Abstract
The molecular mechanisms used by regulatory T cells (Treg) to inhibit the effector phase of adaptive immune responses are still elusive. In the present work, we investigated the possibility that Treg may interfere with a basic biological function of T helper cells (TH): polarization of secretory machinery for dedicated help delivery. To address this question, we visualized by confocal microscopy different parameters of activation in TH and Treg cells interacting simultaneously with individual antigen-presenting cells (APC). Our results show that, although productive TCR engagement in TH/APC conjugates was unaffected by the presence of adjacent Treg, the reorientation of TH secretory machinery toward APC was strongly inhibited. Blocking TGF-β completely reverted Treg induced inhibition of TH polarization. Our results identify a previously undescribed mechanism by which Treg inhibit effector T cells. TGF-β produced by adjacent Treg interferes with polarization of TH secretory machinery toward APC, thus affecting a crucial step of TH-mediated amplification of the immune response.
Keywords: confocal microscopy, immunological synapse, T cell activation
Naturally arising CD4+ CD25+ regulatory T cells (Treg) play a pivotal role in the maintenance of peripheral self tolerance. Deficiency of this suppressive T cell subset results in the development of autoimmune lymphoproliferative disorders in mouse models and human patients (1). Treg are also implicated in controlling immune responses against infectious agents and have been shown to be detrimental for antitumor immunity (2, 3).
Treg use different strategies to inhibit immune responses. Among these, an important mechanism involves TGF-β, which is a central cytokine in the homeostasis of the immune system (4, 5). Effector T cells in which the response to TGF-β is abrogated are resistant to Treg suppression (6, 7).
Although the role of Treg in controlling the amplitude and duration of immune responses has been thoroughly documented, and several effector molecules have been identified (8), the mechanisms by which Treg interfere with the early-activation steps of other cells of the immune system are still elusive. In particular, it is not clear whether and how Treg may interfere with the signaling leading to biological responses in effector cells, such as T helper cells (TH) during their interaction with cognate antigen-presenting cells (APC).
Two key features define TH activation and effector function. The first feature is that TH form prolonged conjugates with APC and undergo sustained signaling that is required for induction of cytokine production (9, 10). The second feature is that TH polarize secretory machinery toward APC and selectively activate cognate APC (11, 12).
Although T cell activation is a slow process resulting from sustained TCR engagement and triggering (9, 10, 13), T cell polarization is a rapid and adaptable phenomenon. T cells interacting simultaneously with APC offering different antigenic stimuli rapidly remodel immunological synapses and adjust polarization of Golgi apparatus (14, 15). This mechanism ensuring an exquisite specificity to help delivery may be instrumental to orchestrate and amplify adaptive immune responses.
In the present work, we investigated the impact that Treg may have on rapid polarization responses and on sustained signaling in autologous human TH cells.
We report that although productive TCR engagement in TH/APC conjugates was unaffected by the presence of adjacent Treg, TH polarization response toward APC was impaired via a TGF-β-dependent mechanism.
By showing that Treg interfere with dedicated help delivery, we identify a mechanism by which Treg affect adaptive immune responses.
Results
Isolation, Expansion, and Characterization of Human Treg.
Typical CD4+ CD25+ T cell purification from peripheral blood is shown in supporting information (SI) Fig. 6A. Freshly isolated CD4+ CD25+ T cells were mainly Foxp3+ (SI Fig. 6B) and CD127−/lo (Fig. 6C) as described (16, 17). CD4+ CD25+ T cells were expanded by using beads coated with anti-CD3 and -CD28 antibodies (SI Fig. 6D). After expansion CD4+ CD25+ remained mainly Foxp3+ and CD127−/lo (SI Fig. 6E). CD4+ CD25− TH cells were obtained from the CD4+ fraction of peripheral blood mononuclear cell (PBMC) after sorting of CD25+ T cells and were expanded in parallel; they remained Foxp3− and CD127high (SI Fig. 6F). To test the suppressive potential of CD4+ CD25+ T cells, TH cells loaded with carboxyfluorescein diacetate succinimidyl ester (CFSE) were stimulated by anti-CD3/CD28 mAb-coated beads and cocultured either in the presence or in the absence of CD4+ CD25+ T cells at different cell–cell ratios. As shown in SI Fig. 6 G and H, in vitro expanded CD4+ CD25+ T cells efficiently suppressed the proliferative response of autologous CD4+ CD25− T cells (18). We also investigated whether CD4+ CD25+ T cells could affect TH cell proliferation and IFN-γ production after 72-h coculture with APC pulsed with a mixture of bacterial superantigens. As shown in SI Fig. 7, both IFN-γ production and TH cell proliferation were strongly inhibited in the presence of in vitro expanded CD4+ CD25+ T cells.
