Abstract
Background and purpose:
Vascular endothelial growth factor (VEGF) is the most important proangiogenic protein. We have demonstrated that ATL-1, a synthetic analogue of aspirin-triggered lipoxin A4, inhibits VEGF-induced endothelial cell (EC) migration. In the present study, we investigated the effects of ATL-1 in several other actions stimulated by VEGF.
Methods:
Human umbilical vein ECs were treated with ATL-1 for 30 min before stimulation with VEGF. Cell proliferation was measured by thymidine incorporation. Adherent cells were determined by fluorescence intensity using a Multilabel counter. Expression and activity of matrix metalloproteinases (MMP) were analysed by western blot and zymography.
Key results:
ATL-1 inhibited EC adhesion to fibronectin via interaction with its specific receptor. Furthermore, VEGF-induced MMP-9 activity and expression were reduced by pretreatment with ATL-1. Because the transcription factor NF-κB has been implicated in VEGF-mediated MMP expression and EC proliferation, we postulated that ATL-1 might modulate the NF-κB pathway and, indeed, ATL-1 inhibited NF-κB nuclear translocation. Pretreatment of EC with ATL-1 strongly decreased VEGF-dependent phosphorylation of phosphainositide 3-kinase (PI3-K) and extracellular signal-regulated kinase-2 (ERK-2), two signalling kinases involved in EC proliferation. Inhibition of VEGF-induced EC proliferation by ATL-1 was antagonized by sodium orthovanadate, suggesting that this inhibitory activity was mediated by a protein tyrosine phosphatase. This was confirmed by showing that ATL-1 inhibition of VEGF receptor-2 (VEGFR-2) phosphorylation correlates with SHP-1 association with VEGFR-2.
Conclusions and implications:
The synthetic 15-epi-lipoxin analogue, ATL-1, is a highly potent molecule exerting its effects on multiple steps of the VEGF-induced angiogenesis.
Keywords: lipoxins, angiogenesis, VEGF, SHP-1
Introduction
Angiogenesis, the growth of new capillaries from sprouting of pre-existing ones, occurs through dynamic functions of endothelial cells (ECs) including migration, proliferation and maturation. The angiogenic process is involved in physiological responses, for instance, embryonic development, corpus luteum formation and wound healing. Otherwise, de-regulated angiogenesis plays a critical role in various pathological conditions such as rheumatoid arthritis, diabetic retinopathy, solid tumour formation and metastasis (Folkman, 1971, 1995). During angiogenesis, ECs become activated and can degrade the extracellular matrix surrounding the vessels, which serves to liberate EC from their basement membrane and to create a path by which the cells can migrate. Matrix degradation depends on expression and activation of matrix metalloproteinases (MMPs), a family of zinc-dependent enzymes. Subsequently, EC proliferate, forming a sprout towards a specific stimulus (Kalluri, 2003).
The angiogenic process can be stimulated by different growth factors such as platelet-derived growth factor (PDGF), fibroblast growth factor and vascular endothelial growth factor (VEGF). Among these, VEGF has been shown as the most important regulator of EC physiology (Carmeliet and Jain, 2000). VEGF exerts its effects mainly by binding two specific tyrosine kinase receptors, VEGFR-1 (also named Flt-1) and VEGFR-2 (KDR/Flk-1). Activation of VEGFR-1 has been reported to be important in embryo development, whereas VEGFR-2 plays a central role in mitosis, angiogenesis and permeability of EC (Ferrara et al., 2003). Once activated, VEGFR dimerize and become autophosphorylated, leading to phosphorylation of several cytoplasmic signalling molecules. VEGF has been reported to inhibit EC apoptosis and induce proliferation by activating phosphatidylinositol 3-kinase (PI3-K) and extracellular signal-regulated protein kinase (ERK), respectively.
Two non-receptor protein tyrosine phosphatases (PTPs), Src homology 2 domain-containing PTP 1 (SHP-1) and SHP-2, were reported to physically associate with VEGFR-2 (Kroll and Waltenberger, 1997). SHP-2 has been proposed to positively regulate receptor tyrosine kinases (RTK), including epidermal growth factor (Qu et al., 1999), PDGF (Lu et al., 1998) and fibroblast growth factor receptor signalling (Hadari et al., 1998). Conversely, SHP-1 acts as a negative regulator of RTK by binding to their SH2 (Src homology-2) domain (Östman et al., 2006).
Non-steroidal anti-inflammatory drugs, such as aspirin, have been implicated in preventing lung and colon tumours (Marcus, 1995; Vane, 2000; Shtivelband et al., 2003). In addition, epidemiological data suggest that the use of aspirin and other non-steroidal anti-inflammatory drugs may contribute to reduce the risk of stomach and oesophagus cancer (Baron, 2003; Gonzalez-Perez et al., 2003). These inhibitory effects could be associated with the ability of aspirin to reduce angiogenesis (Hla et al., 1993; Pearce et al., 2003; Gerrah et al., 2004). Aspirin's well-known mechanism of action involves acetylation of COX-2, thereby blocking its ability to produce prostanoids (Vane, 2000). Notwithstanding, the acetylated COX-2 protein retains other enzymic activities and catalyses the biosynthesis of aspirin-triggered 15-epi-lipoxins (ATL) (Clària and Serhan, 1995), the carbon 15-epimers of the native lipoxins (LX), which mimic some of their bioactivities. As LXs are biosynthesized and rapidly enzymically inactivated, stable and more potent analogues were constructed (Parkinson, 2006). Native LXs and their stable analogues exert their biological effects by binding to a G-protein-coupled receptor, denoted ALX (Fiore et al., 1994; Chiang et al., 2006), acting as a downregulatory signal at sites of inflammation, mediating inhibition of neutrophil and eosinophil chemotaxis (Serhan, 2006), and stimulation of human monocyte chemotaxis (Maddox and Serhan, 1996; Simões and Fierro, 2005). Currently, our group has shown that an ATL analogue induced the expression of HO-1 (haeme oxygenase-1) in EC, suggesting a new anti-inflammatory mechanism for these novel lipid mediators (Nascimento-Silva et al., 2005).
In addition, a synthetic analogue of ATL, 15-epi-16-(para-fluoro)-phenoxy-LX A4 (named ATL-1), inhibited VEGF-induced EC migration and proliferation, in a concentration-dependent manner, identifying a novel and potent action for ATL. The mechanism by which ATL-1 inhibits VEGF-induced EC migration involves inhibition of FAK (focal adhesion kinase) phosphorylation and its subsequent association with the actin cytoskeleton (Cezar-de-Mello et al., 2006). In this study, we sought to understand the mechanism by which ATL-1 may modulate other VEGF-stimulated proangiogenic actions, particularly, EC adhesion and proliferation, as well as MMP release. We found that ATL-1 modulated EC adhesion and MMP activity and expression. Furthermore, this 15-epi-LXA4 analogue inhibited EC proliferation through SHP-1 activation. The present study provides new insights into the inhibition of angiogenesis by ATL-1, indicating a possible role in the treatment of pathologies associated with angiogenesis, including cancer.
