Abstract
Microencapsulation offers a unique potential for high cell density, high productivity mammalian cell cultures. However, for successful exploitation there is the need for microcapsules of defined size, properties and mechanical stability. Four types of alginate/poly-l-Lysine microcapsules, containing recombinant CHO cells, have been investigated: (a) 800 μm liquid core microcapsules, (b) 500 μm liquid core microcapsules, (c) 880 μm liquid core microcapsules with a double PLL membrane and (d) 740 μm semi-liquid core microcapsules. With encapsulated cells a reduced growth rate was observed, however this was accompanied by a 2–3 fold higher specific production rate of the recombinant protein. Interestingly, the maximal intracapsular cell concentration was only 8.7 × 107 cell mL-1, corresponding to a colonization of 20% of the microcapsule volume. The low level of colonization is unlikely to be due to diffusional limitations since reduction of microcapsule size had no effect. Measurement of cell leaching and mechanical properties showed that liquid core microcapsules are not suitable for continuous long-term cultures (>1 month). By contrast semi-liquid core microcapsules were stable over long periods with a constant level of cell colonization (ϕ = 3%). This indicates that the alginate in the core plays a predominant role in determining the level of microcapsule colonization. This was confirmed by experiments showing reduced growth rates of batch suspension cultures of CHO cells in medium containing dissolved alginate. Removal of this alginate would therefore be expected to increase microcapsule colonization.
Keywords: Alginate; Animal cell culture; Mechanical resistance; Microencapsulation, Perfusion culture; Poly-l-lysine
Introduction
The use of animal cell cultures is presently the only way to produce highly glycosylated recombinant proteins and antibodies for medical applications (Duff 1985). However, by comparison with yeast and bacteria, mammalian cells are relatively fragile and, as a result, high cell density cultures may be limited by mechanical and shear forces resulting from agitation and aeration (Papoutsakis 1991). As a result the specific productivity and maximum cell density achieved are generally low (1–10 pg cell−1 h−1 and 1–5 × 106 cells mL−1 respectively). Thus, the only way to increase productivity is to increase the cell density in the culture by the use of perfusion cultures with cell retention systems (Posillico 1986).
As animal cells have a natural tendency to aggregate, immobilization of cells in microcapsules may be a promising technique (King et al. 1987), to provide optimal-shear stress free-growth conditions. The application of microcarriers and hollow membrane fibers for the immobilization of animal cells has indeed been reported to effectively enhance growth and cell viability (Griffiths 1990), but may present diffusion limitations (Ng et al. 1995). Microencapsulation may offer an efficient protection for the cells, while retaining them in the bioreactor without the requirement for a separation device. They can be produced with a narrow size distribution (<3%), and it is possible to control the capsular membrane permeability, such that cell products may be retained or released into the medium (Thu et al. 1996a; Thu et al. 1996b). In this way it should be possible to facilitate downstream processing by concentrating the product within the microcapsules.
Several types of microcapsules have been used for mammalian cells cultures (Scheirer et al. 1984; King et al. 1987; Miller et al. 1988; Al-Rubeai et al. 1990; Yoshioka et al. 1990; Uludag et al. 1993; Heald et al. 1994; Seifert and Phillips 1997; Cruise et al. 1998; Park et al. 1998). However, it has yet to be demonstrated whether such systems offer advantages over classical suspension cultures in terms of specific growth rate or specific productivity. Another reason for the lack of successful industrial application in large-scale culture of mammalian cells (Duff 1985; Rupp 1985) is the poor mechanical stability of hydrogel microcapsules. Long-term stability is a prerequisite for microcapsules intended for bioreactor cultures or artificial organs. Cell culture media or the physiological environment surrounding microcapsules are usually rich in diverse ions, which can have an adverse effect on stability by destabilizing the polyelectrolyte interactions involved in microcapsule integrity.
In this work, the widely used alginate/poly-l-lysine/CaCl2 system has been studied as a reference for cell encapsulation. The kinetic and stoichiometric parameters of CHO cells have been determined for different kinds of microcapsules, with respect to size (500–900 μm), core structure (liquid, solid or semi-liquid) and membrane structure (single or double membrane). Attention was also focused on the variation of the burst force with time, on microcapsule integrity and on the number of cells released into the surrounding medium.
Materials and methods
Cell cultures
CHO SSF3 (suspension and serum-free adapted Chinese hamster ovary cells) was kindly supplied by Novartis (Basel, Switzerland). These cells were transfected to secrete recombinant human secretory component (rhSC), a glycoprotein of molecular weight 66 kD. Cells, from a working cell bank stored at −196 °C, were rapidly thawed at 37 °C and used to inoculate a T-flask (75 mL, Falcon, Beckton Dickinson, Basel, Switzerland) containing 15 mL of a protein-free culture medium (Chomaster HP−1, Cell Culture Technologies, Zürich, Switzerland) to an initial cell density of 2 × 105 cell mL−1. After incubation at 37 °C, under a humidified atmosphere of air containing 5% CO2, cells were harvested upon reaching a density of 106 cells mL−1 and used to inoculate a spinner flask (Integra Biosciences GmbH, Fernwald, Germany) containing 100 mL of culture medium. Upon reaching a cell density of 106 cells mL−1 the cells were harvested by centrifugation and used either to prepare microcapsules or to inoculate bioreactors.
