Abstract
Pulmonary vaccination is a promising immunization route. However, there still remains a crucial need to characterize the different parameters affecting the efficacy of inhaled vaccination. This study aimed at assessing the impact of antigen distribution within the respiratory tract on the immune response to a monovalent A/Panama/2007/99 H3N2 influenza split virus vaccine administered to BALB/c mice. Varying the administration technique allowed the targeting of the vaccine to different sites of the mouse respiratory tract, i.e. the nasal cavity, the upper or central airways, or the deep lung. This targeting was verified by using ovalbumin as a tracer compound. The immune responses generated following influenza vaccine administration to the different respiratory tract sites were compared to each other and to those elicited by intramuscular and peroral intragastric immunization. Delivery of the vaccine to the different respiratory regions generated systemic, local and cellular virus-specific immune responses, which increased with the depth of vaccine deposition, culminating in deep-lung vaccination. The latter induced virus-specific serum immunoglobulin G and neutralizing antibody titres as elevated as intramuscular vaccination, whereas the production of mucosal secretory immunoglobulin A was significantly superior in deep-lung-vaccinated animals. The analysis of cytokines secreted by mononuclear cells during an in vitro recall response indicated that deep-lung vaccination induced a local shift of the cellular immune response towards a T helper type 1 phenotype as compared to intramuscular vaccination. In conclusion, antigen distribution within the respiratory tract has a major effect on the immune response, with the deep lung as the best target for inhaled influenza vaccination.
Keywords: antigen delivery site, influenza virus, mucosal/cellular immunity, pulmonary vaccination
Introduction
Yearly outbreaks of influenza cause significant morbidity in the general population and mortality in high-risk patient groups.1 The World Health Organization indicates that 5–15% of the world population fall ill with influenza every year, resulting in a significant socio-economic burden.2 Vaccination can limit influenza virus infection as well as spread.
The vast majority of currently licensed influenza vaccines are unadjuvanted inactivated split virus preparations that are administered seasonally by injection principally to people at high-risk of influenza complications. Although these vaccines are known to induce serum immunoglobulin G (IgG) antibodies, they are poor stimulators of secretory IgA (S-IgA) at respiratory mucosal sites. This may be considered as a lack because S-IgA has been shown to increase protection through its role in viral clearance and its effective intracellular and extracellular neutralization of influenza virus at the point of viral entry.3 Existing injectable influenza split vaccines also show sporadic CD8+ cytotoxic T lymphocyte activation, particularly in the elderly.4,5 Therefore, there is a need for improvement, not only in vaccine efficacy but also in ease of administration and immunization coverage.
To overcome the limitations of existing parenteral vaccines, alternative vaccination strategies have been evaluated. One of these strategies is mucosal vaccination because the advantages of generating both a mucosal and a systemic immunity have been recognized.6 In addition, delivery to mucosa is needle-free and painless, and might therefore improve patient compliance. Mucosal vaccination is also more suitable for mass vaccination campaigns and thereby might help to increase the herd immunity.7,8 This is particularly critical in children because it has been shown that the risk for acquiring influenza infection in all age groups could be reduced substantially by community-wide routine vaccination in young children.9
Different types of influenza vaccines have been evaluated for delivery to the respiratory mucosa. Some studies made with non-replicative influenza virus vaccines showed them to be poorly immunogenic when administered intranasally (i.n.) and this prompted subsequent studies with adjuvanted vaccines.10–12 The choice of adjuvant remains, however, critical because safety concerns have been observed in some cases (e.g. heat-labile Escherichia coli toxin13). The development of cold-adapted live attenuated influenza vaccines for nasal administration has been more successful.14 An intranasal vaccine of live attenuated virus has been licensed (Flumist®) and its potential to induce a broad cross-reactive immunity represents a clear asset. Yet, its use remains limited because of its restricted target group of healthy people aged from 5 to 49 years, its frozen storage and its high cost.
Delivery of vaccines to the lungs is a potential alternative for mucosal immunization of the respiratory tract. The utility, effectiveness and safety of pulmonary vaccination have been especially well established for measles, with the pioneering studies of Sabin in the early 1980s and more recently with the mass vaccination campaigns in Mexico and South Africa.15–17 One laboratory has previously investigated the pulmonary administration of influenza vaccines.18,19 Their goal was to optimize an inhalation dry powder of the inactivated whole or split influenza virus using surfactant lipids as well as IgG for targeting phagocytes, or a detergent for facilitating antigen release. The immunity induced by pulmonary delivery of the dry powder could be higher than that generated by injection of the vaccine.
Pulmonary vaccination is a promising immunization route. However, there still remains a crucial need to characterize the different parameters affecting the efficacy of inhaled vaccination. A critical parameter might be the site of antigen deposition within the respiratory tract.20 Targeting within human lungs can be accurately controlled by aerosol particle size and the choice of the optimal aerosol particle size might be a potential question in future clinical trials.21,22 This was illustrated by Menzel et al., who showed that alveolar targeting of a pneumococcal polysaccharide vaccine in humans induced a trend towards increased serum antibody responses as compared to vaccine delivery to large airways.23
The aim of the present study was to assess the impact of the delivery site of a monovalent split influenza vaccine within the respiratory tract on the intensity and on the type of immune responses induced in mice. First, we verified that different techniques of administration were able to target different respiratory tract regions by using ovalbumin (OVA) as a marker of antigen distribution. Second, we administered the monovalent influenza vaccine to the different respiratory tract regions, identified the optimal delivery site and compared the immune responses with those induced by intramuscular (i.m.) or oral administration of a same dose of the vaccine.
Materials and methods
Mice
Female NMRI mice, aged between 6 and 8 weeks, from specific-pathogen-free colonies bred at the Université catholique de Louvain (Brussels, Belgium) were used for the lung deposition studies. Specific-pathogen-free BALB/c female mice (Elevage Janvier, Le Genest-St-Isle, France), 6–8 weeks old, were used for the immunization studies. Different mouse strains were used for the deposition and immunization study for practical reasons of accessibility to the mice and for reasons of cost. However, mice in the deposition study were controlled for equivalent body weight to those in the immunization study. All experimental protocols in mice were approved by the Institutional Animal Care and Use Committee of the Université catholique de Louvain.
