Abstract
Polymicrobial sepsis induces the suppression of macrophage function as determined by a reduction of pro-inflammatory cytokine production upon re-exposure to lipopolysaccharide (LPS) in vitro. Here, we examined whether macrophages were refractory to only LPS or if they were unable to respond to other stimuli such as CD40 ligand (CD40L). Monocytic cells exposed in vitro to LPS showed a dose-dependent reduction of their ability to produce interleukin-12 and tumour necrosis factor-α upon subsequent CD40L stimulation, as compared to cells stimulated with CD40L alone. Similarly, LPS interfered with the up-regulation of CD40, CD80 and CD86 induced by CD40L in monocytic cells. The effect of LPS on the response of monocytes to CD40L was similar whether these cells were directly exposed to LPS or cocultured with LPS-pretreated cells, indicating that soluble factors released by LPS stimulation could mediate tolerance to CD40L. We also show that the functional alterations induced by LPS in monocytes can be reversed by indomethacin, thus suggesting a role for inducible cyclooxygenase in mediating the LPS-induced hyporesponsive state of monocytes to CD40L. In conclusion, we propose that in vitro CD40L tolerance may be an appropriate model of monocyte alteration observed during septic immunosuppression and may help in the development of novel therapeutic strategies.
Keywords: CD40 ligand, lipopolysaccharide, macrophages, sepsis
Introduction
During the late stages of sepsis, massive deterioration of the immune response occurs, which is characterized by suppression of both macrophage and lymphocyte immune function. Indeed, a significant percentage of survivors of sepsis have an elevated risk of succumbing to bacterial superinfection.1–4
CD40 is a molecule with molecular weight 50 000 that is expressed on different cell types including monocyte–macrophages.5 The human form of a ligand for CD40 (CD40L) is a type II integral membrane protein expressed primarily on activated CD4+ T cells.6,7 Upon CD40 engagement, monocytic cells secrete a vast array of cytokines, among them, interleukin-12 (IL-12) and tumour necrosis factor-α (TNF-α), which are important in promoting and maintaining T helper type 1 (Th1) and pro-inflammatory responses during bacterial infection.8–12 Activation of macrophages by CD40L also results in the up-regulation of surface molecules, such as CD40, CD80 and CD86, which play a critical role in T-cell activation.13 Thus, CD40–CD40L interaction is an essential step in triggering the adaptive immune response14,15 and is very likely to play a prominent role during sepsis, as demonstrated by the increased mortality observed in septic animals with mutations of the CD40L gene.16,17
Lipopolysaccharide (LPS), a major cell wall component of Gram-negative bacterial organisms, can alone instigate many of the pathophysiological events that occur within the host during a septic episode.18,19 Endotoxin tolerance was first described as the temporary insensitivity of a host to a repeated LPS challenge with respect to systemic inflammation parameters. This phenomenon is associated with functional alterations to the monocytes and can be imitated in vitro. After pretreatment with an initial activating LPS dose, a second LPS stimulation of monocytic cells causes reduced production of pro-inflammatory cytokines, when compared with non-LPS-prestimulated controls.20–23
In contrast to previous studies, which defined the functional state of monocytic cells during endotoxin tolerance only in terms of LPS desensitization, we intended to characterize LPS-tolerized monocytes in terms of their capacity to respond to CD40L stimulation. Here we show the altered production of IL-12 and TNF-α, and the reduced expression of molecules that are important for T-cell stimulation by human monocytes stimulated by CD40L after an initial exposure to LPS. Furthermore, we tested the role of endogenous mediators produced during the initial LPS exposure in the alterations expressed by the monocytes.
Materials and methods
Cells
Peripheral blood obtained from healthy donors was enriched for peripheral blood mononuclear cells by centrifugation over Ficoll–Hypaque. These peripheral blood mononuclear cells were then further enriched for monocytes by elutriation as previously described.24 Cells obtained by this method are >90% monocytes as determined by fluorescence-acitvated cell sorting (FACS) analysis. The cells were cultured in RPMI-1640 medium supplemented with 20% heat-inactivated fetal calf serum, 2 mm l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, referred to as complete medium. The cells were kept at 37° in a humidified atmosphere of 5% CO2 in air, in 96-well V-bottom plates (Corning Incorporated, Corning, NY) at a concentration of 5 × 105 cells/well/250 μl.
Compounds
Immunex (Seattle, WA) provided soluble trimeric recombinant CD40L. Escherichia coli 0111/B4 LPS and indomethacin were purchased from Sigma Chemical Co. (St Louis, MO). Recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF) was obtained from Sandoz Research Institute (East Hanover, NJ) and contained 5·4 × 106 chronic myelogenous leukaemia units per milligram of glycoprotein. Interleukin-4 was obtained from Genzyme (Cambridge, MA).
Limulus amoebocyte lysate test
All the compounds and media used in this study were analysed for endotoxin contamination by the Limulus amoebocyte lysate test (QCL-1000, BioWhittaker, Inc, Walkersville, MD). All the samples analysed were found to be free of endotoxin contamination (less than 0·1 EU/ml).
Antibodies
For FACS analysis, the following monoclonal antibodies (mAbs) were used: anti-CD14, anti-CD40, anti-CD86, anti-CD80, anti-IL-12 (this antibody measures the IL-12 p40 and p70 heterodimer, but not the p35 subunit) and anti-TNF-α and anti-CD40L (all from PharMingen, San Diego CA). Staining was performed with antibodies coupled with fluorescein isothiocyanate, phycoerythrin or CyChrome. Neutralizing mouse anti-human transforming growth factor-β[TGF-β; immunoglobulin G1 (IgG1)] and anti-human IL-10 (IgG2) mAbs were purchased from R & D Systems (Minneapolis, MN). According to the manufacturer's specifications these antibodies neutralize the bioactivity of TGF-β1, TGF-β2, TGF-β3 and IL-10, respectively.
