Abstract
Caloric restriction (CR) is the most potent intervention known to both protect against carcinogenesis and extend lifespan in laboratory animals. A variety of anticarcinogens and CR mimetics induce and activate the NF-E2-related factor 2 (Nrf2) pathway. Nrf2, in turn, induces a number of antioxidative and carcinogen-detoxifying enzymes. Thus, Nrf2 offers a promising target for anticarcinogenesis and antiaging interventions. We used Nrf2-disrupted (KO) mice to examine its role on the biological effects of CR. Here, we show that Nrf2 is responsible for most of the anticarcinogenic effects of CR, but is dispensable for increased insulin sensitivity and lifespan extension. Nrf2-deficient mice developed tumors more readily in response to carcinogen exposure than did WT mice, and CR was ineffective in suppressing tumors in the KO mice. However, CR extended lifespan and increased insulin sensitivity similarly in KO and WT mice. These findings identify a molecular pathway that dissociates the prolongevity and anticarcinogenic effects of CR.
Keywords: aging, carcinogenesis, oxidative stress
Almost a century ago, Moreschi (1) and Rous (2) published their observations on the beneficial impact of caloric restriction (CR) on transplanted and induced tumors. Years later, McCay and colleagues first observed lifespan extension in laboratory rats maintained on CR (3). Since then, CR has been studied intensively with consistent results showing its beneficial effects on longevity, age-associated diseases, attenuation of functional decline, and carcinogenesis across a variety of species and diet formulations (4–8). However, the mechanism(s) underlying the effects of CR protection still remain unknown. Nevertheless, it is safe to say that the three most extensively studied hallmarks of CR are enhanced protection against induced and spontaneous carcinogenesis, reduced insulin/insulin-like growth factor 1 (IGF-1) signaling, and increased median and maximum lifespan (5–9).
Even if CR was shown to benefit human health, confer cancer protection, and increase longevity, it would be extremely difficult to achieve adherence to such a stringent diet that might require a reduction of 20–40% in caloric intake. To this end, considerable investment has been focused on dissecting the pathways that regulate the beneficial effects of CR to spur development of pharmacological agents potentially acting as CR mimetics (10). Several of the currently proposed CR mimetics are phytochemicals (resveratrol, quercetin, and curcumin) that act, at least in part, through the activation of the NF-E2-related factor 2 (Nrf2) pathway (11–14). Nrf2 is a transcription factor that binds to the antioxidant response element (ARE) of target genes in response to oxidative stress and increases the transcription of a variety of antioxidative and carcinogen-detoxification enzymes. Stress can result from a variety of causes, including fasting, overfeeding, endogenous compounds, exposure to chemicals, or environmental agents, which generally leads to increased production of reactive oxygen species (ROS). Under normal conditions, Nrf2 is bound to Keap1 in the cytoplasm, where it undergoes proteolytic degradation and rapid turnover. However, upon ROS exposure, Nrf2 is released and translocates to the nucleus, where it binds to AREs to induce expression of multiple cytoprotective enzymes, including NAD(P)H-quinone oxidoreductase 1 (NQO1), glutathione S-transferases (GSTs), and heme oxygenase 1 (HO-1) (15, 16).
Nrf2 can be linked to each of the three hallmarks of CR: protection against spontaneous and induced carcinogenesis, the insulin/IGF-1 signaling pathway, and prolonged survival. A variety of environmental carcinogens, including low levels of UV-B irradiation, also activate Nrf2 and up-regulate ARE-responsive gene products to enhance protection against cellular and molecular damage that can induce cancer (17). In addition, changes in insulin levels during fasting induce a mild oxidative stress and an alteration of the expression of Nrf2-induced enzymes (18).
Nrf2 expression has shown an age-related decrease in rodent tissues (19, 20), possibly leading to higher levels of ROS and an increased risk of cancer. However, CR has been shown to attenuate the age-related loss of antioxidative capacity due to aging and to increase levels of NQO1 and GST in brain and liver, respectively (21–23). The lack of functional Nrf2 in mice increases sensitivity to carcinogenesis through a lowered constitutive expression of antioxidative and detoxifying enzymes including NQO1 and GSTs (24–26). NQO1 KO mice are also more susceptible to chemical carcinogens as measured by a higher incidence of tumors and more tumors per animal (26). They are also insulin resistant, suggesting that NQO1 is involved in insulin signaling (27). Addition of exogenous insulin to cells causes the translocation of Nrf2 from the cytoplasm to the nucleus and leads to increased expression of HO-1 and GSTs (28, 29).
