Abstract
ASK1 (apoptosis signal-regulating kinase 1), a MKKK (mitogen-activated protein kinase kinase kinase), is activated in response to cytotoxic stresses, such as H2O2 and TNFα (tumour necrosis factor α). ASK1 induction initiates a signalling cascade leading to apoptosis. After exposure of cells to H2O2, ASK1 is transiently activated by autophosphorylation at Thr845. The protein then associates with PP5 (protein serine/threonine phosphatase 5), which inactivates ASK1 by dephosphorylation of Thr845. Although this feedback regulation mechanism has been elucidated, it remains unclear how ASK1 is maintained in the dephosphorylated state under non-stressed conditions. In the present study, we have examined the possible role of PP2Cϵ (protein phosphatase 2Cϵ), a member of PP2C family, in the regulation of ASK1 signalling. Following expression in HEK-293 cells (human embryonic kidney cells), wild-type PP2Cϵ inhibited ASK1-induced activation of an AP-1 (activator protein 1) reporter gene. Conversely, a dominant-negative PP2Cϵ mutant enhanced AP-1 activity. Exogenous PP2Cϵ associated with exogenous ASK1 in HEK-293 cells under non-stressed conditions, inactivating ASK1 by decreasing Thr845 phosphorylation. The association of endogenous PP2Cϵ and ASK1 was also observed in mouse brain extracts. PP2Cϵ directly dephosphorylated ASK1 at Thr845 in vitro. In contrast with PP5, PP2Cϵ transiently dissociated from ASK1 within cells upon H2O2 treatment. These results suggest that PP2Cϵ maintains ASK1 in an inactive state by dephosphorylation in quiescent cells, supporting the possibility that PP2Cϵ and PP5 play different roles in H2O2-induced regulation of ASK1 activity.
Keywords: apoptosis, apoptosis signal-regulating kinase 1 (ASK1), hydrogen peroxide (H2O2), mitogen-activated protein kinase (MAPK), protein phosphatase 2C (PP2C), protein serine/threonine phosphatase 5 (PP5), stress-activated protein kinase (SAPK)
Abbreviations: AP-1, activator protein 1; ASK1, apoptosis signal-regulating kinase 1; DSP, dual-specificity phosphatase; GST, glutathione S-transferase; HA, haemagglutinin; HEK-293 cell, human embryonic kidney cell; HRP, horseradish peroxidase; IL-1, interleukin-1; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MBP, myelin basic protein; MEKK1, MAPK/extracellular-signal-regulated kinase kinase kinase 1; MKK, MAPK kinase; MKKK, MKK kinase; NHS, N-hydroxysuccinimido; PP, protein serine/threonine phosphatase; PP2C, protein phosphatase 2C; PTP, protein tyrosine phosphatase; SAPK, stress-activated protein kinase; TAK1, transforming-growth-factor-β-activated kinase 1; TNF, tumour necrosis factor; Trx, thioredoxin
INTRODUCTION
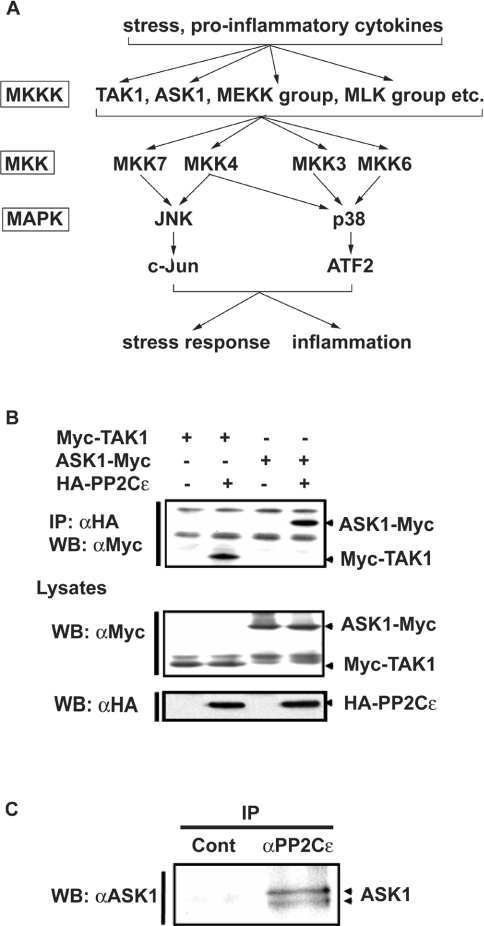
SAPKs (stress-activated protein kinases), a subfamily of the MAPK (mitogen-activated protein kinase) superfamily, are highly conserved from yeast to mammals. SAPKs relay signals from various extracellular stimuli, including environmental stresses and inflammatory cytokines [1]. In mammalian cells, two distinct classes of SAPKs have been described: the c-Jun N-terminal kinases (JNK1, JNK2 and JNK3) and the p38 MAPKs (p38α, p38β, p38γ and p38δ) (Figure 1A) [1,2]. SAPKs are activated by phosphorylation of conserved tyrosine and threonine residues in the catalytic domain by dual-specificity protein kinases of the MKK (MAPK kinase) family. MKK3 and MKK6 phosphorylate p38, MKK7 phosphorylates JNK, and MKK4 can phosphorylate both targets. These MKKs in turn are activated by phosphorylation of conserved serine and threonine residues [1]. Several MKK-activating MKKKs (MKK kinases) have been identified [1], some of which, including ASK1 (apoptosis signalregulating kinase 1), TAK1 (transforming-growth-factor-β-activated kinase 1), MEKK1 (MAPK/extracellular-signal-regulated kinase kinase kinase 1) and MLK3 (mixed-lineage kinase 3) are also activated by phosphorylation [3–7].