Encapsulated cells were grown in either a 2 litre (1.5 litre working volume) standard laboratory bioreactor (Biolafitte, St-Germain-en-Laye, France) or 1 litre (500 mL working volume) spinner flasks (Integra Biosciences GmbH, Fernwald, Germany). The bioreactor was operated in batch or continuous (perfusion) mode at a temperature of 37 °C, agitation (marine turbine) of 150 rpm with bubble-free aeration controlled by the use of 1–2 m Teflon tubing (W. L. Gore & Associates GmbH, Putzbrunn, Germany). Using a recently developed regulation system (Ruffieux et al. 1998; Ducommun et al. 2000), the dissolved oxygen concentration, determined using a polarographic oxygen electrode, (Ingold, Messtechnik AG, Urdorf, Switzerland) was maintained at 80% air saturation while simultaneously enabling the on-line measurement of the specific oxygen consumption rate. The pH was maintained constant at a value of 7.2 by the automatic addition of CO2 to the inlet airflow. For perfusion cultures, the volume was maintained constant (1.5 L) by the use of a simple overflow device.
For encapsulated cell cultures, a microcapsule volume of 100 mL per 1,500 mL culture medium (ratio 1:15) was used for bioreactor cultures and a microcapsule volume of 50 mL per 500 mL medium (ratio of 1:10) was used for spinner-flask cultures.
Microcapsule formation
The encapsulation method used was a modification of the technique originally developed by Lim and Sun (Lim 1984) and was undertaken under completely sterile conditions using an encapsulation device (Model IEM®, Inotech AG, Dottikon, Switzerland) as described elsewhere (Serp et al. 2000). Alginate beads were first formed, by extrusion of a pre-sterilised (0.2 μm microfiltration) sodium alginate solution (1.5% w/v, Inotech AG, Switzerland), containing CHO cells (2 × 105 cell mL−1alginate), through the encapsulator nozzle (300 μm or 200 μm diameter) into 400 mL of an aqueous solution of CaCl2 (110 mM). After 5 min, the CaCl2 solution was removed and microcapsules were formed by the addition of 400 mL of a 0.05% (w/v) poly-l-lysine 34 kDa (PLL, Sigma, St-Louis, MO, USA) in 110 mM CaCl2 solution. After incubation for 30 min at room temperature the microcapsules were washed with saline buffer (0.9% NaCl in 10 mM MOPS, Sigma, St-Louis, MO, USA) followed by incubation in 400 mL of 0.03% (w/v) sodium alginate for 10 min. After washing with saline buffer the solid alginate core of the microcapsules was liquefied by incubation for 15 min in 0.05 M sodium citrate. Semi-liquid core microcapsules were produced as described for liquid core microcapsules however, these microcapsules were washed with a solution containing 150 mM NaCl and 0.5 mM CaCl2 in place of sodium citrate. For double membrane microcapsules, a similar procedure was used, except that in the first PLL incubation step, PLL with a Mw of 210 kDa was used for a reduced period of 7.5 min. After core liquefaction with citrate, the microcapsules were incubated for a further 7.5 min in 0.05%(w/v) of PLL 34 kDa. All of the resulting microcapsules were subsequently washed twice with saline buffer and immediately used to inoculate spinner flasks containing the standard culture medium. These cultures were grown until the cell number reached a value sufficient to inoculate a 1.5 liter bioreactor at an initial cell concentration of 5 × 104 cell mL−1reactor.
Sample analyses
Homogeneous culture samples (4 mL) containing microcapsules were regularly removed from the bioreactor/spinners cultures to determine intra/extra-capsular cell number, concentration of culture metabolites, microcapsule size and burst force.
The concentration of cells (extracapsular biomass) was determined microscopically using the Trypan blue extrusion method. After mixing with Trypan blue (80 μL sample + 20 μL Trypan-blue, 0.4%), the total and viable cell numbers were counted microscopically using a Neubauer haemocytometer. Encapsulated cells were first liberated by gentle extrusion of the microcapsules through a 300 μm capillary 3 times, in order to dissociate cell clusters. Microcapsules with a semi-liquid core were incubated for 10 min in a sodium citrate solution (0.05 M) in order to dissolve the alginate core, before disruption by extrusion through a capillary.
Glucose and lactate concentration were determined by HPLC analysis using a Supelco H column, H2SO4 0.035% as solvent and a RI detector (HP 1047 A, Hewlett Packard GmbH, Waldbronn, Germany). Ammonium was measured enzymatically using a standard method (Boehringer Mannheim GmbH, Mannheim, Germany). Secretory component (SC), was determined by an ELISA technique, as described previously (Ducommun et al. 2002).
The size of microcapsules was determined microscopically using a standard laboratory microscope (Axiolab, Carl Zeiss Jena GmbH, Jena, Germany) incorporating a digital camera (Sony CCD Iris Camera, Sony Corporation, Tokyo, Japan) coupled to a PC operating with an image analysis program (Cyberview, Cervus International, Courtaboeuf, France). A sample of thirty microcapsules was measured and the mean diameter, and standard deviation, determined. The mechanical resistance of microcapsules was determined using a Texture Analyzer (TA−2xi, Stable Micro Systems, Goldaming, England). The mechanical deformation tests were performed using a mobile probe speed of 0.05 mm sec−1 until bursting was observed. The probe area was 36.3 mm2 which enabled measurement of several microcapsules simultaneously. The average resistance of at least 200 microcapsules was determined to obtain statistically relevant data. The mean burst force is given in grams with a standard deviation of 15%. Compression plots also provide an indication of the elasticity of the membrane (strain at bursting) and the presence of gelling ions in the core.
