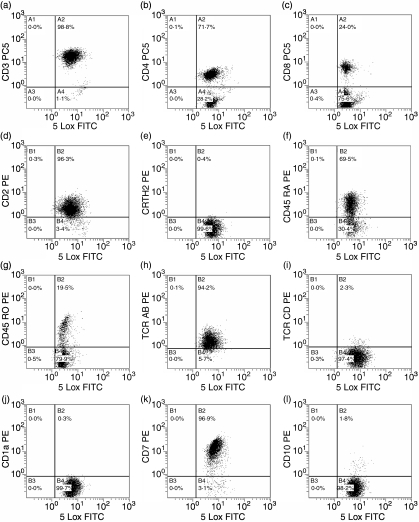
FACS analysis of double-stained cells also demonstrated the presence of 5-LOX in T cells (Fig. 3a). F(ab)′2 secondary antibody alone and isotypic controls gave no signal. Almost all CD3+, CD2+ and CD7+ cells were positive for 5-LOX. In parallel, staining with anti-CD45RO, anti-CD45RA, anti-CD4, anti-CD8, anti-T helper type 2 (Th2), anti-TCR-αβ and anti-TCR-γδ antibodies revealed the presence of 5-LOX in all subpopulations examined. 5-LOX was also present in immature T cells (CD1a and CD10 positive cells). No 5-LOX-negative cells were detected.
Figure 3.
Analysis of peripheral blood T lymphocyte populations by flow cytometry. The acquisition panels represent T cells gated on the CD19-negative, CD45-positive population. Cells were incubated with anti-5-LOX monoclonal antibody and F(ab)′2 goat anti-mouse fluorescein isothiocyanate (FITC) followed by staining with anti-CD3 (a), CD4 (b), CD8 (c), CD2 (d), CRTH2 (e), CD45RA (f), CD45RO (g), TCR-αβ (h), TCR-γδ (i), CD1a (j), CD7 (k) and CD10 (l) antibodies. The x-axis represents 5-LOX FITC and the y-axis represents the antibodies defining the different subpopulations. Results are from one experiment and are representative of five.
Western blot analysis of protein from Jurkat, MOLT4 and peripheral blood T cells
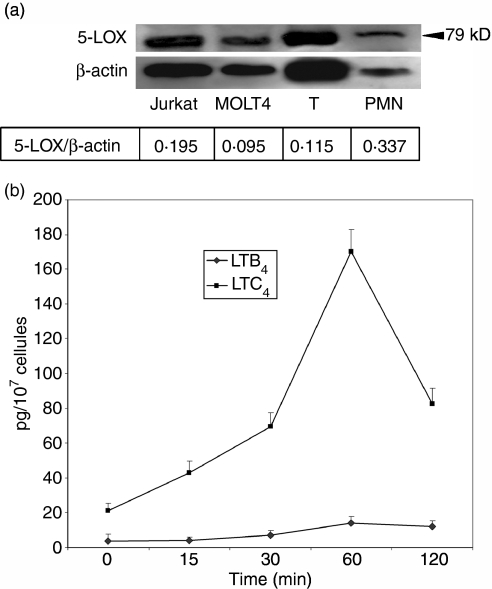
Western blot analysis demonstrated the presence of a 79 000 MW protein in purified blood T cells at the same level as that in the neutrophils that served as a positive control (Fig. 4a). In MOLT4 and Jurkat cells the molecular weight of the band observed was slightly lower. β-actin served as a loading control and the 5-LOX : β-actin ratio indicated that T lymphocytes had approximately three times less 5-LOX protein than did neutrophils.
Figure 4.
5-LOX protein expression and metabolite production. (a) Western blot of 5-LOX in MOLT4 and Jurkat T-cell lines and peripheral blood T lymphocytes. Cells were lysed and the cytosolic fraction was retrieved by ultracentrifugation. Equal amounts of protein (25 μg) were subjected to SDS–PAGE on 4–15% gradient gels, then transferred and immunoblotted using anti-5-LOX monoclonal antibody. Lane 1, molecular weight standard; lane 2, Jurkat cells; lane 3, MOLT4 cells; lane 4, peripheral blood T cells; lane 5, neutrophils. (b) Synthesis of LTB4 and LTC4 by Jurkat cells. Cells were activated by CD2–CD3–CD28 crosslinking. Supernatants were harvested at different times and production of LTB4 and LTC4 was quantified by EIA.
