Abstract
Context: Control of aromatase expression in uterine leiomyoma has significant clinical implications because aromatase inhibitors reduce tumor growth and associated irregular uterine bleeding. The mechanisms that regulate aromatase expression in leiomyoma are unknown.
Objectives: We previously demonstrated that the cAMP-responsive proximal promoters I.3 and II regulate aromatase expression in vivo in uterine leiomyoma tissue. Here, we investigated the cellular and molecular mechanisms responsible for promoter I.3/II usage.
Results: In smooth muscle cells isolated from leiomyoma (LSMCs), dibutyryl cAMP significantly induced aromatase mRNA and enzyme activity. Reporter constructs of promoter I.3/II deletion and site-directed mutants with selective disruption of cis-regulatory elements in the −517/−16 bp region revealed that five out of seven elements, including three CCAAT/enhancer binding protein (C/EBP) binding sites and two cAMP response elements, were essential for cAMP-induced promoter activity. EMSAs demonstrated that nuclear extracts from LSMCs contain complexes assembled on four of the five cis-elements, with C/EBP binding sites, including a novel −245/−231 bp sequence, clearly associating with C/EBPβ. Chromatin immunoprecipitation assays revealed that C/EBPβ binds specifically to the promoter I.3/II region in intact cells. Dibutyryl cAMP significantly induced nuclear C/EBPβ protein levels in LSMCs in a time-dependent manner. Conversely, knockdown of C/EBPβ dramatically suppressed cAMP-induced aromatase mRNA and enzyme activity.
Conclusions: C/EBPβ, which binds to multiple cis-regulatory elements in promoter I.3/II, is a key factor in the transcriptional complex controlling aromatase expression in uterine leiomyoma cells. Definition of this mechanism further may assist in designing inhibitors of aromatase specific for leiomyoma tissue.
In uterine leiomyoma cells, CCAAT/enhancer binding protein (C/EBP) binds to three specific cis-regulatory elements in promoter I.3/II of the aromatase gene. These findings may assist in designing future aromatase inhibitors specific for leiomyoma tissue.
Recent clinical data indicate that aromatase plays a significant role in the pathophysiology of uterine leiomyomata because therapeutic targeting of aromatase effectively reduces the size of these tumors and associated symptoms such as uterine bleeding in premenopausal and perimenopausal women (1,2,3,4). Uterine leiomyomata, benign smooth muscle tumors originating from the uterus, are the most common solid tumors in reproductive age women and the most frequently reported indication for surgery in women. Leiomyoma generally causes abnormal uterine bleeding, menorrhagia, pressure-related symptoms, and recurrent pregnancy loss. Abnormal uterine bleeding represents the major complaint in women and is the primary indication for surgical intervention (5). Each leiomyoma represents a monoclonal proliferation of a transformed myocyte derived from myometrium (6). Although the pathogenesis of leiomyoma transformation from myometrium remains unknown, laboratory and clinical evidence support that sex steroids contribute to leiomyoma growth and development. Leiomyoma develops only after puberty with the commencement of menstrual cycles and regresses after menopause. Induction of hypoestrogenism by oophorectomy or treatment with GnRH analogs also results in a reduction in uterine leiomyomata size but does not achieve complete regression. Although the ovary is thought to be the major source of sex steroids that support leiomyoma growth, we and others reported the possible contribution of estrogen synthesized locally to leiomyoma growth within leiomyoma cells. Leiomyomata per se express aromatase at significantly higher levels than the surrounding myometrium and are able to synthesize estrogen (7,8).
Aromatase, the key enzyme for estrogen synthesis, is encoded by the CYP19A1 gene and expressed in a number of human tissues, including uterine leiomyomata (9,10). We previously described local estrogen biosynthesis via aromatase expression in tissue samples and cultured smooth muscle cells from uterine leiomyomata, but not in normal myometrium or cells from disease-free women (7). Tissue concentrations of estrogen were elevated in leiomyoma nodules compared with those in surrounding myometrium (11). Moreover, it was shown in vitro that estrogen synthesized in cultured leiomyoma smooth muscle cells (LSMCs) was sufficient to promote cell proliferation in an intracrine fashion: stimulation of aromatase activity increased cellular proliferation that was inhibited by an aromatase inhibitor (8). Thus, aberrant aromatase expression in leiomyoma may in part be responsible for the persistence and growth of this tissue.
Aromatase gene expression is regulated by the tissue-specific activation of a number of promoters via alternative splicing (9). Each promoter is regulated by a distinct set of hormones and transcription factors. For example, in vitro studies showed that prostaglandin E2 (PGE2) or cAMP analogs stimulate aromatase expression via the proximally promoters I.3/II, whereas treatment with a glucocorticoid plus IL-6 or IL-1β switches promoter use to I.4 (12,13). We and others previously reported that aromatase activity in LSMCs was stimulated by a cAMP analog, PGE2, or a combination of glucocorticoid and IL-1β. Dibutyryl cAMP (Bt2cAMP), a cAMP analog, has also stimulated aromatase expression in LSMCs (7,14). We also demonstrated that aromatase expression in leiomyoma tissue in vivo is primarily regulated by the promoter I.3/II region rather than I.4 (7,15). However, the mechanism of this preferential promoter usage remains unknown.
We initiated the current study in an unbiased fashion to identify the cis-regulatory elements that modulate aromatase promoter activity and responsiveness to various hormones in cultured LSMCs. Here, we report that CCAAT/enhancer binding protein (C/EBP) binding sites and cAMP response elements (CREs) are important for aromatase expression and activity in leiomyoma. Our findings are significant because the pattern of cis-regulatory element use for aromatase expression in uterine leiomyoma seems to be distinct from those observed in another pathological müllerian-derived tissue, such as endometriosis (16). Because local aromatase expression and subsequent estrogen synthesis in uterine leiomyoma are critical for supporting leiomyoma growth, our findings represent a key molecular and clinical advance in our knowledge of the pathogenesis of this common tumor type. This may also lead to the development of strategies for tissue-specific aromatase inhibition.
Materials and Methods
Cell culture
Human uterine leiomyoma and surrounding normal myometrium were obtained at surgery from women (n = 68) undergoing hysterectomy or myomectomy following a protocol approved by the Institutional Review Board for Human Research of Northwestern University, Chicago, IL. Primary human LSMCs (passage 0–3) were prepared as previously described (17). PGE2, cAMP analog (e.g. Bt2cAMP), protein kinase C inducer [e.g. phorbol diacetate (PDA)], and dexamethasone (DEX) were purchased from Sigma-Aldrich (St. Louis, MO).
