Abstract
High mobility group box protein 1 (HMGB1) was previously considered a strict nuclear protein, but lately data are accumulating on its extranuclear functions. In addition to its potent proinflammatory capacities, HMGB1 has a prominent role in a number of processes of specific interest for the placenta. Our overall aim was to investigate the expression of HMGB1 in human term placenta and elucidate a potential difference in HMGB1 expression comparing vaginal deliveries with elective Caesarean sections. In addition, placentas from normal pregnancies were compared with placentas from pregnancies complicated by pre-eclampsia. Twenty-five placentas, 12 from normal term pregnancies and 13 from pregnancies complicated by pre-eclampsia were analysed with immunohistochemistry for HMGB1 and its putative receptors; receptor for advanced glycation end-products (RAGE), Toll-like receptor 2 (TLR2) and TLR4. We present the novel finding that in addition to a strong nuclear HMGB1 expression in almost all cells in investigated placentas, an individual variation of cytoplasmic HMGB1 expression was detected in the syncytiotrophoblast covering the peripheral chorionic villi, by cells in the decidua and in amnion. Production of HMGB1 was confirmed by in situ hybridization. Although labour can be described as a controlled inflammatory-like process no differences in HMGB1 expression could be observed comparing active labour and elective Caesarean sections. However, a tendency towards a higher expression of cytoplasmic HMGB1 in the decidua from women with pre-eclampsia was demonstrated. The abundant expression of the receptors RAGE, TLR2 and TLR4 implicates a local capability to respond to HMGB1, although the precise role in the placenta remains to be elucidated.
Keywords: high mobility group box protein 1 (HMGB1), placenta, pre-eclampsia, receptor for advanced glycation end-products (RAGE), Toll-like receptor 2, Toll-like receptor 4
Introduction
Pregnancy is an immunological challenge in which the mother carries the fetus—essentially a non-self invader—for an extended period of time. The barrier between the two entities is the placenta. The fetal part of the placenta consists of the two membranes, amnion and chorion, the umbilical cord and the chorionic villi covered by the syncytiotrophoblast, while the decidua and the intervillous space belong to the maternal part of the placenta.1
The process of human labour involves contraction-associated proteins, inflammatory cytokines, prostaglandins and extracellular matrix remodelling.2 Furthermore, the nuclear factor-κB (NF-κB) family is an upstream regulator of multiple labour-associated processes. An increase in gene expression of multiple cytokines and chemokines known to be involved in acute inflammation has been observed in chorioamniotic membranes from patients in labour compared to membranes from those not in labour.3
Pre-eclampsia is a pregnancy-related disorder that affects women all over the world and is a major cause of maternal and fetal morbidity and mortality. Major maternal symptoms include hypertension, abnormal amounts of protein in the urine, as well as clotting and liver dysfunction, where eclampsia is the end stage of the disease characterized by generalized seizures. The fetus may suffer increasing nutritional and respiratory insufficiency, asphyxia or death (reviewed in ref. 4). Despite recent progress in research, the biology of pre-eclampsia is still poorly understood, although it is suggested that the condition may originate in an insufficiently developed placenta, referred to as poor placentation.5 Suboptimal placentation results in an inadequate placental blood supply, which creates a hypoxic situation accompanied by oxidative stress, resulting in the maternal symptoms as well as fetal growth retardation during the second half of pregnancy (reviewed in ref. 4). It has further been proposed that pre-eclampsia is an excessive maternal inflammatory response to pregnancy, involving proinflammatory cytokines such as tumour necrosis factor (TNF) and interleukin-6 (IL-6).6
High mobility group box protein 1 (HMGB1) is abundantly expressed in all cell nuclei, where it binds to DNA and facilitates the transcription of several genes. Recently, it was demonstrated that HMGB1 also possesses important extranuclear functions as a potent cytokine mediating the late response to infection, injury and inflammation (reviewed in ref. 7). HMGB1 is a leaderless cytokine, i.e. it is not processed through the Golgi apparatus but is secreted through a non-classical pathway involving regulated exocytosis of secretory lysosomes.8 HMGB1 can be translocated and actively secreted by activated macrophages,8 mature plasmacytoid dendritic cells9 and natural killer cells.10 It can also be passively released during disintegration of necrotic cells.11 Receptor for advanced glycation end-products (RAGE) was the first receptor identified for extracellular HMGB112 but other receptors, such as Toll-like receptor 2 (TLR2) and TLR4, have also been shown to contribute to HMGB1-mediated signaling.13,14 HMGB1 induces NF-κB activation and release of proinflammatory cytokines and chemokines such as TNF, IL-1, IL-6 and IL-8.15
We have investigated the expression of HMGB1 and its suggested signalling receptors; RAGE, TLR2 and TRL4 in human term placenta. Since labour is an inflammatory-like process we wanted to elucidate if HMGB1 was up-regulated by labour. We further investigated HMGB1 expression in pathological placentas from women with pre-eclampsia; a pregnancy-related disease characterized by poor placentation resulting in a hypoxic placental condition and an exaggerated inflammatory response.
