Abstract
Complete resolution of an acute inflammatory response and its return to homeostasis are essential for healthy tissues. Here, we overview ongoing efforts to characterize cellular and molecular mechanisms that govern the resolution of self-limited inflammation. Systematic temporal analyses of evolving inflammatory exudates using mediator lipidomics-informatics, proteomics, and cellular trafficking with murine resolving exudates demonstrate novel endogenous pathways of local-acting mediators that share both anti-inflammatory and pro-resolving properties. In murine systems, resolving-exudate leukocytes switch their phenotype to actively generate new families of mediators from major omega-3 fatty acids EPA and DHA termed resolvins and protectins. Recent advances on their biosynthesis and actions are reviewed with a focus on the E-series resolvins (RvE1, RvE2), D series resolvins (RvD1, RvD2) and the protectins including neuroprotectin D1/protectin D1 (NPD1/PD1) as well as their aspirin-triggered epimeric forms. Members of each new family demonstrate potent stereo-specific actions, joining the lipoxins as endogenous local signals that govern resolution and endogenous anti-inflammation mechanisms. In addition to their origins and roles in resolution biology in the immune system, recent findings indicate that these new mediator families also display potent protective actions in lung, kidney, and eye as well as enhance microbial clearance. Thus, these endogenous agonists of resolution pathways constitute a novel genus of chemical mediators that possess pro-resolving, anti-inflammatory, and antifibrotic as well as host-directed antimicrobial actions. These may be useful in the design of new therapeutics and treatments for diseases with the underlying trait of uncontrolled inflammation and redox organ stress.
Keywords: leukocytes, eicosanoids, resolvins, acute inflammation, ω-3 fatty acids, protectins
Introduction
Acute inflammation has several outcomes that include progression to chronic inflammation, scarring and fibrosis or complete resolution (Cotran et al., 1999). Resolution was defined at the histologic/tissue level and was thought of as a passive process in vivo as, for example, with the dwindling with time of chemotaxic stimuli at the site of inflammation. With the isolation of endogenous anti-inflammatory and pro-resolving mediators and their characterization, it became clear that resolution is an active process involving biochemical circuits that actively biosynthesize local mediators within the resolution phase, such as the resolvins (Serhan, 1997; Serhan et al., 2000, 2002). The resolution phase has emerged as a new terrain for drug design and resolution-directed therapeutics (Gilroy et al., 2004; Lawrence et al., 2005). Resolution by precise definition is not the same as endogenous anti-inflammation. For example, a pro-resolving small molecule can, in addition to serving as an agonist of anti-inflammation, also promote the uptake and clearance of apoptotic neutrophils (polymorphonuclear leukocyte, PMN) from the site of inflammation by macrophages (Maderna and Godson, 2005; Serhan, 2005). A recent consensus report from investigators at the forefront of this emerging area has addressed these definitions to help delineate this new terrain (Serhan et al., 2007). Moreover, because many currently used drugs were developed without an appreciation of their potential impact in resolution, some agents such as the widely used COX-2 inhibitors proved to be resolution toxic (Gilroy et al., 1999; Bannenberg et al., 2005; Serhan et al., 2007), whereas others can possess pro-resolving actions, such as glucocorticoids (Rossi and Sawatzky, 2007), cyclin-dependent kinase inhibitors (Rossi et al., 2006) and aspirin (Serhan et al., 1995; Serhan, 2007).
Interest in natural resolving mechanisms has been heightened in recent years (Henson, 2005; Luster et al., 2005; Serhan and Savill, 2005) because inflammation (characterized by the cardinal symptoms dolor, calor, rubor and loss of function) is now recognized as a central feature in the pathogenesis of many prevalent diseases in modern Western civilization, such as atherosclerosis (Libby, 2002), cancer (Coussens and Werb, 2002), asthma (Barnes, 1999), autoimmune disease, and various neuropathological disorders including stroke, Alzheimer's and Parkinson's diseases (Majno and Joris, 1996; Nathan, 2002; Erlinger et al., 2004; Hansson et al., 2006). Resolution of inflammation is required for the return from inflammatory disease to health, that is, catabasis (Bannenberg et al., 2005). New evidence from this laboratory and others indicates that the catabasis from inflammation to the ‘normal' noninflamed state is not merely passive termination of inflammation but rather an actively regulated program of resolution (Serhan et al., 2007). This event is accompanied by lipid mediator class switching from pro-inflammatory prostaglandins (PGs) and leukotrienes (LT) to the biosynthesis of anti-inflammatory mediators, such as lipoxins (LXs) (Levy et al., 2001), as well as the appearance of new families of pro-resolving mediators biosynthesized in exudates from ω-3 polyunsaturated fatty acid (PUFA) precursors (Serhan et al., 2000, 2002; Hong et al., 2003) (Figure 1a).
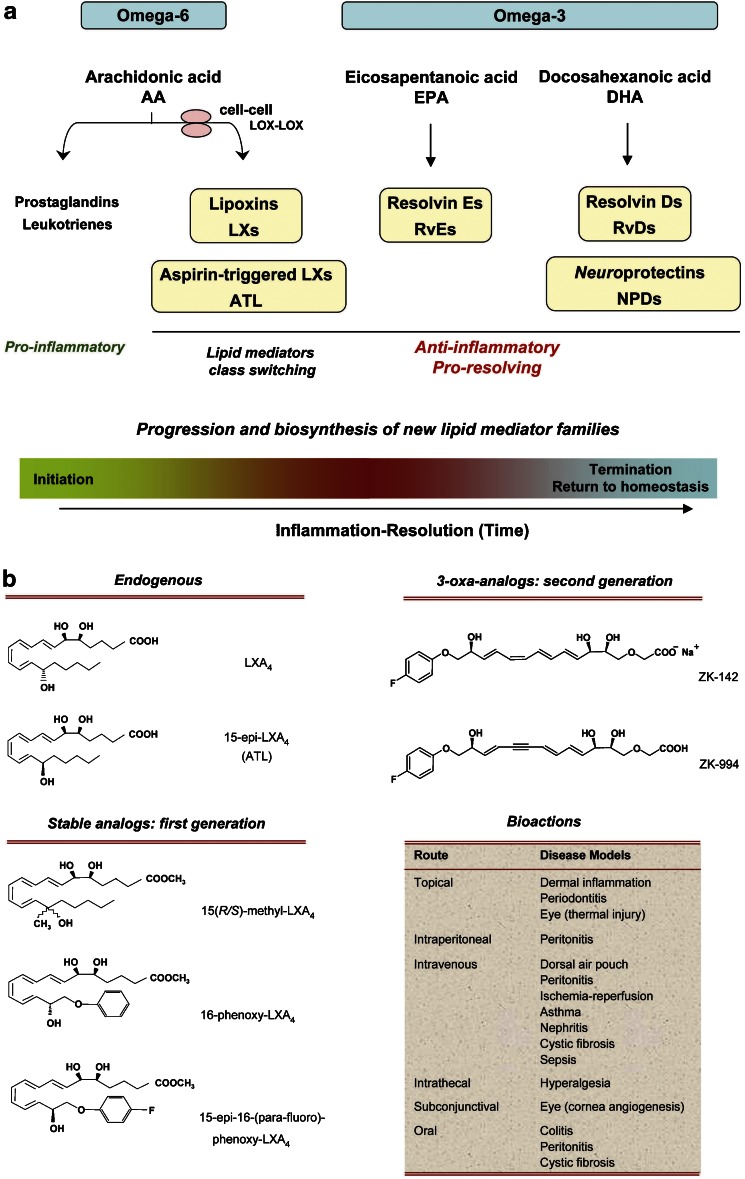
Figure 1.
New families of PUFA-derived lipid mediators and their roles in the progression of acute Inflammation. (a) Specialized chemical mediators and signals appear to be programmed at the tissue level to actively participate in leukocyte responses (PMN, monocytes and macrophages) required for resolution. The chemical mediators are involved in the initiation of acute inflammation, such as PGs and LT. With time, a class switching occurs toward pro-resolving lipid mediators. These mediators include (1) ω-6 PUFA arachidonic acid (AA)-derived lipoxins (LXs) and aspirin-triggered LXs (ATL); (2) ω-3 PUFA eicosapentaenoic acid (EPA)-derived resolvin E-series (RvEs); (3) docosahexaenoic acid (DHA)-derived resolvin D-series (RvDs) and protectins (PDs). PDs are also termed neuroprotectins when generated in neural tissues. LXA4, ATL, resolvins and protectins share anti-inflammatory and pro-resolving properties, yet have distinct actions within the resolution orchestra. (b) Structures of LXA4, ATLa and their representative stable analogs (Bannenberg et al., 2004; Chiang et al., 2006). For detailed bioactions in disease models, see Table 1.
The essential roles of omega-3 PUFAs in preventing disease in rodents were established in 1929 (Burr and Burr, 1929). In humans, the mechanism of action underlying the many decades of reports since Burr and Burr (1929) showing beneficial actions of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the major omega-3 PUFA, remains a topic of interest and considerable discussion (Lands, 1987; Bazan, 1990; Simopoulos et al., 1999; Salem et al., 2001) because structure–activity relationships remained to be established. Hence, elucidating the molecular mechanisms of DHA- and EPA-reported beneficial actions is an important challenge for molecular and translational medicine. One prevailing and popular theory suggests that the omega-3 PUFA compete with the storage of arachidonic acid (AA), replacing it and blocking the production of pro-inflammatory eicosanoids (Lands, 1987). However, none of the new mediators reviewed here and their actions were known at the time. Along with the pro-inflammatory PGs and LT, the n−6 essential fatty acid AA is precursor to LX and aspirin-triggered LX (Figure 1), which possess potent anti-inflammatory and pro-resolving actions (Table 1). These findings emphasize that the popular view of essential n−6 and n−3 PUFA actions in inflammation and homeostasis was incomplete.