Methods
Cell culture
Human umbilical vein ECs (HUVECs) were isolated by collagenase digestion and propagated on gelatin-coated (0.1%) tissue culture plates in medium 199 supplemented with 20% heat-inactivated fetal bovine serum, 8 U ml−1 heparin, 50 U ml−1 penicillin and 15 μg ml−1 streptomycin. HUVEC between second and third passages were used for all experiments. Before each experiment, cells were serum-starved for 12 h.
Adhesion assay
HUVECs were stained with CMFDA (5-chloromethylfluorescein diacetate; 2.5 μM) for 45 min at 37 °C, pre-treated with Boc-2 and followed by treatment with ATL-1. Cells were then allowed to adhere onto 96-well plates (2 × 104 cells per well) coated with fibronectin (1 μg per well) or 1% BSA—negative adhesion control. Adhesion was allowed to proceed for 1 h at 37 °C. Adherent cells were determined by fluorescence intensity using a Multilabel counter (Wallac 1420 VICTOR3; PerkinElmer, Turku, Finland).
Zymography
Conditioned media containing 1% fetal bovine serum from HUVECs pre-treated with ATL-1 and stimulated with VEGF for 24 h were collected and protein amount was determined by BCA (bicinchoninic acid) kit reagent (Pierce, Rockford, IL, USA). The conditioned media were separated on a SDS-PAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis) gel containing 1 mg ml−1 gelatin (Sigma, St Louis, MO, USA). Rainbow-coloured protein molecular weight markers (Amersham Pharmacia Biotech, Uppsala, Sweden) and lysates from placenta tissue (Niu et al., 2000) were run in parallel to estimate molecular weights and pattern of gelatinolytic activity, respectively. After electrophoresis, sodium dodecyl sulphate was removed by washing the gel with 2.5% Triton X-100 for 1 h. Then, the gel was incubated with digestion buffer (50 mM Tris-HCl, pH 8.0, 0.2 M NaCl, 10 mM CaCl2.2H2O, 0.02% sodium azide, 1 μM ZnCl2) for 18 h at 37 °C. To visualize digestion bands, the gel was stained with 0.2% Coomassie Brilliant Blue R-250 and then destained with isopropanol (20%) and acetic acid (10%). A clear zone against the blue background indicated the presence of gelatinolytic activity. The bands were quantified by densitometry, using Scion Image Software (Scion Co., Frederick, MD, USA).
Immunocytochemistry
To investigate nuclear factor-κB (NF-κB), nuclear translocation HUVECs (5 × 105 cells) were incubated with ATL-1 for different times and stimulated with VEGF for 6 h. Cells were then fixed with 4% paraformaldehyde and 4% sucrose in phosphate-buffered saline (PBS) for 20 min. After fixation, cells were permeabilized in PBS containing 0.2% Triton X-100 for 5 min and washed with PBS before incubation overnight with anti-p65 NF-κB subunit antibody (1:50) at 4 °C. Cells were sequentially incubated with anti-rabbit IgG biotin-conjugated for 1 h, followed by streptavidin-conjugated CY3 (1:50) incubation and examined under confocal microscopy.
Nuclear extracts
Cells were treated with ATL-1 and stimulated with VEGF for 6 h. Intranuclear proteins were obtained by lysing the cells with 10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid), pH 7.9, 10 mM KCl 10 mM, 0.1 mM EDTA, 0.1 mM EGTA (ethylene glycol bis(β-aminoethylether)-N,N,N′,N′,-tetraacetic acid), 1 mM DTT (dithiothreitol), aprotinin (10 μg ml−1), leupeptin (10 μg ml−1), pepstatin (2 μg ml−1) and 1 mM PMSF (phenylmethanesulphonyl fluoride) for 15 min at 4 °C. NP40 (nonidet P40) (5%) was added and the nucleus was pelleted at 12 000 × g for 5 min at 4 °C. The pellet was resuspended in a buffer containing 20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, aprotinin (10 μg ml−1), leupeptin (10 μg ml−1), pepstatin (2 μg ml−1) and 1 mM PMSF, and incubated at 4 °C for 30 min. The whole lysate was centrifuged at 12 000 × g for 10 min at 4 °C. Only the supernatant was used. Total protein amount was measured by the Bradford method (Bradford, 1976).
Immunoprecipitation
To investigate PI3-K, ERK-2 and VEGFR-2 phosphorylation, as well as the association of SHP-1 with VEGFR-2, HUVECs (106 cells per well) were pre-incubated with ATL-1 and stimulated with VEGF for 15 min. After treatment, cells were suspended in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 1.5 mM EDTA, Triton X-100 (1% v/v), glycerol (10% v/v), aprotinin (10 μg ml−1), leupeptin (10 μg ml−1), pepstatin (2 μg ml−1) and 1 mM PMSF). Lysates were incubated overnight with specific antibodies (1:200) at 4 °C and protein A/G agarose was added (4:100) followed by incubation for 2 h at 4 °C under rotation. The content of total and phosphorylated protein was analysed by immunoblotting.
Western blots
Cell lysates or conditioned media were denatured in sample buffer (50 mM Tris-HCl, pH 6.8, 1% sodium dodecyl sulphate, 5% β-mercaptoethanol, 10% glycerol, 0.001% bromophenol blue) and heated in a boiling water bath for 3 min. Samples were resolved by SDS-PAGE and proteins were transferred to PVDF membranes. Rainbow-coloured protein molecular weight markers (Amersham Pharmacia Biotech) were run in parallel to estimate molecular weights. Then, membranes were blocked for 30 min with Tween-PBS (0.05% Tween 20) containing 2% BSA and probed overnight with specific antibodies at 4 °C. The membranes were then washed and incubated with IgG antibody biotin-conjugated for 1 h, followed by incubation with streptavidin-conjugated horseradish peroxidase. Bound antibodies were detected by enhanced chemiluminescence (ECL; Pierce, Rockford, IL, USA). Membranes blotted with P-Tyr (phosphorylated tyrosine) were stripped with a strip buffer (62.5 mM Tris, pH 6.7, 2% sodium dodecyl sulphate, 100 mM β-mercaptoethanol) and reprobed to determine ERK-2, PI3-K and VEGFR-2 total content. The bands were quantified by densitometry, using Scion Image Software (Scion Co., Frederick, MD, USA).
