Abstract
Maintaining the proper balance between cell apoptosis and proliferation is required for normal tissue homeostasis; when this balance is disrupted, disease such as pulmonary arterial hypertension (PAH) can result. Activity of K+ channels plays a major role in regulating the pulmonary artery smooth muscle cell (PASMC) population in the pulmonary vasculature, as they are involved in cell apoptosis, survival and proliferation. PASMCs from PAH patients demonstrate many cellular abnormalities linked to K+ channels, including decreased K+ current, downregulated expression of various K+ channels, and inhibited apoptosis. K+ is the major intracellular cation, and the K+ current is a major determinant of cell volume. Apoptotic volume decrease (AVD), an early hallmark and prerequisite of programmed cell death, is characterized by K+ and Cl− efflux. In addition to its role in AVD, cytosolic K+ can be inhibitory toward endogenous caspases and nucleases and can suppress mitochondrial cytochrome c release. In PASMC, K+ channel activation accelerates AVD and enhances apoptosis, while K+ channel inhibition decelerates AVD and inhibits apoptosis. Finally, inhibition of K+ channels, by increasing cytosolic [Ca2+] as a result of membrane depolarization-mediated opening of voltage-dependent Ca2+ channels, leads to PASMC contraction and proliferation. The goals of this review are twofold: (1) to elucidate the role of K+ ions and K+ channels in the proliferation and apoptosis of PASMC, with an emphasis on abnormal cell growth in human and animal models of PAH, and (2) to elaborate upon the targeting of K+ flux pathways for pharmacological treatment of pulmonary vascular disease.
Keywords: pulmonary artery smooth muscle cell, pulmonary hypertension, apoptosis, proliferation
Introduction
A proper balance between cell death and proliferation is necessary for the normal development and function of tissues. Disturbing this equilibrium can lead to disease states, such as cancer (Green and Evan, 2002), neurodegenerative disorders (Yuan and Yankner, 2000) and pulmonary hypertension (Mandegar et al., 2004). Studies have begun to elucidate the roles of ions, including K+, Ca2+, Na+ and Cl−, and their channels, in both apoptosis and cell proliferation, although much remains unknown. K+ channels are found on both the plasma membranes and internal membranes of many cell types where they respond to a variety of stimuli. The four main functional classes are (i) voltage-gated (KV) K+ channels; (ii) Ca2+-activated (KCa) K+ channels, which are further divided into small-conductance (SK), intermediate-conductance (IK) and large-conductance (BK or maxiK) subfamilies; (iii) inwardly rectifying (KIR) K+ channels, of which ATP-sensitive (KATP) K+ channels are a member, composed of KIR6.x and sulphonylurea subunits; and (iv) two-pore domain (K2P) K+ channels. Diversity of K+ channels is due to a number of factors, including a multitude of encoding genes (over 75 have been identified so far), the heterotetrameric structure of functional channels, and the fact that channels can associate with both accessory β regulatory subunits and electrically silent membrane subunits that alter channels' physiological properties (Coetzee et al., 1999; Amberg et al., 2003).
Traditionally, K+ channels are associated with repolarization after action potentials and setting the resting membrane potential of excitable cells (Nelson and Quayle, 1995; Yuan, 1995; Yuan et al., 1998c; Hille, 2001; Amberg et al., 2003), but they are also implicated in T-lymphocyte activation (Chandy et al., 1984; DeCoursey et al., 1984), hypoxic pulmonary vasoconstriction (Yuan et al., 1993; Smirnov et al., 1994; Archer et al., 1998; Coppock et al., 2001; Yuan, 2001), myocardial protection against ischaemia (Nichols and Lederer, 1991; Yokoshiki et al., 1998), controlling insulin release from pancreatic β cells (Yokoshiki et al., 1998; Thevenod, 2002) and neurotransmitter release (Rudy, 1988; Toro et al., 1998). Findings within the last two decades have also implicated K+ channels and K+ flux in cell volume regulatory mechanisms, as well as both programmed cell death (apoptosis) and cellular proliferation. Inhibition of channels is associated with proliferation of pulmonary artery smooth muscle cells (PASMCs) (Platoshyn et al., 2000), whereas in certain other cell types, proliferation has been found to be linked with K+ efflux (Wonderlin and Strobl, 1996; Pardo, 2004). Activation of K+ channels, leading to K+ efflux, is associated with apoptosis through two main mechanisms. Although activity of the electrogenic Na+/K+ pump plays a major role in regulating the transmembrane K+ gradient, apoptotic volume decrease (AVD), a necessary prerequisite for cells undergoing apoptosis, is caused mainly by K+ loss (Bortner et al., 1997; Maeno et al., 2000). Additionally, a loss of K+ releases inhibition of cytoplasmic caspases (Hughes et al., 1997; Dallaporta et al., 1998). A proper balance between proliferation and apoptosis is required for normal tissue homeostasis; when this balance is disturbed in favour of proliferation or too little apoptosis, the resultant increase in cell population can cause pathophysiological problems, as in pulmonary arterial hypertension (PAH).
The pulmonary circulation is a high-flow, low-resistance and low-pressure system, the vascular resistance of which is determined by the contractility of the PASMCs of the medial layer. Sustained pulmonary vasoconstriction can cause increased pulmonary vascular resistance by narrowing the lumen of pulmonary arteries. Another of the major pathologies of PAH is pulmonary vascular medial thickening, caused by PASMC hypertrophy and hyperplasia. Abnormal apoptotic or cell proliferation regulation has been observed in PASMC from PAH patients (Rubin, 1997; Mandegar et al., 2004). This review focuses on K+ channels in PASMC proliferation and apoptosis, with attention given to the dysregulation of K+ equilibrium in PAH and the possibility of therapeutic targeting of K+ channels to treat PAH.
Role of K+ channels in PASMC
Intracellularly, K+ is the major cationic species because of the activity of the Na+/K+ ATPase. At rest, the plasma membrane is most permeable to K+; therefore, the activity of K+ channels plays a large role in setting the resting membrane potential (Em) (Nelson and Quayle, 1995; Yuan, 1995; Peng et al., 1996; Archer et al., 1998; Yuan et al., 1998c; Amberg et al., 2003). Although KV channels have been studied most extensively in this context (Nelson and Quayle, 1995; Yuan, 1995; Peng et al., 1996; Archer et al., 1998; Yuan et al., 1998c), KCa, KATP and K2P channels have also been implicated in setting Em in PASMC (Smirnov et al., 1994; Nelson and Quayle, 1995; Peng et al., 1996; Yokoshiki et al., 1998; Gurney et al., 2003; Olschewski et al., 2006). Partly due to the electrogenic nature of the Na+/K+ ATPase, pumping three Na+ out of the cell for every two K+ it brings into the cell, the membrane potential is negative at rest. The following equation describes whole-cell KV currents: IK(V)=N × i × Popen, where ‘N' is the total number of functional KV channels in the plasma membrane, ‘i' is the amplitude of single-channel current and ‘Popen' is the steady-state open probability of the channel. The electrochemical gradient of K+ is directed out of the cell, so when K+ channels open, K+ flows out, leading to a membrane hyperpolarization. Conversely, a decrease in the outward current through K+ channels, due to decreased open probability, single-channel current and/or number of available channels, will lead to membrane depolarization.
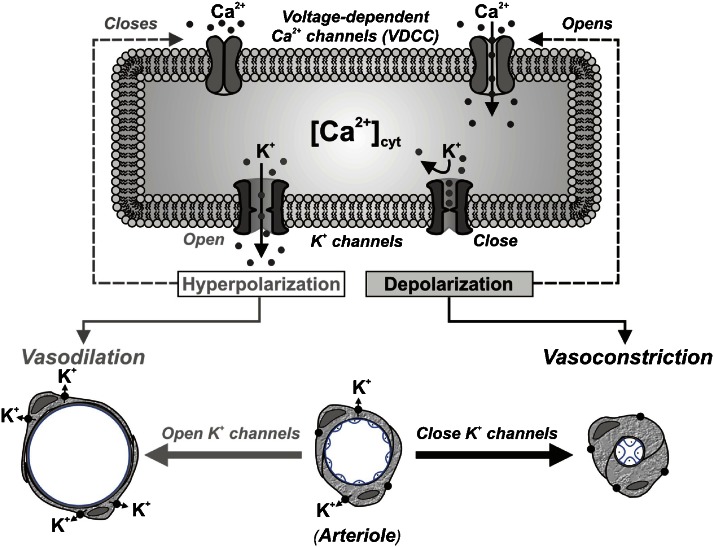
Voltage-gated K+ channels play an important role in regulating pulmonary vascular tone through the control of Em, an important determinant of cytosolic Ca2+ ([Ca2+]cyt) in PASMC (Fleischmann et al., 1994; Smirnov et al., 1994; Nelson and Quayle, 1995; Yuan, 1995; Carl et al., 1996; Archer et al., 1998; Yuan et al., 1998c), although other K+ channels are also known to play a role in controlling vascular tone in other cell types (Yokoshiki et al., 1998). When IK(V) is inhibited pharmacologically, for example, by 4-aminopyridine (4-AP), the resulting membrane depolarization opens L-type voltage-dependent Ca2+ channels (VDCC), causing Ca2+ influx and an increase in [Ca2+]cyt (Nelson et al., 1990; Yuan, 1995; Yuan et al., 1996; Platoshyn et al., 2000; Cribbs, 2006), and subsequent smooth muscle cell contraction through Ca2+–calmodulin activation of myosin light-chain kinase (Somlyo and Somlyo, 1994; Ratz et al., 2005). Conversely, activation of K+ channels in PASMC, such as that induced by nitric oxide (NO), hyperpolarizes the membrane and decreases [Ca2+]cyt (Nelson et al., 1990; Yuan et al., 1996). Furthermore, it has been shown that blockade of KV channels with 4-AP in isolated pulmonary arterial rings is sufficient to increase arterial tension and to inhibit NO-induced relaxation (Peng et al., 1996; Yuan et al., 1996; Zhao et al., 1997). These data support the hypothesis that KV channels are important contributors to Em in PASMC, which in turn, is a major determinant of [Ca2+]cyt (Figure 1).
Figure 1.
Proposed mechanisms showing the role of K+ channel activity and cytosolic [Ca2+] in vasoconstriction. When K+ channels are blocked (or K+ channel expression is downregulated), the resulting membrane depolarization opens voltage-dependent Ca2+ channels (VDCC), promotes Ca2+ influx, increases [Ca2+]cyt and causes vasoconstriction. When K+ channels are activated (or K+ channel gene expression is upregulated), the membrane hyperpolarization closes VDCC, inhibits agonist-mediated Ca2+ influx and causes vasodilation.