Results and discussion
Mechanical resistance
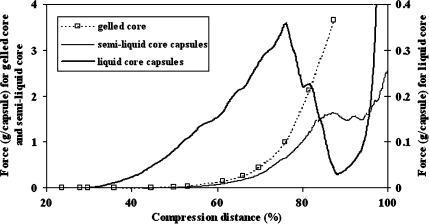
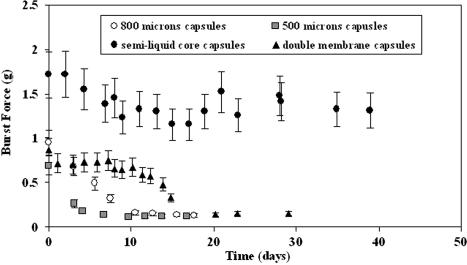
By finely regulating the calcium concentration in the medium, three types of microcapsule could be generated with the PLL/alginate/CaCl2 system: gelled core microcapsules, semi-liquid core microcapsules and liquid core microcapsules. Mechanical resistance profiles of these three types of microcapsules vary significantly (Fig 1). The core structure differs, which is due to the entrapped alginate reacting with divalent cations. When the Ca2+ concentration in the medium is above 0.1 M, microcapsules have a gelled core, and exhibit mechanical properties similar to alginate beads. However, in contrast to beads, the gelled core microcapsules have an external membrane, the permeability of which may be regulated (Goosen et al. 1985). Among the three types of microcapsules, gelled core microcapsules show the highest resistance (3.7 g capsule−1) for a compression of 88%. In the case of liquid core microcapsules, all the calcium ions were removed by washing with citrate. Consequently, bursting under pressure is observed and the mechanical resistance can thus be described by measurement of the burst force. Semi-liquid core microcapsules have a slightly gelled interior core that is formed by the presence of traces of calcium (20 mg L−1 of Ca2+) in the surrounding medium. By contrast, semi-liquid core microcapsules show a 5−fold higher resistance (at 88 % compression) and are therefore very attractive for the encapsulation of animal cells that grow in the presence of low levels of calcium. At the end of the cultures, it was observed that the hydrogel structure was partially or totally damaged (Fig. 4). This resulted in cell leaching during the stationary phase (φ max). By measuring the burst force (Fig. 2) it was determined that the liquid core microcapsule resistance decreased constantly from 0.7–1 g capsule−1 to 0.1 ± 0.02 g capsule−1 with time and the lowest limit was reached within a few days (5–10 days). The resistance of the small microcapsules (500 μm) decreased exponentially, probably due to the shorter incubation time in PLL (10 min) compared with the larger ones (800 μm, 30 min), and it was not possible to maintain the integrity of the microcapsules. The double membrane microcapsules showed a radical decrease in resistance (Fig. 2) by day 16, demonstrating that the use of two PLL solutions with different molecular weights did not result in an increased resistance.
Fig. 1.
Compression diagram for microcapsules (740 μm–800 μm) stored in three media containing increasing amounts of calcium chloride. The burst force for gelled core (medium with 4 g l−1 Ca2+) and semi-liquid core microcapsules (medium with 20 mg l−1 Ca2+) is given on the left axis and for liquid core microcapsules (medium with 0 g l−1 Ca2+) on the right axis
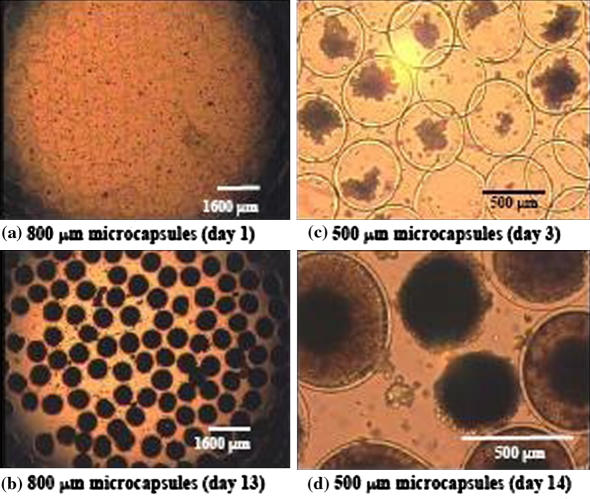
Fig. 4.
Effect of size on the growth of CHO cells in liquid core microcapsules of 800 μm (a, b) and 500 μm diameter (c, d). The capsule colonisation is given with respect to the sampling day. By day 13 (b) the larger microcapsules were homogeneously filled whereas only 36 ± 10% of the small microcapsules were filled with cells (d)
Fig. 2.
Mechanical properties of microcapsules as a function of time. The burst force is given in g per microcapsule. The two types of liquid core microcapsules (800 μm and 500 μm) were cultivated in perfusion mode in a 1.5 L reactor. The semi-liquid core microcapsules were cultivated in a 500 mL spinner-flask and the medium changed 14 times
These results confirm that stirring forces are not the only parameters influencing microcapsule stability. Hence, the constant displacement of the equilibrium between the medium and the microcapsules is destructive for alginate-PLL gels. One reason for this is the accelerated separation of the polyelectrolytes, and cation diffusion from the matrix (Schoichet et al. 1996). Partial destruction of hydrogel and polyelectrolytic complex might also be due to proteolytic enzymes released by the cells (Levy and Edwards-Levy 1996). Due to the low stability of the polyelectrolyte system with cells in ionic media, the application of such microcapsules to the cultivation of cells is limited to a duration of less than one month.