RNA extraction and quantitative analysis using real-time RT-PCR
RNA extraction and real-time RT-PCR were performed as described previously (18). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts were measured as an internal control. Primer pairs for each PCR reaction are listed in Table 1.
Table 1.
Oligonucleotide sequences used for PCR
| Target gene | Objective | Primer pairs’ name: sequence (5′–3′) | Amplicon size (bp) |
|---|---|---|---|
| Aromatase | Real-time PCR | Exon II F: CAC ATC CTC AAT ACC AGG TCC | 143 |
| Exon III R: CAG AGA TCC AGA CTC GCA TG | |||
| C/EBPβ | Real-time PCR | C/EBPβ 1588F: CGT GTG TAC ACG GGA CTG AC | 86 |
| C/EBPβ 1674R: CAA CAA GCC CGT AGG AAC AT | |||
| GAPDH | Real-time PCR | GAPDH F: GAA GGT GAA GGT CGG AGT C | 224 |
| GAPDH R: GAA GAT GGT GAT GGG ATT TC | |||
| Aromatase promoter I.3/II | ChIP-PCR | PII −383 F: TTG GTC AAAAAG GGG AGT TG | 126 |
| PII −257 R: AGC TCA TTC CAG AGG TGG AG | |||
| Aromatase promoter I.3/II | ChIP-PCR | PII −275 F: TCC ACC TCT GGA ATG AGC TT | 98 |
| PII −177 R: ATC ATC TTG CCC TTG AGT GG | |||
| Aromatase promoter | Multiplex RT-PCR | PII F: TCC CTT TGA TTT CCA CAG GAC TC | 303 |
| I.1 F: CTG TGC TCG GGA TCT TCC | 312 | ||
| I.2 F: GGC TTC CTG ACT TTC AAC AG | 321 | ||
| I.3 F: CCT TCT TTT GAC TTG TAA CCA | 330 | ||
| I.4 F: CCT TGT TTT GAC TTG TAA CCA | 339 | ||
| I.6 F: TAAATT GAT TGT CTT GCA CAG G | 347 | ||
| I.7 F: GAA GTA AGA CCG GAG AAA GGG | 365 | ||
| I.8 F: TCA TAT TGG GAG GAG CTT GG | 373 | ||
| Aromatase promoter | Multiplex RT-PCR | Exon III R: [5FAM] CTC CAT ACA CCC GGT TGT AG |
F, Forward; R, reverse.
Multiplex RT-PCR for promoter usage in the CYP19 gene
For the study of CYP19A1 gene promoter usage, eight different amplicons from 303–373 bp were designed (Table 1). The reverse promoter was located at common exon III and labeled with 6-carboxyfluorescein [FAM] to quantify promoter-specific mRNA species. After PCR amplification, products were separated on an ABI3100 capillary sequencer and quantified by GeneScan software (Applied Biosystems, Foster City, CA).
Aromatase assay
Aromatase enzyme activity was measured by radiolabeled [3H]water release assay as described previously (19). Protein concentration was determined using the BCA protein assay kit (Pierce, Rockford, IL). Results were expressed as picomoles of converted androstenedione per mg of protein per 6 h.
Plasmid construction and site-directed mutagenesis
Luciferase plasmids containing serial deletions (−694, −517, −278, −214, −140, and −100 bp) of the 5′-flanking region of human aromatase promoter I.3/II in the pGL3-basic vector were described previously (20). To generate constructs bearing mutated cis-regulatory sequences, site-directed mutagenesis was performed using the QuickChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA). Each mutant position and oligonucleotide is listed in Table 2. The presence of the expected mutation was confirmed by DNA sequencing.
Table 2.
Oligonucleotide sequences used for site-directed mutagenesis
| Potential cis-acting element (site of promoter I.3/II) | Mutated sequencea | Primer sequences for site-directed mutagenesis (5′–3′) |
|---|---|---|
| C/EBP binding site (−350/−337 bp) | TTGTTTTGAAATT →TTGTTTTGcccTT | GTT GGG AGA TTG CCT TTT TGT TTT GCC CTT GAT TTG GCT TCA AGG GAA GAA GA |
| C/EBP binding site (−317/−304 bp) | AGATTGCCTAAACA →AGcccGCCTAAACA | GAT TTG GCT TCA AGG GAA GAA GCC CGC CTA AAC AAA ACC TGC TGA TG |
| C/EBP binding site (−245/−231 bp) | TAATTTGGCAACAA →TAcggTGGCAAGAA | GAC TCC ACC TCT GGA ATG AGC TTT ATT TTC TTA TAC GGT GGC AAG AAA TTT GGC TT |
| CRE (−292/−285 bp) | TGAAGTCA →TGccccCA | TGC CTA AAC AAA ACC TGC TGA TGC CCC CAC AAA ATG ACT CCA CCT CTG GA |
| CRE (−211/−197 bp) | TGCACGTCACTCT →TGgAatTCACTCT | TTT GGC TTT CAA TTG GGA ATG GAA TTC ACT CTA CCC ACT CAA GGG C |
| NRHS (−263/−251 bp) | ATGAGCTTTATTT →ATGgGaaTTATTT | GCT ACA AAA TGA CTC CAC CTC TGG AAT GGG AAT TAT TTT CTT ATA ATT TGG CAA GAA ATT T |
| NRHS (−136/−124 bp) | AGGTCAGAAA→cccTCAGAAA | TAA AGG AAC CTG AGA CTC TAC CAC CCT CAG AAA TGC TGC AAT TCA AG |
Underlined nucleotides were mutated to lowercase nucleotides.
Transient transfections and luciferase reporter gene assay
Transfection was performed using FuGENE HD (Roche Applied Science, Indianapolis, IN) as described previously (21). After transfection for 48 h, cells were starved for 6 h in serum-free media, and then switched to treatment conditions for another 24 h. The reporter gene assay was performed using the Dual-Luciferase reporter assay system (Promega, Madison, WI). Results are expressed as the ratio of firefly luciferase to the internal standard renilla luciferase. Experiments were repeated from six different subjects with reproducible results.
EMSA
Nuclear proteins were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce) (21). Double-stranded oligonucleotide probes were obtained by annealing sense and antisense sequences listed in Table 3. Probes were end labeled with [γ-32P]ATP using T4 kinase (Invitrogen, Carlsbad, CA). EMSA was performed as described previously (22). Antibodies against C/EBPα (sc-61x), C/EBPβ (sc-150x), C/EBPδ (sc-151x), cAMP response element binding protein (CREB) 1 (sc-186x), activating transcription factor (ATF) 2 (sc-187x), or cAMP response element binding protein-binding protein (CBP) (sc-583x) were used for supershift assay. Specific antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and nonimmune IgG (Upstate Biotechnology, Inc., Lake Placid, NY) was used as a negative control.
