Abstract
The tumor suppressor PTEN is mutated or deleted in many tumors, causing the activation of the PI3K pathway. Here, we show that the loss of PTEN increases the transcriptional activity of hypoxia-inducible factor 1 (HIF-1) through the inactivation of Forkhead transcription factors (FOXO) in PTEN-null cells. Reintroduction of PTEN into the nucleus, overexpression of a nonphosphorylatable FOXO3a, which accumulates in the nucleus, or inhibition of nuclear export of FOXO3a by leptomycin B represses HIF-1 transcriptional activity in PTEN-null cells. HIF-1 transcriptional activity increases in PTEN-positive cells depleted of FOXO3a with siRNA. PTEN and FOXO3a regulate the transactivation domain of HIF-1α. Chromatin immunoprecipitation indicates that FOXO3a complexes with HIF-1α and p300 on the Glut-1 promoter, a HIF-1 target gene. Overexpression of p300 reverses FOXO3a-mediated repression of HIF-1 transcriptional activity. Coimmunoprecipitation and GAL4-HIF-1α transactivation assays reveal that FOXO3a interferes with p300-dependent HIF-1 transcriptional activity. Thus, FOXO3a negatively regulates HIF-1 transcriptional activity.
Keywords: PI3-kinase, VEGF, glioblastoma
The PTEN tumor suppressor first was identified as a gene mutated or deleted in multiple primary malignant tumors, including prostate cancers and glioblastomas (1–7). PTEN is a lipid phosphatase that functions as a tumor suppressor by antagonizing the PI3K/AKT-dependent signaling pathway (8–10). Loss of PTEN, in both murine embryonic stem cells or in human cancer cell lines, allows the accumulation of PIP3 and thus the activation of downstream effectors, one major factor being AKT (11–14). The downstream targets of AKT phosphorylation include the mammalian members of the Forkhead transcription factors (FOXO), which include FOXO1a, FOXO3a, and FOXO4 (also referred as FKHR, FKHRL1, and AFX, respectively). Phosphorylation of FOXO factors by AKT prevents their transcriptional activity by inducing the binding of 14-3-3 proteins, thereby promoting export to the cytoplasm (15–21). In PTEN-null cells, FOXO factors are constitutively phosphorylated and consequently predominantly cytoplasmic. The forcible localization of FOXO1a to the nucleus can reverse tumorigenicity of PTEN-null cells (22).
Many PTEN-null tumors are highly vascularized, including prostate cancers and glioblastomas. In particular, glioblastomas are one of the most vascularized tumors and display increased expression of VEGF (23). Hypoxia is a potent stimulus for triggering the “angiogenic switch.” The master transcription factor that regulates the cellular responses to hypoxia is hypoxia-inducible factor 1 (HIF-1) (24). HIF-1 is composed of two subunits, an oxygen-sensitive HIF-1α subunit, and a constitutively expressed HIF-1β subunit. HIF-1 activity depends on the availability of the HIF-1α subunit. Under normoxic (21% O2) conditions, HIF-1α is targeted for ubiquitin-mediated degradation by an E3 ubiquitin ligase complex that contains the von Hippel–Lindau tumor suppressor protein (pVHL), elongin B, elongin C, Cul2, and Rbx (25). This process depends on the hydroxylation of two proline residues by a family of prolyl hydroxylase (PHD) enzymes (26–28). Under hypoxic conditions, hydroxylation is presumed to be inhibited and HIF-1α is stabilized, thereby allowing HIF-1α to localize in the nucleus where it can dimerize with HIF-1β and recruit p300 and CBP, allowing the transcriptional activation of HIF-1 target genes. Previous data show that loss of PTEN can increase HIF-1 activity in glioma and prostate cancer cell lines (29–31). Therefore, exaggerated HIF-1 activity in PTEN-null cancers may explain the aggressiveness of these tumors. Together, these studies suggest a link between the loss of PTEN and the hypoxic activation of HIF-1. However, the mechanism by which loss of PTEN increases HIF activity is not fully understood. In this study, we investigated how the loss of PTEN increases HIF-1 transcriptional activity.