Enzyme-linked immunosorbent assay
Commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kits from R & D Systems were used to determine the concentration of IL-12 (this assay recognizes the p70 heterodimer) and TNF-α. The detection limits of these ELISAs are 15·6 pg/ml and 7·8 pg/ml, respectively. According to the manufacturer's specifications, these ELISAs are specific for the relative interleukin. All the samples were determined in duplicate, in a single analytical set. The intra-series variation coefficient was <15%.
Cell stimulation
To evaluate the ability of LPS to induce endotoxin tolerance, fresh elutriated monocyte–macrophages were cultured for 24 hr in the presence or absence of different LPS concentrations. Then, the cells were extensively washed and fed with fresh medium containing or not 100 ng/ml LPS. Thirty minutes after LPS stimulation, 1 μg/ml of the protein transport inhibitor brefeldin A was added. Sixteen hours after the addition of brefeldin A the cells were analysed by FACS for intracellular cytokine production.
To evaluate the ability of LPS to induce tolerance to CD40L, freshly elutriated cells were cultured for 24 hr in the presence or absence of different LPS concentrations. Then, after extensive washing, the cultures were fed with fresh complete medium containing 500 ng/ml CD40L. Thirty minutes after CD40L stimulation, 1 μg/ml brefeldin A was added. Sixteen hours after brefeldin A addition the cells were analysed either by FACS, for intracellular cytokine production, or by ELISA, in which case the supernatants from each sample were collected and stored at −80° for subsequent analysis. As shown by Brossart et al.,13 7 days exposure to CD40L induces maximal expression of CD40, CD80 and CD86 on monocytes. As previously demonstrated, 16 hr of stimulation by CD40L induces optimal cytokine production by monocytes. Coculture experiments were performed as follows. Fresh elutriated monocyte–macrophages (106) were plated in the bottom of a 24-well plate and were cocultured, separated by a microporous membrane (0·45 μm in diameter; Falcon 3095, Becton Dickinson, Mountain View, CA, USA), with monocyte–macrophages from the same donor, pre-exposed for 45 min to different concentrations of LPS (mock pre-exposed cells were used as control). Just before plating, LPS-treated cells were extensively washed to remove excess LPS. After 24 hr of culture, LPS-treated cells were discarded and non-LPS-treated cells were collected, washed and stimulated with 500 ng/ml CD40L. Thirty minutes after CD40L stimulation, 1 μg/ml brefeldin A was added. Sixteen hours after brefeldin A addition the cells were analysed by FACS for intracellular cytokine production. As shown by Brossart et al.,13 7 days exposure to CD40L induces maximal expression of CD40, CD80 and CD86 on monocytes. Thus, to evaluate the effect of LPS on the ability of CD40L to modulate the expression of CD40, CD80 and CD86, monocyte–macrophages were cultured after LPS challenge or cocultured with LPS-exposed cells as described above, for 7 days in the presence of 500 ng/ml CD40L. At the end of the incubation period the non-LPS-treated cells were analysed by FACS for CD40, CD80 and CD86 expression.
Surface marker and intracellular cytokine staining
After incubation, the cells were washed and stained for surface markers by incubation with the appropriate surface marker for 30 min in the dark on ice. Cells were then washed twice and either analysed by FACS to determine cell surface antigen expression or resuspended in Cytofix/Cytoperm solution (Pharmingen) for 20 min in the dark on ice. The permeabilized cells were washed twice and stained for intracellular cytokines in the dark on ice for 30 min. After intracellular cytokine staining, the cells were washed and resuspended in 200 μl phosphate-buffered saline with 0·4% paraformaldehyde for FACS analysis.
FACS analysis
Flow cytometry was performed using a FACScan flow cytometer and analysed with CellQuest software (Becton Dickinson). For each analysis, 104 events were gated on CD14 expression and a light scatter gate was designed to include only viable cells. Isotype-matched negative controls antibodies (Pharmingen) were used to verify the staining specificity.
Assessment of cell viability
The number of live cells was determined by trypan blue dye assay. In all the experiments performed the number of non-viable cells was consistently <3%.
Statistics
All numerical data were expressed as means ± standard errors of the means. P-values were derived from a two-tailed Student t-test. A P-value ≤0·05 was considered significant.
Results
LPS induces tolerance to CD40L stimulation
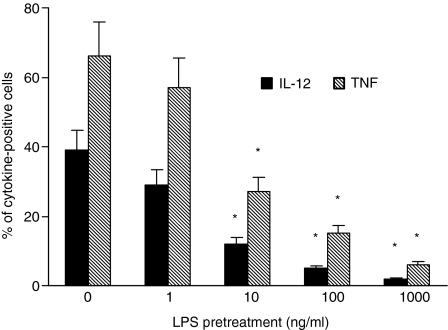
First, we confirmed the capacity of LPS to induce endotoxin tolerance in monocytes. As depicted in Fig. 1, monocytes exposed to LPS showed a decreased ability to produce IL-12 and TNF-α in response to subsequent LPS exposure. This suppression was significant with LPS concentrations of 10 ng/ml, whereas pretreatment with LPS at concentrations of 100–1000 ng/ml induced maximum suppression.
Figure 1.