Figure 1. PP2Cϵ associates with ASK1.
(A) Schematic diagram showing the SAPK signalling pathways. ATF2, activating transcription factor 2; MLK, mixed lineage kinase. (B) HEK-293 cells were transiently transfected with an expression plasmid encoding Myc–TAK1 or ASK1–Myc in the presence or absence of an expression plasmid encoding HA–PP2Cϵ. Proteins were immunoprecipitated (IP) from cell lysates with an anti-HA antibody (αHA); precipitates were immunoblotted (WB) with an anti-Myc antibody (αMyc). The cell lysates were also immunoblotted with anti-Myc or anti-HA antibodies. The results are from one of two reproducible experiments. (C) Proteins immunoprecipitated (IP) from mouse brain cell extracts with normal rabbit IgG (Cont) or an anti-PP2Cϵ antibody (αPP2Cϵ) were immunoblotted (WB) with an anti-ASK1 antibody (αASK1). The results are from one of two reproducible experiments.
In the absence of an activating signal, the constituents of the SAPK cascade return to an inactive dephosphorylated state, suggesting a role for phosphatases in the regulation of the SAPK pathway. Protein phosphatases are classified into three subgroups based on phospho-amino-acid specificity: PPs (protein serine/threonine phosphatases), protein serine/threonine/tyrosine phosphatases [DSPs (dual-specificity phosphatases)] and PTPs (protein tyrosine phosphatases). Dephosphorylation of SAPK signalling pathway components on serine/threonine and threonine/tyrosine residues requires the participation of multiple phosphatase subtypes. JNK and p38 can both be dephosphorylated by PTPs and DSPs [8]. Both p38 and MKK4 can be dephosphorylated by PP2Cα, a member of the PP2C family [9]. The PP2C family contains at least 12 different gene products: PP2Cα, PP2Cβ, PP2Cγ/FIN13, PP2Cδ/ILKAP, PP2Cϵ, PP2Cζ, PP2Cη, Wip1, CaMKPase/hFEM2/POPX2, CaMKP-N, NERPP-2C and SCOP/PHLPP [10–34]. In the regulation of MKKKs, both PP2Cβ and PP2Cϵ associate with and dephosphorylate TAK1 directly in quiescent cells [30,35]. Interestingly, PP2Cϵ transiently dissociates from TAK1 upon IL-1 (interleukin-1) treatment, suggesting that PP2Cϵ associates with and dephosphorylates TAK1 to maintain the TAK1 signalling pathway in an inactive state [30]. In an examination of the role of PP2Cϵ in the regulation of MKKKs, we observed that ASK1 expressed in HEK-293 cells (human embryonic kidney cells) had an affinity for PP2Cϵ that was similar to that of TAK1 (J.-i. Saito, T. Kobayashi and S. Tamura, unpublished work).
ASK1 is activated in response to various cytotoxic stresses, including H2O2, Fas ligation, TNF (tumour necrosis factor), serum withdrawal and antitumour reagents [36–40]. ASK1 kinase activity is tightly regulated within cells. Under non-stressed conditions, ASK1 is inhibited by association with its physiological inhibitor Trx (thioredoxin) [41]. Upon exposure of cells to H2O2 or TNFα, ROS (reactive oxygen species)-dependent oxidation of Trx induces dissociation of ASK1 from Trx, allowing the activation of the kinase by autophosphorylation at Thr845 [7]. Morita et al. [42] have demonstrated that PP5, a member of the PP family, could dephosphorylate ASK1 at Thr845. PP5 also associates with ASK1 following H2O2 or TNFα treatment, suggesting that PP5 participates in the feedback inhibition of ASK1 activity.
In the present study, we have determined the role of PP2Cϵ in the regulation of ASK1 activity. We have also investigated the difference in the roles of PP2Cϵ and PP5 in the regulation of ASK1 activity. We provide evidence that PP2Cϵ negatively regulates ASK1 in a manner different from that of PP5.
EXPERIMENTAL
Materials
Restriction enzymes and DNA-modifying enzymes used for DNA manipulation were obtained from TaKaRa. Human TNFα and Lipofectamine™ were purchased from Techne and Gibco BRL respectively. The luciferase and β-galactosidase enzyme assay systems were supplied by Promega. Glutathione–Sepharose-4B, Protein G–agarose beads, NHS (N-hydroxysuccinimido)-activated Sepharose 4B, PVDF membranes, ECL® (enhanced chemiluminesence) plus kits and [γ-32P]ATP were obtained from GE Healthcare. Anti-HA (haemagglutinin) and anti-FLAG antibodies were purchased from Roche Molecular Biochemicals and Sigma–Aldrich respectively. Anti-(phospho-JNK), anti-(phospho-p38), anti-(phospho-MKK4), anti-(phospho-MKK3/6) and anti-[phospho-ASK(Thr845)] antibodies and HRP (horseradish peroxidase)-conjugated secondary antibodies were obtained from Cell Signaling Technology. Anti-Myc and anti-ASK1 antibodies were from Santa Cruz Biotechnology. All other reagents were purchased from Wako Pure Chemicals.
Construction of expression plasmids
Full-length cDNA clones encoding mouse PP2Cϵ and a dominant-negative point mutant [PP2Cϵ(DA)] were prepared as described previously [30]. The preparation of expression plasmids encoding ASK1 and TAK1 has also been described previously [30,41,43]. To express MKK6, p38 and PP2Cϵ in bacteria, cDNAs encoding these proteins were subcloned into the pGEX vector (GE Healthcare) to generate GST (glutathione S-transferase) fusion proteins.