Results and Discussion
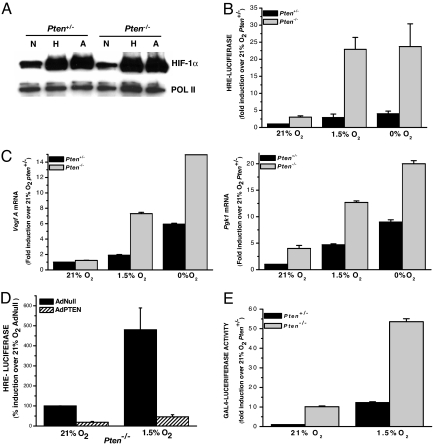
The loss of PTEN activity is associated with the induction of HIF-1 activity and an increase in the expression of HIF-1 inducible genes in prostate and glioblastoma cell lines (29–31). To determine whether loss of PTEN increases HIF-1α protein levels, Pten+/− and Pten−/− murine embryonic fibroblasts (MEFs) were exposed to normoxia (21% O2), hypoxia (1.5% O2), or anoxia (0% O2). The loss of PTEN had no effect on HIF-1α protein levels (Fig. 1A). However, the Pten−/− cells displayed a robust increase in HIF-1-dependent luciferase (HRE-Luciferase) induction compared with the Pten+/− cells under both hypoxic (1.5% O2) and anoxic (0% O2) conditions (Fig. 1B). HIF-1 transcriptional activity was further assessed by examining the induction of HIF-1 target genes, vascular endothelial growth factor (Vegf A), and phosphoglycerate kinase 1 (Pgk1) with real-time quantitative RT-PCR. Pten−/− cells exposed to hypoxia and anoxia displayed a dramatic increase in both Vegf A and Pgk1 levels compared with the Pten+/− cells (Fig. 1C). The transcriptional activity of HIF-1, as assessed by HRE-Luciferase, was significantly decreased by the reintroduction of PTEN using adenovirus (Fig. 1D). Immunoblot analysis demonstrates that the adenovirus-reconstituted cells express PTEN [supporting information (SI) Fig. 7].
Fig. 1.
Loss of PTEN increases transcriptional activity of HIF-1 but has no effect on HIF-1α protein levels. (A) HIF-1α protein levels were analyzed in Pten+/−, and Pten−/− cells were exposed to 21% O2 (N), 1.5% O2 (H), or 0% O2 for 2 h. (B) Pten+/− and Pten−/− cells were transfected with an HRE-Luciferase reporter gene construct and exposed to 21% O2, 1.5% O2, or 0% O2 for 16 h before being harvested. Relative luciferase expression is the ratio of luciferase/total protein levels normalized to Pten+/− cells. (C) Pten+/− and Pten−/− cells were cultured for 16 h under 21% O2, 1.5% O2, or 0% O2 and harvested, and transcription levels of the target genes Vegf A and Pgk1 were determined by quantitative real-time RT-PCR analysis. (D) Pten−/− cells were transfected with an HRE-Luciferase reporter gene construct. At 24 h after transfection, cells were infected with adenoviruses and subsequently exposed to 21% O2 or 1.5% O2 for 16 h. Relative luciferase expression is the ratio of luciferase/total protein levels normalized to cells expressing AdNull. (E) Pten+/− and Pten−/− cells were transfected with a GAL4 (amino acids 1–147) DNA-binding domain fused to HIF-1α (amino acids 531–826) construct and a reporter gene construct encoding five GAL4-binding sites. Cells were incubated at 21% O2 for 20 h, followed by 36 h at 21% O2, 1.5% O2, or 0% O2. Relative luciferase expression is the ratio of luciferase/total protein levels normalized to Pten+/− cells.
To test whether the increase in HIF-1 transcriptional activity, in the Pten−/− cells, is independent of the β-subunit, a specific GAL4 DNA binding domain (amino acids 1–147) fused to HIF-1α (amino acids 531–826) fusion construct was used. Transactivation by GAL4-HIF-1α (amino acids 531–826) increased by 5-fold in the Pten−/− cells compared with the normal transactivation seen in the Pten+/− cells (Fig. 1E). These results indicate that the increase in HIF-1 transcriptional activity caused by the loss of PTEN can be narrowed down to the transactivation domain (TAD) specifically within the HIF-1α subunit. Interestingly, the C-TAD region of HIF-1α is where p300 interacts with the α-subunit to fully activate the HIF-1 transcription factor. To confirm that the increase in transcriptional activity of HIF-1 was indeed attributable to the loss of PTEN, we reintroduced PTEN into the Pten−/− cells with an adenovirus. Collectively, these data demonstrate that the loss of PTEN increases HIF-1 transcriptional activity but has no effect on HIF-1α protein levels.
Recently, a growing body of work has implicated that the tumor suppressor PTEN is not only found in the cytoplasm but also localizes to the nucleus. Therefore, because the loss of PTEN had no effect on the protein levels of the α-subunit, and increased the transcriptional activity of HIF-1 via the TAD of the α-subunit and not the β-subunit, we examined whether nuclear PTEN regulates HIF-1 transcriptional activity. Both the wild-type (WT) PTEN and the phosphatase mutant PTEN (G129R) were expressed in the cytoplasm and the nucleus, whereas PTEN targeted to the nucleus (NLS-PTEN) was significantly enriched in the nucleus (SI Fig. 8). This result is consistent with previously reported data (32). As shown in Fig. 2, WT PTEN, and the NLS-PTEN-repressed HIF-1 activity, as measured by HRE-Luciferase. In contrast, the PTEN-G129R mutant had no effect on HIF-1 activity (Fig. 2). These data suggest a role for PTEN in the nucleus and that the phosphatase function of PTEN is required for the repression of HIF-1 transcriptional activity.