Deposition study
Deposition of the instillate in the nasal passages, the trachea, and the central and peripheral parts of the lung lobes was measured using OVA as a distribution marker. NMRI mice were anaesthetized using ketamine/xylazine (90/10 mg/kg) intraperitoneal injection. Fifty micrograms OVA in phosphate-buffered saline were then instilled using various administration methods (Table 1). Intranasal and intratracheal (i.t.) instillations were performed using a micropipette (Finnpipette) and a 100-μl precision microsyringe (Hamilton, Bonaduz, Switzerland), respectively. The mice were killed by a lethal injection of pentobarbital immediately after OVA administration. A nasal wash was performed by cannulating the trachea towards the nasal cavity and rapidly instilling 3 ml Hanks' balanced salt solution (HBSS). The fluid emerging from the nostrils was collected and stored at −20°. The lungs were then removed and divided into trachea (with main bronchi) and pulmonary lobes. Each lobe was cut in two equal parts by mass: one central part cut round in shape around the bronchus hilum, and one peripheral part. The subdivision was made visually and allowed to obtain a relatively constant weight ratio of the central to the peripheral part. The trachea and central parts of the lung lobes were pooled and represent the central airways fraction. The peripheral parts of the lung lobes were also pooled and constitute the deep-lung fraction. The different tissue fractions were ground for release of the marker in 2 ml ultrapure water with a tissue grinder Potter (Merck Eurolab, Leuven, Belgium) for 2 min and the tissue grinder was rinsed with 1 ml ultrapure water. The tissue homogenates were then centrifuged at 8000 g and 4° for 15 min. The supernatants were stored at −20° until they were assayed for OVA content. When known OVA quantities were added to lung tissue or nasal wash, they were entirely recovered by enzyme-linked immunosorbent assay (ELISA), indicating that degradation or tissue binding was insignificant during sample preparation. The amount of OVA recovered in each fraction was expressed as a percentage of the administered OVA dose.
Table 1.
Administration techniques for targeting the different sites of the respiratory tract
| Targeted site of deposition | Delivery route | Vol. (μl) solution delivered | Angle of tilt of mouse | Air bolus |
|---|---|---|---|---|
| Nasal passages | i.n. | 10 | 90° | none |
| Upper airways | i.n. | 50 | 90° | none |
| Central airways | i.t. | 10 | 0° | none |
| Deep lung | i.t. | 25 | 45° | 1 |
Targeting was achieved by varying the delivery route, the volume of solution and the position of the mouse and by the possible insufflation of a 200 μl air bolus following administration.
Vaccines
Monovalent split influenza virus and monovalent whole inactivated influenza virus were provided by GlaxoSmithKline Biologicals (Rixensart, Belgium). They were prepared from the strain A/Panama/2007/99 (H3N2). Briefly, the virus was inactivated with organic solvents to produce whole inactivated virus. It was then disrupted with detergents and centrifuged, and the split virus was extracted. Vaccine doses were expressed in terms of haemagglutinin (HA) content. The HA was assayed by single radial immunodiffusion as described previously.24 All immunogens were prepared in phosphate-buffered saline.
Immunization
BALB/c mice were immunized as described in Table 2. On day 0, mice were lightly anaesthetized and i.n. primed by instillation of 20 μl (10 μl in each nostril) monovalent A/Panama/2007/99 H3N2 influenza whole virus sterile suspension containing 5 μg HA. This was performed to generate background immunity and thereby to more closely mimic the natural priming that occurs in humans. On day 28, mice were anaesthetized by intraperitoneal injection of ketamine/xylazine. They were then vaccinated with a 1·5-μg HA dose of monovalent split virus sterile suspension of the same influenza strain used for priming, and administered either i.m. (25 μl in both quadriceps), orally (intragastric injection) or to the different sites of the respiratory tract using the different techniques described in Table 1. Control mice only received the priming dose of whole virus. The groups were named according to the targeted site of deposition within the respiratory tract (Table 1). As the deposition in the peripheral lobe parts was significantly higher in the 25-μl i.t. group compared to the other groups, this group represented the ‘deeper’ lung deposition and was named ‘deep lung’ for clarity.
Table 2.
Immunization protocols
| Priming | Vaccination | ||||||
|---|---|---|---|---|---|---|---|
| Vaccination group | Day | Route | HA dose (µg) | Day | Route | Vol. (µl) | HA dose (µg) |
| Priming control | 0 | i.n. | 5 | – | – | – | – |
| Intramuscular | 0 | i.n. | 5 | 28 | i.m. | 50 | 1·5 |
| Nasal cavity | 0 | i.n. | 5 | 28 | i.n. | 10 | 1·5 |
| Upper airways | 0 | i.n. | 5 | 28 | i.n. | 50 | 1·5 |
| Central airways | 0 | i.n. | 5 | 28 | i.t. | 10 | 1·5 |
| Deep lung | 0 | i.n. | 5 | 28 | i.t. | 25 | 1·5 |
| Oral | 0 | i.n. | 5 | 28 | p.o. | 25 | 1·5 |
On day 0, mice were i.n. primed by instillation of 20 μl monovalent A/Panama/2007/99 H3N2 influenza whole virus sterile suspension containing 5 μg HA. On day 28, mice were vaccinated with a 1·5 μg HA dose of monovalent split virus sterile suspension of the same influenza strain used for priming, and administered either i.m., orally or to different sites of the respiratory tract using the different techniques described in Table 1 and validated in Fig. 1 by localization of ovalbumin. i.n., intranasal; i.t., intratracheal; p.o., per os.
Sample collection
Sera were collected from the retro-orbital plexus on days 0, 28 and 42 and stored at −20° until assayed. On day 42, mice were killed with an overdose of pentobarbital and nasal washes and bronchoalveolar lavages (BAL) were performed. A nasal wash was performed using 1 ml HBSS, as described above. The BALs were performed by inversing the cannula in the trachea towards the lungs, slowly injecting 1 ml HBSS and withdrawing the fluid. After centrifugation (1000 g at 4° for 10 min), the resultant cell-free supernatant was stored at −20°.