Metabolite production by Jurkat, MOLT4 and peripheral T lymphocytes
T lymphocytes activated through CD2, CD3 and CD28 cross-linking did not show any detectable LTB4 or LTC4 production. In contrast Jurkat cells stimulated in an identical manner were able to synthesize LTC4 and production was maximal at 1 hr (170 + 13 pg/107 cells, Fig. 4b). Minor amounts of LTB4 were detected (approximately 10 pg/107 cells) regardless of stimulation times. Preincubation with 10−6m and 10−5m MK-886 and AA861 completely abolished LTC4 production. When CD3 crosslinked T lymphocytes were incubated with exogenous AA (100 μm), they synthesized peak amounts of 181 pg LTB4 and 345 pg LTC4/107 cells after 10 min (data not shown). Cells that received AA but were not stimulated showed no detectable LTB4 or LTC4 production. Pharmacologically stimulated cells (including MOLT4), in the presence of exogenous arachidonic acid synthesized both LTB4 and LTC4 (data not shown).
Discussion
The question of 5-LOX expression in T lymphocytes has been unanswered for more than a decade. As this enzyme leads to highly biologically active molecules having immunomodulatory properties, we wanted to address this controversial question. The converging set of data we present in this article clearly defends the presence of 5-LOX both at the transcriptional and translational levels in highly purified peripheral blood T lymphocytes as well as in human leukaemic T-cell lines (MOLT4, Jurkat cells). RT-PCR performed on MOLT4 and Jurkat and sorted circulating T cells, amplified a 295-bp band of the PCR product corresponding to that obtained when 5-LOX cDNA was used as a positive control. Restriction enzyme digestion of the PCR product gave the expected fragments and sequencing showed that it was identical to the published sequence. This is, as far as we know, the first report of 5-LOX mRNA expression in T-cell lines. Other authors failed to find evidence of 5-LOX mRNA30,31 and for many years LTs were thought not to be synthesized in these cell types. Reasons for this divergence of results can be attributed to the use of different primer sets as well as amplification conditions or even to technological improvements in thermocyclers. Jacobsson et al.30 used primers spanning exons 13/14 while we used primer pairs spanning exons 11/12 and 13/14.39
Even though the purity of peripheral blood T lymphocytes was greater than 99%, to overcome the problem of possible contamination by monocytes, a major source of 5-LOX metabolites, we used in situ RT-PCR coupled with anti-CD3 labelling. We found that 5-LOX mRNA was indeed expressed in T lymphocytes. The presence of protein translation was confirmed by Western blot, metabolite production and indirect immunofluorescence. Immunoblotting gave a 79 000 MW band for circulating T lymphocytes and neutrophil lysates. A slightly lower molecular weight was observed for MOLT4 and Jurkat cells. Cifone et al.32 detected a band at 78 000 in Jurkat cells. However, no Western blots have been published for purified blood T lymphocytes. It may be that 5-LOX post-translational modifications such as glycosylation are different in lymphocyte cell lines compared to neutrophils and peripheral T cells, which could alter the molecular weight of the protein as has been described for other cells.40–42
Our immunofluorescence studies showed specific labelling of 5-LOX protein, which was predominantly located in the cytosol of both T-cell lines and peripheral T lymphocytes with a lesser amount in the nucleus as confirmed by confocal microscopy. The significance of 5-LOX localization is unclear even though much has been published recently concerning its compartmentalization,43 which varies according to cell type. 5-LOX is cytosolic in unstimulated blood neutrophils,44 peritoneal macrophages,45 eosinophils46 and monocytes.47 It is both nuclear and cytoplasmic in mast cells48 and alveolar macrophages49 and nuclear in Langerhans