Abstract
G protein-coupled receptors are one of the most actively studied families of proteins. However, despite the ubiquity of protein dimerization and oligomerization as a structural and functional motif in biology, until the last decade they were generally considered as monomeric, non-interacting polypeptides. For the metabotropic glutamate-like group of G protein-coupled receptors, it is now firmly established that they exist and function as dimers or, potentially, even within higher-order structures. Despite some evidence continuing to support the view that rhodopsin-like G protein-coupled receptors are predominantly monomers, many recent studies are consistent with the dimerization/oligomerization of such receptors. Key roles suggested for dimerization of G protein-coupled receptors include control of protein maturation and cell surface delivery and providing the correct framework for interactions with both hetero-trimeric G proteins and arrestins to allow signal generation and its termination. As G protein-coupled receptors are the most targeted group of proteins for the development of therapeutic small molecule medicines, recent indications that hetero-dimerization between co-expressed G protein-coupled receptors may be a common process offers the potential for the development of more selective and tissue restricted medicines. However, many of the key experiments have, so far, been limited to model cell systems. Priorities for the future include the generation of tools and reagents able to identify unequivocally potential G protein-coupled receptor hetero-dimers in native tissues and detailed analyses of the influence of hetero-dimerization on receptor function and pharmacology.
Keywords: G protein-coupled receptor, G protein, signal transduction, receptor trafficking, receptor pharmacology, drug design, therapeutic target
Guanine nucleotide-binding protein (G protein)-coupled receptors (GPCRs) are frequently described as the most tractable target class for the development of novel, small molecule therapeutic medicines (Schlyer and Horuk, 2006). In part, this reflects the historical success of a wide range of medicines that target GPCRs, including those that recognize and respond to neurotransmitters including adrenaline/nor-adrenaline, dopamine, histamine and 5-hydroxytryptamine. It is also likely that drugs that bind to GPCRs that respond to agents as diverse as ions, chemokines, endocannabinoids, free fatty acids, nucleotides, peptide hormones and vitamins will offer the potential to treat a wide range of disorders where there is current unmet need (Wise et al., 2004). The development of radioligand-binding experiments, based on the selectivity and high affinity of antagonist drugs, allowed the direct detection and quantitation of GPCR expression (Hill, 2006). While the complex shapes of [3H]antagonist/agonist competition-binding experiments were instrumental in the appreciation of interactions between GPCRs and their cognate, signal transducing heterotrimeric G proteins (Milligan and Kostenis, 2006), the monophasic nature of [3H]antagonist/antagonist competition-binding experiments promoted the concept of GPCRs as single, non-interacting species. Until the last decade, despite a number of earlier suggestions to the contrary (see Salahpour et al., 2000 for review), it was generally accepted that GPCRs, therefore, existed as monomers. Over the last 10 years, this dogma has gradually reversed, such that despite a number of recent reports indicating that functional GPCRs either can be (Bayburt et al., 2007; Whorton et al., 2007), or routinely are, (James et al., 2006; Meyer et al., 2006) monomers, the vast majority of the literature favours the concept that GPCRs exist, and potentially function, as either dimers or higher-order oligomers (Milligan, 2004, 2007). As well as the expectation that individual GPCRs will exist as homo-dimers, that is, that both protomers of the dimer will be the same polypeptide, there has been growing evidence of the potential of many GPCRs to hetero-dimerize when co-expressed (Milligan, 2006). This suggests the probability of novel and distinct pharmacology and mechanisms of regulation, and hints at means to design more selective therapeutic ligands with reduced side effects (Milligan, 2006). The International Union of Basic and Clinical Pharmacology has recently presented recommendations for the recognition and nomenclature of GPCR hetero-dimers/multimers (Pin et al., 2007) and the G Protein-Coupled Receptor Oligomerization Knowledge Base (GPCR-OKB) ontology project (http://pbtest.med.cornell.edu/wiki/index.php/GPCR_OKB) curates information on this topic (Skrabanek et al., 2007). The aim of this review will be to consider the evidence that supports GPCR dimerization/oligomerization and to address the functional and pharmacological consequences thereof. As the review is related to the Life Sciences 2007 symposium ‘A day in the life of a GPCR', the dimeric/oligomeric status of GPCRs at various stages in their life history from synthesis to destruction has also been considered.
Metabotropic glutamate-like receptors exist and function as dimers
Although relatively small in number, the metabotropic glutamate-like, or family C GPCRs (Brauner-Osborne et al., 2007) clearly exist and function as dimers (Bonde et al., 2006). A defining feature of the de-orphanized members of this family is the presence of a long N-terminal domain that binds the physiological, orthosteric ligand. Early studies on the quaternary structure of each of mGluR5 (Romano et al., 1996), mGluR1a (Robbins et al., 1999) and the Ca2+-sensing receptor (Ray et al., 1999) demonstrated these to be dimers linked via intermolecular, extracellular disulphide bonds, while initial analysis of the molecular basis of interactions between the two polypeptides that generate the functional γ-aminobutyric acid (GABA)B receptor indicated a likely key role for so-called ‘coiled–coil' interactions involving the intracellular C-terminal tails (White et al., 1998). Although clearly important, subsequent studies have also implicated roles for further, non-covalent interactions in dimerization of all family C GPCRs (Romano et al., 2001; Zhang et al., 2001) that reflect interactions between the transmembrane elements of the individual polypeptides. Most importantly, the ability to generate and purify the isolated extracellular domain of mGluR receptors has resulted in the production of crystals and the analysis of structural detail at atomic level resolution that has confirmed the key contribution of the extracellular domain to dimerization (Tsuchiya et al., 2002; Muto et al., 2007).
Although there have been reports of hetero-dimerization between the mGluR1 and the Ca2+-sensing receptor (Gama et al., 2001) and, recently, between the Ca2+-sensing receptor and the subunits of the GABAB receptor (Chang et al., 2007), the general consensus is that the various mGluR subtypes exist and function predominantly as homo-dimers. In contrast, both the functional GABAB receptor (Marshall et al., 1999) and both the sweet and umami T1 taste receptors (Chandrashekar et al., 2006; Palmer, 2007) reflect the constitutive hetero-dimerization of pairs of co-expressed family C GPCRs. These are currently the most compelling, and functionally best-defined examples of GPCR hetero-dimerization (Pin et al., 2007). In the case of the GABAB receptor, initial identification of a seven transmembrane domain GPCR (GABAB R1) that bound a radiolabelled antagonist with high affinity but displayed low affinity for and lack of response to GABA (Kaupmann et al., 1997), resulted in a search for partner polypeptides that might alter the pharmacology and function of GABABR1. Combinations of informatic approaches, co-immunoprecipitation and yeast two-hybrid screens lead to the identification of GABABR2, a distinct class C GPCR, which, although unable to bind GABA itself, was able to uncover functional responses to GABA and the anticipated pharmacology of the GABAB receptor when co-expressed with GABAB R1. Detailed investigation demonstrated that a key role of the GABABR2 polypeptide was to mask an endoplasmic reticulum (ER) retention motif within the intracellular C-terminal tail of GABAB R1 (Margeta-Mitrovic et al., 2000) and allow cell surface delivery, and hence function, of the hetero-dimeric complex. In a similar vein, sweet tastes are identified by cells of the taste papillae that co-express the class C T1R2 and T1R3 taste receptors, while perception of umami sensations requires the co-expression of the T1R1 and T1R3 receptors. Mouse knockout studies have been of particular use in confirming the physiology of these pairings (Zhao et al., 2003) and the food and flavours industry has been highly active in exploring the detailed pharmacology of these GPCR hetero-dimers.
Do rhodopsin-like GPCRs also exist and function as dimers/oligomers?
In recent years, a vast literature has emerged to support the concept that family A, rhodopsin-like GPCRs also exist, and potentially function, as dimers/oligomers. In addition, an emerging literature suggests that hetero-dimerization might be almost as common as homo-dimerization. However, at the current time, most of the reported examples of family A GPCR hetero-dimerization fail to meet each and all of the strict requirements suggested by Pin et al. (2007), particularly in relation to clear demonstration of their existence in native tissues. Furthermore, a number of recent studies have questioned both the existence of dimers at low expression levels (James et al., 2006) and suggestions that dimerization is inherently required for function (Bayburt et al., 2007; Whorton et al., 2007).
Methods to assess GPCR quaternary structure
Central to efforts to explore (and to question) GPCR dimerization is the approaches that have been employed (Milligan and Bouvier, 2005). Particularly, due to a dearth of selective high affinity anti-GPCR antibodies, early studies relied heavily on the ability to co-immunoprecipitate forms of co-expressed GPCRs, which had been differentially epitope-tagged and then co-expressed transiently in heterologous cell lines. Despite controls that involved the mixing of detergent extracts from cell populations, each expressing only one form of the GPCR and/or either ultra centrifugation or passage through 0.22 μM filters to ensure that extracts were truly soluble and did not contain membrane fragments that contained both, but monomeric, forms of the receptors being studied, exclusive reliance on co-immunoprecipitation is now generally considered insufficient. The mainstay of both homo- and hetero-dimerization studies on rhodopsin-like receptors is various forms of resonance energy transfer techniques, based on the non-radiative transfer of energy between an energy donor linked to one GPCR and an energy acceptor linked to a second GPCR (Milligan and Bouvier, 2005; Pfleger and Eidne, 2006). As the biophysical basis of these approaches restricts detection of signals to distances approximately twice the dimensions of a GPCR protomer, positive signals are consistent with, but do not necessarily prove that, the GPCRs containing the energy transfer donor and acceptor molecules interact physically. As high level co-expression of pairs of GPCRs tagged with resonance energy transfer competent donors and acceptors can result in energy transfer that simply reflects proximity and crowding and is described as ‘bystander' effects (Mercier et al., 2002), many recent studies have employed ‘saturation' resonance energy transfer techniques (Mercier et al., 2002; Wilson et al., 2005; James et al., 2006). In these, varying ratios of the acceptor and donor species are co-expressed and energy transfer measured. These are also useful in assessing the relative propensity of pairs of GPCRs to interact, as it is assumed that the dimerization potential of distinct but co-expressed GPCRs is not purely stochastic (Bush et al., 2007), and to further validate selectivity of ‘dimerization'. Despite recent discussion of the most appropriate way to perform and control such experiments (James et al., 2006; Bouvier et al., 2007; Salahpour and Masri, 2007), there is general agreement that, for dimers, such ‘saturation' techniques should generate hyperbolic curves in which the extent of energy transfer approaches a maximum value asymptotically as the ratio of (energy acceptor) to (energy donor) increases and all available donors become complexed with acceptors (Milligan and Bouvier, 2005; Vrecl et al., 2006). By contrast, random collisions or ‘bystander' effects are expected to generate signals that (at reasonable expression levels) increase in an essentially linear manner with increasing (acceptor)/(donor) ratio.