[3H] thymidine incorporation
HUVECs (8 × 103 cells) were cultured in a gelatin-coated 96-well plate and allowed to attach overnight. Cells were washed with PBS and placed in M199 1% fetal bovine serum. Then, HUVECs were pre-treated with Na3VO4 (sodium orthovanadate) or Boc-2 for 1 h and 30 min, respectively, treated with ATL-1 and simulated with VEGF for 24 h. During the last 6 h of incubation, 1 μCi per well [3H] thymidine was added to the cells. After washing the cells with PBS, [3H] thymidine was extracted with 10% trichloroacetic acid (ice-cold) for 15 min, washed with 95% ethanol solubilized in 0.2 N NaOH and measured in a liquid scintillation counter.
Statistical analysis
Statistical significance was assessed by ANOVA followed by Bonferroni's t-test, and P<0.05 was taken as statistically significant.
Reagents
VEGF-A165 was obtained from R&D Systems (Minneapolis, MN, USA). Boc-2 peptide (Boc-Phe-Leu-Phe-Leu-Phe) was purchased from Phoenix Pharmaceuticals Inc. (Belmont, CA, USA). Trypsin was purchased from Amersham Biosciences (Buckinghamshire, UK). Collagenase, gelatin, streptomycin and penicillin were obtained from Sigma. Antibodies and protein A/G agarose were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and streptavidin from Caltag Laboratories (Burlingame, CA, USA). FBS was purchased from Cultilab (Campinas, SP, Brazil) and CMFDA from Invitrogen (Carlsbad, CA, USA). ATL-1, the stable 15-epi-LXA4 analogue (Parkinson, 2006), was a generous gift from Berlex Biosciences (Richmond, CA, USA).
Results
ATL-1 inhibited ECs adhesion to fibronectin via ALX
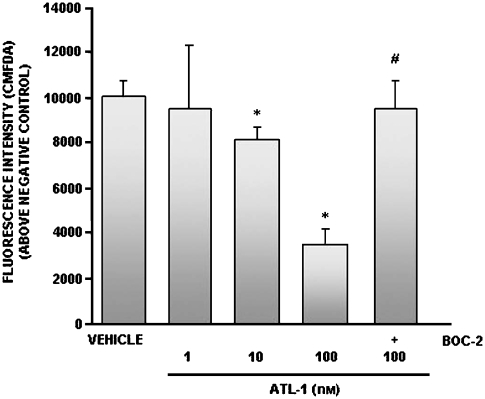
The signalling pathways that control EC growth are dependent on the degree of cell–matrix adhesion, thus the angiogenic response requires conditions of increased cell–matrix adhesiveness (Kalluri, 2003). As fibronectin is an important provisional matrix component, we investigated whether ATL-1 could modulate EC adhesion to immobilized fibronectin. Cells were stained with CMFDA and treated with ATL-1 (1–100 nM) for 30 min before being allowed to adhere for 1 h. ATL-1 significantly decreased EC adhesion peaking at 100 nM (Figure 1).
Figure 1.
ATL-1 inhibited HUVEC adhesion to fibronectin via ALX. HUVECs were stained with CMFDA (2.5 μM) for 45 min at 37 °C before pretreatment with Boc-2 (100 μM) for 15 min and ATL-1 (1–100 nM) for 30 min. Cells (2 × 104 per well) were then allowed to adhere to 96-well plates coated with fibronectin (1 μg per well) for 1 h at 37 °C. Adherent cells were determined by fluorescence intensity using a Multilabel counter (VICTOR3). Negative control (1% BSA) value: 3748. The data show mean±s.e.mean from three independent experiments performed in quadruplicate for each test group. *P<0.05 vs vehicle (0.05% ethanol). #P<0.05 vs ATL-1 (100 nM). ATL-1, 15-epi-16-(para-fluoro)-phenoxy-lipoxin A4; CMFDA, 5-chloromethylfluorescein diacetate; HUVECs, human umbilical vein endothelial cells.
The LX and ATL biological effects are mediated by ALX, the specific G-protein-coupled receptor (Chiang et al., 2006). To determine whether ATL-1 was acting via ALX, we pre-treated the cells for 15 min with Boc-2 (100 μM), an ALXR antagonist, before the treatment with ATL-1. Our results show that Boc-2 reversed the effect of ATL-1 (Figure 1), demonstrating that the analogue inhibits EC adhesion to extracellular matrix via interaction with its specific receptor on the cell surface. Furthermore, Boc-2 did not have any effect when used alone (fluorescence intensity (A.U) 8164±902).
VEGF-induced MMP activity and expression were inhibited by ATL-1
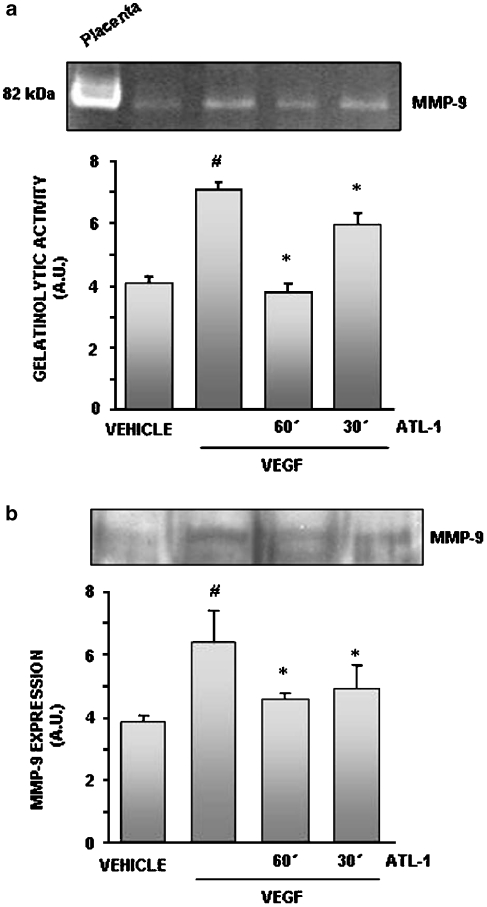
The ability to degrade extracellular matrix proteins is an important step of the angiogenic process and depends on MMP release and activity. A strong correlation between VEGF and MMP-9 expression has been established (Lee et al., 2006). Therefore, we first examined whether ATL-1 was able to modulate MMP activity and/or expression. Conditioned medium from cells treated with VEGF (10 ng ml−1) for 24 h and pre-treated or not with the 15-epi-LXA4 analogue (100 nM) was assayed for total gelatinolytic activity. Although HUVEC constitutively expressed MMP-9 activity, significantly increased activity was detected in VEGF-stimulated EC, which was abolished by pre-incubation with ATL-1 for 60 min (Figure 2a).
Figure 2.
