Abstract
Summary: Retroviruses are an important group of pathogens that cause a variety of diseases in humans and animals. Four human retroviruses are currently known, including human immunodeficiency virus type 1, which causes AIDS, and human T-lymphotropic virus type 1, which causes cancer and inflammatory disease. For many years, there have been sporadic reports of additional human retroviral infections, particularly in cancer and other chronic diseases. Unfortunately, many of these putative viruses remain unproven and controversial, and some retrovirologists have dismissed them as merely “human rumor viruses.” Work in this field was last reviewed in depth in 1984, and since then, the molecular techniques available for identifying and characterizing retroviruses have improved enormously in sensitivity. The advent of PCR in particular has dramatically enhanced our ability to detect novel viral sequences in human tissues. However, DNA amplification techniques have also increased the potential for false-positive detection due to contamination. In addition, the presence of many families of human endogenous retroviruses (HERVs) within our DNA can obstruct attempts to identify and validate novel human retroviruses. Here, we aim to bring together the data on “novel” retroviral infections in humans by critically examining the evidence for those putative viruses that have been linked with disease and the likelihood that they represent genuine human infections. We provide a background to the field and a discussion of potential confounding factors along with some technical guidelines. In addition, some of the difficulties associated with obtaining formal proof of causation for common or ubiquitous agents such as HERVs are discussed.
INTRODUCTION
Retroviruses are a large and diverse group of human and animal pathogens that cause a wide variety of diseases including many cancers and various immunological and neurological conditions (102, 176, 522). In animals, retrovirus-associated diseases have been known for well over a century. For example, pulmonary adenocarcinoma in sheep was first documented in 1825 (495), and bovine leukosis was described in 1870 (238), while filterable agents were associated with infectious anemia of horses in 1904 (499) and with erythroid leukemia of chickens in 1908 (131). Although it was several decades before the causative agents were identified as retroviruses, studies on animal systems such as these have provided an enormous amount of information on retrovirus biology and revealed some fundamental aspects of the cellular mechanisms of disease, particularly in cancer. The recognition of retroviruses as tumor-inducing agents in animals initially led to their designation as “RNA tumor viruses” (522).
Given the preponderance of retroviruses in animals, much effort has been applied to find related viruses in human disease, and so far, four infectious human retroviruses have been identified (Table 1). The first to be discovered, human T-lymphotropic virus type 1 (HTLV-1), was isolated in 1980 (399) and has been shown to cause cancer (adult T-cell leukemia [ATL]) or neurological disease (HTLV-associated myelopathy/tropical spastic paraperesis [HAM/TSP]) in a small percentage of infections (32). Shortly thereafter, a related virus, HTLV-2, was reported in a patient with hairy cell leukemia (242), although its association with disease remains tentative (14, 423). Subsequently, human immunodeficiency viruses type 1 (HIV-1) and HIV-2, which both cause AIDS, were identified in 1983 and 1986, respectively (33, 101).
TABLE 1.
Confirmed infectious human retroviruses
| Virus | Yr identified | Associated disease(s)a | Reference |
|---|---|---|---|
| HTLV-1 | 1980 | ATL, HAM/TSP, polymyositis, HAA | 399 |
| HTLV-2 | 1982 | HAM/TSPb | 242 |
| HIV-1 | 1983 | AIDS | 33 |
| HIV-2 | 1986 | AIDS | 101 |
HAA, HTLV-associated arthropathy.
The association of HTLV-2 with disease is tentative.
In addition to these four viruses, retroviruses have frequently been cited as being potential etiological agents in other human diseases, and numerous putative retroviruses in human tissues and cultured cells have been reported (Table 2). Some of these claims were later disproved, but a number of others remain unconfirmed and controversial. In fact, there have been so many putative human retroviruses described that each new report has come to be received with skepticism among virologists and dismissed as merely another human “rumor” virus. Early work on this subject was comprehensively reviewed in 1984 (523), but the discovery of HIV and HTLV and the disastrous impact of AIDS on human health have significantly raised the profile of retroviruses as human pathogens, and new reports on “novel” human retroviruses have continued to appear.
TABLE 2.
Putative association of human diseases with retrovirusesa
| Disease | Reported evidence | Retrovirus(es) implicated | Reference(s) |
|---|---|---|---|
| Cancer | |||
| Breast cancer | EM, PCR, RT, FISH, Ab | MPMV, MMTV, HERV-K | 34, 132, 135, 137, 145, 287, 328, 514, 516, 517, 523, 545 |
| Lymphoma | PCR | MPMV, HRV-5 | 225, 260, 266, 350, 414 |
| Lung adenocarcinoma | Ag | Betaretrovirus | 115, 116 |
| Thymoma | EM | Unknown | 16 |
| Ovarian carcinoma | EM, PCR, Ab, Ag | HIV-like, HERVs | 405, 518 |
| Melanoma | EM, PCR, RT, Ab | HERV-K | 25, 50, 81, 82, 223, 354, 431 |
| Myeloproliferative disease | EM, PCR, RT | HERV-K | 66, 69, 339 |
| Testicular tumors | EM, PCR, RT, Ab | HERV-K | 58, 60, 71, 118, 156, 174, 257, 404, 430 |
| Prostate cancer | PCR, Ag, FISH | XMRV | 125, 497 |
| Neurological disease | |||
| Schizophrenia | PCR | HERV-W, HERV-K | 113, 246, 375, 521 |
| Motor neuron disease | PCR, RT | HERV-W | 11, 371 |
| MS | EM, PCR, RT, Ab, Ag | HERV-W, HERV-H | 13, 93-96, 98, 99, 194, 258, 385, 390, 391 |
| Chronic fatigue syndrome | EM, RT | “JHK-retrovirus” | 192 |
| Autoimmune and inflammatory | |||
| RA | EM, PCR | HRV-5, HERVs | 67, 188, 356, 469 |
| SLE | EM, PCR, Ab | HRV-5, HERVs, HIAPs | 67, 188, 212, 478, 480 |
| Mixed connective tissue disease | Nab | HIV-related? | 127 |
| SS | EM, PCR, RT, Ag | HIAPs, HRV-5 | 161, 189, 444, 477, 540 |
| PBC | EM, PCR, Ab, Ag | MMTV-like | 317, 318, 538 |
| Graves' disease | EM, PCR, Ab | HFV, HIAP | 100, 235, 270 |
| IDDM | PCR, RT | HERV-K | 105, 463, 472 |
| Psoriasis | EM, PCR, Ab, Ag | HERV-E | 43, 110, 214, 230, 335, 336 |
| Systemic sclerosis | Ab | HIAP-I | 273 |
| Alopecia areata | Ab | HIAP-I | 274 |
| Other | |||
| ICL | EM, Ab, RT | HIAP-II, HICRV | 162, 193, 196 |
| Osteopetrosis | EM | Unknown | 68, 267 |
Ab, antiretroviral antibodies; Nab, neutralizing antibodies; Ag, retroviral antigen.
Interest in retrovirus discovery has been stimulated in part by the advent of PCR and related gene amplification techniques, which have provided improved tools to search for retroviruses (and other pathogens) in human tissues. However, although the increased sensitivity of these methods has permitted the identification of several new candidate retroviruses, their confirmation as genuine human infections remains difficult. Here, we describe these recent developments and discuss some general themes related to the identification of novel human retroviruses. In addition, we highlight a number of technical issues that should be addressed by those still attracted by the notion of retrovirus hunting. While our focus is retroviruses, some of these issues also apply to putative links between other viruses and human disease. A notable example is the macaque papovavirus simian virus 40, which has been linked with a variety of human tumors, largely on the basis of PCR evidence (168, 442).
STRUCTURE AND REPLICATION OF RETROVIRUSES
Retroviruses are enveloped viruses with single-stranded, positive-sense RNA genomes (102, 176, 522) (Fig. 1). The defining features of retrovirus replication are reverse transcription of the viral RNA soon after the infection of a target cell and integration of the DNA product into the cellular chromosomal DNA. These two steps in replication are catalyzed by the virus-encoded enzymes reverse transcriptase (RT) and integrase (IN), respectively. Once integrated, the DNA provirus replicates by directing the synthesis of viral proteins and particles using cellular mechanisms for transcription and translation. Capsid assembly occurs either in the cytoplasm or at the plasma membrane, and new progeny virions are released by budding. The replication strategy of retroviruses is important for their role in disease because reverse transcription and integration confer unique pathogenic mechanisms including insertional mutagenesis and oncogene transduction (365). Previous classification schemes for retroviruses have been based on disease association or morphological features. Currently, seven genera are recognized and distinguished by the genetic relatedness of the RT protein (Table 3) (286).
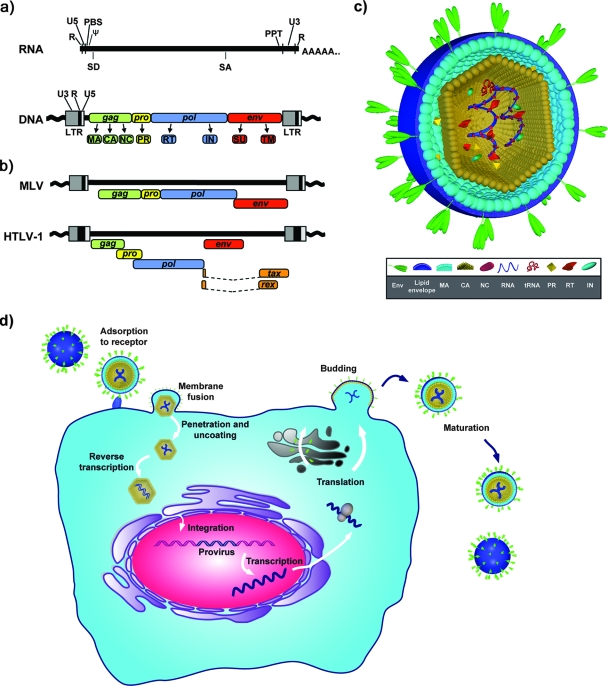
FIG. 1.
Retrovirus structure and replication. (a) Genome organization. The RNA and DNA forms of a generalized retrovirus genome are shown with conserved features. R, repeated region at termini of RNA genome; U5 and U3, unique elements close to the 5′ and 3′ ends, respectively, of the RNA genome; PBS, primer binding site used for initiation of reverse transcription; Ψ, encapsidation signal; PPT, polypurine tract. All infectious retroviruses have at least one splice donor (SD) and one splice acceptor (SA) site used for expression of a spliced transcript encoding env; some retroviruses have additional splice sites. During reverse transcription, the LTR is formed, which contains gene promoter and enhancer elements. At least four genes are present in all infectious retroviruses, gag, pro, pol, and env. Retroviral proteins are synthesized as large polyprotein precursors and later cleaved into the mature viral proteins matrix (MA), capsid (CA), nucleocapsid (NC), protease (PR), reverse transcriptase (RT), and integrase (IN) and into-the-surface (SU) and transmembrane (TM) glycoproteins. Specific retroviruses encode additional proteins with specialized functions in the viral life cycle or pathogenesis. (b) Comparison of proviral structures of MLV and HTLV-1 showing arrangement of ORFs for viral genes. (Panels a and b are adapted from reference 345 with permission from Elsevier.) (c) Structure of a generalized retrovirus particle indicating virus capsid containing two copies of the RNA genome associated with NC protein, viral enzymes, and a cellular tRNA molecule. The capsid is contained within the viral lipid envelope, which is associated with the envelope glycoproteins. (d) Replication. Retroviruses infect their target cells by adsorption to one or more specific cell surface receptors. Binding leads to conformational changes in the envelope and receptor molecules that trigger fusion of the viral and cell membranes. Depending on the specific virus, this may occur at the plasma membrane or within endosomes following endocytosis. Fusion releases the viral core into the cytoplasm (uncoating), and reverse transcription is initiated, during which the single-stranded RNA genome is converted into a double-stranded DNA form. This DNA subsequently becomes integrated into the chromosomal DNA of the cell to form the provirus. The expression of viral genes and proteins requires the host cellular machinery for transcription and translation, although some retroviruses also encode proteins that can regulate these processes. The cellular specificity of expression is dependent on enhancer elements located in the LTR. Assembly of retroviral capsids occurs either in the cytoplasm prior to budding (betaretroviruses and spumaviruses) or at the plasma membrane concomitant with budding (all other retroviral genera). Once released, the retroviral protease is activated, and the viral polyproteins become cleaved into their mature forms. This maturation step is required for infectivity.
TABLE 3.
Classification of retroviruses
| Subfamily and genusa | Previous nomenclature | Species infected | Example(s) |
|---|---|---|---|
| Orthoretrovirinae | |||
| Alpharetrovirus | Avian C-type oncoretrovirus | Birds | Avian leukosis viruses, Rous sarcoma virus |
| Betaretrovirus | B-type oncoretrovirus | Mice | MMTV |
| D-type oncoretrovirus | Primates | MPMV | |
| Sheep | JSRV | ||
| Gammaretrovirus | Mammalian C-type oncoretrovirus | Mice | MLVs |
| Cats | Feline leukemia viruses | ||
| Primates | Gibbon ape leukemia virus | ||
| Birds | Reticuloendotheliosis virus | ||
| Deltaretrovirus | C-type oncoretrovirus | Cattle | Bovine leukemia virus |
| Primates | Human T-lymphotropic viruses | ||
| Epsilonretrovirus | None | Fish | Walleye dermal sarcoma virus |
| Lentivirus | Lentivirus | Primates | HIV and SIV |
| Sheep | Maedi/visna virus | ||
| Cats | Feline leukemia virus | ||
| Horses | Equine infectious anemia virus | ||
| Spumaretrovirinae | |||
| Spumavirus | Foamy virus | Primates | HFV and SFV |
| Cats | Feline foamy virus | ||
| Cattle | Bovine foamy virus |
Refers to exogenous retroviruses only (286), but note that ERVs related to extant alpharetroviruses, betaretroviruses, gammaretroviruses, and spumaretroviruses are present in many vertebrate species.
HUMAN ENDOGENOUS RETROVIRUSES: CONFOUNDING FACTORS FOR HUMAN RETROVIRUS DISCOVERY
Occasionally, retroviruses infect germ cells, and this enables them to colonize the host's germ line DNA by forming endogenous retroviruses (ERVs) (29, 30, 57, 172). This has occurred numerous times during evolution, and ERVs have been detected in all vertebrate species examined. ERVs can be recognized by their sequence similarity with modern-day infectious retroviruses even though millions of years have passed since they integrated. In some host species, exogenous, infectious counterparts are still circulating as infectious viruses, e.g., jaagsiekte sheep retrovirus (JSRV) (377) and mouse mammary tumor virus (MMTV) (2).
In humans, estimates from the genome sequencing project suggest that ERVs now comprise some 8% of our DNA (285), representing around 4,000 proviruses and thousands more solitary long terminal repeats (LTRs) (29). Human ERVs (HERVs) have been divided into three classes, classes I, II, and III, based on their sequence similarity to animal gammaretroviruses, betaretroviruses, or spumaviruses, respectively. Each class contains several families, each representing an independent integration event (172). There is no standard nomenclature for HERVs, but one system refers to the tRNA specificity of the primer binding site used to initiate reverse transcription (Fig. 1). Thus, HERV-K would use lysine and HERV-H would use histidine if they were replicating viruses. There are no HERVs related to lentiviruses or deltaviruses. Although some very short regions of sequence similarity are present in human DNA (218, 383), they appear unlikely to represent genuine ancestral infections by these retroviruses (492). Note, however, that ERVs related to lentiviruses have recently been detected in rabbits (248).