Although combinations of co-immunoprecipitation and resonance energy transfer-based studies are employed most commonly, other approaches that have been used to argue in favour of GPCR dimerization and/or oligomerization include agonist-induced co-internalization studies (Yesilaltay and Jenness, 2000; Jordan et al., 2001; Sartania et al., 2007), analysis of radioligand-binding studies (Albizu et al., 2006; Kopanchuk et al., 2006) and ‘dominant negative' and ER trapping studies (Salahpour et al., 2004; Wilson et al., 2005; Herrick-Davis et al., 2006; Pidasheva et al., 2006; Sanchez-Laorden et al., 2006).
A day in the life of a GPCR dimer
It has been suggested that GPCRs are ‘born as dimers and die as dimers'. Does the available experimental data support this claim?
Synthesis of GPCR dimers
Both for the rhodopsin-like and metabotropic glutamate-like GPCRs, dimerization is initiated during protein synthesis in the ER (Margeta-Mitrovic et al., 2000; Salahpour et al., 2004; Wilson et al., 2005). As with many other transmembrane proteins, interactions of GPCRs with various chaperonins appear to be an essential step in protein folding. Misfolded proteins fail to pass cellular quality control and are processed for subsequent proteosomal destruction (Petaja-Repo et al., 2001). As introduced earlier, clear evidence of the early assembly of GPCR dimers developed from studies on the GABAB receptor. Expression of the GABABR1 subunit in isolation results in ER retention, while co-expression with the GABABR2 subunit relieves this by masking the ER retention motif of the GABABR1 subunit and hence allows cell surface delivery of the hetero-dimer (Margeta-Mitrovic et al., 2000). This appears to be a specialized example of a general phenomenon. A series of studies have taken advantage of the requirement for correct assembly of GPCR dimers, prior to maturation and transport to the cell surface, to explore the selectivity of GPCR dimerization. For example, Salahpour et al. (2004) replaced the intracellular C-terminal tail of the β2-adrenoceptor with the corresponding region of the GABABR1 subunit to generate an ER retained form of the β2-adrenoceptor. This export deficient GPCR prevented cell surface delivery of the wild type β2-adrenoceptor when the two forms were co-expressed. An equivalent approach was adopted by Wilson et al. (2005) to examine CXCR1/CXCR2 homo- and hetero-dimerization, using the C-terminal 14 amino acids of the α2C-adrenoceptor that also contains a strong ER-retention motif. When this sequence was added to the C-terminal tail of the human CXCR1 receptor, it produced an ER-retained form, and co-expression of this variant with either wild-type CXCR1 or CXCR2 functioned to prevent their delivery to the cell surface. Importantly, co-expression of the ER-retained CXCR1 receptor with the α1A-adrenoceptor did not modulate cell surface delivery of the adrenoceptor, which a range of other approaches had shown not to interact with CXCR1 (Wilson et al., 2005). Such studies demonstrated that protein–protein interactions in the ER are selective. Although not employing an ER-retained mutant, Bush et al. (2007) recently employed a broad spectrum screen for GPCRs that might interact with the olfactory receptor M71 by measuring cell surface delivery of M71 when co-expressed with a range of other GPCRs. A wide range of mutant GPCRs also fail to traffic effectively through the ER and Golgi compartments. However, these are not necessarily dimerization-deficient mutants and, in all but a few potential cases, ER trapping reflects ineffective protein folding, which can, in some cases, be recovered by the use of so-called ‘pharmacological chaperones' (Leskela et al., 2007), small molecule ligands that may have potential in the treatment of diseases that stem from the production of misfolded, mutant GPCRs (Ulloa-Aguirre et al., 2004; Robben and Deen, 2007).
Do ligands alter the quaternary structure of GPCR dimers?
The experiments discussed above clearly indicate that the quaternary structure of GPCRs can be established at an early stage in biogenesis, and further studies have indicated that, at least for the β2-adrenoceptor, the G protein α-subunit and the β/γ-complex become associated with the receptor dimer/oligomer prior to membrane delivery (Dupre et al., 2006). This general view is opposed, however, by experiments that support a model in which delta opioid peptide (DOP)-mu opioid peptide (MOP) receptor hetero-dimers only form at the cell surface and that such interactions require the presence of a G protein that these receptors are able to interact with and activate (Law et al., 2005). Furthermore, there is little available direct information on the affinity of interactions between pairs of GPCR protomers and it is hence possible that GPCR quaternary structure can be modulated by ligand binding. Although a wide range of studies on dimerization of rhodopsin-like GPCRs have reported minimal effects of ligands (see Milligan, 2004 for review), a number of reports have demonstrated alterations of resonance energy transfer signals subsequent to the addition of receptor ligands (Milligan, 2004). Both increases and decreases of signal have been reported but appropriate interpretation of such observations is more complex (Milligan and Bouvier, 2005). As both distance and dipole orientation of resonance energy transfer donor and acceptor species alter the effectiveness of energy transfer, then relatively small changes in conformation of a receptor may be detected as an alteration in signal. Indeed, studies employing intramolecular fluorescence resonance energy transfer between two reporters incorporated into the sequence of a single GPCR polypeptide have been used to examine movements between the third intracellular loop and the C-terminal tail in response to the binding of both agonist and inverse agonist ligands (Vilardaga et al., 2003; Rochais et al., 2007). Furthermore, the exact location that energy transfer reporter constructs are introduced into, at least, hetero-trimeric G protein subunits has recently been shown to influence the extent and even direction of signal alteration upon activation. This has resulted in different conclusions being reached as to whether these subunits dissociate from one another or, rather, only re-orientate upon activation (Frank et al., 2005; Digby et al., 2006; Gales et al., 2006). Thus, although resonance energy reporters are invariably linked to the C-terminal tail of the receptor(s) in question for GPCR dimerization studies, the conformation of this region might be altered in the presence of ligands and hence cause an alteration in signal. With such caveats in mind, and although the majority of studies suggest otherwise, it remains unclear whether ligand binding truly alters the dimerization/oligomerization state of at least certain GPCRs. However, data from atomic level structures of the extracellular domain of mGluR receptors in the presence or absence of ligands demonstrate ligand-dependent movements in and between these elements without inherently modifying overall protein–protein interactions. Given such uncertainties, it has been proposed that ligand-induced alterations in the organization of GPCR dimers should only be considered as compelling if agonist and inverse agonists produce contrasting data and if the concentrations of ligands required to produce such effects are consistent with pre-existing pharmacological knowledge (Percherancier et al., 2005).
The use of cysteine cross-linking experiments to explore potential dimer interfaces of the D2 dopamine receptor has demonstrated that agonist and inverse agonist ligands alter the relative orientation of amino acids on the outward facing elements of transmembrane domain IV (Guo et al., 2005). These studies undoubtedly demonstrate relative movements of the protomers within the D2 dopamine receptor dimer/oligomer in response to binding of ligands and allowed the authors to speculate on a model of organizational structure based, in part, on the organization of rhodopsin protomers viewed in situ via atomic force microscopy in mouse rod outer segment discs (Fotiadis et al., 2004, 2006). Although inherently true for the GABAB receptor in which only the GABAB R1 subunit is able to bind the agonist GABA, and in which although the GABAB R2 subunit does not bind the ligand, it is the key subunit in G protein activation (Havlickova et al., 2002; Pin et al., 2005), recent studies have also indicated that binding of a single molecule of agonist to the BLT1 leukotriene B(4) receptor dimer results in conformational alterations of the protomers, and that this is dependent upon interaction with G protein (Damian et al., 2006). As will be discussed later, a number of studies are beginning to appear that are consistent with binding of a ligand to one protomer of a GPCR hetero-dimer altering the pharmacology or function of the partner protomer. As such, allosteric effects across the dimer interface in response to ligand binding may provide a useful means to probe the presence of GPCR hetero-dimers in physiologically relevant settings (Milligan and Smith, 2007). It may thus require the application of approaches distinct from those based on resonance energy transfer to more fully explore the effects of ligand binding on GPCR quaternary structure. In this regard, it is interesting that the active metabolite of the anti-thrombotic drug clopidogrel has been reported to dissociate oligomers of the P2Y12 receptor in both transfected cells and in platelets (Savi et al., 2006), while atomic force microscopy has been employed to indicate that agonist-activation of the mating factor receptor Ste2p from the budding yeast Saccharomyces cerevisiae results in an increase in its dimerization/oligomerization state (Shi et al., 2007). Such observations are not, however, consistent with early reports indicating this receptor to be a dimer/oligomer in the absence of ligand and this being unaffected by ligand binding (Overton and Blumer, 2000, see Overton et al., 2005 for review). Constitutive formation of dimers/oligomers also appears to be the case for the equivalent receptor system in the fission yeast Schizosaccharomyces pombe (Ladds et al., 2005).
Is dimerization of rhodopsin-like GPCRs required for function?
If GPCRs are transported to the plasma membrane as dimers/oligomers, then it might be anticipated that they function as dimers. Certain modelling studies have attempted to rationalize this on the basis that the footprint of, for example, a rhodopsin dimer may be more appropriate than a monomer of the receptor to bind the transducin α/β/γ G protein hetero-trimer (Fotiadis et al., 2004). This is supported by reconstitution and biophysical studies on the BLT1 leukotriene B4 receptor complexed to a G protein hetero-trimer consisting of the Gαi2-, β1- and γ2-subunits (Baneres and Parello, 2003) and, although in a much less direct manner, by studies co-expressing both the wild-type and inactive forms of the serotonin 5-HT2C receptor (Herrick-Davis et al., 2005). The generality of such conclusions has been questioned, however, in recent studies by Dell'Orco et al. (2007), who employed computational analyses to indicate that a 1:1 rhodopsin:transducin stoichiometry was the most likely organization. In attempts to explore if GPCR monomers can directly interact with and activate G proteins both Bayburt et al. (2007) and Whorton et al. (2007) have recently employed nanodiscs; high-density lipoprotein particles sufficiently small to allow incorporation of a very limited number of protein molecules. After demonstrating the presence of only a monomer of the β2-adrenoceptor in such particles, Whorton et al. (2007) added a Gs hetero-trimer and demonstrated both activation of the G protein by the β2-adrenoceptor agonist isoproterenol and the type of ‘2 site' [3H]antagonist/agonist-binding competition indicative of GPCR–G protein interaction. Similar conclusions on the capacity of monomeric rhodopsin to interact with transducin were obtained by Bayburt et al. (2007). Earlier studies had shown a capacity of monomeric rhodopsin to activate transducin but suggested this to be less effective than for rhodopsin dimers and oligomers (Jastrzebska et al., 2006). The key question, however, is not whether isolated GPCR monomers are able to activate G proteins but rather, if GPCRs are delivered to the cell surface as dimers/oligomers and are pre-associated with their relevant G protein complex, if such demonstrations of monomer function are of direct physiological relevance. In one attempt to examine this question, Hernanz-Falcon et al., 2004 initially employed an informatic approach to identify amino acids in transmembrane helices I and IV that they predicted to be at the interface of the chemokine CCR5 receptor dimer/oligomer. Following mutation of these residues, both resonance energy transfer signals and signal transduction capability was lost but cell surface delivery of the receptor was reported to be maintained. This would suggest that a monomer of the CCR5 receptor is not functional.