cells.50 5-LOX has been reported to be nuclear in B lymphocytes51 although Werz et al.52 were later unable to detect it in nuclear fractions but found it in the cytosolic compartment. Therefore 5-LOX distribution according to our study seems to be similar in B and T lymphocytes. Upon incubation of peripheral blood T cells in Chang MF medium for 3 days, 5-LOX accumulated at the nuclear membrane, indicating a possible translocation of the enzyme. This was not surprising because it appears that the topographic distribution could influence LT biosynthetic capacity. For example, in NIH3T3 cells, nuclear 5-LOX, which translocated to the inner membrane of the nuclear envelope, generated higher quantities of LTB4 than cytosolic enzyme53 whereas nuclear 5-LOX imported from the cytoplasm in eosinophils appeared to reduce LT production.54
Using FACS analysis we confirmed the presence of 5-LOX by double labelling. Almost all CD3+, CD2+ and CD7+ cells were positive for 5-LOX, suggesting that it was expressed in all subpopulations. To further characterize these subpopulations, cells were incubated with anti-CD45RA, anti-CD45RO, anti-CD4, anti-CD8, anti-Th2, anti-TCR-αβ and anti-TCR-γδ antibodies. In addition, immature T cells expressing CD1a or CD10 were also studied. Again, all cells appeared to be 5-LOX-positive, indicating that 5-LOX is a ubiquitous enzyme in T cells.
No LTB4 or LTC4 synthesis was detected by EIA in supernatants from purified circulating T cells activated by CD2–CD3–CD28 crosslinking. These results are similar to those reported for B lymphocytes. Biosynthesis of LTB4 by intact B leukaemia cells has only been described when arachidonic acid and calcium ionophore were added to the culture medium or in sonicated B cells.51,55 In our study, when exogenous arachidonic acid was supplied, T lymphocytes produced both LTB4 and LTC4. Cifone et al.32 also reported cytseinyl LT production by purified T cells following crosslinking of TCR/CD3 and labelling with 3H-labelled AA. This production peaked at 5 min, which is similar to our results in which maximal amounts were obtained at 10 min. They did not detect any LTB4 synthesis but this may be because they did not stimulate cells with anti-CD28 antibodies, a major costimulatory signal for T-cell activation. Los et al. detected only LTB4 following CD28 ligation and peptidyl LT production was not enhanced when the TCR complex was stimulated by anti-CD3. These discrepancies may be the result of differences in stimulation protocols.
In contrast to peripheral blood T lymphocytes, physiologically stimulated Jurkat cells were able to synthesize LTC4 in the absence of AA but amounts of LTB4 were barely detectable, which is interesting because cysteinyl LT production has not been described in B cells. Production was maximal at 1 hr and declined thereafter. Preincubation of Jurkat cells with MK-886 and AA861, at concentrations considered to inhibit 5-LOX activity, before activation with antibody-coated beads, totally abolished LTC4 production. To our knowledge, this is the first time that Jurkat cells have been clearly shown to produce LTC4 without the addition of exogenous substrate. This indicates that the signal transduction pathway is complete and that Jurkat cells have sufficient amounts of AA incorporated in their cell membranes for the synthesis of detectable quantities of metabolites. MOLT4 cells were not stimulated by CD2–CD3–CD28 cross-linking because they have been reported to lack CD3.
Jurkat and MOLT4 cells were stimulated pharmacologically by calcium ionophore and AA, which bypasses the requirement for cytosolic PLA2, to determine whether or not 5-LOX was functional in MOLT4 cells. Both cell lines produced LTB4 and LTC4. The fact that Jurkat cells synthesized LTB4 only when exogenous AA was supplied may indicate a difference in priority for 5-LOX. In the presence of limited substrate, the formation of cysteinyl LTs may be favoured.