In some animal species such as mice (468), chickens (222), and pigs (380), a few ERV proviruses have retained the ability to encode replication-competent viruses. Such viruses, while transmitted in germ line DNA as endogenous elements, are also capable of infectious transmission and can therefore behave as infectious viruses, infecting either the same species or other species. An example is the endogenous feline retrovirus RD114, which was identified after it infected human rhabdomyosarcoma cells that had been passaged through the brain of a fetal kitten and which was originally thought to be a human virus (322). Similarly, foreign tissue grafts in nude mice are commonly infected by endogenous murine viruses (5, 108, 416, 486). In contrast to these animal ERVs, none of the HERVs characterized so far are capable of producing infectious particles. In vitro transmission has been reported for some HERVs (e.g., see references 97 and 354), but further work is needed to clone the infectious genomes (discussed below).
HERVs and Disease
The majority of HERV proviruses contain mutations that prevent the expression of viral particles. Despite this, transcription of HERV RNA occurs in many normal human tissues and cultured cells (326, 395, 438, 464). In a few instances, viral proteins or particles may be produced (Table 4). The level and pattern of expression may be modulated under pathological conditions, particularly in cancer and inflammatory disease (293, 529). There has been considerable debate as to whether HERV expression has pathogenic consequences, and HERVs have been proposed to be etiological cofactors in numerous diseases on the basis of increased RNA and protein expression (30, 293, 498). However, as yet, there is no conclusive proof that HERVs have a causative role in any disease (discussed below).
TABLE 4.
HERV proteins expressed in tissues and cultured cellsa
| HERV family | Protein(s) expressed | Tissue | References |
|---|---|---|---|
| HERV-K | Gag-Pro-Pol | Testicular cancer, melanomas, myeloproliferative disease, breast cancer, placenta | 30, 46, 61, 82, 294, 339, 354, 430, 488 |
| Env | GCTs, melanoma, ovarian cancer | 120, 488, 518 | |
| Rec, Np9 | GCTs, melanoma | 17, 18, 58, 81, 118, 156, 305, 354, 541 | |
| SAg | EBV-infected B cells, IDDM(?) | 105, 219, 471, 472, 476 | |
| Particles | GCTs, melanoma, myeloproliferative disease | 46, 61, 82, 294, 339, 354 | |
| HERV-W | Gag | Brain, normal tissue, MS lesions, schizophrenia | 391, 426, 521 |
| Env (syncytin-1) | Brain, placenta, breast cancer | 13, 52, 55, 56, 153, 309, 310, 333, 391 | |
| Particlesb (MSRV, LM7) | MS patient B cells and leptomeningeal cells | 387, 390, 393 | |
| HERV-E | Env | Ovarian cancer, psoriasis, normal skin, interstitial lung disease | 43, 479, 518 |
| HERV-R | Env | Placenta, ovarian cancer | 502, 518 |
| HERV-H | Intact ORFs for Env proteins | Unknown but immunosuppressive in experimental systems | 118, 312 |
| Particlesb (RGH-2) | Transformed lymphocyte cultures from MS patients | 94, 194 | |
| HERV-FRD | Env (syncytin-2) | Placenta | 53, 54, 307, 308 |
| HRES-1c | Gag-related protein | Brain, liver, T-lymphoblastoid cell lines | 28, 383 |
In the context of novel retrovirus discovery, HERVs can be a problem for researchers hunting for retroviral nucleic acid or protein signatures in disease tissues because they produce a high level of background “noise” that can mask the signal from an infectious retrovirus or, alternatively, produce false-positive results. While this issue is now well established, it may not have been recognized in earlier reports of novel human retroviruses (523). Several of the recently described candidate human retroviruses are indistinguishable from HERVs (94, 105, 385), and much of the data implicating new retroviruses in disease could be a result of increased HERV expression under pathological conditions. However, given the apparent inability of any HERV to form replicating particles, new paradigms are required to explain how HERVs might contribute to disease (467). While a number of mechanisms have been suggested based on data from inbred mouse models and other transposable elements, there is only limited evidence that they are active for HERVs (Table 5). Indeed, an overtly pathogenic HERV would tend to be lost through negative selection (172). Nevertheless, this does not preclude the possibility that HERVs may comprise cofactors for disease in some circumstances, and recent work on specific proteins of HERV-K and HERV-W has provided new advances in this area (see below) (13, 58, 117, 156, 476).
TABLE 5.
Potential mechanisms for HERV-induced disease
| Mechanism | Agent | Reference(s) |
|---|---|---|
| Direct action of HERV protein | HERV-K Rec and Np9 | 18, 58, 117, 156 |
| HERV-K SAg | 219, 463, 471, 472, 476 | |
| HERV-W Env | 13, 389, 420 | |
| HERV-H Env | 312 | |
| Autoimmune/immune response | HERV-K | 24, 60, 208, 404, 430 |
| HIAP | 161, 235, 273, 274, 318, 477, 478 | |
| Insertional mutagenesisa | LINEs | 89, 334, 344 |
| Murine ERVs | 527, 536 | |
| Modulation of cellular | HERV-E | 271, 325, 435 |
| gene expression | HERV-L | 129 |
| Interaction with | HERV-K | 219, 471, 472 |
| exogenous viruses | HERV-W | 76, 77, 393 |
Insertional mutagenesis by HERVs has not been described, but analogous mutations have been reported for murine ERVs and for long interspersed nuclear elements (LINEs) in humans.
HUMAN DISEASES WITH SUSPECTED RETROVIRAL ETIOLOGY
The association of retroviruses with cancers and inflammatory disorders of animals naturally led to a search for related agents in analogous human diseases. A great deal of evidence has now accumulated to suggest that retroviruses other than HIV and HTLV may be human pathogens. However, much of this evidence is circumstantial, and we argue that a strong case has yet to be made for an etiological role for any specific candidate retrovirus. In a few instances, nucleotide sequences from putative retroviruses have been cloned from human tissues by PCR (94, 189, 246, 385, 514, 538), but these have so far all been proven to be closely related to ERVs of either human or animal origin.
Retroviruses in Human Cancer
As noted above, retroviruses were first discovered as tumor-inducing agents in animals (131, 424). Early studies in mice and chickens defined two general mechanisms by which retroviruses induce tumors; oncogene capture and insertional mutagenesis (102, 176, 365, 501). Oncogene capture involves the insertion of cellular proto-oncogene sequences into the retroviral genome by recombination during reverse transcription. The resulting virus is usually defective and requires coinfection with a helper (wild-type) virus for transmission. Oncogenesis is a result of the constitutive expression of the captured oncogene under the control of the viral LTR. Such viruses are known as acute-transforming viruses, reflecting the rapid growth of tumors in animals in which they arise. Insertional mutagenesis arises by the chance integration of retroviruses at a site in the host DNA adjacent to a cellular proto-oncogene. The promoter and enhancer elements in the viral LTR then activate the expression of the oncogene, thereby enhancing cellular proliferation, which may ultimately lead to the development of a tumor. Viruses that induce tumors in this way are replication competent and are known as cis-activating retroviruses. In this case, the tumors generally take much longer to develop than with acutely transforming viruses.
In addition to these two well-characterized oncogenic mechanisms, a small number of retroviruses that encode their own oncogenic protein or that directly stimulate cells through signaling motifs contained within the Env proteins have been described. Examples include the Tax protein of HTLV-1, which promotes cellular proliferation by activating the expression of a number of cellular genes (184), and the Env protein of JSRV, which activates signaling pathways including the MEK/extracellular signal-regulated kinase and Akt protein kinase cascades (288). Thus, retroviruses have a variety of strategies for inducing cellular proliferation and cancer, and novel human retroviruses might use any of these, or they might use entirely new strategies.
The discovery of HTLV-1 and its role in ATL confirmed that retroviruses can be oncogenic in humans. HIV-1 is indirectly implicated in cancer by creating an immunosuppressive environment that permits the growth of opportunistic tumors (65). In addition, studies on B- and T-cell lymphomas in AIDS patients have suggested that oncogene activation by insertional mutagenesis might be another mechanism by which HIV-1 could cause cancer (206, 448), although direct tumorigenesis by HIV in this way appears to be very rare.
While both HIV and HTLV can be oncogenic, neither is closely related to the large groups of well-characterized oncogenic gammaretroviruses and alpharetroviruses of animals. It has been suggested that human cancers could potentially be caused by the cross-species transmission of these retroviruses from animals (547). Such zoonotic viruses may not necessarily induce tumors in their natural host or replicate efficiently in human cells. Numerous studies in the 1960s and 1970s searched for retroviruses in human cancers (245, 523). In some cases, the viruses identified turned out to be cell culture contaminants of animal viruses, such as HL-23 virus, later found to be gibbon ape leukemia virus, and “HeLa” virus, which was actually Mason-Pfizer monkey virus (MPMV) (523). The provenance of some other viruses is still unexplained. More recently, PCR has been used to search for retrovirus sequences expressed in human tumors, and these have been found either to be HERVs (69, 82, 294, 440) or to be closely related to animal viruses (350, 414, 497, 514). HERVs have been implicated in a variety of tumors including melanoma (82, 354), germ cell tumors (30), breast cancer (516), and leukemia (66, 339), but it is unclear whether the increased expression of these elements precedes the cancer or whether it is a result of altered gene regulation in the tumor cells.
Retroviruses in Human Inflammatory Disease
As with studies of cancer, a search for retroviruses in human inflammatory diseases followed the description of related conditions during infection with some animal retroviruses. For example, murine models for glomerulonephritis, a feature of systemic lupus erythematosus (SLE), were previously thought to be triggered by immune complexes containing retroviral antigens (158). Similarly, small ruminant lentiviruses cause a chronic inflammatory disease that can involve several tissues including lung, brain, and joints (528). In addition, transgenic mice expressing human retroviral proteins such as HTLV-1 Tax and Env exhibit inflammatory symptoms reminiscent of human disease (185, 233). However, the tissues involved and the severity of the inflammation that occurs in these models depend to some degree on the exact structure of the transgene, so it is difficult to be certain how relevant such models are to human disease.
HTLV-1 and HIV-1 can both cause inflammatory symptoms, and this has reinforced the concept that other retroviruses might have a role in human inflammatory disease. A subgroup of individuals infected with HIV-1 develop a salivary gland inflammation similar to that seen in Sjögren's syndrome (SS), known as diffuse inflammatory lymphocytosis syndrome (229). Additional features of inflammatory disease such as autoantibody production, arthropathy, and vasculitis also occur in patients infected with HIV-1 (reviewed in references 163 and 249). Inflammatory reactions in HTLV-1 infection are even more striking, and while those of HAM/TSP are the most clinically overt, HTLV-1 is also associated with SS, arthropathy, uveitis, polymyositis, and myelitis in up to 5% of infections (367).
These clinical observations and animal models demonstrate that human retroviruses can cause inflammatory reactions and have led many workers to investigate other groups of patients for evidence of retrovirus expression (reviewed in references 103, 163, and 498). In addition, a number of models have been proposed to describe how retroviruses might trigger autoimmunity. These include general mechanisms such as lymphocyte activation and the upregulation of major histocompatibility complex (MHC) molecules and proinflammatory cytokines. However, the potential direct effects of retroviral proteins acting through molecular mimicry or as superantigens (SAgs) have received the greatest amount of attention (154, 163, 263, 498).
Molecular mimicry.
The concept of virus pathogenesis due to molecular, or antigenic, mimicry has been around for several decades and is characterized as an immune response to an infectious agent that cross-reacts with a host antigen (reviewed in reference 154). Despite the establishment of immune tolerance by the removal of self-reactive T lymphocytes during thymic maturation, it is clear that some self-reactive T cells persist in healthy individuals (243). Similarly, self-reactive B lymphocytes are also present. Molecular mimicry implies that these self-reactive T cells are activated in susceptible individuals following infection with a pathogen encoding a protein with a shared epitope. Subsequently, additional self-reactive lymphocytes might then be activated through epitope spreading, thereby exacerbating the autoimmune pathology (154). A number of diseases represent good candidates for initiation by molecular mimicry; however, while there is tantalizing evidence to support these examples, at present, they remain unproven (40, 154).
For retroviruses, the development of computer programs for comparing protein sequences led to a number of predicted epitopes shared between viral and host proteins, including some known autoantigens (160, 163, 320, 355, 378, 403). Epitopes identified in this way do not always prove to be biologically significant (154, 355), but for some retroviruses, the presence of cross-reacting antibodies has been established in a number of studies. For example, the CA (p30) proteins of mammalian gammaretroviruses such as feline leukemia virus and murine leukemia virus (MLV) share common epitopes with the U1 small nuclear ribonucleoprotein-associated 70K autoantigen (403) and DNA topoisomerase I (320), which are autoantigens in SLE and systemic sclerosis, respectively. Molecular mimicry between a C-terminal epitope of the HTLV-1 Tax protein and a neuron-specific ribonuclear antigen has also been demonstrated (283). Patients with HAM/TSP have antibodies that bind to this epitope on both proteins, as do monoclonal antibodies to the Tax peptide. Similarly, there is antigenic mimicry between the HIV-1 TM (gp41) protein and human leukocyte antigen class II molecules (178). Antibodies recognizing the cross-reactive epitopes are predicted to contribute to the functional impairment of CD4+ T lymphocytes in HIV patients (179).
These findings provide a basis for retrovirally induced autoimmunity through antigenic mimicry, although whether such mimicry extends to T-cell-mediated autoimmunity is currently unclear. Due to their similarity with exogenous retroviruses, HERV proteins have been cited as being potential autoantigens (103, 163, 498). While it is true that some individuals (with and without disease) do have T cells and antibodies that recognize HERV proteins (24, 60, 159, 208, 404), currently, no HERV protein is a recognized autoantigen in any disease.
It is worth mentioning that an alternative outcome of antigenic mimicry is that the cross-reactive epitope on a pathogen may be recognized as “self” by the immune system, effectively creating a “hole” in the immune response. An example is the HIV-1 TM (gp41) protein, some epitopes of which mimic sites on phospholipids such as cardiolipin and phosphatidylserine (201). Since these are ubiquitous host antigens, antibodies to these epitopes are rarely produced in HIV-1-infected individuals. Such mimicry may therefore be responsible for partially protecting these cross-reactive HIV epitopes from immune response, thereby impairing neutralization of the virus and contributing to pathogenesis.
SAgs.