Do GPCRs internalize as dimers?
A further suggestion for a key role of GPCR dimerization in function is to provide an appropriate footprint for interactions with arrestins. The arrestins are viewed predominantly as polypeptides involved in the turn-off and desensitization of G protein-mediated signalling. However, in recent years, a substantial body of data has emerged to suggest that they can also function to initiate a distinct series of signal transducing events (Reiter and Lefkowitz, 2006; DeWire et al., 2007) and that ligand-induced GPCR conformations suitable for the binding of arrestins can be teased apart from those involved in G protein recognition. Based on crystal structures and the molecular dimension of arrestins (Han et al., 2001; Milano et al., 2006), it appears possible that a single arrestin might bind to a GPCR dimer. Furthermore, it has recently become clear that arrestins are themselves able to dimerize (Storez et al., 2005; Milano et al., 2006) and, that as well as homo-dimerization, hetero-dimerization between arrestin 2 and arrestin 3 can occur (Storez et al., 2005; Milano et al., 2006). Although the regulation between monomer and dimer forms is suggested to alter nuclear to cytoplasmic distribution of the arrestin, if arrestin dimers moved to the cell surface to interact with agonist-activated GPCRs, then such an arrestin dimer might be anticipated to be able to interact with a string, or array, of GPCRs and hence might scaffold higher-order oligomers of GPCRs, such as those of the α1b-adrenoceptor observed recently using multicolour fluorescence resonance energy transfer (FRET) imaging (Lopez-Gimenez et al., 2007). Philip et al. (2007) have also recently detected a fraction of the bradykinin B2 receptor with characteristics of a higher-order oligomeric structure.
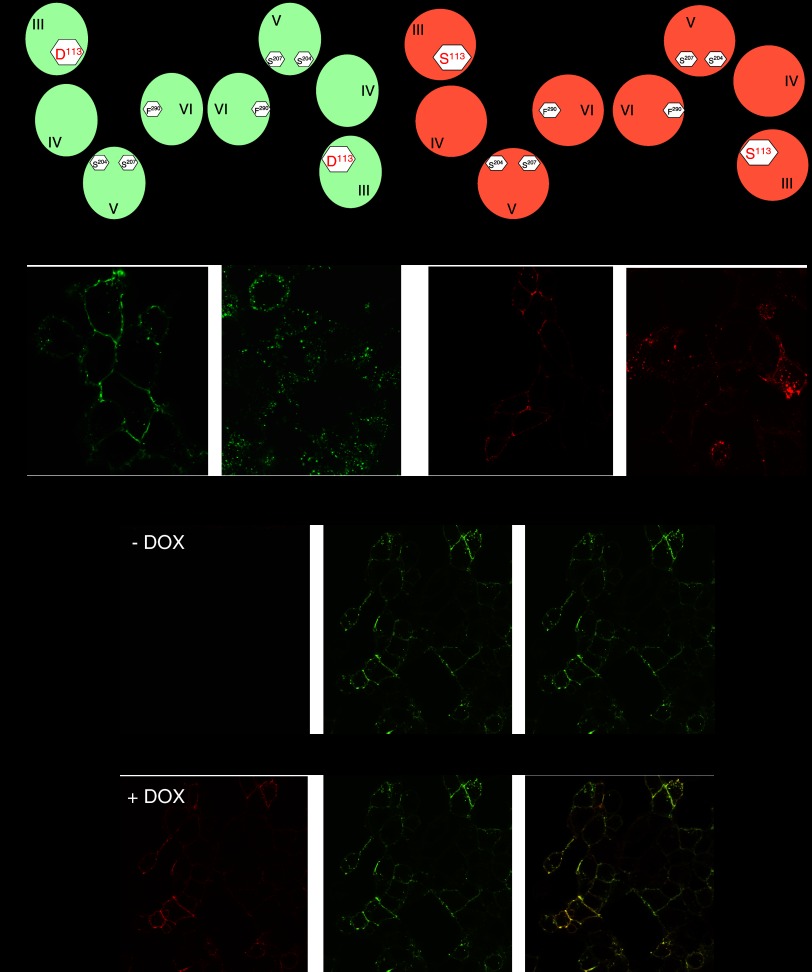
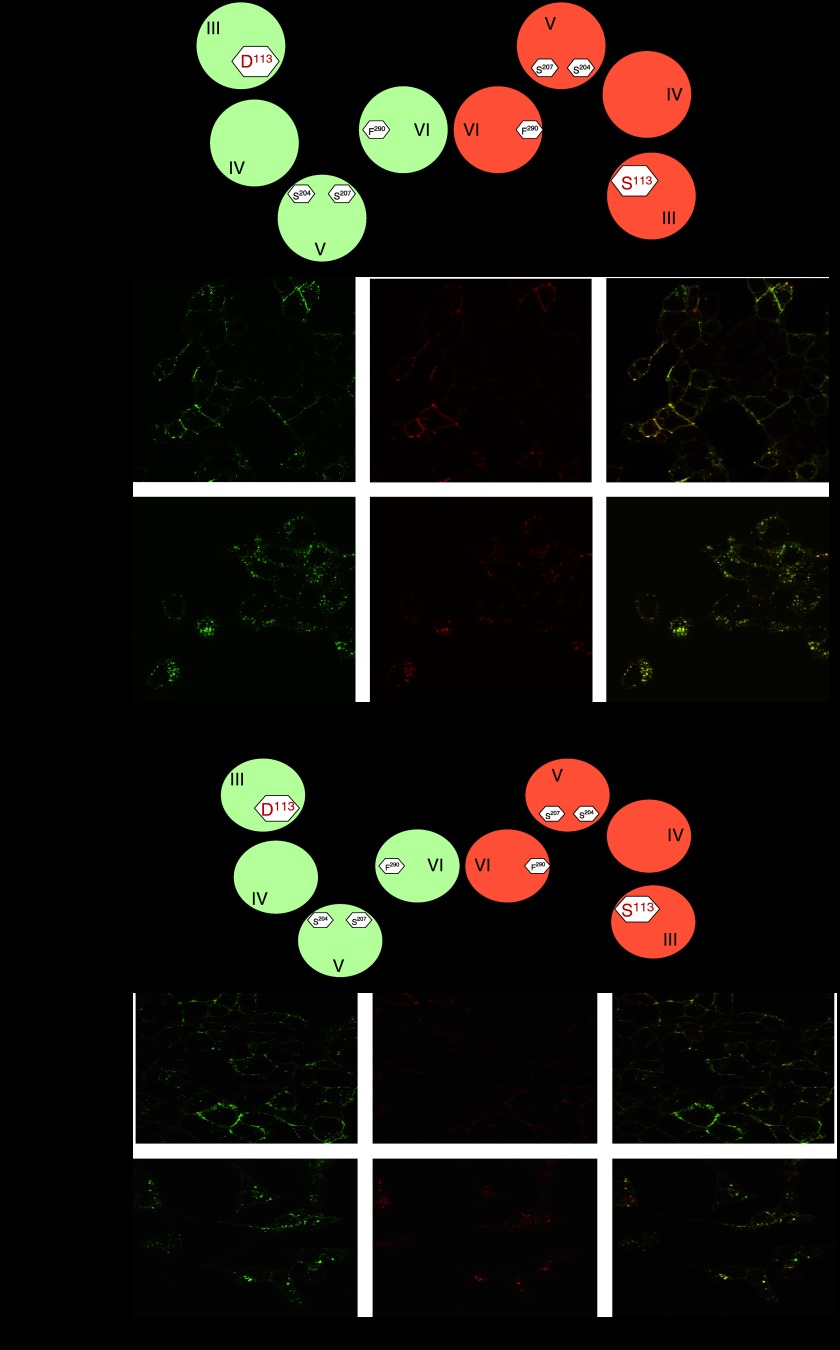
If an arrestin monomer interacts with dimeric GPCRs, then it should be expected that GPCRs, which internalize in an arrestin-dependent manner will do so as dimers. This should certainly be the case for strong interactions that result in the co-internalization of the receptor(s) and the arrestin and may also be the case for weaker interactions in which the arrestin and GPCR separate soon after entry into the endocytic process. Both Yesilaltay and Jenness (2000) and Overton and Blumer (2000) demonstrated co-internalization of the wild-type Saccharomyces cerevisiae mating factor receptor Ste2p and co-expressed mutants that were either unable to bind the ligand (Yesilaltay and Jenness, 2000) or were C-terminally truncated to eliminate agonist-mediated endocytosis (Overton and Blumer, 2000). The former studies were also consistent with the binding of a single molecule of agonist being sufficient to drive internalization of the GPCR dimer. In an extension of this basic concept, Sartania et al. (2007) demonstrated that, when each variant was expressed individually, the agonist isoprenaline was able to cause internalization of the wild-type β2-adrenoceptor but not of an Asp113Ser mutant that does not bind catecholamine ligands with high affinity (Figure 1). By contrast, 1-(3′4′-dihydroxyphenyl)-3-methyl-1-butanone, that is able to interact with and activate Asp113Serβ2-adrenoceptor but not the wild-type β2-adrenoceptor, caused internalization only of the mutant (Figure 1). However, following co-expression of the wild-type and Asp113Ser forms of the β2-adrenoceptor, either isoprenaline or 1-(3′4′-dihydroxyphenyl)-3-methyl-1-butanone caused internalization of both forms of the receptor (Figure 1). These data indicate that wild-type and Asp113Ser β2-adrenoceptors dimerize and that agonist occupancy of only one protomer within the dimer is sufficient to cause internalization. As internalization of the β2-adrenoceptor is dependent on arrestins then, although indirect, these results might also be extrapolated to indicate that arrestins interact with a GPCR dimer. Cao et al. (2005) also examined potential internalization and trafficking of a β2-adrenoceptor dimer by co-expression of wild-type and C-terminally mutated, recycling deficient forms of this receptor. They showed that the presence of a mutated form of the receptor inhibited the recycling of the wild-type β2-adrenoceptor and targeted the wild-type receptor to lysosomal compartments for destruction. Combining data from a number of studies, there is good evidence, therefore, for the β2-adrenoceptor at least existing as a dimer/oligomer from synthesis to destruction.