Fig. 2.
Nuclear PTEN represses HIF-1 transcriptional activity. Pten−/− cells were cotransfected with the HRE-Luciferase reporter gene construct and either pLNCX vector control, pLNCX-PTEN (WT), pLNCX-NLS-PTEN, or pLNCX-PTEN (G129R). At 24 h after transfection, cells were exposed to 21% O2 or 1.5% O2 for 16 h. Relative luciferase expression is the ratio of luciferase/total protein levels normalized to pLNCX vector control.
In PTEN-null cells, FOXO transcription factors are exported from the nucleus (15–18). The three known phosphorylation sites on FOXO3a can be mutated to alanine [FOXO3a (AAA)], and this mutant therefore cannot be phosphorylated and accumulates in the nucleus, allowing the constitutive activity of FOXO3a in PTEN-null cells (33, 34). Indeed, loss of PTEN decreased FOXO transcriptional activity (Fig. 3A). Cells expressing the constitutively active FOXO3a (AAA) mutant repressed HIF-1 transcriptional activity, whereas expression of the WT FOXO3a had no effect on HIF-1 (Fig. 3B). Furthermore, transactivation of HIF-1α was also severely impaired by nuclear FOXO3a compared with WT FOXO3a (Fig. 3C). The expression of FOXO3a (AAA) triple mutant was abundant in the nucleus of Pten−/− cells (see SI Fig. 9). Leptomycin B (LMB) inhibits the nuclear export of proteins that are escorted to the cytoplasm by the nuclear export receptor CRM1 (35). It has been shown that FOXO transcription factors are sequestered in the nucleus upon treatment with LMB (15–17, 36). To determine whether HIF-1 transcriptional activity could be inhibited by preventing endogenous FOXO3a from exiting the nucleus, PTEN-null cells were treated with LMB. LMB represses HIF-1 transcriptional activity, as assessed by the HRE-Luciferase (Fig. 3D). Together, these data indicate that either by overexpressing a nonphosphorylatable FOXO3a, which accumulates in the nucleus, or by inhibiting the nuclear export of FOXO3a by LMB, HIF-1 transcriptional activation can be prevented in PTEN-null cells.
Fig. 3.
Nuclear FOXO3a represses transcriptional activity. (A) Pten−/− and Pten+/− cells were cotransfected with the luciferase reporter construct containing a 6× repeat of the Daf16-binding elements (6×DBE-Luciferase) and the normalization construct (pTK-rLuc). At 24 h after transfection, cells were harvested and data were reported as the ratio of firefly/Renilla luciferase activity. (B) Pten−/− cells were cotransfected with the HRE-Luciferase reporter gene construct and the normalization construct (pTK-rLuc). At 24 h after transfection, cells were infected with null adenovirus or adenovirus expressing WT FOXO3a or the constitutively active FOXO3a (AAA) (100 pfu/μl) and subsequently exposed to 21% O2 or 1.5% O2 for 16 h. Firefly luciferase activity was normalized to Renilla luciferase activity and data reported as fold induction over 21% O2 AdNull. (C) Pten−/− cells were transfected with a GAL4 (amino acids 1–147) DNA-binding domain fused to HIF-1α (amino acids 531–826) construct and a reporter gene construct encoding five GAL4-binding sites. After transfection, cells were infected with adenoviruses. Relative luciferase expression is the ratio of luciferase/total protein levels normalized to AdNull infected cells. (D) Pten−/− cells were cotransfected with the HRE-Luciferase reporter gene construct and the normalization construct (pTK-rLuc) and subsequently exposed to 21% O2 or 1.5% O2 for 16 h ± 20 nM LMB. Firefly luciferase activity was normalized to Renilla luciferase activity, and data are reported as fold induction over untreated (−LMB) normoxic cells.
To directly investigate whether endogenous FOXO3a is in a complex with HIF-1 on HREs of HIF-1-dependent target genes, we performed chromatin immunoprecipitation (ChIP) on hypoxic Pten−/− cells treated with LMB to sequester FOXO3a in the nucleus. Glut-1 is a common HIF-1α target gene with a well characterized HRE. Therefore, an anti-HIF-1α, an anti-p300, and an anti-Foxo3a antibody were used to precipitate HRE-containing genomic DNA fragments from the Glut-1 promoter of hypoxic Pten−/− cells. The immunoprecipitated samples then were assessed by quantitative SYBR green-based real-time PCR and regular PCR. As expected, IP with the anti-HIF-1α antibody generated a strong signal under hypoxic conditions regardless of LMB treatment (Fig. 4A). IP with an anti-p300 also generated a signal under hypoxia regardless of LMB treatment, whereas IP with the FOXO3a antibody only generated a signal in the hypoxic cells that were treated with LMB (Fig. 4A). Both mock IgG antibodies that were used did not detect the Glut-1 promoter. These ChIP results indicate that HIF-1α, p300, and FOXO3a are all in a complex on the HRE of Glut-1 under hypoxic conditions in PTEN-null cells when FOXO3a is kept in the nucleus. During hypoxic conditions in PTEN-null cells, FOXO3a is not found on the HRE of Glut-1 because FOXO3a is continually being exported out of the nucleus and into the cytoplasm in PTEN-deficient cells. In contrast, FOXO3a is found on the Glut-1 promoter during hypoxia in Pten+/− cells (Fig. 4B). These data support the argument that under hypoxia, when FOXO3a is sequestered in the nucleus of PTEN-null cells, FOXO3a can complex with p300 and HIF-1α on the HRE to suppress HIF-1 target genes.