Lipopolysaccharide induces endotoxin tolerance in monocyte–macrophages. Monocytes were treated with graded doses of LPS or medium for 24 hr, washed, and then stimulated with 100 ng/ml LPS. Intracellular cytokine production was assessed by FACS. Less than 1% IL-12 and TNF-α production was found in mock-LPS treated cells (data not shown). Appropriate controls with isotype-matched irrelevant mAbs were carried out and consistently showed <1% positive cells. The data represent the means of three experiments carried out with cells from three different donors. The error bars represent the standard errors. The asterisk indicates P < 0·05. The cytokine intensities of fluorescence (mean ratio ± SD, arbitrary units), respectively, for TNF-α and IL-12 were as follows: monocytes mock-pretreated with LPS, 34·47 ± 8·3 and 26·81 ± 7·2; monocytes pretreated with 1 ng/ml LPS, 30·16 ± 7·9 and 22·44 ± 4·6; monocytes pretreated with 10 ng/ml LPS, 21·30 ± 5·1 and 15·4 ± 4·3; monocytes pretreated with 100 ng/ml LPS, 12·8 ± 3·6 and 6·2 ± 2; monocytes pretreated with 1000 ng/ml LPS, 5·8 ± 3·9 and <5.
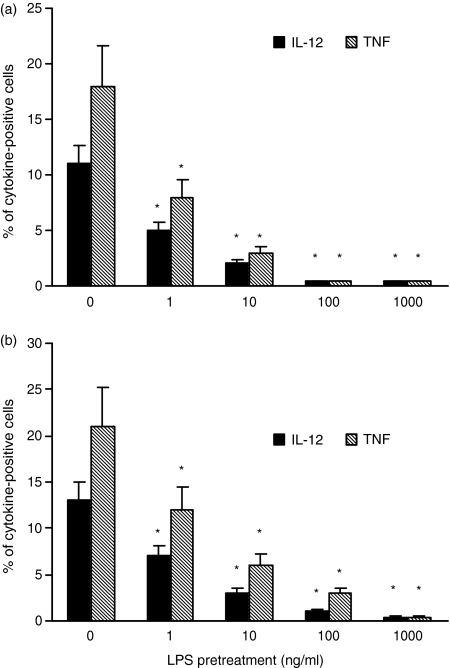
Further experiments were carried out to determine whether LPS could also induce tolerance to CD40L stimulation. As shown in Fig. 2(a), monocytic cells pre-exposed to LPS showed a dose-dependent reduction of their ability to produce IL-12 and TNF-α upon subsequent CD40L stimulation compared to cells stimulated with CD40L alone. Significant suppression of IL-12 and TNF-α was observed in cells pretreated with LPS at concentrations as low as 1 ng/ml. More than 90% suppression of IL-12 and TNF-α was found in monocytes pre-exposed to 100–1000 ng/ml LPS.
Figure 2.
Effect of LPS pretreatment on CD40L-induced intracellular cytokine production. (a) Monocytes were treated with graded doses of LPS or medium for 24 hr, washed, and then stimulated with 500 ng/ml CD40L. (b) Monocytes were cocultured, separated by a microporous membrane, with cells from the same donor pre-exposed to LPS or medium for 45 min, and then washed. After 24 hr of coculture the cells were stimulated with 500 ng/ml CD40L. Intracellular cytokine production was assessed by FACS. Less than 1% IL-12 and TNF-α production was found in mock-CD40L stimulated control samples (data not shown). The data represent the means of three experiments carried out with cells from three different donors. The error bars represent the standard errors. The asterisk indicates P < 0·05. The cytokine intensities of fluorescence (mean ratio ± SD, arbitrary units), respectively, for TNF-α and IL-12 were as follows. (a) monocytes mock-pretreated with LPS, 18·45 ± 7·3 and 13·6 ± 2·9; monocytes pretreated with 1 ng/ml LPS, 8·4 ± 2·5 and 5·6 ± 1·8; monocytes pretreated with 10 ng/ml LPS, <5 and <5; monocytes pretreated with 100 ng/ml LPS, <5 and <5; monocytes pretreated with 1000 ng/ml LPS, <5 and <5; and (b) monocytes mock-pretreated with LPS, 21·5 ± 8 and 15·3 ± 3·2; monocytes pretreated with 1 ng/ml LPS, 10·67 ± 3·7 and 6·9 ± 1·5; monocytes pretreated with 10 ng/ml LPS, 5·1 ± 1·1 and <5; monocytes pretreated with 100 ng/ml LPS, <5 and <5; monocytes pretreated with 1000 ng/ml LPS, <5 and <5.
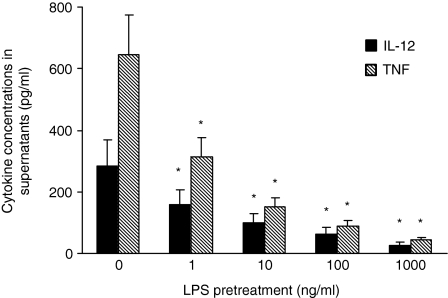
We then asked whether soluble factors released by LPS stimulation could mediate tolerance to CD40L. In these experiments, before CD40L stimulation, monocytes were cocultured separated by a microporous membrane, with cells from the same donor exposed or not to LPS. As shown in Fig. 2(b), the indirect effect of LPS on the cytokine response of monocytes to CD40L strictly paralleled that observed in cells directly pretreated by LPS, with more than 50% and 90% reduction of IL-12 and TNF-α production obtained by 1 ng/ml LPS and 100–1000 ng/ml LPS, respectively. These data were confirmed and expanded by testing cytokine release in supernatants. As shown in Fig. 3, in monocytes cocultured with LPS-pretreated cells the secretion of both IL-12 and TNF-α, was significantly reduced by pretreatment with 1 ng/ml LPS, and almost completely suppressed by 1000 ng/ml LPS.