Materials and methods
Study population
Twenty-five women recruited at the delivery unit at the Karolinska University Hospital, Stockholm, Sweden participated in the study. Twelve women had normal term pregnancies while 13 women suffered from pre-eclampsia (five with moderate pre-eclampsia and eight with severe pre-eclampsia). Demographic data of the study population are shown in Table 1. Moderate pre-eclampsia was defined as >0·3 g proteinuria, systolic/diastolic blood pressure >140/90 (n = 5) and severe pre-eclampsia was defined as >3 g proteinuria, systolic/diastolic blood pressure >160/110 (n = 8). Twelve women had vaginal deliveries (seven healthy controls, five pre-eclampsia cases) and 13 women underwent elective Caesarean section (five healthy controls and eight pre-eclampsia cases). There were no significant differences in age between healthy and pre-eclamptic women. Women with pre-eclampsia delivered earlier than the healthy control women (week 36 versus week 40, P = 0·009) and nulliparity was more common in this group (P = 0·003). All women gave their informed consent to participate in the study. The Ethics Committee of the Karolinska Institutet, Stockholm, Sweden, approved the study.
Table 1.
Demographic data of the study population
| Group | Cases | Maternal age (years) | Parity | Gestation (weeks) | Mode of delivery (vaginal/Caesarean) |
|---|---|---|---|---|---|
| Normal pregnancy | 12 | 33 (24–38)1 | 2 (1–4)1 | 40 (36–42)1 | 7/5 |
| Pre-eclampsia2 | 13 | 32 (24–37)1 | 1 (1–2)1 | 36 (30–42)1 | 5/8 |
Median (range).
The women with pre-eclampsia had either moderate (n = 5, >0·3 g proteinuria, systolic/diastolic blood pressure >140/90) or severe (n = 8, >3 g proteinuria, systolic/diastolic blood pressure >160/110) pre-eclampsia).
Processing and immunohistochemical staining of placental slices
Placental tissue was taken immediately after delivery or kept refrigerated (at 4°) until processed (0–22 hr). Three or four placental slices were taken in a circular fashion around the insertion point of the umbilical cord. The slices spanned from the fetal membranes to the decidua. Slices were washed in NaCl, fixed in formalin and then paraffin embedded.
To investigate whether a longer storage time before fixation influenced placental histology or morphology we performed a separate kinetic study in which placental specimens were washed and either fixed immediately after delivery or kept in the fridge after which specimens were prepared 1, 2, 4, 6 and 24 hr after delivery.