Mammalian cap 'n' collar transcription factors, such as Nrf2, are thought to be most closely related to the Caenorhabditis elegans gene skn-1. SKN-1 is functionally similar to Nrf2 in that it responds to oxidative stress and up-regulates detoxifying enzymes (30, 31). In addition, overexpression of GSTs increases median lifespan in C. elegans (32, 33), and skn-1 mutants have shorter survival and reduced stress response compared with WT worms (31, 34). The presence of skn-1 in only two neurons is necessary for CR to increase median and maximum lifespan in C. elegans (35). Because the regulation of lifespan appears dependent on Nrf2-homologous pathways in C. elegans, the survival effects of CR in mammals could be also regulated through Nrf2 transcription factor networks. We hypothesized that the advantageous effects of CR on carcinogenesis and lifespan would require Nrf2. To explore the role of Nrf2 on CR-mediated effects, KO mice were placed on ad libitum (AL) and CR diets and were evaluated in a two-stage skin carcinogenesis model, for insulin tolerance, and survival.
Results and Discussion
CR Induces Nrf2 Downstream Effectors.
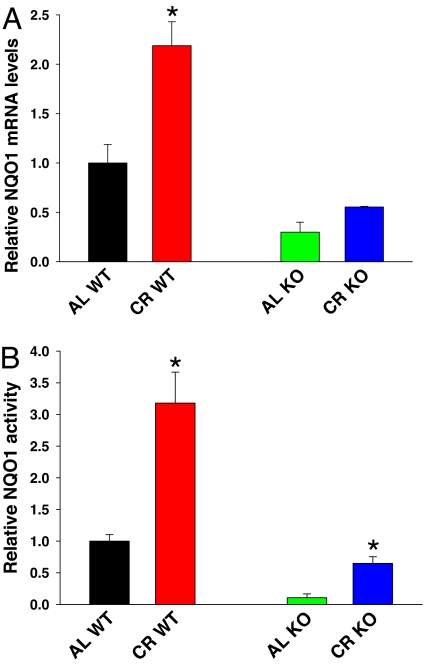
To explore the activation of Nrf2 by CR, mRNA levels of several downstream effectors were measured in AL- and CR-fed WT and KO mice. NQO1 mRNA levels were significantly increased by CR in WT livers (P < 0.05). However, CR produced a small increase in NQO1 levels in KO mice, but this increase was not statistically significant (Fig. 1A). There were trends in the same direction for HO-1, glutamate-cysteine ligase, catalytic subunit (GCLC), GST A1, and glutathione peroxidase-1 (GPx-1), but they did not reach significance when comparing AL to CR WT livers [supporting information (SI) Fig. 5 A and D]. There were no trends toward increased mRNA expression in the AL- vs. CR-fed KO mouse livers, suggesting that these genes are not inducible under CR conditions without activation of Nrf2. To ascertain the increases on NQO1, we performed activity assays, which correlated well with the differences seen in NQO1 mRNA levels (Fig. 1B). NQO1 activity was significantly increased in CR-fed WT and KO liver cytosol compared with AL-fed WT and KO mice, respectively (P < 0.001 in both comparisons). In agreement with our findings, CR attenuated the age-related loss of antioxidative capacity due to aging and increased levels of NQO1 and GST in brain and liver, respectively (21–23).
Fig. 1.
NQO1 levels are increased in response to CR. (A) Relative RNA levels of NQO1 were determined by real-time PCR. Liver homogenates from AL and 40% CR-fed WT and KO mice were tested. CR significantly increased NQO1 levels in WT mice (P < 0.05), and there was a trend toward an increase in CR-fed KO livers compared with AL-fed KO mice (P < 0.07). n = 3 for all groups. (B) NQO1 activity was determined and CR-fed WT liver homogenates showed increased activity compared with AL (P < 0.001). Also, there was a significant increase in the CR-fed KO mice compared with the AL-fed KO (P < 0.001). n = 5 for all groups. Black, AL WT; red, CR WT; green, AL KO; blue, CR KO.
CR Requires Nrf2 for Protection Against Induced Tumors.