Figure 3.
Effect of LPS pretreatment on CD40L-induced cytokine secretion. Monocytes were cultured and stimulated as described in the legend to Fig. 2(b). Cytokine release in supernatants was assessed by ELISA. No intracellular sequestration of cytokines was detected by ELISA testing of cells lysed by repeated cycles of freezing and thawing (data not shown). Shown is the amount of the different cytokines as the mean of three experiments, with three different donors, each carried out in triplicate. The error bars represent the standard errors. The asterisk indicates P < 0·05.
LPS prevents expression of costimulatory surface molecules induced by CD40L
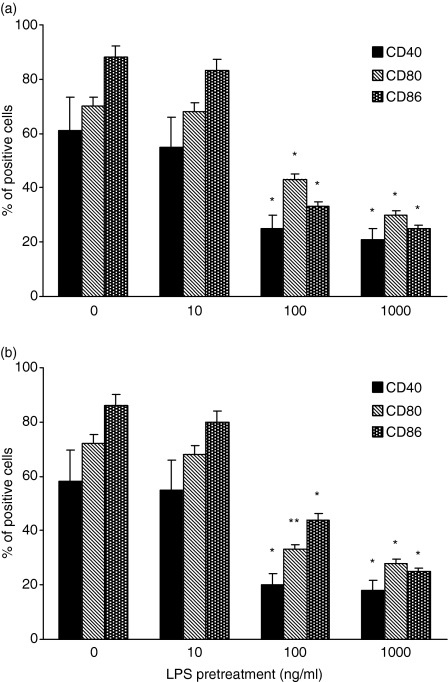
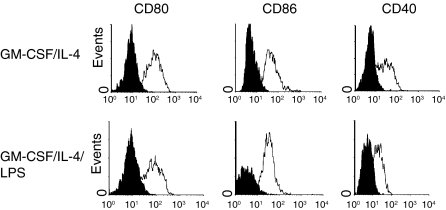
As shown in Fig. 4, LPS was able to interfere with the up-regulation of CD40, CD80 and CD86 induced by CD40L in monocytic cells. Again, the effect of LPS on the response of monocytes to CD40L was similar whether these cells were directly exposed to LPS (Fig. 4a) or cocultured with LPS-pretreated cells (Fig. 4b). In particular, a significant reduction of CD40, CD80 and CD86 expression was observed in LPS-treated cultures with LPS concentrations ≤100 ng/ml, as compared to cells stimulated with CD40L alone. Both GM-CSF and IL-4 are currently used to generate dendritic cells from blood monocytes.13 Differentiation of monocytes into dendritic cells results in the up-regulation of (along with many other molecules) CD40, CD80 and CD86.13 To evaluate whether the interference of LPS in CD40L-triggered activation of surface marker expression was specific or not, further experiments were carried out in which modulation of CD40, CD80 and CD86 was induced by GM-CSF/IL-4 in the presence or not of LPS. Figure 5 shows that LPS failed to affect the up-modulation of CD40, CD80 and CD86 induced by GM-CSF/IL-4.
Figure 4.
Effect of LPS on the expression of costimulatory surface molecules induced by CD40L. Monocytes were cocultured for 7 days with cells pre-exposed to LPS or medium, in the presence of 500 ng/ml CD40L. The expression of CD40, CD86 and CD80 on CD14+ cells was analysed by FACS. Appropriate controls with isotype-matched irrelevant mAbs were carried out and consistently showed <1% of positive cells. The data represent the mean of three experiments carried out with cells from three different donors. The error bars represent the standard errors. The asterisk indicates P < 0·05.
Figure 5.
Lipopolysaccharide does not interfere with the expression of CD80, CD86 and CD40 induced by GM-CSF/IL-4 stimulation. Histograms show expression of indicated cell surface molecules after 7 days of culture. Elutriated monocytes were cultured in the presence of 100 U/ml GM-CSF plus 1000 IU/ml IL-4 in the presence or in the absence of 100 ng/ml LPS. Solid histograms: labelling with isotype-matched irrelevant mAbs. The data represent a typical experiment out of three carried out with similar results.
Indomethacin alters the suppressive effect of LPS on the response of monocytes to CD40L
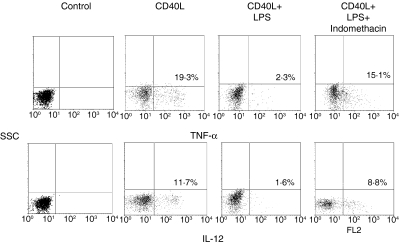
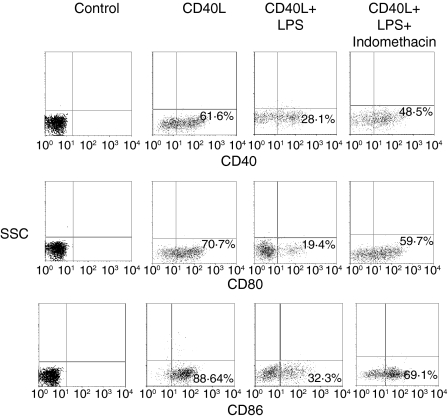
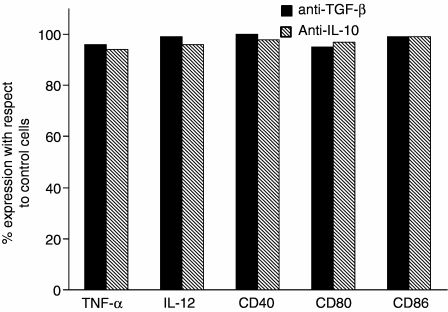
When the cyclooxygenase (COX) inhibitor indomethacin (1 μm), was added to monocytes cocultured with cells pre-exposed to LPS, the production of both IL-12 and TNF-α induced by CD40L increased by more than 80% (P < 0·05 and P < 0·05, respectively) (Fig. 6). Similarly, a significant increase of the expression of CD40 (60%, P < 0·05), CD80 (51%, P < 0·05) and CD86 (55%, P < 0·05) was observed in indomethacin-treated samples compared to untreated cells (Fig. 7). In contrast, experiments using neutralizing anti-TGF-β or anti-IL-10 antibodies showed that none of these mAbs could restore the CD40L-induced cytokine production or the surface marker expression in monocytes cocultured with LPS-activated cells (Fig. 8). Finally, Fig. 9 shows a representative example of how gating on the scatter plots and CD14+ cells was performed.