Taken together our results unequivocally show that, like other blood leucocytes, T cells also express 5-LOX. For the first time we demonstrated the presence of 5-LOX mRNA at the transcriptional level. Even though 5-LOX protein is present, T cells are incapable of LT formation unless AA is added. Our results confirm those of Cifone et al.32 who showed evidence for 5-LOX in peripheral blood T lymphocytes by stimulating these cells collectively through the TCR–CD3 complex. We also showed for the first time that physiologically stimulated Jurkat cells were capable of synthesizing from endogenous substrate. Several explanations may be proposed concerning the difference in LT synthesis by normal peripheral T lymphocytes and Jurkat cells. One possibility is the difference in cellular AA content between the two cell types. T lymphocytes may contain a lower amount of AA compared to Jurkat cells. If this is the case, limited substrate may lead to undetectable LT production in the absence of exogenous AA. Another possibility could involve different regulatory mechanisms. For example, this could be the result of differences in DNA methylation of the 5-LOX promoter. Methylation of CpG sites in the promoter region of 5-LOX is an epigenetic mechanism to silence the expression of 5-LOX. Demethylation of these particular 5-methyl-C residues permits the expression of 5-LOX to proceed, increasing quantities of 5-LOX primary transcripts and mature mRNA and increasing the synthesis of LTB4, its trans isomers and 5-HETE.56
Lymphoid cells do not produce large amounts of LT compared to inflammatory cell types. It is likely that the role of LT in lymphocytes is more an immunomodulatory one rather than an inflammatory one. Arcoleo et al.57 preincubated adherent-cell-depleted murine splenocytes with LTB4 for 3 hr followed by concanavalin A stimulation and noted an increase in synthesis of the Th1 and Th2 lymphokines IL-2, IL-4, IL-10 and IFN-γ. This seems to be related to 5-LOX activity since incubation of Jurkat cells with nordihydroguaiaretic acid (NDGA) and MK-886 at concentrations considered to inhibit LT production impaired tumour necrosis factor-induced NF-κΒ-mediated transactivation.58 In another study, ONO-4057, an LTB4 receptor antagonist, inhibited T-cell proliferation and abolished IL-2, IFN-γ and IL-4 production by CD3-stimulated cells as well as IL-12-induced IFN-γ production.33 Cysteinyl LTs have been implicated in T-cell chemotaxis to lymph nodes and renal allograft rejection but studies are needed to further characterize their role in T-lymphocyte function.59,60
Recently, degradation of 5-LOX after the splitting of BL41-E95-A cells, an Epstein–Barr-virus-infected Burkitt's lymphoma B-cell line, was strongly correlated with the activation of caspases 6 and 8.61 This decrease in 5-LOX seemed to be necessary for cells to resume proliferation. The authors did not observe this phenomenon in other 5-LOX-positive cell types, indicating that it may be unique to BL41-E95-A cells or B lymphocytes. It would be interesting to study this further in T lymphocytes because the lack of metabolite production in non-proliferating cells contrasts with production by those in constant division. This could contribute to the major physiological differences between the two cell types. It will probably be difficult to clearly define the biological roles of 5-LOX in T lymphocytes. Nevertheless, this question is an important one because 5-LOX modulation of adaptive immunity may lead to new therapeutic strategies.
Acknowledgments
The authors would like to thank Drs F. Terro, A. Rametti and Y. Mousseau for technical assistance and photography and J. L. Faucher for flow cytometry analysis.