Fig. 4.
Endogenous FOXO3a complexes with HIF-1α and p300 on the HRE of Glut-1 promoter. (A) Chromatin fragments were immunoprecipitated with anti-HIF-1α, anti-p300, anti-FOXO3a, or control antibodies (mouse IgG for the anti-HIF-1α and rabbit IgG for both the anti-p300 and anti-FOXO3a) in cross-linked hypoxic Pten−/− cells ± 20 nM LMB. DNA from input and immunoprecipitated samples were detected by using PCR and run on a 2% agarose gel. Primers specific for the HRE of the common HIF-1α target gene Glut-1 were used. A gel representative of three independent experiments is shown. DNA from input and immunoprecipitated samples also were detected by using SYBR green real-time PCR. Primers specific for the HRE of the common HIF-1α target gene Glut-1 were used. The data presented are the result of triplicate analyses, and the error bars indicate SEM. (B) ChIP was performed in hypoxic Pten+/− cells as in A. DNA from input and immunoprecipitated samples also were detected by using SYBR green real-time PCR. Primers specific for the HRE of the common HIF-1α target gene Glut-1 were used. The data presented are the result of triplicate analyses, and the error bars indicate SEM.
PTEN is commonly mutated or lost in prostate cancers and in glioblastomas. LnCaP prostate carcinoma cells are PTEN-null (Fig. 5A). Reintroduction of PTEN in LnCaP cells had no effect on the protein levels of HIF-1α (Fig. 5B). In contrast, the presence of PTEN decreased HIF-1 transcriptional activity in the LnCaP cells, as measured by HRE-Luciferase (Fig. 5C). These results coincide with the above data in the Pten−/− MEFs. Next, to investigate whether nuclear PTEN could regulate HIF-1 activity, PTEN mutants were used, just as in Pten−/− MEFs. As shown in Fig. 5D, WT PTEN, as well as the NLS-PTEN, repressed HIF-1 activity. In contrast, the PTEN-G129R mutant actually increased HIF-1 activity compared with the vector control cells. WT PTEN, as well as NLS-PTEN, also decreased HIF-1 transcriptional activity in human malignant glioma cells, U251 cells (Fig. 5I). U251 cells also are PTEN-null cancer cells, and reintroduction of PTEN with an adenovirus had no effect on HIF-1α protein levels (Fig. 5 G and H). The expression of the triple mutant FOXO3a (AAA), which is predominately nuclear, and treatment of LMB, which prevents nuclear export of FOXO3a, significantly decreased HIF-1 transcriptional activity in the LnCaP cells (Fig. 5 E and F). Moreover, treatment of LMB also decreased HIF-1 transcriptional activity in the U251 cells (Fig. 5J). These data indicate that by inhibiting FOXO nuclear export in PTEN-null cancers cells, HIF-1 activity can be repressed.
Fig. 5.
Nuclear PTEN/FOXO pathway regulates HIF-1 transcriptional activity in PTEN-null cancer cells. (A) LnCaP cells infected with adenoviruses. Cell lysates were analyzed by immunoblotting with an anti-PTEN antibody. (B) HIF-1α protein levels in LnCaP cells infected with adenoviruses and subsequently exposed to 21% O2 (N) or 1.5% O2 (H) for 2 h. (C) LnCaP cells were transfected with an HRE-Luciferase reporter gene construct. At 24 h after transfection, cells were infected with adenoviruses and subsequently exposed to 21% O2 or 1.5% O2 for 16 h. Relative luciferase expression is the ratio of luciferase/total protein levels normalized to cells expressing AdNull. (D) LnCaP cells were cotransfected with the HRE-Luciferase reporter gene construct and pLNCX vector control, pLNCX-PTEN (WT), pLNCX-NLS-PTEN, or pLNCX-PTEN (G129R). At 24 h after transfection, cells were exposed to 21% O2 or 1.5% O2 for 16 h. Relative luciferase expression is the ratio of luciferase/total protein levels normalized to pLNCX vector control. (E) LnCaP cells were transfected with an HRE-Luciferase reporter gene construct. At 24 h after transfection, cells were infected with adenoviruses and subsequently exposed to 21% O2 or 1.5% O2 for 16 h. Relative luciferase expression is the ratio of luciferase/total protein levels normalized to cells expressing AdNull. (F) LnCaP cells were transfected with the HRE-Luciferase reporter gene construct and exposed to 21% O2 or 1.5% O2 for 16 h ± 20 nM LMB. Relative luciferase expression is the ratio of luciferase/total protein levels normalized to untreated normoxic cells. (G) U251 cells infected with adenoviruses. Cell lysates were analyzed by immunoblotting with an anti-PTEN antibody. (H) HIF-1α protein levels in U251 cells infected with adenoviruses and subsequently exposed to 21% O2 (N) or 1.5% O2 (H) for 2 h. (I) U251 cells were cotransfected with the HRE-Luciferase reporter gene construct and pLNCX vector control, pLNCX-PTEN (WT), pLNCX-NLS-PTEN, or pLNCX-PTEN (G129R). (J) U251 cells were transfected with the HRE-Luciferase reporter gene construct and exposed to 21% O2 or 1.5% O2 for 16 h ± 20 nM LMB.