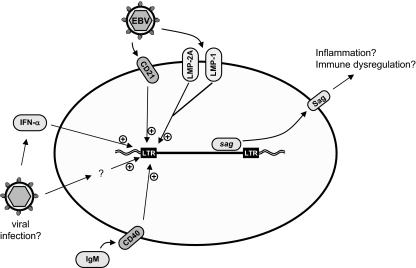
SAgs are a class of immune-stimulating proteins encoded by some bacteria and viruses that activate T lymphocytes in a non-antigen-restricted manner by interacting directly with the Vβ chain of the T-cell receptor (90, 315, 526). This results in massive polyclonal T-cell activation and cytokine release. The activation of T cells in the absence of specific antigen may lead to anergy, and so the longer-term consequences of SAg activity can be the peripheral depletion of a specific T-cell Vβ subset and/or local tissue proliferation of the same T-cell subset (2). This ability to dysregulate the immune system presents SAgs as potential mediators of inflammatory and autoimmune disease.
SAgs have been described in a number of bacteria, e.g., Streptococcus pyogenes and Staphylococcus aureus, where they cause diseases such as toxic shock syndrome and food poisoning (289). Several viruses have been proposed to encode SAgs, but the best-characterized example is the SAg of MMTV, which has a central function in virus dissemination in the early stages of infection (1, 2, 90). SAg activity has also been linked with HIV, rabies virus, the human herpesviruses Epstein-Barr virus (EBV), and human cytomegalovirus, but in these cases, the SAg genes and proteins have not been identified (534). In addition, the presence of a SAg-encoding retrovirus has sometimes been invoked to explain inappropriate T-cell activation in autoimmune diseases where there is evidence for the deletion of specific Vβ T-cell subsets (104, 163, 400). The controversial description of a SAg encoded by a specific HERV-K provirus (105, 463, 472) has provided a model by which various unrelated viruses might induce SAg activity on infection (discussed below). Interestingly, in the MMTV system, SAg activity and T-cell activation do not commonly elicit autoimmune inflammatory symptoms.
Epidemiology
Novel human viruses that have been discovered in recent years have been found in diseases where there was good epidemiological support for an infectious cause (250). For example, in the mid-1980s, it was obvious that an additional infectious agent was present in non-A, non-B hepatitis, and this justified the search that led to the discovery of hepatitis C virus (91). In contrast, many of the chronic immunological and neurological conditions that have been linked to retroviral infection do not have strong epidemiological evidence for a simple contagious etiology. Instead, these diseases are proposed to have a multifactorial etiology requiring the interaction of a number of genetic and nongenetic causative factors, with infection representing just one environmental component (e.g., see references 173, 181, and 200). Therefore, disease may be a rare outcome of infection in susceptible individuals. For some diseases, this model is supported by studies of monozygotic twins and patterns of disease incidence in migrant populations (265). Although infectious agents represent attractive targets for environmental factors, their role remains speculative in the absence of a clearer understanding of how all the contributory factors are linked. Interestingly, for a number of diseases where an infectious retrovirus has been sought, HERVs (i.e., genetic factors) have been identified (94, 105, 336, 384).
For diseases where there are no epidemiological data supporting a role for a virus, a “hit-and-run” mechanism in which acute infection with a specific virus elicits a chronic pathological response that persists after the original infection has been cleared might be proposed. Possible mechanisms include the activation of autoreactive T cells by antigenic mimicry (154) and the initiation of tumorigenesis that no longer requires the presence of the viral oncogene once the tumor is established (10). There is some experimental evidence supporting hit-and-run mechanisms, e.g., in adenovirus and herpesvirus transformation (364, 445) and in the development of chronic inflammatory disease following paramyxovirus infection (216); however, as yet, there are no confirmed examples in human disease. Subacute sclerosing panencephalitis in measles virus infection might perhaps be proposed as one example, although a defective form of the virus persists in the brain. Gastric adenocarcinoma induced by Helicobacter pylori is another possible example, but this too can persist. It is currently unclear whether hit-and-run mechanisms apply to retroviral infections. Because retroviruses integrate into the host DNA and therefore persist for the lifetime of that cell, the likelihood of them eliciting disease by a hit-and-run mechanism appears to be lower than that for other microbes.
LABORATORY METHODS FOR IDENTIFYING RETROVIRAL INFECTIONS
A wide range of techniques has been used to identify and characterize novel retroviruses. In addition to traditional methods such as cell culture, electron microscopy (EM), and serological assays, RT activity has been exploited as a biochemical marker of retroviral infection and is routinely used to quantify retrovirus production in vitro. The introduction of PCR provided new opportunities for the identification and analysis of virus infections. In addition, advances in bioinformatics and microarray technologies have provided further novel and potentially high-throughput approaches for new virus discovery. The relative merits and disadvantages of these techniques were reviewed elsewhere previously (303, 523). Here, we describe some aspects of particular relevance to novel retrovirus discovery and highlight problems that can arise due to the presence of ERVs.
Electron Microscopy
EM is of great value for directly visualizing candidate novel viruses in human tissues and cultured cells. Retroviruses have been grouped into four morphological types, denoted A-, B-, C-, and D-type particles (42, 111), and there have been numerous reports of retroviral infection in humans based on EM evidence (Fig. 2). Putative virions have been described in tissues or blood from patients with SS (161, 540), multiple sclerosis (MS) (194, 390), breast cancer (137), psoriasis (230), myeloproliferative diseases (66), malignant melanoma (25, 50), benign osteopetrosis and sporadic para-articular osteoma (267), and thymomas (16). Similarly, retrovirus-like particles (RVLP) have been found in body fluids from patients with rheumatoid arthritis (RA) (469), SLE (480), and epidemic neuropathy (418) and in cell cultures established from individuals with testicular tumors (46, 71) and chronic fatigue syndrome (192). In addition, RVLPs have also been observed in healthy human tissues and fluids including placenta (241, 504) and breast milk (138, 338) and in cultured cells (126, 328, 348). Although reported as being retroviruses, such particles do not always have the expected morphology or size of retroviruses; for example, particles described in RA (469) have an apparent diameter of 200 nm, which is roughly double that expected for retroviruses, and their identity as such is therefore doubtful.
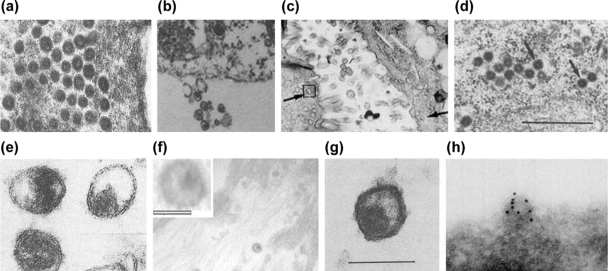
FIG. 2.
Retrovirus-like particles described in diseased human tissues and cultured cells. (a) LM7 particles from leptomeningeal cells from MS induced by ICP0 protein of herpes simplex virus type 1. (Reprinted from reference 393 with permission of the publisher.) (b) Particles in cultured lymphocytes from MS. (Reprinted from reference 194 with permission from Elsevier.) (c) Particles in SS salivary gland (see arrows). (Reprinted from reference 540 with permission of the publisher.) (d) HICRV in ICL. Bar, 0.5 μm. (Reprinted from reference 193 with permission.) (e) Virus-like particles in human milk. (Reprinted from reference 429 by permission from Macmillan Publishers Ltd.) (f) Particles in PBC. (Reprinted from reference 538 with permission of the publisher. Copyright 2003 National Academy of Sciences, U.S.A.) (g) Particles in myeloproliferative disease. Bar, 100 nm. (Reprinted from reference 66 with permission from Elsevier.) (h) HERV-K in teratocarcinoma-derived cell lines labeled with gold anti-HERV-K Gag. (Reprinted from reference 61 with permission from Elsevier.)
EM is subject to a number of artifacts, and it may sometimes be difficult to accurately interpret the appearance of extracellular particles. Virion morphology can be dependent on sample preparation methods, with the result that the interpretation of the images obtained can be rather subjective. Moreover, the likelihood of identifying rare particles is proportional to the length of time spent studying micrographs. Establishing the specificity of expression to diseased tissues therefore requires that identical attention is applied to both diseased and control specimens. In addition, several cellular components can resemble retroviral particles when seen in cross section; e.g., a cellular membrane protrusion of ∼100 nm diameter, if cut transversally, might appear as a 100-nm-diameter extracellular RVLP.
Another potential disadvantage of using EM for virus discovery is that this method cannot detect latent retroviral infection where few or no particles are being produced. Indeed, the technique is generally rather insensitive, and several hours of scrutinizing sections may be required to identify virions even for a moderately productive infection. Despite these issues, micrographs of sections taken directly through a retrovirus particle can be very convincing, particularly if they are stained with gold-labeled antibodies to the suspected virus. This approach has been used successfully to confirm the identity of HERV-K particles produced by teratocarcinoma cell lines (61). A similar approach may be successful in determining the identity of RVLPs in other human cell lines and tissues under either pathological or normal physiological conditions (339).
Detection of Virus Antigens
Several investigators have attempted to detect the presence of retroviral proteins in human tissues by immunohistochemistry using antibodies raised against other retroviruses. A number of studies in the 1970s found antigens related to mammalian betaretroviruses and gammaretroviruses in healthy human tissues, in some cancers, and in inflammatory diseases (reviewed in reference 523). More recent examples include an antigen that is reactive with a monoclonal antibody to the HTLV matrix protein (MA) (p19) in salivary gland tissue from a patient with SS (444) and an antigen in some human lung adenocarcinomas that is reactive with an antiserum to the CA protein of JSRV (115). It has been hypothesized on the basis of such data that a related antigen, and possibly a retrovirus, is present. While it is true that cross-reactive epitopes are present in Gag and (less commonly) Env proteins, the significance of such reactivity is unclear unless the antigen is purified or otherwise identified, and this has generally not been achieved. Therefore, while the detection of cross-reactive antigens with antiretroviral antibodies can provide support for data obtained by other means, this technique is not very informative when used alone. Recently developed proteomics technologies that facilitate the identification of peptides and proteins isolated from complex mixtures may permit the antigens detected in this way to be characterized more rapidly in the future.
Detection of Serum Antibodies to Retroviruses
The detection of serum antibodies to viruses is a well-established method for determining prior or current infection. Serological assays can be employed either in large cross-sectional studies to monitor the prevalence of infection in a population or in longitudinal studies to track the humoral immune response through the course of infection. For genuine human retroviral infections, this has been applied very successfully, and patients infected with HTLV or HIV generally produce strong antibody responses to Gag and Env proteins and to regulatory proteins such as Tat and Tax. Antibodies to Pol proteins are also common (269, 292). While many retrovirus infections of animals also elicit strong humoral responses, some do not (217, 374), and for a putative novel human retrovirus, it is therefore not possible to predict with certainty whether antibodies would be produced in infected individuals, especially if the virus is closely related to a HERV.
Several reports described antibodies to animal retroviruses in humans, e.g., to MMTV in breast cancer patients (530), to MLV in patients with psoriasis and in healthy individuals (335), and to bovine leukemia virus in blood donors (78) (also see reference 523). However, the epitopes responsible have been characterized in relatively few studies, and in some of the older reports, this seroreactivity turned out to be to carbohydrate antigens present on the viruses due to their production in nonhuman cell lines (31, 455). Antibodies to HERV proteins have also been described, particularly in patients with testicular tumors (60, 430) and melanoma (82), but such antibodies can also be present in healthy individuals (24, 208).
Antibodies reactive with HIV or HTLV Gag (CA, p24) are common in patients with autoimmune diseases such as MS, SS, and SLE (369, 407, 477, 478). In general, anti-Env antibodies are not present in these individuals, although sera from patients with mixed connective tissue disease are reported to contain neutralizing antibodies that block HIV infection (127). These groups of patients have no other markers that would indicate a genuine infection with HIV or HTLV, so it has been proposed that this seroreactivity reflects the expression of another, cross-reactive retrovirus, which might be exogenous or endogenous. Alternatively, such antibodies could be elicited by cross-reactive epitopes on nonretroviral host antigens such as ribonucleoprotein antigens, or they may simply represent low-affinity antibodies generated by nonspecific B-cell activation, a common feature of some autoimmune diseases.
Reactivity to a single retroviral protein provides only weak evidence of the presence of a retrovirus since this could be due to low-affinity “nonspecific” binding. However, reactivity to multiple viral proteins or to multiple epitopes on the same protein suggests that a viral antigen is actually driving antibody production and gives greater support to the case for infection. Additional characterization of antibody reactivity can also be persuasive in favor of infection. In the Borna virus system, human infection was cast into doubt by suggestions that positive detection was due to PCR contamination (461). Subsequently, epitope-mapping studies found low titers but high-avidity antibodies in patients with schizophrenia, and this raised the profile of human infections again (48). Approaches such as this might be useful if applied to putative retroviral infections. Similarly, longitudinal studies of antibody titer, if correlated with disease severity, can provide additional support for an association between virus replication and disease (257).
Although antiretroviral antibodies have been described by several groups, cell-mediated immune responses to retroviral antigens in autoimmune patients have been examined only very recently. This is surprising since inflammatory reactions in some of the diseases, notably RA, MS, and psoriasis, are thought to be largely T-cell driven. One report described T cells that are specific for HERV-K peptides in patients with seminoma and in healthy individuals (404), but it is not yet clear whether this response has any physiological significance in disease outcome.
RT Assays
Since its discovery in 1970, the demonstration of RT activity has often been used as a biochemical marker for retroviral infection (523). Initial assays monitored the synthesis of radiolabeled DNA using synthetic homopolymeric primer-template complexes, and it is possible that these assays did not adequately distinguish retroviral RT activity from that of cellular enzymes such as DNA-dependent DNA polymerases (298, 509) and terminal deoxynucleotidyl transferase (87). These early assay formats were also used to discriminate the RT activity of different groups of retroviruses; for example, gammaretroviruses preferentially use Mn2+ over Mg2+ as a cation, while alpharetroviruses and lentiviruses work more efficiently with Mg2+ (503). Modified versions of this assay, such as the “simultaneous detection” of viral 70S RNA, were devised to provide additional evidence that the detected activity was retroviral in origin (432), although this assay was not adopted as a universal standard. In the context of retrovirus discovery, RT assays have been important because the detection of genuine RT activity provides a basis for additional characterization of the putative agent; e.g., the detection of RT activity in T-cell cultures from patients with lymphadenopathy and ATL was a significant step in the discovery of HIV-1 (33) and of HTLV-1 (399).
In the PCR age, RT assays have been updated. The polymerase-enhanced RT (PERT) assay is an RT-PCR-based technique that detects the presence of RT activity with up to 106-fold-greater sensitivity than conventional assays (402, 450) and is reported to be capable of detecting a single particle per assay (450). False-positive results due to cellular DNA polymerase activity were initially an issue here also, but recent adaptations have largely overcome this problem (298, 509). PERT assays have been employed to detect RT activity in diseases such as SS (189), MS (99, 195), and motor neuron disease (11). Positive results could be taken as evidence of the presence of a retrovirus, but so far, none of these examples has been confirmed as a genuine infectious virus. In some studies (11, 99, 189, 328, 385), RT assays have been performed on virus preparations that have been purified on sucrose density gradients or by ultracentrifugation, procedures thought to remove cellular contaminants. However, such preparations may also contain high levels of cellular material (Fig. 3) (also see reference 338). Because there are several cellular sources of genuine RT activity, including HERVs, non-LTR retrotransposons (41, 121, 319), and telomerase (87), the detection of RT activity alone cannot be relied upon to formally prove the presence of a retrovirus. Conversely, the absence of such activity in a specimen does not necessarily mean that a retrovirus is absent because it may be present in latent form.