VEGF-stimulated MMP-9 activity and expression were inhibited by ATL-1. Cells were pre-treated with ATL-1 (100 nM) for 30 or 60 min and stimulated with VEGF (10 ng ml−1) for 24 h. (a) Conditioned media were collected and subjected to a gelatinolytic assay, as described in the Methods. (b) Conditioned media were collected and resolved by SDS-PAGE and blotted for MMP-9. A representative blot is shown and the densitometry shows mean±s.e.mean from three independent experiments performed with similar results. #P<0.05 vs vehicle (0.05% ethanol). *P<0.05 vs VEGF alone. ATL-1, 15-epi-16-(para-fluoro)-phenoxy-lipoxin A4; MMP, matrix metalloproteinase; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis; VEGF, vascular endothelial growth factor.
We next analysed MMP expression as a result of VEGF stimulation by western blot. As shown in Figure 2b, along with the activity, VEGF-induced MMP-9 expression was reduced by ATL-1.
ATL-1 abolished VEGF-induced NF-κB nuclear translocation
The transcription factor NF-κB is well established as a regulator of genes encoding cytokines, proteins involved in the control of the cellular proliferation (Yamamoto and Gaynor, 2001) and MMP-9 expression (Ko et al., 2005). To address the hypothesis that the inhibitory effects of ATL-1 involved NF-κB, ECs were pre-treated with the analogue (100 nM) for different periods of time and then stimulated with VEGF (10ng ml−1) for 6 h. We first examined the translocation of p65 subunit of NF-κB into the nucleus by immunocytochemistry. Treating cells with ATL-1 led to a remarkable reduction of the fluorescence into the nucleus. To confirm these data, we performed western blot analyses of nuclear extracts and as illustrated in Figure 3, ATL-1 abolished NF-κB translocation.
Figure 3.
NF-κB nuclear translocation induced by VEGF was inhibited by ATL-1. Cells were pre-treated with ATL-1 (100 nM) for different times and stimulated with VEGF (10 ng ml−1) for 6 h. Nuclear extracts were resolved by SDS-PAGE and immunoblotted for p65 and histone. Blots were analysed by densitometry and the NF-κB/histone ratio content was expressed in arbitrary units. A representative blot is shown and the densitometry shows mean±s.e.mean from three independent experiments performed with similar results. #P<0.05 vs vehicle (0.05% ethanol). *P<0.05 vs VEGF alone. ATL-1, 15-epi-16-(para-fluoro)-phenoxy-lipoxin A4; NF-κB, nuclear factor-κB; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis; VEGF, vascular endothelial growth factor.
VEGF-induced phosphorylation of kinase cascades was inhibited by ATL-1
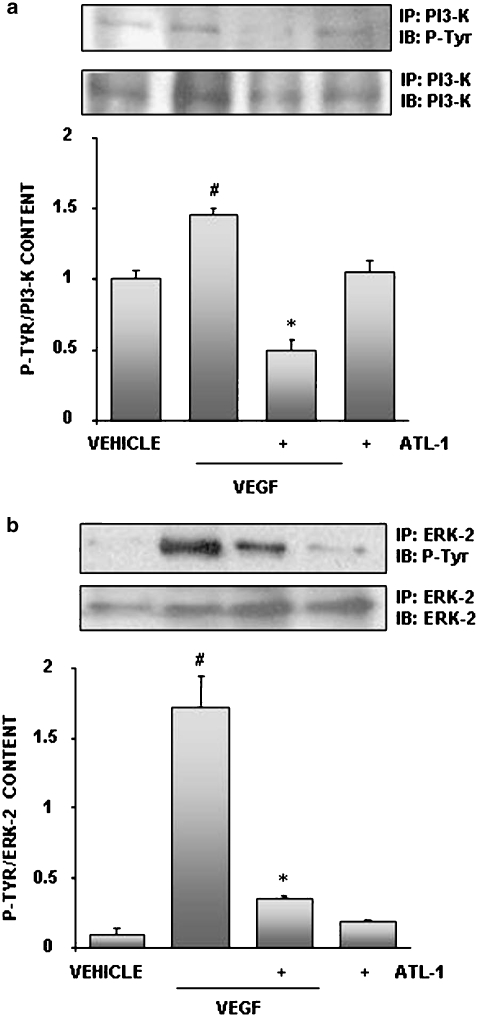
The VEGF signalling pathway involves various key components to the angiogenic process. The proliferation and survival responses stimulated by VEGF evoke activation, through phosphorylation, of ERK-2 and PI3-K, respectively (Zachary, 2003). We therefore tested the effect of ATL-1 on the ability of VEGF to induce PI3-K and ERK-2 phosphorylation. The cells were either left untreated or incubated with ATL-1 (100 nM) before VEGF (10 ng ml−1). As show in Figure 4, the analogue completely blocked both PI3-K (Figure 4a) and ERK-2 (Figure 4b) phosphorylation. These results point to an interesting mechanism for the antiangiogenic effects exerted by ATL-1, that is the activation of PTPs.
Figure 4.
ATL-1 inhibited VEGF-induced PI3-K and ERK-2 phosphorylation. Cells were pre-treated with ATL-1 (100 nM) for 30 min and stimulated with VEGF (10 ng ml−1) for 15 min. Cell lysates were immunoprecipitated with an anti-PI3-K antibody (a) or anti-ERK-2 antibody (b) and immunoblotted with an anti-phosphotyrosine antibody. Membranes were stripped and blotted to PI3-K or ERK-2, respectively. Blots were analysed by densitometry, and the phosphokinase/total kinase ratio was expressed in arbitrary units. A representative blot is shown and the densitometry shows mean±s.e.mean from three independent experiments performed with similar results. #P<0.05 vs vehicle (0.05% ethanol). *P<0.05 vs VEGF alone. ATL-1, 15-epi-16-(para-fluoro)-phenoxy-lipoxin A4; ERK-2, extracellular signal-regulated kinase-2; PI3-K, phosphainositide 3-kinase; VEGF, vascular endothelial growth factor.
ATL-1 antiproliferative activity was dependent on phosphatase activation
In this study, we used a pharmacological approach to understand the mechanism by which the analogue exerts its antiproliferative effect. ECs were treated for 1 h with Na3VO4 (5 μM), a nonspecific phosphatase inhibitor, followed by ATL-1 (100 nM) incubation for 30 min before EC stimulation with VEGF (10 ng ml−1) for 24 h. The data in Figure 5 show that VEGF promoted thymidine incorporation into DNA and, as expected, this effect was decreased by ATL-1. The phosphatase inhibitor antagonized the ability of ATL-1 to reduce DNA synthesis stimulated by VEGF. These data suggest that ATL-1 mediates its inhibitory effects, at least in part, through activation of a PTP.
Figure 5.
ATL-1 inhibition of VEGF-induced HUVEC proliferation was mediated by a protein phosphatase. Cells (8 × 103) were pre-treated with Na3VO4 (5 μM) for 1 h, treated with ATL-1 (100 nM) for 30 min and stimulated with VEGF (10 ng ml−1) for 24 h. During the last 6 h of incubation, 1 μCi per well [3H] thymidine was added to the cells and measured in a liquid scintillation counter. The data show mean±s.e.mean from three independent experiments performed in quadruplicate for each test group. *P<0.05 vs VEGF. #P<0.05 vs VEGF plus ATL-1. ATL-1, 15-epi-16-(para-fluoro)-phenoxy-lipoxin A4; HUVEC, human umbilical vein endothelial cells; Na3VO4, sodium orthovanadate; VEGF, vascular endothelial growth factor.