Table 1. In vivo actions of LXA4 and aspirin-triggered ATL.
| Disease model | Action(s) | Dosage (route) | Duration of postexposure | References |
|---|---|---|---|---|
| Dermal inflammation | Inhibit neutrophil recruitment into ear skin and prevent vascular permeability | 240 nmol (topical) | 8 h | Takano et al. (1997, 1998) |
| 20 μg cm−2 | Bannenberg et al. (2004) | |||
| Schottelius et al. (2002) | ||||
| Skin (dorsal air pouch) | Block TNF-α induced leukocyte recruitment | 10 μg (i.v.) | 4 h | Clish et al. (1999) |
| Block P gingivalis-elicited leukocyte infiltration and lower PGE2 levels within exudates | 10 μg (intrapouch) | 4 h | Pouliot et al. (2000) | |
| I/R injury | Attenuate hindlimb I/R-induced lung injury | 10 μg (i.v.) | 3 h | Chiang et al. (1999) |
| 500 μg kg−1 (i.v.) | Bannenberg et al. (2004) | |||
| Detachment of adherent leukocytes in mesenteric I/R | 10 nmol l−1 (superfusion) | 2 h | Scalia et al. (1997) | |
| Peritonitis | Inhibit neutrophil recruitment and regulate chemokine/cytokine production | 300 ng (i.p.) | 4–24 h | Bannenberg et al. (2005) |
| 50 ng–50 μg kg−1 (oral) | Bannenberg et al. (2004) | |||
| Inhibit vascular leakage | 60 μg kg−1 (i.p.) | 4 h | Chiang et al. (2003) | |
| Promote phagocytosis of neutrophil by macrophage | 1–2 μg (i.p.) | <1 h | Mitchell et al. (2002) | |
| Promote lymphatic removal of microbial particles (zymosan) | 300 ng (i.p.) | 24 h | Schwab et al. (2007) | |
| Colitis (IBD) | Attenuate pro-inflammatory gene expression and reduce severity of colitis | 10 μg ml−1 (oral) | 0–20 days | Gewirtz et al. (2002) |
| Inhibit weight loss, inflammation and immune dysfunction | 300–1000 μg kg−1 (oral, daily) | 7 days | Fiorucci et al. (2004) | |
| Mesangioproliferative nephritis | Reduce proteinuria, IL-1β, IL-6 and proliferation score of mesangial cells | i.v., every 8 h | 4 days | Wu et al. (2007) |
| Kidney | Antagonizes LTD4-induced falls in glomerular filtration rate | 1–2 μg kg−1 min−1 (infused into renal arteries) | 20 min | Katoh et al. (1992) |
| Protects from ischemic acute renal failure | 15 μg per mouse (perfusion) | 24 h | Leonard et al. (2002) | |
| Pleuritis | Shorten the duration of pleural exudation | 0.5–5 μg (local) | 24 h | Bandeira-Melo et al. (2000) |
| Asthma | Inhibit airway hyperresponsiveness and pulmonary inflammation | 10 μg per mouse per day (i.v.) | 5 days | Levy et al. (2002) |
| Modulates airway obstruction in humans | 100 μM (inhalation) | Christie et al. (1992) | ||
| Cystic fibrosis | Decrease neutrophilic inflammation, pulmonary bacterial burden and disease severity | 10 μg ml−1 (oral) plus 1 μg (i.v.) | 6 days | Karp et al. (2004) |
| Sepsis | Attenuate LPS-induced acute lung injury | 0.7 mg kg−1 (i.v.) | Jin S-W et al. (2007) | |
| Angiogenesis | Reduce angiogenic phenotype: endothelial cell proliferation and migration | 10 μg per pouch (local) | 6 days | Fierro et al. (2002) |
| Periodontitis (oral inflammation and bone loss)* | Reduce microbe-initiated, neutrophil-mediated tissue damage and bone destruction | 6 μg twice per week (topical application around the second premolar) | 6 weeks | Serhan et al. (2003) |
| Eye | Accelerate cornea reepithelialization, limit sequelae of thermal injury (i.e., neovascularization, opacity) and promote host defense | 1 μg three times per day (topical application on eye) | 2–4 days | Gronert et al. (2005) |
| Suppress corneal angiogenesis, inhibiting inflammatory cytokines and VEGF/VEGFR-2 expression | 100 ng per 10 μl (subconjunctivally, once daily) | 3 days | Jin Y et al. (2007) | |
| BMT | Protect against BMT-induced GvHD | 50 μg kg−1 (host), 100 ng ml−1 (donor) | 9 days | Devchand et al. (2005) |
| Carrageenan-induced hyperalgesia | Prolong paw withdraw latency, reducing hyperalgesic index; reduce pain | 24 nmol kg−1 (i.v.) | 0–8 h | Svensson et al. (2007) |
| Reduce paw edema | 0.3 nmol (intrathecal) |
Abbreviations: BMT, bone marrow transplant; GvHD, graft-vs-host diseases; IBD, inflammatory bowel disease; IL, interleukin; I/R, ischemia/reperfusion; LPS, lipopolysaccharide; PGE2, prostaglandin E2; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
*All animal models were designed and carried out in either mice or rats, except for periodontitis, which was carried out in rabbits.
In this review, we summarize the evidence available to date that indicates that ω-3 PUFAs are precursors to potent novel mediators that are enzymatically generated and identified in the resolving inflammatory exudates (Serhan et al., 2000, 2002; Hong et al., 2003) and are also neuroprotective (Marcheselli et al., 2003; Mukherjee et al., 2004). The term resolvins (resolution-phase interaction products) was first introduced to signify that the new structures were endogenous mediators possessing potent anti-inflammatory and immunomodulatory actions demonstrated in the nanogram dose range in vivo (Serhan et al., 2002). These include reducing neutrophil traffic and pro-inflammatory cytokines, as well as lowering the magnitude of the inflammatory response in vivo (Serhan et al., 2000, 2002). The terms protectin and neuroprotectin (when generated in neural tissues) (Serhan et al., 2006a) were introduced given the anti-inflammatory (Hong et al., 2003) as well as the protective actions of the DHA-derived mediator NPD1/PD1 in neural systems (Mukherjee et al., 2004), stroke (Marcheselli et al., 2003) and Alzheimer's disease (Lukiw et al., 2005).
The earlier oxygenated products characterized as being formed from ω-3 PUFA are similar in structure to known AA-derived eicosanoids, but either devoid of bioactions or far less potent than their AA counterparts (for examples, see Lee et al., 1984). This sharply contrasts the biosynthesis and actions of specific members of the resolvin and protectin families that carry potent biological actions demonstrable in the nanomolar and picomolar ranges in vitro and in vivo (Serhan et al., 2000, 2002; Hong et al., 2003; Marcheselli et al., 2003; Mukherjee et al., 2004). In this review, we summarize the bioactivities of pro-resolving mediators and underlying biochemical pathways of the ω-3 PUFA-derived mediators. These include (1) resolvins of the E series (resolvin E1 or RvE1) from EPA, (2) resolvins of the D series (resolvin D1 or RvD1) and their aspirin-triggered (AT) epimeric forms from DHA and (3) the neuroprotectins/protectins (PDs) from DHA. These novel mediators are endogenous agonists controlling inflammation via stimulating resolution. These novel endogenous anti-inflammatory and pro-resolving mediators provide the biotemplates to produce stable analogs and mimetics as in Serhan et al. (1995); together with the LXs they constitute a pharmacologic genus of pro-resolving and anti-inflammatory molecules. Armed with a quantitative set of resolution indices (Bannenberg et al., 2005) and a firm consensus of the cellular, molecular and tissue events and precise terms defining resolution from leading scholars in the area (Serhan et al., 2007), we may find this new genus of pro-resolving agonists of resolution to be a useful therapeutic approach to treat many diseases characterized by uncontrolled inflammation in their pathogenesis and progression.
Endogenous mediators in initiating inflammation and its resolution
The role of chemical mediators in inflammation became apparent with the discovery of the PGs, named by the Swedish laureate von Euler (for recent review, see Flower, 2006), and LT, from seminal studies of Borgeat et al. (1976). Bergström (1982), Samuelsson and colleagues established in earlier studies that AA is transformed into many potent bioactive compounds such as PGs, LT, and the more recent addition, the LXs (Samuelsson, 1982; Samuelsson et al., 1987). This departure of PUFA from playing structural roles in cell membranes or as energy stores came with recognition that AA is transformed by both cyclooxygenase (COX) and lipoxygenase (LOX) mechanisms to potent products, which led to a Nobel prize in 1982 (Bergström, 1982; Samuelsson, 1982; Vane, 1982). Peripheral blood neutrophils release mediators derived from AA via COX and LOXs that are mostly pro-inflammatory (Samuelsson, 1983). Recent results, however, have heightened our awareness that neutrophils (PMN), which are first to arrive on the scene, in addition to initially releasing pro-inflammatory mediators, can with time switch their product profile of lipid mediators to produce ‘protective' lipid mediators that serve as agonists to actively limit inflammation and promote resolution (Serhan, 2004).
Within this framework, LXs were the first mediators biosynthesized from AA identified to possess both anti-inflammatory and pro-resolving actions (Serhan, 2005). In general, this is a departure from the well-appreciated pro-inflammatory roles of lipid mediators (Samuelsson, 1983). LXs are generated from endogenous sources of the n−6 AA (Figure 1) and are potent agonists of resolution in vivo in many disease models (Table 1), thus serving as checkpoint controllers of inflammation (Chiang et al., 2006). Of interest, an early human trial with lipoxin A4 (LXA4) conducted by Professor TH Lee and colleagues in London demonstrated that LXA4 was safe and blocked airway challenge and hyperreactivity (Christie et al., 1992). Increasing evidence demonstrating that resolution of inflammation is actually an active biochemical process came with the identification of specialized chemical mediators biosynthesized during the resolution phase such as the resolvins and protectins, which actively promote resolution (Serhan et al., 2000; Levy et al., 2001; Gilroy et al., 2004) and the return to homeostasis (Serhan, 2002) (vide infra).