Figure 6.
Indomethacin alters the suppressive effect of LPS on the CD40-mediated cytokine response. Monocytes were cultured and stimulated as described for Fig. 2(b). The cells were stained with anti-CD14, anti-IL-12 or anti-TNF-α, and analysed by FACS. The cells were gated on CD14+ population. The data are displayed as dot plots. Appropriate controls with isotype-matched irrelevant mAbs were carried out and consistently showed <1% positive cells. The data represent a typical experiment out of three carried out with similar results. Cytokine levels (pg/ml) ± SD in supernatants for IL-12 and TNF-α, respectively, were as follows: CD40L, 236 ± 76 and 112 ± 34; CD40L + LPS, 26 ± 6 and 13 ± 2; CD40L + LPS + indomethacin, 209 ± 68 and 96 + 28·2
Figure 7.
Indomethacin alters the suppressive effect of LPS on the CD40-mediated surface marker expression. Monocytes were cocultured for 7 days with cells pre-exposed to LPS or medium in the presence of 500 ng/ml CD40L. The cells were stained with anti-CD14, anti-CD40, anti-CD86 or anti-CD80, and analysed by FACS. The cells were gated on a CD14+ population. The data are displayed as dot plots. Appropriate controls with isotype-matched irrelevant mAbs were carried out and consistently showed <1% positive cells. The data represent a typical experiment out of three carried out with similar results.
Figure 8.
Failure of neutralizing mAbs to TGF-β and IL-10 to restore the CD40L-induced cytokine production and surface marker expression in LPS-pretreated cells. Monocytes were cultured as previously described (see legends to Figs 2 and 7) in the presence or absence of 5 μg/ml anti-TGF-β or anti IL-10 mAbs. Results were calculated as (% of expression in the presence of the neutralizing mAbs)/(% of expression in the absence of neutralizing mAbs) × 100. Data represent the arithmetic means of two experiments carried out with cells from two different donors.
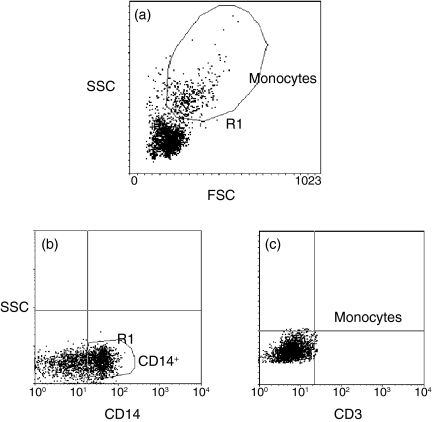
Figure 9.
Forward- and side-scatter profiles and CD14+ gating of the cells. (a) Monocytes were selected on the basis of their forward- and side-scatter profiles. (b, c) The side scatter gain was then appropriately lowered and the threshold for forward- and side-scatter was increased to exclude lymphocytes, dead cells and cellular debris and the cells that stained positive for CD14 (as compared with the CD3-stained control) were gated to analyse cytokine or surface markers expression.
Discussion
We have characterized LPS-tolerized monocytes in terms of their capacity to respond to CD40L stimulation. Our findings demonstrate that tolerance in such cells involves a distinct functional state of activation and/or differentiation, which is not restricted to LPS tachyphylaxis. Indeed, pretreatment with LPS substantially reduced the response of monocytic cells to CD40L in terms of both cytokine production and expression of costimulatory molecules. We also show that these functional alterations induced by LPS in monocytes can be reverted by indomethacin, suggesting a role for inducible COX in mediating the LPS-induced hyporesponsive state of monocytes to CD40L.