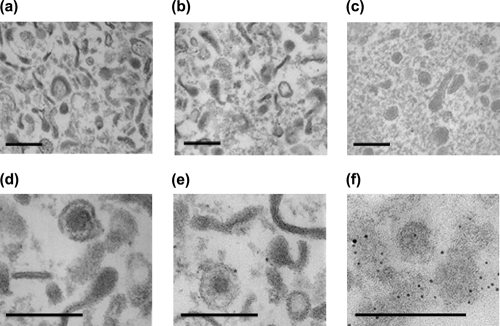
FIG. 3.
Electron micrographs of cell supernatants purified by sucrose density gradient centrifugation. Culture supernatants from EBV-transformed human B lymphocytes (a, b, d, and e) and HTLV-1-infected MT-2 cells (c and f) were concentrated by ultracentrifugation and then recentrifuged through a 10 to 60% sucrose density gradient. Fractions with a density typical of retroviruses (1.15 to 1.18 g/ml) were pooled, fixed in 2.5% paraformaldehyde-0.4% glutaraldehyde, and embedded in Epon resin. Ultrathin sections were stained with 1% uranyl acetate for 1 h and 1% lead citrate for 4 min and analyzed by transmission EM. Multiple vesicular particle-like structures can be observed. The origin of these structures is unclear, although they may be derived from cellular components such as polyribosomes, exosomes, and apoptotic blebs. Alternatively, because these cells were transformed with EBV, it is also possible that these structures are viral in origin (470). Panel f shows immunogold labeling of a Unicryl-embedded section with an anti-HTLV CA antiserum. Bar, 200 nm (C. Voisset, B. Mandrand, and G. Paranhos-Baccalà, unpublished data).
Virus Culture
The ability to grow a virus in cultured cells in vitro confirms that it is a genuine infectious replicating agent, and the rescue of infectious virus from disease tissue can provide convincing evidence of its existence as a real entity. On the other hand, there are several well-documented examples of human cell lines that have become contaminated with animal retroviruses. These viruses, such as HL26 virus, HeLa virus (see above), and ESP-1 virus (actually MLV), were some of the first contributions to the list of human RNA rumor viruses (reviewed in reference 523). Virus culture has been of enormous value for human retrovirology, as both HTLV-1 and HIV-1 were first identified in cultured lymphocytes from infected patients (33, 399). In contrast, some candidate human retroviruses have so far proved to be recalcitrant to culture, usually producing so little virus that it has been difficult to convincingly demonstrate transmission (97, 112, 161, 189, 388, 538). The lack of transmissibility in vitro could have numerous explanations but may suggest that the viruses are in fact defective particles encoded by HERVs, which could be activated by the conditions in the culture system, for example, in mixed cell cultures where cell lines are exposed to primary tissue biopsies that may express an undefined profile of cytokines. Indeed, several HERVs have been shown to be upregulated by proinflammatory cytokines (239, 311).
A lack of transmissibility in culture may also be due to the lack of a suitable cell substrate, and a judicious (or fortuitous) choice of host cell is therefore important. Failure of replication can occur for many reasons, e.g., the absence of an appropriate surface receptor, transcription factor, or other required cellular component (reviewed in reference 175). Alternatively, infection may be restricted by the presence of dominant cellular factors that interfere with viral replication. Recent work has led to the characterization of a number of these restriction factors in mammalian cells, which can exhibit cell type or species specificity or be selective against particular retrovirus strains (reviewed in reference 177). Such proteins include members of the APOBEC family, which target reverse transcription and result in highly mutated virus genomes, and TRIM (tripartite motif) proteins, which interfere with virus trafficking at an early postentry stage. Some restriction factors in sheep and mice are derived from ERVs (44, 349). Additional restriction factors are also present in mammalian cells (433) but require further characterization. A more detailed understanding of retroviral restriction factors and their interactions with other mechanisms of innate immunity to infection, such as interferon (IFN) response pathways, may lead to improved culture systems for those viruses that do not currently grow well in vitro.
Detection of Retroviral Sequences by PCR
PCR is a powerful technique for studying novel viral infections. The combination of sensitivity and specificity allows the detection of rare viral sequences amid a large excess of host nucleic acid, and the ability to sequence the amplified fragments has permitted numerous studies on molecular epidemiology and phylogeny. PCR can be used in the initial stages of identification of a retroviral genome and in later studies of molecular epidemiology to determine the degree of association with disease. A crucial advantage of PCR over the methods described above is that the amplicons can be sequenced, and the identity of the product can be unequivocally determined.
A variation of PCR for pathogen discovery is the use of consensus (degenerate) primers targeted at sequence motifs conserved between several microbes, which allows researchers to search for related sequences in diseases with a suspected infectious etiology (250). This general strategy has been used successfully to identify a number of novel microbes including bacteria, e.g., Tropheryma whippelii (409), and viruses, e.g., herpesviruses (413) and papillomaviruses (197). Several groups have described degenerate oligonucleotides that are applicable for identifying retroviral genomes (Table 6). Some of these primer sets target the highly conserved pol gene and can detect a broad range of retrovirus genera, but others are selective for a specific retroviral genus, allowing reduced degeneracy and higher sensitivity in the amplification reaction.
TABLE 6.
Degenerate PCR primers based on conserved motifs in retroviruses
| Protein(s) | Motif(s)a | Genus | Reference(s) |
|---|---|---|---|
| RT | LPQG, YXDD | All | 106, 124, 189, 214, 251, 301, 421, 440, 447 |
| RT | Several | Lentivirus | 122 |
| RT (nested primers) | Several | All | 284 |
| RT (heminested primers) | LPQG, YXDD | All | 246, 385, 493 |
| PR | DTG | Betaretrovirus | 189 |
| PR, RT | GRD, LPQG | Betaretrovirus | 324 |
| PR, RT | DTG, YXDD | All | 207, 380, 491 |
| PR, RT | DTGA, VLPQG, YMDD | All | 381 |
| IN (nested primers) | Several | Spumaretrovirus | 436 |
| NC, IN | Several | Betaretrovirus | 190 |
| Pol | Several | Deltaretrovirus | 128, 394 |
| Pol (nested primers) | Several | Lentivirus | 169 |
| Gag, Pol | Several | Spumavirus (of primates) | 47, 73 |
| Gag, Pol | Several | Non-HERV | 80 |
| Gag, Pol | Several | Betaretrovirus (of primates) | 282 |
Consensus peptide motifs used.
A trade-off for the increased sensitivity of PCR is a greater potential for sample contamination and false-positive amplification, and this has contributed to a number of controversial reports linking viruses and disease. For example, PCR has been used to link simian virus 40 with a number of human malignancies (168, 442). Similarly, Borna disease virus, which naturally infects horses and sheep, has been implicated in schizophrenia on the basis of PCR and serological evidence (461), and the human herpesvirus EBV has been proposed to have a role in breast cancer (15, 140, 313). For these viruses, additional evidence is still required to provide a solid case for a causal relationship with these diseases.
In comparison with other virus families, the identification of a new human retrovirus by PCR presents particular difficulties due to the high number of HERV sequences in the human genome. Where genuinely new retroviral sequences are obtained, care must be taken that they do not result from ERVs, either human (385) or nonhuman (190). This is particularly true for degenerate primer PCR because HERVs share the same conserved motifs as exogenous retroviruses. Therefore, while this has been a powerful technique for the characterization of specific ERV families in several species (316, 380, 447), putative exogenous retroviral sequences cloned in this way from diseases such as MS, diabetes, and SS have later also been found to be endogenous retroviral sequences (105, 189, 385). To address this issue, primer sets have been devised for betaretroviruses, gammaretrovirus, and deltaretroviruses that are specifically designed to exclude the amplification of HERVs (80). The use of these primers in attempts to identify novel retroviruses in human lymphoma has so far been unsuccessful (80), but their use in other disease contexts may yet be fruitful. However, should an exogenous retrovirus closely related to a HERV be circulating in human populations, it would of course be excluded by these primers.
One refinement to the degenerate PCR strategy has been to purify and concentrate retrovirus virions prior to degenerate RT-PCR (66, 96, 105, 189, 246, 379, 385, 452). Purification may be achieved by centrifuging the homogenized sample through a density gradient such that retroviruses migrate to their typical buoyant density of between 1.16 and 1.18 g/ml. These gradients are traditionally prepared with sucrose, but other compounds such as iodixanol can also be employed (337). The purpose of the gradient procedure is to physically separate encapsidated viral RNA (which migrates through the gradient) from unpackaged soluble RNA (typically defective HERV RNA), which is not expected to enter the gradient to an appreciable extent. Similar purifications have been employed prior to the detection of RT activity using the PERT assay (11, 99, 189, 328). Nuclease treatment can also be used to degrade extraneous cellular DNA and RNA prior to amplification (164, 440), because in theory, encapsidated RNA genomes should be protected.
The purification of retroviruses on density gradients is thought to remove cellular contaminants, and it has generally been accepted that any sequence amplified from a gradient was packaged inside purified virions. However, it is unclear whether this is a valid assumption because material migrating at 1.16 to 1.18 g/ml might also include polyribosomes, microsomal vesicles, or other cellular components, which can migrate through the gradient (Fig. 3). Large complexes such as these could potentially protect cellular nucleic acids (including HERV sequences) from nuclease digestion. Thus, to confirm specificity, density gradients of control tissues and cultures must be analyzed in parallel. The choice of control tissue may also be important in this respect. Cell death in disease lesions or cultured cells can result in the release of large quantities of free DNA and RNA into the extracellular environment, providing a template for amplification by degenerate primers. Therefore, to determine disease specificity, the ideal control tissues for such experiments would have a similar level of cell death and not simply be “normal” tissues.
Bioinformatics and Genomics in Retrovirus Discovery
Computerized analysis of virus sequence data has long been an essential tool in the characterization of viruses (428). However, the availability of whole-genome sequence databases now means that bioinformatics methods can be used directly in the identification of new retroviral elements without any laboratory experimentation. For example, a novel family of endogenous gammaretrovirus sequences was identified by sequence analysis of murine genomic and expressed-sequence-tag databases (70). This was achieved entirely in silico with the BLASTN program using MLV as a query sequence. Other groups have devised algorithms for interrogating sequence databases by searching for sequence motifs that are conserved among retroviral elements (236, 323, 505), and these may be useful in identifying novel human retroviruses in sequence data.
The availability of the complete human genome sequence is also helping to better define the repertoire of HERVs. Turner and coworkers described two HERV-K proviruses, HERV-K113 and HERV-K115, which are present in around 30% and 15% of humans, respectively, and are therefore insertionally polymorphic alleles (494). The HERV-K113 provirus is of particular interest since it contains complete open reading frames (ORFs) for the viral proteins and may therefore be replication competent (although the Env protein appears to be nonfusogenic in vitro) (119). Whether the presence of either provirus is associated with any specific disease can be assessed by PCR of the integration sites on patient DNA, since the flanking sequences are known (79, 346, 375). In addition, since both proviruses were first identified in the genome of a single individual, it appears likely that additional polymorphic HERV-K proviruses exist in humans, and this is being confirmed as the genomes of more individuals are analyzed (38, 221, 300). Because polymorphic HERVs are more likely to have recently integrated, these are perhaps the most likely HERV proviruses still to be biologically active and pathogenic.
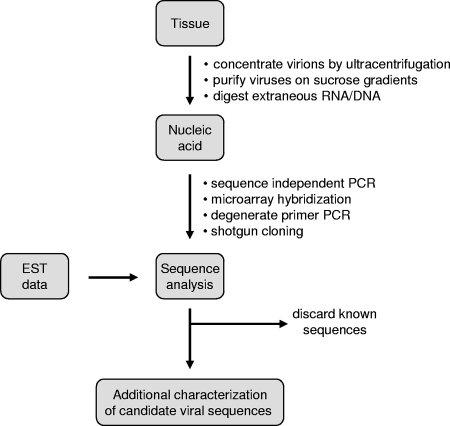
The approaches described above are useful for characterizing ERVs, but they are not generally applicable for detecting infectious exogenous viruses present in only a small population of somatic cells. However, the human genome sequence has also provided new strategies for the discovery of infectious viruses by facilitating the rapid identification of sequences obtained from large-scale “shotgun cloning” experiments. Weber and colleagues have described a general method for identifying novel virus genomes in which sequences obtained from randomly sequenced cDNA libraries are searched using the BLAST algorithm against sequence databases to filter out all host sequences, known infectious agents, and artifactual sequences (519). The few sequences remaining after this “computational subtraction” can be reasonably assumed to represent novel candidate infectious agents and further validated for their function in disease using laboratory methods. So far, this technique has been successfully applied only in control experiments, and a limitation is that it is heavily dependent on sequencing, requiring upwards of 10,000 clones to be sequenced to have a reasonable chance of detecting a rare cDNA (539). However, related approaches that include an enrichment step in which putative viruses are first concentrated by ultracentrifugation and then treated with nucleases to deplete the pellets of extraneous nonviral nucleic acid have been described. Sequence-independent PCR (“random” PCR) is then applied to amplify any remaining DNA and RNA, which is characterized by shotgun cloning, sequencing, and database comparison. This strategy has been used to identify novel human parvoviruses, coronaviruses, and polyomaviruses in human tissue samples (8, 9, 167, 240, 500). Figure 4 summarizes the various approaches that might be taken. Application of these techniques to other diseases could deliver further new viruses, possibly including retroviruses, although an exogenous retrovirus with high similarity to HERVs might be excluded by the screening procedure.
FIG. 4.
Identification of novel viral sequences. A generalized scheme summarizing the approaches taken by a number of groups is shown (e.g., see references 9, 240, 500, 519, and 539). Sequence data can be generated experimentally or collected directly from expression sequence databases. Bullet points indicate alternative procedures at each stage. EST, expressed sequence tag.
Future approaches to the identification of novel viruses are likely to be based on DNA array technology. Seifarth and colleagues described a method where 90-mer oligonucleotides representing a variable region of the RT gene from all known exogenous and endogenous retroviruses were blotted onto a nylon membrane (439). These blots were then used to probe RNA or RT-PCR products amplified from cell lines or tissues to identify which classes of retroviral sequences are being expressed. More recently, this retrovirus array has been used in a microarray format to catalog HERV transcription in healthy tissues (438), in breast cancer (151), and in schizophrenia and bipolar disorders (150). Microarrays have also been employed on a larger scale using oligonucleotides from all known infectious virus families (Virochip) (511, 512). In those studies, a remarkable innovation has been the recovery of hybridized nucleic acid from the microarrays by a micromanipulator, enabling cloning and further characterization (512) (Fig. 5). This method offers great potential for the future discovery of novel viruses and has already been used to detect a virus related to MLV in human prostate cancer (discussed in detail below) (497). Additional viruses are likely to be identified in this way, but as with PCR, the challenge will be to prove a role in disease.
FIG. 5.
Scheme for recovery of viral nucleic acid from microarray spots. Hybridized viral sequences were physically scraped from a DNA microarray spot using a tungsten wire probe mounted on a micromanipulator, while the spots were visualized under fluorescence microscopy. Subsequently, the virus was identified by nucleic acid amplification, cloning, and sequencing. (Reprinted from reference 512 with permission.)