Table 3.
Oligonucleotide sequences used for EMSA
| Potential cis-acting element (site of promoter I.3/II) | Sequences for EMSAa (5′–3′) |
|---|---|
| C/EBP binding site (−350/−337 bp) | GCC TTT TTG TTT TGAATT GAT TTG GC |
| C/EBP binding site (−317/−304 bp) | GGG AAG AAG ATT GCC TAA ACA AAAC CT |
| C/EBP binding site (−245/−231 bp) | ATT TTC TTA TAA TTT GGC AAG AAA TTT GGC T |
| CRE (−292/−285 bp) | CCT GCT GAT GAA GTC ACAAAA TGA C |
| CRE (−211/−197 bp) | ATT GGG AAT GCA CGT CAC TCT ACC CAC TC |
Underline represents potential cis-regulatory elements.
Chromatin immunoprecipitation (ChIP)-PCR
The in situ binding of specific transcription factors to the promoter I.3/II region was analyzed using ChIP-PCR as described previously (21). After treatment with Bt2cAMP, ChIP assays were performed using the ChIP assay kit (Upstate Biotechnology). The same antibodies were used for EMSAs and ChIP assays. PCR was performed using primers listed in Table 1.
Immunoblotting
Nuclear and cytoplasmic proteins from cultured LSMCs were prepared as described previously. Immunoblotting was performed as described previously (21). Antibodies against C/EBPβ (C-19; 1:5000, sc-150x; Santa Cruz Biotechnology), C/EBPβ-liver-enriched activating protein (LAP) (1:500, no. 3087; Cell Signaling Technology, Danvers, MA), and phospho-C/EBPβ (Thr235; 1:500, no. 3084; Cell Signaling Technology) were used for the detection of proteins. The signal was detected by Supersignal West Femto Maximum Sensitivity Substrate (Pierce).
Small interfering RNA (siRNA)
To knock down the expression of C/EBPβ, LSMCs were transfected with C/EBPβ siRNA (Dharmacon, Chicago, IL) using Lipofectamine RNAiMAX (Invitrogen). Nontargeting control siRNA (Dharmacon) and transfection reagents only (mock transfection) were transfected as negative controls. The siRNA was diluted to 50 nm in Opti-MEM I reduced-serum medium (Invitrogen). After transfection for 36 h, cells were serum starved for 12 h and treated with or without Bt2cAMP for 48 h. To confirm the effect of C/EBPβ knockdown on aromatase expression, both mRNA expression levels and enzyme activity were determined.
Statistical analysis
Statistical analysis for comparison between different treatments or over time was performed by ANOVA, followed by the Tukey multiple comparisons procedure. Differences in the presence or absence of treatment were evaluated using the Wilcoxon signed rank test. A P value less than 0.05 was considered significant. All values are given as the mean ± sem.
Results
The proximal promoter I.3/II region directs Bt2cAMP-stimulated aromatase expression in LSMCs
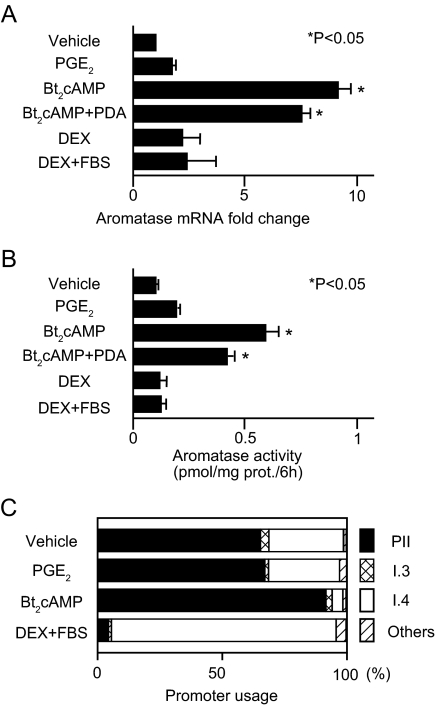
First, we determined the effect of various well-known inducers of aromatase on LSMCs (Fig. 1, A and B). Bt2cAMP was the most potent inducer of both aromatase mRNA expression and enzyme activity, and addition of PDA had little effect. PGE2 and DEX also induced aromatase activity, but change from baseline was not significant. Aromatase response to the same inducers was entirely different in primary cultured myometrial smooth muscle cells (MSMCs) (the aromatase response can be seen in supplemental Fig. 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Although Bt2cAMP significantly induced aromatase mRNA in MSMCs, mRNA levels and enzyme activity were lower than those stimulated by DEX or DEX plus fetal bovine serum (FBS).
Figure 1.
Aromatase mRNA, enzyme activity, and promoter usage in LSMCs treated with various aromatase inducers. Primary cultured LSMCs were starved in serum-free DMEM/F12 (1:1) medium for 12 h and treated with PGE2 (100 nm), Bt2cAMP (500 μm), Bt2cAMP plus PDA (100 nm), DEX (250 nm), or DEX plus 10% FBS for 24 h. A, Aromatase mRNA expression levels were measured by real-time RT-PCR and were normalized to GAPDH mRNA. Levels are reported as fold change compared with cells containing vehicle only (vehicle) and represent the average result from LSMCs from eight subjects. B, Data shown are representative results from aromatase enzyme activity assays of LSMCs from one subject, performed in triplicate. The results from six subjects were reproducible, but the range of activity was different for each subject. All values are reported as the mean results of assays performed in triplicate, with the bars indicating the sem. *, P < 0.05 (ANOVA). C, CYP19 gene promoter usage with or without treatment in LSMCs was determined using multiplex RT-PCR. The relative usage of each promoter is reported as an average from six different subjects.
Next, we characterized CYP19A1 gene promoter usage in LSMCs using multiplex RT-PCR. As expected, Bt2cAMP stimulated aromatase mRNA expression levels primarily via promoter I.3/II, whereas DEX plus FBS also activated promoter I.4 (Fig. 1C). Because Bt2cAMP is the most potent inducer of aromatase in LSMCs, we hypothesized that cAMP-dependent activation of the proximal promoter I.3/II region is the primary pathway leading to stimulation of aromatase expression in LSMCs.