ATL-1 inhibited VEGF-induced VEGFR-2 phosphorylation
VEGF effects involve activation of its specific RTK in EC, VEGFR-2. The binding of VEGF to the receptor induces receptor dimerization and autophosphorylation of specific intracellular tyrosine residues (Ferrara et al., 2003).
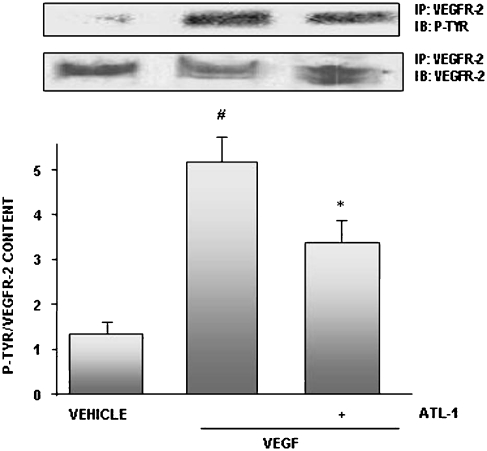
Recently, McMahon et al. (2002) have shown that LXA4 was able to antagonize leukotriene D4 (LTD4) trans-activation of PDGF receptors, suggesting that LX might have the ability to modulate RTKs. This observation prompted us to examine whether ATL-1 might impair VEGFR-2 activation. As illustrated in Figure 6, ATL-1 (100 nM) pretreatment for 30 min prevented VEGF-induced tyrosine phosphorylation of VEGFR-2.
Figure 6.
ATL-1 inhibited VEGFR-2 phosphorylation. Cells were pre-treated with ATL-1 (100 nM) for 30 min and exposed to VEGF (10 ng ml−1) for 15 min. Cell lysates were immunoprecipitated with an anti-VEGFR-2 antibody and immunoblotted with an anti-phosphotyrosine antibody. Membranes were stripped and blotted to VEGFR-2. Blots were analysed by densitometry, and phosphoVEGFR-2/total VEGFR-2 ratio content was expressed in arbitrary units. A representative blot is showed and the densitometry shows mean±s.e.mean from three independent experiments performed with similar results. #P<0.05 vs vehicle (0.05% ethanol). *P<0.05 vs VEGF alone.ATL-1, 15-epi-16-(para-fluoro)-phenoxy-lipoxin A4; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
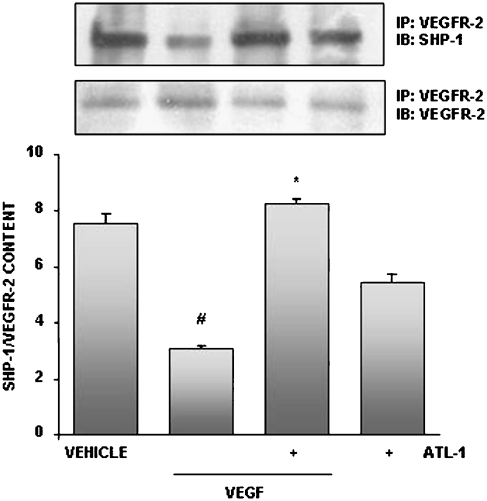
ATL-1 modulated SHP-1 association with VEGFR-2
PTPs play an important role in modulating RTK activity and the biological responses that they regulate (Schlessinger, 2000). It has already been shown that SHP-1 physically associates with VEGFR-2, antagonizing its downstream signalling. We then attempted to elucidate whether SHP-1 could be a target for ATL-1 inhibition of VEGFR-2 phosphorylation.
We performed a VEGFR-2 immunoprecipitation from untreated cells, EC pre-treated with ATL-1 (100 nM) for 30 min and stimulated with VEGF (10 ng ml−1) for 15 min or incubated with these agents alone. Addition of the analogue resulted in a significant augmentation of SHP-1 association with VEGFR-2 (Figure 7), showing for the first time that LX can activate a PTP.
Figure 7.
ATL-1 promoted association of SHP-1 with VEGFR-2. Cells were pre-treated with ATL-1 (100 nM) for 30 min and exposed to VEGF (10 ng ml−1) for 15 min. Cell lysates were immunoprecipitated with an anti-VEGFR-2 antibody and immunoblotted with an anti-SHP-1 antibody. Membranes were stripped and blotted to VEGFR-2. Blots were analysed by densitometry, and SHP-1/VEGFR-2 ratio content was expressed in arbitrary units. A representative blot is showed and the densitometry shows mean±s.e.mean from two independent experiments performed with similar results. #P<0.05 vs vehicle (0.05% ethanol). *P<0.05 vs VEGF alone. ATL-1, 15-epi-16-(para-fluoro)-phenoxy-lipoxin A4; SHP-1, Src homology 2 domain-containing protein tyrosine phosphatase 1; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Discussion
There is substantial evidence that lipid mediators formed by lipoxygenases and COX-2 participate in angiogenesis and cancer development. Certain types of cancer, including colorectal, lung, bone and brain display a characteristic enhancement of the expression of these enzymes (Avis et al., 1996; Ohd et al., 2003; Romano and Clària, 2003). In agreement with these observations, the use of non-steroidal anti-inflammatory drugs, such as aspirin, reduced cellular proliferation, tumour growth and angiogenesis (Shtivelband et al., 2003; Gao et al., 2004). In this study, we reported that a stable aspirin-triggered LXA4 analogue, ATL-1, inhibits multiple steps of the angiogenic process, particularly adhesion, MMP activity and EC proliferation.
During angiogenesis, when EC migrate towards a stimulus, a provisional matrix offers a support framework, guiding EC to their targets (Carmeliet, 2003). Considering that fibronectin is an important provisional matrix component, we investigated the effect of ATL-1 in EC adhesion to fibronectin matrix. Our findings show that ATL-1 inhibition of EC adhesion to immobilized fibronectin occurred in a concentration-dependent manner. Because LX and synthetic analogues exert their effects by binding to a specific receptor, we demonstrated, using a receptor antagonist, that this effect depends on the interaction of the analogue with ALXR. ECs have transmembrane adhesion receptors, such as integrins, which when linked to adhesion proteins provide survival and/or migration signalling to the cells. Thus, our results suggest that ATL-1 could be modulating the first steps of the angiogenic process.