SPECIFIC CANDIDATE HUMAN RETROVIRUSES
As we have described above, there is a wealth of circumstantial evidence implicating retroviruses other than HIV and HTLV in human disease. Although in some instances, specific agents have not been identified, in recent years, several putative retroviruses have emerged as candidate human pathogens. In this section, we discuss these specific examples in some detail, including those that have been characterized by PCR and gene sequencing.
Human Mammary Tumor Virus
The suggestion that a retrovirus related to MMTV might be involved in breast cancer in humans is one of the longest-running controversies in human retrovirology (313, 523). MMTV is a betaretrovirus that causes mammary tumors in mice by the insertional activation of proto-oncogenes (1, 84). The virus is transmitted in breast milk and infects dendritic cells and B lymphocytes in the gut of the suckling pup. MMTV encodes a SAg in its 3′ LTR, which, when presented to cognate T lymphocytes in the gut lymphoid tissue, triggers their activation (90). The specific subset of T cells activated depends on the particular strain of MMTV, and the resulting T-cell response leads to the activation of the infected B cell, which proliferates sufficiently that the virus is carried to the mammary gland, where it infects the mammary epithelium. SAg-dependent activation of T cells is essential for infection of the mammary gland since replication in B cells is otherwise inefficient, and transmission to mammary tissue is unsuccessful (202).
Once the mammary epithelium is infected, estrogen-driven activation of the MMTV LTR mediates the mammary-specific activation of the virus and its accumulation in milk. Mammary tumors are induced by the insertional activation of cellular proto-oncogenes (84). The genes activated by MMTV were originally designated as int loci and later identified as members of the Wnt, notch, and fibroblast growth factor (fgf) families. In addition to its role in mammary tumors, a variant of MMTV with a rearranged U3 sequence in the viral LTR is associated with T-cell lymphomas (26). A functional SAg gene is not required for lymphoma production (353).
A link between MMTV and breast cancer in humans was first proposed following the detection of B-type virus particles in healthy human milk by EM (137, 138, 338). Similar particles were also detected in a number of cell lines derived from breast tumors (20, 155, 252, 459) and directly in tumor tissue biopsies (137). Subsequently, several laboratories attempted to characterize this putative virus. RT activity and 70S RNA were detected using a “simultaneous detection” assay (136, 170, 432), and molecular hybridization studies appeared to identify MMTV-related sequences in RNA from breast tumors (21, 459), although the specificity of these reactions is uncertain given that HERVs had not been characterized at that time. In addition, breast tissue was reported to contain antigens related to the major core protein (CA, p27) of MMTV and MPMV and to SU (gp52) of MMTV (253, 332, 460, 542). Human infection by MMTV was further supported by the detection of anti-Gag and anti-Env antibodies in sera from breast cancer patients (215, 313, 530).
Despite these data, others could not reproduce the detection of MMTV-related nucleic acid (51) or antigens (88, 203), and many viewed the evidence as unconvincing at best (425). One problem was that much of this work did not sufficiently address the question of disease association, and a comparison of data from malignant and healthy tissue revealed inconsistent findings; e.g., B-type particles were identified in healthy milk, but antigen reactivity and RT activity were specific to tumors (for a more detailed description of the early work on this subject, see reference 523).
Despite these inconsistencies, by the mid-1980s, there was a good deal of circumstantial evidence to support the idea of a human breast cancer virus, but rigorous proof had not been obtained. The advent of PCR provided an opportunity to revisit the question with more specific and sensitive probes. In 1995, Wang and colleagues reported the detection of MMTV-like env sequences in 38.5% of unselected breast cancer DNA samples but in only 2% of normal breast tissues (514). The sequenced PCR products had 95 to 99% similarity with murine MMTV viruses. Subsequent RT-PCR analysis indicated that these sequences are expressed in 66% of MMTV-positive tumors and are never expressed in normal tissue (513). The validity of these data has been questioned (49, 314, 531, 546), but at least two other groups obtained similar positive PCR amplifications (132, 145). In one study, all regions of the MMTV genome were detected in breast tumor DNA by PCR, and these sequences appeared to be integrated into chromosomal DNA of a tumor-derived cell line on the basis of fluorescent in situ hybridization (FISH) with an env-LTR probe (287). However, the data from those FISH experiments showed staining of only one sister chromatid of each positive metaphase chromosome, which is quite anomalous in FISH chromosome spreads (37). Nevertheless, subsequent studies employing in situ PCR (144) and PCR on laser-capture microdissected tumor cells (545) support the specific association of MMTV-like DNA and RNA with tumor cells and strengthen the argument that MMTV-like sequences are present in some human breast cancers.
Recent unpublished data suggest that human MMTV can be transmitted in vitro from infected metastatic cells to cultured human lymphocytes and mammary epithelial cell lines (S. Melana, B. Pogo, et al., presented at the 19th International Workshop on Retroviral Pathogenesis, Vienna, Austria, 25 to 28 September 2007). In other studies, a putative MMTV accessory protein (designated p14) was reported to be expressed in human breast cancer and to localize in the nucleolus (34). The function of this protein is unknown, although the antiserum used may cross-react with the MMTV Rem protein with which it shares some amino acid sequence (228, 331). There have also been several attempts to identify other cellular markers that correlate with the presence of the MMTV sequence. Faedo and coworkers found that the accumulation of p53 and progesterone receptor positivity were both more common in MMTV-positive tumors (135), although this contradicted one earlier report (398).
In addition to the laboratory analyses, an intriguing hypothesis relating geographical variations in the prevalence of breast cancer to the natural range of certain species of mice, particularly Mus domesticus, has been proposed (466). Areas where M. domesticus mice are endemic coincide to a large extent with those countries that have the highest incidence of breast cancer, leading to the hypothesis that M. domesticus may harbor, and transfer, a human-tropic strain of MMTV. This theory is supported by data from Ford and colleagues, who studied breast cancer DNA from patients from Australia (high prevalences of breast cancer and M. domesticus) and Thailand (low prevalences of cancer and M. domesticus). MMTV-positive samples were found only in the Australian group (145). That hypothesis was expanded recently by the suggestion that domestic cats may represent an intermediate host for MMTV and increase human exposure to the virus (475).
The molecular evidence now provides a reasonable case supporting human infection with MMTV and its association with breast carcinoma. However, a number of issues remain. In particular, the majority of human breast malignancies do not share histopathological properties with the murine tumors (313), and the epidemiology of human breast cancer does not support a link to breastfeeding (485). Therefore, if MMTV is involved in human breast cancer, the mechanisms involved may be quite different from those in mice. The current understanding of the MMTV life cycle also casts doubt on its ability to infect humans, since there is no evidence of SAg activity in patients with breast cancer. A SAg encoded by MMTV sequences amplified from human DNA extracts by PCR is active in vitro (515), but this means little if the origin of the sequence is contaminating mouse DNA. If MMTV infection in humans results in SAg expression as it does in mice, it should be possible to measure the proliferation or deletion of specific T-cell subsets in patients and to track these same subsets through familial cases of cancer. If SAg activity were not required by a human MMTV, this would imply that the replication of this virus is more efficient in human lymphocytes than in murine lymphocytes.
Another issue relates to the MMTV cell surface receptor, which has been identified as the murine transferrin receptor (422). The human homolog of this protein does not support MMTV infection in transient transfection experiments (422), but MMTV can nevertheless infect human fibroblasts and mammary cells in culture (226, 227), although it is not known whether this is via the same receptor. While humans may harbor an alternative receptor molecule for MMTV, it is striking that the MMTV sequences reported for humans do not appear to have any distinguishing features that mark them as human isolates rather than viruses obtained from mice. With differential receptor usage, we might expect variations in the Env sequence.
The current status of MMTV as an etiological agent in human breast cancer therefore remains unproven. PCR data have added some weight to the link, but at present, this might equally be explained as contamination. A comparison of MMTV sequences in breast cancer and non-Hodgkin's lymphoma in the same patient suggested that different clones of the virus are present in each type of cancer (132). However, the sequences of some of the clones were identical to sequences of endogenous MMTV proviruses; therefore, these data are also consistent with contamination with murine DNA. Here, it may be worth noting that contamination of human biological samples with animal ERV sequences was described previously (see below) (147, 190, 397). Irrefutable proof of human infection could be ascertained by cloning the integration sites from breast tissue. Alternatively, if the integration sites were identified as being murine sequences, it would confirm contamination with murine DNA. If integration sites were identified close to oncogenes activated in the tumor, this would greatly help to resolve this controversy. It would also be interesting to analyze the expression of those genes which are activated in infected mice (e.g., Wnt, notch, and fgf) in normal and malignant human breast tissues and correlate it with MMTV detection. For example, fgf10 has been identified as a common insertion site in MMTV-induced tumors in mice and is also activated in a subset of human breast cancers (482). If these same human cancers were also positive for MMTV sequences, this would directly implicate MMTV in tumorigenesis.
A recent development in this area has been reports linking MMTV with primary biliary cirrhosis (PBC), an autoimmune liver disease characterized by the progressive granulomatous destruction of small intrahepatic bile ducts and the presence of autoantibodies to mitochondrial proteins (362). An MMTV-related genome (designated human betaretrovirus) has been cloned from patients with PBC, and RVLPs have been observed in biliary epithelial cells by EM (537, 538). Immunoblotting data indicate that patients with PBC have antibodies to retroviral proteins, and MMTV infection of biliary epithelial cells in vitro leads to the cell surface expression of the mitochondrial E2 complex, a pathological characteristic of PBC (538). While some of these data have been questioned (441), a trial of PBC patients on antiretroviral drugs appears to be clinically beneficial (317), although these drugs may be acting through another (nonviral) mechanism. Recently, human integration sites have been reported in PBC lymph node (S. Indik, A. Mason, et al., presented at the 18th International Workshop on Retroviral Pathogenesis, Palm Springs, California, 6 to 10 December 2006). If confirmed, this would demonstrate incontrovertibly that MMTV can infect humans and would provide a stronger basis for an investigation of its potential etiological role in PBC. A similar confirmation of infection in human breast cancer tissue is now a priority.
Retroviruses Associated with Multiple Sclerosis
MS is a chronic, inflammatory disease of the central nervous system (CNS) characterized by demyelinated lesions that form large plaques in the brain and spinal cord (173). The cause of MS is unknown, and although immunological and genetic factors have been investigated, they do not yet provide a definitive etiological explanation, and there has been much speculation that an infectious agent may contribute to MS (191). A role for environmental factors such as infection is consistent with the epidemiology of MS, which has geographical areas of high and low risk, with the prevalence increasing with distance from the equator. Migration studies and twin studies also support a role for an environmental component (reviewed in reference 173). Over the last 40 years, the potential infectious etiology of MS has become highly controversial, with numerous agents being suggested as the culprit, including herpesviruses (451), coronaviruses (352), paramyxoviruses (182), rabies and measles viruses (396), and retroviruses (93).
HTLV-1.
A retroviral involvement in MS was first proposed following the observation that retroviruses can be neuropathogenic in animals (376, 449). This idea was strengthened by the association of HTLV-1 with HAM/TSP, which has clinical similarities with MS (171). Subsequently, antibodies that are reactive with HIV and HTLV antigens were identified in MS patients but were found only rarely in people with other neurological diseases or healthy controls (259, 369, 386, 406). Although those serological data appeared to provide evidence to support the presence of a retrovirus in MS patients, it is now recognized that such antibody-based techniques can be unreliable in autoimmune diseases such as MS (164, 392), and indeed, a number of groups were unable to confirm these data (199, 247, 291, 366, 520).
The suggested role of HTLV-1 in MS encouraged several groups to test MS patients and their family members for retroviral sequences by PCR. Initial reports described the presence of DNA sequences from HTLV-1 or a related virus in MS patient tissue (186, 408), but the data were questioned by others who were unable to repeat the positive amplification of HTLV-1 DNA by PCR (27, 114, 199, 237, 244, 366, 370, 412). In retrospect, the differing results possibly reflect the contamination of the samples with HTLV sequences, although difficulties in distinguishing MS from HAM/TSP may also have accounted for some of the positive data. When a large-scale blinded study failed to identify a statistical correlation between positive PCR detection and MS, the issue appeared to have been resolved (130). However, the enthusiasm generated by this issue led to a search for other retroviruses in MS, and the field took a new course when two laboratories independently reported evidence of retrovirus activity in MS patient-derived cell cultures. These viruses were designated MS-associated retrovirus (MSRV) and HERV-H(RGH-2) (reviewed in reference 93).
MSRV.
Perron and coworkers described extracellular RVLPs and RT activity in the supernatants of cultured leptomeningeal cells from an MS patient (designated LM7 cells) (387, 390, 393). The production of this putative “LM7 virus” was increased by mitogens such as phorbol myristate acetate and by infection of LM7 cells with human herpes simplex virus type 1 (393). In contrast, analysis of a leptomeningeal culture (LM11) from a different neurological disease (Strumpell-Lorrain disease) produced no evidence of a virus (388).
In an attempt to clone the virus genome encoding the LM7 virus, heminested RT-PCR with degenerate pol primers was performed on extracts from density gradient-purified culture supernatants of MS-derived B cells producing RVLP and RT activity (385, 493). Amplicons of the predicted size were obtained from the gradient fractions that were positive for RT activity. B cells from non-MS donors were used as controls and did not produce RT activity or RVLPs. Sequence analysis identified a novel gammaretroviral sequence, which was designated MSRV (385). MSRV sequences were partially characterized, and a consensus PR-RT sequence was created from several smaller overlapping clones (385). Additional MSRV-related sequences encompassing the whole genome were later amplified from MS patient plasma and cell culture supernatants by RT-PCR (258).
Subsequently, the MSRV sequence was found to be closely related to a novel HERV family, designated HERV-W (55). The human genome contains dozens of HERV-W loci (107, 382, 507), although no complete coding-competent provirus has been described (507). The sequence reported as MSRV (258) has over 90% sequence similarity to HERV-W, but no identical provirus has yet been found in the human genome. One explanation could be that MSRV represents an exogenous homolog of HERV-W. However, the available “MSRV” sequences are all chimeric and derived from multiple PCR products. Moreover, the published MSRV genome does not possess complete ORFs due to the presence of stop codons or frameshifts in gag, pol, and env (258), indicating that it does not encode a replication-competent virus. Despite this, the transmission of MSRV from irradiated LM7 cells to LM11 cells has been reported (388). Unfortunately, at that time, the MSRV pol sequence had not been cloned, and genetic methods for monitoring the infectious process (such as PCR for 2-LTR circles or identification of preintegration complexes) were not available, so it is not certain that a virus was transmitted.
It is worth noting that no direct evidence has been presented to confirm that the LM7 “virions” were actually encoded by MSRV/HERV-W or even that these sequences were encapsidated by the RVLPs. This link appears to have been made simply because the production of RVLPs and amplification of MSRV RNA were both observed in MS patients, even though the LM7 RVLPs and MSRV sequences were identified in cultures of different tissues from different patients (385, 387, 390). Given that the only complete ORFs described so far for MSRV/HERV-W are for env and pro (107, 258, 507), it appears unlikely that the particles are encoded by this defective HERV. It is therefore possible that the RVLPs and the “MSRV” sequence represent different entities. Confirmation that MSRV/HERV-W encodes the RVLPs produced by MS cell lines could be obtained by immuno-EM using gold-labeled antibodies raised against Gag or Env proteins (61).