Cell culture and transfection
HEK-293 cells were grown in Dulbecco's modified Eagle's medium (Gibco BRL) supplemented with 10% (v/v) fetal bovine serum. At approx. 80% confluency, cells in 12-well plates were transfected with 1 μg of total DNA per well using Lipofectamine™. After transfection, cells were cultured for 24–48 h before harvesting.
Production of an anti-PP2Cϵ antibody
The pGEX-4T construct encoding GST–PP2Cϵ was transfected into Escherichia coli BL21 cells, and the transformant was grown in the presence of 0.1 mM IPTG (isopropyl β-D-thiogalactoside) for 3 h at 30 °C. The cells were harvested and lysed by sonication, and GST–PP2Cϵ was collected using glutathione–Sepharose beads. The PP2Cϵ portion was then cleaved from the GST carrier by incubation with the site-specific protease thrombin (25 units/ml) for 1 h at 25 °C. Purified protein (1 mg) was used to immunize rabbits. The PP2Cϵ antiserum generated was affinity-purified by NHS-activated Sepharose 4B covalently coupled with PP2Cϵ.
Immunoprecipitation and immunoblot analysis
After two washes in PBS, cells transfected with the indicated expression plasmids were lysed in ice-cold lysis buffer [20 mM Tris/HCl (pH 7.5), 1% (v/v) Triton X-100, 150 mM NaCl, 1 mM EGTA, 1 mM sodium orthovanadate, 50 mM NaF, 1 mM dithiothreitol and 1 mM PMSF]. Proteins were immunoprecipitated using the indicated antibodies and Protein G–agarose beads. Immunoprecipitates were subjected to SDS/PAGE [10% (w/v) gels] and transferred on to PVDF membranes. Membranes were incubated with the indicated primary antibodies for 1 h at 25 °C, treated with HRP-conjugated secondary antibodies for 1 h at 25 °C and visualized using an ECL® plus kit.
In vitro association assay
HEK-293 cells were transfected with empty vector or the expression plasmid encoding ASK1–FLAG and cultured for 48 h at 37 °C before harvesting. ASK1–FLAG in cell lysates was immunopurified using Protein G–agarose beads with an anti-FLAG antibody. ASK1-bound Protein G–agarose beads were incubated with recombinant PP2Cϵ for 2 h at 4 °C. The association of PP2Cϵ with ASK1 in vitro was identified by Western blot analysis of proteins bound to the Protein G–agarose beads.
Reporter gene assay
Reporter gene activity assays were performed as described previously [30]. AP-1 (activator protein 1)-dependent transcriptional activity was measured using the pGL3-AP-1/luciferase reporter gene. Luciferase activity was determined using the Luciferase Assay System (Promega). A β-galactosidase reporter plasmid, in which expression of the enzyme was controlled by the β-actin promoter, was co-transfected to facilitate normalization of transfection efficiencies.
In vitro coupled kinase assay
Following expression in HEK-293 cells, ASK1 was immunoprecipitated from cell lysates. The precipitated proteins were incubated with 40 μl of pre-incubation mixture [50 mM Tris/HCl (pH 7.5), 0.1% (v/v) 2-mercaptoethanol, 1 μM microcystin-LR, 0.1 mM sodium orthovanadate, 0.1 mM EGTA, 10 mM MgCl2, 0.1 mg/ml BSA, 7.5 μg/ml recombinant GST–MKK6, 100 μg/ml recombinant GST–p38 and 0.1 mM unlabelled ATP] for 15 min at 30 °C. A portion of the reaction mixture (3 μl) was then incubated with 47 μl of incubation mixture {50 mM Tris/HCl (pH 7.5), 0.1% (v/v) 2-mercaptoethanol, 1 μM microcystin-LR, 0.1 mM sodium orthovanadate, 0.1 mM EGTA, 10 mM MgCl2, 0.1 mg/ml BSA, 0.4 mg/ml MBP (myelin basic protein) and 0.1 mM [γ-32P]ATP} for 10 min at 30 °C. The reaction was terminated by applying the reaction mixture on to phosphocellulose P81 paper, followed by washing with 75 mM phosphoric acid. The radioactivity incorporated into MBP was quantified by Cerenkov counting.
RESULTS
PP2Cϵ associates with ASK1 in vivo
ASK1 expressed in HEK-293 cells was able to bind to ectopically expressed PP2Cϵ with a similar affinity as TAK1 (Figure 1B). We therefore investigated whether endogenous PP2Cϵ associated with endogenous ASK1 in cells. PP2Cϵ was immunoprecipitated from mouse brain extracts with an anti-PP2Cϵ antibody, and the immunoprecipitates were then examined by immunoblotting with an anti-ASK1 antibody. The results demonstrated that endogenous PP2Cϵ associated with ASK1 in the brain (Figure 1C).
PP2Cϵ suppresses the ASK1-induced activation of both MKK3 and MKK4
To study the effect of PP2Cϵ expression on ASK1 function, we investigated the effect of PP2Cϵ expression on ASK1-induced activation of the AP-1 reporter gene. We observed a 9-fold increase in AP-1 activity when ASK1 was expressed in HEK-293 cells (Figure 2, lane 1 compared with lane 2). PP2Cϵ expression suppressed ASK1-mediated AP-1 activation in a concentration-dependent manner (Figure 2, lanes 2–5). In contrast, expression of a dominant-negative mutant of PP2Cϵ, PP2Cϵ(DA), stimulated AP-1 activity (Figure 2, lane 2 compared with lane 6), indicating that PP2Cϵ phosphatase activity was required for the inactivation of the ASK1–AP-1 signalling pathway. These results also suggest that endogenous PP2Cϵ suppresses the ASK1 signalling pathway.