Having characterized the regulatory function of in vitro expanded CD4+ CD25+ T cells (Treg), we used these cells to study their impact on the dynamics of TH interaction with APC in all of the further experiments described in this study.
Treg Do Not Interfere with [Ca2+]i Increase in TH Cells Conjugated with APC.
We initially investigated whether Treg could affect TH conjugation with APC or sustained signaling in TH cells. We measured [Ca2+]i increase in TH cells at different time points after conjugation with APC pulsed with different concentrations of superantigens (either in the presence of bystander Treg or of bystander TH, as a control).
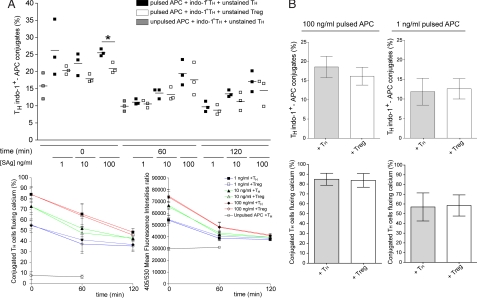
As shown in Fig. 1A at the different superantigen concentrations used Treg either did not affect or only marginally affected TH/APC conjugation. Interestingly, the fraction of conjugated TH cells undergoing calcium mobilization and the intensity of [Ca2+]i increase in conjugated TH cells were unaffected in the presence of Treg (Fig. 1A).
Fig. 1.
Sustained [Ca2+]i increase in TH cells is unaffected in the presence of Treg. Indo-1-loaded TH cells were conjugated with EBV-B cells in the presence of either unstained TH or unstained Treg. The following cellular ratio was used 1:1:1 (TH Indo-1+ : APC : Treg or control TH). (A) APC were either unpulsed or pulsed with 100 ng/ml, 10 ng/ml, or 1 ng/ml superantigens. (Upper) Percentage of Indo-1-loaded TH cells in conjugate at the indicated time points. (Lower Left) Percentage of TH in conjugate that exhibit [Ca2+]i increase. (Lower Right) Mean fluorescence intensity of 405/530 emission ratio in TH cells at different time points after conjugate formation. (B) APC pulsed with either 100 ng/ml or 1 ng/ml superantigens were precultured with Treg or control TH for 2 h 30 min. Indo-1-loaded TH were conjugated at the ratio 1:1:1 (TH Indo-1+ : APC : Treg or control TH). (Upper) Percentage of Indo-1-loaded TH cells in conjugate. (Lower) Percentage of TH in conjugate that exhibit [Ca2+]i increase. Data are from three independent experiments performed by using cells from two different donors. Bars represent SD values. Statistical significance of differences between groups was evaluated by an unpaired Student's t test with GraphPad Prism software. *, P < 0.05.
Preincubation of Treg with APC for 2 h 30 min did not inhibit the number of TH/APC conjugates nor the fraction of conjugated TH cells undergoing calcium mobilization (Fig. 1B).
Taken together the above results indicate that Treg do not inhibit productive TCR engagement and signaling initiation in TH cells conjugated with APC.
Polarization of TH Secretory Machinery Is Impaired in the Presence of Treg.
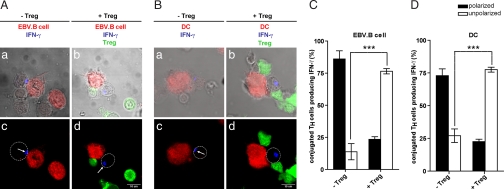
Because in our cellular system Treg did not block early signaling in TH/APC conjugates, we investigated whether they could interfere with additional steps of the TH/APC cognate interaction. A key function of TH cells is the rapid and selective polarization of secretory machinery toward APC (12, 14, 15, 19). To investigate whether Treg may affect this basic function of TH cells, we visualized intracellular IFN-γ in TH cells conjugated either with EBV-transformed-B (EBV-B) cells or with autologous mature dendritic cells (DC) pulsed with bacterial superantigens.