Abbreviations:
- AA
arachidonic acid
- cDNA
complementary DNA
- Cy5
Cychrome 5
- DIG
digoxygenin
- 5-HETE
5-hydroxy-6,8,11,14-eicosatetraenoic acid
- 5-LOX
5-lipoxygenase
- FLAP
five lipoxygenase activating protein
- IFN
interferon
- IL
interleukin
- LT
leukotriene
- mRNA
messenger RNA
- MW
molecular weight
- NDGA
nordihydroguaiaretic acid
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- PGE2
prostaglandin E2
- RT-PCR
reverse transcription–polymerase chain reaction
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- SSC
saline sodium citrate
- TBS
Tris-buffered saline
- TCR
T-cell receptor
- TE
Tris-EDTA
- Th
T helper
References
- 1.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 2.Ruegg C, Dormond O, Mariotti A. Endothelial cell integrins and COX-2: mediators and therapeutic targets of tumor angiogenesis. Biochim Biophys Acta. 2004;1654:51–67. doi: 10.1016/j.bbcan.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin JS, Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- 4.Stjernschantz J. The leukotrienes. Med Biol. 1984;62:215–30. [PubMed] [Google Scholar]
- 5.Rouzer CA, Kargman S. Translocation of 5-lipoxygenase to the membrane in human leukocytes challenged with ionophore A23187. J Biol Chem. 1988;263:10980–8. [PubMed] [Google Scholar]
- 6.Miller DK, Gillard JW, Vickers PJ, et al. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 1990;343:278–81. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- 7.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–55. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 8.Lam S, Chan H, LeRiche JC, Chan-Yeung M, Salari H. Release of leukotrienes in patients with bronchial asthma. J Allergy Clin Immunol. 1988;81:711–17. doi: 10.1016/0091-6749(88)91043-3. [DOI] [PubMed] [Google Scholar]
- 9.Tagari P, Rasmussen JB, Delorme D, Girard Y, Eriksson LO, Charleson S, Ford-Hutchinson AW. Comparison of urinary leukotriene E4 and 16-carboxytetranordihydro leukotriene E4 excretion in allergic asthmatics after inhaled antigen. Eicosanoids. 1990;3:75–80. [PubMed] [Google Scholar]
- 10.Bonizzi G, Piette J, Merville MP, Bours V. Distinct signal transduction pathways mediate nuclear factor-kappaB induction by IL-1beta in epithelial and lymphoid cells. J Immunol. 1997;159:5264–72. [PubMed] [Google Scholar]
- 11.Marcinkiewicz J, Grabowska A, Bryniarski K, Chain BM. Enhancement of CD4+ T-cell-dependent interleukin-2 production in vitro by murine alveolar macrophages: the role of leukotriene B4. Immunology. 1997;91:369–74. doi: 10.1046/j.1365-2567.1997.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Los M, Schenk H, Hexel K, Baeuerle PA, Droge W, Schulze-Osthoff K. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. Embo J. 1995;14:3731–40. doi: 10.1002/j.1460-2075.1995.tb00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dornand J, Sekkat C, Mani JC, Gerber M. Lipoxygenase inhibitors suppress IL-2 synthesis: relationship with rise of [Ca++]i and the events dependent on protein kinase C activation. Immunol Lett. 1987;16:101–6. doi: 10.1016/0165-2478(87)90115-5. [DOI] [PubMed] [Google Scholar]
- 14.Brach MA, de Vos S, Arnold C, Gruss HJ, Mertelsmann R, Herrmann F. Leukotriene B4 transcriptionally activates interleukin-6 expression involving NK-chi B and NF-IL6. Eur J Immunol. 1992;22:2705–11. doi: 10.1002/eji.1830221034. [DOI] [PubMed] [Google Scholar]
- 15.Johnson HM, Torres BA. Leukotrienes: positive signals for regulation of gamma-interferon production. J Immunol. 1984;132:413–16. [PubMed] [Google Scholar]