Immunohistochemical stainings were performed on formaldehyde-fixed paraffin-embedded serial 4·5-μm sections of placentas. For detection of HMGB1 expression, slides were preheated at 60° overnight before deparaffinization and rehydration with ethanol. Subsequently, antigen retrieval was achieved by microwave irradiation in ethylenediamine tetraacetic acid buffer (pH 9·0). To block endogenous peroxidase activity, sections were treated with 1% H2O2 followed by a serum block with 2% human AB sera for 30 min and an avidin–biotin blocking step (Vector Laboratories Inc., Burlingame, CA). The slides were thereafter incubated overnight with an affinity-purified monoclonal mouse immunoglobulin G2b anti-HMGB1 antibody (concentration 2 μg/ml, 2G7, Critical Therapeutics Inc. Lexington, MA). A biotin-labelled horse anti-mouse antibody (Vector Laboratories Inc.) containing 2% normal horse serum was used for detection. Phosphate-buffered saline supplemented with 0·1% saponin was used in all subsequent washes and incubation steps to permeabilize the cells. In each assay, controls for specificity of the HMGB1 staining were included, based on parallel staining studies omitting the primary antibody and using a primary isotype-matched immunoglobulin of irrelevant antigen-specificity (negative mouse immunoglobulin G2b control, DAKO Cytomation, Glostrup, Denmark). The specificities of intracellular HMGB1 immunoreactivities were further verified by blocking experiments with preabsorption of the HMGB1-specific antibody with recombinant HMGB1 before staining.
Staining for RAGE, TLR2 and TLR4 was performed with some modifications of the staining protocol, as stated below. Pre-heating was excluded, because it was not needed for good staining performance. To block endogenous peroxidase activity, sections were treated with 0·3% H2O2, followed by incubation with 10% normal horse serum. Primary antibodies from Santa Cruz Biotechnology Inc (Santa Cruz, CA); goat anti-human RAGE (concentration 2·5 μg/ml; cat. no. sc-8230), goat anti-human TLR2 (concentration 5 μg/ml; cat. no. sc-8689) and goat anti-human TLR4 (concentration 2·5 μg/ml; cat. no. sc-8694) were absorbed against 25% human serum for 2 hr before being added to the specimen. Subsequently, the slides were incubated with the respective primary antibody for 45 min at room temperature. Biotin-labelled horse anti-goat antibody (Vector Laboratories Inc.) was used for detection. Staining without the primary antibody and staining with the primary antibodies preincubated with blocking peptides specific for respective antibody (Santa Cruz Biotechnology Inc.) were used as negative controls.
All stainings were developed using a DAB-kit (Vector Laboratories Inc.) according to the instruction of the manufacturer. Sections were counterstained with Mayer's haematoxylin.
Evaluation of the immunohistochemical stainings
The evaluated sections spanned the whole placental tissue, from the fetal membranes to the decidual plate. A semiquantitative scale from 0 to ++ + (where 0 is no positive cells, + is <25% positive cells, ++ is 25–75% positive cells and ++ + is >75% positive cells) was used for the evaluation of HMGB1 staining and that of the receptors RAGE, TLR2 and TLR4. For HMGB1 only cells with cytoplasmic expression were regarded as positive cells. The evaluation was performed blindly by three independent investigators (U.H., N.B. and E.S-E.). The agreement between the different investigators was >90%. The discrepancy was never more than one scale step, and consensus was obtained by re-evaluation.
In situ hybridization
A 50-base-pair oligonucleotide probe for HMGB1 and a random probe with no similarities to known sequences (from GenBank, National Institutes of Health) were synthesized (DNA Technology, Århus, Denmark). In situ hybridization was performed as described previously.16 Briefly, oligonucleotide probes were labelled with 33P-dATP (DuPont NEN, Boston, MA) at the 3′-end using terminal deoxynucleotidyltransferase (Amersham, Bucks, UK) and purified through QIAquick spin columns (QIAGEN, GmbH, Hilden, Germany). Sections were hybridized overnight at 42° in humidified boxes with 0·5 ng labelled probe (1 × 106 to 4 × 106 counts per minute/l) per slide in a hybridization mix and rinsed five times for 15 min each time in sodium saline citrate at 60°. Controls for specificity were performed with parallel experiments in which labelled random probe or an excess (100 ×) of cold probe was added to the hybridization mix. Tissue sections were dehydrated, air-dried, dipped in NTB2 nuclear track photographic emulsion (Kodak, Rochester, NY) and exposed for 7–14 days at 8°. Dipped slides were developed for 4 min in D19 (Kodak), fixed in Unifix (Kodak) for 7 min and rinsed in tap water for 20 min. After air-drying, sections were counterstained with haematoxylin and eosin.