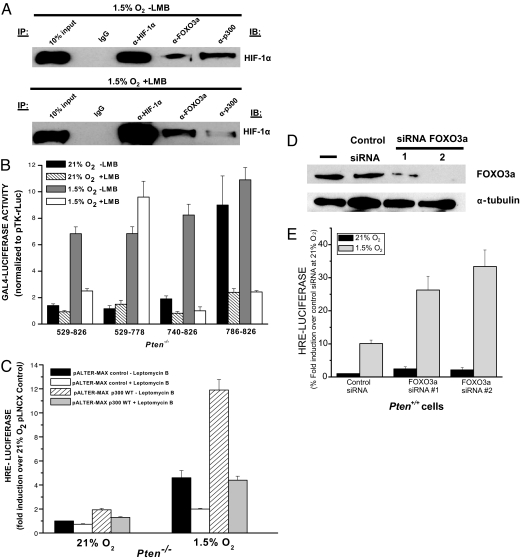
HIF-1 induction of gene expression depends on the coactivator p300. To determine whether FOXO3a interferes with the ability of p300 to function as a coactivator by forming a complex with p300 and HIF-1α, we coimmunoprecipitated nuclear extracts from hypoxic cells in the presence of LMB. FOXO3a coimmunoprecipitates with the HIF-1α protein complex in the presence of LMB (Fig. 6A). In contrast, proportionally less p300 coimmunoprecipitates with the HIF-1α protein complex compared with cells not treated with LMB (Fig. 6A). There is no detectable HIF-1α protein to immunoprecipitate from nuclear extracts from normoxic cells (SI Fig. 10). To further test the dependence of FOXO3a on p300–HIF-1α interaction, we performed GAL4-HIF-1α assays that contain different HIF-1α TADs. HIF-1α contains two oxygen-regulated TADs, which are termed N-TAD and C-TAD (37). The C-TAD is regulated by the hydroxylation of an asparagine residue by factor-inhibiting HIF-1 (FIH) to prevent the interaction of the HIF-1α protein with transcriptional coactivators such as p300. The N-TAD is not regulated by p300. LMB decreased transcriptional activity of GAL4-HIF-1α (amino acids 740–826), which contains C-TAD in the Pten−/− cells (Fig. 6B). This domain allows for p300 binding to the HIF-1α protein in a hypoxic-dependent manner. LMB also decreased transcriptional activity of GAL4-HIF-1α (amino acids 786–826), which contains part of the C-TAD that is not hypoxia-dependent but is still p300-dependent (Fig. 6B). By contrast, LMB did not decrease transcriptional activity of GAL4-HIF-1α (amino acids 529–778), which contains exclusively N-TAD. To determine whether overexpression of p300 in Pten−/− cells in the presence LMB would rescue HIF-1 transcriptional activity, cells were treated with LMB in the Pten−/− cells. Cotransfection of p300 increased HRE-Luciferase activity under both normoxia and hypoxia (Fig. 6C). Interestingly, the overexpression of p300 in the LMB-treated cells reversed the inhibition of HIF-1 transcriptional activity seen by the treatment of LMB in PTEN-null cells (Fig. 6C). We conclude that even though FOXO3a is sequestered in the nucleus by LMB treatment, HIF-1 activity can be rescued by increasing the amount of p300. Furthermore, decreasing endogenous levels of FOXO3a protein by siRNA increases HIF-1 transcriptional activity in PTEN-positive HEK-293 cells (Fig. 6 D and E). Collectively, our findings support the argument that FOXO3a is a negative regulator of HIF-1 transcriptional activity by interfering with the ability of p300 to serve a transcriptional coactivator.
Fig. 6.