Molecular links to ω-3 PUFA and resolution of inflammation
It has often been questioned whether essential fatty acids, such as the ω-3 EPA or DHA, are converted to potent lipid mediators, as is the case with AA. In view of the compelling results from the GISSI study, which showed improvements in >11 000 cardiovascular patients, that is, reduction in the incidents of sudden death to ∼45% in those taking almost 1 g of ω-3 per day (GISSI-Prevenzione Investigators, 1999; Marchioli et al., 2002). However, the mechanism of this ω-3 action in humans with vascular disease was not established, and the impact of ongoing aspirin therapy was not accounted for in the analyses. Inspection of their methods indicated that all the patients also took daily aspirin; the contribution of aspirin to the beneficial outcomes was not taken into account (GISSI-Prevenzione Investigators, 1999). Also, an abundant literature with ω-3 PUFA given at doses of milligrams to grams per day pointed to beneficial actions in many diseases including inflammatory diseases as well as cancer (Lands, 1987; Bazan, 1992). Each of the three major human LOXs (5-LOX, 12-LOX and 15-LOX) can convert ω-3 PUFA to various monohydroxy-containing products. The biological importance of these products, if any, was not known (Hamberg and Samuelsson, 1967; Lee et al., 1984; Sawazaki et al., 1994). DHA is also subject to nonenzymatic oxygenation to isoprostane-like compounds termed neuroprostanes that reflect oxidative stress in the brain (Reich et al., 2000) or, when mishandled, can rapidly auto-oxidize to monohydroxy racemates (VanRollins et al., 1984). However, despite the many decades of research with ω-3 PUFA, the cellular and molecular mechanisms accounting for their reported in vivo immunoprotective actions remained to be established, and their direct connection to human disease and treatment potential is still an important biomedical challenge.
Aspirin initiates biosynthesis of endogenous pro-resolving and anti-inflammatory lipid mediators
Although it is clear that aspirin inhibits PG formation and hence a key mechanism in anti-inflammatory therapy (Vane, 1982), and that aspirin has well-appreciated clinical uses and ability to limit leukocyte traffic into sites of inflammation, the mechanism remained unknown. In this context, aspirin impinges on the endogenous LX-generating system during cell–cell interactions in the vasculature, triggering formation of epimeric forms of LXs, termed aspirin-triggered LXs (ATL) (Clària and Serhan, 1995; Chiang et al., 2006).
As is the case for other autacoids, both LXA4 and ATL are rapidly generated in response to stimuli, act locally and then are rapidly inactivated by metabolic enzymes, namely 15-hydroxyprostaglandin dehydrogenase (15-PGDH) and eicosanoid oxidoreductase (EOR) (also known as LXA4/PGE 13,14-reductase/LTB4 12-hydroxydehydrogenase (PGR/LTB4DH)). To this end, stable analogs were constructed with specific modifications of the native structures of LXA4 and ATL (Serhan et al., 1995) to resist enzymatic metabolism. These analogs not only display prolonged half-life in blood but also enhanced bioavailabilities as well as potency in vivo (Clish et al., 1999). As a class, LXs, ATL and their stable analogs share potent protective actions in controlling inflammation (Table 1).
In light of these results, attention was focused next on understanding the biochemical events activated during healthy resolution of acute inflammatory responses. The LX and ATL stable analogs are potent and are effective in many experimental disease models (Table 1). Of interest, the first-generation LX/ATL analogs proved to be active when administrated i.v., i.p., topically and orally available (Schottelius et al., 2002; Serhan et al., 2003; Bannenberg et al., 2004). The second generation of LX/ATL analogs (Guilford et al., 2004) are also bioavailable and have proven effective in murine models of colitis (Wallace and Fiorucci, 2003; Fiorucci et al., 2004) and asthma (Levy et al., 2007b). In asthma, the ZK-994 LX/ATL analog (see Figure 1b for structures) was more effective than Singulair in reducing airway inflammation and airway bronchoconstriction (Levy et al., 2007b). In view of these compelling findings in experimental animal systems demonstrating anti-inflammatory and pro-resolving actions in vivo (Mitchell et al., 2002; Kieran et al., 2004), a proof of concept was established, namely, that treatment with a pro-resolving and anti-inflammatory lipid mediator or its stable analog mimetic can reduce inflammation and disease symptoms in animals (Table 1). Of interest, recent results demonstrate that LX and their ATL analogs block pain signals (Svensson et al., 2007) and regulate neural stem cells (Wada et al., 2006). Hence, it will be of interest to learn whether these or related agonists of resolution can reduce disease symptoms in humans and promote the return of the tissues to homeostasis in a human trial.
Impact of anti-inflammatory agents on PUFA-derived mediators
Aspirin, in addition to its well-appreciated ability to inhibit the pro-inflammatory PG, can also ‘switch on' the body's own anti-inflammatory lipid mediators, namely aspirin-triggered lipid mediators. This new action of aspirin involves COX-2-bearing cells such as vascular endothelial cells or epithelial cells and their coactivation with PMN (Clària and Serhan, 1995; Chiang et al., 2006). Briefly, inflammatory stimuli (for example, tumor necrosis factor (TNF)-α, lipopolysaccharide (LPS)) induce COX-2 to generate 15R-HETE when aspirin is administered. Aspirin irreversibly acetylates COX-2 and changes the enzyme's products from intermediates for PG and thromboxane to precursors for ATL, namely 15R-HETE. This precursor carries a carbon-15 alcohol in the R configuration and is rapidly converted by 5-LOX in activated PMN to 15 epimeric-LXs, or ATL, that carry their 15 position alcohol in the R configuration rather than 15S (native LX). This biosynthetic mechanism is also operative with EPA-derived RvEs and DHA-derived RvDs (Serhan et al., 2000, 2002) in that aspirin initiates formation of these ω-3-derived mediators by acetylating vascular COX-2. Therefore, these aspirin-triggered (AT) protective mediators provide a novel mechanism underlying aspirin's clinical benefits.
Other commonly used nonsteroidal anti-inflammatory drugs (NSAIDs) do not acetylate COX-2. Some do, however, with recombinant COX-2 and EPA, permit formation of 18R-hydroxyeicosapentaenoic acid (18R-HEPE), a precursor for EPA-derived RvEs (see below), even though 18R-HEPE levels are lower than those in the absence of these NSAIDs (Serhan et al., 2000). In comparison, when incubated with recombinant COX-2 and DHA, these NSAIDs did not give appreciable amounts of 17R-HDHA, a precursor for DHA-derived RvDs (Serhan et al., 2002). Of interest, some NSAIDs inhibit enzymes involved in the inactivation of PUFA-derived mediators, namely 15-PGDH and EOR, thus likely leading to accumulation of LXs and Rvs (Table 2). The widely used anti-inflammatory glucocorticoids may also facilitate the formation and action of PUFA-derived mediators via regulating not only the biosynthetic and metabolic enzymes, but also one of the receptors for LXA4/ATL, namely ALX (Sawmynaden and Perretti, 2006; Hashimoto et al., 2007). Along these lines, both thiazolidinediones and statins have anti-inflammatory actions; however, the underlying mechanisms are not clear. Of interest, a recent report by Birnbaum et al. (2006) demonstrated that both pioglitazone and atorvastatin increase the myocardial content of LXA4 and 15-epi-LXA4, which in turn can likely contribute to the anti-inflammatory properties of thiazolidinediones and statins.
Table 2. Impact of anti-inflammatory agents on LX, RV and PD: biosynthesis, inactivation and receptors.
| Anti-inflammatory agent | Impact |
|---|---|
| NSAID | |
| Aspirin | Switches COX-2 activity to produce 15R-HETE (from AA; Clària and Serhan, 1995), 18R-HEPE (from EPA; Serhan et al., 2000), and 17R-HDHA (from DHA; Serhan et al., 2002) |
| Increases ATL formation in vivo in experimental animals (reviewed in Chiang et al., 2006) and in human (Chiang et al., 2004) | |
| Initiates RvE1 formation in vivo in experimental animals (Serhan et al., 2000) and in human (Arita et al., 2005a) | |
| Initiates RvD formation in vivo in experimental animals (Serhan et al., 2002) | |
| Inhibits 15-PGDH (Hansen, 1974) | |
| Indomethacin | Permits 18R-HEPE generation with recombinant COX-2, yet reduces its levels (Serhan et al., 2000) |
| Inhibits 15-PGDH (Cho and Tai, 2002; Hansen, 1974) and EOR (Clish et al., 2001) | |
| Acetaminophen | Permits 18R-HEPE generation with recombinant COX-2, yet reduces its levels (Serhan et al., 2000) |
| Diclofenac | Inhibits EOR (Clish et al., 2001) |
| Steroid | |
| Dexamethasone | Upregulates 5-LOX expression (Colamorea et al., 1999; Uz et al., 2001) |
| Inhibits 15-PGDH (cell-type dependent) (Tong and Tai, 2000) | |
| Upregulates ALX receptor (Hashimoto et al., 2007; Sawmynaden and Perretti, 2006) | |
| Thiazolidinedione | |
| Pioglitazone | Increases LXA4 and 15-epi-LXA4 formation (Birnbaum et al., 2006) |
| Ciglitazone | Inhibits 15-PGDH (Cho and Tai, 2002) |
| Statin | |
| Atorvastatin | Increase LXA4 and 15-epi-LXA4 formation (Birnbaum et al., 2006) |
Abbreviations: 15-PGDH; 15-hydroxyprostaglandin dehydrogenase; 18R-HEPE, 18R-hydroxyeicosapentaenoic acid; AA, arachidonic acid; ATL, aspirin-triggered LX; COX-2, cyclooxygenase-2; EOR, eicosanoid oxidoreductase; DHA, docosahexaenoic acid; 5-LOX, 5-lipoxygenase; LXA4, lipoxin A4; NSAID, nonsteroidal anti-inflammatory drug; Rv, resolvin.
Mediator lipidomics: profiling and complete structure elucidation of novel endogenous mediators
We developed mediator informatics and lipidomics employing liquid chromatography-ultraviolet-tandem mass spectrometry (LC-UV-MS-MS)-based analyses of inflammatory exudates. We also constructed lipid mediator libraries with physical properties, such as MS and MS/MS spectra, elution times and UV spectra for matching studies (Lu et al., 2005, 2006). These databases and informatics were geared to evaluate whether novel lipid mediators are indeed generated during the resolution phase of inflammation (Serhan et al., 2000, 2002, 2006b). An experimental model of contained inflammation was used for these studies, namely, murine dorsal air pouch. In this model, 4 h after TNF-α injection, PMN numbers drop within the exudates (Serhan et al., 2000, 2002), which is the cellular definition of resolution (Cotran et al., 1999). Exudates were collected in this ‘spontaneous resolution' phase (Winyard and Willoughby, 2003). In this phase, lipid mediator profiles were recorded using tandem LC-UV-MS-MS to identify lipid mediators present within the exudates. When novel bioactive mediators were encountered, their structures were elucidated. This was accomplished by carrying out retrograde analysis for both biogenic (that is, using recombinant enzymes) and total organic synthesis (for example, see Petasis et al., 2005). Also, this approach permitted assessment of structure–activity relationships as well as the scale-up required to confirm bioactions characterized for the novel compounds identified (Arita et al., 2005a; Lu et al., 2006).