Virus neutralization assay
Sera were heat inactivated at 56° for 30 min and two-fold dilutions were prepared in a 50-μl volume. One hundred tissue infectious dose 50 of wild-type A/Panama/2007/99 virus was added in a volume of 50 μl and the mixture was incubated at room temperature for 90 min. After incubation, 100 μl MDCK cells (4·8 × 105 cells/ml) were added to each well. The cells were incubated at 35° with 5% CO2. After 7 days of incubation, cytopathic effect was visually scored in each well under the microscope. Controls for viral activity and cellular survival were included on each plate. The neutralization titre of a serum was expressed as the reciprocal of the highest dilution of serum that completely inhibited cytopathic effect. As the first dilution of sera was 1 : 20, an undetectable level was scored as a titre equal to 10.
Antibody ELISA assays
Virus-specific antibody ELISA
Virus-specific IgG, IgG1, IgG2a and IgA titres were measured by ELISA in sera, BALs and nasal washes. Plates (96-well, Nunc-Immunoplate Maxisorb Surface, Gibco BRL Life Technologies, Merelbeke, Belgium) were coated with influenza HA (split A/Panama/2007/99 virus suspension). After washing and blocking, serial dilutions of sera, BALs or nasal washes were added. Plates were washed again, then incubated with horseradish-peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) or IgA (Sigma, St Louis, MO). For IgG titre determination, plates were developed by incubation with o-phenylenediamine substrate and optical densities were read at 492/620 nm (SpectraMax 190, Molecular Devices Corp, Sunnyvale, CA). For IgA titre determination, plates were developed by incubation with tetramethylbenzidine substrate and optical densities were read at 450/620 nm (SpectraMax 190). The endpoint titres were defined as the reciprocal dilution corresponding to an optical density value of 0·6. To correct for variations in lavage efficiency, the nasal and bronchoalveolar anti-influenza virus immunoglobulin responses were expressed as a ratio of specific response (end-point titre) in the lavage to the total amount of immunoglobulin (in micrograms) measured in the same sample.
Total antibody ELISA
Total IgG and IgA titres in BALs or nasal washes were determined by ELISA as above, substituting influenza HA with goat anti-mouse IgG (Jackson ImmunoResearch) or goat anti-mouse IgA (Sigma). Serial dilutions of BALs or nasal washes, and standard curves constituted from mouse IgG (Chrompure, Jackson ImmunoResearch) or purified mouse myeloma protein IgA (MP Biomedicals, Aurora, OH) were added to the plates. The quantification limits of the IgG and IgA assays were 0·9 and 2·4 ng/ml, respectively.
Evaluation of proliferative responses and cytokine production in vitro
The spleens as well as the parathymic and posterior mediastinal lymph nodes (LN) were removed aseptically from mice after killing on day 42. Spleen and LN cells were centrifuged on Lympholyte M cell separation medium gradient (Cedarlane, Hornby, Canada) for the isolation of viable lymphocytes. Spleen and lung-draining LN mononuclear cells (MNCs) were resuspended in Dulbecco's modified Eagle's medium supplemented with 10% volume/volume heat-inactivated fetal bovine serum, 0·05 mmβ-mercaptoethanol, 0·55 mm l-arginine, 0·24 mm l-asparagine, 1·5 mm l-glutamine. Proliferation assays were set up in flat-bottom 96-well plates (Nunc) with 5 × 105 spleen MNCs and 1·6 × 105 LN MNCs in each well. The stimulants used were 3 μg/ml influenza HA (split A/Panama/2007/99 virus suspension) or 5 μg/ml concanavalin A. Negative control cultures received no stimulants. All cultures were incubated for 48 hr and then pulsed overnight with 0·5 μCi [3H]thymidine per well. Cell pellets were filtered onto microplate unifilters and the activity was counted using a TopCount scintillation counter (PerkinElmer, Zaventem, Belgium). The results were expressed as stimulation index, calculated using the following formula: stimulation index = (mean c.p.m. of antigen-stimulated cultures)/(mean c.p.m. of relevant negative control cultures), where c.p.m. is counts/min. Supernatants were harvested for interferon-γ (IFN-γ) and interleukin-4 (IL-4) assays (mouse IFN-γ and IL-4 DuoSet ELISA Developments kits, R & D Systems Europe LTD, Abingdon, UK).25 The detection limits were 3·1 pg/ml and 0·6 pg/ml for the IFN-γ and IL-4 assays, respectively.
Statistics
Values are expressed as means ± the standard error of the mean. In the deposition study, groups of mice comprised five or six animals and, in the immunization study, groups included seven to nine mice. Data were analysed by using the JMP version 4·0.2 and GraphPad Prism version 4·00 software programs. The normal distribution of the data was checked by applying the Shapiro–Wilk W-test (Goodness-of-fit test, JMP version 4·0.2) on the residual differences between the individual data and their respective partial means, allowing us to check normality on a larger data population. Log transformation was applied in case of log-normal distribution.
Statistical analysis was made by one-way analysis of variance with Tukey's multiple comparison post-test (Figs 1–5). Comparison of the antigen-specific proliferative responses of the i.m. and deep-lung groups with the priming control group was made using Student's t-test (Fig. 5a).
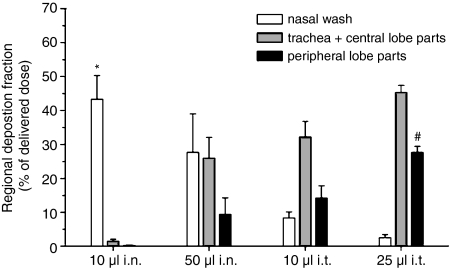
Figure 1.
Regional deposition in NMRI mice of instilled ovalbumin following delivery using the techniques described in Table 1. * Indicates higher regional deposition fraction versus the corresponding fraction of 10 µl i.t. (P < 0·05) and 25 µl i.t. (P < 0·01). # Indicates higher regional deposition fraction as compared to the corresponding fraction of 10 µl i.n. (P < 0·001), 50 µl i.n. (P < 0·01) and 10 µl i.t. (P < 0·05).