Studies on the expression of MSRV/HERV-W in MS and other diseases have shown that HERV-W is transcribed in some healthy tissues, including brain (278, 438, 543), but that its expression is increased during inflammation. Extracellular “particle-associated” RNA has been reported in patients with MS (123, 164, 368), RA (165), and schizophrenia (246). Taken together, these reports suggest that the presence of MSRV/HERV-W sequences may be a marker for inflammation in general rather than for MS specifically. This would be consistent with in vitro work that demonstrates the activation of HERV-W, HERV-H, and HERV-K by proinflammatory cytokines such as tumor necrosis factor, interleukin-6 (IL-6), and gamma IFN (IFN-γ) (13, 239). Interestingly, these cytokines are thought to be important mediators of inflammation in MS. In addition, one study found that the detection of MSRV/HERV-W correlated with disability progression in MS patients (458).
A number of groups have examined possible mechanisms by which MSRV might be linked to inflammatory reactions in MS. MSRV/HERV-W proteins have been detected in the brain using immunohistochemistry with antibodies to Gag and Env (13, 391, 521). The culture supernatant from cells producing MSRV RVLPs has been reported to have a toxic effect on glial cells (329, 384) and to cause T-lymphocyte-dependent death with brain hemorrhage in humanized SCID mice (142). In addition, the Env protein is proposed to exhibit SAg activity (389). However, since there is no standard infectious molecular clone of MSRV, it is not clear whether those studies describe properties of the same entity or unrelated phenomena, and further work is needed to confirm these pathogenic activities.
The most consistent evidence for a role for MSRV/HERV-W in MS comes from studies carried out with the HERV-W Env protein. One HERV-W provirus on chromosome 7 encodes an intact Env protein (designated syncytin and later syncytin-1) that has been shown to have fusogenic properties (56, 333) and may have a physiological role in the formation of the placental syncytiotrophoblast (56, 153, 309, 333). In a landmark study, Antony and coworkers demonstrated that syncytin-1 is up-regulated in glial cells within acute demyelinated lesions of MS patients and that the expression of this protein in astrocytes induces the release of redox reactants, which are cytotoxic to oligodendrocytes (13). This paper therefore provides a direct link between MSRV/HERV-W and the immunopathological events in MS. Consistent with those findings, in vitro stimulation of peripheral blood mononuclear cells (PBMCs) by the HERV-W Env protein induces the production of IL-12-p40, IL-6, and IFN-γ, with divergent reactivities between cells from MS patients and those from controls (419). These cytokines are proposed to be elicited by the stimulation of monocytes by MSRV/HERV-W Env acting through Toll-like receptor 4 (420). Taken together, those results suggest that the inflammatory properties of syncytin-1 could contribute in some part to the immunopathogenic events in MS. Therefore, while there is no strong evidence that MSRV functions as a replication-competent virus, the initial characterization of RVLPs and RT activity in MS ultimately led to the identification of HERV-W and syncytin-1, which is now a credible candidate as an inflammatory mediator in this disease. Since syncytin-1 is ubiquitous in humans, it is now important to determine what triggers its unusual overexpression in the CNS of MS patients.
HERV-H(RGH-2).
A second retrovirus associated with MS was also first detected as extracellular RVLPs and RT activity in mitogen-stimulated T and B lymphocytes from MS patients and healthy individuals (96, 99, 194, 348, 456). Subsequently, RT-PCR with primer sets specific for several different HERV families was employed to identify the viral genome encoding these purified RVLPs. Positive amplification was obtained with gag and env primers specific for RGH-2, a member of the HERV-H superfamily (94, 95). A number of different HERV-H sequence variants were identified, suggesting a general activation of several endogenous proviruses rather than the presence of a single infectious form. PCR on gradient-purified “virions” from plasma found that these were specific to MS patients with active disease but not healthy controls. Interestingly, MSRV/HERV-W-related sequences were not detected despite the use of primers specific for this HERV family (94).
As with MSRV, no link between the RVLPs and HERV-H(RGH-2) has been formally established. In addition, only defective HERV-H genomes are associated with the RVLPs, and no coding-competent virus genome has been identified (93, 95). Nevertheless, rats immunized with RVLPs prepared from MS patient B cells produced antibodies to HERV-H Gag and Env peptides (98), which is at least consistent with a HERV-H origin for these particles.
Aside from the sequence characterization, relatively little on the pathological potential of HERV-H(RGH-2) has been reported. The transmission of HERV-H in vitro using a retroviral vector marker rescue strategy has been described (97). However, the level of infection was extremely low, and it was not formally demonstrated that infectious HERV-H was present. In terms of individual viral proteins, there are at least three HERV-H proviruses in the genome that could encode an intact Env protein (118), but unlike HERV-W Env, there is no evidence that any of these is specifically expressed in MS or has inflammatory properties. However, in a murine tumor model, HERV-H Env was found to have immunosuppressive properties (312), suggesting that it could have a role in immune dysregulation in some circumstances.
Over the last 20 years, there has been a great deal of work focused on retroviruses in MS, and although this has not yet established a consensus in the field that either MSRV/HERV-W or HERV-H is an etiological agent in this disease, recent findings may at last be beginning to resolve the issue. It is now clear that several HERVs are activated by inflammatory events in MS and that some of the proteins (such as syncytin-1) may themselves have inflammatory properties and contribute to pathogenesis. In addition, several groups have shown that HERVs are activated by infection with other viruses either in immune cells or in the CNS (76, 77, 93, 276, 359, 393, 426). These include common infections that have been independently linked with MS, including human herpesvirus 6, herpes simplex virus type 1, EBV, and influenza virus. Together, these studies could bring together several disparate findings into one general model implicating viruses and HERVs in MS.
In contrast, the hypothesis that there is an infectious retrovirus linked with MS is not well supported. In particular, the failure to identify proviruses of HERV-W or HERV-H with ORFs for all major viral proteins tests the plausibility of these agents as genuine infectious retroviruses, and it appears unlikely that an exogenous version of either virus exists. Because both HERVs are activated by cytokines present in MS, the RVLPs and RT activity observed in disease lesions could represent the coexpression of several HERVs under inflammatory conditions. For example, defective HERV-H and HERV-W genomes could potentially be encapsidated by functional Gag proteins from HERV-K. Alternatively, the functional Gag proteins could derive from a so-far-unidentified exogenous retrovirus. Cross-packaging of heterologous retroviral genomes in this way is well characterized. Nevertheless, even if they turn out to be unrelated to disease, the original description of these particles has led to exciting developments in the study of MS, and the identification of their origin remains of great interest.
Human Intracisternal A-Type Particles
Intracisternal A-type particles (IAPs) are ERV particles of rodents that are encoded by defective proviruses related to betaretroviruses (264). During particle assembly, IAPs bud into the endoplasmic reticulum rather than through the plasma membrane and typically appear on electron micrographs as “doughnut”-shaped structures in the endoplasmic reticulum lumen. The greatest expression of IAPs has been reported for murine plasmacytoma cell lines (340, 446), while other studies have linked the expression of IAP RNA and Gag proteins with a form of autoimmune diabetes (279, 280). Most IAP elements have no env gene, and the few that do are highly defective in gag and pol (411); thus, these elements are not thought to be transmissible. Between 1990 and 1996, several IAPs were described in human tissues and linked to disease, particularly SS and idiopathic CD4 lymphocytopenia (ICL).
IAPs and SS.
SS is a systemic autoimmune condition characterized by chronic inflammation and destruction of exocrine glands, principally the salivary and lachrymal glands (149, 401). Reduced glandular secretion results in dryness of the eyes and mouth, leading to symptoms such as corneal ulceration and dental decay. The clinical diagnosis of primary SS requires evidence of lymphocytic infiltration of (or proliferation in) salivary glands, while the systemic production of autoantibodies to the ribonucleoproteins SS-A(Ro) and SS-B(La) is also a common feature (149). Retroviruses were invoked as potential etiological agents in SS after salivary gland inflammation in individuals infected with HIV-1 and HTLV-1 was described (229, 357, 401). In addition, approximately one-third of patients with SS have serum antibodies that are reactive with Gag proteins of HIV-1 or HTLV-1 (477), and antigens related to HTLV and HIV have been detected in SS patients by immunohistochemistry (444, 540). These patients were not infected with either virus, suggesting that another (related) retrovirus might be present.
Human IAPs were first described in SS in cultures of salivary gland tissue that had been cocultivated with a human T-lymphoblastoid cell line (H9) (161). RVLPs similar to rodent IAPs were detected by transmission EM in two of six cocultures and designated human IAP (HIAP) (later HIAP-I). Lysates of H9 cells expressing HIAP-I contained Mn2+-dependent RT activity with a density of 1.22g/ml on sucrose gradients, which is typical of unenveloped retroviral cores and of IAPs. In addition, the fractionated RT-positive material contained HIV-related antigens (161). In contrast, HIAP-I was not observed in H9 cells before cocultivation or in H9 cells infected by HIV-1. A similar analysis of normal salivary gland biopsies was not performed, so the specificity of these particles to SS could not be assessed. Also, it was not established whether the HIAPs originated in the salivary gland tissue and were transferred to the H9 cells or whether cytokines or other factors present in the biopsies had activated an endogenous retrovirus in the H9 cells. Some allogeneic stimulation of the H9 cells would be expected due to the presence of lymphocytes in the salivary gland tissue.
That initial study did not examine SS salivary gland tissue directly for the presence of HIAPs prior to culture, but this was reported subsequently by Yamano and colleagues, who detected similar RVLPs in epithelial cells in uncultured tissue from 3 of 10 salivary gland specimens from patients with primary SS (540). Such particles were not found in salivary glands from control subjects, suggesting that the RVLPs were disease specific. Similar to studies of HIAP-I (161), Mn2+-dependent RT activity was detected in salivary gland extracts. Furthermore, the three patients that were positive for SS were seroreactive against HIV-1 p24 (Gag, CA), and their salivary epithelia reacted with anti-p24 antibodies (540). Morphologically, the RVLPs consisted of a distinct, eccentrically located internal core and an outer surface envelope. However, in contrast to HIAP-I, these particles were generally found in the cytoplasm. The authors speculated that the retrovirus that they detected might be encoded by HERV-K, although this was not directly tested.
The genome encoding HIAP-I has not been characterized; however, cells expressing these RVLPs are reported to have reduced protein kinase C activation and IL-2 production and to express reduced levels of CD4 and increased levels of MHC class II in comparison to normal H9 cells (143). In addition, stimulation of the same cells with phorbol myristate acetate resulted in an increased production of RVLPs, although these RVLPs appeared as cytoplasmic clusters of particles of smaller diameter (approximately 33 nm) (112) and are therefore unlikely to be the same particles observed originally.
An interesting aspect of the studies on HIAP-I is that although the particles have not been characterized at the protein or nucleotide level, a number of serological studies demonstrated reactivity to HIAP-I proteins in human autoimmune diseases. The initial study found that sera from 16 out of 18 patients with SS or SLE reacted with one or more proteins of molecular masses of 45, 61, and 70 kDa in “infected” cell lysates (161). Subsequently, reactivity to HIAP-I cell lysates was described for Graves' disease (235), PBC (318), systemic sclerosis (273), arthritis (330), SLE (318), and alopecia areata (274). The significance of these findings is unclear, since it is unknown whether the proteins are genuinely retroviral (although a rabbit serum to HIV CA reacted with the 61- and 70-kDa proteins) (161). Collectively, those studies suggest that the same virus (or a group of related agents) is expressed in all these diseases, which could be explained by HERV activation. Alternatively, such antibodies may be a result of the polyclonal B-cell activation that is a characteristic of some of these diseases. Significantly, no link has been established between the HIAPs observed by EM and the proteins detected on immunoblots. Application of proteomics methods would permit these antigens to be identified and could lead to the cloning of the genes that encode them.
IAPs and ICL.
ICL is a rare immunodeficiency syndrome characterized by the severe depletion of CD4-positive T lymphocytes but in which HIV is not present (213, 454). ICL patients may develop some of the opportunistic infections and rare cancers associated with AIDS, but in contrast to patients with HIV, they can recover after several years of illness. Due to the similarities with AIDS, a retroviral etiology was proposed, and there have been two independent reports of retrovirus expression in ICL patients.
In the first study (193), PBMCs from a patient with severe CD4+ T-cell deficiency and Pneumocystis carinii infection were cocultured with H9 cells. This patient had no serological or PCR evidence of infection with HIV-1, HIV-2, HTLV-1, or HTLV-2. An acute cytopathic effect was observed in the cocultured cells, and EM examination revealed the presence of numerous RVLPs with an appearance typical of IAPs in cisternae (193). Similar particles were present in cocultures of H9 cells with PBMCs from the patient's daughter, who also had signs of CD4+ T-cell dysfunction. These RVLPs were named human intracisternal retroviral particles (HICRV). Immunoblot analysis showed that the patient's serum contained antibodies that reacted with a 24-kDa protein present in extracts of cells producing HICRV but not in control cultures. Also, healthy individuals did not have antibodies that were reactive with this protein.
The relationship of the HICRV to HIAP-I is unclear. In common with HIAP-I (196), the supernatant of H9 cells that expressed HICRV contained Mn2+-dependent RT activity (193), although extracellular particles were not observed. However, in contrast to HIAP-I, lysates of HICRV-producing cells did not react with anti-HIV antibodies. This suggests that the particles are not the same, although a different antibody was used, so these results are not directly comparable. In contrast to many of the other candidate human retroviruses, numerous HICRV particles were observed on electron micrographs (193), providing convincing evidence of their retroviral origin. Given this abundance, it is unclear why the genome encoding them has not been cloned and why no other group has reproduced the findings.
A second IAP in ICL was described by Garry and colleagues (162) and designated HIAP-II to distinguish it from the morphologically distinct HIAP-I found in SS (161) and from HICRV (193). HIAP-II particles were smaller than HIAP-I, with a diameter of approximately 60 nm. Despite this, sera from SLE patients reacted with proteins from cells in which HIAP-I and HIAP-II were observed, suggesting that these cells possess antigenically related proteins (162). Antibodies reacting with a 97-kDa protein in HIAP-II-producing cell lysates were identified in 50% of ICL patients but in only 10% of healthy matched controls (162). These sera did not react with control cell lysates. As with the two previous descriptions of IAPs in humans (161, 193), the disease specificity of the particles could not be determined since PBMCs from healthy individuals were not studied, and controls for cytokine-induced HERV activation in H9 cells or PBMCs from healthy donors were not included.