Resolvins: the 18R E-series resolvins from EPA
Formation and structure elucidation
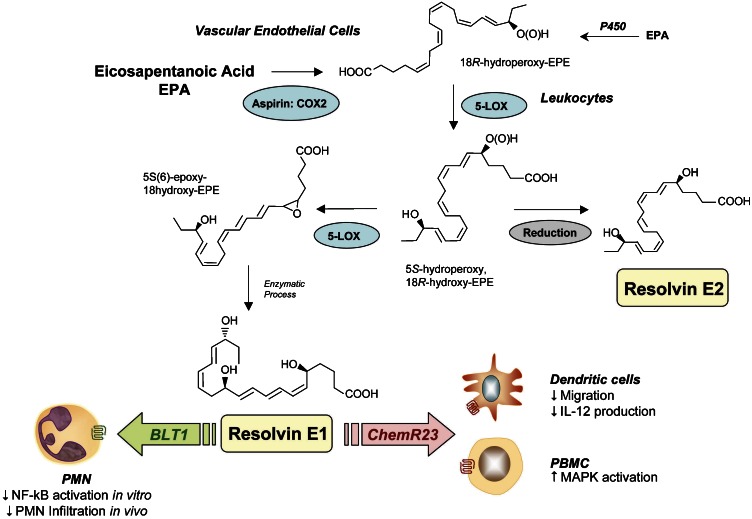
The exudates collected from murine air pouches in the resolution phase contained 18R-HEPE as well as several other related bioactive compounds (Serhan et al., 2000). These novel compounds were generated from EPA. The first bioactive compound, termed resolvin E1 (RvE1), was isolated from exudates and found to reduce inflammation in vivo and blocks human PMN transendothelial migration in vitro. Structural elucidation was carried out using both GC-MS and LC-MS-MS analyses of bioactive fractions obtained following extraction and reversed phase-high pressure liquid chromatography. The basic structure of this potent bioactive product generated from EPA proved to be 5,12,18R-trihydroxyeicosapentaenoic acid (EPE) (Serhan et al., 2000). To recapitulate these in vivo events with human cells, isolated human vascular endothelial cells treated with aspirin convert EPA to 18R-HEPE that is released and then rapidly converted by activated human PMN to a 5(6)-epoxide-containing intermediate that is converted to bioactive RvE1 (Figure 2).
Figure 2.
E-series resolvins. Aspirin impacts the formation of resolvin E1 (RvE1) by acetylating COX-2 in vascular endothelial cells that stereoselectively generate 18R-hydroperoxy-EPE (18R-H(p)EPE). 18R-HEPE is further converted via sequential actions of leukocyte 5-LOX, leading to formation of RvE1. The complete stereochemistry of RvE1 was recently established. Microbial P-450s can also contribute to RvE biosynthesis via converting eicosapentaenoic acid (EPA) to 18-HEPE (Arita et al., 2005b). Human recombinant 5-LOX also generates resolvin E2 (RvE2) from 18-HEPE. At least two separate GPCRs can specifically interact with RvE1: (1) ChemR23, on mononuclear cells and DCs, and (2) BLT1, on human PMN. Also, when expressed on epithelial cells ChemR23 and RvE1 stimulated CD-55-dependent clearance of PMN from the mucosal surface (Campbell et al., 2007).
RvE1 possesses an interesting and novel distinct structure consisting of a conjugated diene plus conjugated diene chromophore present within the same molecule. Both biogenic (Serhan et al., 2000) and total organic syntheses were achieved and its complete stereochemical assignment was established along with that of several related natural isomers (Arita et al., 2005a). RvE1 proved to be 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoicacid (Figure 2). RvE1 production in healthy human volunteers, marked by mass spectra-based identification in plasma, is increased with aspirin therapy (Arita et al., 2005a). However, biologically significant amounts of RvE1, that is, concentrations that can evoke biological responses, are produced and present in plasma without taking aspirin.
Human recombinant 5-LOX generates resolvin E2 (RvE2) from a common precursor of E-series resolvins, namely 18-HEPE. RvE2, which is 5S,18-dihydroxyeicosapentaenoic acid, stopped zymosan-induced PMN infiltration, displaying potent anti-inflammatory properties in murine peritonitis (Tjonahen et al., 2006). In addition, RvE1 and RvE2, when given together, displayed additive action in controlling PMN infiltration. These results demonstrate that RvE2, together with RvE1, may contribute to the beneficial actions of ω-3 fatty acids in human diseases (Table 3). Moreover, they indicate that the 5-LOX, in human leukocytes, is a pivotal enzyme that is temporally and spatially regulated in vivo to produce either pro- or anti-inflammatory local chemical mediators. Thus, the production of these mediators is dependent on the local environment of the leukocytes; for example, within the inflammatory exudates. Also of interest are results indicating that microbial p450 can generate 18-HEPE from EPA (Serhan et al., 2000) that can be converted by human PMN to RvE1 and RvE2 (Arita et al., 2005b). Hence, the microbial content in the local environment can also be a critical factor in the production of E-series resolvins in vivo in humans.
Table 3. In vivo actions of resolvin E series.
| Disease model | Action | Dosage (route) | Duration of postexposure | Reference |
|---|---|---|---|---|
| Resolvin E1 | ||||
| Skin (dorsal air pouch) | Reduces PMN infiltration | 100 ng per mouse (i.v.) | 4 h | Serhan et al. (2000, 2002) |
| Peritonitis | Reduces PMN infiltration and regulates chemokine/cytokine production | 300 ng per mouse (i.p.) | 4–24 h | Bannenberg et al. (2005) |
| 100 ng per mouse (i.v.) | 2 h | Arita et al. (2005a) | ||
| Reduces PMN infiltration | 10 ng per mouse (i.p.) | 2–4 h | Serhan et al. (2006a) | |
| Colitis | Decreases PMN recruitment and pro-inflammatory gene expression | 50 μg kg−1 (i.p.) on days −8, −1 and 0 before induction of colitis | 4–12 days | Arita et al. (2005c) |
| Improves survival | ||||
| Reduces weight loss | ||||
| Oral (periodontitis) | Reduces PMN infiltration, stops inflammation-induced tissue and bone loss | 4 μg per tooth (topical application, every other day) | 6 weeks | Hasturk et al. (2006) |
| Eye (retinopathy) | Reduces vaso-obliteration and neovascularization | 10 ng per day (i.p.), from postnatal day 6 to day 17 | 17 days | Connor et al. (2007) |
| Resolvin E2 | ||||
| Peritonitis | Reduces PMN infiltration | 10 ng per mouse (i.v., i.p.) | 2 h | Tjonahen et al. (2006) |
Abbreviation: PMN, polymorphonuclear leukocyte.
Receptors
It was of interest to determine the receptors involved in the actions of RvE1 bioactions. To this end, a panel of G-protein-coupled receptors (GPCR) was screened (Arita et al., 2005a). An orphan receptor, denoted earlier as ChemR23, was found to attenuate nuclear factor (NF)-κB in response to RvE1. Specific binding of RvE1 to this receptor was confirmed using synthetic [3H]-labeled RvE1. The main second messenger for RvE1 agonist actions via ChemR23 appears to be activation of intracellular phosphorylation pathways rather than mobilization of intracellular calcium or cyclic AMP, as with most pro-inflammatory mediators (Arita et al., 2005a, 2005c). Recently, a second GPCR that interacts with RvE1 was identified, that is, the leukotriene B4 receptor (BLT)1 (Arita et al., 2007). RvE1 specifically interacts with BLT1 on human PMN, demonstrated by radioligand binding and G-protein signaling. Also, RvE1 attenuates LTB4-dependent pro-inflammatory signals, such as calcium influx and NF-κB activation. RvE1–BLT1 interaction in vivo was demonstrated using BLT1-deficient mice. Thus, RvE1 selectively interacts with two distinct GPCRs on different cell types to control inflammation and promote resolution.
Actions
Resolvins of the E-series comprise several molecules. Among them, RvE1 was the first isolated and studied in depth. RvE1 displayed potent stereoselective actions in vivo and with isolated cells (Table 3; Figure 2). At nanomolar levels in vitro, RvE1 dramatically reduced human PMN transendothelial migration, dendritic cell (DC) migration and interleukin (IL)-12 production (Serhan et al., 2002; Arita et al., 2005a).
In several animal models of inflammatory diseases, RvE1 displays potent counterregulatory actions that protect against leukocyte-mediated tissue injury and excessive pro-inflammatory gene expression (Arita et al., 2005a, 2005c; Hasturk et al., 2006). For example, administration of synthetic RvE1 blocks PMN infiltration in periodontal disease in a rabbit model (Hasturk et al., 2006) and protects against the development of 2,4,6-trinitrobenzene sulfonic acid-induced colitis (Arita et al., 2005c). Most recently, RvE1 was shown to reduce neovascularization in oxygen-induced retinopathy (Connor et al., 2007) and enhance CD55-dependent clearance of PMN from epithelial surfaces (Campbell et al., 2007). These new findings provide evidence for endogenous mechanism(s) that may account for some of the widely touted beneficial actions noted with dietary supplementation with ω-3 PUFA (EPA and DHA), thereby providing new approaches for the treatment of gastrointestinal mucosal and oral inflammation.
Metabolic inactivation
One route of enzymatic inactivation of RvE1 was recently established (Arita et al., 2006). In murine lung, apparently the 15-PGDH, which in abundant in lung tissues, can catalyze the conversion of RvE1 to 18-oxo-RvE1, representing the major initial metabolic route for RvE1 in lung tissues. At the same doses as RvE1, 18-oxo-RvE1 was devoid of activity in regulating/stopping PMN recruitment in zymosan-induced peritonitis. In human neutrophils, C-20 hydroxylation of RvE1 was the main route of conversion. An RvE1 analog, namely 19-(p-fluorophenoxy)-RvE1, was synthesized that resisted rapid metabolic inactivation and proved to retain biological activity, reducing PMN infiltration and pro-inflammatory cytokine/chemokine production in vivo. Thus, the designer RvE1 mimetics that resist further metabolism/inactivation could be useful tools to evaluate the agonist actions of RvE1 in complex disease models.