After 2 h 30 min of incubation (a time sufficient to activate TH for IFN-γ production) (20), cells were stained with anti-IFN-γ mAb. T cell/APC conjugates were visualized by using confocal microscopy.
Images depicting three-cell conjugates in which one APC simultaneously interacted with a TH and a Treg were registered. Conjugated TH cells were scored for IFN-γ accumulation beneath the cell–cell contact site (14).
In the absence of Treg a major fraction of TH conjugated with APC exhibited polarized IFN-γ toward the APC (both EBV-B cells and autologous DC) in agreement with reported data (12, 14, 15) (Fig. 2). Interestingly, in the presence of Treg a profound inhibition of IFN-γ polarization was observed in TH cells interacting with EBV-B cells as well as DC (Fig. 2). To further quantify this inhibitory effect we measured in individual TH/APC conjugates the distance between IFN-γ accumulation and the center of the immunological synapse (IS) using the Profile function of the Zeiss software. This analysis showed that the distance between IFN-γ intracellular accumulation, and the IS was significantly increased in the presence of Treg (SI Fig. 8 A and B).
Fig. 2.
Treg inhibit polarization of IFN-γ toward APC. (A and B) EBV-B cells (red, A) or mature autologous DC (red, B) were conjugated with either TH alone (unstained a, c) or together with Treg (green b, d) at 1:1:1 ratio. After 2-h 30-min incubation at 37°C, cells were stained for IFN-γ. (B) White arrows indicate IFN-γ accumulation; white circles mark T cell contour. (C and D) Quantification of TH IFN-γ polarization toward EBV-B cells (C) or toward DC (D) by visual inspection. In C, 72 three-cell conjugates plus Treg and 75 conjugates without Treg were scored. In D, 43 conjugates plus Treg and 46 conjugates without Treg were scored. The histograms represent the mean +/− SD values of three independent experiments using cells from three different donors. Statistical significance of difference between groups was evaluated by an unpaired Student's t test using the GraphPad Prism software. ***, P < 0.001.
To investigate whether Treg could affect IFN-γ production by TH during the 2-h 30-min coculture, we measured intracellular IFN-γ by FACS analysis. As shown in SI Fig. 9, 2-h 30-min coculture with Treg resulted only in a partial reduction of IFN-γ production in TH cells.
Taken together, these results highlight a mechanism used by Treg to interfere with the early steps of TH cell effector function. During the first few hours of cell–cell interaction, Treg reduce only IFN-γ production. Yet by affecting polarization toward APC, they avoid dedicated help delivery.
Treg Acquire Their Inhibitory Potential in a Time-Dependent Fashion.
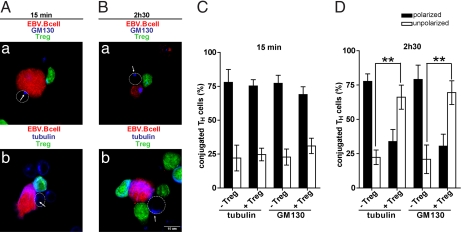
Activation of IFN-γ production requires a sustained signaling and cannot be detected after a few minutes of cell–cell conjugation (20). Yet TH cell polarization is a very rapid phenomenon accomplished within a few minutes after TH/APC encounter (14, 15). To determine whether Treg could affect swift polarization responses we investigated the effect of Treg on the polarization of additional markers of the secretory machinery such as tubulin cytoskeleton and Golgi apparatus that are known to relocate very rapidly toward the TH/APC contact site (14, 15).
TH/APC were cocultured for either 15 min or 2 h 30 min (either in the presence or absence of Treg), conjugates were stained with either anti-β tubulin mAb or a rabbit polyclonal Ab against the GM130 protein (to visualize Golgi apparatus). Images depicting three-cell conjugates in which one APC simultaneously interacted with a TH and a Treg were registered. Conjugated TH cells were scored for Microtubule Organization Center (MTOC) or Golgi apparatus polarization.