- 16.Dugas N, Dugas B, Kolb JP, Yamaoka K, Delfraiss JF, Damais C. Role of leukotriene B4 in the interleukin-4-induced human mononuclear phagocyte activation. Immunology. 1996;88:384–8. doi: 10.1046/j.1365-2567.1996.d01-658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jozefowski S, Biedron R, Bobek M, Marcinkiewicz J. Leukotrienes modulate cytokine release from dendritic cells. Immunology. 2005;116:418–28. doi: 10.1111/j.1365-2567.2005.02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stankova J, Rola-Pleszczynski M. Leukotriene B4 stimulates c-fos and c-jun gene transcription and AP-1 binding activity in human monocytes. Biochem J. 1992;282:625–9. doi: 10.1042/bj2820625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stankova J, Gagnon N, Rola-Pleszczynski M. Leukotriene B4 augments interleukin-2 receptor-beta (IL-2R beta) expression and IL-2R beta-mediated cytotoxic response in human peripheral blood lymphocytes. Immunology. 1992;76:258–63. [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaoka KA, Claesson HE, Rosen A. Leukotriene B4 enhances activation, proliferation, and differentiation of human B lymphocytes. J Immunol. 1989;143:1996–2000. [PubMed] [Google Scholar]
- 21.Payan DG, Missirian-Bastian A, Goetzl EJ. Human T-lymphocyte subset specificity of the regulatory effects of leukotriene B4. Proc Natl Acad Sci USA. 1984;81:3501–5. doi: 10.1073/pnas.81.11.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gualde N, Atluru D, Goodwin JS. Effect of lipoxygenase metabolites of arachidonic acid on proliferation of human T cells and T cell subsets. J Immunol. 1985;134:1125–9. [PubMed] [Google Scholar]
- 23.Rola-Pleszczynski M. Differential effects of leukotriene B4 on T4+ and T8+ lymphocyte phenotype and immunoregulatory functions. J Immunol. 1985;135:1357–60. [PubMed] [Google Scholar]
- 24.Irvin CG, Tu YP, Sheller JR, Funk CD. 5-Lipoxygenase products are necessary for ovalbumin-induced airway responsiveness in mice. Am J Physiol. 1997;272:L1053–8. doi: 10.1152/ajplung.1997.272.6.L1053. [DOI] [PubMed] [Google Scholar]
- 25.Ambrus JL, Jr, Jurgensen CH, Witzel NL, Lewis RA, Butler JL, Fauci AS. Leukotriene C4 produced by a human T-T hybridoma suppresses Ig production by human lymphocytes. J Immunol. 1988;140:2382–8. [PubMed] [Google Scholar]
- 26.Goetzl EJ. Selective feed-back inhibition of the 5-lipoxygenation of arachidonic acid in human T-lymphocytes. Biochem Biophys Res Commun. 1981;101:344–50. doi: 10.1016/0006-291x(81)91266-3. [DOI] [PubMed] [Google Scholar]
- 27.Abraham RT, McKinney MM, Forray C, Shipley GD, Handwerger BS. Stimulation of arachidonic acid release and eicosanoid biosynthesis in an interleukin 2-dependent T cell line. J Immunopharmacol. 1986;8:165–204. doi: 10.3109/08923978609028614. [DOI] [PubMed] [Google Scholar]
- 28.Goldyne ME, Burrish GF, Poubelle P, Borgeat P. Arachidonic acid metabolism among human mononuclear leukocytes. Lipoxygenase-related pathways. J Biol Chem. 1984;259:8815–19. [PubMed] [Google Scholar]
- 29.Poubelle PE, Borgeat P, Rola-Pleszczynski M. Assessment of leukotriene B4 synthesis in human lymphocytes by using high performance liquid chromatography and radioimmunoassay methods. J Immunol. 1987;139:1273–7. [PubMed] [Google Scholar]
- 30.Jakobsson PJ, Steinhilber D, Odlander B, Radmark O, Claesson HE, Samuelsson B. On the expression and regulation of 5-lipoxygenase in human lymphocytes. Proc Natl Acad Sci USA. 1992;89:3521–5. doi: 10.1073/pnas.89.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.el Makhour-Hojeij Y, Baclet MC, Chable-Rabinovitch H, Beneytout JL, Cook J. Expression of 5-lipoxygenase in lymphoblastoid B and T cells. Prostaglandins. 1994;48:21–9. doi: 10.1016/0090-6980(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 32.Cifone MG, Cironi L, Santoni A, Testi R. Diacylglycerol lipase activation and 5-lipoxygenase activation and translocation following TCR/CD3 triggering in T cells. Eur J Immunol. 1995;25:1080–6. doi: 10.1002/eji.1830250433. [DOI] [PubMed] [Google Scholar]