Resolvins: the 17R and 17S D-series resolvins from DHA
Formation and structure elucidation
17R D-series resolvins
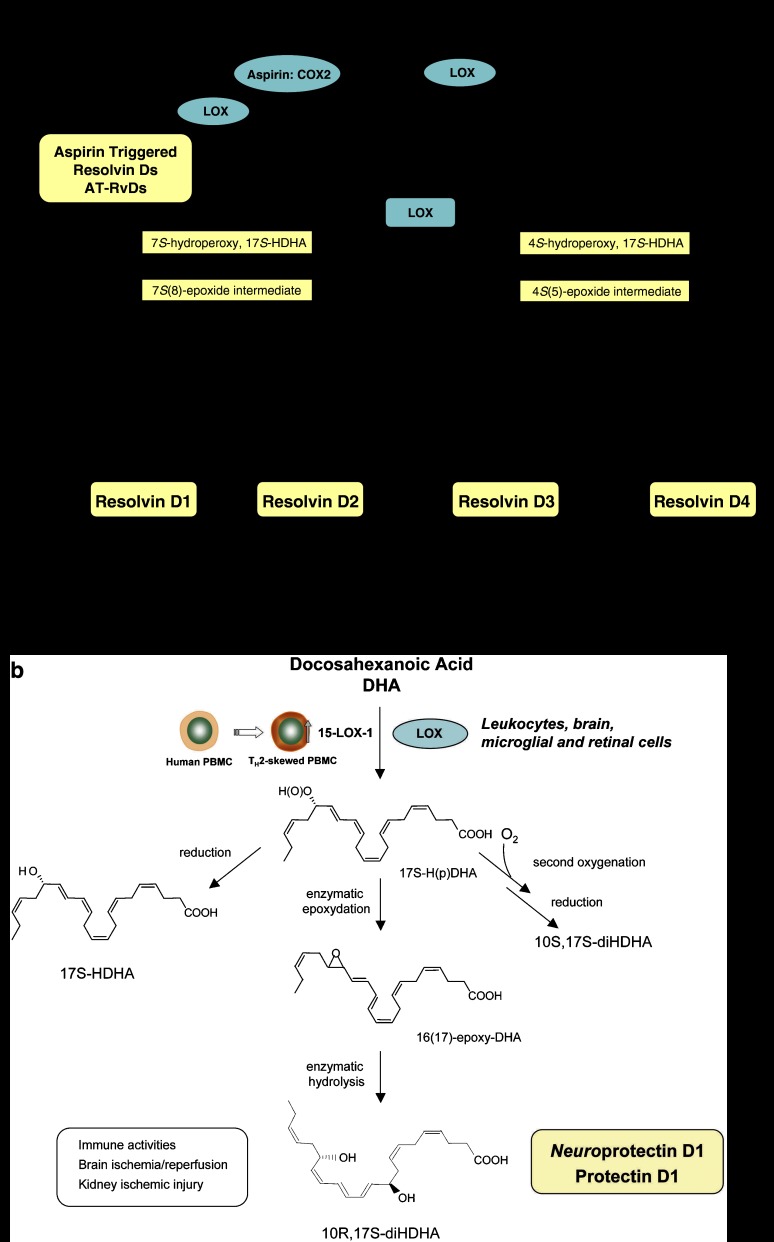
The resolving exudates from mice given aspirin plus DHA also contained novel 17R-hydroxy-DHA (17R-HDHA) and several related bioactive compounds (Figure 3a). The biosynthetic pathways were reconstructed in vitro to establish a potential origin for these novel compounds found in vivo. Human microvascular endothelial cells, when treated with aspirin in hypoxia, released 17R-HDHA produced from DHA. Human recombinant COX-2 converts DHA to 13-hydro(peroxy)-DHA, which is monitored as 13-hydroxydocosahexaenoic acid. With aspirin, this switches to 17R-oxygenation to give a group of AT resolvins (AT-RvD1 through RvD4) that were also found in brain (Serhan et al., 2002; Hong et al., 2003).
Figure 3.
D-series resolvins and protectins. (a) Resolvin Ds: formed from docosahexaenoic acid (DHA), the proposed biosynthetic pathways reconstructed in vitro involve the lipoxygenase (LOX) product 17S-H(p)DHA, which is rapidly transformed by the LOX activity in human polymorphonuclear leukocyte (PMN) into two epoxide intermediates. These two novel epoxide intermediates open to form bioactive products denoted 17S-resolvin D series (RvD1-4). Aspirin also impacts the formation of resolvin D series by catalytically switching COX-2 to a 17R-lipoxygenase-like mechanism that generates 17R-H(p)DHA, and subsequently 17R-resolvin D series (AT-RvDs). (b) Protectins: the initial enzymatic product 17S-H(p)DHA is converted to neuroprotectin D1/PD1. The complete stereochemistries of the bioactive mediators and related natural isomers are established (see text for further details).
17S D-series resolvins
Brain, synapses and retina are highly enriched in DHA, a major ω-3 fatty acid (Bazan, 1992; Salem et al., 2001). Deficiencies in this essential fatty acid are reportedly associated with neuronal function, cancer and inflammation (Coussens and Werb, 2002; Lawrence, 2007). Using the mediator lipidomics-informatics approach (Hong et al., 2007) employing tandem LC-PDA-MS-MS, it was exciting to find that neither aspirin nor exogenous DHA was required to monitor the production of these new structures in vivo (Hong et al., 2003; Duffield et al., 2006). The endogenous DHA was converted in vivo to a 17S alcohol-containing series of resolvins via LOX-initiated mechanisms (Hong et al., 2003; Marcheselli et al., 2003). The stereochemistry of both 17R- and 17S-series of resolvin D1 (RvD1 and AT-RvD1) was established and total organic synthesis achieved (Sun et al., 2007). The synthetic compounds matched the physical and biological properties of those enzymatically generated. RvD1 proved to be 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid, and AT-RvD1 matched 7S,8R,17R-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid. Also, the total organic synthesis of resolvin D2 (RvD2) was reported (Rodriguez and Spur, 2004), confirming the physical properties of the original compound identified in exudates (Serhan et al., 2002).
Actions
RvD1 and AT-RvD1 each proved to be potent regulators of both human and murine PMN. They stopped transendothelial migration of human neutrophils (EC50 approximately 30 nM) (Serhan et al., 2002). In microglial cells, they block TNF-α-induced IL-1β transcripts (Hong et al., 2003). In murine peritonitis in vivo, RvD1 and AT-RvD1 proved equipotent (at nanogram dosages), limiting PMN infiltration in a dose-dependent fashion (Table 4). Direct comparisons between RvE1, AT-RvD1 (triggered by aspirin treatment) and RvD1 (via LOX-initiated mechanisms) at equal doses of 100 ng per mouse demonstrated that RvD1 and AT-RvD1 display essentially similar actions, reducing PMN infiltration by ∼50% in peritonitis and air pouch, compared to ∼75–80% inhibition by RvE1 in air pouch (Serhan et al., 2002; Hong et al., 2003; Sun et al., 2007). Thus, DHA is precursor to potent protective mediators. Both the RvDs (17S-series) and AT RvDs (17R-series) are potent regulators of PMN infiltration in vivo, and the S to R switch with aspirin treatment in the biosynthesis of the 17 position alcohol in the ω side chain does not diminish their activity. Recently, RvD1 was generated in response to bilateral ischemia/reperfusion injury in mouse kidneys (Duffield et al., 2006). Administration of RvDs before or after ischemia resulted in a reduction in functional and morphological kidney injury. Interstitial fibrosis after ischemia/reperfusion was also reduced in mice treated with RvDs. In addition, RvDs reduced infiltrating leukocytes and blocked Toll-like receptor-mediated activation of macrophages. These findings demonstrated previously unrecognized endogenous anti-inflammatory and antifibrotic responses of RvDs in protecting acute kidney injury, confirmed with synthetic RvD1 and PD1.
Table 4. In vivo actions of resolvin D series.
| Disease model | Action | Dosage (route) | Duration of postexposure | Reference |
|---|---|---|---|---|
| Skin (dorsal air pouch) | Reduces PMN infiltration | 100 ng per mouse (intrapouch) | 4 h | Serhan et al. (2002) |
| Hong et al. (2003) | ||||
| Peritonitis | Reduces PMN infiltration | 100 ng per mouse (i.v.) | 2 h | Hong et al. (2003) |
| 0.05–50 μg kg−1 (i.v.) | 4 h | Sun et al. (2007) | ||
| Kidney ischemia-reperfusion | Protects in renal ischemic injury by limiting PMN infiltration | 5 μg per mouse (i.v., s.c.) | 24–48 h | Duffield et al. (2006) |
| Eye (retinopathy) | Reduces vaso-obliteration and neovascularization | 10 ng per day (i.p.), from postnatal day 6 to day 17 | 17 days | Connor et al. (2007) |
Abbreviation: PMN, polymorphonuclear leukocyte.
Metabolic inactivation
RvD1 was converted by EOR to novel 8-oxo- and 17-oxo-RvD1. 17-oxo-RvD1 gave dramatically reduced bioactivity, and the enzymatic conversion of AT-RvD1 was sharply reduced, indicating that the AT form resists rapid inactivation, suggesting that it is likely to be longer acting in vivo (Sun et al., 2007). 8-oxo-RvD1 retained activity in reducing peritonitis.
Protectins from DHA
Formation and structure elucidation
Endogenous DHA is also converted in vivo to a triene-containing structure via LOX-initiated mechanisms (Hong et al., 2003; Marcheselli et al., 2003). The LOX product 17S-H(p)DHA is converted to a 16(17)-epoxide that is enzymatically converted to the 10,17-dihydroxy-containing bioactive product (Hong et al., 2003), first denoted 10,17S-docosatriene, and recently coined NPD1/protectin D1 (PD1) based on its potent actions in vivo. PD1 possesses a conjugated triene-containing structure as the key feature of this new family of compounds derived from DHA. The complete stereochemistry of PD1, namely chirality of the alcohol groups and geometry of the conjugated triene, was recently established. Total organic synthesis of PD1 and related isomers and matching studies with biologically derived materials showed that endogenous PD1, signified as neuroprotectin D1 when produced by neural tissues, was recently established in isolated human cells in vitro and murine cells in vivo as 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid (Serhan et al., 2006a).