Multiple and novel cis-regulatory elements within promoter I.3/II are essential for cAMP-dependent aromatase expression in LSMCs
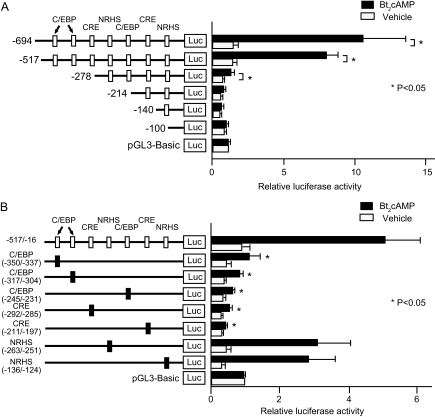
We next identified the elements within the aromatase promoter I.3/II region that mediate cAMP responsiveness in LSMCs. We transiently transfected reporter constructs containing deletion mutants of the promoter I.3/II region between −694 and −16 bp fused to a luciferase reporter gene into LSMCs (Fig. 2A). Treatment with Bt2cAMP induced luciferase activity of the −694/−16 and −517/−16 bp constructs over 5-fold. Although the −278/−16 bp construct mediated a 2-fold induction of luciferase activity by Bt2cAMP, this treatment had no effect on activity of the −214, −140, or −100 to −16 bp constructs. Thus, the −517/−214 bp region contains critical elements required for cAMP induction of aromatase promoter I.3/II activity in LSMCs.
Figure 2.
Identification of regulatory regions responsible for cAMP-dependent aromatase promoter I.3/II activity in LSMCs. A, Reporter plasmids containing the 5′-flanking region of human aromatase promoter I.3/II with serial deletions (−694, −517, −278, −214, −140, and −100 to −16 bp) were transfected into LSMCs. Relative positions of the cis-regulatory elements are indicated as white boxes. Statistical analysis was performed for each construct comparing promoter activity with or without Bt2cAMP treatment. *, P < 0.05 (Wilcoxon signed rank test). B, Site-directed mutagenesis for each cis-regulatory element was performed as described in Materials and Methods, and each reporter plasmid was transfected into LSMCs. White boxes represent positions of each cis-regulatory element within the promoter I.3/II region, and black boxes represent the cis-regulatory sequence selectively disrupted by site-directed mutagenesis in each construct. Numbers below the name of each cis-regulatory element indicate the distance from the transcription start site in the promoter I.3/II region. Statistical analysis was performed comparing Bt2cAMP induction of each mutant construct with the −517/−16 bp construct. *, P < 0.05 (ANOVA). Luciferase (Luc) activity was normalized to cotransfected renilla luciferase activity and is reported as the average of data from triplicate experiments + sem. The empty luciferase vector, pGL3-Basic, was arbitrarily assigned a unit of one. Results are reported as an average from LSMCs from six subjects.
We and others previously reported that five distinct cis-regulatory elements in the promoter I.3/II bind transcription factors: two C/EBP binding sites, a CRE, and two nuclear receptor half-sites (NRHSs) (20,23,24). We now identified two novel cis-regulatory elements in the promoter I.3/II: a C/EBP binding site located at −245/−231 bp and a CRE located at −292/−285 bp. Based on these findings, we prepared a series of site-directed mutant constructs of the −517/−16 promoter region fused to a luciferase reporter gene, each of which contains mutations in one of the seven identified cis-regulatory elements, and transfected them into LSMCs. Mutation of C/EBP binding sites or CREs significantly reduced the responsiveness of the −517/−16 region of promoter I.3/II to Bt2cAMP in LSMCs. In contrast, mutating of either of the two NRHS motifs had little effect on Bt2cAMP responsiveness (Fig. 2B). Although Bt2cAMP responsiveness of the aromatase promoter was generally lower in MSMCs compared with LSMCs, similar results with the deletion and site-directed mutants were observed (results can be seen in supplemental Fig. 2). These results demonstrate that multiple but selected cis-regulatory elements play key roles in the regulation of aromatase transcription. In particular, three C/EBP binding sites located at −350/−337, −317/−304, and −245/−231 bp, and two CREs located at −292/−285 and −211/−197 bp are necessary for cAMP-induced aromatase expression in both LSMCs and MSMCs.
C/EBPβ binds multiple cis-regulatory elements in the aromatase promoter I.3/II region in LSMCs
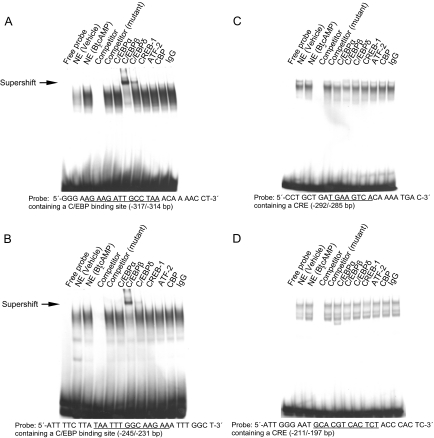
We performed EMSAs to determine the cis-regulatory elements that showed increased nuclear protein binding in response to treatment of LSMCs with Bt2cAMP. Based on the results of our luciferase reporter gene assays, we designed five oligonucleotide probes representing the three C/EBP binding sites and two CREs within the −517/−16 region of aromatase promoter I.3/II. We identified distinct LSMC nuclear protein-DNA complexes that occupy sites on the −317/−304 and −245/−231 bp C/EBP binding motif probes, and −292/−285 and −211/−197 bp CRE probes (Fig. 3). Complex assembly increased upon treatment with Bt2cAMP and was abolished with the addition of cold competitor, but not mutant competitor oligonucleotides. Addition of an anti-C/EBPβ antibody contributed to distinct supershifts and slower migrating positions of the complexes bound to the −317/−304 and −245/−231 bp probes. The addition of an anti-C/EBPδ antibody also resulted in a distinct but less prominent supershift (Fig. 3, A and B). In contrast, none of nuclear protein-DNA complexes binding the CRE probes were supershifted in the presence of antibodies against C/EBPα, -β, -δ, CREB1, ATF2, or CBP (Fig. 3, C and D). A less prominent complex occupied the −350/−337 bp C/EBP binding site; however, its intensity was not affected by Bt2cAMP treatment or addition of antibodies directed against each of the C/EBP transcription factors (results can be seen in supplemental Fig. 3).
Figure 3.