- 33.Morita H, Takeda K, Yagita H, Okumura K. Immunosuppressive effect of leukotriene B(4) receptor antagonist in vitro. Biochem Biophys Res Commun. 1999;264:321–6. doi: 10.1006/bbrc.1999.1523. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh J. Inhibition of arachidonate 5-lipoxygenase triggers prostate cancer cell death through rapid activation of c-Jun N-terminal kinase. Biochem Biophys Res Commun. 2003;307:342–9. doi: 10.1016/s0006-291x(03)01201-4. [DOI] [PubMed] [Google Scholar]
- 35.Esa AH, Converse PJ. Nordihydroguaiaretic acid blocks IL-2-independent lymphocyte proliferation and enhances responses to PPD. Scand J Immunol. 1996;43:127–33. doi: 10.1046/j.1365-3083.1996.d01-20.x. [DOI] [PubMed] [Google Scholar]
- 36.Papadogiannakis N, Barbieri B. Lipoxygenase inhibitors counteract protein kinase C mediated events in human T lymphocyte proliferation. Int J Immunopharmacol. 1997;19:263–75. doi: 10.1016/s0192-0561(97)00068-4. [DOI] [PubMed] [Google Scholar]
- 37.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 38.Matsumoto T, Funk CD, Radmark O, Hoog JO, Jornvall H, Samuelsson B. Molecular cloning and amino acid sequence of human 5-lipoxygenase. Proc Natl Acad Sci USA. 1988;85:26–30. doi: 10.1073/pnas.85.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Funk CD, FitzGerald GA. Eicosanoid forming enzyme mRNA in human tissues. Analysis by quantitative polymerase chain reaction. J Biol Chem. 1991;266:12508–13. [PubMed] [Google Scholar]
- 40.Pettersen I, Andersen JH, Bjornland K, Mathisen O, Bremnes R, Wellman M, Visvikis A, Huseby NE. Heterogeneity in gamma-glutamyltransferase mRNA expression and glycan structures. Search for tumor-specific variants in human liver metastases and colon carcinoma cells. Biochim Biophys Acta. 2003;1648:210–18. doi: 10.1016/s1570-9639(03)00146-8. [DOI] [PubMed] [Google Scholar]
- 41.Kamemura K, Hayes BK, Comer FI, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem. 2002;277:19229–35. doi: 10.1074/jbc.M201729200. [DOI] [PubMed] [Google Scholar]
- 42.Magro G, Schiappacassi M, Perissinotto D, et al. Differential expression of mucins 1–6 in papillary thyroid carcinoma: evidence for transformation-dependent post-translational modifications of MUC1 in situ. J Pathol. 2003;200:357–69. doi: 10.1002/path.1360. [DOI] [PubMed] [Google Scholar]
- 43.Peters-Golden M, Brock TG. Intracellular compartmentalization of leukotriene biosynthesis. Am J Respir Crit Care Med. 2000;161:S36–40. doi: 10.1164/ajrccm.161.supplement_1.ltta-8. [DOI] [PubMed] [Google Scholar]
- 44.Brock TG, Paine R, 3rd, Peters-Golden M. Localization of 5-lipoxygenase to the nucleus of unstimulated rat basophilic leukemia cells. J Biol Chem. 1994;269:22059–66. [PubMed] [Google Scholar]
- 45.Peters-Golden M, McNish RW. Redistribution of 5-lipoxygenase and cytosolic phospholipase A2 to the nuclear fraction upon macrophage activation. Biochem Biophys Res Commun. 1993;196:147–53. doi: 10.1006/bbrc.1993.2227. [DOI] [PubMed] [Google Scholar]
- 46.Brock TG, Anderson JA, Fries FP, Peters-Golden M, Sporn PH. Decreased leukotriene C4 synthesis accompanies adherence-dependent nuclear import of 5-lipoxygenase in human blood eosinophils. J Immunol. 1999;162:1669–76. [PubMed] [Google Scholar]