The geometry of the double bonds in PD1 and their positions during biosynthesis from the key intermediates in its biosynthesis in situ, namely via an epoxide intermediate at the 16(17) position, indicated that PD1 biosynthesis requires enzymatic steps to generate the potent bioactive molecule from DHA (Figure 3b). Other dihydroxy-docosanoids (both positional and geometric isomers) were identified natural isomers, but proved to be substantially less bioactive than native PD1 in vivo (Hong et al., 2003; Mukherjee et al., 2004; Serhan et al., 2006a). The double LOX product 10S,17S-diHDHA formed by sequential enzymatic oxygenation with molecular oxygen is less active than PD1 (Serhan et al., 2006a), and its conjugated triene is in the trans-, cis-, trans-, configuration, which is different from the configuration in PD1. Of note in humans, NPD1/PD1 is also generated by human T cells skewed toward Th2 phenotype and is biosynthesized via a 16(17)-epoxide intermediate in these human cells (Ariel et al., 2005; Figure 3b).
Actions
Identification of the resolvins and protectins generated from DHA now opens exploration into the essential roles of these pathways and mediators. PD1 proved to be log orders of magnitude more potent than its precursor DHA, demonstrated in several animal models of diseases (Table 5). Synthetic PD1 at 10 nM attenuates human neutrophil transmigration by ∼50% in vitro, whereas its Δ15-trans-isomer is essentially inactive. PD1 is also a potent regulator of PMN in vivo by reducing PMN infiltration (∼40% at 1 ng per mouse) in murine peritonitis. Of importance to potential treatments and uses, PD1 also reduced PMN infiltration when administered after the initiation of inflammation in vivo as well as acts in an additive fashion with RvE1 to stop PMN infiltration. These results indicate that PD1 is a potent, stereoselective anti-inflammatory molecule (Serhan et al., 2002, 2006a; Hong et al., 2003). PD1 displays potent actions in the eye, promoting corneal epithelial cell wound healing and limiting sequelae of thermal injury (Gronert et al., 2005), and also protects against retinopathy (Connor et al., 2007). In kidney ischemia/reperfusion injury, mouse kidneys produce RvDs and PD1. Administration of RvDs or PD1 to mice before the ischemia resulted in a reduction in functional and morphological kidney injury (Duffield et al., 2006). In addition, PD1 is generated in human asthma in breath condensates and in mouse lungs in vivo. Administration of PD1 in an allergic airway inflammation model dampens airway hyperresponsiveness and accelerated the resolution of airway inflammation (Levy et al., 2007a).
Table 5. In vivo actions of protectin D1.
| Disease model | Action | Dosage (route) | Duration of postexposure | Reference |
|---|---|---|---|---|
| Peritonitis | Reduces PMN infiltration | 100 ng per mouse (i.v.) | 2 h | Hong et al. (2003) |
| 0.1–100 ng per mouse (i.p.) | 2–4 h | Serhan et al. (2006a) | ||
| Shortens resolution interval and regulates cytokines/chemokines | 300 ng per mouse (i.p.) | 4–24 h | Bannenberg et al. (2005) | |
| Limits T-cell recruitment in peritonitis | 100 ng per mouse (i.v.) | 2 h | Ariel et al. (2005) | |
| Stroke | Limits stroke damage and PMN entry into the brain | 0.4 μg per mouse (perfusion) | 48 h | Marcheselli et al. (2003) |
| Retinal injury | Protects cellular damage from retinal injury | Mukherjee et al. (2004) | ||
| Alzheimer disease | Promotes neural cell survival, and reduces amyloid β-42-induced neurotoxicity | Lukiw et al. (2005) | ||
| Eye (wound healing) | Promotes corneal epithelial cell wound healing and repair Limits sequelae of thermal injury | 1 μg per mouse three times daily (topical) | 48 h | Gronert et al. (2005) |
| Eye (retinopathy) | Reduces vaso-obliteration and neovascularization | 10 ng per day (i.p.), from postnatal day 6 to 17 | 17 days | Connor et al. (2007) |
| Kidney ischemia- reperfusion | Protects in renal ischemic injury by limiting PMN infiltration | 5 μg per mouse (i.v., s.c.) | 24–48 h | Duffield et al. (2006) |
| Asthma | Decreases airway eosinophil, T-lymphocyte and pro-inflammatory mediators | 2–200 ng per mouse (i.v.) | 4 days | Levy et al. (2007a) |
Abbreviation: PMN, polymorphonuclear leukocyte.
Ongoing collaborative efforts with N Bazan and colleagues at LSU (Marcheselli et al., 2003; Mukherjee et al., 2004; Lukiw et al., 2005) have documented the formation of NPD1 in neural tissues and identified its potent immunomodulatory actions (Serhan et al., 2002, 2006a; Hong et al., 2003) and neuroprotective actions (Marcheselli et al., 2003; Mukherjee et al., 2004; Lukiw et al., 2005) (Table 5). NPD1 limits stroke brain injury in part by reducing the entry of activated PMN to the stroke area (Marcheselli et al., 2003) and retinal pigmented cellular damage and promotes brain cell survival, suppressing Aβ42-induced neurotoxicity (Mukherjee et al., 2004) (Table 5). The protection obtained with NPD1 in the presence of oxidative stress involves upregulation of the antiapoptotic Bcl-2 and Bcl-x(L) in response to exposure to nanomolar amounts of NPD1, whereas the proapoptotic Bax and Bad are downregulated, resulting in reduced caspase-3 cleavage (Lukiw et al., 2005).
In summation, when taken together these results are the first to indicate that novel potent lipid mediators are generated and present within resolving exudates that are derived from the precursors, ω-3 fatty acids EPA and DHA. This work also established that these novel compounds derived from EPA and DHA carry anti-inflammatory and pro-resolving properties and that they are log orders more potent than their precursors in vivo. Most importantly, their actions are stereoselective and cell-type specific.
Conclusion
Endogenous lipid mediators (autacoids) play key roles in local controlling and programming of the acute innate inflammatory response and its resolution as an active biosynthetic process in vivo (Serhan, 1994; Serhan et al., 2000). Systematic studies focusing on mechanisms of resolution demonstrated that PMN change their profile of lipid mediators in exudates and switch from producing initiators to stop signals that are also pro-resolving mediators in a process termed ‘eicosanoid class switching' (Figure 1). This turns on production of LX biosynthetic machinery (Levy et al., 2001). LX and ATL possess both anti-inflammatory and pro-resolving actions, stopping PMN infiltration and stimulating the nonphlogistic recruitment of monocytes in vivo (Maddox and Serhan, 1996; Romano et al., 1996; Maddox et al., 1997, 1998; Hachicha et al., 1999) (for reviews, see Serhan, 1994, 1997). These systematic, temporal and differential analyses using combined cell trafficking, lipid-mediator lipidomics informatics and proteomic analyses of the resolving exudates uncovered ω-3 PUFA-derived resolvins (Rv) and protectins (PD). These small molecules have unique structures, are biosynthesized by independent pathways and share anti-inflammatory actions in vivo, but each member evokes specific and temporally distinct pro-resolving responses in vivo. The epimeric forms of these families of compounds, namely, their AT epimers (ATL, AT-Rv and AT-PD), are endogenously generated during aspirin treatment. These AT forms share the characteristic features of reducing inflammation and PMN-mediated injury from within, key culprits in many widespread human diseases, as well as stimulating resolution. The results summarized here underscore the role(s) of PUFA-derived local mediators in resolution and tissue catabasis (Tables 3, 4 and 5).
The new families of EPA- and DHA-derived chemical mediators, namely the resolvins and protectins, qualify as ‘resolution agonists' along with the n−6 derived agonists of resolution, the LX, in this new arena of immunomodulation and tissue protection. These are conserved structures in evolution, because rainbow trout biosynthesize resolvins and protectins, which are present in their neural and hematopoietic tissues (Hong et al., 2005). Their functional roles in fish and lower phyla remain to be established, but are likely to involve cell trafficking, motility and protection. Additionally, they now open new avenues to design ‘resolution-targeted'-based therapies where aberrant uncontrolled inflammation and/or impaired resolution are components of the disease pathophysiology. Of interest, one of the authors of this review (CNS) recently served as discussion chair for a National Institute of Dental and Craniofacial Research–hosted programmatic conference of experts to contemplate the role of controlled resolution of inflammatory responses by LX, Rv and PD and their potential in the molecular design of nanomaterials useful in engineering tissue healing and regeneration (see the NIH commentary in Lumelsky, 2007) as novel means to harness uncontrolled inflammation in biomaterials and engineered tissues. Since LXs act on neural stem cells (Wada et al., 2006), and PD1 is neuroprotective (Mukherjee et al., 2004), it is likely that this new genus of endogenous pro-resolving mediators (Figure 1), their mimetics and stable analogs reviewed herein will not only prove of interest in the control of inflammation and fibrosis but will also open new opportunities for therapies and engineered materials for regenerative medicine. Thus, agonists of inflammatory resolution are likely to have a promising future in drug development for inflammatory diseases and regenerative medicine. This is best exemplified to the BJP readership by the recent findings that two of the most widely used drugs worldwide, aspirin and glucocorticoids, have as their in vivo mechanism of action the specific ability to generate endogenous mediators of resolution (Perretti et al., 2002; Paul-Clark et al., 2004; Gilroy, 2005; Gilroy and Perretti, 2005; Rossi and Sawatzky, 2007).
Acknowledgments
This work was supported in part by National Institutes of Health grant numbers GM38675, DK074448 and P-50 DE-016191. We thank Mary Halm Small for expert assistance with manuscript preparation.