Figure 1.
The β2-adrenoceptor is internalized as a dimer following the binding of a single molecule of agonist ligand to either protomer. (a) Differentially epitope-tagged forms of either the wild-type β2-adrenoceptor (green) or an Asp113Ser β2-adrenoceptor mutant (red) were expressed in Flp-In, T-Rex HEK293 cells. The wild-type receptor was internalized in response to challenge with isoprenaline but not to L158,870 (1-(3′4′-dihydroxyphenyl)-3-methyl-1-butanone), while the reverse was true for the Asp113Ser β2-adrenoceptor. The cartoons illustrate both forms of the β2-adrenoceptor forming dimers via interactions involving transmembrane domain VI (Hebert et al., 1996) and the assumption that each promoter within the dimer is able to bind a molecule of the appropriate agonist. Docking of the ligands is based on mutational analysis of how the β2-adrenoceptor binds ligands. (b) With N-terminally HA-tagged wild-type β2-adrenoceptor (HA-β2-AR) (green) expressed stably and constitutively and N-terminally VSV-G tagged Asp113Ser β2-adrenoceptor (VSV-G-Asp113Ser-β2-AR) (red) cloned into the Flp-In locus, HA-β2-AR is expressed in both the absence (−) and presence (+) of the antibiotic DOX, while VSV-G-Asp113Ser-β2-AR is expressed only following exposure of the cells to DOX. Merge, indicated the extent of overlap of the distribution (yellow) of the two forms of the receptor when they are both expressed. (c) Following co-expression of HA-β2-AR (green) and VSV-G-Asp113Ser-β2-AR (red), isoprenaline causes internalization of both forms of the β2-adrenoceptor and the co-internalized receptors overlap in distribution (merge, yellow). The cartoon illustrates that a HA-β2-AR/VSV-G-Asp113Ser-β2-AR dimer can only bind a single molecule of isoprenaline. (d) Following co-expression of HA-β2-AR (green) and VSV-G-Asp113Ser-β2-AR (red), L158,870 also causes internalization of both forms of the β2-adrenoceptor and the co-internalized receptors overlap in distribution (merge, yellow). The cartoon illustrates that a HA-β2-AR/VSV-G-Asp113Ser-β2-AR dimer can only bind a single molecule of L158,870. These data are consistent with the β2-adrenoceptor being internalized as a dimer and in response to agonist occupancy of either protomer within the dimer. Data are adapted and extended from Sartania et al., 2007. DOX, doxycycline; HA, hemagglutinin; VSV, vesicular stomatitis virus.
Does the co-internalization of pairs of co-expressed GPCRs define their hetero-dimerization?
A substantial number of studies have observed co-internalization of pairs of distinct but co-expressed GPCRs in response to ligands with significant affinity for only one of the pair. Such studies have generally been interpreted as further evidence of hetero-dimerization. For example, co-expression of D1 and D2 dopamine receptors has been reported to result in the co-internalization of both receptors in response to addition of agonist ligands selective for either protomer (So et al., 2005). Equally, following co-expression of the β2-adrenoceptor and the DOP receptor in HEK293 cells, Jordan et al. (2001) demonstrated that the opioid agonist etorphine caused internalization of the β2-adrenoceptor as well as of the DOP receptor, although etorphine does not have inherent affinity for the β2-adrenoceptor and did not cause internalization of the β2-adrenoceptor when it was expressed alone. Equally, the β2-adrenoceptor agonist isoprenaline caused internalization of the DOP receptor only when it was co-expressed with the β2-adrenoceptor (Jordan et al., 2001). These authors also showed that co-expression of the kappa opioid peptide (KOP) receptor with the β2-adrenoceptor resulted in limitation of isoprenaline-induced internalization of the β2-adrenoceptor, again potentially an effect related to opioid receptor-β2-adrenoceptor hetero-dimerization, because the KOP receptor is resistant to internalization even in response to agonist selective for this GPCR. By contrast, a related study by Cao et al. (2005) indicated that the reciprocal regulation of β2-adrenoceptor and DOP receptor internalization by selective ligands could only be observed following co-expression of high levels of these two receptors. As such, although observation of dual regulation of pairs of receptors by ligands with significant affinity for only one is certainly consistent with hetero-dimerization (Laroche et al., 2005), the levels of expression of the receptors need to be carefully controlled to limit low affinity, potentially non-physiological interactions. In a recent study on interactions between the cannabinoid CB1 receptor and the orexin-1 receptor, Ellis et al. (2006) noted that when expressed individually in HEK293 cells, the orexin-1 receptor was present largely at the plasma membrane, whereas the cannabinoid CB1 recycled continuously and, at steady state, was predominantly in recycling endosomes. As a means to regulate expression levels of one of the partner GPCRs, the orexin-1 receptor was expressed from an inducible locus. When expression of the orexin-1 receptor was induced in the presence of the cannabinoid CB1 receptor, now both receptors were present largely in recycling endosomes and were shown to be within the same protein complex. Treatment of these cells with either the cannabinoid CB1 receptor antagonist/inverse agonist rimonabant or the orexin-1 receptor blocker SB-674042 caused the trapping of both receptors at the cell surface (Ellis et al., 2006). These results are again consistent with the two GPCRs forming hetero-dimers and maintaining this status throughout endocytosis and recycling to the cell surface. Importantly, this was a specific interaction. Turning on expression of the MOP receptor from the inducible locus in the presence of the recycling cannabinoid CB1 receptor did not result in the MOP receptor adopting the recycling phenotype (Ellis et al., 2006). While these results are not supportive of hetero-dimerization between the cannabinoid CB1 and MOP receptor, it must be noted that other workers have indeed reported hetero-dimerization between this pair of receptors and functional consequences thereof (Rios et al., 2006). Such variations in conclusions between studies are not uncommon in this area, as it is a relatively immature field and a great deal of extra work will be required to unravel the details of the selectivity and basis of GPCR hetero-dimerization.
What is the molecular basis for GPCR dimerization and can it be disrupted?
Although it is now widely accepted that GPCRs are able to homo-dimerize and that certain pairs are able to hetero-dimerize, particularly for the rhodopsin-like GPCRs, understanding of the molecular basis of these contacts remains limited. Previous reviews (Milligan, 2004, 2007) have described a number of the early efforts to explore this issue, including potential contributions of the extracellular N-terminus and the intracellular C-terminal tail to protein–protein interactions. Evidence has begun to favour direct contacts between residues on the lipid-facing elements of the transmembrane helices. Although both ‘contact' and ‘domain-swap' models of dimerization have been proposed, direct evidence in support of the ‘domain-swap' model, in which it is suggested that transmembrane domains I–V and VI–VII that are separated by the third intracellular loop of the receptor are exchanged between the promoters of a dimer, is limited (Vohra et al., 2007). In the ‘contact' model of dimerization, where such unravelling of the structural organization of the bundle of transmembrane helices is not required, transmembrane domains IV and/or V have gained most support as dimerization interfaces in recent times. Transmembrane domain IV was implicated in the dimerization of the dopamine D2 receptor in early studies by Lee et al. (2003) that employed various truncated forms of the receptor and by Guo et al. (2003), who employed a cysteine cross-link approach. Further studies by Guo et al. (2005) extended these studies by replacing residues all along transmembrane helix IV with cysteine residues and then performing cross-linking experiments in the presence of agonist or inverse agonist ligands. Differences in the rates of cross-linking of individual sites were indicative of alterations in the dimer interface associated with active and inactive receptor states and were consistent with the concept of allosteric effects of ligands being communicated between protomers across the dimer interface (Guo et al., 2005). Endogenous cysteine residues located in transmembrane domains III and IV have also been shown to play an important role in dimerization of the 5-HT4 receptor (Berthouze et al., 2007). A key role for transmembrane helix IV in homo-interactions of the α1b-adrenoceptor was also uncovered by combinations of receptor fragmentation (Carrillo et al., 2004) and mutagenesis (Lopez-Gimenez et al., 2007). As in earlier studies employing the complement C5a receptor (Klco et al., 2003), self-association between transmembrane domain IV helices was insufficient to explain the comprehensive interaction map for the α1b-adrenoceptor fragments and resulted in initially models (Carrillo et al., 2004) and subsequently direct experimental support (Lopez-Gimenez et al., 2007), for an oligomeric organization of this receptor. It remains unclear if certain aspects of the observed packing and potential interaction interfaces of rhodopsin in native rod outer segments simply reflect the high expression levels of rhodopsin. However, oligomeric complexes of rhodopsin have been identified in detergent extracts (Jastrzebska et al., 2006) and transmembrane helix IV–transmembrane helix V interactions modelled from the images obtained via atomic force microscopy (Fotiadis et al., 2006). A crystal structure of a photo-activated rhodopsin dimer at a resolution of 4.15 A (Salom et al., 2006) indicated roles for transmembrane helices I and II as well as the intracellular helix 8. These interactions were suggested to be in addition to the previously reported transmembrane helix IV/V interactions and to potentially provide a framework to produce the oligomeric arrays of rhodopsin observed in situ. The lack of uniformity in which transmembrane helices are implicated as dimerization interfaces for different GPCRs may reflect that multiple helices play roles, as predicted in various models (Vohra et al., 2007), but that experimental scientists have concentrated on limited regions simply because the task of exploring this on a global and systematic level would be challenging. The contribution to GPCR dimerization of individual amino acids within transmembrane helices has been even more difficult to explore or to generalize. Although sequences such as the glycophorin A like GXXXG motif have been identified in a range of GPCRs and in a range of distinct transmembrane helices, only for the yeast pheromone receptor has disruption of such a motif indicated it to play a key role (Overton et al., 2003; Gehret et al., 2006). By contrast, in the α1b-adrenoceptor, disruption of such motifs in two separate transmembrane helices was without significant effect (Stanasila et al., 2003; Carrillo et al., 2004) and this was also the case following mutation of a potential GXXXG motif in transmembrane helix VII of the class B secretin receptor (Lisenbee and Miller, 2006). Other motifs that have been suggested to contribute to stabilization and specificity of interactions between transmembrane helices include QXXS (Sal-Man et al., 2005) and Aromatic-xx-Aromatic (Sal-Man et al., 2007).