As shown in Fig. 3 and SI Fig. 8, in the absence of Treg, a large fraction of TH cells polarized tubulin cytoskeleton and Golgi apparatus toward superantigen pulsed EBV-B cells both at early and at late times after conjugation (Fig. 3 C and D and SI Fig. 8 C and D). This result further supports the notion that TH rapidly polarize toward APC and stay polarized for a sustained time (12, 14, 15). Interestingly, whereas 15 min after conjugation, polarization of the secretory machinery was unaffected in the presence of Treg (Fig. 3 A and C and SI Fig. 8C), at 2 h 30 min, polarization of both tubulin cytoskeleton and Golgi apparatus was impaired (Fig. 3 B and D and SI Fig. 8D).
Fig. 3.
Inhibition of secretory machinery polarization occurs in a time-dependent fashion. EBV-B cells were conjugated with TH for different times either in the presence or in the absence of Treg. (A and B) EBV-B cells (red) were conjugated with TH (unstained) in the presence of Treg (green) at 1:1:1 ratio. After 15-min incubation at 37°C (A) or 2-h 30-min incubation at 37°C (B), cells were stained for either GM130 (Aa and Ba) or tubulin (Ab and Bb). White arrows indicate localization of MTOC or GM130; white circles mark T cell contour. (C and D) Quantification of TH GM130 and tubulin polarization toward APC after 15 min incubation (C) or after 2-h 30-min incubation (D). In C, 66 three-cell conjugates plus Treg and 85 conjugates without Treg were scored for GM130; 60 three-cell conjugates plus Treg and 63 conjugates without Treg were scored for tubulin. In D, 82 three-cell conjugates plus Treg and 60 conjugates without Treg were scored for GM130; 88 three-cell conjugates plus Treg and 91 conjugates without Treg were scored for tubulin. The histograms represent the mean and SD values of three independent experiments using cells from three different donors. Statistical significance of differences between groups was evaluated by an unpaired Student's t test using the GraphPad Prism software. **, P < 0.01.
These results indicate that the inhibitory effect of Treg on TH polarization is observed only after a sustained interaction with APC. This delayed effect may be due to a time delay required for Treg activation. Alternatively, Treg may need to condition APC so that interaction of conditioned APC with TH results in a failure of effector cells to polarize.
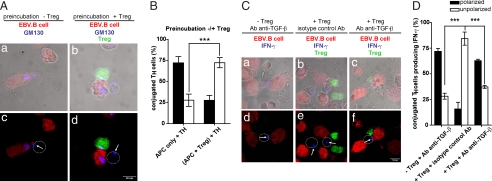
To address these questions, we first investigated whether APC could be conditioned during interaction with Treg by measuring polarization responses in TH interacting with chemically fixed APC either in the presence or in the absence of Treg. SI Fig. 10 A and B show that, in these conditions, Treg exhibited an inhibitory effect on TH polarization. These results suggest that in our cell system the APC mainly play the role of cellular platforms for TH/Treg encounter and are not implicated in mediating the observed Treg inhibition.
We next investigated whether preactivation of Treg resulted in a rapid inhibition of polarization responses in TH cells. To so, EBV-B cells were either cultured alone or cocultured with Treg for 2 h 30 min. TH cells were then added to the culture for an additional 15 min. In these conditions, a profound effect of Treg on the polarization of TH Golgi apparatus was observed in three-cell conjugates even though the cells were cocultured for only 15 min (Fig. 4 A and B and SI Fig. 8E). In TH/APC, conjugates without Treg a moderate inhibition of TH polarization was observed (SI Fig. 8E).
Fig. 4.
Inhibition of polarized IFN-γ secretion in TH cells is mediated by TGF-β production. (A) EBV-B cells (red) were either kept in culture alone (a, c) or conjugated with Treg (green, b, d) for 2 h 30 min. TH (unstained) were added for an additional 15 min at 1:1:1 ratio. Cells were stained for GM130. White arrows indicate GM130 localization; white circles mark T cell contour. (B) Quantification of TH GM130 polarization toward APC in the culture conditions described in A. A total of 48 three-cell conjugates (plus Treg) and 48 conjugates (without Treg) were scored. (C) EBV-B cells (red) were conjugated with either TH alone (unstained, a, d) or with TH plus Treg (green, b, c, e, f) at 1:1:1 ratio. After 2-h 30-min incubation at 37°C, cells were stained for IFN-γ. In a, d, c, and f, cells were treated with anti-TGF-β mAb at time 0 of conjugate formation. In b and e, cells were treated with an isotype control Ab. White arrows indicate IFN-γ accumulation; white circles mark T cell contour. (D) Quantification of TH IFN-γ polarization toward APC in the culture conditions described in C. Forty-four conjugates TH alone plus anti-TGF-β Ab, 43 conjugates TH plus Treg plus the isotype control Ab, and 41 conjugates TH plus Treg and plus anti-TGF-β Ab were scored. The histograms represent the mean and SD values of three independent experiments using cells from three different donors. Statistical significance of differences between groups was evaluated by an unpaired Student's t test using the GraphPad Prism software. ***, P < 0.001.