Cells growth in suspension and in liquid core microcapsules
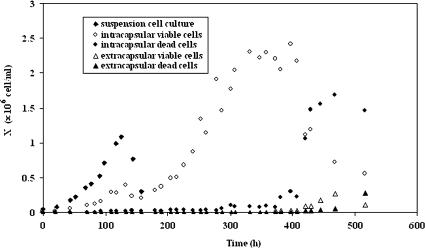
The microcapsules used in these experiments had a size of 800 or 500 μm with a liquid core, and were incubated for 4 days in a spinner-flask before being used to inoculate the bioreactor at an initial cell concentration of 5 × 104 cell mL−1reactor. Microcapsules were heavy enough not to be washed out by the overflow device. A batch suspension cell culture was performed in parallel with the same initial cell concentration (5 × 104 cell mL−1reactor) and under similar conditions. The encapsulated cells were grown in batch mode for 140 h before initiation of medium feed. For both cultures, no lag phase was observed with the cells growing exponentially immediately after inoculation (Fig. 3). Growth of encapsulated cells could be divided into four distinct phases: (1) an exponential growth phase during batch (0 h–140 h), (2) a second semi-exponential growth phase with continuous medium supply and removal (140 h–310 h), (3) a stationary phase (320 h–400 h) and (4) a decline phase (medium feed was stopped at 400 h). In contrast to the suspension cell culture (μ = 0.028 h−1, Table 1), the specific growth rate decreased with time (from 0.027 to 0.013 h−1, Table 1). In addition, the growth of encapsulated cells was linear at the end of the second growth phase. The microcapsules were completely retained within the bioreactor, becoming colonized with cells (Fig. 3) to a maximum concentration of 2.2 × 106 cell mL−1reactor (6.6 × 107 cell mL−1caps). These values are similar to those reported for other cell lines (4 × 107–108 cell mL−1)caps), (Posillico 1986; King et al. 1987). The suspension cells grew to a maximum concentration of 1.41 × 106 cell mL−1reactor and 8.5 × 105 cell mL−1reactor in the presence of dissolved alginate (Table 1), at which point all of the glucose in the medium had been consumed. Moreover, the doubling time in the presence of alginate is nearly double that found in the cell culture medium, with values of 24 and 35 h respectively. The viability of both suspension cells and encapsulated cells remained above 95% until depletion of glucose.
Fig. 3.
Growth of CHO cells in a suspension cell culture and in 800 μm microcapsules. Cultures were performed in a continuous 1.5 L bioreactor, and cell concentration is given with respect to the volume of the reactor. The initial microcapsule volume was 1/15 of the reactor volume. The inoculum concentration was 5 × 104 cell mL−1reactor for both cultures. The suspension cell culture was undertaken in batch mode. Microapsules were first cultured for 140 h in batch mode before medium feed was started
Table 1.
Kinetic and stoichiometric parameters of suspension and encapsulated CHO cells cultures
| Value | Units | Suspension cells (batch) | Suspension cells + 1.5% alginate (batch) | 800 μm microcapsules liquid core | 500 μm microcapsules liquid core |
|---|---|---|---|---|---|
| μ | (h−1) | 0.028 | 0.021 | 0.027/0.013 | 0.020/0.006 |
| Culture time | (h) | 160 | 80 | 650 | 690 |
| Xreactor, max | (cell mL−1reactor) | 1.41 × 106 | 8.5 × 105 | 2.2 × 106 | 2.9 × 106 |
| Xcaps, max | (cell mL−1caps) | – | – | 6.6 × 107 | 8.7 × 107 |
| Max cells | (cell caps−1) | – | – | 17000 | 6100 |
| Colonization (ϕ) | (%) | – | – | 17 | 20 |
| qS | (mmol cell−1 h−1) | 6.4 × 10–10 | 3.3 × 10−10 | 7.0 × 10−10 | 4.7 × 10−10 |
| qlactate | (mmol cell−1 h−1) | 1 × 10−9 | 6 × 10−10 | 9.7 × 10−10 | 7 × 10−10 |
| qO2 | (mmol cell−1 h−1) | 3.2 × 10−10 | - | - | 5.9 × 10−10 |
| qNH3 | (mmol cell−1 h−1) | 4.7 × 10−11 | - | 8.0 × 10−11 | - |
| qP | (mg cell−1 h−1) | 1.7 × 10−9 | 2.0 × 10−9 | 5.3 × 10−9 | 4.3 × 10−9 |
| YX/S | (cell mmol−1) | 4.0 × 107 | 5.9 × 107 | 7.9 × 106 | 2.3 × 107 |
| Ylactate/S | (mol mol−1) | 1.64 | 1.72 | 1.41 | 1.45 |
The suspension cells were cultivated in batch mode. The 800 μm microcapsules were incubated for 30 min in PLL and the 500 μm microcapsules for 10 min. The specific growth rate (μ) is given for the two growth phases of the perfusion cultures (first phase corresponds to the batch mode and the second phase begins when the feed is initiated). The biomass yield with respect to glucose (YX/S), corresponds to the yield during the growth phase
After 300 h the encapsulated cells reached a stationary state but continued to consume glucose (7.0 × 10−10 mmol h−1 cell−1), produce lactate (9.7 × 10−10 mmol h−1 cell−1) and produce SC (5.3 × 10−9 mg h−1 cell−1) at higher rates than suspension cells (Table 1). The yield of lactate with respect to glucose (YX/S = 1.41) was lower for encapsulated cells than for suspension cells (1.64 and 1.72 with alginate) indicating a more efficient utilization of glucose via respiration. After the stationary phase, cell viability decreased and a non-negligible fraction of cells was found in suspension. The extracapsular concentration reached 2.7 × 105 cell mL−1reactor after 3 weeks and, from the sharp increase in the concentration of free cells in suspension (Fig. 4), it can be concluded that the cells were not present at the start of the culture but were released from microcapsules.