In addition to KV channels, KCa and KIR channels have also been shown to contribute to regulating Em of arterial smooth muscle cells (Nelson and Quayle, 1995). Additionally, the expression of different K+ channel subtypes may also influence cell phenotypes. In fully differentiated aortic smooth muscle cells (that is, those having the contractile phenotype), large-conductance BK (BKCa) currents predominate, whereas in immature cells (that is, of the proliferative phenotype), voltage-insensitive intermediate-conductance IKCa currents are the dominant type (Neylon et al., 1999; Köhler et al., 2003; Jackson, 2005). Ivanov et al. (2006) found that epidermal growth factor (EGF) caused membrane hyperpolarization of freshly isolated arterial smooth muscle cells through iberiotoxin-sensitive BKCa channels. Similarly, KV channels are also found to be differentially expressed according to the cell's phenotype. KV1 channels were found to be associated with the contractile phenotype of human uterine vascular smooth muscle cells, whereas KV3.4 was associated with the proliferating phenotype (Miguel-Velado et al., 2005).
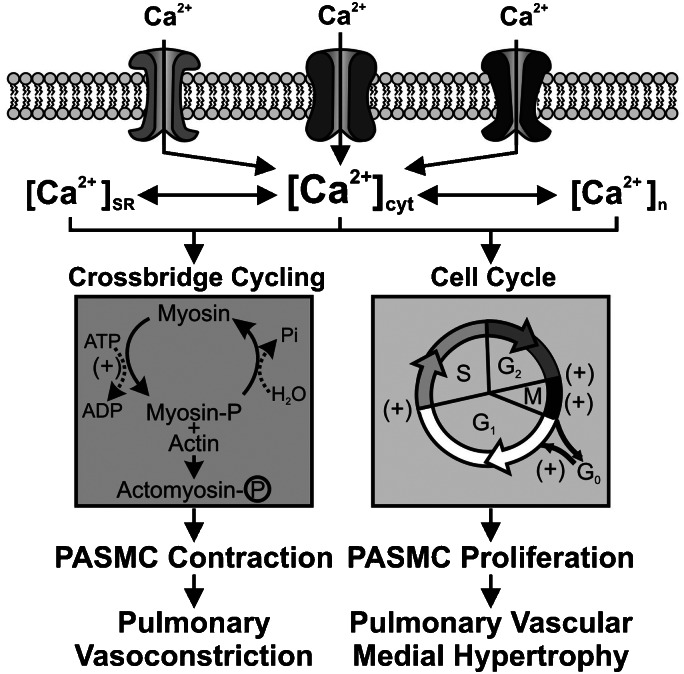
When KV channels close and [Ca2+]cyt increases, smooth muscle contraction is not the only functional change that occurs. Intracellular Ca2+, in addition to its role in muscle contraction, is also an important second messenger for cell migration and proliferation. Ca2+ influx through L-type VDCC triggers CRE- and c-Jun-mediated gene transcription, and activates transcription factors, such as CREB, NF-AT and NF-κB that are involved in cell proliferation, protein synthesis and inflammation (Sheng et al., 1990; Bading et al., 1997; Hardingham et al., 1997, 1998; Hardingham and Bading, 1999). Ca2+ is also required for cell-cycle progression, for example, at the G0 to G1 transition, DNA synthesis and mitosis (Dubois and Rouzaire-Dubois, 1993; Berridge, 1995; Clapham, 1995). In PASMC, inhibition of KV channels is associated with cell proliferation, consistent with the idea that the depolarization-induced increase in [Ca2+]cyt allows for progression through the cell cycle. Specifically, Platoshyn et al. (2000) showed that in proliferating PASMC compared to growth-arrested PASMC, resting [Ca2+]cyt was higher and the Em more depolarized. Furthermore, IK(V) was diminished in proliferating PASMC, consistent with the hypothesis that Ca2+ influx following inhibition of K+ currents leads to proliferation. Thus, a rise in [Ca2+]cyt also functions as a signal for stimulating PASMC proliferation and gene expression (Figure 2).
Figure 2.
Ca2+ causes pulmonary artery smooth muscle cell (PASMC) contraction and stimulates cell proliferation. An increase in [Ca2+]cyt due to Ca2+ influx through voltage-dependent Ca2+ channels (such as L-type and T-type VDCC) causes PASMC contraction by promoting myosin and actin interaction and stimulates PASMC proliferation by propelling the cell through the cell cycle. (+) Ca2+-sensitive steps in the cell cycle. In addition, increases in [Ca2+] in the sarcoplasmic reticulum (SR, [Ca2+]SR), cytosol ([Ca2+]cyt) and nucleus ([Ca2+]n), all contribute to activating cytoplasmic signalling proteins and nuclear transcription factors (for example, CREB, NF-AT, c-Jun and NF-κB) and stimulating cell proliferation.
It should be noted that evidence as to which K+ channels are involved in specific physiological roles, such as setting the Em or cell volume control, can be extremely cell specific, even within PASMC, as cultured proliferating cells may express a K+ channel profile that is distinct from that expressed by freshly isolated PASMC (Neylon et al., 1999; Moudgil et al., 2006). Furthermore, different segments (for example, conduit vs resistance arteries) of the vessels within the pulmonary circulation, and even different PASMC from the same segment (for example, in different cell-cycle phases or in different phenotypes), express varying profiles of K+ channels (Archer et al., 1996). Additionally, effects of ion channels in response to apoptotic or proliferative stimuli are likely to be highly time dependent, as initial short-term effects develop within minutes, whereas long-term effects can take hours or days to occur.
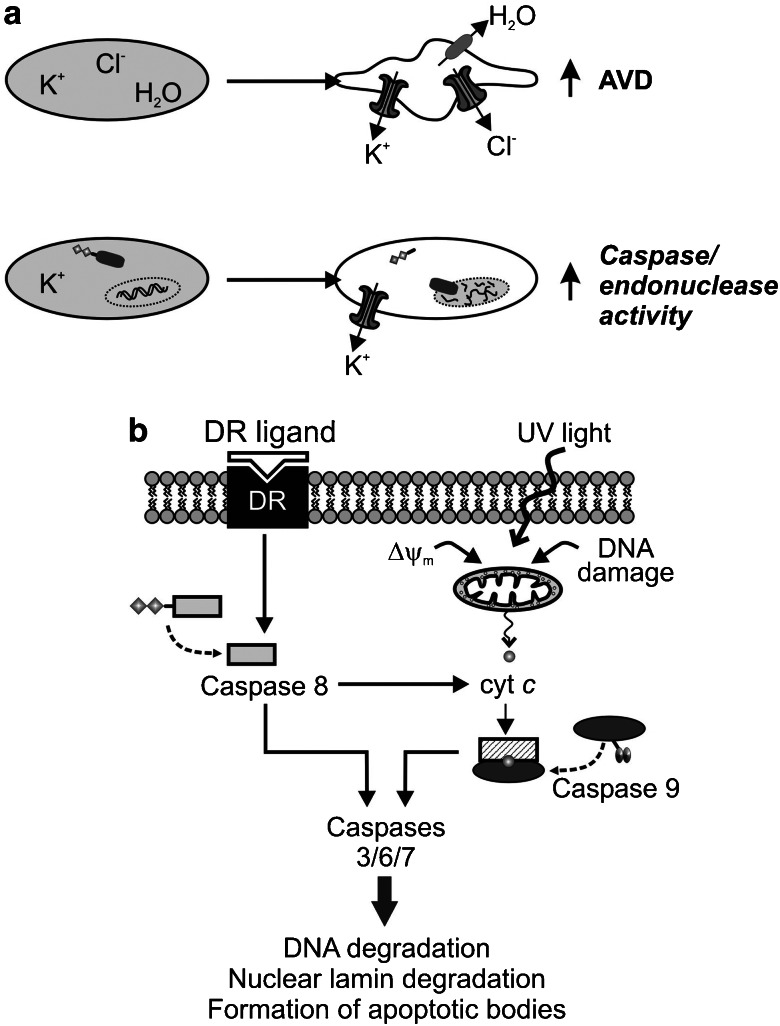
Cell volume is directly related to the movement of ions, with homeostasis being achieved by a balance of osmotic pressure across the plasma membrane. Most cells achieve and maintain their osmotic balance due to the continuous activity of the Na+/K+ ATPase pump, which creates an intracellular environment rich in K+ and low in Na+, which is in contrast with the extracellular space where high levels of Na+ and low levels of K+ exist. Despite the net transmembrane potential that is generated, a net electrochemical gradient is established that favours the passive movement of K+ out of the cell (al-Habori, 1994). Because most cell types have a high resting permeability to K+, resting Em values are generally close to those of the actual Nernstian K+ potential (EK≈−80 mV). However, the distinct permeability of the membrane to other ions (for example, Ca2+, Na+ and Cl−) in different cell types, such as smooth muscle cells, can render Em more positive than EK. Nonetheless, it is not surprising that K+ channels are also involved in the regulation of cell volume (Figure 3a). Cells have active volume regulatory mechanisms, including regulatory volume decrease (RVD), to maintain a constant volume in response to environmental challenges such as hypo- or hypertonic challenge and the gain or loss of osmotically active substances by transport or metabolism (Chamberlin and Strange, 1989). Interestingly, lymphocytes lack a volume regulatory mechanism and undergo apoptosis in response to hypertonic conditions, whereas other cell types that initially undergo shrinkage upon hypertonic challenge are able to then maintain their volume or regulate back to normal volumes without undergoing apoptosis (Bortner and Cidlowski, 1996). Imbalances in osmolarity across cell membranes trigger the movement of water through aquaporins, with the resulting hydrostatic pressure gradient compensated by the movement of ions, namely K+ and Cl− (Figure 3a) (Lang et al., 1998).
Figure 3.
Role of K+ efflux in regulation of apoptotic volume decrease (AVD) and apoptosis. (a) Opening of K+ channels in the plasma membrane leads to K+ (and Cl−) efflux. The resultant gradient of osmolarity causes outward transportation of water and eventually leads to AVD (upper panel). Decreased cytosolic [K+] due to increased K+ efflux would relieve the inhibitory effect of cytoplasmic K+ on cytoplasmic cascases and endonucleases, and promote apoptosis (lower panel). (b) Two pathways of apoptosis. When death receptors (DRs) are activated (for example, by Fas ligand), cleavage of procaspase 8 (and/or 10) to active caspase 8 is an important initial step to induce apoptosis. Cytochrome c, which can be released from the mitochondria to the cytosol when cells are exposed to UV light or when mitochondrial membrane potential (ΔΨm) is depolarized, activates cytoplasmic caspase 9. Active caspases 8 and 9 then activate caspases 3/6/7 and cause DNA fragmentation and nuclear breakage, and eventually cell death.