- 47.Woods JW, Evans JF, Ethier D, et al. 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J Exp Med. 1993;178:1935–46. doi: 10.1084/jem.178.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen XS, Naumann TA, Kurre U, Jenkins NA, Copeland NG, Funk CD. cDNA cloning, expression, mutagenesis, intracellular localization, and gene chromosomal assignment of mouse 5-lipoxygenase. J Biol Chem. 1995;270:17993–9. doi: 10.1074/jbc.270.30.17993. [DOI] [PubMed] [Google Scholar]
- 49.Woods JW, Coffey MJ, Brock TG, Singer II, Peters-Golden M. 5-Lipoxygenase is located in the euchromatin of the nucleus in resting human alveolar macrophages and translocates to the nuclear envelope upon cell activation. J Clin Invest. 1995;95:2035–46. doi: 10.1172/JCI117889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spanbroek R, Stark HJ, Janssen-Timmen U, et al. 5-Lipoxygenase expression in Langerhans cells of normal human epidermis. Proc Natl Acad Sci USA. 1998;95:663–8. doi: 10.1073/pnas.95.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jakobsson PJ, Shaskin P, Larsson P, et al. Studies on the regulation and localization of 5-lipoxygenase in human B-lymphocytes. Eur J Biochem. 1995;232:37–46. doi: 10.1111/j.1432-1033.1995.tb20778.x. [DOI] [PubMed] [Google Scholar]
- 52.Werz O, Klemm J, Radmark O, Samuelsson B. p38 MAP kinase mediates stress-induced leukotriene synthesis in a human B-lymphocyte cell line. J Leukoc Biol. 2001;70:830–8. [PubMed] [Google Scholar]
- 53.Luo M, Jones SM, Peters-Golden M, Brock TG. Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. Proc Natl Acad Sci USA. 2003;100:12165–70. doi: 10.1073/pnas.2133253100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cowburn AS, Holgate ST, Sampson AP. IL-5 increases expression of 5-lipoxygenase-activating protein and translocates 5-lipoxygenase to the nucleus in human blood eosinophils. J Immunol. 1999;163:456–65. [PubMed] [Google Scholar]
- 55.Runarsson G, Liu A, Mahshid Y, Feltenmark S, Pettersson A, Klein E, Bjorkholm M, Claesson HE. Leukotriene B4 plays a pivotal role in CD40-dependent activation of chronic B lymphocytic leukemia cells. Blood. 2005;105:1274–9. doi: 10.1182/blood-2004-07-2546. [DOI] [PubMed] [Google Scholar]
- 56.Uhl J, Klan N, Rose M, Entian KD, Werz O, Steinhilber D. The 5-lipoxygenase promoter is regulated by DNA methylation. J Biol Chem. 2002;277:4374–9. doi: 10.1074/jbc.M107665200. [DOI] [PubMed] [Google Scholar]
- 57.Arcoleo F, Milano S, D'Agostino P, Cillari E. Effect of exogenous leukotriene B4 (LTB4) on BALB/c mice splenocyte production of Th1 and Th2 lymphokines. Int J Immunopharmacol. 1995;17:457–63. doi: 10.1016/0192-0561(95)00038-4. [DOI] [PubMed] [Google Scholar]
- 58.van Puijenbroek AA, Wissink S, van der Saag PT, Peppelenbosch MP. Phospholipase A2 inhibitors and leukotriene synthesis inhibitors block TNF-induced NF-kappaB activation. Cytokine. 1999;11:104–10. doi: 10.1006/cyto.1998.0404. [DOI] [PubMed] [Google Scholar]
- 59.Honig SM, Fu S, Mao X, Yopp A, Gunn MD, Randolph GJ, Bromberg JS. FTY720 stimulates multidrug transporter- and cysteinyl leukotriene-dependent T cell chemotaxis to lymph nodes. J Clin Invest. 2003;111:627–37. doi: 10.1172/JCI16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spurney RF, Ibrahim S, Butterly D, Klotman PE, Sanfilippo F, Coffman TM. Leukotrienes in renal transplant rejection in rats. Distinct roles for leukotriene B4 and peptidoleukotrienes in the pathogenesis of allograft injury. J Immunol. 1994;152:867–76. [PubMed] [Google Scholar]
- 61.Werz O, Tretiakova I, Michel A, et al. Caspase-mediated degradation of human 5-lipoxygenase in B lymphocytic cells. Proc Natl Acad Sci USA. 2005;102:13164–9. doi: 10.1073/pnas.0505991102. [DOI] [PMC free article] [PubMed] [Google Scholar]