FOXO3a impedes p300-dependent HIF-1 transcriptional activity. (A) U251 cells were exposed to 1.5% O2 ± 20 nM LMB for 16 h, and, subsequently, nuclear fractions were collected. IP was carried out on the U251 nuclear lysates by using anti-HIF-1α, anti-FOXO3a, anti-p300, or an IgG control antibody followed by immunoblotting for HIF-1α. (B) Pten−/− cells were transfected with a reporter gene construct encoding five GAL4-binding sites, the normalization construct (pTK-rLuc), and a GAL4 (amino acids 1–147) DNA-binding domain fused to HIF-1α (amino acids 529–826), (amino acids 531–778), (amino acids 740–826), or (amino acids 786–826) constructs. Cells were incubated at 21% O2 for 20 h, followed by 36 h at 21% O2, 1.5% O2, or 0% O2. Data reported as the ratio of firefly/Renilla luciferase activity. (C) Pten−/− cells were transfected with the HRE-Luciferase reporter gene, the normalization construct (pTK-rLuc), and either the pALTER-MAX vector control or pALTER-MAX p300 WT. At 24 h after transfection, cells were exposed to 21% O2 or 1.5% O2 for 16 h ± 20 nM LMB. Firefly luciferase activity was normalized to Renilla luciferase activity, and data are reported as fold induction over untreated (−LMB) normoxic cells. (D) FOXO3a protein levels in PTEN-positive HEK-293 cells transfected with control siRNA or two different FOXO3a siRNA (1 or 2). (E) PTEN-positive HEK-293 cells were transfected with control siRNA or two different FOXO3a siRNA (1 or 2) along with HRE-Firefly luciferase and TK-Renilla luciferase. Cells were exposed to 21% O2 or 1.5% O2 for 16 h. Data reported as the ratio of firefly/Renilla luciferase activity.
The physiological connotation of FOXO3a negatively regulating HIF-1 has important implications. HIF-1 is required for angiogenesis and the shift to glycolysis during hypoxia. In rapidly growing tumors where the cell proliferation exceeds blood supply, the activation of HIF-1 is important for adaptation. FOXO3a could inhibit The ability of HIF-1 to promote angiogenesis and the shift to glycolysis resulting in impairment of tumorigenesis. However, HIF-1 does have other functions, notably in regulating apoptosis as cells become anoxic. Hypoxia does not result in apoptosis. HIF-1 activation during hypoxia promotes tumorigenesis, but as cells approach anoxia, the function of HIF-1 may impair tumorigenesis by inducing apoptosis. There are multiple targets of HIF-1—including BNIP3, NOXA, and RTP801—that have been implicated in the induction of apoptosis (38–40). In this context, FOXO3a would promote survival of cells by inhibiting The ability of HIF-1 to induce apoptosis. Thus, depending on oxygen level, FOXO3a could have different outcomes with respect to HIF-1-dependent tumorigenesis. HIF-1 has been shown to both promote and inhibit tumorigenesis (41, 42). Similarly, we predict that FOXO3a may either promote or impair tumorigenesis by interfering with p300-dependent HIF-1 transcriptional activity.
Materials and Methods
Cell Culture and Reagents.
Pten+/− and Pten−/− MEFs (supplied by T.W.M.) and U251 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS (Gibco) and antibiotics. LnCaP cells were grown in RPMI medium 1640 supplemented with 10% FBS (Gibco), 1% sodium pyruvate, and antibiotics. All cells were maintained at 37°C in 5% CO2-humidified incubators. Hypoxic conditions (1.5% O2) were achieved in a humidified variable aerobic workstation (INVIVO O2; BioTrace). Anoxic conditions (0% O2) were achieved by using the Bugbox workstation (BioTrace). LMB was purchased from Sigma. The adenovirus expressing PTEN (AdPTEN) was purchased from the Vector Core Facility at the University of Pittsburgh. The adenoviruses, AdFOXO3a (WT) and AdFOXO3a (AAA) were purchased from Vector Biolabs. For siRNA studies, PTEN-positive HEK-293 cells were transfected by using Lipofectamine 2000 (Invitrogen) with a negative control (medium GC) and two different human FOXO3a. Stealth siRNA were from Invitrogen. Small inhibitory RNAs were used at a final concentration of 100 nM. Transfection efficiency was verified by using BLOCK-IT fluorescent oligo (Invitrogen).
Immunoblot Analysis.
HIF-1α protein was analyzed in nuclear extracts as previously in ref. 43. The anti-HIF-1α antibody (Cayman Chemical) was used and an anti-Pol II antibody (Santa Cruz Biotechnology) as the loading control. For total cell lysates, the following antibodies were used for immunoblotting: PTEN and an anti-α-tubulin antibody (Sigma-Aldrich) to control for loading. A representative blot is shown above of three independent experiments.
IP Analysis.