Characterization of protein binding to the cis-regulatory elements on the aromatase promoter I.3/II region in LSMCs. EMSA was performed using oligonucleotide probes containing a C/EBP or CRE cis-regulatory element from the aromatase promoter I.3/II region, as indicated. All nuclear extracts (except the vehicle lane) were obtained from LSMCs treated with Bt2cAMP (100 μm, 24 h). Unlabeled oligonucleotide was used as cold probe competitor, and competitors of mutated consensus sequences of the C/EBP binding site and CRE were used to confirm specificity of binding activity. Antibodies against C/EBPα, C/EBPβ, C/EBPδ, CREB-1, ATF-2, and CBP were used in supershift assays to identify proteins binding to each cis-regulatory element probe, and nonimmune IgG was used as a negative control. Distinct nuclear protein-DNA complexes occupied two C/EBP binding sites within the −317/−304 bp (A) and −245/−231 bp (B) probes. One of these complexes was supershifted upon addition of anti-C/EBPβ antibody and, although less prominently, anti-C/EBPδ antibody (arrow). Distinct nuclear protein-DNA complexes also occupied two CREs located at −292/−285 bp (C) and −211/−197 bp (D), but these complexes could not be supershifted with addition of any antibody tested. NE, nuclear extract; IgG, nonimmune rabbit IgG.
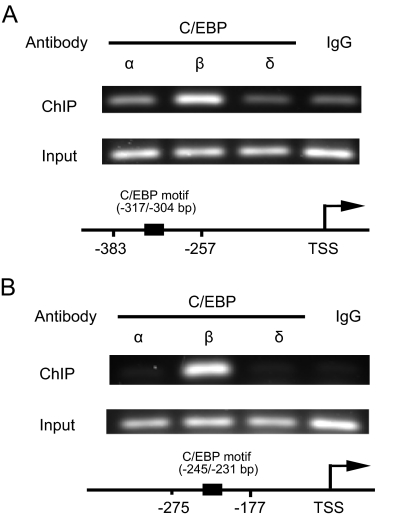
To verify the results of EMSA that is a cell-free assay, we performed ChIP-PCR to identify the C/EBP proteins that bind to the Bt2cAMP-responsive aromatase promoter I.3/II region in intact LSMCs. We used antibodies against C/EBPα, C/EBPβ, and C/EBPδ for immunoprecipitation, and amplified two distinct regions of the aromatase promoter I.3/II containing the C/EBP binding sites located at −317/−304 and −245/−231 bp. Only the anti-C/EBPβ antibody bound in situ to both promoter regions (Fig. 4). In contrast, the anti-C/EBPα or the anti-C/EBPδ antibodies did not immunoprecipitate DNA fragments detected by oligonucleotide pairs specific for either region. These results suggest that cAMP-dependent aromatase expression in LSMCs is mediated in part via the interaction of C/EBPβ with C/EBP binding sites at −317/−304 and −245/−231 bp sequences.
Figure 4.
C/EBPβ bound to the aromatase promoter I.3/II region in situ in LSMCs. ChIP-PCR was performed using LSMCs treated with Bt2cAMP to verify specific binding between C/EBP family proteins (C/EBPα, β, and δ) and the aromatase promoter I.3/II region in intact cells. Nonimmune rabbit IgG (IgG) was used as a negative control. A, Amplification of the −383/−257 bp region of aromatase promoter I.3/II, which contains a C/EBP binding site at −317/−304 bp. B, Amplification of the −275/−177 bp region of promoter I.3/II, which contains another C/EBP binding site at −245/−231 bp. Diagrams indicate the relative positions of each primer pair within the promoter I.3/II region. Input, Product before immunoprecipitation; TSS, transcription start site.
cAMP up-regulates C/EBPβ levels in LSMCs
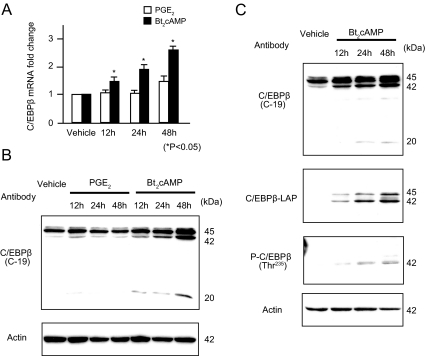
We performed real-time RT-PCR and immunoblotting to evaluate mRNA and protein expression levels of C/EBPβ upon PGE2 or Bt2cAMP treatment of LSMCs. Levels of C/EBPβ mRNA significantly increased after treatment of LSMCs with Bt2cAMP, but not PGE2, in a time-dependent manner (Fig. 5A). Similarly, nuclear C/EBPβ protein levels increased after treatment with Bt2cAMP in a time-dependent manner, but levels did not change after treatment with PGE2 (Fig. 5B). Interestingly, using an antibody (C-19) that recognizes all isoforms of C/EBPβ (1, 2, and 3), we detected three major bands: 45-kDa full-length C/EBPβ (C/EBPβ-1), 42-kDa LAP (C/EBPβ-2), and 20-kDa liver-enriched inhibitory protein (LIP) (C/EBPβ-3, Fig. 5B). Bt2cAMP induced levels of both C/EBPβ-2 and 3. Using more specific antibodies against C/EBPβ-2 (LAP) or its phosphorylated form at Thr235, we demonstrated that Bt2cAMP strikingly induces both total and phosphorylated forms of C/EBPβ-2 in LSMCs (Fig. 5C). In contrast, no changes were observed in cytoplasmic C/EBPβ protein levels (results can be seen in supplemental Fig. 4). These results suggest that cAMP up-regulates C/EBPβ expression, increases nuclear C/EBPβ levels, and leads to C/EBPβ phosphorylation in LSMCs.
Figure 5.
C/EBPβ expression levels in LSMCs increased after treatment with Bt2cAMP. Primary cultured LSMCs were pretreated with PGE2 or Bt2cAMP for 12, 24, and 48 h, and total RNA and nuclear protein were extracted. A, C/EBPβ mRNA expression levels were detected using real-time RT-PCR and standardized to GAPDH mRNA. The average + sem of data from triplicate experiments is reported. *, P < 0.05 (ANOVA). B, Changes in nuclear C/EBPβ protein levels upon treatment of LSMCs with PGE2 or Bt2cAMP were detected by immunoblotting. Using an antibody (C-19) that recognizes three C/EBPβ isoforms (C/EBPβ-1, 45 kDa; C/EBPβ-2, 42 kDa; and C/EBPβ-3, 20 kDa), cAMP was shown to induce all of these isoforms. C, Immunoblotting was performed with more specific anti-C/EBPβ antibodies, as indicated. A C/EBPβ-LAP antibody could be used to detect C/EBPβ-2 (42 kDa) more distinctly, whereas phosphorylation of C/EBPβ-2 could be determined using an antibody against C/EBPβ-2 phosphorylated at Thr235 [P-C/EBPβ (Thr235)]. Equal loading was confirmed by immunoblotting with an anti-actin antibody.