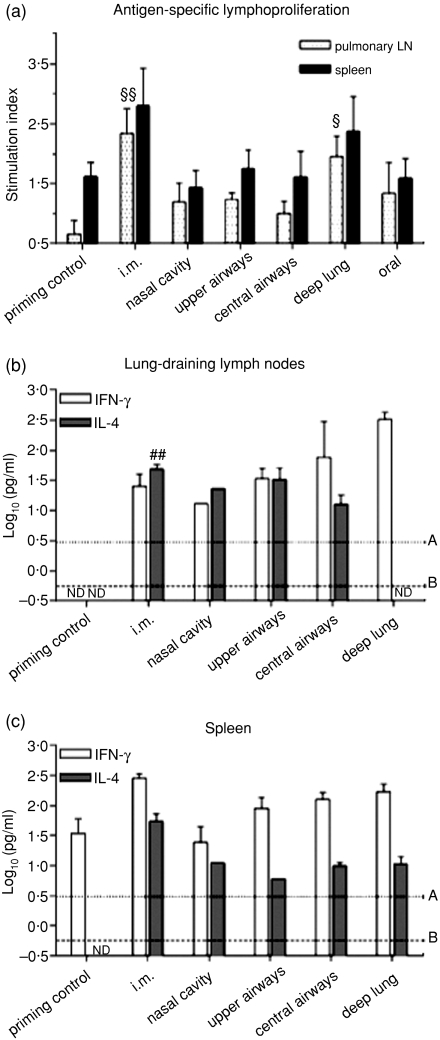
Figure 5.
Impact of the delivery site of a monovalent A/Panama/2007/99 H3N2 influenza split virus vaccine on cellular immune responses. BALB/c mice were immunized as detailed in Table 2. (a) Proliferation of spleen and pulmonary lymph node (LN) mononuclear cells (MNCs) following in vitro split A/Panama/2007/99 virus stimulation. The results are expressed as stimulation indexes. § Indicates significant differences from the priming control group. One symbol indicates P < 0·05, two symbols indicate P < 0·01. The supernatants from the cultures of (b) pulmonary LN MNCs and (c) spleen MNCs were assayed for IFN-γ and IL-4. ## Indicates highly sifgnificant difference from the pulmonary LN IL-4 production [Student's t-test versus LD(IL-4), P < 0·01]. ND: below limit of detection (LD); line A, LD for IFN-γ = 0·49; line B, LD for IL-4 = 0·25.
Results
Deposition study
To target the different regions of the respiratory tract in mice, we developed different administration techniques. Targeting was achieved by varying the administration route, the volume of solution, the position of the mouse and possibly by insufflating a 200-μl air bolus following administration (Table 1). Figure 1 shows the regional deposition of the poorly absorbed marker, OVA, in the respiratory tract of NMRI mice following its administration by the different techniques. Instillation of a small volume of solution in the nostrils (10 μl i.n.) resulted in deposition of the majority of the OVA dose in the nasal cavity. Increasing the volume administered i.n. (50 μl i.n.) yielded a lower deposition as well as a more homogeneous distribution of OVA throughout the respiratory tract with most of the dose deposited in the nasal cavity, the trachea and the central airways. The i.t. delivery of a small volume of solution to the mouse laid on its back (10 μl i.t.) pushed the OVA solution further down into the respiratory tract, with deposition of the largest fraction of the OVA dose in the trachea and central airways. The fraction of the OVA dose deposited in the deep lung was significantly raised by increasing the volume of solution to 25 μl and by insufflating a 200-μl air bolus following administration (25 μl i.t.). The total percentages of the OVA dose recovered from the respiratory tract following administration with the different techniques were equivalent (P > 0·05) and within a range of 45–75%.
Serum antibody responses
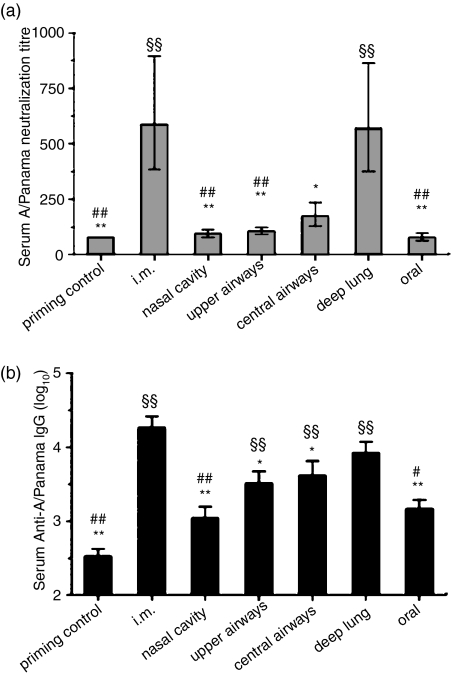
BALB/c mice were primed i.n. with the monovalent A/Panama/2007/99 H3N2 influenza whole virus to mimic the natural priming that occurs in humans. They were then immunized by delivering the monovalent split virus vaccine of the same influenza strain to the different regions of the respiratory tract using the techniques described above. Unvaccinated primed mice were used as controls. For comparison, the monovalent vaccine was also delivered by i.m. injection or orally (Table 2). Delivery of the monovalent split virus to the deep lung or to the muscle induced a marked and equivalent rise in serum virus-neutralization titres (Fig. 2a). In contrast, vaccine delivery to the nasal cavity, upper or central airways or oral inoculation did not induce a significant increase in neutralization titres as compared to the control group (which only received the priming). Serum anti-A/Panama/2007/99 IgG levels were significantly increased following i.m. vaccine administration or its delivery to the upper or central airways or to the deep lung (Fig. 2b). An upward trend in serum IgG levels was observed with the depth of deposition of the vaccine within the lungs. Intramuscular and deep-lung vaccination were statistically equivalent, while i.m. vaccination induced significantly superior serum anti-A/Panama/2007/99 IgG levels compared to vaccine inoculation to the upper or central airways.
Figure 2.
Impact of the delivery site of a monovalent A/Panama/2007/99 H3N2 influenza split virus vaccine on serum antibody responses. BALB/c mice were immunized as detailed in Table 2. (a) A/Panama/2007/99 virus neutralization titre. (b) Anti-A/Panama/2007/99 IgG titres. § Indicates significant difference from the priming control group. # Indicates significant difference from the deep-lung-immunized group. * Indicates significant difference from the i.m. immunized group. One symbol indicates P < 0·05, two symbols indicate P < 0·01.