Statistical analysis
The Stata 8 software (StataCorp LP, College Station, TX) was used for statistical analysis. The Mann–Whitney test was used to test for differences between groups regarding extranuclear HMGB1 expression and either Mann–Whitney or Fisher's exact test was used to test for differences between groups regarding demographic data. The sample size and number of ties were small so a Monte Carlo permutation test was used when calculating the P-values after performing the Mann–Whitney test. To verify which of the parameters: delivery method (vaginal/Caesarean) or disease, had the greatest influence on the HMGB1 expression, score data were also analysed with a proportional odds logit model for ordinal data17 with delivery and status (healthy/pre-eclamptic) as explanatory factors. The difference was considered significant if P < 0·05.
Results
HMGB1 is expressed on protein and messenger RNA level in human term placenta
To investigate if HMGB1 is expressed and produced in human term placenta we performed immunohistochemistry and in situ hybridization on placental sections from normal healthy pregnancies.
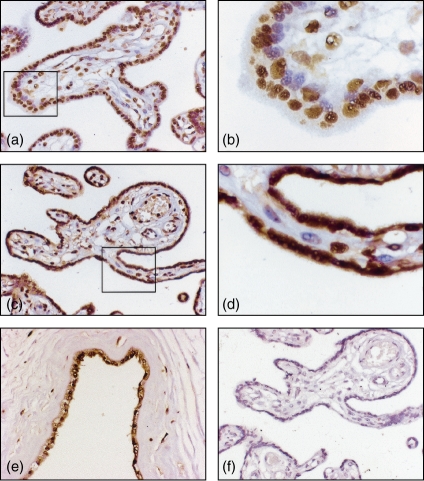
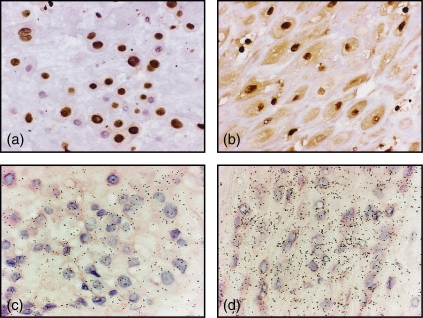
We could demonstrate strong nuclear expression and a homogeneous cytoplasmic expression of HMGB1 in the placental syncytiotrophoblast covering the peripheral chorionic villi (Fig. 1a–d), in cells in the decidua (Fig. 2a,b) and in the amniotic membranes (Fig. 1e) of all placentas investigated; however, individual variation in cytoplasmic HMGB1 expression was observed (Table 2). Nuclear expression of HMGB1 could also be seen in cells in the mesenchymal part of the chorionic villi. Production of HMGB1 in the placental syncytiotrophoblast and decidua was confirmed with in situ hybridization with positive transcripts in the syncytiotrophoblast, in cells in the decidua and in amnion. The in situ hybridization correlated well with the extranuclear HMGB1 expression obtained with immunohistochemistry, where an up-regulation of messenger RNA (mRNA) expression corresponded to abundant cytoplasmic expression in tissue specimens (Fig. 2c,d). No reactivity was seen in control hybridization with random or cold probes. A longer storage time before fixation did not influence placental histology, morphology or the cytoplasmic expression of HMGB1 (data not shown). There was no staining when the placental slides were incubated with an isotype-matched control (Fig. 1f) or when the HMGB1-specific antibody was preabsorbed with recombinant HMGB1 before staining. In addition, similar morphology of HMGB1 expression was obtained using a polyclonal rabbit-anti-HMGB1 antibody (cat. no. 556528, PharMingen, San Diego, CA) compared to the staining demonstrated in this report using the monoclonal anti-HMGB1 antibody. These two antibody preparations recognize separate epitopes of the HMGB1 molecule.
Figure 1.