Remodelling and extracellular matrix breakdown requires proteinase activity, such as MMP (Kalluri, 2003; Carmeliet, 2003). In this study, we found that VEGF enhanced MMP-9 activity and this effect is correlated with high levels of the enzyme. The pretreatment of EC with ATL-1 reduced MMP-9 activity and expression in conditioned media, suggesting that the analogue might regulate the gene encoding MMP-9, thereby causing a significant reduction of MMP-9 release from EC stimulated by VEGF. Different members of the MMP family are required for invasive activity and mounting evidence suggest a role for those MMPs, especially MMP-2 and MMP-9, which degrade type IV collagen, the major structural collagen of the basement membrane (Egeblad and Werb, 2002). Supporting our data, earlier work showed that LXs modulate MMP-3 activity in human fibroblasts through augmenting tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 (Sodin-Semrl et al., 2000). Whether ATL-1 inhibits MMP-9 activity by upregulating TIMP is an important question that ongoing experiments will elucidate.
The number of functions attributed to the transcription factor NF-κB is rapidly increasing. Inappropriate NF-κB activity has been reported in several pathological situations, and the involvement of most of the NF-κB target genes in several disease conditions makes inhibitors of NF-κB attractive candidates as therapeutic agents. Several studies reported that MMP-9 expression can be induced via NF-κB activation (Bond et al., 1998; Esteve et al., 2002; Ko et al., 2005). On the basis of these data, we sought to investigate whether ATL-1 might have any impact in VEGF-stimulated NF-κB translocation into the nucleus. Our results using two different techniques demonstrated that pretreatment of EC with the analogue caused a striking inhibition of VEGF-induced NF-κB translocation, suggesting that ATL-1 reduction of MMP-9 expression could be mediated through inhibition of NF-κB. These data are in accordance with known modulation of NF-κB activation by LX in different cell types (Jozsef et al., 2002, Fierro et al., 2003; Sodin-Semrl et al., 2004; Nascimento-Silva et al., 2007).
The binding of VEGF to its receptor, VEGFR-2, triggers receptor dimerization, phosphorylation and recruitment of SH2 domain-containing signalling molecules (Zachary, 2003). PI3-K is a heterodimer of p85 adaptor subunit and p110 catalytic subunit. The p85 subunit contains two SH2 domains, and is constitutively associated with VEGFR-2 undergoing phosphorylation upon stimulation with VEGF (Thakker et al., 1999). This kinase is involved in EC survival (Zachary, 2003; Ferrara et al., 2003) and proliferation (Thakker et al., 1999). Moreover, VEGFR-2 activation classically induces proliferation through activation of ERK, culminating in gene transcription (Cross et al., 2003). We therefore examined the effect of ATL-1 on kinase pathways involved in signalling during EC proliferation mediated by VEGF. We found that VEGF-induced PI3-K and ERK-2 phosphorylation was strongly inhibited by the previous treatment with the LX analogue, indicating that VEGF-mediated cell survival and proliferation is a target for the downregulatory activity of this compound. These data corroborate recent findings showing ATL-1 inhibition of VEGF-induced EC proliferation (Fierro et al., 2002). Furthermore, it has been shown that LXs play a critical role in the regulation of proliferation in many cell types. LXA4 antagonizes mitogenic effects of LTD4 in human mesangial cells via inhibition of PI3-K activation (McMahon et al., 2000). This lipid also inhibits proliferation of human lung fibroblasts by regulation of PI3-K and ERK-2 inhibition, as well as cell cycle arrest (Wu et al., 2006). Control of the cell cycle concomitant with the inhibition of proliferation and kinase phosphorylation by LX is a phenomenon observed in several systems, including human (Mitchell et al., 2004) and rat mesangial cells (Wu et al., 2005) in addition to human lung fibroblasts (Wu et al., 2006). Whether the downregulatory effects of ATL-1 in EC proliferation may be due to the regulation of cell cycle is a hypothesis currently under investigation.
The transcription factor NF-κB is involved in many cellular responses, including proliferation (Yamamoto and Gaynor, 2001). Recently, Grosjean et al. (2006) reported that VEGF-induced HUVEC survival via NF-κB activation is triggered by the PI3-K pathway. These observations prompted us to suggest that LX analogue inhibition of NF-κB translocation may be implicated in its antiproliferative action, as well.
Tyrosine phosphorylation induced by the activation of RTKs promotes cell proliferation, and PTPs can counterbalance the activities of RTKs. In the present study, we showed that a nonspecific inhibitor of PTP, Na3VO4, reversed ATL-1 inhibition of EC proliferation, suggesting that this analogue was able to activate a PTP, probably due to its interaction with ALXR. In agreement with our findings, recent reports demonstrated that tumour necrosis factor-α inhibition of VEGF-mediated EC proliferation was also reversed by prior incubation with sodium orthovanadate (Guo et al., 2000).
The ability of one receptor system to communicate with and impair the activity of a second, a process of heterologous receptor inactivation has been proposed by several reports. LTD4 induces proliferation of mesangial cells by binding to its specific receptor, Cys-LT2, followed by trans-activation of the PDGF receptor (McMahon et al., 2002). This effect was antagonized by LXA4, which inhibited LTD4 trans-activation of PDGF receptors. Moreover, RTK activation can be modulated by a G-protein-coupled receptor agonist in several systems (Linseman et al., 1995; Daub et al., 1996). Our data indicate that ATL-1 trans-inactivated VEGFR-2 phosphorylation, an effect equivalent to the trans-inactivation of the epidermal growth factor receptor mediated by bradykinin (Graness et al., 2000).
PTP families are divided into receptor-like forms and non-receptor forms, SHP-2 and SHP-1 (also called SH-PTP-1/PTP1C/HCP), being the best studied of the classical non-receptor PTP (Poole and Jones, 2005). SHP-1 is restricted mainly to haematopoietic cells, and has been proposed to be a candidate tumour suppressor gene and an antagonist of growth factor signalling in haematopoietic and ECs (Östman et al., 2006). In this study, we provide evidence that ATL-1 promotes SHP-1 association with VEGFR-2, although a direct activation of the phosphatase by the analogue may not be excluded. These observations are consistent with a previous report, showing that tumour necrosis factor-α inhibition uses a PTP to inhibit VEGFR-2 activation (Guo et al., 2000).
Our study provides evidence that the inhibitory activity of ATL-1 might be tightly linked with the putative activation of SHP-1. Furthermore, we also speculate that this lipid analogue could be able to inhibit SHP-2, because, as reported previously, LXs inhibit mesangial cell proliferation via SHP-2 inhibition (Wu et al., 2005). In addition, it has been shown that ALX is coupled to activation and recruitment of SHP-2 (Mitchell et al., 2007). Further experiments are required to confirm this hypothesis and whether other phosphatases could be involved, such as PTEN (phosphatase and tensin homologue), as this phosphatase can negatively modulate VEGF-induced angiogenic effects, including cell survival, proliferation and migration (Huang and Kontos, 2002).