In conclusion encapsulation permitted cell retention in the bioreactor without the need for a cell or microcapsule separation device. It proved to be efficient for reaching higher densities than batch cultures of suspension cells and the number of cells per microcapsule reached 17,000 (Table 1). An advantage of this system is that much higher cell densities may be attained in the bioreactor, greater than 107 cell mL−1reactor, simply by increasing the volume of microcapsules in the bioreactor. This assumes that there are no medium limitations, such as through perfusion operation (Seifert and Phillips 1997). Furthermore the SC productivity could be increased three-fold despite the reduced specific growth rate.
Size of microcapsules is a critical parameter for the encapsulation of micro-organisms and animal cells, since large microcapsules (>1mm) will suffer from diffusion limitations resulting in reduced cell growth and necrosis within the core (Glacken et al. 1983; Robitaille et al. 1999). Nevertheless, decreasing the microcapsule size from 800 to 500 μm did not improve colonization by cells (Table 1, Fig. 4). These unexpectedly low values of ϕ indicate that cells were not able to grow freely within the core, but the similar value of qS and qlactate suggests that there was no diffusion limitation.
The specific growth rate in the 500 μm microcapsules was lower than for the 800 μm microcapsules. Cell viability was also consistently lower (60 ± 10%) than within the 800 μm microcapsules (>95%), although the biomass yield, YX/S, was not affected by microcapsule size (3%, Table 1). By comparison to suspension cell cultures (Table 1), the 500 μm microcapsules behaved similarly to 800 μm microcapsules, with a lower specific growth rate but higher specific SC production rate (4.3 × 10−9 mg cell−1 h−1). The behaviour of encapsulated cells is similar to cells grown in suspension in the presence of alginate, since in both case the doubling time is longer and protein production higher than for simple suspension cultures. From these results, it can be deduced that alginate slows down the growth of CHO cells but does not seem to affect significantly the main metabolic pathways. This suggests that cells could not proliferate freely within the microcapsule, the result being a stimulation of metabolic activity and SC production.
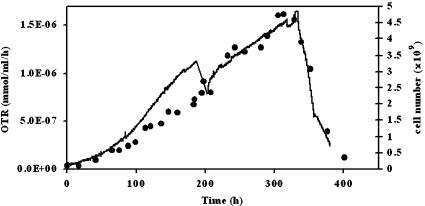
By continuous on-line monitoring of the oxygen consumption rate of the cultures it was possible to follow the growth of cells (Fig. 5). The results demonstrate that the oxygen uptake rate is an efficient way to measure encapsulated biomass with an indirect and non-destructive method. The specific oxygen uptake rate, qO2, for encapsulated cells (5.9 × 10−10 mmol cell−1 h−1) was 45% higher than for suspension cells and correlates with the higher values of YX/S and qS.
Fig. 5.
Oxygen transfer rate for a culture of 500 μm liquid core microcapsules (solid line). The growth of cells inside the microcapsules (•) can be followed online by the OTR signal. The slowing down of the growth when nutrients are depleted is directly monitored as displayed at t = 340 h. The step in the signal at t = 190 h corresponds to the removal and replacement of the whole culture medium
Double membrane microcapsules
The motivation for multiple layers is two-fold: (1) permeability and microcapsule strength may be uncoupled and (2) the formation of a first, very porous membrane may facilitate the liberation of the internal core alginate before application of subsequent layers. The latter may be important since it has been shown that cell growth may be inhibited by the reduced intracapsular volume (Chang et al. 1994). Thus, double membrane microcapsules may be an alternative for improving ϕ and for improving long-term stability (King et al. 1987). By creating more porous membranes, the rate of alginate diffusion from the core would be facilitated and would be expected to lead to high levels of colonization by providing more free volume for the cells to proliferate and thereby increase ϕ.
Multi-membrane microcapsules were cultured in 500 mL spinner flasks in repeated batch mode and compared to single membrane microcapsules of the same size and cultured under the same conditions. The specific growth rate (0.021 h−1) and the specific rates of metabolite consumption and production reached higher values (10–25%) for the double layer microcapsules (Table 2). This suggests that higher membrane permeability may offer higher rates of nutrient exchange. Interestingly, the intracapsular cell concentration (5.5 × 107 cell mL−1capsule) and ϕ (12%) of the double layer microcapsules were equal to the values obtained with the single membrane microcapsules (Table 2). This means that the final intracapsular free volume was similar for both types of microcapsules and alginate did not diffuse out to any greater extent. In conclusion, a double membrane led to an acceleration of metabolic activity but did not improve the colonization potential of the microcapsules (ϕ).
Table 2.