Various K+ channels are involved in regulation of cell volume, including KV channels (for example, KV1.3 and KV1.5), KCa channels (for example, SKCa, IKCa and BKCa), and K2P (for example, TREK1, TRAAK and TASK2) (Felipe et al., 1993; Lang et al., 1998). An increased IKCa current was observed during the RVD response to hypotonic challenge in human epithelial small intestine cells; treatment with clotrimazole, an IKCa channel-specific blocker, both inhibited the currents and prevented the RVD response (Wang et al., 2003). Additionally, BKCa currents in human bronchial epithelial cells were augmented in response to hypotonicity (Fernández-Fernández et al., 2002). As K+ flows out of the cell in response to hypotonicity, water follows and the osmotic balance between the intra- and extracellular environments is restored. In addition to maintaining cell volume, K+ channels and volume regulation also play a role in controlling the progression of apoptosis (Remillard and Yuan, 2004; Burg et al., 2006). The role of K+ in volume regulation in relation to apoptosis will be discussed in further detail below.
Overview of apoptosis
Apoptosis, or programmed cell death, is an important part of normal tissue development and function that allows cells to die by a regulated series of events. Morphological changes observed in apoptotic cells include loss of cell volume, termed AVD, nuclear condensation, DNA fragmentation and apoptotic body formation. Apoptosis can be divided into three general stages: (1) the initiation event, when the signal to apoptose is first received by the cell and AVD begins; (2) the effector phase when the mitochondrial membrane potential (ΔΨm) is depolarized, cytochrome c is released and caspases are activated; and (3) the last phase in which DNA is degraded, apoptotic bodies pinch off from the plasma membrane, the nuclear lamina is degraded, the cytoskeleton is broken down and phosphatidyl serine, normally found only on the internal face of the plasma membrane, is exposed to the external environment. The two major apoptotic pathways, the mitochondrial (intrinsic) and death receptor (extrinsic) pathways, culminate in the activation of cytoplasmic effector caspases (caspases 3, 6 and 7) from their inactive procaspase forms (Haunstetter and Izumo, 1998). Once activated, effector caspases cleave chromatin, proteins and the nuclear lamin (Thornberry and Lazebnik, 1998). Although the effector caspases activated by the two pathways are identical, the initiator caspases, which activate effector caspases, are distinct: the extrinsic pathway involves initiator caspase 8 (and 10), while the intrinsic pathway involves caspase 9 (Vu et al., 2001; Gustafsson and Gottlieb, 2007) (Figure 3b).
The extrinsic pathway starts with the activation of transmembrane death receptors by ligands such as Fas, TNF-α or CD95 that ultimately leads to the activation of effector caspases through the recruitment and activation of death domain proteins and initiator caspase 8 (Gulbins et al., 2000; Vu et al., 2001; Remillard and Yuan, 2004; Gustafsson and Gottlieb, 2007). This pathway can also cause the release of cytochrome c from the mitochondria.
The intrinsic pathway involves the release of cytochrome c from the mitochondrial matrix and can be induced by such mediators as staurosporine (ST), UV radiation and actinomycin D (Green and Reed, 1998; Duchen, 1999). Release of cytochrome c is linked to ΔΨm disruption and the formation of the mitochondrial permeability transition (MPT) pore (Kroemer et al., 1998). Formation of the MPT and ΔΨm disruption mark a point of no return after which the cell is committed to apoptose (Kroemer et al., 1998; Duchen, 1999). Opening of the MPT results in cytochrome c release, uncoupling of the respiratory chain, reduction of ΔΨm and swelling of the mitochondrial matrix (Gulbins et al., 2000). Once released into the cytosol, cytochrome c associates with APAF-1 and initiator caspase 9 to form the apoptosome, which activates effector caspases that lead to chromatin degradation and apoptosis. The role of intracellular K+ in inhibiting caspases will be discussed in the next section.
The MPT can also associate with members of the Bcl-2 family of proteins (Gulbins et al., 2000). Proapoptotic Bax and Bak form a pore through which, upon ΔΨm depolarization, cytochrome c can pass from the matrix to the cytosol (Shimizu et al., 2000). Anti-apoptotic proteins such as Bcl-2 and Bcl-xL have their effects by promoting mitochondrial hyperpolarization and preventing the opening of the MPT, which attenuates cytochrome c release (Vander Heiden et al., 1999; Gustafsson and Gottlieb, 2007). Overexpression of Bcl-2 can inhibit mitochondrial membrane depolarization and cytochrome c release in response to tert-butylhydroperoxide challenge, but does not protect against diamide-induced ΔΨm depolarization or cytochrome c release (Zamzami et al., 1998), indicating that different apoptotic inducers function with distinct mechanisms. Bcl-2 can also suppress the activity of proteases activated by apoptotic signals. In epithelial cells, which normally undergo apoptosis in response to disruption of cell adhesions in a process called anoikis, Bcl-2 overexpression was found to suppress activity of an interleukin-converting enzyme-related cysteine protease in response to cell suspension (Frisch et al., 1996). In PASMC, the anti-apoptotic effect of Bcl-2 has been linked to a decrease in IK(V) (Ekhterae et al., 2001). In cells treated with ST, a potent apoptotic inducer, Bcl-2 both prevented the ST-induced increase in IK(V) and significantly inhibited apoptosis; furthermore, the mRNA expression of pore-forming KV channel α subunits (KV1.1, KV1.5 and KV2.1) was found to be attenuated when Bcl-2 was overexpressed (Ekhterae et al., 2001). Overall, the importance of the mitochondria to apoptosis lies mainly in its release of cytochrome c, which activates the final mediators of programmed cell death.
The mitochondrial inner membrane is also host to a variety of K+ channels that control mitochondrial K+ uptake, background conductance, volume regulation, and that play a role in protection against ischaemia (Murata et al., 2001; Lee and Thevenod, 2006). K+ channels, including mitoKCa (Siemen et al., 1999; Xu et al., 2002) and mitoKATP (Inoue et al., 1991), are similar to their plasmalemmal counterparts in that they are sensitive to increased [Ca2+]cyt and ATP, respectively. Activity of mitoKCa channels play a role in protection against myocardial infarction (Xu et al., 2002). Mitochondrial and plasmalemmal KATP channels can be distinguished from one another pharmacologically, as mitochondrial channels are selectively stimulated and inhibited by diazoxide and 5-hydroxydecanoate, respectively (Inoue et al., 1991; Liu et al., 1998; Siemen et al., 1999; Deçbska et al., 2001; Xu et al., 2002).
Apoptotic volume decrease and intracellular K+ in the early stages of apoptosis
K+ fluxes have been implicated in both early and late stages of apoptosis. AVD, one of the earliest morphological changes observed in cells undergoing apoptosis, is a requisite for apoptosis (Bortner and Cidlowski, 1996). AVD is accomplished in nearly the same manner as RVD. As K+ efflux through open K+ channels increases in the early stages of AVD, Cl− ions follow, moving down their electrochemical gradient. Water exits the cell through aquaporins to maintain the osmotic pressure balance between the intracellular and extracellular compartments, thus achieving cell shrinkage. Given the similarity of the molecular mechanisms of AVD and RVD, it is not surprising that in certain instances AVD is coupled to facilitated RVD (Maeno et al., 2000). AVD can occur within minutes or a few hours of apoptotic induction and occurs before caspase activation, DNA fragmentation and, in some instances, disruption of the mitochondrial membrane potential (Bortner and Cidlowski, 1996; Bortner et al., 1997; Yu et al., 1997; Bortner and Cidlowski, 1999; Maeno et al., 2000; Krick et al., 2001a; Platoshyn et al., 2002). In some studies, K+ loss was observed to occur only in cells in which the mitochondrial membrane potential had already been disrupted; however, DNA fragmentation was observed only in cells that had undergone K+ leakage (Dallaporta et al., 1998).
Interestingly, some studies have found that certain apoptotic characteristics (that is, cell shrinkage, K+ efflux and altered ΔΨm) can occur independently of both DNA degradation and caspase activity (Bortner and Cidlowski, 1999; Platoshyn et al., 2002). In PASMC, ST augments IK(V), induces ΔΨm depolarization and causes apoptosis (Krick et al., 2001a; Platoshyn et al., 2002). However, when cells were exposed to a high concentration of external K+, ST-induced ΔΨm still occurred while the ST-induced IK(V) increase and apoptosis were both inhibited (Krick et al., 2001a). Furthermore, cytochrome c, when introduced into PASMC cytoplasm, was found to increase K+ currents before inducing nuclear breakage; the rise in K+ current was independent of caspase 9 activity (Platoshyn et al., 2002).
The requirement of K+ efflux for apoptosis was initially shown in lymphocytes (Bortner et al., 1997; Hughes et al., 1997), but was later demonstrated in neurons (Yu et al., 1997), thymocytes (Dallaporta et al., 1998), liver cells (Nietsch et al., 2000), cardiomyocytes (Ekhterae et al., 2003) and PASMC (Krick et al., 2001a, 2001b). In lymphocytes treated with various apoptotic inducers, including Fas ligand, dexamethasone, ST or anisomysin, only the cells that were shrunken exhibited DNA fragmentation, increased caspase activity and depolarized ΔΨm (Bortner et al., 1997; Bortner and Cidlowski, 1999). Furthermore, apoptosis is inhibited when K+ efflux is prevented, either by raising the extracellular K+ concentration (that is, decreasing the driving force on K+ efflux) or by pharmacologically blocking K+ channels (Bortner et al., 1997; Gómez-Angelats et al., 2000; Krick et al., 2001b). That K+ efflux, rather than mitochondrial membrane depolarization itself, is necessary for apoptosis in PASMC was demonstrated by Krick et al. (2001a), who showed that ST-induced K+ efflux and apoptosis, but not mitochondrial membrane depolarization, were inhibited by high levels of extracellular K+. Additionally, PKC has been shown to be inhibitory towards Fas ligand-induced apoptosis through its effects on K+ loss and cell shrinkage (Gómez-Angelats et al., 2000).
K+ efflux as a requisite for the occurrence of apoptosis may be cell and mediator specific. For example, UV-induced apoptosis of myeloblastic leukaemia cells was found to depend on K+ channel activation, whereas etoposide induced apoptosis regardless of K+ channel suppression (Wang et al., 1999). Additionally, amyloid β (Aβ) was found to increase outward K+ currents in certain neuronal cell types; toxicity in a cholinergic cell line was found to depend on activation of tetraethylammonium (TEA)-sensitive K+ channels, whereas glucose deprivation did not have an effect on K+ currents, and TEA treatment did not protect the cells from hypoglycaemia death (Colom et al., 1998). Furthermore, a dopaminergic cell line was found to be sensitive to Aβ in that the cells died when exposed; however, K+ current density was not affected by Aβ and TEA did not prevent cell toxicity (Colom et al., 1998).