Mice were treated topically once with the tumor initiator 7,12-dimethylbenz(a)anthracene (DMBA) and then twice weekly with topical treatments of the tumor promoter 12-O-tetradecanoyl phorbol-13-acetate (TPA) (SI Fig. 6A). DMBA has been shown to bind to DNA and cause mutations particularly in the H-ras gene, which is normally involved with proliferation and differentiation (36). The tumor promoter TPA triggers ROS production and causes hyperplasia in skin cells with H-ras mutations, which leads ultimately to the formation of papillomas (36, 37). CR is protective against the promotion phase of the two-stage carcinogenesis model and decreases tumor incidence and multiplicity (38). A typical papilloma is an overgrowth of keratinocytes and stromal cells (SI Fig. 6B). TPA treatment was terminated after a single papilloma >1 mm2 was observed on the mouse, because initial tumor incidence was the main focus of this study. Representative tumors were fixed and H&E stained (SI Fig. 6C).
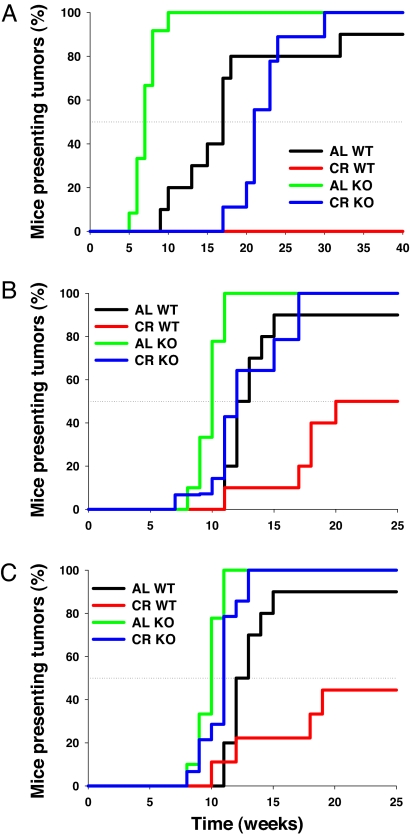
Male WT, heterozygous (HT), and KO mice were maintained on a standard diet. They were separated into AL and 40% CR groups at 10 weeks of age. CR food weights were determined by AL food intake and adjusted weekly to meet 40% of the AL intake (of their respective genotype). Body weights dropped markedly in the first 5 weeks after restriction in CR-fed WT and KO mice (SI Fig. 7A). After body weights stabilized on the CR diet, DMBA was administered at time 0, and TPA was then given topically twice weekly beginning in week 2 until an initial tumor was observed. AL-fed KO mice were more susceptible to two-stage carcinogenesis than the AL-fed WT mice, corroborating data published in refs. 25 and 39. AL-fed KO mice showed very rapid onset of tumorigenesis; both AL- and CR-fed KO mice reached 100% tumor incidence by 30 weeks (Fig. 2A). Tumor incidence in AL-fed WT mice was similar to that of the CR-fed KO mice. Regarding tumor incidence in CR-fed WT mice, the results were striking. In contrast to the CR-fed KO mice, even at the end of the study with 42 weeks of treatment, no papillomas were present in the CR-fed WT mice. Further evidence of the importance of Nrf2 activation by CR is shown by heterozygous mice that had intermediate tumor incidence when compared with the WT and KO mice (SI Fig. 8 A and B).
Fig. 2.
Lack of protection against induced tumors in CR KO mice. (A) Forty percent CR WT, 40% CR KO. (B) Thirty percent CR WT, 30% CR KO. (C) Twenty percent CR WT, 20% CR KO. Shown are the percentages of mice with at least one papilloma for AL and 40% CR-fed WT and KO (A) AL and 30% CR-fed WT and KO (B) and AL and 20% CR-fed WT and KO (C) mice presenting tumors after DMBA treatment at time 0. Black, AL WT; red, CR WT (20, 30, or 40% restriction); green, AL KO; blue, CR KO (20, 30, or 40% restriction).
Tumor incidence in the CR-fed KO mice was not significantly different from the AL-fed WT mice under stringent conditions of 40% CR. However, given the slight delay in onset of carcinogenesis, we investigated less-harsh CR conditions at 20% and 30% restriction in WT and KO mice. The body weights of the 20% and 30% CR-fed mice (SI Fig. 7 B and C) were decreased when compared with the AL-fed mice. The incidence of tumors in the 20% CR KO mice was not significantly different from the AL-fed KO mice, and both groups were more susceptible than the AL-fed WT mice to carcinogenesis (Fig. 2C). Meanwhile, the 30% CR-fed KO mice overlapped the AL-fed WT mice for the duration of the study (Fig. 2B). The 20% and 30% CR-fed WT mice were still significantly delayed in their development of tumors compared with the AL-fed WT mice (Fig. 2 B and C). The tumor onset results are also summarized in Table 1. Tumor multiplicity was decreased in CR-fed KO mice compared with AL-fed KO mice, but there were no significant differences between the CR-fed KO mice and the AL-fed WT mice (SI Fig. 8C). The number of tumors per mouse was divided by the number of weeks tumor development continued (from time of first tumor) until the animal was euthanized to obtain a tumor occurrence rate. Therefore, the anticarcinogenic effects of CR are significantly impaired in Nrf2 KO mice. This impairment in chemical-induced carcinogenesis was also observed in female mice (SI Fig. 9 A and C).