HMGB1 is abundantly expressed in human term placenta. Formaldehyde-fixed paraffin-embedded sections of placenta were immunohistochemically stained for expression of HMGB1. Representative micrographs illustrate how the morphology of HMGB1 expression differed in different tissue specimens, where presence of extranuclear expression of HMGB1 was evaluated and graded semiquantitatively with a score from 0 to ++ +. (a, b) HMGB1 expression was restricted to cell nuclei and so illustrating a score of 0. (c, d) A predominant cytoplasmic expression of HMGB1 is evident in the syncytiotrophoblast, illustrating a score of ++ + (> 75% of cells expressing extranuclear HMGB1). (e) Expression of HMGB1 in amnion. (f) No staining was observed with an isotype matched control. Original magnifications 250 × (a, c, e, f) and 800 × (b, d).
Figure 2.
Micrographs illustrating HMGB1 expression in decidual cells where mRNA expression assessed by in situ hybridization correlated well with the extranuclear HMGB1 expression obtained with immunohistochemistry. The nuclear HMGB1 expression in (a) and the abundant cytoplasmic HMGB1 expression in (b) corresponded to (c) a low mRNA expression, and (d) up-regulated mRNA expression, respectively. Original magnification 400 ×.
Table 2.
Results from immunohistochemistry – extranuclear HMGB1
| Trophoblast | Decidua2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | + | + + | + + + | Total | + | + + | + + + | Total |
| Normal pregnancies | ||||||||
| HMGB11 | 5 | 6 | 1 | 12 | 5 | 5 | 1 | 11 |
| Vaginal | 3 | 4 | 0 | 7 | 2 | 3 | 1 | 6 |
| EC | 2 | 2 | 1 | 5 | 3 | 2 | 0 | 5 |
| Pre-eclampsia | ||||||||
| HMGB11 | 4 | 5 | 4 | 13 | 2 | 4 | 4 | 10 |
| Vaginal | 1 | 3 | 1 | 5 | 0 | 3 | 0 | 3 |
| EC | 3 | 2 | 3 | 8 | 2 | 1 | 4 | 7 |
A semiquantitative scale from 0 to ++ + (0 = no positive cells, + = < 25% positive cells, ++ = 25–75% positive cells, ++ + = > 75% positive cells) was used for the evaluation of the HMGB1 staining.
All sections did not contain decidual tissue.
Vaginal, vaginal deliveries; EC, elective Caesarean sections.
RAGE, TLR2 and TLR4 expression in human term placenta
HMGB1 has been proposed to signal through several receptors including RAGE,12 TLR2 and TLR4.13,14 To elucidate if the placenta has the cellular machinery for mediating local cell responses to HMGB1 we performed immunohistochemistry with antibodies against RAGE, TLR2 and TLR4 on the placental slides.
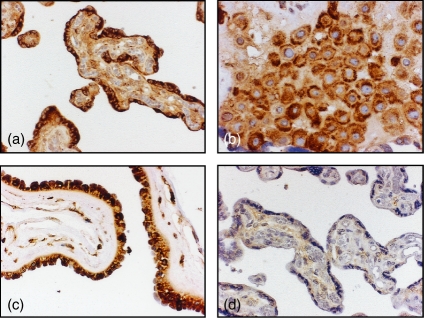
RAGE was highly and homogeneously expressed on the syncytiotrophoblast covering the peripheral chorionic villi (Fig. 3a), in cells in the decidua (Fig. 3b) and in the amniotic membranes (Fig. 3c) in all placentas investigated (Table 3). We also stained the placental slides with anti-TLR2 and anti-TLR4 antibodies, which resulted in prominent homogeneous staining of the syncytiotrophoblast covering the peripheral chorionic villi, cells in the decidua (Table 3) and in amniotic membranes. The results correlated well with the expression of RAGE and with our previously published data.18,19 The staining was extinct when the anti-RAGE (Fig. 3d), anti-TLR2 and anti-TLR4 antibodies had been preincubated with blocking peptides specific for respective antibody.
Figure 3.
RAGE is highly and evenly expressed by human term placenta. Formaldehyde-fixed paraffin-embedded sections of placenta were immunohistochemically stained for expression of RAGE, a central receptor for HMGB1 signalling. Representative micrographs are illustrating RAGE expression in (a) the syncytiotrophoblast, (b) decidual cells and (c) amnion. (d) Staining was extinct when the anti-RAGE antibody had been preincubated with blocking peptide specific for the anti-RAGE antibody. Original magnifications 250 × (a, c, d) and 400 × (b).