Respiratory tract IgA and IgG responses
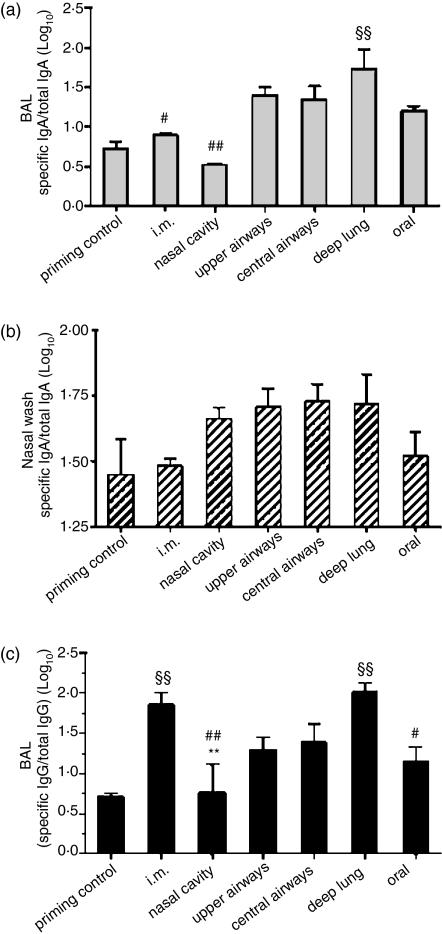
Similar to serum anti-A/Panama/2007/99 IgG levels, BAL anti-A/Panama/2007/99 IgA levels increased with the depth of deposition of the vaccine within the respiratory tract and reached a maximum following delivery of the influenza vaccine to the deep lung (Fig. 3a). However, except for deep-lung vaccination, BAL IgA titres in the other vaccination groups were not significantly different from those of the control group. There was a trend towards increased nasal-wash virus-specific IgA levels following delivery of the split influenza vaccine to the different sites of the respiratory tract (Fig. 3b). Intramuscular or oral administration of the vaccine did not produce nasal-wash IgA.
Figure 3.
Impact of the delivery site of a monovalent A/Panama/2007/99 H3N2 influenza split virus vaccine on the local respiratory antibody response. BALB/c mice were immunized as detailed in Table 2. (a) BAL and (b) nasal-wash anti-A/Panama/2007/99 IgA titres, (c) BAL anti-A/Panama/2007/99 IgG titres. § Indicates significant difference from the priming control group. # Indicates significant difference from the deep-lung-immunized group. * Indicates significant difference from the i.m. immunized group. One symbol indicates P < 0·05, two symbols indicate P < 0·01.
The BAL virus-specific IgG levels closely paralleled serum IgG levels (Figs 2b and 3c). An upward trend in BAL anti-A/Panama/2007/99 IgG levels was observed with the depth of deposition of the vaccine within the lungs. Intramuscularly and deep-lung-vaccinated mice presented highly significant and equivalent BAL anti-A/Panama/2007/99 IgG levels (Fig. 3c).
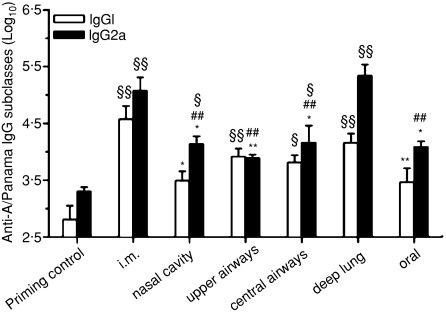
Serum IgG subclasses
Serum IgG1 and IgG2a subclasses were analysed as an indirect assessment of the polarization of T-helper cell populations. The highest IgG1 and IgG2a levels were observed following i.m. and deep-lung administration of the monovalent split vaccine (Fig. 4). Both IgG1 and IgG2a production tended to increase with the depth of deposition of the vaccine within the respiratory tract. When compared to i.m. immunization, deep-lung vaccination induced a trend towards lower IgG1 and slightly higher IgG2a levels, suggesting a slight leaning of the immune response towards T helper type 1 (Th1).
Figure 4.
Impact of the delivery site of a monovalent A/Panama/2007/99 H3N2 influenza split virus vaccine on specific serum immunoglobulin G (IgG) subclasses response. BALB/c mice were immunized as detailed in Table 2. § Indicates significant difference from the priming control group. # Indicates significant difference from the deep-lung-immunized group. * Indicates significant difference from the i.m. immunized group. One symbol indicates P < 0·05, two symbols indicate P < 0·01.
Cellular immune responses
Intramuscular and deep-lung vaccination against influenza significantly increased the antigen-specific proliferative responses in pulmonary lymph nodes (Fig. 5a). Moreover, the splenocytes derived from these animals showed a trend towards increased proliferation as compared to the control group or to the other groups. The i.m. and deep-lung group were the only groups to show stimulation index values superior to the threshold commonly used to express positive proliferation (i.e. >2·0). Inoculation of the monovalent split vaccine in the nasal cavity, upper or central airways, or in the stomach did not significantly affect proliferative responses. Yet, these groups showed a tendency towards increased uptake of thymidine into MNCs derived from the lung-draining lymph nodes. When exposed to the mitogenic stimulus, concanavalin A, a significant uptake of [3H]thymidine (P < 0·05, data not shown) was measured, confirming that the replicative ability of the cells had not been compromised during isolation.
A 100-fold reduction in IL-4 (P < 0·01) and a 10-fold increase in IFN-γ levels in antigen-stimulated pulmonary LN MNCs indicated that deep-lung vaccination, as compared to i.m. vaccination, induced a significant local shift towards a Th1 cellular response (Fig. 5b). However, at the central level, this shift was only noticeable by a non-significant five-fold reduction in IL-4 levels in spleen MNCs of deep-lung-vaccinated mice (Fig. 5c). The pulmonary LN cellular response showed a trend to increasingly shift towards a Th1 response with the depth of vaccine inoculation within the respiratory tract, whereas the central response was not so affected by antigen distribution within the respiratory tract (Fig. 5b,c).