A series of computational modelling studies have also tried to consider likely homo-dimer interfaces. For the adenosine A3 receptor, Kim and Jacobson (2006) concluded that, among a number of possibilities, interactions involving transmembrane helix IV and transmembrane helix V were more favoured. In a related study, Fanelli (2007) employed computational modelling to conclude a likely key role for intermolecular transmembrane helix IV–transmembrane helix IV interactions in dimerization of the lutropin receptor. Although apparently counter-intuitive, modelling studies have also suggested that structurally highly related receptors, such as the dopamine D2, D3 and D4 receptors, may have different homo-dimer interfaces (Nemoto and Toh, 2005). If this is true, then prospects for finding an underlying, common pattern seem poor. A series of studies have attempted to disrupt GPCR dimerization. One strategy has been to employ peptides corresponding to specific transmembrane domains. Wang et al. (2006) demonstrated that a synthetic peptide corresponding to transmembrane domain IV of the chemokine CXCR4 receptor reduced FRET signals between co-expressed energy transfer competent forms of this receptor and blocked a number of CXCR4-mediated effects. By contrast, for the cholecystokinin receptor, peptides corresponding to transmembrane helices VI and VII appeared to interfere with dimerization (a peptide corresponding to transmembrane helix IV was not tested) but this did not result in an alteration of function (Harikumar et al., 2006). As such, the current lack of clarity on the key interaction interfaces of individual homo-dimers has restricted studies on the effects of disrupting GPCR-dimerization. It is also possible that because numerous elements seem to contribute to the basis and selectivity of GPCR dimerization, single point mutations may be insufficient to prevent dimerization.
Although the bulk of studies to date have concentrated on homo-dimeric interactions, both modelling and experimental studies have also addressed aspects of the interfaces of hetero-dimers. An early example in which potential hetero-dimer interfaces were modelled and predicted indicated that although closely related, hetero-dimers between opioid receptor subtypes might employ different contact interfaces than the corresponding homo-dimers (Filizola et al., 2002). Although interesting, this remains to be tested experimentally. One example of efforts to explore the interface between protomers within a hetero-dimer is for interactions between the adenosine A2a and dopamine D2 receptors (Torvinen et al., 2004), a pairing that has been much studied (Canals et al., 2003) and is of potential physiological and therapeutic significance (Fuxe et al., 2005). As an experimental approach to interfere with GPCR hetero-dimerization, McLaughlin et al. (2007) ‘knocked down' protease activated receptor 3 levels in endothelial cells and, by doing so, moderated function of the protease activated receptor 1, which they demonstrated to form a hetero-dimer with protease activated receptor 3. Similarly, Levoye et al. (2006a) also utilized a small interfering RNA-based strategy to knock down levels of the orphan GPCR GPR50 and, by doing so, uncovered binding to, and signalling via, the melatonin MT1 receptor that they proposed to form a non-functional hetero-dimer with GPR50 in cells in which they are co-expressed. Such studies have also allowed these authors to speculate whether all ‘orphan' GPCRs actually have natural orthosteric ligands, or whether some might act simply as hetero-dimer partners to alter the functionality of other, specific co-expressed GPCRs (Levoye et al., 2006b).
Does GPCR dimerization have relevance to disease?
Alteration in the function and activity of many GPCRs is targeted by the pharmaceutical industry in attempts to treat a wide range of common diseases and disorders. A substantial number of diseases, even though many are relatively rare, are associated with mutations in GPCRs (Tao, 2006) and polymorphic variation can alter disease susceptibility (Thompson et al., 2005). Given the importance of effective GPCR dimerization in cellular quality control prior to plasma membrane delivery, then forms of GPCRs that fold incorrectly might be anticipated to interfere with dimerization of wild-type GPCRs and hence interfere with their cell surface delivery and function. This has been observed for a substantial number of GPCRs. For example, mutants of the melanocortin 1 receptor that are retained intracellularly act, in a dominant negative manner, to limit the function of the wild-type receptor (Sanchez-Laorden et al., 2006). A number of mutants of this receptor are associated with red hair and the exact effect of the mutant on the extent of malfunction and dimerization with a wild-type allele was suggested to modulate the complexity of skin phenotypes. In a similar manner, mutations of the calcium-sensing receptor are known that are associated with familial hypocalciuric hypercalcaemia and severe neonatal hyperthyroidism. These are able to dimerize but not to transit through the ER (Pidasheva et al., 2006). Certain mutations of the thyroid stimulating hormone receptor that are associated with dominant resistance to thyroid stimulating hormone also seem to produce these effects by interacting with and entrapping the wild-type form of this receptor intracellularly (Calebiro et al., 2005), while other examples of similar effects include mutants of the ghrelin/growth hormone-releasing hormone receptor (McElvaine and Mayo, 2006; Leung et al., 2007), the gonadotropin-releasing hormone receptor (Brothers et al., 2004) and others. These examples hint at commonality in such mechanisms. Although Nemoto and Toh (2005) noted that a missense mutation in transmembrane helix IV of the dopamine D2 is associated with myoclonus dystonia, and that this was within a region they had predicted as a dimerization interface, no direct data have yet indicated this to be a GPCR dimerization defect.
Hetero-dimers may also be relevant to disease states. Although still requiring independent verification, studies from Quitterer and co-workers have implicated alterations in the relative amounts of hetero-dimers involving the angiotensin AT1 receptor and, for example, the bradykinin B2 receptor in disease and suggested that both preeclampsia and some forms of experimental hypertension might be disorders associated with altered GPCR hetero-dimerization (AbdAlla et al., 2001, 2005). Furthermore, interactions between the angiotensin AT1 receptor and the β2-adrenoceptor have been reported to result in either AT1 receptor blockers or β2-adrenoceptor antagonists blocking function at both receptors (Barki-Harrington et al., 2003). In a similar vein, hetero-dimerization between the β2-adrenoceptor and prostaglandin receptors of the E class in airway smooth muscle has been reported to result in reduced bronchodilator response to β2-adrenoceptor agonists and thus, potentially, to impact on asthma (McGraw et al., 2006). As such, although a research area still in its infancy, there may be numerous examples of ways in which GPCR hetero-dimerization may contribute to the actions (and perhaps side effects) of therapeutic medicines.
Do GPCR hetero-dimers offer a novel set of pharmacological targets?
One of the most common questions asked of those exploring GPCR hetero-dimerization is whether they provide a distinct group of targets for novel drug design. This question has been addressed to some extent in previous reviews (Maggio et al., 2005; Prinster et al., 2005; Milligan, 2006). Three aspects of GPCR function offer potential in this regard. Firstly, it may be possible to identify ligands that are selective for GPCR hetero-dimers. Secondly, it is becoming clear that GPCR hetero-dimers can generate very distinct signals from the corresponding homo-dimers and thirdly, hetero-dimers may offer a means to improve tissue selectivity of function. In terms of the potential to generate hetero-dimer selective ligands, it has been clear for many years that multiple observed opioid pharmacologies cannot be explained easily by consideration only of the three major opioid receptors acting in isolation (Gupta et al., 2006). Furthermore, co-expression of pairs of opioid receptors has, at least in part, helped to unravel such effects. Portoghese and co-workers have actively explored the actions of a series of opioid ligands in which two distinct pharmacophores are separated by a linker arm of varying length. Using such reagents, although they are tool compounds rather than inherent drug-like molecules, has suggested that, for example, hetero-dimers between DOP and KOP receptors may reflect the pharmacologically, rather than molecularly, defined delta(1) and kappa(2) receptors (Xie et al., 2005). Similarly, the marked pharmacological differences observed following co-expression of pairs of opioid receptors is likely to reflect a level of hetero-dimerization or interaction (Fan et al., 2005; Snook et al., 2006). An orthosteric ligand that has the capacity to regulate a GPCR hetero-dimer is bovine adrenal medulla peptide 22. The DOP receptor and sensory neuron-specific receptor-4 are co-expressed in dorsal root ganglia and are able to hetero-dimerize/oligomerize (Breit et al., 2006). Bovine adrenal medulla peptide 22 contains pharmacophores for each of the individual receptors, but at opposite ends of the bovine adrenal medulla peptide 22 peptide sequence. Hence, without further processing, bovine adrenal medulla peptide 22 is able to interact with both elements of the DOP receptor/sensory neuron-specific receptor-4 hetero-dimer, and in doing so produce a distinct pharmacology that allows selective ligands at these two receptors to produce effects across the hetero-dimer.
To develop a true capacity to examine the physiological importance of GPCR hetero-dimerization requires the identification and use in animal models of small molecule ligands that are highly hetero-dimer selective. This will clearly not be a trivial challenge, and hence the report of 6′ guanidinonaltrindole as a highly selective agonist at DOP/KOP receptor hetero-dimers (Waldhoer et al., 2005) has attracted a great deal of attention (Park and Palczewski, 2005). Not least, this reflects the in vivo action of 6′ guanidinonaltrindole as a spinally selective analgesic (Waldhoer et al., 2005) rather than effects being reported only in heterologous, transfected cells. These results clearly require independent confirmation, but offer hope for the identification of further selective ligands via large-scale screens. Strategies to screen for such ligands have been discussed recently (Milligan, 2006). Although it is not entirely clear how 6′ guanidinonaltrindole might bind selectively to the DOP/KOP hetero-dimer, attempts to dock this ligand within a suitable computational model and a comparison of this with the KOP receptor ligand 5′ guanidinonaltrindole could be revealing.
There is also emerging evidence that GPCR hetero-dimers may generate different signals than the corresponding homo-dimers. Once again, co-expressed pairs of opioid receptors that are able to interact physically have contributed significantly to understanding. For example, DOP–MOP receptor interactions are reported to alter G protein coupling selectivity as George et al. (2000) reported a loss of pertussis toxin sensitivity of signal transduction following co-expression of these two receptors. One possible explanation for this is a selective recruitment of Gz to the DOP–MOP hetero-dimer. A different alteration in function has recently also been reported for DOP–MOP hetero-dimers as they have been shown to result in β-arrestin 2-dependent activation of the extracellular regulated kinases mitogen-activated protein kinases (Rozenfeld and Devi, 2007). D1/D2 dopamine receptor hetero-dimers are also reported to generate novel Ca2+ signals, via activation of Gq/G11 G proteins when the two receptors are co-expressed and such signals can be observed in the striatum (Rashid et al., 2007). Interestingly, such responses become desensitized by agonist occupancy of either protomer (So et al., 2007). It appears likely that more examples of differential or biased signal transduction from GPCR hetero-dimers will be uncovered as this issue is explored more fully. Such effects may also contribute to an ability to utilize previously characterized ligands in distinct ways or to help explain unanticipated effects of ligands in physiologically relevant cells and tissues, and in both animal models and patients. Finally, even if ligands display only relatively modest differences in affinity and potency, rather than complete specificity, at GPCR hetero-dimers compared to the homo-dimers, higher affinity and potency may allow these to function in a cell or tissue-selective manner if the hetero-dimer in question is expressed only in tissues of therapeutic interest. For all of these reasons, further research into the extent, molecular basis and functional consequences of GPCR hetero-dimerization is likely to be pursued actively.