IL-12 is an immunoregulatory cytokine that is critical to the orchestration of cell-mediated immune responses in both the innate and adaptive immune systems. IL-12 augments the production of interferon-γ and other cytokines from natural killer cells and T cells.25 Furthermore, IL-12 appears to be a vital component of the host defence against both Gram-positive and Gram-negative bacteria, as shown by the heightened host resistance conferred by IL-12 administration in several models of bacterial infection.8–12 IL-12 is produced largely by monocyte–macrophages and dendritic cells upon LPS or CD40L triggering.26 Therefore, the likely relevance of endotoxin tolerance-related dysregulation of IL-12 production to the increased risk of bacterial superinfection in survivors of sepsis is of considerable interest. In this context, it is worth noting that macrophage-derived dendritic cells from septic mice were found to be unable to secrete IL-12 upon stimulation with CD40L.27 On the other hand, the role of TNF-α in combating infections has recently been underscored by the finding that sepsis and other infectious complications developed in patients with rheumatoid arthritis who were treated with TNF antagonists.28 Moreover, in clinical trials, immunotherapy against TNF-α significantly increased mortality.29
The most popular model of T-cell activation postulates a requirement for two distinct signals for T-cell activation.30,31 The first signal is believed to be the interaction of the T-cell receptor with the major histocompatibility complex/peptide complex on the surface of the antigen-presenting cells (APC), and the second signal comes from costimulatory molecules such as CD80 and CD86 on the surface of APC stimulating CD28 on the surface of T cells. Both CD80 and CD86 interact with CD28 and direct T cells to release cytokines. Therefore, their down-regulation affects not only the individual macrophage but also the other cells of the immune systems with which it comes in contact. It is crucial that T cells receive both signals from the APC when foreign antigen is presented; otherwise, T-cell anergy or death will result. A number of different cell types perform APC functions, including macrophages. However, macrophages that express low-level costimulatory molecules constitutively are not competent APC and require activation by CD40L first to up-regulate costimulatory activity and to become competent APC.32 Here, we found that LPS prevents the CD40L-mediated up-regulation of CD80 and CD86. In addition, LPS significantly affected the ability of CD40L to up-modulate the expression of CD40, which can further reduce the responsiveness of monocyte–macrophages to CD40L. With respect to experimental sepsis, recent studies indicate that the macrophage antigen-presenting capacity appears to become dysfunctional by 24 hr after induction of sepsis33 and remains at subnormal levels for up to 14 days after.34 More interestingly, Sugimoto et al. reported an inverse relationship between CD40 expression and susceptibility to infections and outcome in bacteraemic patients.35 However, other authors have reported that CD86 expression is decreased on peritoneal macrophages after sepsis, while CD80 and CD40 expression is unaltered.36 Differences between our in vitro results and in vivo studies may be in part the result of the presence, in vivo, of additional mediators able to affect co-receptor expression. Alternatively, it should be noted that in our experimental system the effect on co-receptor expression was observed at LPS concentrations equal or higher than 100 ng/ml. Such amounts of circulating LPS are not usually detected during sepsis, even though they can be found in the microenvironment at the site of infection.37
It has been recently demonstrated that the decreased TNF-α production capacity of monocytes observed during endotoxin tolerance is mediated by IL-10 and TGF-β produced during the primary LPS stimulation.38 However, we found that neutralization of endogenous IL-10 and TGF-β failed to prevent the LPS-induced reduced monocyte susceptibility to CD40L, suggesting that other factors are involved as the cause of this phenomenon. LPS is a potent trigger of prostaglandin E2 (PGE2) in monocytes.39 The interaction of PGE2 with the PGE2 receptor on monocyte–macrophages results in reduced activation of the Janus family protein tyrosine kinase JAK3.40,41 Recent findings indicate that CD40 engagement in human monocytes induces JAK3 phosphorylation and subsequent activation of transcription factors, suggesting a critical role of JAK3 as signal transducer after CD40L stimulation.42 Indeed, in CD40-triggered monocytes, the inhibition of JAK3 with the specific inhibitor WHI-P-154 drastically prevented cytokine production and the expression of maturation markers.43 Therefore, it is conceivable that CD40L-response suppression by LPS is mediated by PGE2. This hypothesis is consistent with the ability of indomethacin to ameliorate the ability of LPS-exposed monocytic cells to respond to CD40L stimulation. In vivo and in vitro experiments indicate that the induction of inducible COX by endotoxins results in elevated production of PGE2 that is linked to the immunological abnormalities seen during sepsis.44–47 Several studies of the effects of non-selective COX inhibition during sepsis have been conducted with the intent of determining whether COX inhibition is beneficial and safe. Available data indicate that COX inhibitors are generally safe and may ameliorate several clinical and immunological parameters.46,48–50 However, treatment with COX inhibitors did not appear to improve the rate of survival in patients with sepsis even though it has been shown to ameliorate the extent of IL-12 reduction and to provide survival advantage in septic animals.45,51,52
Lipopolysaccharide-containing endotoxins are potent activators of the innate immune system. As such, endotoxins are central to the pathogenesis of Gram-negative sepsis.18,53–55 Massive LPS-induced release of pro-inflammatory cytokines in vivo has been associated with the induction of acute septic shock. In this context, it is worth stressing the role of CD40 engagement in contributing to the host early inflammatory response to bacterial infection.16,17 However, death as a result of hyperactivation of the innate immune system is an uncommon result of exposure to endotoxins. Moreover, immunomodulatory therapy directed at inhibition of the inflammatory response has been largely unsuccessful.29,56 Indeed, it has been observed that subsequent to septic shock there often follows a clinical state characterized by profound immunosuppression. Modern medicine is now able potentially to control acute hyperinflammatory situations, but there is no established therapy to deal with the high risk associated with immunosuppression. We propose that in vitro CD40L tolerance may be an appropriate model of monocyte alteration observed during septic immunosuppression and may help in the development of novel therapeutic strategies.