Table 1.
Tumor incidence by percentage of mice with at least one tumor, reported in weeks
| Tumor incidence, % | AL |
20% CR |
30% CR |
40% CR |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT* | HT | KO* | WT | KO | WT | KO | WT | HT | KO | |
| 10 | 10 | 7 | 7 | 10 | 9 | 11 | 10 | n/a | 18 | 17 |
| 25 | 13 | 7 | 8 | 18 | 10 | 18 | 11 | n/a | 20 | 21 |
| 50 | 15 | 8 | 9 | n/a | 11 | 20 | 12 | n/a | n/a | 21 |
| 75 | 16 | 9 | 9 | n/a | 11 | n/a | 15 | n/a | n/a | 23 |
| 90 | 24 | 9 | 10 | n/a | 13 | n/a | 17 | n/a | n/a | 24 |
| 100 | n/a | 9 | 11 | n/a | 13 | n/a | 17 | n/a | n/a | 30 |
n/a indicates that tumor incidence did not reach this percentage during study.
*Results averaged between two studies.
CR Does Not Require Nrf2 for Insulin Sensitivity or Increased Longevity.
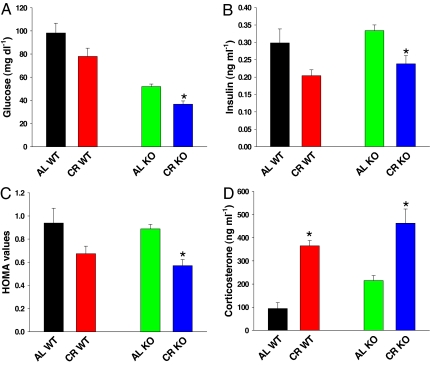
We next tested whether the delay of onset in tumorigenesis was correlated with CR-induced changes in serum levels of glucose, insulin, and IGF-1, which have been associated previously with tumor incidence. In another cohort of mice on CR for 4 weeks, WT mice had higher levels of fasting glucose than KO mice. In both WT and KO mice, CR decreased glucose and insulin levels (Fig. 3 A and B) and improved insulin sensitivity, as measured by the homeostasis model assessment (HOMA) method (Fig. 3C). Although these trends were consistent after a 4-week treatment among all groups involved in this study, the changes reached statistical significance only for the KO mice. We performed an insulin tolerance test in which glucose was measured after an i.p. insulin challenge. Glucose levels normally fall in response to insulin and at a critical level of glucose, counterregulatory mechanisms are activated that increase glucose levels (gluconeogenesis under the control of epinephrine and glucagon, mainly). CR mice did not change their initial response to insulin but both CR groups induced similar levels of counterregulatory activity in response to insulin-induced hypoglycemia in both the WT and KO mice (data not shown). Fasting IGF-1 levels were also measured in serum from mice on CR for 4 weeks (data not shown). There were no significant differences between the AL and CR-fed mice of either genotype; thus, it is likely that 4 weeks of CR may not have been sufficient time to observe the predicted decrease in IGF-1 levels. The adrenal gland and particularly corticosterone levels have been shown to be important factors in the development of induced tumors (40). Corticosterone was increased in CR-fed mice of both genotypes compared with AL-fed mice (Fig. 3D). Thus, it is possible that some of the delay in tumor development in the CR-fed KO mice may be due to higher levels of corticosterone observed in these mice.
Fig. 3.