Discussion
This work emphasizes the importance of targeting an inactivated influenza vaccine to the deep lung to induce the broadest and most intense immunity. The immune response to the monovalent A/Panama/2007/99 H3N2 influenza split virus vaccine increased and shifted towards a local Th1 phenotype with the depth of vaccine deposition within the respiratory tract in mice (Figs 2, 4 and 5). Deep-lung vaccination induced systemic immune responses as intense as those induced by i.m. vaccination (Fig. 2). However, in contrast to i.m. immunization, delivery of the monovalent split vaccine to the deep lung resulted in secretion of mucosal S-IgA (Fig. 3a,b) and in a local immunity that was more shifted towards Th1 (Figs 4, 5).
Delivery of the monovalent A/Panama/2007/99 H3N2 influenza split virus vaccine to the lung periphery was more effective in generating specific immunity than its delivery to the nasal cavity or to the upper or central airways (Figs 2–5). Targeting was attained by varying the technique of administration and was confirmed by quantifying OVA in the different regions of the mouse respiratory tract (Fig. 1). The impact of the site of deposition of vaccines within the respiratory tract had not been systematically investigated previously. However, our results are consistent with the limited previous studies. Alveolar delivery of a pneumococcal polysaccharide vaccine induced slightly superior antibody levels than its bronchial targeting in humans.23 Specific hepatitis B antibodies were produced following i.t. instillation (25 μl) of the hepatitis B surface antigen in mice, but no antibodies could be detected following i.n. instillation (10 μl)25. The i.n. application of a large volume (≥ 50 μl) in mice yielded significantly higher immune responses than the application of a small volume (20 μl) because large volumes flow into the trachea and lung while small ones remain within the nasal cavity.26
The increased efficacy of deep-lung vaccination might be explained by the ensuing long residence time of the split influenza virus within the lungs. In fact, the deeper the deposition of macromolecules and particles within the respiratory tract, the slower their clearance.27,28 Haemagglutinin and neuraminidase have respective molecular weights of 220 000 and 240 000 and large proteins are poorly absorbed systemically following inhalation.29 Exogenous substances are cleared within minutes from the nasal mucosa by mucociliary clearance (nasal mucociliary transit time is 12–15 min in humans30). The tracheobronchial region clears them with half-times of a few hours (4 hr in mice and 3 hr in humans31,32). In contrast, macromolecules and particles can remain in the alveoli for several days,28,29 although alveolar macrophages can endocytose a fraction of the amount delivered.33,34 A long residence time of antigens within the respiratory tract will permit their prolonged contact with local lymphoid cells and structures, such as dendritic cells and the bronchus- and nasal-associated lymphoid tissues.35,36 These lymphoid cells and tissues play an important role in recruiting and priming lymphocytes in response to inhaled immunization. The widespread use of mucoadhesive polymers as adjuvants in mucosal vaccination demonstrates the interest in increasing the duration of presence of an antigen at mucosal surfaces to optimize immune responses.37
The immune response induced by deep-lung vaccination was significantly superior to that induced by oral immunization (Figs 2, 4 and 5). This supports the possibility that the effectiveness of deep-lung vaccination is the result of local administration of the antigen and not of mere swallowing of antigen after clearance from the respiratory tract. Differences in S-IgA levels following deep-lung or oral vaccination were less significant (Fig. 3a,b), possibly illustrating the concept of ‘common mucosal immune system’ whereby immunocytes activated at one site disseminate immunity to remote mucosal tissues rather than to systemic sites.38
The analysis of IgG subclasses and cytokines secreted by MNCs in culture indicated that deep-lung vaccination induced a trend of the cellular immune response to shift towards a Th1 phenotype as compared to the mixed response induced by i.m. vaccination (Figs 4 and 5b,c). This Th1 shift was mostly marked in the local pulmonary compartment. This is unexpected because the lung microenvironment has been shown to intrinsically favour priming for the differentiation of Th2 CD4+ T cells.39 This especially holds true for BALB/c mice. However, in these previous studies, antigen delivery was carried out by i.n. instillation of large volumes.40–42 In our study, i.n. instillation of 50 μl resulted in antigen deposition in the upper airways (Fig. 1) and also caused a Th2 polarized immunity (Figs 4 and 5b,c). Th1 responses have been shown to be essential for efficient recovery from virus infection.40 In fact, IFN-γ production and complement-activating IgG2a participate in viral clearance.40,43 The local Th1 polarization of deep-lung immunization might therefore improve vaccine efficacy.
Different hypotheses could be presented to explain the local Th1-shifted immune response against influenza following delivery to the deep lung. Differentiation to Th1 or Th2 phenotypes can be significantly influenced by a variety of factors, including the microenvironment (e.g. cytokines) at the actual antigen-presenting site, the type of antigen-presenting cell (APC), the amount and nature of the antigen, the presence of adjuvants, the genetic background of the subject (including major histocompatibility complex), and the duration and strength of both T-cell receptor and costimulatory signals.44 In the present study, the only difference between the deep-lung-vaccinated group and the other groups was the site of antigen deposition. Therefore, preferential activation of specific Th1 subsets must be linked to microenvironmental factors. A key factor in this respect appears to be the type of APC subpopulation that will encounter the antigen and produce certain cytokines. Studies have identified considerable heterogeneity in surface marker expression and also major functional differences between APC subpopulations from the conducting airways and the alveoli.45 It has been suggested, for example, that alveolar macrophages are implicated in the induction of a Th1 microenvironment.42 It is therefore likely that the site at which the vaccine/antigens are introduced may affect the differentiation of T cells, as indicated by our findings.
Delivery of the monovalent split influenza vaccine to the deep lung generated virus-specific serum antibody responses and respiratory IgG levels as elevated as i.m. vaccination did (Fig. 2). Moreover, it also resulted in secretion of S-IgA in the airways, in contrast to i.m. vaccination which did not (Fig. 3a,b), in line with previous findings.46,47 Influenza is primarily an infection of the upper respiratory tract which can spread to the lower respiratory tract and cause viral pneumonia or enhance susceptibility to bacterial infections.43 The respective role of IgA versus IgG in controlling influenza infection has previously been highlighted in mice.48 Passive transfer of polymeric IgA fully protected mice from influenza virus infection in the nose and trachea while IgG did not at three-fold higher doses. Secretory IgA is five to 10 times more efficient than IgG in in vivo viral neutralization, it is actively transported across respiratory epithelia and it is the most stable antibody of the immune system.49,50 However, polymeric IgA was unable to protect alveoli from viral infection while IgG did so.48 This is because alveolar cells do not express the polymeric immunoglobulin receptor and polymeric IgA is therefore not transported into the alveolar lumen. In contrast, IgG passively diffuses from serum into the lungs. Serum IgG transudation into the lungs is also suggested by our study because BAL IgG levels paralleled serum levels (Figs 2c and 3b). Therefore, an effective vaccination strategy should produce mucosal S-IgA in the upper airways for immune exclusion and IgG systemically for avoiding virus spread. Our study shows that this could be attained by deep-lung vaccination.