C/EBPβ modulates cAMP-induced aromatase expression and enzyme activity
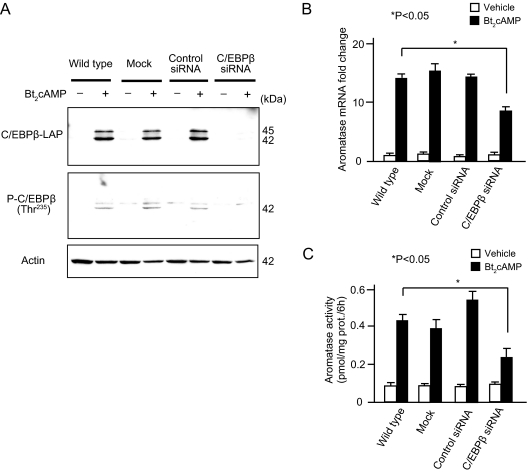
Finally, we used siRNA to knockdown C/EBPβ to establish a functional role for C/EBPβ in mediating cAMP-induced aromatase expression in LSMCs. Transfection of LSMCs with C/EBPβ siRNA reduced basal C/EBPβ mRNA levels more than 70% (data not shown) and abolished the stimulatory effect of Bt2cAMP on C/EBPβ protein levels (Fig. 6A). Consequently, the stimulatory effect of Bt2cAMP on both aromatase mRNA expression and enzyme activity was also significantly reduced in the presence of C/EBPβ siRNA (Fig. 6, B and C). Basal aromatase expression levels were not affected by C/EBPβ siRNA transfection. These results strongly suggest that C/EBPβ directly modulates cAMP-induced aromatase expression and enzyme activity in LSMCs.
Figure 6.
Knockdown of C/EBPβ reduces Bt2cAMP-induced aromatase expression and enzyme activity. LSMCs were transfected without oligonucleotide (mock), with a nontargeting, control siRNA or C/EBPβ siRNA for 36 h, then starved for 12 h, followed by treatment with or without Bt2cAMP for 48 h. A, Immunoblotting for C/EBPβ was performed to determine the knockdown efficiency by C/EBPβ siRNA. Equal loading was confirmed by immunoblotting with anti-actin antibody. Both C/EBPβ-1 (45 kDa) and C/EBPβ-2 (42 kDa) were detected using the anti-C/EBPβ antibodies: C/EBPβ-LAP and C/EBPβ phosphorylated at Thr235 [P-C/EBPβ (Thr235)]. B, Aromatase mRNA expression levels after transfection of C/EBPβ siRNA were compared with wild type (without transfection), mock (transfection reagent only), and control siRNA (nontargeting control siRNA) treated with Bt2cAMP (black bars) or vehicle (white bars). Aromatase mRNA expression levels were quantified by real-time RT-PCR and normalized to GAPDH mRNA. Data are represented as the average + sem from triplicate experiments. Transcripts from only vehicle, untransfected cells were arbitrarily assigned a unit of one, against which expression from transfected cells was compared. *, P < 0.05 (ANOVA). C, Aromatase enzyme activity was compared as in B using a [3H]water releasing assay. Data are represented as the average + sem from triplicate experiments. *, P < 0.05 (ANOVA). All of the results were reproducible in at least three subjects.
Discussion
Aromatase is important for leiomyoma growth because aromatase inhibitors reduce leiomyoma size in premenopausal and perimenopausal women (1,2,3,4). Furthermore, locally produced estrogen via an aromatase-dependent pathway within leiomyoma was shown to contribute to tumor growth (7,8). We previously reported that the cAMP-responsive promoter I.3/II but not the glucocorticoid-responsive I.4 was the primary regulator of aromatase expression in leiomyoma tissue (15), however, the underlying molecular mechanisms remained unclear. Here, we demonstrate that cAMP-induced binding of C/EBPβ to multiple motifs in the promoter I.3/II region is a critical mechanism regulating aromatase expression in LSMCs in primary culture.
The cAMP-responsive promoter I.3/II region plays a critical role for aromatase expression in a number of other pathological estrogen-dependent tissues including, endometriosis and breast cancer (16,20,21). We concluded here that three C/EBP binding sites and two CREs in this region were the key cis-regulatory elements that regulate aromatase expression in uterine leiomyoma cells. Two out of three C/EBP binding sites, including a newly identified one, bind C/EBPβ, suggesting that C/EBPβ is a key factor regulating aromatase expression in uterine leiomyoma cells. Some of the five cis-regulatory elements reported in this study were previously shown to bind various transcription factors in other sex steroid-dependent tumors and tissues (20,24,25). Interestingly, the two NRHSs, which were previously shown to be extremely critical for the regulation of aromatase expression in endometriotic stromal cells or gonadal cells, had little effect on aromatase expression in leiomyoma cells (16,23). One of these sites was shown to bind steroidogenic factor-1 (SF-1) or liver receptor homolog-1 (LRH-1) in ovarian granulosa cells, endometriotic stromal cells, and breast cancer fibroblasts (16,26,27). We recently found only low levels of SF-1 or LRH-1 that are not differentially expressed in leiomyoma vs. normal myometrial tissue (unpublished observations). This is in contrast to extremely high levels of SF-1 or LRH-1 elevated specifically in endometriotic and breast tumor fibroblasts and ovarian granulosa cells (16,18,26,27). This conclusion should be made with some caution because we did not include a mutant construct that carries double mutations of both of the NRHSs. Nevertheless, C/EBP binding cis-regulatory elements in the promoter I.3/II region and C/EBPβ as a transactivating factor emerge as key regulators of aromatase expression in uterine leiomyoma in contrast to other aromatase-overexpressing pathological tissues.
C/EBPs are basic leucine zipper transcription factors that function as regulators of cell growth and differentiation in numerous cell types (28). There are six C/EBP family members (C/EBPα, β, γ, δ, ε, and ξ) that recognize a common but specific sequence within target promoters. As a consequence of the high similarity in the basic region among the six C/EBP family members, C/EBPα, C/EBPβ, and C/EBPδ have interacted with virtually identical DNA sequences (28). C/EBPβ, also named nuclear factor IL-6, was originally identified as a mediator of IL-6 signaling that binds to IL-6-responsive elements in the promoters of acute phase response genes TNF, IL-8, and granulocyte colony-stimulating factor (29). In humans, three isoforms of C/EBPβ have been identified: C/EBPβ-1, -2, and -3. The C/EBPβ-1 is a full-length protein of 346 amino acids. C/EBPβ-2 (also called LAP in rodents) is an N-terminal truncated form, whereas C/EBPβ-3 (also called LIP in rodents) is a C-terminal truncated form (30). Human C/EBPβ-1 and C/EBPβ-2 function as transcriptional activators, whereas C/EBPβ-3 may act as a transcriptional repressor because it lacks the transcription activating domain. C/EBPβ plays an essential role in female reproduction (31). C/EBPβ-deficient female mice are viable but completely infertile and exhibit reduced ovulation in response to gonadotropins (32). Because estrogen production plays a key role in reproduction, perturbation of aromatase expression in the gonads and other tissues of these C/EBPβ-deficient mice may be in part responsible for this phenotype.