Together the above results suggest that preactivation of Treg may elicit the secretion of a soluble factor acting in a paracrine fashion.
TGF-β Mediates Treg-Induced Inhibition of TH Polarization.
It has been shown that Treg inhibit TH and CTL effector function via a mechanism that requires functional TGF-β signaling in effector cells (6, 7, 21). We investigated the possibility that TGF-β may mediate the observed inhibitory effect on TH polarization response.
We included in our experimental approach a blocking mAb against TGF-β. Conjugated TH cells were scored for IFN-γ polarization. As shown in Fig. 4 C and D and SI Fig. 8F, anti-TGF-β mAb alone did not affect the polarization of TH cells. Conversely, addition of the anti-TGF-β mAb to the cultures containing Treg markedly reverted the inhibitory effect exerted by Treg. In control experiments the anti-TGF-β used in this study inhibited TGF-β1 mediated induction of Foxp3 expression in human T cells (data not shown).
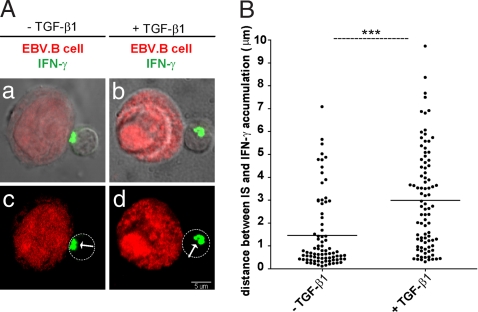
To investigate whether TGF-β could affect polarization of secretory machinery also in antigen-specific T cells, cloned TH1 cells were conjugated with MHC-matched EBV-B cells (pulsed with the antigenic peptide) in the presence of 20 ng/ml soluble TGF-β1. As shown in Fig. 5, treatment with soluble TGF-β1 resulted in a clear inhibition of T cell polarization toward cognate APC, thus further supporting the role of this cytokine in affecting dedicated help delivery.
Fig. 5.
Exogenous TGF-β1 inhibits polarization of antigen specific T cells. (A) Peptide-pulsed EBV-B cells (red) were conjugated with either 6396p5.1.2 cells in the absence (a, c) or presence of 20 ng/ml TGF-β (b, d). After 2-h 30-min incubation at 37°C, cells were stained for IFN-γ (green). (B) Distances of IFN-γ from the center of the T cell/APC contact site were measured by using the Profile function of the Zeiss software. Each dot corresponds to a TH/APC conjugate. Ninety conjugates plus TGF-β and 81 conjugates without TGF-β were scored. Statistical significance of differences between groups was evaluated by an unpaired Student's t test by using the GraphPad Prism software. ***, P < 0.001.
Taken together, the above results indicate that Treg inhibit polarization in TH cells via a TGF-β-dependent mechanism.
Discussion
Although the suppressive role of Treg in controlling immune responses has been thoroughly investigated, the molecular mechanisms used by Treg to control the activation of the different cells of the immune system are still elusive. In particular, how Treg may affect the biological function of effector T cells is still an unresolved question. In the present work, we provide a stepping stone to address this challenging question. Using Treg isolated from human peripheral blood and expanded in culture, we investigated whether and how these cells may affect TH cell polarization toward APC. Our results show that Treg inhibit polarization of TH cells toward APC via a TGF-β-dependent mechanism.
In a recent study Sumoza-Toledo et al. (22) investigated the effect of murine Treg on CD4+ T cells IS formation. They found that although synaptic PKCθ recruitment was altered in T cells in the presence of Treg, polarization of MTOC toward APC was unaffected (22). However, in this study, polarization of tubulin cytoskeleton was investigated only a short time after conjugate formation. Therefore, our results extend these previous observations.