IP assays were performed by using the Nuclear Complex Co-IP kit (Active Motif). U251 cells were exposed to 1.5% O2 ± 20 nM LMB (Sigma) for 16 h, and nuclear extracts were prepared by using the kit's extraction reagents (Active Motif). Then, 100 μg of nuclear protein was used per IP reaction and incubated with 2 μg of HIF-1α antibody (BD Biosciences), 2 μg of FOXO3a antibody (Santa Cruz Biotechnology), 2 μg of p300 antibody (Santa Cruz Biotechnology), or 2 μg of IgG control antibody (Sigma). Fifty microliters of Protien A/G PLUS-Agarose (Santa Cruz Biotechnology) was added to each IP reaction. After the IP, 2× sample buffer was added to each IP reaction, samples were boiled and run on an SDS/PAGE gel. The samples then were subjected to immunoblotting as described above.
ChIP Analysis.
ChIP assays were performed with the EZ ChIP Assay Kit and protocol (Upstate). Pten−/− cells were grown at 1.5% O2 ± 20 nM LMB (Sigma), and Pten+/− cells were grown at 1.5% O2. A total of 4.5 × 107 cells were fixed in 1% formaldehyde at room temperature for 20 min. Isolated nuclei were lysed followed by chromatin shearing with the Enzymatic Shearing Kit (Active Motif). An anti-HIF-1α monoclonal antibody (Novus Biological), anti-FOXO3a polyclonal antibody (Santa Cruz Biotechnology), and anti-p300 polyclonal antibody (Santa Cruz Biotechnology) were used. A mouse IgG antibody (Upstate) and rabbit IgG antibody (Sigma) were used as controls. After reverse cross-linking and DNA purification, DNA from input (1:20 diluted) or immunoprecipitated samples were assayed with PCR, and products were separated by agarose gel electrophoresis. The following were the primers used to detect HRE-containing Glut-1: Glut-1, 5′-GGGCTGTGTTACTCACTCTTACTCC-3′ (forward); Glut-1, 5′-CTCTTCCTGGGTTGTGTTCAAGCTG-3′ (reverse). DNA from input and immunoprecipitated samples also was amplified by using the Bio-Rad iCycler iQ system (Bio-Rad Laboratories) and quantified by using the iQ SYBR Green SuperMix (Bio-Rad Laboratories). The following primers were used to detect HRE-containing Glut-1 by using quantitative SYBR-green real-time PCR: Glut-1, 5′-ATTTCTAAGGCCCTGGGTCC-3′ (forward); Glut-1, 5′-CCTGCCTGATGCGTGTCA-3′ (reverse). All cycle threshold (Ct) values were compared with the input amounts to normalize for variations. The data were analyzed by using the Pfaffl method (44). The results were graphed as fold changes relative to the control IgG antibodies.
Transfections and Reporter Assays.
Transfections were performed by using the Mirus TransIT Transfection reagent (Mirus Bio Corporation) according to the manufacturer's protocol. HRE-reporter assays and GAL4-reporter assays were performed as described in ref. 43. The HRE-Luciferase is a pGL2 vector containing three hypoxia response elements from the Pgk-1 gene upstream of firefly luciferase. TK-Renilla luciferase was cotransfected to control for transfection efficiency. The GAL4-HIF-1α (amino acids 531–826) fusion construct was obtained from Gregg Semenza (Johns Hopkins School of Medicine, Baltimore) and the GAL4-luciferase reporter construct was obtained from R. A. Maurer (Oregon Health and Science University, Portland, OR). The GAL4-HIF-1α (amino acids 529–826), (amino acids 531–778), (amino acids 740–826), and (amino acids 786–826) fusion constructs were obtained from Jaime Caro (Thomas Jefferson University, Philadelphia) and Nianli Sang (Thomas Jefferson University). The luciferase reporter construct containing a 6× repeat of the Daf16-binding elements (6×DBE-Luciferase) was used to assess FOXO transcriptional activity. The pALTER-MAX p300 WT construct was provided by Terry Unterman (University of Illinois College of Medicine, Chicago). The data presented are the result of triplicate analyses, and the error bars indicate SEM.
Real-Time RT-PCR Analysis.
Total RNA was isolated from cells exposed to various conditions by using the Aurum Mini Kit (Bio-Rad Laboratories). First-strand cDNA was synthesized from 1 μg of total RNA by using the RETROscript cDNA synthesis kit (Ambion, Inc.) with the random decamer primers. Prepared cDNA was amplified by using the Bio-Rad iCycler iQ system (Bio-Rad Laboratories) and analyzed by using the iQ SYBR Green SuperMix (Bio-Rad Laboratories). The following primer sequences were used: for Vegf A, 5′- GTACCCCGACGAGATAGAGT-3′ (forward) and 5′- ATGATCTGCATGGTGATGTTG-3′ (reverse); for Pgk1, 5′-TCTGTTCTTGAAGGATTGTGTGG-3′ (forward) and 5′-CTCTACATGAAAGCGGAGGTTT-3′ (reverse); and for L19, 5′-CATCAAGCGATCAGGGAATG-3′ (forward) and 5′-GAGGATTATACAGTTCAAAGCAAAT-3′ (reverse). Cycle threshold (Ct) values were normalized for amplification of the mitochondrial ribosomal protein L19, and the data were analyzed by using the Pfaffl method (44).