Phosphorylation of C/EBPβ is required for its activation pathway in adipogenesis, monocytic differentiation, and cortical neurogenesis (33,34,35). We demonstrated that nuclear C/EBPβ protein was induced by Bt2cAMP in a time-dependent manner in LSMCs, and the level of phosphorylated C/EBPβ (Thr235) also increased. Intriguingly, phosphorylation was reduced upon knockdown of C/EBPβ protein by siRNA. These results suggest that phosphorylation of C/EBPβ may be important for the interaction between C/EBPβ and the cis-regulatory elements in promoter I.3/II, and mediation of cAMP-dependent aromatase expression in LSMCs. This notion is also supported by the report that agents (e.g. 1-methyl-3-isobutylxanthine), which increase cAMP levels, enhance adipogenic differentiation (36).
Because cAMP strikingly induced C/EBPβ levels, we chose to demonstrate the role of this transcription factor in aromatase expression via a knockdown approach, but not through overexpression. C/EBPβ knockdown reduced cAMP-regulated aromatase activity only by 50% but did not abolish it. It is likely that the CREs in promoter I.3/II, which can interact with members of the ATF/CREB family, may account for the rest of cAMP-dependent aromatase induction.
PGE2 is a potent inducer of aromatase in human breast adipose fibroblasts, endometriosis-derived stromal cells, and rat granulosa cells, which are dominantly regulated by the promoter I.3/II region (37,38,39,40). Contrary to our expectations, PGE2 showed little effect on aromatase expression in LSMCs. We determined mRNA levels of the four PGE2 receptors, namely, EP1, EP2, EP3, and EP4 (the mRNA levels can be seen in supplemental Fig. 5). We found very high levels of EP3 that reduces cAMP formation and barely detectable levels of EP2 that induces cAMP formation. This is in contrast to stromal cells of endometriosis and breast adipose tissue, which aromatase expression is regulated primarily via EP2 (12,16). Future studies will investigate the role of alternative factors upstream of cAMP-dependent aromatase expression in LSMCs.
In summary, our data suggest that cAMP and C/EBPβ are thus far the best characterized regulators of aromatase expression in primary uterine leiomyoma cells. Currently, targeting of aromatase enzyme (protein) via specific inhibitors reduces the size of uterine leiomyomata (3). However, this approach induces a total deprivation of estrogen in the whole body with important side effects such as bone loss and hot flashes. It is our earnest hope that determining signaling mechanisms for local aromatase expression in leiomyoma cells will lead to targeted therapies, which might have therapeutic effects comparable to total estrogen deprivation.
Supplementary Material
Acknowledgments
We thank Dr. Masashi Demura for establishing the multiplex RT-PCR experiment.
Footnotes
This work was supported by National Institutes of Health Grant HD46260, Avon Foundation, Lynn Sage Foundation, and Friends of Prentice.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 8, 2008
Abbreviations: ATF, Activating transcription factor; Bt2cAMP, dibutyryl cAMP; CBP, cAMP response element binding protein-binding protein; C/EBP, CCAAT/enhancer binding protein; ChIP, chromatin immunoprecipitation; CRE, cAMP response element; CREB, cAMP response element binding protein; DEX, dexamethasone; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LAP, liver-enriched activating protein; LIP, liver-enriched inhibitory protein; LRH-1, liver receptor homolog-1; LSMC, leiomyoma smooth muscle cell; MSMC, myometrial smooth muscle cell; NRHS, nuclear receptor half-site; PDA, phorbol diacetate; PGE2, prostaglandin estradiol; SF-1, steroidogenic factor-1; siRNA, small interfering RNA.
References
- Attilakos G, Fox R 2005 Regression of tamoxifen-stimulated massive uterine fibroid after conversion to anastrozole. J Obstet Gynaecol 25:609–610 [DOI] [PubMed] [Google Scholar]
- Rivera JA, Christopoulos S, Small D, Trifiro M 2004 Hormonal manipulation of benign metastasizing leiomyomas: report of two cases and review of the literature. J Clin Endocrinol Metab 89:3183–3188 [DOI] [PubMed] [Google Scholar]
- Varelas FK, Papanicolaou AN, Vavatsi-Christaki N, Makedos GA, Vlassis GD 2007 The effect of anastrazole on symptomatic uterine leiomyomata. Obstet Gynecol 110:643–649 [DOI] [PubMed] [Google Scholar]
- Shozu M, Murakami K, Segawa T, Kasai T, Inoue M 2003 Successful treatment of a symptomatic uterine leiomyoma in a perimenopausal woman with a nonsteroidal aromatase inhibitor. Fertil Steril 79:628–631 [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Steiner CA 2002 Hysterectomy rates in the United States 1990–1997. Obstet Gynecol 99:229–234 [DOI] [PubMed] [Google Scholar]
- Stewart EA 2001 Uterine fibroids. Lancet 357:293–298 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Simpson ER, Word RA 1994 Expression of the CYP19 gene and its product aromatase cytochrome P450 in human uterine leiomyoma tissues and cells in culture. J Clin Endocrinol Metab 78:736–743 [DOI] [PubMed] [Google Scholar]
- Sumitani H, Shozu M, Segawa T, Murakami K, Yang HJ, Shimada K, Inoue M 2000 In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology 141:3852–3861 [DOI] [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun SE 1994 Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev 15:342–355 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S 2005 Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev 57:359–383 [DOI] [PubMed] [Google Scholar]
- Otubu JA, Buttram VC, Besch NF, Besch PK 1982 Unconjugated steroids in leiomyomas and tumor-bearing myometrium. Am J Obstet Gynecol 143:130–133 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Agarwal VR, Mendelson CR, Simpson ER 1996 Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology 137:5739–5742 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Nichols JE, Bulun SE, Mendelson CR, Simpson ER 1995 Aromatase P450 gene expression in human adipose tissue. Role of a Jak/STAT pathway in regulation of the adipose-specific promoter. J Biol Chem 270:16449–16457 [DOI] [PubMed] [Google Scholar]