In conclusion, our results identified antigen distribution within the respiratory tract as a major parameter affecting the efficacy of inhaled vaccination against influenza. We showed that deep-lung immunization with an inactivated influenza vaccine induced not only serum antibodies and viral neutralization comparable to i.m. immunization, but also S-IgA and a local Th1-biased response. This broad response illustrates the promising potential of pulmonary vaccination to further increase the efficacy of existing inactivated split virus injectable vaccines. Therefore, we suggest further investigations to confirm these murine data. Trials using trivalent vaccines containing other influenza strains could be conducted, and safety and efficacy trials in humans should be initiated.
Acknowledgments
This work was supported by a ‘FIRST EUROPE Objectif 3’ grant no. EPH3310300R0382/215297 subsidized by the European Social Fund and the Walloon Region of Belgium. Rita Vanbever is a Chercheur Qualifié of the Fonds National de la Recherche Scientifique (FNRS, Belgium). We thank GlaxoSmithKline Biologicals (Rixensart, Belgium) for kindly donating the monovalent influenza virus.
References
- 1.Glezen WP. Serious morbidity and mortality associated with influenza epidemics. Epidemiol Rev. 1982;4:25–44. doi: 10.1093/oxfordjournals.epirev.a036250. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Influenza Fact Sheet. Geneva: World Health Organization; 2003. no. 211. [Google Scholar]
- 3.Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59:1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 4.McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine. 2005;23(Suppl. 1):S10–S25. doi: 10.1016/j.vaccine.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157–60. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogra PL, Fishaut M, Gallagher MR. Viral vaccination via the mucosal routes. Rev Infect Dis. 1980;2:352–69. doi: 10.1093/clinids/2.3.352. [DOI] [PubMed] [Google Scholar]
- 7.Friede M, Aguado MT. Need for new vaccine formulations and potential of particulate antigen and DNA delivery systems. Adv Drug Deliv Rev. 2005;57:325–31. doi: 10.1016/j.addr.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Nat Med. 2003;9:99–103. doi: 10.1038/nm0103-99. [DOI] [PubMed] [Google Scholar]
- 9.Jordan R, Connock M, Albon E, et al. Universal vaccination of children against influenza: are there indirect benefits to the community? A systematic review of the evidence. Vaccine. 2006;24:1047–62. doi: 10.1016/j.vaccine.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Tamura S, Samegai Y, Kurata H, Nagamine T, Aizawa C, Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988;6:409–13. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- 11.Gluck U, Gebbers JO, Gluck R. Phase 1 evaluation of intranasal virosomal influenza vaccine with and without Escherichia coli heat-labile toxin in adult volunteers. J Virol. 1999;73:7780–6. doi: 10.1128/jvi.73.9.7780-7786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langley JM, Halperin SA, McNeil S, et al. Safety and immunogenicity of a Proteosome™-trivalent inactivated influenza vaccine, given nasally to healthy adults. Vaccine. 2006;24:1601–8. doi: 10.1016/j.vaccine.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 13.Fujihashi K, Koga T, Van Ginkel FW, Hagiwara Y, McGhee JR. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine. 2002;20:2431–8. doi: 10.1016/s0264-410x(02)00155-x. [DOI] [PubMed] [Google Scholar]
- 14.Johnson PR, Feldman S, Thompson JM, Mahoney JD, Wright PF. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J Infect Dis. 1986;154:121–7. doi: 10.1093/infdis/154.1.121. [DOI] [PubMed] [Google Scholar]
- 15.Sabin AB, Flores AA, Fernandez de CJ, et al. Successful immunization of children with and without maternal antibody by aerosolized measles vaccine. I. Different results with undiluted human diploid cell and chick embryo fibroblast vaccines. JAMA. 1983;249:2651–62. [PubMed] [Google Scholar]
- 16.Dilraj A, Cutts FT, de Castro JF, et al. Response to different measles vaccine strains given by aerosol and subcutaneous routes to schoolchildren: a randomised trial. Lancet. 2000;355:798–803. doi: 10.1016/s0140-6736(99)95140-1. [DOI] [PubMed] [Google Scholar]
- 17.Bellanti JA, Zeligs BJ, Mendez-Inocencio J, et al. Immunologic studies of specific mucosal and systemic immune responses in Mexican school children after booster aerosol or subcutaneous immunization with measles vaccine. Vaccine. 2004;22:1214–20. doi: 10.1016/j.vaccine.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Smith DJ, Bot S, Dellamary L, Bot A. Evaluation of novel aerosol formulations designed for mucosal vaccination against influenza virus. Vaccine. 2003;21:2805–12. doi: 10.1016/s0264-410x(03)00224-x. [DOI] [PubMed] [Google Scholar]
- 19.Bot AI, Smith DJ, Bot S, et al. Receptor-mediated targeting of spray-dried lipid particles coformulated with immunoglobulin and loaded with a prototype vaccine. Pharm Res. 2001;18:971–9. doi: 10.1023/a:1010988311640. [DOI] [PubMed] [Google Scholar]
- 20.Hensel A, Lubitz W. Vaccination by Aerosols: Modulation of Clearance Mechanisms in the Lung. Marburg/Lahn: Behring Institute Mitt; 1997. pp. 212–19. [PubMed] [Google Scholar]
- 21.Heyder J, Gebhart J, Rudolf G, Schiller CF, Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0·005–15 μm. J Aerosol Sci. 1986;17:811–25. [Google Scholar]
- 22.Schulz H. Mechanisms and factors affecting intrapulmonary particle deposition: implications for efficient inhalation therapies Holger Schulz. Pharmaceut Sci Technol Today. 1998;1:326–44. [Google Scholar]