Figure 2. PP2Cϵ inhibits ASK1-induced AP-1 activation.
HEK-293 cells were transfected with an AP-1 luciferase reporter plasmid (0.1 μg/well) in combination with empty vector (0.3 μg/well; lane 1) or an expression plasmid encoding ASK1–Myc (0.3 μg/well; lanes 2–6) in the presence or absence of various amounts of expression plasmid encoding HA–PP2Cϵ (lanes 1 and 2, none; lane 3, 0.1 μg/well; lane 4, 0.2 μg/well; and lane 5, 0.3 μg/well) or HA–PP2Cϵ(DA) (lane 6, 0.3 μg/well). The luciferase activities of cell lysates were determined and normalized on the basis of β-galactosidase expression 48 h after transfection. Results are the means±S.E.M. (n=3). Cell lysates were also immunoblotted with anti-HA (αHA) or anti-Myc (αMyc) antibodies. The results are from one of three reproducible experiments. *P<0.05 and **P<0.01 compared with the control experiment in which only ASK1 was expressed.
ASK1 activates AP-1 by stimulating MKK4/7–JNK-mediated pathways [36]. ASK1 has also been reported to be responsible for activation of the MKK3/6–p38 pathway [36]. In our previous study [30], we determined that PP2Cϵ was not able to dephosphorylate either MKK3 or MMK4. On the basis of these observations, suppression by PP2Cϵ of ASK1-enhanced activation of both MKK3 and MKK4 would suggest that PP2Cϵ directly or indirectly inactivates ASK1. We expressed ASK1 alone or in combination with PP2Cϵ in HEK-293 cells and determined the phosphorylation status of endogenous MKK3 and MKK4 by immunoblot analysis using anti-(phospho-MKK3) and anti-(phospho-MKK4) antibodies respectively. The phosphorylation levels of these two signalling components were substantially decreased by the co-expression of PP2Cϵ (Figure 3), which is consistent with the hypothesis that ASK1 is the target of PP2Cϵ.
Figure 3. PP2Cϵ inhibits ASK1-enhanced phosphorylation of MKK3 and MKK4.
HEK-293 cells were transfected with empty vector or an expression plasmid encoding ASK1–Myc in the presence or absence an expression plasmid encoding HA–PP2Cϵ. Immunoblotting of cell lysates was performed using anti-(phospho-MKK3) (αpMKK3), anti-MKK3 (αMKK3), anti-(phospho-MKK4) (αpMKK4), anti-MKK4 (αMKK4), anti-Myc (αMyc) or anti-HA (αHA) antibodies. The results are from one of two reproducible experiments.
PP2Cϵ inactivates ASK1 by dephosphorylating Thr845
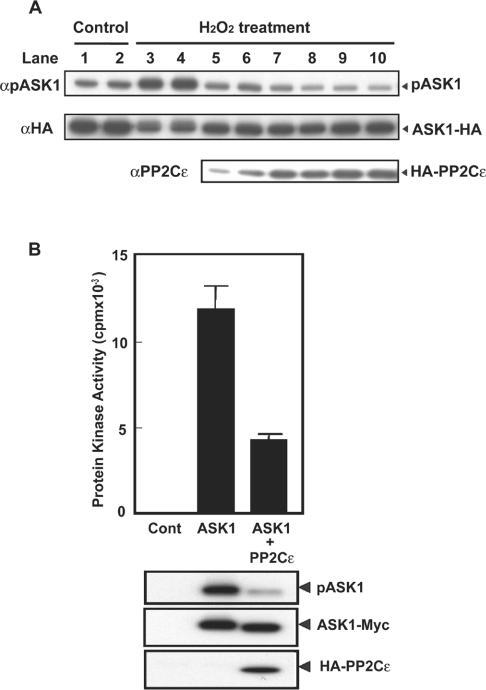
H2O2 treatment of cells activates ASK1 by inducing ASK1 autophosphorylation at Thr845 [7]. To clarify the mechanism by which PP2Cϵ suppresses ASK1 signalling pathway in more detail, we examined the effect of PP2Cϵ expression on the H2O2-enhanced phosphorylation of ASK1 at Thr845. H2O2 treatment of HEK-293 cells enhanced exogenous ASK1 phosphorylation at Thr845 (Figure 4A, lanes 1 and 2 compared with lanes 3 and 4). HA–PP2Cϵ expression decreased Thr845 phosphorylation in a concentration-dependent manner (Figure 4A, lanes 3–10).
Figure 4. PP2Cϵ induces decreased phosphorylation of ASK1 at Thr845.
(A) HEK-293 cells (5×105 cells/well) were transfected with an expression plasmid encoding ASK1–HA (0.3 μg/well) in the presence or absence of various amounts of an expression plasmid encoding HA–PP2Cϵ (lanes 1–4, none; lane 5, 0.05 μg/well; lane 6, 0.1 μg/well; lane 7, 0.2 μg/well; lane 8, 0.3 μg/well; lane 9, 0.4 μg/well; and lane 10, 0.5 μg/well). After treatment of cells with 1 mM H2O2 for 15 min, cell lysates were analysed by immunoblot analyses with anti-[phospho-ASK(Thr845)] (αpASK1), anti-HA (αHA) or anti-PP2Cϵ (αPP2Cϵ) antibodies. The results are from one of three reproducible experiments. (B) HEK-293 cells were transfected with an expression plasmid encoding ASK1–Myc in the presence or absence of an expression plasmid encoding HA–PP2Cϵ. ASK1–Myc from cell lysates was immunoprecipitated with an anti-Myc antibody, and isolated proteins were then analysed by an in vitro kinase assay (top panel). Results are the means±S.E.M. (n=3). Cell lysates were also immunoblotted with anti-[phospho-ASK(Thr845)], anti-Myc or anti-PP2Cϵ antibodies. The results are from one of two reproducible experiments.