- Shozu M, Sumitani H, Segawa T, Yang HJ, Murakami K, Kasai T, Inoue M 2002 Overexpression of aromatase P450 in leiomyoma tissue is driven primarily through promoter I.4 of the aromatase P450 gene (CYP19). J Clin Endocrinol Metab 87:2540–2548 [DOI] [PubMed] [Google Scholar]
- Imir AG, Lin Z, Yin P, Deb S, Yilmaz B, Cetin M, Cetin A, Bulun SE 2007 Aromatase expression in uterine leiomyomata is regulated primarily by proximal promoters I.3/II. J Clin Endocrinol Metab 92:1979–1982 [DOI] [PubMed] [Google Scholar]
- Zeitoun KM, Bulun SE 1999 Aromatase: a key molecule in the pathophysiology of endometriosis and a therapeutic target. Fertil Steril 72:961–969 [DOI] [PubMed] [Google Scholar]
- Rossi MJ, Chegini N, Masterson BJ 1992 Presence of epidermal growth factor, platelet-derived growth factor, and their receptors in human myometrial tissue and smooth muscle cells: their action in smooth muscle cells in vitro. Endocrinology 130:1716–1727 [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE 2007 Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab 92:3261–3267 [DOI] [PubMed] [Google Scholar]
- Ackerman GE, Smith ME, Mendelson CR, MacDonald PC, Simpson ER 1981 Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J Clin Endocrinol Metab 53:412–417 [DOI] [PubMed] [Google Scholar]
- Zhou J, Gurates B, Yang S, Sebastian S, Bulun SE 2001 Malignant breast epithelial cells stimulate aromatase expression via promoter II in human adipose fibroblasts: an epithelial-stromal interaction in breast tumors mediated by CCAAT/enhancer binding protein β. Cancer Res 61:2328–2334 [PubMed] [Google Scholar]
- Deb S, Zhou J, Amin SA, Imir AG, Yilmaz MB, Lin Z, Bulun SE 2006 A novel role of sodium butyrate in the regulation of cancer-associated aromatase promoters I.3 and II by disrupting a transcriptional complex in breast adipose fibroblasts. J Biol Chem 281[Erratum (2006) 281:9832]:2585–2597 [DOI] [PubMed] [Google Scholar]
- Cheng YH, Richardson BD, Hubert MA, Handwerger S 2004 Isolation and characterization of the human syncytin gene promoter. Biol Reprod 70:694–701 [DOI] [PubMed] [Google Scholar]
- Gurates B, Sebastian S, Yang S, Zhou J, Tamura M, Fang Z, Suzuki T, Sasano H, Bulun SE 2002 WT1 and DAX-1 inhibit aromatase P450 expression in human endometrial and endometriotic stromal cells. J Clin Endocrinol Metab 87:4369–4377 [DOI] [PubMed] [Google Scholar]
- Yang S, Fang Z, Suzuki T, Sasano H, Zhou J, Gurates B, Tamura M, Ferrer K, Bulun S 2002 Regulation of aromatase P450 expression in endometriotic and endometrial stromal cells by CCAAT/enhancer binding proteins (C/EBPs): decreased C/EBPβ in endometriosis is associated with overexpression of aromatase. J Clin Endocrinol Metab 87:2336–2345 [DOI] [PubMed] [Google Scholar]
- Sofi M, Young MJ, Papamakarios T, Simpson ER, Clyne CD 2003 Role of CRE-binding protein (CREB) in aromatase expression in breast adipose. Breast Cancer Res Treat 79:399–407 [DOI] [PubMed] [Google Scholar]
- Michael MD, Kilgore MW, Morohashi K, Simpson ER 1995 Ad4BP/SF-1 regulates cyclic AMP-induced transcription from the proximal promoter (PII) of the human aromatase P450 (CYP19) gene in the ovary. J Biol Chem 270:13561–13566 [DOI] [PubMed] [Google Scholar]
- Clyne CD, Speed CJ, Zhou J, Simpson ER 2002 Liver receptor homologue-1 (LRH-1) regulates expression of aromatase in preadipocytes. J Biol Chem 277:20591–20597 [DOI] [PubMed] [Google Scholar]
- Lekstrom-Himes J, Xanthopoulos KG 1998 Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem 273:28545–28548 [DOI] [PubMed] [Google Scholar]
- Poli V, Mancini FP, Cortese R 1990 IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell 63:643–653 [DOI] [PubMed] [Google Scholar]
- Descombes P, Schibler U 1991 A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67:569–579 [DOI] [PubMed] [Google Scholar]
- Mantena SR, Kannan A, Cheon YP, Li Q, Johnson PF, Bagchi IC, Bagchi MK 2006 C/EBPβ is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci USA 103:1870–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterneck E, Tessarollo L, Johnson PF 1997 An essential role for C/EBPβ in female reproduction. Genes Dev 11:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkowska E, Garay E, Gocek E, Chrobak A, Wang X, Studzinski GP 2006 Regulation of C/EBPβ isoforms by MAPK pathways in HL60 cells induced to differentiate by 1,25-dihydroxyvitamin D3. Exp Cell Res 312:2054–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin A, Barnabe-Heider F, Kageyama R, Miller FD 2005 CCAAT/enhancer-binding protein phosphorylation biases cortical precursors to generate neurons rather than astrocytes in vivo. J Neurosci 25:10747–10758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD 2005 Sequential phosphorylation of CCAAT enhancer-binding protein β by MAPK and glycogen synthase kinase 3β is required for adipogenesis. Proc Natl Acad Sci USA 102:9766–9771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Zhou J, Sasano H, Suzuki T, Zeitoun KM, Bulun SE 2001 Tumor necrosis factor α and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein alpha and peroxisome proliferator-activated receptor δ: mechanism of desmoplastic reaction. Cancer Res 61:2250–2255 [PubMed] [Google Scholar]
- Cai Z, Kwintkiewicz J, Young ME, Stocco C 2007 Prostaglandin E2 increases cyp19 expression in rat granulosa cells: implication of GATA-4. Mol Cell Endocrinol 263:181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Reierstad S, Lin Z, Lu M, Brooks C, Li N, Innes J, Bulun SE 2007 Prostaglandin E2 induces breast cancer related aromatase promoters via activation of p38 and c-Jun NH2-terminal kinase in adipose fibroblasts. Cancer Res 67:8914–8922 [DOI] [PubMed] [Google Scholar]
- Lu M, Chen D, Lin Z, Reierstad S, Trauernicht AM, Boyer TG, Bulun SE 2006 BRCA1 negatively regulates the cancer-associated aromatase promoters I. 3 and II in breast adipose fibroblasts and malignant epithelial cells. J Clin Endocrinol Metab 91:4514–4519 [DOI] [PubMed] [Google Scholar]
- Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE 1997 Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab 82:600–606 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.