Although increases in K+ channel activity have been widely associated with apoptotic induction, the converse has also been observed. In Jurkat T lymphocytes, treatment with dexamethasone decreases IK(V) (Lampert et al., 2003). These observations do not rule out an early increase in IK(V), however, as cells were pretreated for 2–3 h before observation. Treatment of Jurkat T lymphocytes with ceramide, a metabolite synthesized after Fas receptor activation, decreases IK(V) and phosphorylates and inactivates KV1.3 channels (Szabò et al., 1996; Gulbins et al., 1997). Apoptosis is a dynamic process, with the final biochemical changes, such as DNA degradation, marking the common manifestations of an apoptotic cell. Given the above data and the complexity of K+ channels, it is likely that the exact mechanisms leading up to the final common stages of apoptosis are both highly cell and inducing agent specific. Another example that illustrates this point is the effect of overexpression of KV channels. Interestingly, overexpression of the KV1.5 channel gene (KCNA5) in PASMC enhances apoptosis (Brevnova et al., 2004), whereas transfection of KV10.1 (eag) into CHO cells induces a transformed phenotype (Pardo et al., 1999).
The identity of the K+ currents and channels involved in AVD has been mostly inferred from pharmacological blockade of specific K+ channels: 4-AP and TEA-sensitive KV channels (Yu et al., 1997; Krick et al., 2001a, 2002; Bock et al., 2002; Ekhterae et al., 2003), quinine- and Ba2+-sensitive K+ channels (Maeno et al., 2000; Nietsch et al., 2000) and KCa channels (Nietsch et al., 2000; Krick et al., 2001b, 2002; Ekhterae et al., 2003). FCCP, a mitochondrial protonophore, was shown to cause apoptosis by increasing KCa currents (IK(Ca)) through BKCa channels in rat and human PASMC; blockage of these channels with iberiotoxin or TEA inhibited FCCP-induced apoptosis (Krick et al., 2001b). Cells lacking KV1.3 are resistant to actinomycin D-induced apoptosis, failing to demonstrate DNA fragmentation, release of cytochrome c and depolarization of ΔΨm; retransfection of the missing channel restores the apoptotic response to actinomycin D (Bock et al., 2002). In PASMC, KCa channel opening enhances nitric oxide-mediated apoptosis (Krick et al., 2002), and overexpressing KV1.5 enhances both basal and ST-induced apoptoses (Brevnova et al., 2004), whereas K+ channel blocked with 4-AP, TEA or iberiotoxin inhibits ST- and NO-induced apoptoses (Krick et al., 2002; Brevnova et al., 2004). These studies, taken together, indicate an essential role for increased K+ flux, most notably through KV and KCa channels, in apoptosis.
K+ in suppression of caspases during mid-to-late phase apoptosis
Intracellular K+ is inhibitory towards apoptosis in that physiological levels of K+ inhibit the activation of caspases. Just as blockade of K+ efflux diminishes AVD, it has also been shown to prevent the activation of cytosolic caspases (Krick et al., 2001a). Using dexamethasone-induced thymocyte autodigestion as a model for apoptosis, Hughes et al. (1997) demonstrated that only cells with decreased cytosolic K+ concentration ([K+]cyt) exhibited intracellular caspase and nuclease activity. Maintaining high [K+]cyt suppressed caspase and nuclease activity independently of the mode of apoptotic induction (Hughes et al., 1997). Thompson et al. (2001), working with a cell free system, showed that in activated lysates, APAF-1 oligomerized to both an active caspase processing complex and a biologically inactive complex; in the presence of increasing [K+]cyt, the active complex did not form and caspase activation was inhibited. These findings were extended to show that normal intracellular levels of K+ are enough to suppress active complex formation and that the effects of K+ on apoptosome assembly are antagonized in a concentration-dependent manner by cytochrome c (Cain et al., 2001). The authors postulated that physiological [K+]cyt levels safeguard the cell against inadvertent cytochrome c release, which would lead to irreversible apoptosome formation (Cain et al., 2001). These data suggest that, in addition to contributing to AVD, K+ efflux creates a permissible environment for caspase and nuclease activity by relieving inhibition on these apoptotic mediators. Therefore, closure or block of K+ channels, and consequent reduced K+ efflux, would attenuate apoptosis by both preventing AVD and by maintaining caspase and nuclease inhibition (see Figure 3a, lower panel).
That ion concentrations and fluxes play a role in mediating apoptosis is widely established. However, the role of ions in apoptosis is highly cell specific. This is clearly illustrated with the effects of ouabain, a Na+/K+ ATPase inhibitor, on various cell types. Overall, ouabain has the effect of increasing intracellular Na+ levels and decreasing [K+]cyt. Treatment of rat aortic vascular smooth muscle cells with ouabain attenuates apoptosis induced by serum withdrawal, ST and okadaic acid due to the resulting high intracellular [Na+]/[K+] ratio (Orlov et al., 1999). This effect was found to depend on an early induction of RNA synthesis (Orlov et al., 2000). However, porcine aortic endothelial cells respond to ouabain by undergoing necrotic cell death (Orlov et al., 2004). In cultured cortical neurons, ouabain was found to induce cell death that had features reminiscent of both necrosis and apoptosis, including caspase 3 activation, cytochrome c release and DNA laddering. These effects were found to be mediated by intracellular depletion of K+ and accumulation of Ca2+ and Na+ (Xiao et al., 2002). Ouabain, similarly, induced cell death in canine epithelial kidney cells, but this was found to be independent of the inversion of the intracellular [Na+]/[K+] ratio, as was the case with the ouabain-induced necrosis of porcine aortic endothelial cells (Pchejetski et al., 2003; Orlov et al., 2004).
In addition to their role in apoptosis, caspases also contribute to inflammatory signalling. Early studies showed that IL-1β processing and export in macrophages challenged with LPS depends on K+ efflux (Perregaux and Gabel, 1994; Walev et al., 1995). Bacterial toxin-induced membrane permeabilization of CHO cells leads to decreased [K+]cyt, which promotes both formation of the inflammasome, an innate immune signalling complex, and activation of caspase 1 (Gurcel et al., 2006). Caspase 1 is known to play a role in processing the precursor form of interleukin-1β, a component of inflammation signalling. Interestingly, when activated in the context of bacterial toxin membrane permeabilization and reduced [K+]cyt, caspase 1 promotes membrane repair through the activation of the sterol regulatory element-binding proteins (Gurcel et al., 2006). K+ efflux was found to be both necessary and sufficient to activate sterol regulatory element-binding protein (Gurcel et al., 2006).
Overall, K+ fluxes contribute to apoptosis in distinct chronological phases. In the early stages of apoptosis where AVD is most prominent, increased K+ efflux in response to an apoptotic mediator causes cell shrinkage. Later after K+ efflux has been initiated during AVD and the osmotic balance is disrupted, a low K+ level in the cytoplasm creates a permissible environment for caspase activation, thus leading to chromatin and protein cleavage.
K+ channels in proliferation
The evidence presented until this point is quite strong regarding the role of K+ channels in vascular smooth muscle cell apoptosis. Conversely, there is also mounting evidence that K+ channel activation may also play a significant role in promoting proliferation (Neylon, 2002). Traditionally, K+ channel activation would result in membrane hyperpolarization and decreased Ca2+ influx via voltage-gated Ca2+ channels. However, it appears that, in some cases, K+ channel-mediated hyperpolarization can regulate the spatial and temporal organization of Ca2+ signalling. Since only certain types of Ca2+ signals can lead to the activation of growth-promoting genes (Dolmetsch et al., 1997; Hardingham et al., 1997), the ability of K+ channels to modulate the amplitude and duration of Ca2+ signalling can therefore influence their capacity to alter cell function.
The most common form of intracellular Ca2+ signalling involves Ca2+ release from the sarcoplasmic reticulum. Global Ca2+ transients induced in contractile smooth muscle cells (for example, caffeine causing release from intracellular ryanodine-sensitive Ca2+ stores in the form of Ca2+ sparks) lead to activation of BKCa channels and closure of VDCC. In contrast, sustained [Ca2+]cyt (for example, due to IP3 receptor stimulation) activate IKCa channels, which are not affected by changes in Em. The subsequent hyperpolarization increases the driving force for Ca2+ influx across its chemical gradient via non-selective cation channels such as receptor-operated Ca2+ channels (Golovina, 1999). The resultant sustained or oscillatory increases in [Ca2+]cyt can selectively activate smooth muscle growth mechanisms such as growth factor gene expression, activation of kinases and other processes involved in cell division, and phosphorylation (Dolmetsch et al., 1997; Hardingham et al., 1997; Levitan, 1999).
One of the observations upon which the hypothesis that increased K+ current is needed for proliferation depends is that blocking K+ channels inhibits proliferation. It has been suggested that K+ channel activity is needed specifically for the G1/S transition (Wonderlin and Strobl, 1996; Pardo, 2004). In addition, 4-AP suppressed human myeloblastic ML-1 cell proliferation by arresting cells in G1; however, once cells passed the G1/S boundary, channel blockade had no effect on cell-cycle progression (Xu et al., 1996). Further, blockade of K+ channels prevents the activation of the ERK-2 mitogenic signalling cascade initiated by epidermal growth factor in ML-1 cells (Xu et al., 1999).
Generalizations arising from these studies need to be made with caution for many reasons. First, tumour cells are not normal with respect to their proliferative phenotype. The role of K+ channels in tumour cell proliferation and apoptosis is beyond the scope of this review; however, the interested reader is referred to a recent review by Wang (2004). Second, in many studies involving lymphocytes and various cancer cell lines, the concentrations of K+ channel antagonist needed to inhibit proliferation are higher than the concentrations needed to inhibit the K+ currents (Wonderlin and Strobl, 1996). Third, studies showing that K+ channel blockers inhibit proliferation do not consistently demonstrate that the blockers induce depolarization or have an effect on intracellular Ca2+ (Pardo, 2004). For example, in neuroblastoma cells, blockers inhibited proliferation but did not induce membrane depolarization (Dubois and Rouzaire-Dubois, 1993). Furthermore, studies have shown that charybdotoxin, a BK antagonist, blocks progression of T lymphocytes through the G1 phase; however, this could be due to the fact that charybdotoxin blocked the induction of IL-2 expression, which is needed for G1 progression, rather than a direct requirement for K+ efflux (Wonderlin and Strobl, 1996). These observations suggest that channel blockers that inhibit mitogenesis may have their effects through nonspecific mechanisms.
Intracellular K+ is protective against apoptosis (Hughes et al., 1997; Dallaporta et al., 1998), an effect that has been discussed in the previous section. It must be noted that an increased K+ current would, therefore, set up two opposing forces: one towards an increased driving force for Ca2+ entry and subsequent proliferation; and one towards volume decrease and a release of inhibition on caspases and nucleases, which would favour apoptosis. At any rate, Ca2+ signalling, including spikes, sparks, oscillations and sustained waves, is known to be complex, with both spatial and temporal attributes of the signal contributing to its effect (Berridge, 1995; Carl et al., 1996; Jaggar et al., 2000); therefore, the mechanisms linking K+ channels, Ca2+ influx and proliferation are likely to be highly cell and signal specific.