Acknowledgments
Work on aspects of GPCR dimerization/oligomerization in the authors' laboratory is funded by the Biotechnology and Biosciences Research Council, the Medical Research Council and the Wellcome Trust.
Glossary
- ER
endoplasmic reticulum
- GABA
γ-amino butyric acid
- G protein
guanine nucleotide-binding protein
- GPCR
G protein-coupled receptor
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- AbdAlla S, Abdel-Baset A, Lother M, el Massiery A, Quitterer U. Mesangial AT1/B2 receptor heterodimers contribute to angiotensin II hyperresponsiveness in experimental hypertension. J Mol Neurosci. 2005;26:185–192. doi: 10.1385/JMN:26:2-3:185. [DOI] [PubMed] [Google Scholar]
- AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med. 2001;7:1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- Albizu L, Balestre MN, Breton C, Pin JP, Manning M, Mouillac B, et al. Probing the existence of G protein-coupled receptor dimers by positive and negative ligand-dependent cooperative binding. Mol Pharmacol. 2006;70:1783–1791. doi: 10.1124/mol.106.025684. [DOI] [PubMed] [Google Scholar]
- Baneres JL, Parello J. Structure-based analysis of GPCR function: evidence for a novel pentameric assembly between the dimeric leukotriene B4 receptor BLT1 and the G-protein. J Mol Biol. 2003;329:815–829. doi: 10.1016/s0022-2836(03)00439-x. [DOI] [PubMed] [Google Scholar]
- Barki-Harrington L, Luttrell LM, Rockman HA. Dual inhibition of beta-adrenergic and angiotensin II receptors by a single antagonist: a functional role for receptor–receptor interaction in vivo. Circulation. 2003;108:1611–1618. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]
- Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG. Transducin activation by nanoscale bilayers containing one and two rhodopsins. J Biol Chem. 2007;282:14875–14881. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- Berthouze M, Rivail L, Lucas A, Ayoub MA, Russo O, Sicsic S, et al. Two transmembrane Cys residues are involved in 5-HT4 receptor dimerization. Biochem Biophys Res Commun. 2007;356:642–647. doi: 10.1016/j.bbrc.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Bonde MM, Sheikh SP, Hansen JL. Family C 7TM receptor dimerization and activation. Endocr Metab Immune Disord Drug Targets. 2006;6:7–17. doi: 10.2174/187153006776056594. [DOI] [PubMed] [Google Scholar]
- Bouvier M, Heveker N, Jockers R, Marullo S, Milligan G. (Rigorous) BRET analysis of GPCR oligomerization: Newer does not mean better. Nat Methods. 2007;4:3–4. doi: 10.1038/nmeth0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets. 2007;8:169–184. doi: 10.2174/138945007779315614. [DOI] [PubMed] [Google Scholar]
- Breit A, Gagnidze K, Devi LA, Lagace M, Bouvier M. Simultaneous activation of the delta opioid receptor (deltaOR)/sensory neuron-specific receptor-4 (SNSR-4) hetero-oligomer by the mixed bivalent agonist bovine adrenal medulla peptide 22 activates SNSR-4 but inhibits deltaOR signaling. Mol Pharmacol. 2006;70:686–696. doi: 10.1124/mol.106.022897. [DOI] [PubMed] [Google Scholar]
- Brothers SP, Cornea A, Janovick JA, Conn PM. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol. 2004;18:1787–1797. doi: 10.1210/me.2004-0091. [DOI] [PubMed] [Google Scholar]
- Bush CF, Jones SV, Lyle AN, Minneman KP, Ressler KJ, Hall RA. Specificity of olfactory receptor interactions with other G protein-coupled receptors. J Biol Chem. 2007;282:19042–19051. doi: 10.1074/jbc.M610781200. [DOI] [PubMed] [Google Scholar]
- Calebiro D, de Filippis T, Lucchi S, Covino C, Pangione S, Beck-Peccoz P, et al. Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum Mol Genet. 2005;14:2991–3002. doi: 10.1093/hmg/ddi329. [DOI] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, et al. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- Cao TT, Brelot A, von Zastrow M. The composition of the beta-2 adrenergic receptor oligomer affects its membrane trafficking after ligand-induced endocytosis. Mol Pharmacol. 2005;67:288–297. doi: 10.1124/mol.104.003608. [DOI] [PubMed] [Google Scholar]
- Carrillo JJ, Lopez-Gimenez JF, Milligan G. Multiple interactions between transmembrane helices generate the oligomeric alpha1b-adrenoceptor. Mol Pharmacol. 2004;66:1123–1137. doi: 10.1124/mol.104.001586. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Chang W, Tu C, Cheng Z, Rodriguez L, Chen T, Gassmann M, et al. Complex formation with the type B γ-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor: studies with HEK-293 cells and neurons. J Biol Chem. 2007;282:25030–25040. doi: 10.1074/jbc.M700924200. [DOI] [PubMed] [Google Scholar]
- Damian M, Martin A, Mesnier D, Pin JP, Baneres JL. Asymmetric conformational changes in a GPCR dimer controlled by G-proteins. EMBO J. 2006;25:5693–5702. doi: 10.1038/sj.emboj.7601449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Orco D, Seeber M, Fanelli F. Monomeric dark rhodopsin holds the molecular determinants for transducin recognition: insights from computational analysis. FEBS Lett. 2007;581:944–948. doi: 10.1016/j.febslet.2007.01.074. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Digby GJ, Lober RM, Sethi PR, Lambert NA. Some G protein heterotrimers physically dissociate in living cells. Proc Natl Acad Sci USA. 2006;103:17789–17794. doi: 10.1073/pnas.0607116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre DJ, Robitaille M, Ethier N, Villeneuve LR, Mamarbachi AM, Hebert TE. Seven transmembrane receptor core signalling complexes are assembled prior to plasma membrane trafficiking. J Biol Chem. 2006;281:34561–34573. doi: 10.1074/jbc.M605012200. [DOI] [PubMed] [Google Scholar]
- Ellis J, Pediani JD, Canals M, Milasta S, Milligan G. Orexin-1 receptor-cannabinoid CB1 receptor hetero-dimerization results in both ligand-dependent and -independent co-ordinated alterations of receptor localization and function. J Biol Chem. 2006;281:38812–38824. doi: 10.1074/jbc.M602494200. [DOI] [PubMed] [Google Scholar]
- Fan T, Varghese G, Nguyen T, Tse R, O'Dowd BF, George SR. A role for the distal carboxyl tails in generating the novel pharmacology and G protein activation profile of mu and delta opioid receptor hetero-oligomers. J Biol Chem. 2005;280:38478–38488. doi: 10.1074/jbc.M505644200. [DOI] [PubMed] [Google Scholar]
- Fanelli F. Dimerization of the lutropin receptor: insights from computational modeling. Mol Cell Endocrinol. 2007;260–262:59–64. doi: 10.1016/j.mce.2005.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filizola M, Olmea O, Weinstein H. Prediction of heterodimerization interfaces of G-protein coupled receptors with a new subtractive correlated mutation method. Protein Eng. 2002;15:881–885. doi: 10.1093/protein/15.11.881. [DOI] [PubMed] [Google Scholar]
- Fotiadis D, Jastrzebska B, Philippsen A, Muller DJ, Palczewski K, Engel A. Structure of the rhodopsin dimer: a working model for G-protein-coupled receptors. Curr Opin Struct Biol. 2006;16:252–259. doi: 10.1016/j.sbi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. The G protein-coupled receptor rhodopsin in the native membrane. FEBS Lett. 2004;564:281–288. doi: 10.1016/S0014-5793(04)00194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Thumer L, Lohse MJ, Bunemann M. G Protein activation without subunit dissociation depends on a G{alpha}(i)-specific region. J Biol Chem. 2005;280:24584–24590. doi: 10.1074/jbc.M414630200. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Canals M, Torvinen M, Terasmaa A, Marcellino D, et al. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J Mol Neurosci. 2005;26:209–220. doi: 10.1385/JMN:26:2-3:209. [DOI] [PubMed] [Google Scholar]
- Gales C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, et al. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol. 2006;13:778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- Gama L, Wilt SG, Breitwieser GE. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J Biol Chem. 2001;276:39053–39059. doi: 10.1074/jbc.M105662200. [DOI] [PubMed] [Google Scholar]
- Gehret AU, Bajaj A, Naider F, Dumont ME. Oligomerization of the yeast alpha-factor receptor: implications for dominant negative effects of mutant receptors. J Biol Chem. 2006;281:20698–20714. doi: 10.1074/jbc.M513642200. [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, et al. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- Guo W, Shi L, Filizola M, Weinstein H, Javitch JA. Crosstalk in G protein-coupled receptors: changes at the transmembrane homodimer interface determine activation. Proc Natl Acad Sci USA. 2005;102:17495–17500. doi: 10.1073/pnas.0508950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Shi L, Javitch JA. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem. 2003;278:4385–4388. doi: 10.1074/jbc.C200679200. [DOI] [PubMed] [Google Scholar]
- Gupta A, Decaillot FM, Devi LA. Targeting opioid receptor heterodimers: strategies for screening and drug development. AAPS J. 2006;8:E153–E159. doi: 10.1208/aapsj080118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane translocation. Structure. 2001;9:869–880. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- Harikumar KG, Dong M, Cheng Z, Pinon DI, Lybrand TP, Miller LJ. Transmembrane segment peptides can disrupt cholecystokinin receptor oligomerization without affecting receptor function. Biochemistry. 2006;45:14706–14716. doi: 10.1021/bi061107n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlickova M, Prezeau L, Duthey B, Bettler B, Pin JP, Blahos J. The intracellular loops of the GB2 subunit are crucial for G-protein coupling of the heteromeric gamma-aminobutyrate B receptor. Mol Pharmacol. 2002;62:343–350. doi: 10.1124/mol.62.2.343. [DOI] [PubMed] [Google Scholar]