Table 3.
Results from immunohistochemistry-receptors
| Trophoblast | Decidua2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | + | + + | + + + | Total | + | + + | + + + | Total |
| Normal pregnancies | ||||||||
| RAGE1 | 2 | 3 | 8 | 13 | 1 | 2 | 6 | 9 |
| Vaginal | 2 | 3 | 3 | 8 | 1 | 1 | 4 | 6 |
| EC | 0 | 0 | 5 | 5 | 0 | 1 | 2 | 3 |
| TLR21 | 0 | 5 | 7 | 12 | 0 | 1 | 9 | 10 |
| Vaginal | 0 | 5 | 3 | 8 | 0 | 1 | 6 | 7 |
| EC | 0 | 0 | 4 | 4 | 0 | 0 | 3 | 3 |
| TLR41 | 0 | 2 | 11 | 13 | 0 | 0 | 13 | 13 |
| Vaginal | 0 | 1 | 7 | 8 | 0 | 0 | 8 | 8 |
| EC | 0 | 1 | 4 | 5 | 0 | 0 | 5 | 5 |
| Pre-eclampsia | ||||||||
| RAGE1 | 1 | 4 | 8 | 13 | 1 | 0 | 10 | 11 |
| Vaginal | 0 | 3 | 2 | 5 | 1 | 0 | 3 | 4 |
| EC | 1 | 1 | 6 | 8 | 0 | 0 | 7 | 7 |
| TLR21 | 0 | 4 | 8 | 12 | 0 | 1 | 8 | 9 |
| Vaginal | 0 | 2 | 3 | 5 | 0 | 1 | 3 | 4 |
| EC | 0 | 2 | 5 | 7 | 0 | 0 | 5 | 5 |
| TLR41 | 0 | 5 | 8 | 13 | 0 | 0 | 12 | 12 |
| Vaginal | 0 | 3 | 2 | 5 | 0 | 0 | 5 | 5 |
| EC | 0 | 2 | 6 | 8 | 0 | 0 | 7 | 7 |
A semiquantitative scale from 0 to ++ + (0 = no positive cells, + = < 25% positive cells, ++ = 25–75% positive cells, ++ + = > 75% positive cells) was used for the evaluation of the HMGB1 and RAGE staining.
All sections did not contain decidual tissue.
Vaginal, vaginal deliveries; EC, elective Caesarean sections.
The expression of extranuclear HMGB1 and its signalling receptors is not up-regulated by labour
Since HMGB1 is known as an inflammatory cytokine we wanted to investigate if a difference could be seen in the expression of HMGB1 and its signalling receptors RAGE, TLR2 and TLR4 in placenta from vaginal deliveries and elective Caesarean sections.
Staining was evaluated comparing vaginal deliveries (n = 5) and elective Caesarean sections (n = 7). However, no significant difference in HMGB1 (Table 2), RAGE, TLR2 or TLR4 (Table 3) expression could be seen between placentas from women in labour and from women undergoing elective Caesarean sections, although there were individual variations.
Expression of HMGB1 and its signalling receptors in placenta from women with pre-eclampsia
Pre-eclampsia is characterized by an inadequate placental blood supply leading to a hypoxic situation accompanied by oxidative stress. This results in maternal symptoms as well as fetal growth retardation during the second half of pregnancy. We wanted to investigate the expression of HMGB1 and its signalling receptors in these pathological placentas and compare our findings with the results obtained from normal pregnancies.
A tendency towards a higher expression of extranuclear HMGB1 in cells in the decidua could be observed in placentas from women with pre-eclampsia compared to placentas from normal pregnancies (Table 2). However, it did not reach statistical significance and could not be correlated with disease severity. No significant difference in the expression of RAGE, TLR2 and TLR4 could be seen between pre-eclamptic and normal placentas (Table 3). Furthermore, no correlation between HMGB1, RAGE, TLR2 or TLR4 expression and mode of delivery could be seen in the pre-eclamptic material (vaginal deliveries n = 5, elective Caesarean sections n = 8) or when the placentas from women with normal pregnancies and with pre-eclampsia were grouped together (vaginal deliveries n = 12, elective Caesarean sections n = 13). Furthermore, by using a proportional odds logit model we could verify that pre-eclampsia and not mode of delivery was the factor with greatest influence on the expression of HMGB1 in decidua.