To determine whether ASK1 activity decreased in parallel with the PP2Cϵ-induced decrease in Thr845 phosphorylation, we expressed ASK1–FLAG in HEK-293 cells in the presence or absence of HA–PP2Cϵ. Exogenous ASK1 was then immunoprecipitated with an anti-FLAG antibody. An in vitro kinase assay of the immunoprecipitates using MKK6 as substrate indicated that PP2Cϵ expression resulted in a 65% decrease in ASK1 activity (Figure 4B). In addition to the decrease in ASK1 activity, Thr845 phosphorylation was decreased by approx. 65% following PP2Cϵ expression (Figure 4B).
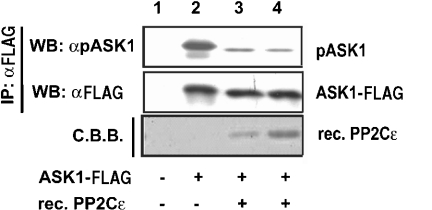
We next determined whether PP2Cϵ was able to dephosphorylate ASK1 Thr845 directly in vitro. Following expression in HEK-293 cells, ASK1–FLAG was immunoprecipitated with an anti-Flag antibody. Immunopurified ASK1–FLAG was then incubated in vitro with bacterially expressed PP2Cϵ. Immunoblotting with the anti-[phospho-ASK(Thr845)] antibody demonstrated that PP2Cϵ was able to dephosphorylate Thr845 directly (Figure 5).
Figure 5. PP2Cϵ directly dephosphorylates ASK1 in vitro.
HEK-293 cells were transiently transfected with an expression plasmid encoding ASK1–FLAG. Exogenously expressed ASK–FLAG was then immunoprecipitated (IP) with an anti-FLAG antibody (αFLAG) and incubated with various amounts of recombinant PP2Cϵ (rec. PP2Cϵ; lanes 1 and 2, none; lane 3, 0.1 μg; and lane 4, 0.3 μg) in vitro. Incubation mixtures were immunoblotted (WB) with anti-[phospho-ASK(Thr845)] (αpASK1) or anti-FLAG (αFLAG) antibodies. Coomassie Brilliant Blue (C.B.B.) staining of recombinant PP2Cϵ following blotting on to PVDF membranes is also shown (bottom panel). The results are from one of two reproducible experiments.
H2O2 induces transient dissociation of PP2Cϵ from ASK1
Morita et al. [42] have demonstrated that ASK1 is negatively regulated by PP5. In a manner similar to that demonstrated for PP2Cϵ, PP5 dephosphorylated ASK1 at Thr845. Expression of PP5, however, had a minimal effect on the basal or H2O2-induced activity of ASK1. ASK1 activity, however, decreased gradually with time in cells expressing exogenous PP5. The time course of ASK1 inactivation correlated well with that of ASK1–PP5 association, suggesting that PP5 participates in negative-feedback regulation of the ASK1 signalling pathway [42]. The H2O2-induced association of PP5 with ASK1 was observed in both HeLa and HEK-293 cells [42].
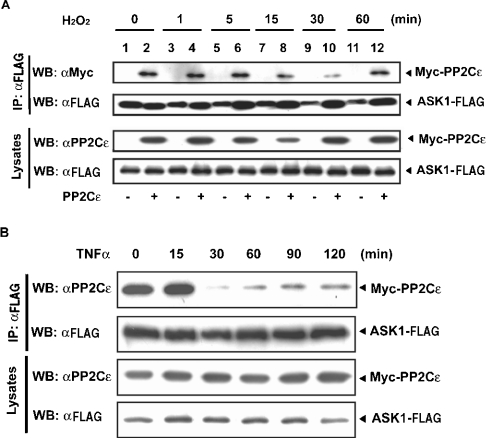
The association of PP2Cϵ with ASK1 under non-stressed conditions prompted us to determine whether PP2Cϵ behaves differently from PP5 following H2O2 treatment of cells. We expressed ASK1–FLAG alone or ASK1–FLAG and Myc–PP2Cϵ together in HEK-293 cells prior to treatment with 1 mM H2O2. We observed a stable association of PP2Cϵ with ASK1 until 5 min after H2O2 treatment (Figure 6A, lanes 2, 4 and 6), followed by transient dissociation of PP2Cϵ from ASK1 (Figure 6A, lanes 8, 10 and 12). The dissociation reached a maximum at 30 min after treatment (Figure 6A, lane 10); re-association was observed by 60 min after treatment (Figure 6A, lane 12). These results indicate that PP2Cϵ and PP5 behave differently upon H2O2 stimulation of cells.
Figure 6. H2O2 induces the transient dissociation of PP2Cϵ from ASK1.
(A) HEK-293 cells were transiently transfected with an expression plasmid encoding ASK1–FLAG alone (lanes 1, 3, 5, 7, 9 and 11) or together with an expression plasmid for Myc–PP2Cϵ (lanes 2, 4, 6, 8, 10 and 12). Transfectants were cultured for 48 h, and then treated with 1 mM H2O2 for the indicated times. Exogenously expressed ASK1–FLAG was immunoprecipitated (IP) with an anti-FLAG antibody (αFLAG); immunoprecipitates were immunoblotted (WB) with anti-Myc (αMyc) or anti-FLAG (αFLAG) antibodies. Cell lysates were also immunoblotted with anti-PP2Cϵ (αPP2Cϵ) or anti-FLAG (αFLAG) antibodies. The results are from one of three reproducible experiments. (B) HEK-293 cells were transiently transfected with expression plasmids encoding ASK1–FLAG and Myc–PP2Cϵ, cultured for 48 h and treated with 50 ng/ml TNFα for the indicated times. Exogenously expressed ASK1–FLAG was immunoprecipitated (IP) with an anti-FLAG antibody (αFLAG); the resulting immunoprecipitates were immunoblotted (WB) with anti-PP2Cϵ (αPP2Cϵ) or anti-FLAG (αFLAG) antibodies. Cell lysates were also immunoblotted with anti-PP2Cϵ or anti-FLAG antibodies. The results are from one of two reproducible experiments.