K+ channels, apoptosis and pulmonary arterial hypertension
The pulmonary vasculature is a high-flow, low-resistance system that depends on arterial distensibility and recruitment to adapt to changing loads of increased cardiac output. Vascular SMCs have low rates of proliferation in the vascular wall, but pathological situations cause vascular SMCs to lose their differentiated state and proliferate; overall, this contributes to vascular remodelling observed in diseases such as PAH (Rubin, 1997; Mandegar et al., 2004; Cribbs, 2006). Medial hypertrophy and muscularization of the pulmonary arterial wall are among the main pathological findings in patients with PAH.
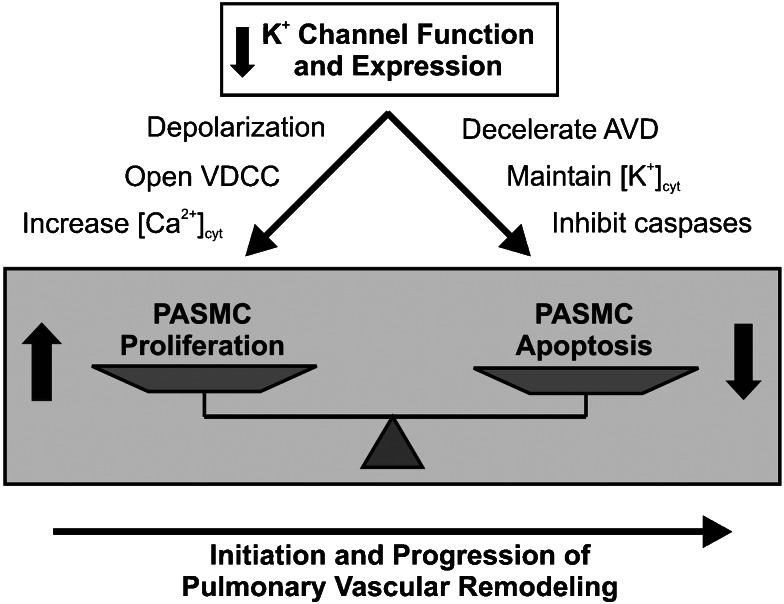
Proliferating PASMCs have higher resting [Ca2+]cyt than growth-arrested PASMCs, and an excessive PASMC cell population is one of the hallmarks of PAH. Consistent with these observations, it has been noted that PASMCs from idiopathic PAH (IPAH) patients have a higher resting [Ca2+]cyt and a more depolarized Em than cells from normal subjects, patients without pulmonary hypertension (NPH) and patients with secondary pulmonary hypertension (SPH) (Yuan et al., 1998a, 1998b). Consistent with the role of KV channels in regulating Em, PASMCs from IPAH patients also have decreased mRNA levels of KV1.5 and KV1.2, decreased IK(V), and more rapid inactivation of IK(V) compared to those cells from patients without pulmonary hypertension and with secondary pulmonary hypertension (Yuan et al., 1998a, 1998b). Taken together, these data suggest that K+ channel dysfunction could lead to increased [Ca2+]cyt and enhanced contraction and proliferation (Figure 4). Both ST- and bone morphogenetic protein (BMP)-induced apoptosis are diminished in PASMCs from IPAH patients compared to cells from patients with secondary pulmonary hypertension (Zhang et al., 2003), further suggesting a high level of [K+]cyt that is protective against cell death. Lastly, sustained membrane depolarization, as may be seen with dysfunctional or inhibited KV channels, leads to increases in [Ca2+]cyt (Fleischmann et al., 1994), which can cause sustained PASMC contraction and promote PASMC proliferation. Taken together, these data suggest an inherent dysfunction in PASMC KV channels from IPAH patients. As has already been discussed, dysfunctional KV channels that decrease IK(V) would cause both membrane depolarization and an increase [Ca2+]cyt, and inhibit apoptotic cell shrinkage and cytoplasmic caspase activity. These altered ion equilibria can result in protection from apoptosis, persistent cell contraction and cellular proliferation, all of which would contribute to sustained pulmonary vasoconstriction and excessive pulmonary medial hypertrophy observed in IPAH.
Figure 4.
Role of K+ channels in pulmonary vascular remodelling. Decreased K+ channel function and expression not only stimulates pulmonary artery smooth muscle cell (PASMC) proliferation by increasing [Ca2+]cyt, but also inhibits PASMC apoptosis by decelerating apoptotic volume decrease (AVD) and attenuating cytoplasmic caspase activity. The increased proliferation and inhibited apoptosis in PASMC may play an important role in initiation and/or progression of pulmonary vascular remodelling. VDCC, voltage-dependent Ca2+ channels.
Mutations in the BMP receptor type II (BMP-RII) gene have been linked to familial and sporadic PAH (Deng et al., 2000; Thomson et al., 2000; Janssen et al., 2002; Rindermann et al., 2003). A member of the TGF-β family of signalling molecules, BMP-RII is needed for recognition of all BMP ligands. Binding of BMP is followed by oligomerization of BMP-RII and RI. BMP receptor activation and the downstream signalling cascade it initiates via Smads and p38 can activate the transcription of genes needed to arrest cell growth and induce apoptosis (Itoh et al., 2000; Nohe et al., 2004). Although it is tempting to conclude that mutations in the receptor would lead to decreased rates of apoptosis in response to normal physiological signalling and thus contribute to medial hypertrophy observed in PAH, the exact mechanism linking BMP-RII mutations and PAH is unknown. PASMCs expressing mutations of BMP-RII that are found in IPAH patients are resistant to BMP-induced apoptosis (Lagna et al., 2006). Furthermore, a link between BMP signalling and K+ channel function has been established in PASMC. Treatment of normal PASMCs with BMP-2 or BMP-7 induces apoptosis by activation of caspases 3, 8 and 9, downregulation of anti-apoptotic Bcl-2 protein and cytochrome c release, whereas PASMCs from IPAH patients are resistant to BMP-induced apoptosis (Zhang et al., 2003; Lagna et al., 2006). Furthermore, IK(V) and protein levels of KV1.5 were increased in normal PASMCs exposed to BMP-2 (Fantozzi et al., 2006; Young et al., 2006), providing a plausible link between dysfunctional BMP signalling, K+ channel function and PASMC apoptosis.
Animal models of PAH have also been fundamental in elucidating the K+/BMP/apoptosis axis in PAH. Upon expression of an inducible smooth muscle cell-targeted dominant-negative version of BMP-RII found in a PAH family, mice develop increased pulmonary arterial pressures, increased right to left ventricle+septum weight ratios and pulmonary artery muscularization without an effect on their systemic pressures (West et al., 2004), supporting a role for dysfunctional BMP signalling specifically in PAH pathogenesis. Furthermore, whole-lung tissue from these mice demonstrated decreased expression of KV1.1, KV1.5 and KV4.3 mRNA as well as KV1.5 protein (Young et al., 2006), echoing the reduced KV transcripts found in human PASMCs from IPAH patients. Treatment of these PAH mice with nifedipine, a specific L-type Ca2+ channel blocker, reduced right systolic pressures, indicating a role for Ca2+ in disease onset (Young et al., 2006). Taken together, these studies suggest that a functional loss of BMP signalling in PASMCs can lead to vasoconstriction and remodelling through reduced IK(V), thereby contributing to the pathogenesis of PAH.
Exposure to chronic hypoxia (CH) has also been used as an animal model for PAH. Hypoxia is known to cause decreased IK(V) and KV gene expression in PASMC (Yuan et al., 1993; Wang et al., 1997). In chronically hypoxic rats, dichloroacetate (DCA), a metabolic modulator that increases mitochondrial oxidative phosphorylation, was able to either reverse or prevent (depending on how soon after CH exposure it was given) haemodynamic abnormalities induced by CH, including the increase in pulmonary vascular resistance, right ventricular hypertrophy and PA remodelling (Michelakis et al., 2002). On a molecular level, DCA increased K+ currents in PASMC and restored CH-induced KV2.1 channel expression (Michelakis et al., 2002). DCA was also found to be effective in reversing monocrotaline (MCT)-induced PAH in rats, leading to similar recovery from haemodynamic symptoms as the CH rats (McMurtry et al., 2004). In the MCT study, it was found that DCA depolarized the mitochondria of PASMC, causing release of cytochrome c, and induced a significant increase in PASMC apoptosis and/or decrease in PASMC proliferation in the pulmonary arterial medial layer (McMurtry et al., 2004). Additionally, DCA rescued the MCT-induced decrease in IK(V), and protein and mRNA expression of KV1.5 (McMurtry et al., 2004). Survivin, an inhibitor of apoptosis protein (Blanc-Brude et al., 2002), was found to be expressed in the pulmonary arteries of patients and rats with MCT-induced PAH, but not in patients or rats without MCT-induced PAH (McMurtry et al., 2005). In the rat model of PAH, administration of a dominant-negative form of survivin reversed PAH and improved survival, leading to many of the same haemodynamic improvements noted above, including decreased pulmonary vascular resistance, right ventricular hypertrophy and PA medial hypertrophy (McMurtry et al., 2005). Survivin treatment also led to increased IK(V), depolarized mitochondria which caused cytochrome c release and PASMC apoptosis (McMurtry et al., 2005). Given the above data, possible future therapeutic avenues for PAH may target KV channels, leading to an increase in channel activity. Additionally, treatment with DCA or survivin inhibition should be explored further given their successes in reversing PAH and the haemodynamic sequelae in experimental rat models of PAH.
Development of future treatments that target K+ channels will benefit from a careful examination of specific K+ channel subtypes. In fully differentiated aortic smooth muscle cells, BKCa currents predominate, whereas in cells of the proliferative phenotype, IKCa currents are the dominant type (Neylon et al., 1999; Köhler et al., 2003; Jackson, 2005). It has been suggested that in vascular injury, a switch towards IKCa (from BKCa) may contribute to excessive intimal vascular smooth muscle cell proliferation (Köhler et al., 2003). Supporting this hypothesis, it was found that specific IKCa channel blockers significantly reduced intimal hyperplasia in a rat model of balloon catheter injury (Köhler et al., 2003). Although an exhaustive characterization of IKCa channels in PASMC from PAH patients has not been performed, it would be interesting to determine whether a similar phenotype is present in the vasculature of IPAH patients, thereby opening the possibility of new avenues of treatment.