Caloric restriction improves insulin sensitivity in WT and KO mice. (A) WT and KO mice were fed AL or 40% CR for 4 weeks. They were fasted overnight (16 h), and glucose levels were determined. There was a trend toward a decrease in the CR-fed WT mice (P < 0.09) and a significant decrease in the CR-fed KO mice (P < 0.01) compared with their respective AL-fed control mice. n = 10 for all groups except CR-fed WT, for which n = 9. (B) Fasting insulin values were determined by ELISA. There was a trend toward a decrease in the CR-fed WT mice (P < 0.10) and a significant decrease in the CR-fed KO mice (P < 0.05) compared with their respective AL-fed control mice. n = 5 for all groups except CR-fed WT, for which n = 4. (C) HOMA values were calculated from fasted glucose and insulin values, using the HOMA2 software available from the Oxford Centre for Diabetes, Endocrinology, and Metabolism Diabetes Trials Unit (www.dtu.ox.ac.uk). The values were from five mice per group (except CR-fed WT, n = 4) after a 16-h fast. There was a significant decrease in the HOMA values for CR vs. AL-fed KO mice (P < 0.01). (D) Fasting corticosterone levels. Corticosterone levels were significantly increased in CR-fed WT and KO mice compared with AL-fed WT and KO mice respectively (P < 0.01 in both cases). These mice were fed CR for 8 weeks. n = 9 for all groups except AL-fed WT, for which n = 7. Black, AL WT; red, CR WT; green, AL KO; blue, CR KO.
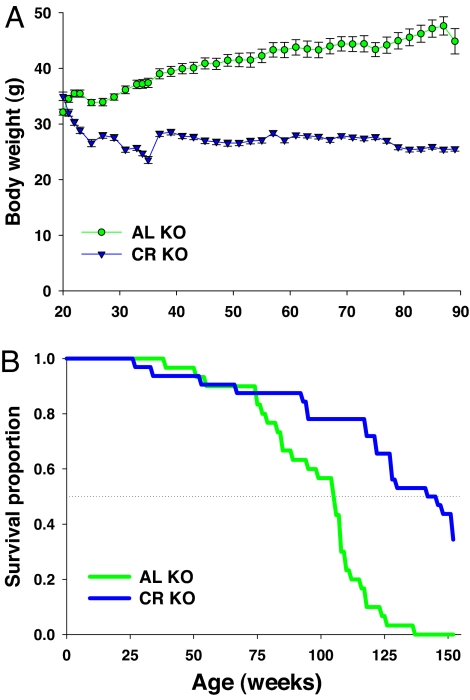
Thus, the beneficial effects of CR on carcinogenesis appear blunted in the absence of Nrf2, but improvement in insulin sensitivity is maintained. We also set out to determine whether this lack of protection by CR was also observed when using longevity as the endpoint. For this study, we did not assess the effects of CR on WT mice, because it would be fully expected that CR would increase lifespan under these conditions as shown by others (41). Rather, we focused on the important question of whether the absence of Nrf2 would mitigate the effects of CR. Male KO mice were split into AL-fed and 40% CR-fed groups for a survival study at 20 weeks of age. The body weights of CR-fed mice dropped over a 5–10 week period (Fig. 4A). AL-fed mice maintained steady body weight until approximately week 90, when their weights began to decrease and eventually intersected with the CR groups (data not shown). The survival curves show that the CR and AL-fed KO mice overlapped until week 80, when they sharply diverged for the remainder of the study with mice on a CR-fed diet surviving longer than mice on an AL-fed diet (Fig. 4B). According to Kaplan–Meier analysis, the survival curves for the AL and CR KO mice differed significantly by the logrank test (χ2 = 28.9, P < 0.0001). CR increased median lifespan by 35.8% (144 vs. 106 weeks). Although maximum lifespan has not yet been recorded in the CR KO group, it would appear that it is certain to be extended. At this point, none of the AL-fed Nrf2 mice are alive, and if the remainder of the CR-fed mice (n = 11) die within the next week, maximum lifespan will have been significantly extended by 15% at a minimum. Increased median and maximum lifespan were also observed in female mice (SI Fig. 9 B and D).
Fig. 4.
Kaplan–Meier survival analyses for KO mice. (A) Body weight of male mice in grams. KO mice were separated into two groups, AL and CR. The food was gradually restricted to 40% less than that of the AL-fed KO mice. (B) Kaplan–Meier survival analyses of AL and CR-fed KO mice were performed. n = 30 and n = 32 for AL and CR-fed mice, respectively. The survival curves for the AL and CR-fed KO mice differ significantly by the logrank test; (χ 2 = 28.9, P < 0.0001). Green, AL KO; blue, CR KO.