ASK1 is also activated by TNFα stimulation of cells [41]. Therefore we determined whether the transient dissociation of PP2Cϵ from ASK1 in HEK-293 cells was also observed following treatment with TNFα. The results indicated that TNFα also induced the transient dissociation of PP2Cϵ from ASK1 (Figure 6B), although the rate of re-association was lower than that seen in cells treated with H2O2.
DISCUSSION
In the present study, we have demonstrated that exogenous PP2Cϵ associated with exogenous ASK1 in HEK-293 cells. This interaction resulted in the inactivation of ASK1 by decreasing Thr845 phosphorylation under non-stressed conditions. PP2Cϵ inactivated ASK1 directly by dephosphorylating Thr845 of ASK1, as confirmed by in vitro assays. In addition, PP2Cϵ transiently dissociated from ASK1 upon H2O2 treatment of cells. Collectively, these results indicate that PP2Cϵ maintains ASK1 in an inactive state by dephosphorylating ASK1 in quiescent cells. These observations are consistent with the hypothesis that PP2Cϵ and PP5 play different physiological roles in the negative regulation of ASK1.
We have demonstrated previously [30] that PP2Cϵ directly associates with and dephosphorylates TAK1 in quiescent cells, transiently dissociating from TAK1 upon IL-1 treatment. In the present study, we have established that PP2Cϵ dissociated transiently from ASK1 upon both H2O2 and TNFα treatment of cells (Figure 6). These observations raise the possibility that the broad substrate specificity of PP2Cϵ for MKKKs contributes to the generalized maintenance of MKKKs in an inactive state in quiescent cells. Supporting this conclusion is the observation that exogenous MEKK1 expressed in HEK-293 cells could be coimmunoprecipitated with exogenous PP2Cϵ (J.-i. Saito, T. Kobayashi and S. Tamura, unpublished work). Dissociation of PP2Cϵ from MKKKs may be a cellular mechanism conserved throughout the stress- or pro-inflammatory-cytokine-induced activation of multiple MKKKs.
The conclusion that PP2Cϵ participates in maintaining ASK1 in an inactive state in quiescent cells is reminiscent of the role of Ptc1, a yeast orthologue of mammalian PP2C, in the osmotic stress response of the yeast Saccharomyces cerevisiae [44]. Three Ptc family members, Ptc1, Ptc2 and Ptc3, have been implicated in the regulation of the MAPK Hog1 signalling pathway after the exposure of cells to high-osmolarity environments [44–47]. Deletion of PTC1 led to a high basal activity of Hog1, indicating that Ptc1 is responsible for maintaining the Hog1 pathway in an inactive state under non-stressed conditions [44]. In contrast, deletion of PTC2 and PTC3 led to increased Hog1 activation upon osmotic stress than was observed in wild-type strains, although no obvious change in basal Hog1 activity was observed [47]. These observations indicate that, in contrast with Ptc1, Ptc2 and Ptc3 limit maximal Hog1 activity. Similarly to Ptc1 in the yeast Hog1 system, PP2Cϵ participates in maintaining ASK1 in an inactive state in quiescent cells. The molecular mechanisms governing the regulation of maximal ASK1 activation following H2O2 treatment are not well understood. It would be interesting to test whether other PP2C family members, similarly to Ptc2 and Ptc3 in the yeast Hog1 pathway, are involved in the regulation of maximal ASK1 activation upon H2O2 treatment of cells.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Davis R. J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 2.Nebreda A. R., Porras A. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 2000;25:257–260. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- 3.Deak J. C., Templeton D. J. Regulation of the activity of MEK kinase 1 (MEKK1) by autophosphorylation within the kinase activation domain. Biochem. J. 1997;322:185–192. doi: 10.1042/bj3220185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Posas F., Saito H. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO. J. 1998;17:1385–1394. doi: 10.1093/emboj/17.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishimoto K., Matsumoto K., Ninomiya-Tsuji J. TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J. Biol. Chem. 2000;275:7359–7364. doi: 10.1074/jbc.275.10.7359. [DOI] [PubMed] [Google Scholar]
- 6.Leung I. W., Lassam N. The kinase activation loop is the key to mixed lineage kinase-3 activation via both autophosphorylation and hematopoietic progenitor kinase 1 phosphorylation. J. Biol. Chem. 2001;276:1961–1967. doi: 10.1074/jbc.M004092200. [DOI] [PubMed] [Google Scholar]
- 7.Tobiume K., Saitoh M., Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J. Cell. Physiol. 2002;191:95–104. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- 8.Tamura S., Hanada M., Ohnishi M., Katsura K., Sasaki M., Kobayashi T. Regulation of stress-activated protein kinase signaling pathways by protein phosphatases. Eur. J. Biochem. 2002;269:1060–1066. doi: 10.1046/j.0014-2956.2002.02754.x. [DOI] [PubMed] [Google Scholar]