References
- 1.Sayeed MM. Alteration in cell signalling and related effectors functions in T lymphocytes in burn/trauma/septic injuries. Shock. 1996;5:157–66. doi: 10.1097/00024382-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Choudhry MA, Ahamad S, Thompson KD, Sayeed MM. T-lymphocyte Ca2+ signaling and proliferative responses during sepsis. Shock. 1994;1:267–72. doi: 10.1097/00024382-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Faist E, Schinkel C, Zimmer S. Update on the mechanisms of immune suppression of injury and immune modulation. World J Surg. 1996;20:454–9. doi: 10.1007/s002689900071. [DOI] [PubMed] [Google Scholar]
- 4.Volk HD, Reinke P, Docke WD. Clinical aspects: from systemic inflammation to ‘immunoparalysis’. Chem Immunol. 2000;74:162–77. doi: 10.1159/000058753. [DOI] [PubMed] [Google Scholar]
- 5.Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–74. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–2. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 7.Graf D, Korthauer U, Mages HW, Senger G, Kroczek RA. Cloning of TRAP, a ligand for CD40 on human T cells. Eur J Immunol. 1992;22:3191–4. doi: 10.1002/eji.1830221226. [DOI] [PubMed] [Google Scholar]
- 8.Chaussabel D, Jacobs F, de Jonge J, de Veerman M, Carlier Y, Thielemans K, Goldman M, Vray B. CD40 ligation prevents Trypanosoma cruzi infection through interleukin-12 upregulation. Infect Immun. 1999;67:1929–34. doi: 10.1128/iai.67.4.1929-1934.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiener PA, Moran-Davis P, Rankin BM, Wahl AF, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble gp 39 induces proinflammatory responses in human monocytes. J Immunol. 1995;155:4917–25. [PubMed] [Google Scholar]
- 10.Greenberger MJ, Kunkel SL, Strieter RM, et al. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J Immunol. 1996;157:3006–12. [PubMed] [Google Scholar]
- 11.Metzger DW, Raeder R, Van Cleave VH, Boyle MD. Protection of mice from group A streptococcal skin infection by interleukin-12. J Infect Dis. 1995;171:1643–5. doi: 10.1093/infdis/171.6.1643. [DOI] [PubMed] [Google Scholar]
- 12.Kincy-Cain T, Clements J, Bost K. Endogenous and exogenous interleukin-12 augments the protective immune response in mice orally challenged with Salmonella dublin. Infect Immun. 1996;64:1437–40. doi: 10.1128/iai.64.4.1437-1440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brossart P, Grunebach F, Sthuler G, Reichardt VL, Mohle R, Kanz L, Brugger W. Generation of functional human dendritic cells from adherent peripheral blood monocytes by CD40 ligation in the absence of granulocyte–macrophage colony-stimulating factor. Blood. 1998;11:4238–47. [PubMed] [Google Scholar]
- 14.Grewal IS, Flavell RA. CD40 and CD154 in cell mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 15.Stout RD, Suttles J. The many roles of CD40 in cell-mediated inflammatory responses. Immunol Today. 1996;17:487–92. doi: 10.1016/0167-5699(96)10060-i. [DOI] [PubMed] [Google Scholar]
- 16.Nolan A, Weiden MD, Hoshino Y, Gold JA. CD40 but not CD154 knockout mice have reduced inflammatory response in polymicrobial sepsis: a potential role for Escherichia coli heat shock protein 70 in CD40-mediated inflammation in vivo. Shock. 2004;22:538–42. doi: 10.1097/01.shk.0000143416.20649.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold JA, Parsey M, Hoshino Y, Hoshino S, Nolan A, Yee H, Tse DB, Weiden MD. CD40 contributes to lethality in acute sepsis: in vivo role for CD40 in innate immunity. Infect Immun. 2003;71:3521–8. doi: 10.1128/IAI.71.6.3521-3528.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison DC, Ryan JL. Endotoxins and disease mechanisms. Annu Rev. 1987;38:417–32. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 19.Remick DG, Strieter RM, Eskandari MK, Nguyen DT, Genord MA, Raiford CL, Kunkel SL. Role of tumor necrosis factor-alpha in lipopolysaccharide-induced pathologic alterations. Am J Pathol. 1990;136:49–60. [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy RC, Chen GH, Tekchandani PK, Standiford TJ. Sepsis-induced immunosuppression: from bad to worse. Immunol Res. 2001;24:273–87. doi: 10.1385/IR:24:3:273. [DOI] [PubMed] [Google Scholar]
- 21.Mengozzi M, Ghezzi P. Cytokine down-regulation in endotoxin tolerance. Eur Cytokine Netw. 1993;4:89–98. [PubMed] [Google Scholar]
- 22.Haas JG, Baeuerle PA, Riethmuller G, Ziegler-Heitbrock HWL. Molecular mechanisms in down-regulation of tumor necrosis factor expression. Proc Natl Acad Sci USA. 1990;87:9563–7. doi: 10.1073/pnas.87.24.9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Morrison DC. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor α and nitric oxide production in mouse peritoneal macrophages. J Exp Med. 1993;177:511–16. doi: 10.1084/jem.177.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Placido R, Mancino G, Amendola A, et al. Apoptosis of human monocytes/macrophages in Mycobacterium tuberculosis infection. J Pathol. 1997;181:31–8. doi: 10.1002/(SICI)1096-9896(199701)181:1<31::AID-PATH722>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Brunda M. Interleukin-12. J Leukocyte Biol. 1994;55:280–8. doi: 10.1002/jlb.55.2.280. [DOI] [PubMed] [Google Scholar]
- 26.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–96. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 27.Flohe SB, Agrawal H, Schmitz D, Gertz M, Flohe S, Schade U. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J Leukocyte Biol. 2006;79:473–81. doi: 10.1189/jlb.0705413. [DOI] [PubMed] [Google Scholar]