- 23.Menzel M, Muellinger B, Weber N, Haeussinger K, Ziegler-Heitbrock L. Inhalative vaccination with pneumococcal polysaccharide in healthy volunteers. Vaccine. 2005;23:5113–19. doi: 10.1016/j.vaccine.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Williams MS, Mayner RE, Daniel NJ, et al. New developments in the measurement of the hemagglutinin content of influenza virus vaccines by single-radial-immunodiffusion. J Biol Stand. 1980;8:289–96. doi: 10.1016/s0092-1157(80)80006-0. [DOI] [PubMed] [Google Scholar]
- 25.Lombry C, Marteleur A, Arras M, et al. Local and systemic immune responses to intratracheal instillation of antigen and DNA vaccines in mice. Pharm Res. 2004;21:127–35. doi: 10.1023/b:pham.0000012160.00222.55. [DOI] [PubMed] [Google Scholar]
- 26.McCluskie MJ, Weeratna RD, Davis HL. Intranasal immunization of mice with CpG DNA induces strong systemic and mucosal responses that are influenced by other mucosal adjuvants and antigen distribution. Mol Med. 2000;6:867–77. [PMC free article] [PubMed] [Google Scholar]
- 27.Foster WM, Walters DM, Longphre M, Macri K, Miller LM. Methodology for the measurement of mucociliary function in the mouse by scintigraphy. J Appl Physiol. 2001;90:1111–17. doi: 10.1152/jappl.2001.90.3.1111. [DOI] [PubMed] [Google Scholar]
- 28.Moller W, Barth W, Kohlhaufl M, Haussinger K, Stahlhofen W, Heyder J. Human alveolar long-term clearance of ferromagnetic iron oxide microparticles in healthy and diseased subjects. Exp Lung Res. 2001;27:547–68. doi: 10.1080/019021401753181827. [DOI] [PubMed] [Google Scholar]
- 29.Hastings RH, Grady M, Sakuma T, Matthay MA. Clearance of different-sized proteins from the alveolar space in humans and rabbits. J Appl Physiol. 1992;73:1310–16. doi: 10.1152/jappl.1992.73.4.1310. [DOI] [PubMed] [Google Scholar]
- 30.Merkus FW, Verhoef JC, Schipper NG, Marttin E. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev. 1998;29:13–38. doi: 10.1016/s0169-409x(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 31.Snipes MB, Boecker BB, McClellan RO. Retention of monodisperse or polydisperse aluminosilicate particles inhaled by dogs, rats, and mice. Toxicol Appl Pharmacol. 1983;69:345–62. doi: 10.1016/0041-008x(83)90258-2. [DOI] [PubMed] [Google Scholar]
- 32.Moller W, Haussinger K, Winkler-Heil R, et al. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J Appl Physiol. 2004;97:2200–6. doi: 10.1152/japplphysiol.00970.2003. [DOI] [PubMed] [Google Scholar]
- 33.Lombry C, Edwards DA, Preat V, Vanbever R. Alveolar macrophages are a primary barrier to pulmonary absorption of macromolecules. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1002–L1008. doi: 10.1152/ajplung.00260.2003. [DOI] [PubMed] [Google Scholar]
- 34.Takenaka S, Karg E, Roth C, et al. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Perspect. 2001;109(Suppl. 4):547–51. doi: 10.1289/ehp.01109s4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–34. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 36.Woodland DL, Randall TD. Anatomical features of anti-viral immunity in the respiratory tract. Semin Immunol. 2004;16:163–70. doi: 10.1016/j.smim.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alpar HO, Somavarapu S, Atuah KN, Bramwell VW. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv Drug Deliv Rev. 2005;57:411–30. doi: 10.1016/j.addr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 39.Constant SL, Lee KS, Bottomly K. Site of antigen delivery can influence T-cell priming: pulmonary environment promotes preferential Th2-type differentiation. Eur J Immunol. 2000;30:840–7. doi: 10.1002/1521-4141(200003)30:3<840::AID-IMMU840>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 40.Moran TM, Park H, Fernandez-Sesma A, Schulman JL. Th2 responses to inactivated influenza virus can be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis. 1999;180:579–85. doi: 10.1086/314952. [DOI] [PubMed] [Google Scholar]
- 41.Constant SL, Brogdon JL, Piggott DA, et al. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest. 2002;110:1441–8. doi: 10.1172/JCI16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones HP, Hodge LM, Fujihashi K, Kiyono H, McGhee JR, imecka JW. The pulmonary environment promotes Th2 cell responses after nasal-pulmonary immunization with antigen alone, but Th1 responses are induced during instances of intense immune stimulation. J Immunol. 2001;167:4518–26. doi: 10.4049/jimmunol.167.8.4518. [DOI] [PubMed] [Google Scholar]
- 43.Schmid DS, Rouse BT. Respiratory viral vaccines. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. 3. London: Elsevier; 2005. pp. 923–36. [Google Scholar]
- 44.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 45.von Garnier C, Filgueira L, Wikstrom M, et al. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol. 2005;175:1609–18. doi: 10.4049/jimmunol.175.3.1609. [DOI] [PubMed] [Google Scholar]
- 46.Moldoveanu Z, Clements ML, Prince SJ, Murphy BR, Mestecky J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995;13:1006–12. doi: 10.1016/0264-410x(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 47.Muszkat M, Yehuda AB, Schein MH, et al. Local and systemic immune response in community-dwelling elderly after intranasal or intramuscular immunization with inactivated influenza vaccine. J Med Virol. 2000;61:100–6. [PubMed] [Google Scholar]
- 48.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–86. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 49.Mazanec MB, Lamm ME, Lyn D, Portner A, Nedrud JG. Comparison of IgA versus IgG monoclonal antibodies for passive immunization of the murine respiratory tract. Virus Res. 1992;23:1–12. doi: 10.1016/0168-1702(92)90063-f. [DOI] [PubMed] [Google Scholar]
- 50.Brandtzaeg P. Role of secretory antibodies in the defence against infections. Int J Med Microbiol. 2003;293:3–15. doi: 10.1078/1438-4221-00241. [DOI] [PubMed] [Google Scholar]