Conclusion
In this study, we have shown that the protective effects of CR against carcinogenesis depend on a pathway that involves Nrf2. However, the lack of Nrf2 did not attenuate lifespan extension nor alter the CR-induced improvement in insulin sensitivity in the KO mice. A disconnect between tumor burden and lifespan was also observed by Van Remmen et al. (42) when they showed that reduction of an antioxidant enzyme could markedly increase DNA damage and spontaneous cancer incidence without affecting survival and lifespan in AL-fed mice. However, our study demonstrates that distinct pathways are involved in the various beneficial effects of CR and suggests that many mechanisms are involved in its protection. Recent data from invertebrates suggested that Nrf2 or at least some of its downstream effectors could hold the key to caloric restriction and longevity. It appears now in mammals that besides the involvement of Nrf2 on anticarcinogenic protection by CR, there must be other pathways or factors regulating longevity. Our data suggest that the lifespan extension seen in CR mice is not solely a consequence of up-regulation of the Nrf2 pathway. Finally, and most importantly, the activation of the Nrf2 pathway is a promising target to evaluate for CR mimetics in the search for preventive strategies against environmentally-induced cancers.
Materials and Methods
Animals.
Survival study.
Nrf2 KO mice on an Institute of Cancer Research (ICR) background were obtained from a colony at The Johns Hopkins University and rederived at the National Institutes of Health (Bethesda, MD). They were then bred to ICR mice (Taconic, Germantown, NY) from KO to WT and then from HT to HO as described by Itoh et al. (43) in a specific pathogen-free (SPF) facility at the National Institute on Aging and genotyped. Male mice were maintained on NIH-31 diet until 20 weeks of age. Mice were then randomly assigned to AL or CR groups with no initial differences in body weights. CR mice were fed 40% less than AL-fed mice until the first 25% of the AL mice were dead or stopped feeding normally. At this point, total grams of food for CR mice were kept unchanged or the restriction was adjusted to maintain the initial body weight differences. The mice were singly housed in duplex caging in an environmentally-controlled vivariam with unlimited access to water and a controlled photoperiod (12 h light; 12 h dark). Body weight and food intake data were recorded biweekly. All mice were maintained between 68 and 72°F according to animal protocols and National Institutes of Health guidelines. Survival curves were plotted by using the Kaplan–Meier method in the MedCalc Program for Windows, Version 9.2.1.0 (MedCalc Software). All mice were included in the survival analysis (n = 30 for AL and n = 32 for CR mice). Survival studies were performed in female mice following the same protocol as for the male mice (n = 30 for AL and n = 30 for CR mice).
Induced carcinogenesis.
Male ICR WT mice were purchased from Taconic, and Nrf2 HT and KO mice were bred in the SPF colony at the National Institute on Aging and were genotyped according to standard methods. The mice were then transferred to a conventional animal facility. They were maintained on Teklad 2018 diet under either AL or CR-fed conditions from 10 weeks of age. Food intake and body weight data were measured weekly for the duration of the study. CR food intake was calculated from AL food consumption for each genotype. All mice were singly housed. All mice were maintained on a 12 h light/12 h dark cycle and between 68 and 72°F according to animal protocols and National Institutes of Health guidelines. After 5–6 weeks of CR, mice began the two-stage carcinogenesis model. Induced carcinogenesis studies were performed in female mice following the same protocol as for the male mice.
Insulin sensitivity.
Male ICR WT mice were purchased from Taconic, and KO mice were bred and maintained under similar conditions to the induced carcinogenesis study above. When they were 10 weeks of age, the mice were fed either AL or 40% CR for 4 weeks before being used for the glucose and insulin assays discussed in detail in SI Text. All diets contained 18–19% protein and 5–6% fat.
Real-Time PCR.
RNA was isolated from frozen liver sections of WT and KO mice on AL and 40% CR diets from the two-stage carcinogenesis mice for NQO1 (n = 3) or untreated mice for HO-1, GPx-1, GST A1, and GCLC (n = 4–5 for each group). Further details are provided in SI Text.
NQO1 Activity Assay.
NQO1 activity was measured in frozen liver homogenates of 16-week-old WT and KO mice on AL and 40% CR diets for 6 weeks (n = 5 for each group) as described in SI Text.
Two-Stage Carcinogenesis.