- 9.Takekawa M., Maeda T., Saito H. Protein phosphatase 2Cα inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J. 1998;17:4744–4752. doi: 10.1093/emboj/17.16.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura S., Li M. G., Komaki K., Sasaki M., Kobayashi T. Roles of mammalian protein phosphatase 2C family members in the regulation of cellular functions. In: Arino J., Alexander D. R., editors. Topics in Current Genetics: Protein Phosphatases, vol. 5. Heidelberg: Springer-Verlag; 2004. pp. 91–105. [Google Scholar]
- 11.Tamura S., Lynch K. R., Larner J., Fox J., Yasui A., Kikuchi K., Suzuki Y., Tsuiki S. Molecular cloning of rat type 2C (IA) protein phosphatase mRNA. Proc. Natl. Acad. Sci. U.S.A. 1989;86:1796–1800. doi: 10.1073/pnas.86.6.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann D. J., Campbell D. G., McGowan C. H., Cohen P. T. Mammalian protein serine/threonine phosphatase 2C: cDNA cloning and comparative analysis of amino acid sequences. Biochim. Biophys. Acta. 1992;1130:100–104. doi: 10.1016/0167-4781(92)90471-b. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto T., Fiorenza M. T., Zhao Y., Hasuike S., Westphal H. Molecular cloning and expression analysis of MPPα-2, a novel mouse transcript detected in a differential screen of pituitary libraries. Biochim. Biophys. Acta. 2002;1577:109–112. doi: 10.1016/s0167-4781(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 14.Wenk J., Trompeter H. I., Pettrich K. G., Cohen P. T., Campbell D. G., Mieskes G. Molecular cloning and primary structure of a protein phosphatase 2C isoform. FEBS Lett. 1992;297:135–138. doi: 10.1016/0014-5793(92)80344-g. [DOI] [PubMed] [Google Scholar]
- 15.Terasawa T., Kobayashi T., Murakami T., Ohnishi M., Kato S., Tanaka O., Kondo H., Yamamoto H., Takeuchi T., Tamura S. Molecular cloning of a novel isotype of Mg2+-dependent protein phosphatase β (type 2Cβ) enriched in brain and heart. Arch. Biochem. Biophys. 1993;307:342–349. doi: 10.1006/abbi.1993.1598. [DOI] [PubMed] [Google Scholar]
- 16.Kato S., Kobayashi T., Terasawa T., Ohnishi M., Sasahara Y., Kanamaru R., Tamura S. The cDNA sequence encoding mouse Mg2+-dependent protein phosphatase α. Gene. 1994;145:311–312. doi: 10.1016/0378-1119(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 17.Kato S., Terasawa T., Kobayashi T., Ohnishi M., Sasahara Y., Kusuda K., Yanagawa Y., Hiraga A., Matsui Y., Tamura S. Molecular cloning and expression of mouse Mg2+-dependent protein phosphate β-4 (type 2Cβ4) Arch. Biochem. Biophys. 1995;318:387–393. doi: 10.1006/abbi.1995.1244. [DOI] [PubMed] [Google Scholar]
- 18.Marley A. E., Kline A., Crabtree G., Sullivan J. E., Beri R. K. The cloning expression and tissue distribution of human PP2Cβ. FEBS Lett. 1998;431:121–124. doi: 10.1016/s0014-5793(98)00708-x. [DOI] [PubMed] [Google Scholar]
- 19.Seroussi E., Shani N., Ben-Meir D., Chajut A., Divinski I., Faier S., Gery S., Karby S., Kariv-Inbal Z., Sella O., et al. Uniquely conserved non-translated regions are involved in generation of the two major transcripts of protein phosphatase 2Cβ. J. Mol. Biol. 2001;312:439–451. doi: 10.1006/jmbi.2001.4967. [DOI] [PubMed] [Google Scholar]
- 20.Guthridge M. A., Bellosta P., Tavoloni N., Basilico C. FIN13, a novel growth factor-inducible serine-threonine phosphatase which can inhibit cell cycle progression. Mol. Cell. Biol. 1997;17:5485–5498. doi: 10.1128/mcb.17.9.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travis S. M., Welsh M. J. PP2Cγ: a human protein phosphatase with a unique acidic domain. FEBS Lett. 1997;412:415–419. doi: 10.1016/s0014-5793(97)00837-5. [DOI] [PubMed] [Google Scholar]
- 22.Tong Y., Quirion R., Shen S. H. Cloning and characterization of a novel mammalian PP2C isozyme. J. Biol. Chem. 1998;273:35282–35290. doi: 10.1074/jbc.273.52.35282. [DOI] [PubMed] [Google Scholar]
- 23.Leung-Hagesteijn C., Mahendra A., Naruszewicz I., Hannigan G. E. Modulation of integrin signal transduction by ILKAP, a protein phosphatase 2C associating with the integrin-linked kinase, ILK1. EMBO J. 2001;20:2160–2170. doi: 10.1093/emboj/20.9.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiscella M., Zhang H., Fan S., Sakaguchi K., Shen S., Mercer W. E., Vande Woude G. F., O'Connor P. M., Appella E. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6048–6053. doi: 10.1073/pnas.94.12.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitani T., Ishida A., Okuno S., Takeuchi M., Kameshita I., Fujisawa H. Molecular cloning of Ca2+/calmodulin-dependent protein kinase phosphatase. J. Biochem. (Tokyo) 1999;125:1022–1028. doi: 10.1093/oxfordjournals.jbchem.a022381. [DOI] [PubMed] [Google Scholar]