We show that TH undergo conjugate formation and sustained signaling when interacting with the APC in the presence of Treg. This indicates that, in our system, Treg do not interfere with the early steps of TH activation, but they rather affect downstream pathways. Accordingly, it has been recently shown, using in vitro time-lapse video microscopy, that [Ca2+]i increase at the single-cell level in murine T cells conjugated with APC is not affected by Treg (23). Taken together, our results and those published results indicate that regulation does not directly affect TCR signaling.
Previous studies investigated the role of Treg in inhibiting the stop signal in vivo in naïve murine T cells. They showed that in the presence of Treg, the length of T cell/APC interactions was reduced (24, 25). Our present results are not in contrast with those previous findings. Indeed, in our experimental approach in vitro expanded human CD4+ T cells were conjugated by centrifugation with APC to measure the extent of calcium responses by FACS analysis. Therefore, our experimental approach did not address the question of whether Treg affect the length of the stop signal in migrating TH cells.
A key function of TH cells is their capacity to rapidly polarize their secretory machinery toward APC. This may allow TH cells to provide dedicated help to other actors of the immune response such as B lymphocytes or macrophages (11, 12, 26). Via this mechanism immune cells that display the strongest antigenic stimulus for T cells can be selectively activated, resulting in a progressive amelioration of specific immune responses (11, 14, 15).
Our results, by showing that Treg rapidly inhibit TH polarization, shed light on the biology of these cells. We show that, in addition to other previously described mechanisms used by Treg to inhibit adaptive immune responses (8), TGF-β1 inhibits polarization responses.
Our morphological quantifications of three-cell conjugates in which one APC was simultaneously in contact with one Treg and one TH show that, although the proximity between Treg and TH facilitates inhibition (SI Fig. 8E), a direct contact between Treg and regulated cells is not required (SI Fig. 11). This observation is in agreement with previously reported studies based on two-photon microscopy approaches in which it was shown that Treg do not need to enter in contact with TH to deliver their inhibitory signals (24, 25). In addition, the observation that anti-TGF-β blocking antibody reverts Treg inhibition of TH polarization and that soluble TGF-β1 mimics this Treg inhibitory effect contributes to support a model of Treg function in which Treg act (independently of cell–cell contact) via the secretion of soluble mediators.
Nevertheless, it should be noted that in our study [and also in the above mentioned previous studies (24, 25)] undetected short-lived contacts between Treg and regulated T cells could contribute to the observed inhibition.
Our observations are in apparent contrast with studies showing that in vitro suppression by Treg of T cell proliferation and cytokine production requires cell–cell contact and is normally independent of soluble factors (27). We also observed that the anti-TGF-β used in this study did not reverse suppression of TH proliferation and IFN-γ production in 72-h cultures (data not shown). Conversely and interestingly when the same expanded Treg population were used in short time cocultures in which TH polarization toward the APC was investigated, the Treg inhibition was TGF-β-dependent (Fig. 4 C and D).
We propose that our results identify an additional mechanism used by Treg to interfere with the effector function of TH cells. Although at late times Treg block TH cell proliferation and cytokine production, during the first few hours of cell–cell interaction they affect T cell polarization toward APC. TGF-β secretion appears to be necessary and sufficient to mediate this last inhibitory effect, whereas other Treg derived inhibitory mechanisms may be responsible of the observed inhibition of TH proliferation in vitro (28).
What could be the biological function of the observed inhibition of TH polarization toward APC? An initial answer to this challenging question comes from the observation that the addition of TGF-β1 to TH/APC conjugates inhibited TH induced increase of CD40 and ICAM-1 expression on the surface of APC, showing an inhibitory effect of TGF-β on APC function (SI Fig. 12). These results also suggest that, in the course of an immune response, our presently described mechanism may contribute to alter the stop signal in migrating T cells, in agreement with reported measurements of cellular dynamics in living tissues (24, 25).
It is tempting to speculate that by inhibiting polarization of TH cells toward APC Treg would limit TH mediated APC activation. This mechanism would not be relevant to control TH activation in vitro; on the contrary, it would affect in vivo the function of APC during further encounters with new T cells, thus contributing to the remarkably efficient control of immune responses exerted by Treg. A scheme presented in SI Fig. 13 summarizes our interpretation of the results.