Male mice were divided into groups according to genotype. All groups began with an n = 10, except for KO on 20 and 30% restriction, where n = 15. Several mice were euthanized or found dead during the study, and their data were included until the time when survival recording was terminated. Mice on the CR-fed diet were restricted for 5–6 weeks until body weights reflected the accurate restriction before tumor induction. Within 24 h before DMBA tumor initiation, a 2 cm × 2 cm area was shaved into the dorsal fur. All mice were treated with a single dose of 25 μg of DMBA dissolved in 100 μl of acetone. Because of the carcinogenicity of DMBA, the mice were not handled for the following 72 h, at which time the cages and bedding were changed. Tumor promotion with TPA (4 μg dissolved in 100 μl of acetone) treatment began 2 weeks after DMBA initiation and continued twice weekly until at least 1 papilloma with a radius >1 mm was recorded for tumor incidence data. The second week of TPA treatment was withheld because of skin reactions on the mice. Individual mice were euthanized upon the following criteria: tumor radius exceeded 1 cm or tumor(s) appeared to be causing discomfort to the mouse. The majority of study groups were euthanized when the tumor incidence exceeded 75% of the mice in a particular group. Upon euthanasia, final tumor number, size, and total weight were measured. Livers were collected and flash frozen in liquid nitrogen and stored at −80°C.
Serum Markers and Hormones.
Statistics.
Unpaired t tests were performed to compare AL and CR results from a given genotype, using the StatView program. Statistics for the tumor incidence were performed by taking the mean of the initial tumor incidence week for each mouse. If a mouse did not develop a tumor throughout the study, its value was designated as the week of sacrifice. Survival analysis was performed with MedCalc statistical software.
Supplementary Material
ACKNOWLEDGMENTS.
We thank O. Carlson, T. H. Moran, and R. Tavaluc for their technical assistance; D. Phillips and the Comparative Medicine Section for animal care; M. S. Bonkowski for assistance with the design of the insulin tolerance test; and M. Gorospe, M. P. Mattson, and J. Mattison for their critical reading of this manuscript. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging, National Institutes of Health Grants HD055030 (K.L.T.) and DK077623 (T. H. Moran), and Spanish MCyT Grant BMC2002-01602 (P.N.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712162105/DC1.
References
- 1.Moreschi C. Beziehungen zwischen Ernahrung und Tumorwachstum. Zeitschrift fur Immunitatsforsch. 1909;2:661–675. [Google Scholar]
- 2.Rous P. The influence of diet on transplanted and spontaneous tumors. J Exp Med. 1914;20:433–451. doi: 10.1084/jem.20.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 4.Tannenbaum A. The initiation and growth of tumors. Introduction. Effects of underfeeding. Am J Cancer. 1940;38:335–350. [Google Scholar]
- 5.Gross L, Dreyfuss Y. Reduction in the incidence of radiation-induced tumors in rats after restriction of food intake. Proc Natl Acad Sci USA. 1984;81:7596–7598. doi: 10.1073/pnas.81.23.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: Longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 7.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kritchevsky D. Caloric restriction and experimental carcinogenesis. Hybrid Hybridom. 2002;21:147–151. doi: 10.1089/153685902317401753. [DOI] [PubMed] [Google Scholar]
- 9.Bartke A, Masternak MM, Al-Regaiey KA, Bonkowski MS. Effects of dietary restriction on the expression of insulin-signaling-related genes in long-lived mutant mice. Interdiscip Top Gerontol. 2007;35:69–82. doi: 10.1159/000096556. [DOI] [PubMed] [Google Scholar]
- 10.Roth GS, Ingram DK, Lane MA. Caloric restriction in primates and relevance to humans. Ann N Y Acad Sci. 2001;928:305–315. doi: 10.1111/j.1749-6632.2001.tb05660.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Comm. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh TC, Lu X, Wang Z, Wu JM. Induction of quinone reductase NQO1 by resveratrol in human K562 cells involves the antioxidant response element ARE, is accompanied by nuclear translocation of transcription factor Nrf2. Med Chem. 2006;2:275–285. doi: 10.2174/157340606776930709. [DOI] [PubMed] [Google Scholar]
- 13.Tanigawa S, Fujii M, Hou DX. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic Biol Med. 2007;42:1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Balogun E, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishii T, Itoh K, Yamamoto M. Roles of Nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol. 2002;348:182–190. doi: 10.1016/s0076-6879(02)48637-5. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Kannan S, Jaiswal AK. Low and high dose UVB regulation of transcription factor NF-E2-related factor 2. Cancer Res. 2006;66:8421–8429. doi: 10.1158/0008-5472.CAN-06-1181. [DOI] [PubMed] [Google Scholar]