- 26.Tan K. M., Chan S. L., Tan K. O., Yu V. C. The Caenorhabditis elegans sex-determining protein FEM-2 and its human homologue, hFEM-2, are Ca2+/calmodulin-dependent protein kinase phosphatases that promote apoptosis. J. Biol. Chem. 2001;276:44193–44202. doi: 10.1074/jbc.M105880200. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi M., Ishida A., Kameshita I., Kitani T., Okuno S., Fujisawa H. Identification and characterization of CaMKP-N, nuclear calmodulin-dependent protein kinase phosphatase. J. Biochem. 2001;130:833–840. doi: 10.1093/oxfordjournals.jbchem.a003055. [DOI] [PubMed] [Google Scholar]
- 28.Koh C. G., Tan E. J., Manser E., Lim L. The p21-activated kinase PAK is negatively regulated by POPX1 and POPX2, a pair of serine/threonine phosphatases of the PP2C family. Curr. Biol. 2002;12:317–321. doi: 10.1016/s0960-9822(02)00652-8. [DOI] [PubMed] [Google Scholar]
- 29.Labes M., Roder J., Roach A. A novel phosphatase regulating neurite extension on CNS inhibitors. Mol. Cell. Neurosci. 1998;12:29–47. doi: 10.1006/mcne.1998.0692. [DOI] [PubMed] [Google Scholar]
- 30.Li M. G., Katsura K., Nomiyama H., Komaki K., Ninomiya-Tsuji J., Matsumoto K., Kobayashi T., Tamura S. Regulation of the interleukin-1-induced signaling pathways by a novel member of the protein phosphatase 2C family (PP2Cϵ) J. Biol. Chem. 2003;278:12013–12021. doi: 10.1074/jbc.M211474200. [DOI] [PubMed] [Google Scholar]
- 31.Kashiwaba M., Katsura K., Ohnishi M., Sasaki M., Tanaka H., Nishimune Y., Kobayashi T., Tamura S. A novel protein phosphatase 2C family member (PP2Cζ) is able to associate with ubiquitin conjugating enzyme 9. FEBS Lett. 2003;538:197–202. doi: 10.1016/s0014-5793(03)00153-4. [DOI] [PubMed] [Google Scholar]
- 32.Komaki K., Katsura K., Ohnishi M., Li M. G., Sasaki M., Watanabe M., Kobayashi T., Tamura S. Molecular cloning of PP2Cη, a novel member of the protein phosphatase 2C family. Biochim. Biophys. Acta. 2003;1630:130–137. doi: 10.1016/j.bbaexp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu K., Okada M., Takano A., Nagai K. SCOP, a novel gene product expressed in a circadian manner in rat suprachiasmatic nucleus. FEBS Lett. 1999;458:363–369. doi: 10.1016/s0014-5793(99)01190-4. [DOI] [PubMed] [Google Scholar]
- 34.Gao T., Furnari F., Newton A. C. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis and suppresses tumor growth. Mol. Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Hanada M., Ninomiya-Tsuji J., Komaki K., Ohnishi M., Katsura K., Kanamaru R., Matsumoto K., Tamura S. Regulation of the TAK1 signaling pathway by protein phosphatase 2C. J. Biol. Chem. 2001;276:5753–5759. doi: 10.1074/jbc.M007773200. [DOI] [PubMed] [Google Scholar]
- 36.Ichijo H., Nishida E., Irie K., ten Dijke P., Saitoh M., Moriguchi T., Takagi M., Matsumoto K., Miyazono K., Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 37.Tobiume K., Inage T., Takeda K., Enomoto S., Miyazono K., Ichijo H. Molecular cloning and characterization of the mouse apoptosis signal-regulating kinase 1. Biochem. Biophys. Res. Commun. 1997;239:905–910. doi: 10.1006/bbrc.1997.7580. [DOI] [PubMed] [Google Scholar]
- 38.Chang H. Y., Nishitoh H., Yang X., Ichijo H., Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- 39.Gotoh Y., Cooper J. A. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-α signal transduction. J. Biol. Chem. 1998;273:17477–17482. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- 40.Nishitoh H., Saitoh M., Mochida Y., Takeda K., Nakano H., Rothe M., Miyazono K., Ichijo H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 41.Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morita K., Saitoh M., Tobiume K., Matsuura H., Enomoto S., Nishitoh H., Ichijo H. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 2001;20:6028–6036. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanada M., Kobayashi T., Ohnishi M., Ikeda S., Wang H., Katsura K., Yanagawa Y., Hiraga A., Kanamaru R., Tamura S. Selective suppression of stress-activated protein kinase pathway by protein phosphatase 2C in mammalian cells. FEBS Lett. 1998;437:172–176. doi: 10.1016/s0014-5793(98)01229-0. [DOI] [PubMed] [Google Scholar]
- 44.Warmka J., Hanneman J., Lee J., Amin D., Ota I. Ptc1, a type 2C Ser/Thr phosphatase, inactivates the HOG pathway by dephosphorylating the mitogen-activated protein kinase Hog1. Mol. Cell. Biol. 2001;21:51–60. doi: 10.1128/MCB.21.1.51-60.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maeda T., Tsai A. Y., Saito H. Mutations in a protein tyrosine phosphatase gene (PTP2) and a protein serine/threonine phosphatase gene (PTC1) cause a synthetic growth defect in Saccharomyces cerevisiae. Mol. Cell. Biol. 1993;13:5408–5417. doi: 10.1128/mcb.13.9.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maeda T., Wurgler-Murphy S. M., Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- 47.Young C., Mapes J., Hanneman J., Al-Zarban S., Ota I. Role of Ptc2 type 2C Ser/Thr phosphatase in yeast high-osmolarity glycerol pathway inactivation. Eukaryotic Cell. 2002;1:1032–1040. doi: 10.1128/EC.1.6.1032-1040.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]