Immunofluorescence Microscopy.
For details, see SI Methods.
Supplementary Material
ACKNOWLEDGMENTS.
We are grateful to Dr. Jaime Caro and Dr. Nianli Sang for the GAL4 constructs and Dr. Terry Unterman for the p300 WT construct. This work is supported in part by National Institutes of Health Grants GM60472-08 and CA123067-01 (to N.S.C.). B.M.E. is supported by a fellowship from American Heart Association Grant 0610044Z.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706790105/DC1.
References
- 1.Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- 2.Li DM, Sun H. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- 3.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 4.Liu W, James CD, Frederick L, Alderete BE, Jenkins RB. Cancer Res. 1997;57:5254–5257. [PubMed] [Google Scholar]
- 5.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 6.Teng DH, Hu R, Lin H, Davis T, Iliev D, Frye C, Swedlund B, Hansen KL, Vinson VL, Gumpper KL, et al. Cancer Res. 1997;57:5221–5225. [PubMed] [Google Scholar]
- 7.Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D, Parsons R. Cancer Res. 1997;57:4183–4186. [PubMed] [Google Scholar]
- 8.Di Cristofano A, Pandolfi PP. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 9.Maehama T, Taylor GS, Dixon JE. Annu Rev Biochem. 2001;70:247–279. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- 10.Simpson L, Parsons R. Exp Cell Res. 2001;264:29–41. doi: 10.1006/excr.2000.5130. [DOI] [PubMed] [Google Scholar]
- 11.Franke TF, Kaplan DR, Cantley LC, Toker A. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 12.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. Proc Natl Acad Sci USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. Proc Natl Acad Sci USA. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biggs WH, III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Proc Natl Acad Sci USA. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brownawell AM, Kops GJ, Macara IG, Burgering BM. Mol Cell Biol. 2001;21:3534–3546. doi: 10.1128/MCB.21.10.3534-3546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 18.Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. J Biol Chem. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- 19.Takaishi H, Konishi H, Matsuzaki H, Ono Y, Shirai Y, Saito N, Kitamura T, Ogawa W, Kasuga M, Kikkawa U, Nishizuka Y. Proc Natl Acad Sci USA. 1999;96:11836–11841. doi: 10.1073/pnas.96.21.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang ED, Nunez G, Barr FG, Guan KL. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 21.Tomizawa M, Kumar A, Perrot V, Nakae J, Accili D, Rechler MM. J Biol Chem. 2000;275:7289–7295. doi: 10.1074/jbc.275.10.7289. [DOI] [PubMed] [Google Scholar]
- 22.Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- 23.Wesseling P, Ruiter DJ, Burger PC. J Neurooncol. 1997;32:253–265. doi: 10.1023/a:1005746320099. [DOI] [PubMed] [Google Scholar]
- 24.Semenza GL. Biochem Pharmacol. 2000;59:47–53. doi: 10.1016/s0006-2952(99)00292-0. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 26.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 27.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 28.Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 30.Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, et al. Genes Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- 32.Liu JL, Sheng X, Hortobagyi ZK, Mao Z, Gallick GE, Yung WK. Mol Cell Biol. 2005;25:6211–6224. doi: 10.1128/MCB.25.14.6211-6224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Proc Natl Acad Sci USA. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudo N, Wolff B, Sekimoto T, Schreiner EP, Yoneda Y, Yanagida M, Horinouchi S, Yoshida M. Exp Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- 36.Kau TR, Schroeder F, Ramaswamy S, Wojciechowski CL, Zhao JJ, Roberts TM, Clardy J, Sellers WR, Silver PA. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 37.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. J Biol Chem. 1997;272:19253–19260. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 38.Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, Moshel Y, Elbaz S, Budanov A, Chajut A, et al. Mol Cell Biol. 2002;22:2283–2293. doi: 10.1128/MCB.22.7.2283-2293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo K, Searfoss G, Krolikowski D, Pagnoni M, Franks C, Clark K, Yu KT, Jaye M, Ivashchenko Y. Cell Death Differ. 2001;8:367–376. doi: 10.1038/sj.cdd.4400810. [DOI] [PubMed] [Google Scholar]
- 40.Kim JY, Ahn HJ, Ryu JH, Suk K, Park JH. BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1α. J Exp Med. 2004;199:113–124. doi: 10.1084/jem.20030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan HE, Lo J, Johnson RS. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, et al. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 43.Emerling BM, Platanias LC, Black E, Nebreda AR, Davis RJ, Chandel NS. Mol Cell Biol. 2005;25:4853–4862. doi: 10.1128/MCB.25.12.4853-4862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfaffl MW. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.