- 28.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 29.Fisher CJ, Jr, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334:1697–702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 30.Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–85. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins MK. The ups and downs of T cell costimulation. Immunity. 1994;1:443–6. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 32.Ho WY, Cooke MP, Goodnow CC, Davis MM. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4C T cells. J Exp Med. 1994;179:1539–49. doi: 10.1084/jem.179.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayala A, Urbanich MA, Herdon CD, Chaudry IH. Is sepsis-induced apoptosis associated with macrophage dysfunction? J Trauma. 1996;40:568–73. doi: 10.1097/00005373-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Gallinaro RN, Naziri W, McMasters KM, Peyton JC, Cheadle WG. Alteration of mononuclear cell immune-associated antigen expression, interleukin-1 expression, and antigen presentation during intra-abdominal infection. Shock. 1994;1:130–4. doi: 10.1097/00024382-199402000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Sugimoto K, Galle C, Preiser J-C, Creteur J, Vincent J-L, Pradier O. Monocyte CD40 expression in severe sepsis. Shock. 2003;19:24–7. doi: 10.1097/00024382-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Newton S, Ding Y, Chung CS, Chen Y, Lomas-Neira JL, Ayala A. Sepsis-induced changes in macrophage co-stimulatory molecule expression: CD86 as a regulator of anti-inflammatory IL-10 response. Surg Infect. 2004;5:375–83. doi: 10.1089/sur.2004.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuchihashi H, Yamamoto H, Maeda K, et al. Concentrations of adiponectin, an endogenous lipopolysaccharide neutralizing protein, decrease in rats with polymicrobial sepsis. J Surg Res. 2006;134:348–53. doi: 10.1016/j.jss.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk HD. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med. 1995;18:1887–92. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowers GJ, MacVittie TJ, Hirsh EF, Conklin JC, Nelson RD, Roethel RJ, Fink MP. Prostanoid production by lipopolysaccharide-stimulated Kupffer cells. J Surg Res. 1985;38:501–8. doi: 10.1016/0022-4804(85)90068-x. [DOI] [PubMed] [Google Scholar]
- 40.Li R, Mouillesseaux KP, Montoya D, Cruz D, Gharavi N, Dun M, Koroniak L, Berliner JA. Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circ Res. 2006;98:642–50. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- 41.Kolenko V, Rayman P, Roy B, et al. Downregulation of JAK3 protein levels in T lymphocytes by prostaglandin E2 and other cyclic adenosine monophosphate-elevating agents: impact on interleukin-2 receptor signaling pathway. Blood. 1999;93:2308–18. [PubMed] [Google Scholar]
- 42.Revy P, Hivroz C, Andreu G, Graber P, Martinache C, Fischer A, Durandy A. Activation of the Janus kinase 3-STAT5a pathway after CD40 triggering of human monocytes but not of resting B cells. J Immunol. 1999;163:787–93. [PubMed] [Google Scholar]
- 43.Säemann MD, Kelemen P, Zeyda M, Böhmig G, Staffler G, Zlabinger GJ. CD40 triggered human monocyte-derived dendritic cells convert to tolerogenic dendritic cells when JAK3 activity is inhibited. Transplant Proc. 2002;5:1407–8. doi: 10.1016/s0041-1345(02)02907-x. [DOI] [PubMed] [Google Scholar]
- 44.Dubois RN, Abramson SB, Crofford L, Gupta RALS, van de Putte LBA, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73. [PubMed] [Google Scholar]
- 45.Schwacha MG, Chung CS, Ayala A, Bland KI, Chaudy IH. Cyclooxygenase 2-mediated suppression of macrophage interleukin-12 production after thermal injury. Am J Physiol Cell Physiol. 2002;282:C263–C270. doi: 10.1152/ajpcell.00357.2001. [DOI] [PubMed] [Google Scholar]
- 46.Michelin MA, Silva JS, Cunha FQ. Inducible cyclooxygenase released prostaglandin mediated immunosuppression in acute phase of experimental Trypanosoma cruzi infection. Exp Parasitol. 2005;111:71–9. doi: 10.1016/j.exppara.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Weikert LF, Bernard GR. Pharmacotherapy of sepsis. Clin Chest Med. 1996;17:289–305. doi: 10.1016/s0272-5231(05)70315-4. [DOI] [PubMed] [Google Scholar]
- 48.Bernard GR, Wheeler AP, Russell JA, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen Sepsis Study Group. N Engl J Med. 1997;336:912–18. doi: 10.1056/NEJM199703273361303. [DOI] [PubMed] [Google Scholar]
- 49.Akbulut H, Celik I, Ayar A, Vural P, Canbaz M. Ibuprofen reduces plasma nitrite/nitrate levels in a rabbit model of endotoxin-induced shock. Neuro Endocrinol Lett. 2005;26:407–12. [PubMed] [Google Scholar]
- 50.Dallal O, Ravindranath TM, Choudhry MA, Kohn A, Muraskas JK, Namak SY, Alattar MH, Sayeed MM. T-cell proliferative responses following sepsis in neonatal rats. Biol Neonate. 2003;83:201–7. doi: 10.1159/000068921. [DOI] [PubMed] [Google Scholar]
- 51.Strong VE, Mackrell PJ, Concannon EM, Naama HA, Schaefer PA, Shaftan GW, Stapleton PP, Daly JM. Blocking prostaglandin E2 after trauma attenuates pro-inflammatory cytokines and improves survival. Shock. 2000;14:374–9. doi: 10.1097/00024382-200014030-00023. [DOI] [PubMed] [Google Scholar]
- 52.Shoup M, He LK, Liu H, Shankar R, Gamelli R. Cyclooxygenase-2 inhibitor NS-389 improves survival and restores leukocyte counts in burn infections. J Trauma. 1998;45:215–20. doi: 10.1097/00005373-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–43. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 54.Ayala A, Herdon CD, Lehman DL, Ayala CA, Chaudry IH. Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency, and the nature of the mediators. Blood. 1996;87:4261–75. [PubMed] [Google Scholar]
- 55.Haimovitz-Friedman A, Cordon-Cardo C, Bayoumy S, et al. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med. 1997;186:1831–41. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Opal SM, Fisher JC, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase II, randomized, double blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25:1115–24. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]