Discussion
We present the novel finding of both nuclear and cytoplasmic expression as well as new synthesis of the pro-inflammatory cytokine HMGB1 in human term placenta.
Since the discovery in the late 1990s that HMGB1, previously solely considered as a nuclear protein, also mediates lethal septicaemia a growing body of data is accumulating on its extracellular cytokine-like functions. Similar to other cytokines, HMGB1 is pleiotropic with differential tissue-specific activities. In addition to its potent proinflammatory capacities, HMGB1 has been demonstrated to have a prominent role in a number of processes of specific interest for the placenta. HMGB1 has been identified as an angiogenetic switch molecule because it initiates endothelial growth as well as endothelial cell migration and sprouting.20,21 Interestingly, HMGB1–RAGE interactions promote invasiveness22 and HMGB1 has also been accredited a role in tissue repair.23,24
HMGB1 has been proposed to signal through several receptors including RAGE,12 TLR2 and TLR4.13,14 We could detect a high expression of RAGE, TLR2 and TLR4 by the syncytiotrophoblast covering the peripheral chorionic villi, by cells in the decidua and in amniotic membranes in all placentas investigated. The abundant expression of RAGE and TLRs in placenta thus provides the cellular machinery for mediating local cell responses to HMGB1 and is highly suggestive for a role of HMGB1 within the placenta.
HMGB1 is an abundant nuclear protein and, as expected, we observed a distinct nuclear HMGB1 expression in almost all cells of the placenta specimens studied. However, although individual variations were discernable, in all placentas investigated a substantial number of cells also expressed HMGB1 cytoplasmically. To be certain that the individual differences seen among the patients were not caused by an ex vivo reaction caused by the handling of the material we performed a kinetic study. However, no correlation between cytoplasmic HMGB1 expression and time between delivery and fixation of the placental tissue could bee seen. In addition, specimens with prominent cytoplasmic HMGB1 expression correlated well with up-regulated HMGB1 mRNA expression by in situ technique. Whether different placental cells are capable of active secretion of HMGB1 remains to be elucidated.
Active labour is associated with a highly regulated inflammatory-like response.2 Since HMGB1 is a potent inflammatory cytokine we wanted to investigate placental HMGB1 expression in relation to mode of delivery. However, when comparing the cytoplasmic expression of HMGB1 in placentas from vaginal deliveries with elective Caesarean sections no differences could be observed. Since HMGB1 mediates the late response to infection, injury and inflammation (reviewed in ref. 7) there might be a difference in placental expression of HMGB1 between normal pregnancies and pregnancies complicated by intrauterine infection. Further, there is a possibility that HMGB1 has a role in tissue repair within the placenta during normal pregnancy and a more proinflammatory role in pregnancies complicated by infection.
Pre-eclampsia is a placenta-dependent disorder affecting 3–5% of all pregnancies. A defective remodelling of the spiral arteries is believed to cause high-resistance vessels and reduced placental perfusion, leading to hypoxia and oxidative stress. We could observe a tendency towards a higher extranuclear expression of HMGB1 in cells in the decidua in pre-eclamptic placentas compared to placentas from healthy pregnancies. Although the cause is still unknown, there is growing evidence that an imbalance between factors promoting angiogenesis and factors antagonizing angiogenesis has a fundamental role in the pathogenesis of pre-eclampsia.25 Interestingly, HMGB1 has been suggested to be a pro-angiogenic cytokine. HMGB1 together with its receptor RAGE has been shown to induce a pro-angiogenic phenotype in endothelial cells and neovascularization in the chick embryo.20 Further, Schlueter et al.21 have shown that HMGB1 induces sprouting of endothelial cells. HMGB1 might therefore play an important role in placentation, which remains to be investigated. Interestingly, because pre-eclampsia is characterized by hypoxia and oxidative stress, the tendency towards a higher expression of HMGB1 in pre-eclamptic placentas could be a result of hypoxia and a way for the placenta to try and increase vascularization. However, we cannot exclude that the intrauterine environment differs immunologically between a first pregnancy and subsequent pregnancies. This could have implications for the cytoplasmic HMGB1 expression reported in this study because there is a difference in parity between women with normal pregnancy and women suffering from pre-eclampsia.