Kinetic and stoichiometric parameters for 500 mL spinner-flask cultures of encapsulated CHO cells. The maximum cell number (X) is given with respect to the microcapsule volume (Xcaps, max)
| Value | Units | 800 μm capsules with single PLL membrane | 800 μm capsules with a double PLL membrane | 500 μm capsules with a semi-liquid core |
|---|---|---|---|---|
| μ | (h−1) | 0.019 | 0.021 | linear |
| Culture time | (h) | 516 | 698 | 1050 |
| Xcaps, max | (cell mL−1caps) | 5.5 × 107 | 5.5 × 107 | 1.2 × 107 |
| Max. cells per capsule | (cell caps−1) | 10700 | 14700 | 2600 |
| Occupied vol. (ϕ) | (%) | 12 | 13 | 3 |
| qS | (mmol cell−1 h−1) | 4.0 × 10−10 | 4.8 × 10−10 | 3.0 × 10−10 |
| qlactate | (mmol cell−1 h−1) | 6.9 × 10−11 | 8.2 × 10−10 | 5.3 × 10−10 |
| qNH3 | (mmol cell−1 h−1) | 2.2 × 10−11 | – | – |
| qP | (mg cell−1 h−1) | 3.7 × 10−9 | 4.9 × 10−9 | 3.4 × 10−9 |
| Ylactate/S | (mol mol−1) | 1.70 | 1.75 | 1.73 |
Repeated batch cultures with semi-liquid core microcapsules
A possible approach to solve the problem of microcapsule stability may be to add low concentrations of calcium, or PLL, to the culture medium. Due to the high cost of PLL, only the influence of CaCl2 addition to the culture medium was studied. The addition of calcium to the medium results in the formation of two different types of microcapsules, gelled core and semi-gelled core (semi-liquid). The use of a gelled- core would increase the mechanical resistance of the microcapsules, however the space available for growth would be reduced and growth restricted. Furthermore the calcium concentration required (>100 mM) to achieve complete gelation would be toxic to the cells. In contrast semi-liquid core microscapsules, or partially gelled microcapsules, would have a higher mechanical resistance than liquid core microcapsules yet may be formed by the addition of only trace levels of calcium (<0.5 mM) to the medium.
Semi-liquid core microcapsules were generated using the standard encapsulation process, but the citrate treatment step was left out. The microcapsules were then stored in a saline solution (0.92% w/w) or culture medium, and remaining calcium ions were slowly replaced by Na+ present in medium. Thus, after a few days, these microcapsules would be expected to show the similar mechanical properties as ordinary microcapsules. In order to maintain microcapsule resistance, calcium chloride was added to the culture medium to a final concentration of 20 mg L−1 Ca2+.
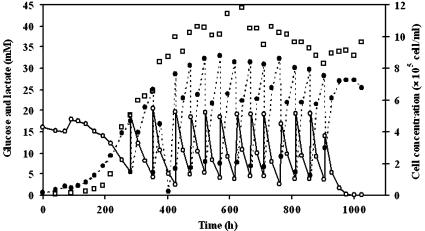
By comparison with liquid core microcapsules, cells grew linearly in the period 200 to 400 h (Fig. 6) attaining a maximal cell density of 1.2 × 106 cell mL−1reactor (1.2 × 107 cell mL−1caps) after 400 h (Table 2). The cell concentration subsequently remained in a stationary state for a further 600 h (8−fold longer than for the liquid core microcapsules) until the medium feed was stopped (Fig. 6). The cells continued to metabolize glucose and produce lactate during the stationary phase, however the specific glucose consumption rate (3.0 × 10−10 mmol h−1 cell−1) and the specific lactate production rate (5.3 × 10−10 mmol h−1 cell−1) were 40% lower than the rates observed with liquid core microcapsules (Table 1). Interestingly, the specific production rate of SC (3.4 × 10−9 mg h−1 cell−1) was still two-fold higher than that found in suspension cultures (Table 1), showing that the diffusion of SC from the microcapsules was not inhibited by the denser core matrix.
Fig. 6.
Evolution of cell number (□), glucose (○) and lactate (•) concentration for a repetitive fed-batch culture of CHO cells encapsulated in semi-liquid core microcapsules. The culture was performed in a 500 mL spinner-flask and the medium was enriched with calcium chloride (20 mg l−1 Ca2+). The cell number is given with respect to the spinner volume
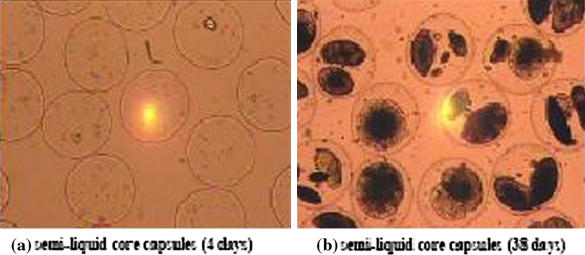
As shown in Fig. 7, the cells grew as spindle shapes and formed independent aggregates within the core. After more than 5 weeks of growth (Fig. 7B) the cells had only colonized certain regions of the core and distinct visual differences could be seen compared with liquid core microcapsules (Fig. 4). The volume fraction colonized by the cells in semi-liquid microcapsules (3%) was effectively lower than for liquid core microcapsules (ϕ = 12–20%), however neither broken microcapsules nor free cells could be detected in the culture medium.
Fig. 7.
Cells in semi-liquid core microcapsules (40 × magnification). (a) Pictures of the microcapsules after 4 days in the spinner-flask (500 mL). Cells only colonize microcapsules up to 3% even after 5 weeks of culture (b)
The resistance of the microcapsules (Fig. 2) could be maintained throughout the 7 week duration of the cultures at a value higher than 1.2 g capsule−1, with the main resistance of the microcapsule being due to the alginate core which was partially gelled. The decrease in the membrane mechanical resistance suggests that poly-l-lysine continuously diffused out into the medium during the culture.
In conclusion, the mechanical properties of alginate/poly-l-lysine microcapsules could be largely improved by adding traces (<1 ppm) of calcium ions to the medium. However, the maximum cell concentration within the microcapsules was 5−fold lower than for liquid-core microcapsules, presumably due to a reduction in available volume. The pressure of the core network did not significantly influence cell metabolism (Table 2) and cell viability (>95%). After 7 weeks the cell metabolism was similar to that after 2 weeks. The culture was deliberately arrested after 7 weeks by stopping the nutrient feed, however it is confidently felt that much longer duration cultures should be possible, with concomitant high productivity of SC. Hence, semi-liquid core microcapsules are a good alternative for encapsulating proliferating organisms, since they show much better long-term stability than liquid core microcapsules (<1 month) while not being as rigid as gelled-core microcapsules. The maximum local cell concentration in semi-liquid core microcapsules was greater than 107 cell mL−1caps thus, by increasing the volume of microcapsules in the reactor, very high cell densities should be attainable, such as using packed bed reactors.