Summarizing, our results point to a substantial antiangiogenic activity of a synthetic analogue of ATL, including inhibition of EC adhesion, MMP-9 activity and proliferation, presumably via SHP-1 activation. Undoubtedly, these findings will improve the experimental and therapeutic options for the treatment of a broad range of pathologies associated with unwanted angiogenesis.
Acknowledgments
We thank Carlos Bizarro Rodrigues for expert assistance in confocal microscopy. This work was supported by grants from UERJ/SR-2, FAPERJ and CNPq.
Abbreviations
- ATL
aspirin-triggered lipoxins
- ATL-1
15-epi-16-(para-fluoro)-phenoxy-lipoxin A4
- ERK
extracellular signal-regulated kinase
- LX
lipoxin
- MMP
matrix metalloproteinase
- NF-κB
nuclear factor-κB
- PDGF
platelet-derived growth factor
- PI3-K
phosphatidylinositol 3-kinase
- PTP
protein tyrosine phosphatases
- RTK
receptor tyrosine kinases
- SHP
Src homology 2 domain-containing protein tyrosine phosphatase
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
Conflict of interest
The authors state no conflict of interest.
References
- Avis IM, Jett M, Boyle T, Vos MD, Moody T, Treston AM, et al. Growth control of lung cancer by interruption of 5-lipoxygenase-mediated growth factor signaling. J Clin Invest. 1996;97:806–813. doi: 10.1172/JCI118480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA. Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res. 2003;37:1–24. doi: 10.1159/000071364. [DOI] [PubMed] [Google Scholar]
- Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Cezar-de-Mello PFT, Nascimento-Silva V, Villela CG, Fierro IM. Aspirin-triggered lipoxin A4 inhibition of VEGF-induced endothelial cell migration involves actin polymerization and focal adhesion assembly. Oncogene. 2006;25:122–129. doi: 10.1038/sj.onc.1209002. [DOI] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlen S, Drazen JM, Hay DWP, Rovati GE, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Clària J, Serhan CN. Aspirin triggered previously undescribed bioactive eicosanoids by human endothelial cell–leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptors signal transduction. Trends Biochem Sci. 2003;28:488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- Daub H, Weiss F, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Esteve PO, Chicoine E, Robledo O, Aoudjit F, Descoteaux A, Potworowski EF, et al. Protein kinase C-zeta regulates transcription of the matrix metalloproteinase-9 gene induced by IL-1 and TNF-alpha in glioma cells via NF-kappa B. J Biol Chem. 2002;277:35150–35155. doi: 10.1074/jbc.M108600200. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber H, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fierro IM, Colgan SP, Bernasconi G, Petasis NA, Clish CB, Arita M, et al. Lipoxin A4 and aspirin-triggered 15-epi-Lipoxin A4 inhibit human neutrophil migration: comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. J Immunol. 2003;170:2688–2694. doi: 10.4049/jimmunol.170.5.2688. [DOI] [PubMed] [Google Scholar]
- Fierro IM, Kutok JL, Serhan CN. Novel lipid mediator regulators of endothelial cell proliferation and migration: aspirin-triggered-15-R-lipoxin A4 and lipoxin A4. J Pharm Exp Ther. 2002;300:385–392. doi: 10.1124/jpet.300.2.385. [DOI] [PubMed] [Google Scholar]
- Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;1:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;21:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Gao J, Niwa K, Sun W, Takemura M, Lian Z, Onogi K, et al. Non-steroidal anti-inflammatory drugs inhibit cellular proliferation and upregulate cyclooxygenase-2 protein expression in endometrial cancer cells. Cancer Sci. 2004;95:901–907. doi: 10.1111/j.1349-7006.2004.tb02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrah R, Fogel M, Gilon D. Aspirin decreases vascular endothelial growth factor release during myocardial ischemia. Int J Cardiol. 2004;94:25–29. doi: 10.1016/j.ijcard.2003.03.007. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez A, Garcia Rodriguez LA, Lopez-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC Cancer. 2003;3:28. doi: 10.1186/1471-2407-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graness A, Hanke S, Boehmer FD, Presek P, Liebmann C. Protein-tyrosine-phosphatase-mediated epidermal growth factor (EGF) receptor transinactivation and EGF receptor-independent stimulation of mitogen-activated protein kinase by bradykinin in A431 cells. Biochem J. 2000;347:441–447. doi: 10.1042/0264-6021:3470441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean J, Kiriakidis S, Reilly K, Feldmann M, Paleolog E. Vascular endothelial growth factor signaling in endothelial cell survival: a role for NFkB. Biochem Biophys Res Commun. 2006;340:984–994. doi: 10.1016/j.bbrc.2005.12.095. [DOI] [PubMed] [Google Scholar]
- Guo DQ, Wu LW, Dunbar JD, Ozes ON, Mayo LD, Kessler KM, et al. Tumor necrosis factor employs a protein-tyrosine phosphatase to inhibit activation of KDR and vascular endothelial growth factor-induced endothelial cell proliferation. J Biol Chem. 2000;275:11216–11221. doi: 10.1074/jbc.275.15.11216. [DOI] [PubMed] [Google Scholar]
- Hadari Y, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol Cell Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T, Ristmäki A, Appleb S, Barriocanal JG. Cyclooxygenase gene expression in inflammation and angiogenesis. Ann N Y Acad Sci. 1993;696:197–204. doi: 10.1111/j.1749-6632.1993.tb17152.x. [DOI] [PubMed] [Google Scholar]
- Huang J, Kontos CD. PTEN modulates vascular endothelial growth factor-mediated signaling and angiogenic effects. J Biol Chem. 2002;277:10760–10766. doi: 10.1074/jbc.M110219200. [DOI] [PubMed] [Google Scholar]
- Jozsef L, Zouki C, Petasis NA, Serhan CN, Filep JG. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NFkB and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc Natl Acad Sci USA. 2002;99:13266–13271. doi: 10.1073/pnas.202296999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- Ko HM, Park YM, Jung B, Kim HA, Choi JH, Park SJ, et al. Involvement of matrix metalloproteinase-9 in platelet-activating factor-induced angiogenesis. FEBS Lett. 2005;579:2369–2375. doi: 10.1016/j.febslet.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Kroll J, Waltenberger J. The vascular endothelial growth factor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem. 1997;272:32521–32527. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- Lee KS, Min KH, Kim SR, Park SJ, Park HS, Jin GY, et al. Vascular endothelial growth factor modulates matrix metalloproteinase-9 expression in asthma. Am J Respir Crit Care Med. 2006;174:161–170. doi: 10.1164/rccm.200510-1558OC. [DOI] [PubMed] [Google Scholar]