PAH treatment: focus on K+ channels and clinically approved PAH drug therapies and a look to the future
In the past decade, the number of clinically used drugs against PAH has increased dramatically. Most of these have been focused at the population of patients diagnosed with a severe form of PAH known as IPAH. Because of its complex aetiology, IPAH patients are now routinely treated with a combination of active drugs, such as prostanoid, phosphodiesterase, vascular endothelial growth factor receptor antagonists, statins and ET-1 receptor antagonists (Ghofrani et al., 2003; Hoeper et al., 2003, 2006; Martin et al., 2006; Souza et al., 2006; Puri et al., 2007). Targeting K+ channels may have potential in the treatment of PAH, especially considering the fact that K+ channel dysfunction is closely linked to many of the known contributing factors to the development of PAH: (a) enhanced K+ channel activity limits membrane depolarization and Ca2+ influx via VDCC to cause pulmonary vasodilation (Yuan, 1995); (b) the phosphodiesterase 5 inhibitor sildenafil enhances cGMP-mediated activation of KV and KCa channels in PASMC, causing vasodilation and improving pulmonary haemodynamics (Michelakis et al., 2003); (c) BMP-2, a BMPR-II ligand, promotes PASMC proliferation by decreasing expression of anti-apoptotic Bcl-2 (Zhang et al., 2003) and by activating IK(V) in normal PASMC (Fantozzi et al., 2006); (d) pulmonary artery vasoconstriction by ET-1 is, in part, mediated by inhibiting KV channel function (Shimoda et al., 2001); (e) iloprost (a stable analogue of prostacyclin) and prostacyclin activate multiple K+ channels in vascular smooth muscle cells (Murphy and Brayden, 1995); (f) anorexigens inhibit KV channel expression and function in PASMC (Wang et al., 1998; Perchenet et al., 2001); (g) anti-survivin therapy reverses the remodelling and haemodynamic effects of PAH, possibly due to the intermediate activation of KV channels; and (h) in vitro gene transfection of KCNA5 in PASMC causes membrane hyperpolarization and enhanced apoptosis (Brevnova et al., 2004), and (i) in vivo gene transfer of KCNA5 in lung tissues reverses both vasoconstriction and pulmonary remodelling in chronically hypoxic rats (Pozeg et al., 2003). The latter represents the first successful example of K+ channel gene therapy for a vascular disease. These studies clearly illustrate that targeting K+ channels' subunit expression and/or function may be an important approach in treatment of PAH. The future holds much promise as far as the development of more effective anti-pulmonary hypertension drugs is concerned. Modulation of K+ channel expression and function appears to be a common thread linking many current PAH therapies. Whether K+ channels will become a focus in the future remains to be seen.
Conclusion
In summary, expression and function of membrane K+ channels play important roles in regulating PASMC proliferation and apoptosis. In apoptosis, they mediate the K+ efflux that is necessary for AVD. Additionally, K+ efflux leading to decreased [K+]cyt releases the inhibition of caspases, the final apoptotic mediators, which cleave proteins and DNA, leading to cell death. Furthermore, in PASMCs, K+ channels are involved in proliferation, mostly through their control of the resting membrane potential. When K+ channels close, the membrane depolarizes, leading to an influx of Ca2+, an obligatory messenger for cell-cycle progression and proliferation. Dysfunctional K+ channels have been implicated in the development of sustained pulmonary vasoconstriction and vascular medial hypertrophy associated with PAH. The three main classes of drugs used in the treatment of PAH are the prostacyclin analogues, endothelin receptor antagonists and phosphodiesterase 5 inhibitors; however, given the wide variation in individuals' responses to these medications, there is not a standardized treatment regimen for PAH. While the success of these drugs had greatly reduced the number of PAH patients receiving lung transplants in the last two decades, some patients fail to respond well even to different combinations of treatments. Therefore, K+ channels as a therapeutic target for PAH remain an exciting possibility for future drug development.
Glossary
- 4-AP
4-aminopyridine
- BMP
bone morphogenetic protein
- DCA
dichloroacetate
- MCT
monocrotaline
- PAH
pulmonary arterial hypertension
- PASMC
pulmonary artery smooth muscle cell
- ST
staurosporine
- VDCC
voltage-dependent Ca2+ channels
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- al-Habori M. Cell volume and ion transport regulation. Int J Biochem. 1994;26:319–334. doi: 10.1016/0020-711x(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Amberg GC, Koh SD, Imaizumi Y, Ohya S, Sanders KM. A-type potassium currents in smooth muscle. Am J Physiol Cell Physiol. 2003;284:C583–C595. doi: 10.1152/ajpcell.00301.2002. [DOI] [PubMed] [Google Scholar]
- Archer SL, Huang JM, Reeve HL, Hampl V, Tolarová S, Michelakis E, et al. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res. 1996;78:431–442. doi: 10.1161/01.res.78.3.431. [DOI] [PubMed] [Google Scholar]
- Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, et al. Molecular identification of the role of voltage-gated K+ channels, KV1.5 and KV2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H, Hardingham GE, Johnson CM, Chawla S. Gene regulation by nuclear and cytoplasmic calcium signals. Biochem Biophys Res Commun. 1997;236:541–543. doi: 10.1006/bbrc.1997.7037. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium signalling and cell proliferation. BioEssays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- Blanc-Brude OP, Yu J, Simosa H, Conte MS, Sessa WC, Altieri DC. Inhibitor of apoptosis protein survivin regulates vascular injury. Nat Med. 2002;8:987–994. doi: 10.1038/nm750. [DOI] [PubMed] [Google Scholar]
- Bock J, Szabó I, Jekle A, Gulbins E. Actinomycin D-induced apoptosis involves the potassium channel KV1.3. Biochem Biophys Res Commun. 2002;295:526–531. doi: 10.1016/s0006-291x(02)00695-2. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. Absence of volume regulatory mechanisms contributes to the rapid activation of apoptosis in thymocytes. Am J Physiol. 1996;271:C950–C961. doi: 10.1152/ajpcell.1996.271.3.C950. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. Caspase independent/dependent regulation of K+, cell shrinkage, and mitochondrial membrane potential during lymphocyte apoptosis. J Biol Chem. 1999;274:21953–21962. doi: 10.1074/jbc.274.31.21953. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Hughes FM, Jr, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem. 1997;272:32436–32442. doi: 10.1074/jbc.272.51.32436. [DOI] [PubMed] [Google Scholar]
- Brevnova EE, Platoshyn O, Zhang S, Yuan JX-J. Overexpression of human KCNA5 increases IK(V) and enhances apoptosis. Am J Physiol Cell Physiol. 2004;287:C715–C722. doi: 10.1152/ajpcell.00050.2004. [DOI] [PubMed] [Google Scholar]
- Burg ED, Remillard CV, Yuan JX-J. K+ channels in apoptosis. J Membr Biol. 2006;209:3–20. doi: 10.1007/s00232-005-0838-4. [DOI] [PubMed] [Google Scholar]
- Cain K, Langlais C, Sun XM, Brown DG, Cohen GM. Physiological concentrations of K+ inhibit cytochrome c-dependent formation of the apoptosome. J Biol Chem. 2001;276:41985–41990. doi: 10.1074/jbc.M107419200. [DOI] [PubMed] [Google Scholar]
- Carl A, Lee HK, Sanders KM. Regulation of ion channels in smooth muscles by calcium. Am J Physiol. 1996;271:C9–C34. doi: 10.1152/ajpcell.1996.271.1.C9. [DOI] [PubMed] [Google Scholar]
- Chamberlin ME, Strange K. Anisosmotic cell volume regulation: a comparative view. Am J Physiol. 1989;257:C159–C173. doi: 10.1152/ajpcell.1989.257.2.C159. [DOI] [PubMed] [Google Scholar]
- Chandy KG, DeCoursey TE, Cahalan MD, McLaughlin C, Gupta S. Voltage-gated potassium channels are required for human T lymphocyte activation. J Exp Med. 1984;160:369–385. doi: 10.1084/jem.160.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, et al. Molecular diversity of K+ channels. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Colom LV, Diaz ME, Beers DR, Neely A, Xie WJ, Appel SH. Role of potassium channels in amyloid-induced cell death. J Neurochem. 1998;70:1925–1934. doi: 10.1046/j.1471-4159.1998.70051925.x. [DOI] [PubMed] [Google Scholar]
- Coppock EA, Martens JR, Tamkun MM. Molecular basis of hypoxia-induced pulmonary vasoconstriction: role of voltage-gated K+ channels. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1–L12. doi: 10.1152/ajplung.2001.281.1.L1. [DOI] [PubMed] [Google Scholar]
- Cribbs LL. T-type Ca2+ channels in vascular smooth muscle: multiple functions. Cell Calcium. 2006;40:221–230. doi: 10.1016/j.ceca.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Dallaporta B, Hirsch T, Susin SA, Zamzami N, Larochette N, Brenner C, et al. Potassium leakage during the apoptotic degradation phase. J Immunol. 1998;160:5605–5615. [PubMed] [Google Scholar]
- Deçbska G, May R, Kicin'ska A, Szewczyk A, Elger CE, Kunz WS. Potassium channel openers depolarize hippocampal mitochondria. Brain Res. 2001;892:42–50. doi: 10.1016/s0006-8993(00)03187-5. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis. Nature. 1984;307:465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Dubois JM, Rouzaire-Dubois B. Role of potassium channels in mitogenesis. Prog Biophys Mol Biol. 1993;59:1–21. doi: 10.1016/0079-6107(93)90005-5. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekhterae D, Platoshyn O, Krick S, Yu Y, McDaniel SS, Yuan JX-J. Bcl-2 decreases voltage-gated K+ channel activity and enhances survival in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C157–C165. doi: 10.1152/ajpcell.2001.281.1.C157. [DOI] [PubMed] [Google Scholar]
- Ekhterae D, Platoshyn O, Zhang S, Remillard CV, Yuan JX-J. Apoptosis repressor with caspase domain inhibits cardiomyocyte apoptosis by reducing K+ currents. Am J Physiol Cell Physiol. 2003;284:C1405–C1410. doi: 10.1152/ajpcell.00279.2002. [DOI] [PubMed] [Google Scholar]
- Fantozzi I, Platoshyn O, Wong AH, Zhang S, Remillard CV, Furtado MR, et al. Bone morphogenetic protein-2 upregulates expression and function of voltage-gated K+ channels in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L993–L1004. doi: 10.1152/ajplung.00191.2005. [DOI] [PubMed] [Google Scholar]
- Felipe A, Snyders DJ, Deal KK, Tamkun MM. Influence of cloned voltage-gated K+ channel expression on alanine transport, Rb+ uptake, and cell volume. Am J Physiol. 1993;265:C1230–C1238. doi: 10.1152/ajpcell.1993.265.5.C1230. [DOI] [PubMed] [Google Scholar]