Limitations due to encapsulation
Encapsulation may generate three major limitations that are not present in suspension cell cultures: diffusional limitations, product inhibition and space limitations. These limitations may explain the low colonization of the microcapsules (max. 20% of theoretical).
However, diffusional limitations do not appear to be responsible to any great extent since cultures undertaken with smaller microcapsules did not lead to higher cell densities. Cell immobilization may induce concentration gradients in the stationary core of microcapsules, with the result that certain concentrations experienced by cells might be temporarily higher than those measured in the surrounding medium. Lactate and ammonia are the major metabolic by-products and, at higher concentrations, may effectively be detrimental to the cells (Schneider 1995). Alginate present within the core of the microcapsules might influence the growth of cells since some of the polysaccharide chains are too long to diffuse out of the microcapsules and the intracapsular concentration remains quite constant (1.5% w/w) throughout the whole culture. Inhibition by alginate, a highly anionic polyelectrolyte, may be due to electrostatic interactions with the cells, with essential cations or simply by reducing the microcapsule volume. It is also possible that there is a reduced oxygen transfer rate since, without agitation in the core of microcapsules, the viscous alginate solution forms a non-agitated layer. However, cells in suspension grew even in presence of alginate, therefore this phenomenon is not entirely responsible for the failure of cells to colonize microcapsules by more than 20%. An alternative explanation for the low colonization of microcapsules may be a space limitation due to inadequate liquefaction of the core alginate (Chang et al. 1994). Residual core alginate could interact with cations present in the culture medium and, depending on whether the cations are mono- or di-valent, decrease or increase the viscosity. Thus, since the alginate itself has been shown to be non-cytotoxic (Robitaille et al. 1999), this suggests that it is the molecular volume of the hydrated alginate which is responsible for limiting the space available for cell colonization.
Conclusions
Quantitative studies of different types of alginate-PLL microcapsules showed the benefits and limitations of encapsulation using these polymers.
Liquid core microcapsules proved to be efficient for achieving high cell densities for cultures of less than one month duration and could be used to 20% (φ) of the maximum colonization potential. Furthermore, the specific SC productivity could be increased 3-fold. However, the low mechanical stability renders such microcapsules unsuitable for long-term bioreactor cultures or for implantation. Reduction of microcapsule size and the formation of a double membrane did not result in increased cell colonization. By contrast, semi-liquid core microcapsules offered an acceptable compromise between long-term stability, cell colonization (φ = 3%) and SC production.
Alginate entrapped within the microcapsules appears to be the main factor responsible for limiting cell growth, since no substrate diffusion limitation or by-product inhibition could be detected. The two-stage process used here for microcapsule production, that is the formation of a polyelectrolyte bead followed by reaction of the pre-formed bead with a second polyelectrolyte to create the microcapsule membrane, is the most commonly used method. The results strongly suggest that entrapment of the bead-forming polymer is the most important single factor for mechanical resistance of the resulting microcapsule and the ability of cells to colonize them. In addition exodiffusion of the membrane polyelectrolytes occurs with a resultant decrease in long-term stability, which can only be overcome by the addition of counter ions in the surrounding medium. Thus it would appear that this technique for microcapsule production, and the use of polyelectrolyte complexes in general for the encapsulation of cells for application in bioreactors or as implants, has severe limitations.
It should be possible to partially overcome this by the use of low molecular weight core polymers for bead formation and the use of polyelectrolytes with very high affinities for one another, which may not be displaced by low molecular weight ions present in the medium.
Abbreviations
- qNH3
specific ammonia production rate mmol cell−1 h−1
- qO2
specific oxygen consumption rate mmol cell−1 h−1
- qP
specific SC production rate mmol cell−1 h−1
- qS
specific glucose consumption rate mg cell−1 h−1
- rcaps
cell radius μm
- rcell
microcapsule radius μm
- X
cell concentration cell mL−1
- Xcaps, max
maximum cell concentration per microcapsule volume cell mL−1capsule
- Xcaps, max, theo.