A recent in vivo study demonstrated a central role for T cell-produced TGF-β1 in controlling differentiation of TH cells and induction of inflammatory diseases (29). Our in vitro observations are compatible with this recent study: we suggest that in the course of an in vivo response, the effect of TGF-β on TH polarization may be by instrumental in fine tuning APC activation to avoid an uncontrolled amplification of the immune response leading to immunopathological lesions.
In conclusion, our results unveil an unknown strategy used by Treg to interfere with the development and sustenance of adaptive immune responses.
Although the inhibitory effect of Treg on the activation of naïve T cells to become effectors has been thoroughly documented, much less is known about the mechanisms by which Treg may efficiently outcompete the TH-mediated amplification of immune responses. Here, we show that Treg can suppress this pivotal TH cell function. By impairing dedicated help delivery, Treg affect a crucial early step of the TH-dependent immune responses (SI Text).
Materials and Methods
T Cells and APC.
T cells were isolated from the whole blood of healthy donors (Centre de Transfusion Sanguine, Centre Hospitalier Universitaire Purpan, Toulouse, France). The CD4+ CD25+ enriched T cell fraction was sorted from PBMC by using magnetic separation (Miltenyi Biotec). CD4+ T cells were isolated by negative depletion followed by positive selection using anti-CD25 coated beads. Two cellular fractions were obtained, the CD4+ CD25− fraction and the CD4+ CD25+ fraction. Cell purity was assessed by FACS analysis (Facscan, Becton Dickinson) using PE-labeled anti-CD25 mAb (clone M-A251, BD PharMingen) and FITC-labeled anti-CD4 mAb (clone RPA-T4, BD PharMingen). CD4+ CD25+ fractions were routinely 91–98% pure. Cells were assessed for CD127 expression using PE-labeled anti-CD127 mAb (clone R34.34, Immunotech) and/or for Foxp3 using FITC-labeled anti-Foxp3 mAb (clone PCH101, eBioscience).
DC were derived from autologous PBMC. Adherent monocytes were cultured in RPMI medium 1640 10% FCS supplemented with IL-4 (1,000 units/ml) and GM-CSF (50 ng/ml, both from R&D Systems) (30). The percentage of CD11c+ cells in monocyte-derived DCs was checked by FACS after 15 days of culture and was routinely 90% pure. EBV-transformed B cells (LG2) were cultured as described (9).
T Cell Expansion.
Freshly isolated T cell populations were cultured and expanded in RPMI medium 1640, 5% human serum, IL-2 (150 units/ml) in the presence of anti-CD3/CD28 mAb-coated Dynabeads (Invitrogen). For CD4+ CD25+ T cells, IL-15 (5 ng/ml, from R&D Systems) was added to the culture medium. Bead: cell ratio was 2:1 for the CD4+ CD25+ T cells and 1:1 for the CD4+ CD25− T cells (31).
Suppression Assay.
CFSE-loaded (5 × 104) CD4+ CD25− cells per well were cultured in 96 U-bottom plates with CD4+ CD25+ T cells at different ratios. Cells were stimulated with CD3/CD28 mAb-coated beads (18). Cells were cocultured for 72 h, CD4+ CD25− cell proliferation was measured by FACS analysis.
Confocal Microscopy.