- Hebert TE, Moffet S, Morello JP, Loisel TP, Bichet DG, Barret C, et al. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem. 1996;271:16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- Hernanz-Falcon P, Rodriguez-Frade JM, Serrano A, Juan D, del Sol A, Soriano SF, et al. Identification of amino acid residues crucial for chemokine receptor dimerization. Nat Immunol. 2004;5:216–223. doi: 10.1038/ni1027. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Harrigan TJ, Mazurkiewicz JE. Inhibition of serotonin 5-hydroxytryptamine 2C receptor function through heterodimerization. Receptor dimers bind two molecules of ligand and one G protein. J Biol Chem. 2005;280:40144–40151. doi: 10.1074/jbc.M507396200. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Weaver BA, Grinde E, Mazurkiewicz JE. Serotonon 5-HT2C receptor homodimer biogenesis in the endoplasmic reticulum: real time visualization with confocal fluorescence resonance energy transfer. J Biol Chem. 2006;281:27109–27116. doi: 10.1074/jbc.M604390200. [DOI] [PubMed] [Google Scholar]
- Hill SJ. G-protein-coupled receptors: past, present and future. Br J Pharmacol. 2006;147 Suppl 1:S27–S37. doi: 10.1038/sj.bjp.0706455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JR, Oliveira MI, Carmo AM, Iaboni A, Davis SJ. A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nat Methods. 2006;3:1001–1006. doi: 10.1038/nmeth978. [DOI] [PubMed] [Google Scholar]
- Jastrzebska B, Fotiadis D, Jang GF, Stenkamp RE, Engel A, Palczewski K. Functional and structural characterization of rhodopsin oligomers. J Biol Chem. 2006;281:11917–11922. doi: 10.1074/jbc.M600422200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with beta 2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc Natl Acad Sci USA. 2001;98:343–348. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, et al. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- Kim SK, Jacobson KA. Computational prediction of homodimerization of the A3 adenosine receptor. J Mol Graph Model. 2006;25:549–561. doi: 10.1016/j.jmgm.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klco JM, Lassere TB, Baranski TJ. C5a receptor oligomerization. I. Disulfide trapping reveals oligomers and potential contact surfaces in a G protein coupled receptor. J Biol Chem. 2003;278:35345–35353. doi: 10.1074/jbc.M305606200. [DOI] [PubMed] [Google Scholar]
- Kopanchuk S, Veiksina S, Mutulis F, Mutule I, Yahorava S, Mandrika I, et al. Kinetic evidence for tandemly arranged ligand binding sites in melanocortin 4 receptor complexes. Neurochem Int. 2006;49:533–542. doi: 10.1016/j.neuint.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Ladds G, Davis K, Das A, Davey J. A constitutively active GPCR retains its G protein specificity and the ability to form dimers. Mol Microbiol. 2005;55:482–497. doi: 10.1111/j.1365-2958.2004.04394.x. [DOI] [PubMed] [Google Scholar]
- Laroche G, Lepine MC, Theriault C, Giguere P, Giguere V, Gallant MA, et al. Oligomerization of the alpha and beta isoforms of the thromboxane A2 receptor: relevance to receptor signaling and endocytosis. Cell Signal. 2005;17:1373–1383. doi: 10.1016/j.cellsig.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Law PY, Erickson-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, et al. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor–G protein interactions. J Biol Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- Lee SP, O'Dowd BF, Rajaram RD, Nguyen T, George SR. D2 dopamine receptor homodimerization is mediated by multiple sites of interaction, including an intermolecular interaction involving transmembrane domain 4. Biochemistry. 2003;42:11023–11031. doi: 10.1021/bi0345539. [DOI] [PubMed] [Google Scholar]
- Leskela TT, Markkanen PM, Pietila EM, Tuusa JT, Petaja-Repo UE. Opioid receptor pharmacological chaperones act by binding and stabilizing newly synthesized receptors in the endoplasmic reticulum. J Biol Chem. 2007;282:23171–23183. doi: 10.1074/jbc.M610896200. [DOI] [PubMed] [Google Scholar]
- Leung PK, Chow KB, Lau PN, Chu KM, Chan CB, Cheng CH, et al. The truncated ghrelin receptor polypeptide (GHS-R1b) acts as a dominant-negative mutant of the ghrelin receptor. Cell Signal. 2007;19:1011–1022. doi: 10.1016/j.cellsig.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P, et al. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 2006a;25:3012–3023. doi: 10.1038/sj.emboj.7601193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P, et al. Do orphan G-protein-coupled receptors have ligand-independent functions? New insights from receptor heterodimers. EMBO Rep. 2006b;7:1094–1098. doi: 10.1038/sj.embor.7400838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisenbee CS, Miller LJ. Secretin receptor oligomers form intracellularly during maturation through receptor core domains. Biochemistry. 2006;45:8216–8226. doi: 10.1021/bi060494y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Canals M, Pediani JD, Milligan G. The α1b-adrenoceptor exists as a higher-order oligomer: effective oligomerization is required for receptor maturation, surface delivery and function. Mol Pharmacol. 2007;71:1015–1029. doi: 10.1124/mol.106.033035. [DOI] [PubMed] [Google Scholar]
- Maggio R, Novi F, Scarselli M, Corsini GU. The impact of G-protein-coupled receptor hetero-oligomerization on function and pharmacology. FEBS J. 2005;272:2939–2946. doi: 10.1111/j.1742-4658.2005.04729.x. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Marshall FH, Jones KA, Kaupmann K, Bettler B. GABAB receptors—the first 7TM heterodimers. Trends Pharmacol Sci. 1999;20:396–399. doi: 10.1016/s0165-6147(99)01383-8. [DOI] [PubMed] [Google Scholar]
- McElvaine AT, Mayo KE. A dominant negative human growth hormone-releasing hormone (GHRH) receptor splice variant inhibits GHRH binding. Endocrinology. 2006;147:1884–1894. doi: 10.1210/en.2005-1488. [DOI] [PubMed] [Google Scholar]
- McGraw DW, Mihlbachler KA, Schwarb MR, Rahman FF, Small KM, Almoosa KF, et al. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest. 2006;116:1400–1409. doi: 10.1172/JCI25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JN, Patterson MM, Malik AB. Protease-activated receptor-3 (PAR3) regulates PAR1 signalling by receptor dimerization. Proc Natl Acad Sci USA. 2007;104:5662–5667. doi: 10.1073/pnas.0700763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier JF, Salahpour A, Angers S, Breit A, Bouvier M. Quantitative assessment of beta 1- and beta 2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J Biol Chem. 2002;277:44925–44931. doi: 10.1074/jbc.M205767200. [DOI] [PubMed] [Google Scholar]
- Meyer BH, Segura JM, Martinez KL, Hovius R, George N, Johnsson K, et al. FRET imaging reveals that functional neurokinin-1 receptors are monomeric and reside in membrane microdomains of live cells. Proc Natl Acad Sci USA. 2006;103:2138–2143. doi: 10.1073/pnas.0507686103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano SK, Kim YM, Stefano FP, Benovic JL, Brenner C. Nonvisual arrestin oligomerization and cellular localization are regulated by inositol hexakisphosphate binding. J Biol Chem. 2006;281:9812–9823. doi: 10.1074/jbc.M512703200. [DOI] [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- Milligan G. GPCR hetero-dimers: pharmacology, function and relevance to drug discovery. Drug Discov Today. 2006;11:541–549. doi: 10.1016/j.drudis.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim Biophys Acta. 2007;1768:825–835. doi: 10.1016/j.bbamem.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Milligan G, Bouvier M. Methods to monitor the quaternary structure of G protein-coupled receptors. FEBS J. 2005;272:2914–2925. doi: 10.1111/j.1742-4658.2005.04731.x. [DOI] [PubMed] [Google Scholar]
- Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol. 2006;147 Suppl 1:S46–S55. doi: 10.1038/sj.bjp.0706405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Smith NJ.2007Allosteric modulation of hetero-dimeric GPCRs Trends Pharmacol Sci(in press). [DOI] [PubMed]
- Muto T, Tsuchiya D, Morikawa K, Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci USA. 2007;104:3759–3764. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto W, Toh H. Prediction of interfaces for oligomerizations of G-protein coupled receptors. Proteins. 2005;58:644–660. doi: 10.1002/prot.20332. [DOI] [PubMed] [Google Scholar]
- Overton MC, Blumer KJ. G-protein-coupled receptors function as oligomers in vivo. Curr Biol. 2000;10:341–344. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- Overton MC, Chinault SL, Blumer KJ. Oligomerization, biogenesis, and signaling is promoted by a glycophorin A-like dimerization motif in transmembrane domain 1 of a yeast G protein-coupled receptor. J Biol Chem. 2003;278:49369–49377. doi: 10.1074/jbc.M308654200. [DOI] [PubMed] [Google Scholar]
- Overton MC, Chinault SL, Blumer KJ. Oligomerization of G-protein-coupled receptors: lessons from the yeast Saccharomyces cerevisiae. Eukaryot Cell. 2005;4:1963–1970. doi: 10.1128/EC.4.12.1963-1970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RK. The pharmacology and signaling of bitter, sweet, and umami taste sensing. Mol Interv. 2007;7:87–98. doi: 10.1124/mi.7.2.9. [DOI] [PubMed] [Google Scholar]
- Park PS, Palczewski K. Diversifying the repertoire of G protein-coupled receptors through oligomerization. Proc Natl Acad Sci USA. 2005;102:8793–8794. doi: 10.1073/pnas.0504016102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percherancier Y, Berchiche YA, Slight I, Volkmer-Engert R, Tamamura H, Fujii N, et al. Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J Biol Chem. 2005;280:9895–9903. doi: 10.1074/jbc.M411151200. [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem. 2001;276:4416–44123. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- Pfleger KD, Eidne KA. Illuminating insights into protein–protein interactions using bioluminescence resonance energy transfer (BRET) Nat Methods. 2006;3:165–174. doi: 10.1038/nmeth841. [DOI] [PubMed] [Google Scholar]
- Philip F, Sengupta P, Scarlata S. Signalling through a G protein-coupled receptor and its corresponding G protein follows a stoichiometrically limited model. J Biol Chem. 2007;282:19203–19216. doi: 10.1074/jbc.M701558200. [DOI] [PubMed] [Google Scholar]
- Pidasheva S, Grant M, Canaff L, Ercan O, Kumar U, Hendy GN. Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CASR mutants retained intracellularly. Hum Mol Genet. 2006;15:2200–2209. doi: 10.1093/hmg/ddl145. [DOI] [PubMed] [Google Scholar]