Given the potential importance of these three human IAPs and the evidence that a retrovirus is expressed in SS and ICL (i.e., EM, RT activity, serology, and antigen reactivity), it is unfortunate that those initial observations have not been expanded upon. Further discussion of HIAP-I, HIAP-II, and HICRV particles and their relationship to each other remains speculative in the absence of the sequences of the proviruses encoding these RVLPs. The preliminary characterization of HIAP-I sequences has been reported (427), but these data have yet to appear in print or in sequence databases. It appears that these particles may represent rare particles produced by a HERV, but this has not been examined experimentally. It is ironic that while PCR has often been used to identify retroviral sequences in diseases where there is only circumstantial evidence of virion production, it has yet to be successfully employed to identify the encapsidated genomes of the various HIAPs.
Human Retrovirus 5
Following reports implicating a retrovirus in SS (163) and the detection of HIAP-I in cultures of SS salivary gland extracts (161), a search for the genome of the putative virus involved was initiated. Using degenerate primer RT-PCR, a short pol sequence was amplified from sucrose gradient-concentrated salivary gland extracts from a patient with SS (189). This sequence was identified in gradient fractions with a density of 1.16 g/ml, typical of mature retroviruses, and these same fractions contained RT activity when tested by PERT assay. Southern blot and PCR analyses revealed that this virus was not endogenous in human DNA (189). Indeed, in samples in which it was detected, the proviral DNA was present at an extremely low load (around 1 copy in 100,000 cells) (189, 415). This viral sequence was later given the provisional name human retrovirus 5 (HRV-5), as it appeared to be the fifth exogenous human retrovirus. Subsequent analysis of tissue from patients with SS or other rheumatic conditions detected the HRV-5 pol sequence in 6 of the 18 tissue samples studied (189). Although the sample size was too small to draw conclusions regarding disease association, the finding that one-third of the first 18 people studied were positive suggested that the virus was a common infection.
Further “chromosome-walking” experiments led to the cloning of the viral LTRs and complete ORFs for gag, pro, and pol as a series of overlapping PCR fragments (190). Sequence analysis indicated that HRV-5 was a betaretrovirus related to MMTV, MPMV, and IAP elements of rodents. The similarity with IAP elements initially suggested that this sequence might encode the HIAP particles described for SS (161, 540), but this was ruled out when cells expressing HIAP-I were found to be negative by PCR with HRV-5-specific primers. Although the betaretroviruses are the closest relatives of HRV-5, the nucleotide similarity in the conserved region of pol was only 55 to 65%, indicating that this was a novel virus and not a new “human” strain of a previously identified virus. Sequences cloned from different individuals had up to 10% sequence divergence with maintenance of the ORFs, suggesting that this was an actively replicating agent. In addition, this variation was interpreted to indicate that PCR contamination was not responsible for the data. Critically, in addition to our own laboratory in London, HRV-5 sequences were identified in human tissue extracts by a number of other laboratories in Europe and the United States (147, 260, 350, 397; also see reference 187). Taking this evidence together, HRV-5 appeared to represent a novel exogenous human retrovirus. Significantly, however, an env gene could not be identified, although several clones containing pol and 3′ LTR sequences were amplified from different individuals, which raised serious questions regarding the transmissibility of this putative virus.
With regard to disease association, HRV-5 RNA was originally detected in salivary gland biopsies from patients with SS, but proviral DNA could only rarely be detected in such tissue (63, 415). A wider survey of other inflammatory diseases detected HRV-5 DNA in blood samples from 12% of patients with RA and 16% of patients with SLE (188). The link with RA was striking in that HRV-5 DNA was also detected in 50% of synovial biopsies from RA joints, although synovial tissue from some patients with osteoarthritis and reactive arthritis were also occasionally positive. In contrast, HRV-5 DNA was hardly ever detected in normal joints and blood from healthy donors (67, 188, 189, 260, 261, 350, 414). One group could not reproduce the positive detection in joint DNA, but the tissue samples were not directly comparable since there was little inflammatory tissue in these samples (166). A survey of human cancers identified HRV-5 DNA sequences in approximately 10% of lymphoma patients (414), and this was confirmed by much larger independent studies (260, 350). Thus, the hypothesis made from the PCR data was that HRV-5 was a common infection with a very low virus load that was increased in some patients with RA, SLE, or lymphoma. Subsequent studies aimed to determine whether HRV-5 had a role in the etiopathogenesis of these diseases or whether the increased frequency of detection was instead a consequence of disease.
Using molecular clones of HRV-5 amplified from DNA from infected patients by PCR, the functional capacities of the various HRV-5 proteins were tested. Thus, the viral protease was subcloned, expressed in Escherichia coli, and shown to be active (508), and Gag proteins were demonstrated to have the ability to form core particles when expressed in transfected human cells (C. Voisset and D. Griffiths, unpublished data). Preliminary immunoblot analyses indicated that some individuals have serum antibodies that react to HRV-5 Gag proteins (C. Hervé, P. J. Venables, and D. Griffiths, unpublished data), but further studies correlating seroreactivity with genome detection by PCR have not been undertaken. Therefore, the available data supported HRV-5 as a new infectious retrovirus, although this had not been formally shown by transmission in culture. However, despite the initial detection of HRV-5 sequences in H9/lip biopsy cocultures, attempts to obtain a permanently infected culture were unsuccessful; viral DNA and RNA was lost from the cultures within 3 to 4 weeks (189). Moreover, the apparent absence of an env gene in all the patients examined did not sit well with the notion that this was an infectious virus.
To determine whether HRV-5 was a genuine human infection, HRV-5 integration sites were cloned from the DNA of infected clinical specimens by PCR-based techniques (190). Three integration sites were identified (from three different individuals), but none of the flanking sequences was present in human genome sequence databases, and they could not be detected experimentally in human DNA by Southern blot or PCR. A survey of other mammalian species demonstrated that HRV-5 is in fact an ERV of the European rabbit (Oryctolagus cuniculus) and that the rabbit genome harbors around 1,000 to 5,000 copies of this element, now designated rabbit ERV-H (148, 190).
Prior to the identification of HRV-5 sequences in the rabbit genome, HRV-5 had been detected only in human DNA extracts. The possibility that HRV-5 is a replication-competent rabbit virus that has crossed species and infected humans is very remote, since flanking rabbit genomic sequences and mitochondrial DNA were also detected in some human samples (148, 190), so this appears to be a simple case of PCR contamination. However, contamination cannot easily explain the initial discovery of HRV-5 as an RNA element or its disproportionate detection in specific diseases. Its detection by a number of laboratories in different countries using different assays also argues against straightforward PCR contamination. Other mechanisms to explain the presence of rabbit sequences in human DNA preparations, such as DNA in the diet entering the peripheral circulation (147, 190, 434), are theoretically possible but unlikely given that rabbit meat is a minor part of the United Kingdom diet. Instead, it appears that contamination is the most likely source, although the route or mechanism is at present difficult to identify.
Although the accumulated data on HRV-5 appeared to present a reasonable case for a new infectious human retrovirus, the truth was found to be quite different. HRV-5 provides several lessons for retrovirus hunters. First, it shows how the exquisite sensitivity of PCR can lead to misleading results even where the initial disease association is corroborated by other data. Second, the difficulty of using degenerate pol primers for identifying novel genomes is highlighted, since the potential for contamination with ERV sequences, human or animal, is high. Third, the cloning and sequencing of integration sites can provide definitive proof for or against human infection and will be valuable in determining the provenance of other putative human retroviruses.
HERV-K
HERV-K is a family of retroviral elements related to exogenous betaretroviruses (30, 172, 295, 489) that was originally detected by low-stringency hybridization using probes derived from MMTV and murine IAPs (372, 373). Related elements were subsequently identified by consensus pol PCR and grouped into 10 groups of human MMTV-like (HML) sequences (29, 324). Of these groups, the HML-2 group includes the most intact HERV-K proviruses and is therefore perhaps the most likely to be pathogenic since some loci have retained complete ORFs for all viral genes (321, 410, 488, 494). There are two types of HERV-K(HML-2) proviruses, which differ by the presence (type 1) or absence (type 2) of a 292-bp deletion at the pol-env boundary. Both types encode nonstructural proteins in the env region. Type 2 proviruses encode Rec (formerly cORF or K-Rev), a functional homolog of RNA nuclear export factors encoded by HIV and HTLV (Rev and Rex) (305, 541). Type 1 proviruses encode Np9, which is of unknown function but is expressed in a variety of tumors and transformed cell lines (17). Rec and Np9 are both expressed from spliced mRNAs from the env region, and they share 14 amino acids at their N termini. HERV-K has been linked with a variety of autoimmune diseases and cancers (30), but particular attention has focused on its potential role in testicular cancer, melanoma, and insulin-dependent diabetes mellitus (IDDM).
HERV-K and testicular cancer.
HERV-K elements are highly expressed in testicular germ cell tumors, in particular seminomas and teratocarcinomas (30, 61, 71). Cell lines derived from these tumors produce noninfectious RVLPs encoded by HERV-K(HML-2), designated human teratocarcinoma-derived retrovirus (HTDV). Although specific proviruses encoding HTDV have not been described, their identities as HERV-K were confirmed by immunogold labeling of electron micrographs (61). The RVLPs expressed by different cell lines have various morphologies indicative of mutations in one or more viral proteins (46). It is therefore possible that these particles represent composite virions formed by the products of multiple proviruses through complementation.
The increased expression of HERV-K in patients with germ cell tumors is reflected by a high frequency of anti-HERV-K antibodies in these individuals. Antibodies directed against HERV-K Gag or Env have been detected in 50 to 60% of seminoma patients around the time of diagnosis (60, 430). In those studies, reactivity to HERV-K proteins in healthy controls was less than 5%. Patients that received chemotherapy treatment showed a decrease in their antibody titers (257, 430), and antibody reactivity declined further after tumor removal (60). Thus, antibodies to HERV-K are detected frequently in these patients and seem to resolve rapidly with effective therapy of the malignancy. While patients with testicular cancer have particularly high titers of anti-HERV-K antibodies, antibodies to HERV-K are also present in some patients with autoimmune disease and in healthy individuals (24, 208).
In addition to antibodies, T-lymphocyte responses to HERV-K Gag and Env peptides have been detected using an enzyme-linked immunospot assay (404). Whether these T cells are active against tumor cells in vivo has not yet been investigated. The same study also found HERV-K-specific T cells in apparently healthy individuals, raising the question of whether such self-reactive lymphocytes are commonly present in humans and, if so, whether they have a pathogenic role in patients with autoimmune disease.
The clinical significance of HERV-K protein expression in testicular cancer is unclear. It is possible that the expression of HERV-K/HTDV is a result of genetic changes associated with tumor development that activate the LTR and drive the expression of multiple proviruses. For example, HERV-K(HML-2) transcription has been shown to be highly regulated by cytosine methylation (275). Nevertheless, there is mounting evidence that the HERV-K Rec and Np9 proteins may play a direct role in tumorigenesis. Both Rec and Np9 can bind to the promyelocytic leukemia zinc finger (PLZF) protein (58, 117), a transcriptional repressor that is critical for spermatogenesis. The impairment of PLZF function is associated with an increased frequency of teratocarcinoma. Therefore, Rec and Np9 could trigger germ cell transformation by interfering with PLZF, thereby promoting cellular proliferation through the activation of transcription factors such as c-Myc (117). This model is supported by evidence from mice that are transgenic for Rec, which develop carcinoma in situ, an early marker of testicular cancer (156). This effect is specific to the testis because other tissues in these mice which also express Rec do not show signs of carcinoma. Further studies on the mechanism of action of Rec and Np9 in this transgenic model will be valuable in determining the role of HERV-K in tumorigenesis in testicular cancer.
HERV-K and melanoma.
Malignant melanoma is an aggressive form of skin cancer that arises from pigment-producing melanocytes in the epidermis (302). The etiology of melanoma is strongly linked to overexposure to UV radiation, although additional cofactors may be required. Retroviruses were linked with melanoma following the detection of RVLPs in cell lines derived from tumor biopsies (25, 50), and a number of recent studies have shown that HERV-K(HML-2) is expressed in primary melanomas and in lymphoid and cutaneous metastases (81, 82, 354). Particles and RT activity have been detected in the supernatants of cultured tumor biopsies and cell lines, and the expression of HERV-K Gag, Env, and Rec proteins has been demonstrated by immunoblotting and immunofluorescence (81, 82, 223, 354). As with testicular tumors, multiple HERV-K proviruses appear to be expressed in melanoma. Intriguingly, Muster and colleagues described the transmission of HERV-K sequences from melanoma supernatants to bovine kidney cells (354). The supernatant used in that study contained a number of distinct HERV-K sequences (211), and additional work is therefore required to clone the transmitted provirus and to determine whether it represents an infectious HERV-K.
In addition to HERV-K(HML-2), other HERV-K elements may be expressed in melanoma. In one study, a short peptide of 9 amino acids derived from the env region of a highly defective HERV-K(HML-6) provirus was demonstrated to be a melanoma-specific antigen (431). Moreover, patients with melanoma have cytotoxic T lymphocytes that can specifically recognize and kill cells expressing this peptide in association with MHC class I molecules. Whether the expression of this HERV peptide has a function in clinical disease or a role in tumor immunosurveillance is currently under investigation. The expression of a short peptide with physiological function demonstrates the potential importance of defective HERV elements, even where the coding capacity is not obvious.
HERV-K and IDDM: the superantigen hypothesis.
IDDM is an autoimmune disease characterized by the destruction of insulin-producing β cells in the pancreatic islets of Langerhans (484). The etiology of IDDM is complex, involving many genetic loci, with the MHC and the insulin gene being the most important (487). A concordance rate in monozygotic twins of only 50% suggests the additional involvement of environmental factors such as viruses or other infections. SAg activity in IDDM was suggested by the finding that Vβ7-bearing T cells are enriched in patients with recent-onset IDDM (104) and raised the question of whether a retrovirus might be involved.
To investigate this, Conrad and colleagues examined short-term cultures of freshly isolated pancreatic islets and splenocytes derived from IDDM patients for the presence of a retrovirus (105). RT activity detected in supernatants from these cultures was up to 100-fold greater than that detected in supernatants from cultures from nondiabetic donors (105). Subsequent experiments led to the cloning of a HERV-K element from the supernatants of the splenocyte cultures as a series of overlapping RT-PCR fragments. The retroviral genome that was isolated was initially designated IDDMK1,222 (105) and was later identified as a specific allele of HERV-K18, an HML-2 provirus located in the first intron of the CD48 gene on chromosome 1 (198). Allele K18.1 corresponds to IDDMK1,222 (105), allele K18.2 is identical to a published sequence (488), and K18.3 is novel (463). Additional rare variants have also been described. None of the K18 alleles has intact gag or pol genes that could encode functional RT activity or capsid proteins. Thus, it does not appear that this is an infectious strain of HERV-K.
Conrad and colleagues suggested a link between HERV-K and IDDM based on RT-PCR data showing the presence of HERV-K18 RNA in sera from 10 of 10 IDDM patients but in none of 10 age-matched non-IDDM controls (105). This disease specificity was later refuted after a number of groups failed to find any association between HERV-K RNA and IDDM (24, 234, 256, 270, 272, 296, 351, 463). In addition, the increased RT activity detected in supernatants of splenocyte cultures from IDDM patients could not be confirmed (234). It is unclear why the initial association with IDDM appeared to be so strong. The presence of contaminating genomic DNA in the serum RNA extracts is one possible explanation, although control reactions were performed to monitor this (105).