- Linseman DA, Benjamim CW, Jones DA. Convergence of angiotensin II and platelet-derived growth factor receptor signaling cascades in vascular smooth muscle cells. J Biol Chem. 1995;270:12563–12568. doi: 10.1074/jbc.270.21.12563. [DOI] [PubMed] [Google Scholar]
- Lu X, Qu CK, Shi ZQ, Feng GS. Downregulation of platelet-derived growth factor receptor-beta in Shp-2 mutant fibroblast cell lines. Oncogene. 1998;4:441–448. doi: 10.1038/sj.onc.1201988. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Serhan CN. Lipoxin A4 e B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus AJ. Aspirin as prophylaxis against colorectal cancer. N Engl J Med. 1995;333:656–658. doi: 10.1056/NEJM199509073331011. [DOI] [PubMed] [Google Scholar]
- McMahon B, Mitchell D, Shattock R, Martin F, Brady HR, Godson C. Lipoxin, leukotriene, and PDGF receptors cross-talk to regulate mesangial cell proliferation. FASEB J. 2002;16:1817–1819. doi: 10.1096/fj.02-0416fje. [DOI] [PubMed] [Google Scholar]
- McMahon B, Stenson C, Mcphillips F, Fanning A, Brady HR, Godson C. Lipoxin A4 antagonizes the mitogenic effects of leukotriene D4 in human renal mesangial cells. J Biol Chem. 2000;275:27566–27575. doi: 10.1074/jbc.M001015200. [DOI] [PubMed] [Google Scholar]
- Mitchell D, O'Meara SJ, Gaffney A, Crean JKG, Kinsella BT, Godson C. The lipoxin A4 receptor is coupled to SHP-2 activation; implications for regulation of receptor tyrosine kinases. J Biol Chem. 2007;282:15606–15618. doi: 10.1074/jbc.M611004200. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Rodgers K, Hanly J, McMahon B, Brady HR, Martin F, et al. Lipoxins inhibit Akt/PKB activation and cell cycle progression in human mesangial cells. Am J Pathol. 2004;164:937–946. doi: 10.1016/S0002-9440(10)63181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb Haemost. 2007;96:88–98. [PubMed] [Google Scholar]
- Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Villela CG, Fierro IM. Novel lipid mediator aspirin-triggered lipoxin A4 induces heme oxygenase-1 in endothelial cells. Am J Physiol Cell Physiol. 2005;289:C557–C563. doi: 10.1152/ajpcell.00045.2005. [DOI] [PubMed] [Google Scholar]
- Niu R, Okamoto T, Iwase K, Nomura S, Mizutani S. Quantitative analysis of matrix metalloproteinases-2 and -9, and their tissue inhibitors-1 and -2 in human placenta throughout gestation. Life Sci. 2000;66:1127–1137. doi: 10.1016/s0024-3205(00)00416-1. [DOI] [PubMed] [Google Scholar]
- Ohd JF, Nielsen CK, Campbell J, Landberg G, Lofberg H, Sjolander A. Expression of the leukotriene D4 receptor CysLT1, COX-2, and other cell survival factors in colorectal adenocarcinomas. Gastroenterology. 2003;124:57–70. doi: 10.1053/gast.2003.50011. [DOI] [PubMed] [Google Scholar]
- Östman A, Hellberg C, Böhmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–320. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- Parkinson JF. Lipoxin and synthetic lipoxin analogs: an overview of anti-inflammatory functions and new concepts in immunomodulation. Inflamm Allergy Drug Targets. 2006;5:91–106. doi: 10.2174/187152806776383125. [DOI] [PubMed] [Google Scholar]
- Pearce HR, Kalia N, Bardhan KD, Brown NJ. Effects of aspirin and indomethacin on endothelial cell proliferation in vitro. J Gastroenterol Hepatol. 2003;18:1180–1187. doi: 10.1046/j.1440-1746.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Poole AW, Jones ML. A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatase by the C-terminal tail. Cell Signal. 2005;17:1323–1332. doi: 10.1016/j.cellsig.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Qu CK, Yu WM, Azzarelli B, Feng GS. Genetic evidence that Shp-2 tyrosine phosphatase is a signal enhancer of the epidermal growth factor receptor in mammals. Proc Natl Acad Sci USA. 1999;96:8528–8533. doi: 10.1073/pnas.96.15.8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano M, Clària J. Cyclooxygenase-2 and 5-lipoxygenase converging functions on cell proliferation and tumor angiogenesis: implications for cancer therapy. FASEB J. 2003;17:1986–1995. doi: 10.1096/fj.03-0053rev. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signalling by receptor tyrosine kinase. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2006;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Shtivelband MI, Juneja HS, Lee S, Wu KK. Aspirin and salicylate inhibit colon cancer medium- and VEGF-induced endothelial tube formation: correlation with suppression of cyclooxygenase-2 expression. Thromb Haemost. 2003;10:2225–2233. doi: 10.1046/j.1538-7836.2003.00446.x. [DOI] [PubMed] [Google Scholar]
- Simões RL, Fierro IM. Involvement of the Rho-kinase/MLCK pathway on human monocyte chemotaxis induced by ATL-1, an aspirin-triggered LX4 synthetic analog. J Immunol. 2005;175:1843–1850. doi: 10.4049/jimmunol.175.3.1843. [DOI] [PubMed] [Google Scholar]
- Sodin-Semrl S, Spagnolo A, Mikus R, Barbaro B, Varga J, Fiore S. Opposing regulation of interleukin-8 and NF-kappaB responses by lipoxin A4 and serum amyloid A via the common lipoxin A receptor. Int J Immunopathol Pharmacol. 2004;17:145–156. doi: 10.1177/039463200401700206. [DOI] [PubMed] [Google Scholar]
- Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. Lipoxin A4 inhibits IL-1b-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J Immunol. 2000;164:2660–2666. doi: 10.4049/jimmunol.164.5.2660. [DOI] [PubMed] [Google Scholar]
- Thakker GD, Hajjar DP, Muller WA, Rosengart TK. The role of phosphatidylinositol 3-kinase in vascular endothelial growth factor signaling. J Biol Chem. 1999;274:10002–10007. doi: 10.1074/jbc.274.15.10002. [DOI] [PubMed] [Google Scholar]
- Vane J. Aspirin and other anti-inflammatory drugs. Thorax. 2000;55 Suppl 2:S3–S9. doi: 10.1136/thorax.55.suppl_2.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Lu C, Dong L, Zhou GP, He ZG, Chen ZQ. Lipoxin-A4 inhibits TNFα-induced production of interleukins and proliferation of rat mesangial cells. Kidney Int. 2005;68:35–46. doi: 10.1111/j.1523-1755.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- Wu SH, Wu XH, Lu C, Dong L, Chen ZQ. Lipoxin A4 inhibits proliferation of human lung fibroblasts induced by connective tissue growth factor. Am J Respir Cell Mol Biol. 2006;34:65–72. doi: 10.1165/rcmb.2005-0184OC. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of NFkB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I. VEGF signaling: interaction and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–1177. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]