theoretical maximum cell number per microcapsule volume cell mL−1capsule
- Xreactor, max
maximum cell concentration per reactor volume cell mL−1reactor
- Ylactate/S
yield of lactate on glucose mol mol−1
- YX/S
yield of biomass on glucose cell mmol−1
- ϕ
volume fraction of capsules occupied by the cells %
- μ
specific growth rate h−1
References
- Al-Rubeai MA, Rookes S, Emery AN (1990) Studies of cells proliferation and monoclonal antibody synthesis and secretion in alginate-entrapped hybridoma cells. In: de Bont JAM, Visser J, Mattiasson B, Tramper J (eds) Physiology of immobilized cells. Elsevier Science Publishers, Wageningen, pp 181–208
- Chang PL, Hortelano G, Tse M, Awrey DE (1994) Growth of recombinant fibroblasts in alginate microcapsules. Biotechnol Bioeng 43:925–933 [DOI] [PubMed]
- Cruise GM, Hegre OD, Scharp DS, Hubbell JA (1998) A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnol Bioeng 57:655–665 [DOI] [PubMed]
- Ducommun P, Kadouri A, von Stockar U, Marison IW (2002) On-line determination of animal cell concentration in two industrial high-density culture processes by dielectric spectroscopy. Biotechnol Bioeng 77:316–323 [DOI] [PubMed]
- Ducommun P, Ruffieux PA, Furter MP, Marison I, von Stockar U (2000) A new method for on-line measurement of the volumetric oxygen uptake rate in membrane aerated animal cell cultures. J Biotechnol 78:139–147 [DOI] [PubMed]
- Duff RG (1985) Microencapsulation technology––a novel method for monoclonal antibody production. Trends Biotechnol 3:167–170 [DOI]
- Glacken MW, Fleischaker RJ, Sinskey AJ (1983) Large-scale production of mammalian cells and their products - engineering principles and barriers to scale-up. Ann New York Acad Sci 413:355–372 [DOI] [PubMed]
- Goosen MFA, O’Shea GM, Gharapetian HM, Chou S, Sun AM (1985) Optimization of microencapsulation parameters: semipermeable microcapsules as a bioartificial pancreas. Biotechnol Bioeng 27:146–150 [DOI] [PubMed]
- Griffiths B (1990) Perfusion systems for cell cultivation. In: Marcel Dekker I (ed) Large scale mammalian. Cell Culture Technology, New York and Basel, 624p
- Heald KA, Jay TR, Downing R (1994) Assessment of the reproducibility of alginate encapsulation of pancreatic islets using the MTT colorimetric assay. Cell Transplant 3:333–337 [DOI] [PubMed]
- King GA, Daugulis AJ, Faulkner P, Goosen MFA (1987) Alginate PLL microcapsules of controlled membrane MWCO for mammalian cell culture engineering. Biotechnol Prog 3:231–240
- Levy M-C, Edwards-Levy F (1996) Coating alginate beads with cross-linked biopolymers: a novel method based on a transacylation reaction. J Microencapsulation 13:169–183 [DOI] [PubMed]
- Lim F (1984) Microencapsulation of living cells and tissues. Appl Biochem Biotechnol 10:81–85 [DOI] [PubMed]
- Miller WM, Blanch HW, Wilke CR (1988) A kinetic analysis of hybridoma growth and metabolism in batch and continuous suspension culture – effect of nutrient concentration, dilution rate, and pH. Biotechnol Bioeng 32:947–965 [DOI] [PubMed]
- Ng A-C, Berry JM, Butler M (1995) Optimization of physical parameters for cell attachment and growth on macroporous microcarriers. Biotechnol Bioeng 50:627–635 [DOI] [PubMed]
- Papoutsakis ET (1991) Fluid mechanical damage of animal cells in bioreactors. Trends Biotechnol 9:427–437 [DOI] [PubMed]
- Park YG, Iwata H, Ikada Y (1998) Microencapsulation of islets and model beads with a thin alginate-Ba2+ gel layer using centrifugation. Polymers Adv Technols 9:734–739 [DOI]
- Posillico EG (1986) Microencapsulation technology for large-scale antibody production. Bio/Technol 4:114–117 [DOI]
- Robitaille R, Pariseau J-F, Leblond FA, Lamoureux M, Lepage Y, Hallé J-P (1999) Studies on small (<350 um) alginate PLL microcapules III: biocompatibility of smaller versus standard microcapsules. J Biomed Mater Res 44:116–120 [DOI] [PubMed]
- Ruffieux P-A, von Stockar U, Marison I (1998) Measurement of volumetric (OUR) and determination of specific (qO2) oxygen uptake rates in animal cell cultures. J Biotechnol 63:85–95 [DOI] [PubMed]
- Rupp RG (1985) Use of cellular microencapsulation in large scale production of monoclonal antibodies. In: Feder J and Tolbert WR (ed) Large scale mammalian cell culture, Academic, New York, pp 19–36
- Scheirer W, Nilsson K, Merten O-W, Katinger HWD, Mosbach K (1984) Entrapment of animal cells for the production of biomolecules such as monoclonal antibodies. Develop Biol Stand 55:155–161 [PubMed]
- Schneider M (1995) Applications of hydrophobic porous membranes in mammalian cell culture technology. In: Chemistry Department. Swiss Federal Institute of Technology, Lausanne, 218p
- Schoichet MS, Li RH, White ML, Winn SR (1996) Encapsulation: an in vitro comparison of alginate and agarose. Biotechnol Bioeng 50:374–381 [DOI] [PubMed]
- Seifert DB, Phillips JA (1997) Porous alginate-poly(ethylene glycol) entrapment system for the cultivation of mammalian cells. Biotechnol Prog 13:569–576 [DOI] [PubMed]
- Serp D, Catana E, Heinzen C, von Stockar U, Marison IW (2000) Characterization of an encapsulation device for the production of monodisperse alginate beads for cell immobilization. Biotechnol Bioeng 70:41–53 [DOI] [PubMed]
- Thu B, Bruheim P, Espevik T, Smidsrod O, Soonshiong P, Skjakbraek G (1996a) Alginate polycation microcapsules. 1. Interaction between alginate and polycations. Biomaterials 17:1031–1040 [DOI] [PubMed]
- Thu B, Bruheim P, Espevik T, Smidsrod O, Soonshiong P, Skjakbraek G (1996b) Alginate polycation microcapsules. 2. Some functional properties. Biomaterials 17:1069–1079 [DOI] [PubMed]
- Uludag H, Kharlip L, Sefton MV (1993) Protein delivery by microencapsulated cells. Adv Drug Del Revs 10:115–130 [DOI]
- Yoshioka T, Hirano R, Shioya T, Kako M (1990) Encapsulation of Mammalian Cells with Chitosan CMC. Biotechnol Bioeng 35:66–72 [DOI] [PubMed]