In conclusion, we have demonstrated that the placenta not only expresses and produces HMGB1 but also has the potential to respond to this inflammatory cytokine through the receptors RAGE, TLR2 and TLR4. However, the precise role of HMGB1 in placenta remains to be elucidated.
Acknowledgments
We would like to thank Birgitta Byström at the Fertility and Reproduction Laboratory and Agneta Rudels-Björkman, Lena Östeman, Vibeke Tham-Monten and the staff at the Delivery Unit at Karolinska University Hospital for collecting placentas. We are also grateful for the assistance of Gunilla Högberg in preparing sections from paraffin-embedded placental material and Jan-Olov Persson for providing excellent statistical support. This work was supported by grants from Freemason Lodge Barnhuset in Stockholm, the Hesselman Foundation, the Swedish Research Council (Medicine), grant no. 74X-15160, the Swedish Society for Medical Research, the Swärd/Eklund's Foundation, the Åhlén Foundation and Åke Wiberg's Foundation.
References
- 1.van den Brûle F, Berndt S, Simon N, et al. Trophoblast invasion and placentation: molecular mechanisms and regulation. Chem Immunol Allergy. 2005;88:163–80. doi: 10.1159/000087833. [DOI] [PubMed] [Google Scholar]
- 2.Lappas M, Rice GE. The role and regulation of the nuclear factor kappa B signalling pathway in human labour. Placenta. 2007;28:543–56. doi: 10.1016/j.placenta.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394.e1–24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 5.Redline RW, Patterson P. Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Hum Pathol. 1995;26:594–600. doi: 10.1016/0046-8177(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 6.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 7.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 8.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumitriu IE, Baruah P, Bianchi ME, Manfredi AA, Rovere-Querini P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur J Immunol. 2005;35:2184–90. doi: 10.1002/eji.200526066. [DOI] [PubMed] [Google Scholar]
- 10.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–16. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 11.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 12.Hori O, Brett J, Slattery T, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of RAGE and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–61. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 13.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 14.Yu M, Wang H, Ding A, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–9. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 15.Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diez M, Koistinaho J, Dearmond SJ, Groth D, Prusiner SB, Hökfelt T. Marked decrease of neuropeptide Y Y2 receptor binding sites in the hippocampus in murine prion disease. Proc Natl Acad Sci USA. 1997;94:13267–72. doi: 10.1073/pnas.94.24.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullagh P. Regression models for ordinal data. J R Statist Soc B. 1980;42:109–42. [Google Scholar]
- 18.Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekström ES, Scheynius A. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002;107:145–51. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rindsjö E, Holmlund U, Sverremark-Ekström E, Papadogiannakis N, Scheynius A. Toll-like receptor-2 expression in normal and pathologic human placenta. Hum Pathol. 2007;38:468–73. doi: 10.1016/j.humpath.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, Melloni E, Presta M. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol. 2006;176:12–5. doi: 10.4049/jimmunol.176.1.12. [DOI] [PubMed] [Google Scholar]
- 21.Schlueter C, Weber H, Meyer B, Rogalla P, Röser K, Hauke S, Bullerdiek J. Angiogenetic signalling through hypoxia: HMGB1: an angiogenetic switch molecule. Am J Pathol. 2005;166:1259–63. doi: 10.1016/S0002-9440(10)62344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE–amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 23.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–9. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limana F, Germani A, Zacheo A, et al. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. 97:e73–83. doi: 10.1161/01.RES.0000186276.06104.04. Circ Res. [DOI] [PubMed] [Google Scholar]
- 25.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–85. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]