Glossary
- 10S,17S-diHDHA
double dioxygenase product of DHA
- AA
arachidonic acid
- ATL
aspirin-triggered lipoxin A4 (5S,6R,15R-trihydroxyl-7,9,13-trans-11-cis-eicosatetraenoic acid)
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- LC-MS-MS
liquid chromatography-tandem mass spectrometry
- LOX
lipoxygenase
- LXA4
lipoxin A4 (5S,6R,15S-trihydroxyl-7,9,13-trans-11-cis-eicosatetraenoic acid)
- NPD1
neuroprotectin D1; PD1 generated in neural systems
- PD
Protectins, the family of DHA-derived mediators possessing a conjugated triene structure as a distinguishing feature
- NPD1/PD1
protectin D1 (10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid)
- PMN
polymorphonuclear leukocyte
- PUFA
polyunsaturated fatty acids
- Rv
resolvins, resolution phase interaction products carrying bioactivity
- RvD1
resolvin D1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid)
- RvD2
resolvin D2 (7S,16R,17S-trihydroxy-docosa-4Z,8E,10Z,12E,14E,19Z-hexaenoic acid)
- RvE1
resolvin E1 (5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid)
- RvE2
resolvin E2 (5S,18-dihydroxyeicosapentaenoic acid)
Footnotes
Conflict of interest
CNS is inventor on resolvin and lipoxin analog patents that are assigned to Brigham and Women's Hospital. These patents as well as use patents are licensed for clinical development. CNS serves as consultant for companies developing these.
References
- Ariel A, Li P-L, Wang W, Tang W-X, Fredman G, Hong S, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005a;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Clish CB, Serhan CN. The contributions of aspirin and microbial oxygenase in the biosynthesis of anti-inflammatory resolvins: novel oxygenase products from omega-3 polyunsaturated fatty acids. Biochem Biophy Res Commun. 2005b;338:149–157. doi: 10.1016/j.bbrc.2005.07.181. [DOI] [PubMed] [Google Scholar]
- Arita M, Oh S, Chonan T, Hong S, Elangovan S, Sun Y-P, et al. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J Biol Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005c;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira-Melo C, Serra MF, Diaz BL, Cordeiro RSB, Silva PMR, Lenzi HL, et al. Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: relationship with concurrent eosinophilia. J Immunol. 2000;164:1029–1036. doi: 10.4049/jimmunol.164.2.1029. [DOI] [PubMed] [Google Scholar]
- Bannenberg G, Moussignac R-L, Gronert K, Devchand P, Schmidt B, Guilford WJ, et al. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol. 2004;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Therapeutic strategies for allergic diseases. Nature. 1999;402:B31–B38. doi: 10.1038/35037026. [DOI] [PubMed] [Google Scholar]
- Bazan NG.1990Supply of n−3 polyunsaturated fatty acids and their significance in the central nervous systemIn: Wurtman RJ, Wurtman JJ (eds).Nutrition and the Brain Raven Press: New York; 1–22. [Google Scholar]
- Bazan NG. Supply, uptake, and utilization of docosahexaenoic acid during photoreceptor cell differentiation. Nestle Nutr Workshop Ser. 1992;28:121–133. [Google Scholar]
- Bergström S. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Almqvist & Wiksell: Stockholm; 1982. The prostaglandins: from the laboratory to the clinic; pp. 129–148. [Google Scholar]
- Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Nishi SP, Martinez JD, et al. Augmentation of myocardial production of 15-epi-lipoxin-A4 by pioglitazone and atorvastatin in the rat. Circulation. 2006;114:929–935. doi: 10.1161/CIRCULATIONAHA.106.629907. [DOI] [PubMed] [Google Scholar]
- Borgeat P, Hamberg M, Samuelsson B. Transformation of arachidonic acid and homo-gamma-linolenic acid by rabbit polymorphonuclear leukocytes. J Biol Chem. 1976;251:7816–7820. [PubMed] [Google Scholar]
- Burr GO, Burr MM. A new deficiency disease produced by the rigid exclusion of fat from the diet. J Biol Chem. 1929;82:345–367. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, et al. 2007Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution FASEB J(in press), doi:10.1096/fi.1007-8473com. [DOI] [PubMed]
- Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers anti-inflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Gronert K, Clish CB, O'Brien JA, Freeman MW, Serhan CN. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest. 1999;104:309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlén S-E, Drazen JM, Hay DWP, Rovati GE, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Chiang N, Takano T, Arita M, Watanabe S, Serhan CN. A novel rat lipoxin A4 receptor that is conserved in structure and function. Br J Pharmacol. 2003;139:89–98. doi: 10.1038/sj.bjp.0705220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Tai HH. Inhibition of NAD+-dependent 15-hydroxyprostaglandin dehydrogenase (15-PGDH) by cyclooxygenase inhibitors and chemopreventive agents. Prostaglandins Leukot Essent Fatty Acids. 2002;67:461–465. doi: 10.1054/plef.2002.0457. [DOI] [PubMed] [Google Scholar]
- Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am Rev Respir Dis. 1992;145:1281–1284. doi: 10.1164/ajrccm/145.6.1281. [DOI] [PubMed] [Google Scholar]
- Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell–leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clish CB, O'Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc Natl Acad Sci USA. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clish CB, Sun YP, Serhan CN. Identification of dual cyclooxygenase-eicosanoid oxidoreductase inhibitors: NSAIDs that inhibit PG-LX reductase/LTB(4) dehydrogenase. Biochem Biophys Res Commun. 2001;288:868–874. doi: 10.1006/bbrc.2001.5841. [DOI] [PubMed] [Google Scholar]
- Colamorea T, Di Paola R, Macchia F, Guerrese MC, Tursi A, Butterfield JH, et al. 5-Lipoxygenase upregulation by dexamethasone in human mast cells. Biochem Biophys Res Commun. 1999;265:617–624. doi: 10.1006/bbrc.1999.1732. [DOI] [PubMed] [Google Scholar]
- Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran RS, Kumar V, Collins T.1999Robbins Pathologic Basis of Disease WB Saunders: Philadelphia; p1425 [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devchand PR, Schmidt BA, Primo VC, Zhang Q-y, Arnaout MA, Serhan CN, et al. A synthetic eicosanoid LX-mimetic unravels host–donor interactions in allogeneic BMT-induced GvHD to reveal an early protective role for host neutrophils. FASEB J. 2005;19:203–210. doi: 10.1096/fj.04-2565com. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Hong S, Vaidya V, Lu Y, Fredman G, Serhan CN, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- Fierro IM, Kutok JL, Serhan CN. Novel lipid mediator regulators of endothelial cell proliferation and migration: aspirin-triggered-15R-lipoxin A4 and lipoxin A4. J Pharmacol Exp Ther. 2002;300:385–392. doi: 10.1124/jpet.300.2.385. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Wallace JL, Mencarelli A, Distrutti E, Rizzo G, Farneti S, et al. A beta-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc Natl Acad Sci USA. 2004;101:15736–15741. doi: 10.1073/pnas.0404722101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower RJ. Prostaglandins, bioassay and inflammation. Br J Pharmacol. 2006;147:S182–S192. doi: 10.1038/sj.bjp.0706506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, et al. Lipoxin A4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- Gilroy DW. The role of aspirin-triggered lipoxins in the mechanism of action of aspirin. Prostaglandins Leukot Essent Fatty Acids. 2005;73:203–210. doi: 10.1016/j.plefa.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cycloxygenase may have anti-inflammatory properties. Nature Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Perretti M. Aspirin and steroids: new mechanistic findings and avenues for drug discovery. Curr Op Pharmacol. 2005;5:405–411. doi: 10.1016/j.coph.2005.02.006. [DOI] [PubMed] [Google Scholar]
- GISSI-Prevenzione Investigators Dietary supplementation with n−3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Schwartzman ML. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- Guilford WJ, Bauman JG, Skuballa W, Bauer S, Wei GP, Davey D, et al. Novel 3-oxa lipoxin A4 analogues with enhanced chemical and metabolic stability have anti-inflammatory activity in vivo. J Med Chem. 2004;47:2157–2165. doi: 10.1021/jm030569l. [DOI] [PubMed] [Google Scholar]
- Hachicha M, Pouliot M, Petasis NA, Serhan CN. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1α-initiated neutrophil responses and trafficking: regulators of a cytokine–chemokine axis. J Exp Med. 1999;189:1923–1929. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M, Samuelsson B. On the specificity of the oxygenation of unsaturated fatty acids catalyzed by soybean lipoxidase. J Biol Chem. 1967;242:5329–5335. [PubMed] [Google Scholar]
- Hansen HS. Inhibition by indomethacin and aspirin of 15-hydroxyprostaglandin dehydrogenase in vitro. Prostaglandins Leukot Essent Fatty Acids. 1974;8:95–105. doi: 10.1016/0090-6980(74)90072-0. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Robertson AKL, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol Mech Dis. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Murakami Y, Kitasato H, Hayashi I, Endo H. Glucocorticoids co-interact with lipoxin A4 via lipoxin A4 receptor (ALX) up-regulation. Biomed Pharmacother. 2007;61:81–85. doi: 10.1016/j.biopha.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, et al. RvE1 protects from local inflammation and osteoclast mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- Henson PM. Dampening inflammation. Nat Immunol. 2005;6:1179–1181. doi: 10.1038/ni1205-1179. [DOI] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand P, Moussignac R-L, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Hong S, Lu Y, Yang R, Gotlinger KH, Petasis NA, Serhan CN. Resolvin D1, protectin D1, and related docosahexaenoic acid-derived products: analysis via electrospray/low energy tandem mass spectrometry based on spectra and fragmentation mechanisms. J Am Soc Mass Spectrom. 2007;18:128–144. doi: 10.1016/j.jasms.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Tjonahen E, Morgan EL, Yu L, Serhan CN, Rowley AF. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins—mediator lipidomic analysis. Prostaglandins Other Lipid Mediat. 2005;78:107–116. doi: 10.1016/j.prostaglandins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Jin S-W, Zhang L, Lian Q-Q, Liu D, Wu P, Yao S-L, et al. Posttreatment with aspirin-triggered lipoxin A4 analog attenuates lipopolysaccharide-induced acute lung injury in mice: the role of heme oxygenase-1. Anesth Analg. 2007;104:369–377. doi: 10.1213/01.ane.0000252414.00363.c4. [DOI] [PubMed] [Google Scholar]
- Jin Y, Arita M, Zhang Q, Serhan C, Dana R.2007Aspirin-triggered lipoxin agonist (ATLa) inhibits corneal angiogenesis (abstract)Association for Research in Vision and Ophthalmology Annual Meeting, Fort Lauderdale, May 6–10, 2007.
- Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nature Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- Katoh T, Takahashi K, DeBoer DK, Serhan CN, Badr KF. Renal hemodynamic actions of lipoxins in rats: a comparative physiological study. Am J Physiol. 1992;263:F436–F442. doi: 10.1152/ajprenal.1992.263.3.F436. [DOI] [PubMed] [Google Scholar]
- Kieran NE, Maderna P, Godson C. Lipoxins: potential anti-inflammatory, proresolution, and antifibrotic mediators in renal disease. Kidney Int. 2004;65:1145–1154. doi: 10.1111/j.1523-1755.2004.00487.x. [DOI] [PubMed] [Google Scholar]
- Lands WEM. Proceedings of the AOCS Short Course on Polyunsaturated Fatty Acids and Eicosanoids. American Oil Chemists' Society: Champaign, IL; 1987. [Google Scholar]
- Lawrence T. Inflammation and cancer: a failure of resolution. Trends Pharmacol Sci. 2007;28:162–165. doi: 10.1016/j.tips.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- Lee TH, Mencia-Huerta J-M, Shih C, Corey EJ, Lewis RA, Austen KF. Effects of exogenous arachidonic, eicosapentaenoic, and docosahexaenoic acids on the generation of 5-lipoxygenase pathway products by ionophore-activated human neutrophils. J Clin Invest. 1984;74:1922–1933. doi: 10.1172/JCI111612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard MO, Hannan K, Burne MJ, Lappin DW, Doran P, Coleman P, et al. 15-epi-16-(para-fluorophenoxy)-lipoxin A4-methyl ester, a synthetic analogue of 15-epi-lipoxin A4, is protective in experimental ischemic acute renal failure. J Am Soc Nephrol. 2002;13:1657–1662. doi: 10.1097/01.asn.0000015795.74094.91. [DOI] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nature Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A4. Nature Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyper-responsiveness. J Immunol. 2007a;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Lukacs NW, Berlin AA, Schmidt B, Guilford WJ, Serhan CN, et al. 2007bLipoxin A4 analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism FASEB J(in press), doi:10.1096/fj07-8653com. [DOI] [PMC free article] [PubMed]
- Libby P. Atherosclerosis: the new view. Sci Am. 2002;286:46–55. doi: 10.1038/scientificamerican0502-46. [DOI] [PubMed] [Google Scholar]
- Lu Y, Hong S, Gotlinger K, Serhan CN. Lipid mediator informatics and proteomics in inflammation-resolution. Scientific World Journal. 2006;6:589–614. doi: 10.1100/tsw.2006.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Hong S, Tjonahen E, Serhan CN. Mediator-lipidomics: databases and search algorithms for PUFA-derived mediators. J Lipid Res. 2005;46:790–802. doi: 10.1194/jlr.D400020-JLR200. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumelsky NL. Commentary: engineering of tissue healing and regeneration. Tissue Eng. 2007;13:1393–1398. doi: 10.1089/ten.2007.0100. [DOI] [PubMed] [Google Scholar]
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Colgan SP, Clish CB, Petasis NA, Fokin VV, Serhan CN. Lipoxin B4 regulates human monocyte/neutrophil adherence and motility: design of stable lipoxin B4 analogs with increased biologic activity. FASEB J. 1998;12:487–494. doi: 10.1096/fasebj.12.6.487. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderna P, Godson C. Taking insult from injury: lipoxins and lipoxin receptor agonists and phagocytosis of apoptotic cells. Prostaglandins Leukot Essent Fatty Acids. 2005;73:179–187. doi: 10.1016/j.plefa.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Majno G, Joris I. Cells, Tissues and Disease: Principles of General Pathology. Blackwell: Cambridge, Mass; 1996. [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, Hua Tian X, Gronert K, Musto A, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, et al. Early protection against sudden death by n−3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Paul-Clark MJ, van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A(4) receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petasis NA, Akritopoulou-Zanze I, Fokin VV, Bernasconi G, Keledjian R, Yang R, et al. Design, synthesis and bioactions of novel stable mimetics of lipoxins and aspirin-triggered lipoxins. Prostaglandins Leukot Essent Fatty Acids. 2005;73:301–321. doi: 10.1016/j.plefa.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Lipoxin A4 analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: a role for cyclooxygenase-2 and lipoxins in periodontal disease. Biochemistry. 2000;39:4761–4768. doi: 10.1021/bi992551b. [DOI] [PubMed] [Google Scholar]
- Reich EE, Zackert WE, Brame CJ, Chen Y, Roberts LJ, II, Hachey DL, et al. Formation of novel D-ring and E-ring isoprostane-like compounds (D4/E4-neuroprostanes) in vivo from docosahexaenoic acid. Biochemistry. 2000;39:2376–2383. doi: 10.1021/bi992000l. [DOI] [PubMed] [Google Scholar]
- Rodriguez AR, Spur BW. First total synthesis of 7(S),16(R),17(S)-resolvin D2, a potent anti-inflammatory lipid mediator. Tetrahedron Lett. 2004;45:8717–8720. [Google Scholar]
- Romano M, Maddox JF, Serhan CN. Activation of human monocytes and the acute monocytic leukemia cell line (THP-1) by lipoxins involves unique signaling pathways for lipoxin A4 versus lipoxin B4. J Immunol. 1996;157:2149–2154. [PubMed] [Google Scholar]
- Rossi AG, Sawatzky DA.2007The resolution of inflammationIn: Parnham MJ (ed).Progress in Inflammation Research Birkhäuser Verlag AG: Basel [Google Scholar]
- Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- Salem N, Jr, Litman B, Kim H-Y, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Almqvist & Wiksell: Stockholm; 1982. From studies of biochemical mechanisms to novel biological mediators: prostaglandin endoperoxides, thromboxanes and leukotrienes; pp. 153–174. [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Sawazaki S, Salem N, Jr, Kim H-Y. Lipoxygenation of docosahexaenoic acid by the rat pineal body. J Neurochem. 1994;62:2437–2447. doi: 10.1046/j.1471-4159.1994.62062437.x. [DOI] [PubMed] [Google Scholar]
- Sawmynaden P, Perretti M. Glucocorticoid upregulation of the annexin-A1 receptor in leukocytes. Biochem Biophys Res Commun. 2006;349:1351–1355. doi: 10.1016/j.bbrc.2006.08.179. [DOI] [PubMed] [Google Scholar]
- Scalia R, Gefen J, Petasis NA, Serhan CN, Lefer AM. Lipoxin A4 stable analogs inhibit leukocyte rolling and adherence in the rat mesenteric microvasculature: role of P-selectin. Proc Natl Acad Sci USA. 1997;94:9967–9972. doi: 10.1073/pnas.94.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottelius AJ, Giesen C, Asadullah K, Fierro IM, Colgan SP, Bauman J, et al. An aspirin-triggered lipoxin A4 stable analog displays a unique topical anti-inflammatory profile. J Immunol. 2002;169:7063–7070. doi: 10.4049/jimmunol.169.12.7063. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim Biophys Acta. 1994;1212:1–25. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): a jungle of cell–cell interactions or a therapeutic opportunity. Prostaglandins. 1997;53:107–137. doi: 10.1016/s0090-6980(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: an update and role in anti-inflammation and pro-resolution. Prostaglandins Other Lipid Mediat. 2002;68-69:433–455. doi: 10.1016/s0090-6980(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol. 2004;122:305–321. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LAJ, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006a;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- Serhan CN.guest ed. (2005Special issue on lipoxins and aspirin-triggered lipoxins Prostaglandins Leukot Essent Fatty Acids 73139–321. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Lu Y. Lipid mediator informatics-lipidomics: novel pathways in mapping resolution. AAPS J. 2006b;8:E284–E297. doi: 10.1007/BF02854899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, et al. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP, Leaf A, Salem N., Jr Workshop on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. J Am Coll Nutr. 1999;18:487–489. doi: 10.1080/07315724.1999.10718888. [DOI] [PubMed] [Google Scholar]
- Sun Y-P, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, et al. Resolvin D1 and its aspirin-triggered 17R epimer: stereochemical assignments, anti-inflammatory properties and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin stop inflammatory pain processing. J Exp Med. 2007;204:245–252. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 and LXA4 stable analogs are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, et al. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Tong M, Tai HH. Dexamethasone inhibits the induction of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase by phorbol ester in human promonocytic U937 cells. Biochim Biophys Acta. 2000;1497:61–68. doi: 10.1016/s0167-4889(00)00039-2. [DOI] [PubMed] [Google Scholar]
- Uz T, Dwivedi Y, Qeli A, Peters-Golden M, Pandey G, Manev H. Glucocorticoid receptors are required for up-regulation of neuronal 5-lipoxygenase (5LOX) expression by dexamethasone. FASEB J. 2001;15:1792–1794. doi: 10.1096/fj.00-0836fje. [DOI] [PubMed] [Google Scholar]
- Vane JR. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Almqvist & Wiksell: Stockholm; 1982. Adventures and excursions in bioassay: the stepping stones to prostacyclin; pp. 181–206. [Google Scholar]
- VanRollins M, Baker RC, Sprecher HW, Murphy RC. Oxidation of docosahexaenoic acid by rat liver microsomes. J Biol Chem. 1984;259:5776–5783. [PubMed] [Google Scholar]
- Wada K, Arita M, Nakajima A, Katayama K, Kudo C, Kamisaki Y, et al. Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. FASEB J. 2006;20:1785–1792. doi: 10.1096/fj.06-5809com. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Fiorucci S. A magic bullet for mucosal protection … and aspirin is the trigger. Trends Pharmacol Sci. 2003;24:323–326. doi: 10.1016/S0165-6147(03)00166-4. [DOI] [PubMed] [Google Scholar]
- Winyard PG, Willoughby DA.2003Inflammation protocolsIn: Walker JM (ed).Methods in Molecular Biology Totowa, NJ: Humana; p378 [Google Scholar]
- Wu S-H, Wu X-H, Liao P-Y, Dong L. Signal transduction involved in protective effects of 15(R/S)-methyl-lipoxin A4 on mesangioproliferative nephritis in rats. Prostaglandins Leukot Essent Fatty Acids. 2007;76:173–180. doi: 10.1016/j.plefa.2006.12.006. [DOI] [PubMed] [Google Scholar]