EBV-B cells or DC were pulsed with a mixture of bacterial superantigens (TSST-1, SEE, SEB, 100 ng/ml, Toxin Technology) for 1 h at 37°C. During the last 15 min, cells were loaded with 0.5 μM CMTMR-Orange (Molecular Probes). The superantigens not bound to APC were removed by washing. In some experiments APC loaded with CMTMR-orange were fixed with 1% paraformaldehyde before pulsing with superantigens. In parallel, CD4+ CD25+ Treg were loaded with 0.5 μM CMFDA-green (Molecular Probes) for 15 min at 37°C. Each T cell subset (105) and 2 × 105 APC were conjugated as described (14). At different time points after conjugation, cells were fixed and permeabilized either with 0.1% Triton X-100 (for GM130 staining) or with 0,1% saponin for (IFN-γ and tubulin staining) as described (14). The following primary antibodies were used: anti-GM130 (either rabbit polyclonal Ab, kind gift of A. De Matteis, Istituto Mario Negri Sud, Italy, or mouse mAb, clone 35, from BD PharMingen); anti-IFN-γ monoclonal Ab (clone B27, BD PharMingen); anti-β tubulin mAb (clone TUB 2.1, Sigma). Primary Ab were followed by either goat anti-mouse or goat-anti-rabbit polyclonal Ab labeled with Alexafluor 633 (Molecular Probes). In some experiments anti-TGF-β mAb (clone 2G7) (32) or isotype control Ab (BD PharMingen) were added to the culture medium. In some experiments, a DRB1*0101-restricted TH1 cell clone (6396p5.1.2) specific for the measles virus fusion protein peptide F254–268 and DR-matched EBV-B cells were used. Cells were cocultured either in the presence or in the absence of 20 ng/ml recombinant human TGF-β1 (from R&D Systems). The samples were mounted and examined using a Zeiss LSM 510 (Zeiss) confocal microscope with a 63 Plan-Apochromat objective (1.4 oil), electronic zoom 3, as described (14). Snapshots depicting overlapping of differential interference contrast images with green, red, and blue fluorescence are unprocessed images. In snapshots depicting green, red, and blue fluorescence only, background subtraction and color saturation was applied to the whole image using Adobe Photoshop software (Adobe).
Measurement of [Ca2+]i.
[Ca2+]i was measured in TH cells conjugated with APC, as described (9).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Denis Hudrisier and Iris Caramalho for discussion and critical reading of the manuscript and Isabelle Bernard for technical assistance. This work was supported by grants from la Ligue contre le Cancer “Equipe labellisée 2007.” M.E. was supported by a fellowship by the Association pour la Recherche contre le Cancer.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708350105/DC1.
References
- 1.Sakaguchi S. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 2.Zou W. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 3.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 4.Marie JC, Letterio JJ, Gavin M, Rudensky AY. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorelik L, Flavell RA. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 6.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Proc Natl Acad Sci USA. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Boehmer H. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 9.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldsmith MA, Weiss A. Science. 1988;240:1029–1031. doi: 10.1126/science.3259335. [DOI] [PubMed] [Google Scholar]
- 11.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O'Garra A, Cahalan MD, Cyster JG. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kupfer A, Singer SJ. Annu Rev Immunol. 1989;7:309–337. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- 13.Huppa JB, Gleimer M, Sumen C, Davis MM. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 14.Depoil D, Zaru R, Guiraud M, Chauveau A, Harriague J, Bismuth G, Utzny C, Muller S, Valitutti S. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Huse M, Klein LO, Girvin AT, Faraj JM, Li QJ, Kuhns MS, Davis MM. Immunity. 2007;27:76–88. doi: 10.1016/j.immuni.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, et al. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, et al. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Game DS, Hernandez-Fuentes MP, Lechler RI. Am J Transplant. 2005;5:454–464. doi: 10.1111/j.1600-6143.2005.00758.x. [DOI] [PubMed] [Google Scholar]
- 19.Valitutti S, Muller S, Dessing M, Lanzavecchia A. Eur J Immunol. 1996;26:2012–2016. doi: 10.1002/eji.1830260907. [DOI] [PubMed] [Google Scholar]
- 20.Faroudi M, Zaru R, Paulet P, Muller S, Valitutti S. J Immunol. 2003;171:1128–1132. doi: 10.4049/jimmunol.171.3.1128. [DOI] [PubMed] [Google Scholar]
- 21.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Sumoza-Toledo A, Eaton AD, Sarukhan A. J Immunol. 2006;176:5779–5787. doi: 10.4049/jimmunol.176.10.5779. [DOI] [PubMed] [Google Scholar]
- 23.Tang Q, Krummel MF. Curr Opin Immunol. 2006;18:496–502. doi: 10.1016/j.coi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Proc Natl Acad Sci USA. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyara M, Sakaguchi S. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Li MO, Wan YY, Flavell RA. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, Porter SB. Blood. 2004;104:453–461. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 32.Arteaga CL, Hurd SD, Winnier AR, Johnson MD, Fendly BM, Forbes JT. J Clin Invest. 1993;92:2569–2576. doi: 10.1172/JCI116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.