- Pin JP, Kniazeff J, Liu J, Binet V, Goudet C, Rondard P, et al. Allosteric functioning of dimeric class C G-protein-coupled receptors. FEBS J. 2005;272:2947–2955. doi: 10.1111/j.1742-4658.2005.04728.x. [DOI] [PubMed] [Google Scholar]
- Pin J-P, Neubig R, Bouvier M, Devi L, Filizola M, Javitch JA, et al. International union of basic and clinical pharmacology. LXVII. Recommendations for the recognition and nomenclature of G-protein-coupled receptor heteromultimers. Pharmacol Rev. 2007;59:5–13. doi: 10.1124/pr.59.1.5. [DOI] [PubMed] [Google Scholar]
- Prinster SC, Hague C, Hall RA. Heterodimerization of g protein-coupled receptors: specificity and functional significance. Pharmacol Rev. 2005;57:289–298. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, et al. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K, Hauschild BC, Steinbach PJ, Goldsmith PK, Hauache O, Spiegel AM. Identification of the cysteine residues in the amino-terminal extracellular domain of the human Ca(2+) receptor critical for dimerization. Implications for function of monomeric Ca(2+) receptor. J Biol Chem. 1999;274:27642–27650. doi: 10.1074/jbc.274.39.27642. [DOI] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robben JH, Deen PM. Pharmacological chaperones in nephrogenic diabetes insipidus: possibilities for clinical application. BioDrugs. 2007;21:157–166. doi: 10.2165/00063030-200721030-00003. [DOI] [PubMed] [Google Scholar]
- Robbins MJ, Ciruela F, Rhodes A, McIlhinney RA. Characterization of the dimerization of metabotropic glutamate receptors using an N-terminal truncation of mGluR1alpha. J Neurochem. 1999;72:2539–2547. doi: 10.1046/j.1471-4159.1999.0722539.x. [DOI] [PubMed] [Google Scholar]
- Rochais F, Vilardaga JP, Nikolaev VO, Bunemann M, Lohse MJ, Engelhardt S. Real-time optical recording of beta1-adrenergic receptor activation reveals supersensitivity of the Arg389 variant to carvedilol. J Clin Invest. 2007;117:229–235. doi: 10.1172/JCI30012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Miller JK, Hyrc K, Dikranian S, Mennerick S, Takeuchi Y, et al. Covalent and noncovalent interactions mediate metabotropic glutamate receptor mGlu5 dimerization. Mol Pharmacol. 2001;59:46–53. [PubMed] [Google Scholar]
- Romano C, Yang WL, O'Malley KL. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J Biol Chem. 1996;271:28612–28616. doi: 10.1074/jbc.271.45.28612. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: {beta}-arrestin2-mediated ERK activation by {micro}-{delta} opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Angers S, Bouvier M. Functional significance of oligomerization of G-protein-coupled receptors. Trends Endocrinol Metab. 2000;11:163–168. doi: 10.1016/s1043-2760(00)00260-5. [DOI] [PubMed] [Google Scholar]
- Salahpour A, Angers S, Mercier JF, Lagace M, Marullo S, Bouvier M. Homodimerization of the beta2-adrenergic receptor as a prerequisite for cell surface targeting. J Biol Chem. 2004;279:33390–33397. doi: 10.1074/jbc.M403363200. [DOI] [PubMed] [Google Scholar]
- Salahpour A, Masri B. Experimental challenge to a ‘rigorous' BRET analysis of GPCR oligomerization. Nat Methods. 2007;4:599–600. doi: 10.1038/nmeth0807-599. [DOI] [PubMed] [Google Scholar]
- Sal-Man N, Gerber D, Bloch I, Shai Y. Specificity in transmembrane helix-helix interactions mediated by aromatic residues. J Biol Chem. 2007;282:19753–19761. doi: 10.1074/jbc.M610368200. [DOI] [PubMed] [Google Scholar]
- Sal-Man N, Gerber D, Shai Y. The identification of a minimal dimerization motif QXXS that enables homo- and hetero-association of transmembrane helices in vivo. J Biol Chem. 2005;280:27449–27457. doi: 10.1074/jbc.M503095200. [DOI] [PubMed] [Google Scholar]
- Salom D, Lodowski DT, Stenkamp RE, Le Trong I, Golczak M, Jastrzebska B, et al. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc Natl Acad Sci USA. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Laorden BL, Sanchez-Mas J, Martinez-Alonso E, Martinez-Menarguez JJ, Garcia-Borron JC, Jimenez-Cervantes C. Dimerization of the human melanocortin 1 receptor: functional consequences and dominant negative effects. J Invest Dermatol. 2006;126:172–181. doi: 10.1038/sj.jid.5700036. [DOI] [PubMed] [Google Scholar]
- Sartania N, Appelbe S, Pediani JD, Milligan G. Agonist occupancy of a single monomeric element is sufficient to cause internalization of the dimeric β2-adrenoceptor. Cell Signal. 2007;19:1928–1938. doi: 10.1016/j.cellsig.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Savi P, Zachayus JL, Delesque-Touchard N, Labouret C, Herve C, Uzabiaga MF, et al. The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci USA. 2006;103:11069–11074. doi: 10.1073/pnas.0510446103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlyer S, Horuk R. I want a new drug: G-protein-coupled receptors in drug development. Drug Discov Today. 2006;11:481–493. doi: 10.1016/j.drudis.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Shi C, Paige M, Maley J, Loewen MC.2007Agonist-induced oligomerization of the Saccharomyces cerevisiae G protein-coupled receptor, Ste2p Biochemistry(in press). [DOI] [PubMed]
- Skrabanek L, Murcia M, Bouvier M, Devi L, George SR, Lohse MJ, et al. Requirements and ontology for a G protein-coupled receptor oligomerization knowledge base. BMC-Bioinformatics. 2007;8:177. doi: 10.1186/1471-2105-8-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook LA, Milligan G, Kieffer BL, Massotte D. Mu-delta opioid receptor functional interaction: Insight using receptor-G protein fusions. J Pharmacol Exp Ther. 2006;318:683–690. doi: 10.1124/jpet.106.101220. [DOI] [PubMed] [Google Scholar]
- So CH, Varghese G, Curley KJ, Kong MM, Alijaniaram M, Ji X, et al. D1 and D2 dopamine receptors form heterooligomers and co-internalize after selective activation of either receptor. Mol Pharmacol. 2005;68:568–578. doi: 10.1124/mol.105.012229. [DOI] [PubMed] [Google Scholar]
- So CH, Verma V, O'Dowd BF, George SR. Desensitization of the dopamine D1 and D2 receptor heterooligomer mediated calcium signal by agonist occupancy of either receptor. Mol Pharmacol. 2007;72:450–462. doi: 10.1124/mol.107.034884. [DOI] [PubMed] [Google Scholar]
- Stanasila L, Perez JB, Vogel H, Cotecchia S. Oligomerization of the alpha 1a- and alpha 1b-adrenergic receptor subtypes. Potential implications in receptor internalization. J Biol Chem. 2003;278:40239–40251. doi: 10.1074/jbc.M306085200. [DOI] [PubMed] [Google Scholar]
- Storez H, Scott MG, Issafras H, Burtey A, Benmerah A, Muntaner O, et al. Homo- and hetero-oligomerization of beta-arrestins in living cells. J Biol Chem. 2005;280:40210–40215. doi: 10.1074/jbc.M508001200. [DOI] [PubMed] [Google Scholar]
- Tao YX. Inactivating mutations of G protein-coupled receptors and diseases: structure-function insights and therapeutic implications. Pharmacol Ther. 2006;111:949–973. doi: 10.1016/j.pharmthera.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Thompson MD, Burnham WM, Cole DE. The G protein-coupled receptors: pharmacogenetics and disease. Crit Rev Clin Lab Sci. 2005;42:311–392. doi: 10.1080/10408360591001895. [DOI] [PubMed] [Google Scholar]
- Torvinen M, Kozell LB, Neve KA, Agnati LF, Fuxe K. Biochemical identification of the dopamine D2 receptor domains interacting with the adenosine A2A receptor. J Mol Neurosci. 2004;24:173–180. doi: 10.1385/JMN:24:2:173. [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Kunishima N, Kamiya N, Jingami H, Morikawa K. Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+ Proc Natl Acad Sci USA. 2002;99:2660–2665. doi: 10.1073/pnas.052708599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic. 2004;5:821–837. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Bunemann M, Krasel C, Castro M, Lohse MJ. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat Biotechnol. 2003;21:807–812. doi: 10.1038/nbt838. [DOI] [PubMed] [Google Scholar]
- Vohra S, Chintapalli SV, Illingworth CJ, Reeves PJ, Mullineaux PM, Clark HS, et al. Computational studies of Family A and Family B GPCRs. Biochem Soc Trans. 2007;35:749–754. doi: 10.1042/BST0350749. [DOI] [PubMed] [Google Scholar]
- Vrecl M, Drinovec L, Elling C, Heding A. Opsin oligomerization in a heterologous cell system. J Recept Signal Trans. 2006;26:505–526. doi: 10.1080/10799890600932253. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Fong J, Jones RM, Luzner MM, Sharma SK, Kostenis E, et al. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci USA. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, He L, Combs CA, Roderiquez G, Norcross MA. Dimerization of CXCR4 in living malignant cells: control of cell migration by a synthetic peptide that reduces homologous CXCR4 interactions. Mol Cancer Ther. 2006;5:2474–2483. doi: 10.1158/1535-7163.MCT-05-0261. [DOI] [PubMed] [Google Scholar]
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- Whorton MR, Bokoch MP, Rasmussen SGF, Huang B, Zare RN, Kobilka B, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci USA. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Wilkinson G, Milligan G. The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J Biol Chem. 2005;280:28663–28674. doi: 10.1074/jbc.M413475200. [DOI] [PubMed] [Google Scholar]
- Wise A, Jupe SC, Rees S. The identification of ligands at orphan G-protein coupled receptors. Annu Rev Pharmacol Toxicol. 2004;44:43–66. doi: 10.1146/annurev.pharmtox.44.101802.121419. [DOI] [PubMed] [Google Scholar]
- Xie Z, Bhushan RG, Daniels DJ, Portoghese PS. Interaction of bivalent ligand KDN21 with heterodimeric delta-kappa opioid receptors in human embryonic kidney 293 cells. Mol Pharmacol. 2005;68:1079–1086. doi: 10.1124/mol.105.012070. [DOI] [PubMed] [Google Scholar]
- Yesilaltay A, Jenness DD. Homo-oligomeric complexes of the yeast alpha-factor pheromone receptor are functional units of endocytosis. Mol Biol Cell. 2000;11:2873–2884. doi: 10.1091/mbc.11.9.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Sun S, Quinn SJ, Brown EM, Bai M. The extracellular calcium-sensing receptor dimerizes through multiple types of intermolecular interactions. J Biol Chem. 2001;276:5316–5322. doi: 10.1074/jbc.M005958200. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]