- 18.Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther. 2007;113:88–120. doi: 10.1016/j.pharmthera.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh JH, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci USA. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shih PH, Yen GC. Differential expressions of antioxidant status in aging rats: The role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology. 2007;8:71–80. doi: 10.1007/s10522-006-9033-y. [DOI] [PubMed] [Google Scholar]
- 21.Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci USA. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LH, Hu N, Snyder DL. Effects of age and dietary restriction on liver glutathione transferase activities in Lobund–Wistar rats. Arch Gerontol Geriatr. 1994;18:191–205. doi: 10.1016/0167-4943(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 23.Kim JD, McCarter RJ, Yu BP. Influence of age, exercise, and dietary restriction on oxidative stress in rats. Aging (Milan) 1996;8:123–129. doi: 10.1007/BF03339566. [DOI] [PubMed] [Google Scholar]
- 24.Ramos-Gomez M, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.auf dem Keller U, et al. Nrf transcription factors in keratinocytes are essential for skin tumor prevention but not for wound healing. Mol Cell Biol. 2006;26:3773–3784. doi: 10.1128/MCB.26.10.3773-3784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long DJ, II, Waikel RL, Wang XJ, Roop DR, Jaiswal AK. NAD(P)H:quinone oxidoreductase 1 deficiency and increased susceptibility to 7,12-dimethylbenz(a)-anthracene-induced carcinogenesis in mouse skin. J Natl Cancer Inst. 2001;93:1166–1170. doi: 10.1093/jnci/93.15.1166. [DOI] [PubMed] [Google Scholar]
- 27.Gaikwad A, Long DJ, II, Stringer JL, Jaiswal AK. In vivo role of NAD (P)H:quinone oxidoreductase 1 (NQO1) in the regulation of intracellular redox state and accumulation of abdominal adipose tissue. J Biol Chem. 2001;276:22559–22564. doi: 10.1074/jbc.M101053200. [DOI] [PubMed] [Google Scholar]
- 28.Harrison EM, et al. Insulin induces heme oxygenase-1 through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in renal cells. FEBS J. 2006;273:2345–2356. doi: 10.1111/j.1742-4658.2006.05224.x. [DOI] [PubMed] [Google Scholar]
- 29.Kang KW, Lee SJ, Park JW, Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol Pharmacol. 2002;62:1001–1010. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- 30.Walker AK, et al. A conserved transcription motif suggesting functional parallels between Caenorhabditis elegans SKN-1 and Cap‘n’Collar-related basic leucine zipper proteins. J Biol Chem. 2000;275:22166–22171. doi: 10.1074/jbc.M001746200. [DOI] [PubMed] [Google Scholar]
- 31.An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–1893. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayyadevara S, et al. Lifespan extension in hypomorphic daf-2 mutants of Caenorhabditis elegans is partially mediated by glutathione transferase CeGSTP2–2. Aging Cell. 2005;4:299–307. doi: 10.1111/j.1474-9726.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- 33.Ayyadevara S, et al. Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell. 2005;4:257–271. doi: 10.1111/j.1474-9726.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- 34.An JH, et al. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc Natl Acad Sci USA. 2005;102:16275–16280. doi: 10.1073/pnas.0508105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 36.Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 37.Singh NN, Poirier GG, Cerutti PP. Tumor promoter phorbol-12-myristate-13-acetate induces poly(ADP)-ribosylation in fibroblasts. EMBO J. 1985;4:1491–1494. doi: 10.1002/j.1460-2075.1985.tb03807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birt DF, Pelling JC, White LT, Dimitroff K, Barnett T. Influence of diet and calorie restriction on the initiation and promotion of skin carcinogenesis in the SENCAR mouse model. Cancer Res. 1991;51:1851–1854. [PubMed] [Google Scholar]
- 39.Xu C, et al. Inhibition of 7,12-dimethylbenz(a) anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 40.Pashko LL, Schwartz AG. Reversal of food restriction-induced inhibition of mouse skin tumor promotion by adrenalectomy. Carcinogenesis. 1992;13:1925–1928. doi: 10.1093/carcin/13.10.1925. [DOI] [PubMed] [Google Scholar]
- 41.Tazume S, et al. Effects of germfree status and food restriction on longevity and growth of mice. Jikken dobutsu. 1991;40:517–522. doi: 10.1538/expanim1978.40.4_517. [DOI] [PubMed] [Google Scholar]
- 42.Van Remmen H, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 43.Itoh K, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Comm. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.