- Fernández-Fernández JM, Nobles M, Currid A, Vazquez E, Valverde MA. Maxi K+ channel mediates regulatory volume decrease response in a human bronchial epithelial cell line. Am J Physiol Cell Physiol. 2002;283:C1705–C1714. doi: 10.1152/ajpcell.00245.2002. [DOI] [PubMed] [Google Scholar]
- Fleischmann BK, Murray RK, Kotlikoff MI. Voltage window for sustained elevation of cytosolic calcium in smooth muscle cells. Proc Natl Acad Sci USA. 1994;91:11914–11918. doi: 10.1073/pnas.91.25.11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Kelaita D, Sicks S. A role for Jun-N-terminal kinase in anoikis; suppression by bcl-2 and crmA. J Cell Biol. 1996;135:1377–1382. doi: 10.1083/jcb.135.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghofrani HA, Rose F, Schermuly RT, Olschewski H, Wiedemann R, Kreckel A, et al. Oral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertension. J Am Coll Cardiol. 2003;42:158–164. doi: 10.1016/s0735-1097(03)00555-2. [DOI] [PubMed] [Google Scholar]
- Golovina VA. Cell proliferation is associated with enhanced capacitative Ca2+ entry in human arterial myocytes. Am J Physiol. 1999;277:C343–C349. doi: 10.1152/ajpcell.1999.277.2.C343. [DOI] [PubMed] [Google Scholar]
- Gómez-Angelats M, Bortner CD, Cidlowski JA. Protein kinase C (PKC) inhibits fas receptor-induced apoptosis through modulation of the loss of K+ and cell shrinkage. A role for PKC upstream of caspases. J Biol Chem. 2000;275:19609–19619. doi: 10.1074/jbc.M909563199. [DOI] [PubMed] [Google Scholar]
- Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Jekle A, Ferlinz K, Grassme H, Lang F. Physiology of apoptosis. Am J Physiol Renal Physiol. 2000;279:F605–F615. doi: 10.1152/ajprenal.2000.279.4.F605. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Szabo I, Baltzer K, Lang F. Ceramide-induced inhibition of T lymphocyte voltage-gated potassium channel is mediated by tyrosine kinases. Proc Natl Acad Sci USA. 1997;94:7661–7666. doi: 10.1073/pnas.94.14.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Gurney AM, Osipenko ON, MacMillan D, McFarlane KM, Tate RJ, Kempsill FE. Two-pore domain K channel, TASK-1, in pulmonary artery smooth muscle cells. Circ Res. 2003;93:957–964. doi: 10.1161/01.RES.0000099883.68414.61. [DOI] [PubMed] [Google Scholar]
- Gustafsson AB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292:C45–C51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Calcium as a versatile second messenger in the control of gene expression. Microsc Res Tech. 1999;46:348–355. doi: 10.1002/(SICI)1097-0029(19990915)46:6<348::AID-JEMT3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Cruzalegui FH, Chawla S, Bading H. Mechanisms controlling gene expression by nuclear calcium signals. Cell Calcium. 1998;23:131–134. doi: 10.1016/s0143-4160(98)90111-7. [DOI] [PubMed] [Google Scholar]
- Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998;82:1111–1129. doi: 10.1161/01.res.82.11.1111. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sinauer Associates: Sunderland, MA; 2001. [Google Scholar]
- Hoeper MM, Taha N, Bekjarova A, Gatzke R, Spiekerkoetter E. Bosentan treatment in patients with primary pulmonary hypertension receiving nonparenteral prostanoids. Eur Respir J. 2003;22:330–334. doi: 10.1183/09031936.03.00008003. [DOI] [PubMed] [Google Scholar]
- Hu H, Sung A, Zhao G, Shi L, Qiu D, Nishimura T, et al. Simvastatin enhances bone morphogenetic protein receptor type II expression. Biochem Biophys Res Commun. 2006;339:59–64. doi: 10.1016/j.bbrc.2005.10.187. [DOI] [PubMed] [Google Scholar]
- Hughes M, Jr, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem. 1997;272:30567–30576. doi: 10.1074/jbc.272.48.30567. [DOI] [PubMed] [Google Scholar]
- Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- Itoh S, Itoh F, Goumans MJ, Ten Dijke P. Signaling of transforming growth factor-β family members through Smad proteins. Eur J Biochem. 2000;267:6954–6967. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Gerzanich V, Ivanova S, Denhaese R, Tsymbalyuk O, Simard JM. Adenylate cyclase 5 and KCa1.1 channel are required for EGFR up-regulation of PCNA in native contractile rat basilar artery smooth muscle. J Physiol. 2006;570:73–84. doi: 10.1113/jphysiol.2005.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WF. Potassium channels and proliferation of vascular smooth muscle cells. Circ Res. 2005;97:1211–1212. doi: 10.1161/01.RES.0000196742.65848.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- Janssen B, Rindermann M, Barth U, Miltenberger-Miltenyi G, Mereles D, Abushi A, et al. Linkage analysis in a large family with primary pulmonary hypertension: genetic heterogeneity and a second primary pulmonary hypertension locus on 2q31–32. Chest. 2002;121:54S–56S. doi: 10.1378/chest.121.3_suppl.54s. [DOI] [PubMed] [Google Scholar]
- Köhler R, Wulff H, Eichler I, Kneifel M, Neumann D, Knorr A, et al. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation. 2003;108:1119–1125. doi: 10.1161/01.CIR.0000086464.04719.DD. [DOI] [PubMed] [Google Scholar]
- Krick S, Platoshyn O, McDaniel SS, Rubin LJ, Yuan JX-J. Augmented K+ currents and mitochondrial membrane depolarization in pulmonary artery myocyte apoptosis. Am J Physiol Lung Cell Mol Physiol. 2001a;281:L887–L894. doi: 10.1152/ajplung.2001.281.4.L887. [DOI] [PubMed] [Google Scholar]
- Krick S, Platoshyn O, Sweeney M, Kim H, Yuan JX-J. Activation of K+ channels induces apoptosis in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2001b;280:C970–C979. doi: 10.1152/ajpcell.2001.280.4.C970. [DOI] [PubMed] [Google Scholar]
- Krick S, Platoshyn O, Sweeney M, McDaniel SS, Zhang S, Rubin LJ, et al. Nitric oxide induces apoptosis by activating K+ channels in pulmonary vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2002;282:H184–H193. doi: 10.1152/ajpheart.2002.282.1.H184. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- Lagna G, Nguyen PH, Ni W, Hata A. BMP-dependent activation of caspase-9 and caspase-8 mediates apoptosis in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1059–L1067. doi: 10.1152/ajplung.00180.2006. [DOI] [PubMed] [Google Scholar]
- Lampert A, Muller MM, Berchtold S, Lang KS, Palmada M, Dobrovinskaya O, et al. Effect of dexamethasone on voltage-gated K+ channels in Jurkat T-lymphocytes. Pflügers Arch. 2003;447:168–174. doi: 10.1007/s00424-003-1148-2. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, et al. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lee WK, Thevenod F. A role for mitochondrial aquaporins in cellular life-and-death decisions. Am J Physiol Cell Physiol. 2006;291:C195–C202. doi: 10.1152/ajpcell.00641.2005. [DOI] [PubMed] [Google Scholar]
- Levitan IB. Modulation of ion channels by protein phosphorylation. How the brain works. Adv Second Messenger Phosphoprotein Res. 1999;33:3–22. doi: 10.1016/s1040-7952(99)80003-2. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sato T, O'Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection. Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci USA. 2000;97:9487–9492. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX-J. Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res. 2004;68:75–103. doi: 10.1016/j.mvr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Martin KB, Klinger JR, Rounds SIS. Pulmonary arterial hypertension: new insights and new hope. Respirology. 2006;11:6–17. doi: 10.1111/j.1440-1843.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, et al. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res. 2004;95:830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, et al. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- Michelakis ED, Tymchak W, Noga M, Webster L, Wu X-C, Lien D, et al. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation. 2003;108:2066–2069. doi: 10.1161/01.CIR.0000099502.17776.C2. [DOI] [PubMed] [Google Scholar]
- Miguel-Velado E, Moreno-Domínguez A, Colinas O, Cidad P, Heras M, Pérez-García MT, et al. Contribution of KV channels to phenotypic remodeling of human uterine artery smooth muscle cells. Circ Res. 2005;97:1280–1287. doi: 10.1161/01.RES.0000194322.91255.13. [DOI] [PubMed] [Google Scholar]
- Moudgil R, Michelakis ED, Archer SL. The role of K+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation, and apoptosis: implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension. Microcirculation. 2006;13:615–632. doi: 10.1080/10739680600930222. [DOI] [PubMed] [Google Scholar]
- Murata M, Akao M, O'Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca2+ overload during simulated ischemia and reperfusion: possible mechanism of cardioprotection. Circ Res. 2001;89:891–898. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- Murphy ME, Brayden JE. Apamin-sensitive K+ channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries. J Physiol. 1995;489:723–734. doi: 10.1113/jphysiol.1995.sp021086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Neylon CB. Potassium channels and vascular proliferation. Vascul Pharmacol. 2002;38:35–41. doi: 10.1016/s1537-1891(02)00124-6. [DOI] [PubMed] [Google Scholar]
- Neylon CB, Lang RJ, Fu Y, Bobik A, Reinhart PH. Molecular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle: relationship between KCa channel diversity and smooth muscle cell function. Circ Res. 1999;85:e33–e43. doi: 10.1161/01.res.85.9.e33. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261:H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Nietsch HH, Roe MW, Fiekers JF, Moore AL, Lidofsky SD. Activation of potassium and chloride channels by tumor necrosis factor α: role in liver cell death. J Biol Chem. 2000;275:20556–20561. doi: 10.1074/jbc.M002535200. [DOI] [PubMed] [Google Scholar]
- Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Olschewski A, Li Y, Tang B, Hanze J, Eul B, Bohle RM, et al. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ Res. 2006;98:1072–1080. doi: 10.1161/01.RES.0000219677.12988.e9. [DOI] [PubMed] [Google Scholar]
- Orlov SN, Taurin S, Thorin-Trescases N, Dulin NO, Tremblay J, Hamet P. Inversion of the intracellular Na+/K+ ratio blocks apoptosis in vascular smooth muscle cells by induction of RNA synthesis. Hypertension. 2000;35:1062–1068. doi: 10.1161/01.hyp.35.5.1062. [DOI] [PubMed] [Google Scholar]
- Orlov SN, Thorin-Trescases N, Kotelevtsev SV, Tremblay J, Hamet P. Inversion of the intracellular Na+/K+ ratio blocks apoptosis in vascular smooth muscle at a site upstream of caspase-3. J Biol Chem. 1999;274:16545–16552. doi: 10.1074/jbc.274.23.16545. [DOI] [PubMed] [Google Scholar]
- Orlov SN, Thorin-Trescases N, Pchejetski D, Taurin S, Farhat N, Tremblay J, et al. Na+/K+ pump and endothelial cell survival: [Na+]i/[K+]i-independent necrosis triggered by ouabain, and protection against apoptosis mediated by elevation of [Na+]i. Pflügers Arch. 2004;448:335–345. doi: 10.1007/s00424-004-1262-9. [DOI] [PubMed] [Google Scholar]
- Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 2004;19:285–292. doi: 10.1152/physiol.00011.2004. [DOI] [PubMed] [Google Scholar]
- Pardo LA, del Camino D, Sanchez A, Alves F, Bruggemann A, Beckh S, et al. Oncogenic potential of EAG K+ channels. EMBO J. 1999;18:5540–5547. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pchejetski D, Taurin S, Der Sarkissian S, Lopina OD, Pshezhetsky AV, Tremblay J, et al. Inhibition of Na+,K+-ATPase by ouabain triggers epithelial cell death independently of inversion of the [Na+]i/[K+]i ratio. Biochem Biophys Res Commun. 2003;301:735–744. doi: 10.1016/s0006-291x(02)03002-4. [DOI] [PubMed] [Google Scholar]
- Peng W, Karwande SV, Hoidal JR, Farrukh IS. Potassium currents in cultured human pulmonary arterial smooth muscle cells. J Appl Physiol. 1996;80:1187–1196. doi: 10.1152/jappl.1996.80.4.1187. [DOI] [PubMed] [Google Scholar]
- Perchenet L, Hilfiger L, Mizrahi J, Clément-Chomienne O. Effects of anorexinogen agents on cloned voltage-gated K+ channel hKV1.5. J Pharmacol Exp Ther. 2001;298:1108–1119. [PubMed] [Google Scholar]
- Perregaux D, Gabel CA. Interleukin-1β maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- Platoshyn O, Golovina VA, Bailey CL, Limsuwan A, Krick S, Juhaszova M, et al. Sustained membrane depolarization and pulmonary artery smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2000;279:C1540–C1549. doi: 10.1152/ajpcell.2000.279.5.C1540. [DOI] [PubMed] [Google Scholar]
- Platoshyn O, Zhang S, McDaniel SS, Yuan JX-J. Cytochrome c activates K+ channels before inducing apoptosis. Am J Physiol Cell Physiol. 2002;283:C1298–C1305. doi: 10.1152/ajpcell.00592.2001. [DOI] [PubMed] [Google Scholar]
- Pozeg ZI, Michelakis E, McMurtry MS, Thébaud B, Wu X-C, Dyck JRB, et al. In vivo gene transfer of the O2-sensitive potassium channel KV1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- Puri A, McGoon MD, Kushwaha SS. Pulmonary arterial hypertension: current therapeutic strategies. Nat Clin Pract Cardiovasc Med. 2007;4:319–329. doi: 10.1038/ncpcardio0890. [DOI] [PubMed] [Google Scholar]
- Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol. 2005;288:C769–C783. doi: 10.1152/ajpcell.00529.2004. [DOI] [PubMed] [Google Scholar]
- Remillard CV, Yuan JX-J. Activation of K+ channels: an essential pathway in programmed cell death. Am J Physiol Lung Cell Mol Physiol. 2004;286:L49–L67. doi: 10.1152/ajplung.00041.2003. [DOI] [PubMed] [Google Scholar]
- Rindermann M, Grunig E, von Hippel A, Koehler R, Miltenberger-Miltenyi G, Mereles D, et al. Primary pulmonary hypertension may be a heterogeneous disease with a second locus on chromosome 2q31. J Am Coll Cardiol. 2003;41:2237–2244. doi: 10.1016/s0735-1097(03)00491-1. [DOI] [PubMed] [Google Scholar]
- Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sheng M, McFadden G, Greenberg ME. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4:571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Ide T, Yanagida T, Tsujimoto Y. Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J Biol Chem. 2000;275:12321–12325. doi: 10.1074/jbc.275.16.12321. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Sylvester JT, Booth GM, Shimoda TH, Meeker S, Undem BJ, et al. Inhibition of voltage-gated K+ currents by endothelin-1 in human pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1115–L1122. doi: 10.1152/ajplung.2001.281.5.L1115. [DOI] [PubMed] [Google Scholar]
- Siemen D, Loupatatzis C, Borecky J, Gulbins E, Lang F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem Biophys Res Commun. 1999;257:549–554. doi: 10.1006/bbrc.1999.0496. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Robertson TP, Ward JPT, Aaronson PI. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol. 1994;266:H365–H370. doi: 10.1152/ajpheart.1994.266.1.H365. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Souza R, Sitbon O, Parent F, Simonneau G, Humbert M. Long term imatinib treatment in pulmonary arterial hypertension. Thorax. 2006;61:736. doi: 10.1136/thx.2006.064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabò I, Gulbins E, Apfel H, Zhang X, Barth P, Busch AE, et al. Tyrosine phosphorylation-dependent suppression of a voltage-gated K+ channel in T lymphocytes upon Fas stimulation. J Biol Chem. 1996;271:20465–20469. doi: 10.1074/jbc.271.34.20465. [DOI] [PubMed] [Google Scholar]
- Thevenod F. Ion channels in secretory granules of the pancreas and their role in exocytosis and release of secretory proteins. Am J Physiol Cell Physiol. 2002;283:C651–C672. doi: 10.1152/ajpcell.00600.2001. [DOI] [PubMed] [Google Scholar]
- Thompson GJ, Langlais C, Cain K, Conley EC, Cohen GM. Elevated extracellular [K+] inhibits death-receptor- and chemical-mediated apoptosis prior to caspase activation and cytochrome c release. Biochem J. 2001;357:137–145. doi: 10.1042/0264-6021:3570137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-β family. J Med Genet. 2000;37:741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Toro L, Wallner M, Meera P, Tanaka Y. Maxi-KCa, a unique member of the voltage-gated K channel superfamily. News Physiol Sci. 1998;13:112–117. doi: 10.1152/physiologyonline.1998.13.3.112. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol Cell. 1999;3:159–167. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- Vu CC, Bortner CD, Cidlowski JA. Differential involvement of initiator caspases in apoptotic volume decrease and potassium efflux during Fas- and UV-induced cell death. J Biol Chem. 2001;276:37602–37611. doi: 10.1074/jbc.M104810200. [DOI] [PubMed] [Google Scholar]
- Walev I, Reske K, Palmer M, Valeva A, Bhakdi S. Potassium-inhibited processing of IL-1 beta in human monocytes. EMBO J. 1995;14:1607–1614. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Juhaszova M, Conte JV, Jr, Gaine SP, Rubin LJ, Yuan JX-J. Action of fenfluramine on voltage-gated K+ channels in human pulmonary artery smooth-muscle cells. Lancet. 1998;352:290. doi: 10.1016/s0140-6736(05)60264-4. [DOI] [PubMed] [Google Scholar]
- Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel α subunits in pulmonary artery smooth muscle cells. J Clin Invest. 1997;100:2347–2353. doi: 10.1172/JCI119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Morishima S, Okada Y. IK channels are involved in the regulatory volume decrease in human epithelial cells. Am J Physiol Cell Physiol. 2003;284:C77–C84. doi: 10.1152/ajpcell.00132.2002. [DOI] [PubMed] [Google Scholar]
- Wang L, Xu D, Dai W, Lu L. An ultraviolet-activated K+ channel mediates apoptosis of myeloblastic leukemia cells. J Biol Chem. 1999;274:3678–3685. doi: 10.1074/jbc.274.6.3678. [DOI] [PubMed] [Google Scholar]
- Wang Z. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 2004;448:274–286. doi: 10.1007/s00424-004-1258-5. [DOI] [PubMed] [Google Scholar]
- West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, et al. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res. 2004;94:1109–1114. doi: 10.1161/01.RES.0000126047.82846.20. [DOI] [PubMed] [Google Scholar]
- Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. J Membr Biol. 1996;154:91–107. doi: 10.1007/s002329900135. [DOI] [PubMed] [Google Scholar]
- Xiao AY, Wei L, Xia S, Rothman S, Yu SP. Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurons. J Neurosci. 2002;22:1350–1362. doi: 10.1523/JNEUROSCI.22-04-01350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Wilson BA, Lu L. Induction of human myeloblastic ML-1 cell G1 arrest by suppression of K+ channel activity. Am J Physiol. 1996;271:C2037–C2044. doi: 10.1152/ajpcell.1996.271.6.C2037. [DOI] [PubMed] [Google Scholar]
- Xu D, Wang L, Dai W, Lu L. A requirement for K+-channel activity in growth factor-mediated extracellular signal-regulated kinase activation in human myeloblastic leukemia ML-1 cells. Blood. 1999;94:139–145. [PubMed] [Google Scholar]
- Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, et al. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Physiol. 1998;274:C25–C37. doi: 10.1152/ajpcell.1998.274.1.C25. [DOI] [PubMed] [Google Scholar]
- Young KA, Ivester C, West J, Carr M, Rodman DM. BMP signaling controls PASMC KV channel expression in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2006;290:L841–L848. doi: 10.1152/ajplung.00158.2005. [DOI] [PubMed] [Google Scholar]
- Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, et al. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997;278:114–117. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Yuan JX-J. Oxygen-sensitive K+ channel(s): where and what. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1345–L1349. doi: 10.1152/ajplung.2001.281.6.L1345. [DOI] [PubMed] [Google Scholar]
- Yuan JX-J, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr, Gaine SP, et al. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998a;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- Yuan XJ. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res. 1995;77:370–378. doi: 10.1161/01.res.77.2.370. [DOI] [PubMed] [Google Scholar]
- Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol. 1993;264:L116–L123. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]
- Yuan XJ, Tod ML, Rubin LJ, Blaustein MP. NO hyperpolarizes pulmonary artery smooth muscle cells and decreases the intracellular Ca2+ concentration by activating voltage-gated K+ channels. Proc Natl Acad Sci USA. 1996;93:10489–10494. doi: 10.1073/pnas.93.19.10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan XJ, Wang J, Juhaszova M, Gaine SP, Rubin LJ. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet. 1998b;351:726–727. doi: 10.1016/S0140-6736(05)78495-6. [DOI] [PubMed] [Google Scholar]
- Yuan XJ, Wang J, Juhaszova M, Golovina VA, Rubin LJ. Molecular basis and function of voltage-gated K+ channels in pulmonary arterial smooth muscle cells. Am J Physiol. 1998c;274:L621–L635. doi: 10.1152/ajplung.1998.274.4.L621. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Marzo I, Susin SA, Brenner C, Larochette N, Marchetti P, et al. The thiol crosslinking agent diamide overcomes the apoptosis-inhibitory effect of Bcl-2 by enforcing mitochondrial permeability transition. Oncogene. 1998;16:1055–1063. doi: 10.1038/sj.onc.1201864. [DOI] [PubMed] [Google Scholar]
- Zhang S, Fantozzi I, Tigno DD, Yi ES, Platoshyn O, Thistlethwaite PA, et al. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L740–L754. doi: 10.1152/ajplung.00284.2002. [DOI] [PubMed] [Google Scholar]
- Zhao YJ, Wang J, Rubin LJ, Yuan XJ. Inhibition of KV and KCa channels antagonizes NO-induced relaxation in pulmonary artery. Am J Physiol. 1997;272:H904–H912. doi: 10.1152/ajpheart.1997.272.2.H904. [DOI] [PubMed] [Google Scholar]