Despite the uncertainty of the link with IDDM, the initial report was notable in that it described a SAg activity encoded by the truncated env ORF of IDDMK1,222 that could specifically activate human Vβ7-specific T lymphocytes (105). Subsequent work has shown that HERV-K18 transcription is activated by the treatment of PBMCs with IFN-α and that these IFN-α-treated cells have a mitogenic effect on syngeneic Vβ7-specific T cells (463). Treatment with an antiserum to the N-terminal peptide of the SAg inhibits this effect, indicating that it is indeed due to SAg expression. In a parallel study, Sutkowski and coworkers (472) showed that EBV infection of B lymphocytes leads to the transactivation of HERV-K18 env expression and proposed that HERV-K18 encodes the EBV-associated SAg activity reported previously (473).
It is worth noting that other groups initially failed to demonstrate SAg activity using very similar assays of human or murine cells (22, 272, 296), leading to some skepticism that the HERV-K18 env region encodes a functional SAg. However, more recent work in mice transgenic for the HERV-K18 genomic locus has shown that murine Vβ3 and Vβ7 T-lymphocyte subsets are activated by HERV-K18 SAg in vivo (476). In addition, studies on the mechanism by which EBV stimulates HERV-K18 has implicated the EBV surface glycoprotein gp350 via signaling through CD21 (219) and the latent EBV proteins LMP-1 and LMP2A (471). HERV-K18 expression is also induced in B cells by immunoglobulin M cross-linking of CD40 on the cell surface (476). Therefore, current evidence now provides a more persuasive argument that the HERV-K18 SAg activity is genuine. Further studies are necessary to determine whether HERV-K18 SAg activity is actually relevant to immunopathology in IDDM or other inflammatory diseases. Since EBV infection is almost ubiquitous in humans, it will be important to determine how HERV-K18 SAg activation might cooperate with other cofactors to cause disease in a minority of individuals.
An intriguing aspect of the studies on HERV-K18 SAg is that they provide a model linking virus infection, HERVs, and inflammatory disease (463, 471, 472) (Fig. 6). This model could explain how diverse and perhaps common viral infections might be etiological cofactors in diseases such as IDDM that are not regarded as contagious in the conventional sense. For example, multiple acute enterovirus infections (such as coxsackieviruses) in patients who were newly diagnosed with IDDM have been described, and the timing of these infections appears to be related to an increase in Vβ7 gene transcripts (299). It would therefore be of great interest to know whether enteroviruses have the ability to activate HERV-K18 expression and whether such activation is IFN-α dependent.
FIG. 6.
Activation of HERV-K superantigen. Possible mechanisms for activation of a superantigen encoded by HERV-K18 on chromosome 1 (based on data from references 105, 219, 463, 471, 472, and 476). IgM, immunoglobulin M.
A recent study has shown that endogenous MMTV SAg expression can increase susceptibility to infection by a range of bacterial and viral pathogens through an unidentified mechanism (45). Potentially, HERV-K18 SAg may exert similar properties. As with the studies on MSRV/HERV-W described above, the initially reported HERV-K virus particles in IDDM did not turn out to represent an infectious retrovirus but instead led to the identification of a functional HERV protein that may have pathological effects through an entirely unpredicted mechanism.
Infectious HERV-K viruses.
The coding competence of HERV-K(HML-2) indicates that it invaded the genome relatively recently in our evolutionary history, and this is supported by the presence of polymorphic insertions that are present in only a proportion of individuals (38, 221, 300, 494). Some estimates have suggested that HERV-K may have been infecting our ancestors as recently as 100,000 years ago (494), and analysis of the env gene indicates that it has been under continuous purifying selection, suggesting that the infectious spread of HERV-K has continued until very recently. While it is possible that such spread may have occurred within an individual rather than by human-to-human transmission, this nonetheless hints at the possibility that a closely related virus may still be infecting humans today (39).
Although there are some functional copies of the individual HERV-K(HML-2) proteins present in the human genome, no provirus has so far been shown to encode a fully replication-competent virus. Recently, two groups independently constructed recombinant HERV-K viruses based on a consensus of known HML-2 sequences. These viruses, designated Phoenix (120) and HERV-KCON (277), therefore represent close relatives of the progenitor HERV-K(HML-2) virus. The two viruses differ at several positions in the genome, but both viruses are infectious in cultured cells. Although these viruses are artificial, they provide novel tools for future analysis of HERV-K replication and its possible role in disease.
Betaretrovirus in Bronchioloalveolar Carcinoma
Ovine pulmonary adenocarcinoma (OPA) is a transmissible lung cancer of sheep caused by JSRV, which infects and transforms alveolar type II pneumocytes and bronchiolar Clara cells in the periphery of the lung (377). In addition to its importance as an agricultural disease, OPA has attracted attention due to its histological similarity with some forms of human pulmonary adenocarcinoma, particularly cases involving bronchioloalveolar carcinoma (BAC), which arise from the same cell types as OPA (341). These forms of human lung cancer are not strongly associated with smoking, and other environmental agents have therefore been proposed to play an etiological role.
The mechanism of tumorigenesis by JSRV in sheep is currently under investigation, but the viral Env protein is sufficient to induce cellular transformation in vitro (304) and in vivo (86, 535). The histological and pathological similarities of human lung adenocarcinoma and OPA have led to an examination of human tumors for evidence of JSRV infection. An intriguing finding was that 26% of all lung adenocarcinomas reacted positively to an anti-JSRV Gag antiserum by immunohistochemistry, while only 2.5% of the other tumors studied expressed this antigen (115). Antibodies to other betaretroviral Gag proteins also react with an antigen in the same tumors (116).
PCR with primers specific for JSRV gag do not amplify viral sequences from human tumors, indicating that JSRV itself is not present in humans (209, 544). This antigen may therefore represent a related (possibly novel) retrovirus, a HERV protein activated in some tumors, or a different cross-reacting cellular protein coincidentally up-regulated in these tumors. Analysis of expressed betaretrovirus sequences in BAC by degenerate primer RT-PCR could identify only HERV-K(HML-2) and HERV-K(HML-3) sequences in these tumors, and the same HERVs were also expressed in normal lung tissue (P. Hopwood, M. Norval, and D. Griffiths, unpublished data). JSRV infection in humans has also been suggested by the reported PCR amplification of JSRV gag and pol sequences from blood donors and AIDS patients from Cameroon and Nigeria (342). Notably, the lung cancer cases tested in that study were negative. However, some of the sequences obtained were almost identical to sheep ERV sequences, which suggests that some contamination of the PCRs with sheep DNA was present. This result therefore requires confirmation, particularly since JSRV LTR sequences could not be detected in these individuals. Identification of the Gag-reactive antigen(s) in human lung adenocarcinoma should resolve the issue, while elucidation of the signaling pathways activated in sheep tumors may have relevance to oncogenesis in human disease.
Xenotropic MLV-Related Virus and Prostate Cancer
Prostate cancer is one of the most common cancers in men in developed countries and is a leading cause of death (360). The available evidence on the etiology of prostate cancer implicates diet, inflammation, and androgen hormones as contributory cofactors in addition to a strong hereditary component. Several candidate genes have been identified, including some that have roles in innate immunity to infections. One example is RNase L, an RNase that is activated as part of the IFN response and acts to degrade double-stranded viral RNA. An increased risk of prostate cancer is also associated with sexually transmitted infections, although the inflammatory response to infection, rather than any specific agent, is suspected to be involved in promoting the development of neoplastic cells (360).
In light of the defect in antiviral immunity in some familial cases of prostate cancer, a search was made for viruses in tumor tissue using the Virochip microarray, which contains oligonucleotides for all known virus families of animals and plants (511, 512) (Fig. 5). When tumor RNA from prostate cancer was analyzed with this microarray, 8 of 19 samples hybridized with probes from gammaretroviruses (497). Seven of the eight positive specimens were from individuals homozygous for a defective allele of RNase L designated R462Q. Subsequently, the hybridized RNA was recovered from the array and identified as a relative of xenotropic MLV (>96% similarity) and was designated xenotropic MLV-related virus (XMRV) (497). Full-length XMRV genomes identified by PCR from three different tumors had >98% similarity to each other, and the polymorphisms suggested independent acquisition of the virus by these patients. In comparison to previously described murine viruses, XMRV is distinguishable by unique amino acid substitutions and a short deletion in the gag leader region. The prevalence of XMRV in prostate tumors was examined using nested RT-PCR for gag RNA (497). Strikingly, XMRV was detected in 8 of 20 patients homozygous for the impaired R462Q mutant but in only 1 of 66 patients who carried either one or two functional alleles, consistent with the idea that impaired innate immunity could favor infection with this virus.
Following the identification of the XMRV sequence, evidence confirming this as a bona fide human infection has now been presented. Dong and coworkers cloned three XMRV integration sites from tumor tissues from two patients with prostate cancer (125). Two of the integration sites are close to genes encoding the transcription factors CREB5 and NFATc3, while the third is close to APPBP2, a protein involved in microtubule function and androgen signaling. Further studies are needed to determine whether XMRV integration affects the function of these genes and whether this might contribute to tumorigenesis.
Other evidence that XMRV is active in human tissues includes the demonstration of XMRV DNA in tumors by FISH and the detection of MLV-related Gag protein in the same cells by immunohistochemistry (497). In addition, cell culture studies have confirmed that the cloned provirus is infectious in cultured fibroblasts and epithelial cells and that it utilizes a known receptor for xenotropic MLV (125). Furthermore, the replication of XMRV in culture is inhibited by functional IFN response pathways, including RNase L (125), consistent with findings in patients.
The available data confirm that XMRV is a human infection and provide a coherent model for its presence in individuals with a defective innate immune response that fits with existing data on risk factors for prostate cancer. Clearly, it is now important to determine whether XMRV has an etiological role in prostate cancer in these patients. Significantly, in situ studies have demonstrated that XMRV is present in stromal fibroblasts and hematopoietic cells within the tumor but not in neoplastic epithelial cells (497). The infected cells represent less than 1% of the cells within the tumor. This indicates that if XMRV is involved in tumorigenesis, it does not act through the classical mechanism of cis activation of oncogenes in the tumor cell. Nevertheless, a role for stromal cells in promoting tumor progression and survival through the release of modulatory cytokines and chemokines into the local microenvironment has been established for several tumors including prostate cancer (109). Thus, it is possible that XMRV infection may modulate fibroblast function and indirectly promote tumor growth. Alternatively, XMRV may simply represent a commensal infection that has found a particular niche due to defective RNase L function in these patients. So far, nonneoplastic prostate tissue (either normal tissue or tissue from precancerous inflammatory lesions) from R426Q homozygous individuals has not been studied. If such tissue were found to be positive, this would indicate that infection precedes the tumor, consistent with a causal role. Serological studies could also be of value here, although, as discussed above, it can be difficult to interpret the significance of antiretroviral antibodies.
A further interesting question is the origin of XMRV infection in these patients and whether this represents human-to-human transmission or independent zoonotic events from mice or other species. It is possible that XMRV is commonly circulating among humans but establishes infection only in individuals with defective innate immunity. If this is the case, adaptive immunity to prior XMRV infection might explain some of the serological data on antiretroviral antibodies in various patient groups (e.g., see references 78, 96, 212, 369, 477, and 523). In addition, it will also be of interest to examine immunosuppressed populations such as AIDS patients and transplant recipients for XMRV infection.
These two recent papers describing XMRV (125, 497) have presented a solid case for XMRV infection in prostate cancer and provide exciting data for future studies. In addition, this example demonstrates that despite the controversy and skepticism associated with virus discovery, studies on novel human retroviruses are still relevant today. Furthermore, the use of microarrays such as the Virochip microarray may prove to be more productive than PCR for future virus discovery.
HUMAN INFECTION WITH SIMIAN RETROVIRUSES
Studies on HIV and HTLV have revealed that they originated as cross-species infections of humans from other primates. HIV-1 and HIV-2 are thought to have first evolved from simian immunodeficiency viruses (SIVs) in humans within the last 100 years (443), while HTLV-1 and HTLV-2 crossed into humans several thousand years ago (453). Such zoonotic transfers from primates appear to have occurred many times in the recent past and are likely still continuing today. Recent studies have identified new HTLV-like viruses (HTLV-3 and HTLV-4) in individuals from Cameroon (83, 532), although there is no evidence yet that these new viruses are transmitted from human to human.
There have also been a number of reports of human infection with other primate retroviruses. Some of these infections have been shown to represent zoonotic transfer to individuals exposed to infected animals, such as workers in primate centers or researchers handling cultured virus in the laboratory. For example, several cases of humans infected with SIV have been documented (254, 255). Other studies have examined human infection with simian foamy viruses (SFV) (spumaviruses) and primate betaretroviruses. The authenticity of some of these cases remains unconfirmed, but this has not prevented some authors from speculating on an association with disease.
Human Foamy Virus
A spumavirus designated human foamy virus (HFV) was first isolated from cultured lymphoblastoid cells of a Kenyan patient with nasopharyngeal carcinoma in 1971 (4, 327). Additional isolates were reported later in patients with de Quervain thyroiditis (462) and dialysis encephalitis (85). Several attempts to determine the prevalence of HFV in different populations, both geographically and in relation to disease, particularly in thyroid disease and neurological disorders, have been made (6, 64, 85, 268, 363, 462, 525). However, those studies became controversial as different groups produced conflicting results (reviewed in reference 327).
Early seroepidemiological studies suggested that HFV infection is common in East Africa (Kenya and Uganda) and some Pacific islands and rare in regions of North Africa (Tunisia), North America, and Britain (3, 290, 347). However, this was disputed by Brown and colleagues (74, 75), who failed to detect markers of HFV infection in people from any of these areas. The same group also demonstrated that HFV is antigenically related to SFV-6 of chimpanzees, and this was the first suggestion that HFV may be a rare SFV zoonosis or a nonhuman laboratory contaminant (75, 361). Later, Schweizer and coworkers used immunoblotting, enzyme-linked immunosorbent assay, and immunofluorescence to study sera from 2,688 individuals, many of whom were individuals identified as being at “high risk” of HFV infection on the basis of geographical location or disease (437). All but seven of these sera were negative for antibodies to HFV antigens, and the remainder gave indeterminate results for technical reasons. In contrast, control sera from SFV-infected nonhuman primates or from human subjects that had become accidentally infected with SFV-3 or HFV were positive by these tests, demonstrating that foamy virus infection of humans does elicit an antibody response. Ali and colleagues reported similar results in a study of 6,000 human sera (7). PCR on DNA from 223 individuals by use of HFV pol primers also produced exclusively negative results, while DNA from individuals known to be infected with HFV were positive (437).
In light of these data, it is now widely accepted that HFV in humans represents a rare zoonotic infection by a simian spumavirus, and all confirmed cases of infection have been in persons with occupational or other close contact with nonhuman primates (205, 327, 474, 533). Human-to-human spread of the virus has not been demonstrated, even in recipients of blood transfusions from an HFV-infected donor (62). Thus, the papers citing positive results for human infection appear to have been based largely on false-positive immunological assays or laboratory contamination. It should also be noted that genuine cases of human infection, in common with SFV infections of nonhuman primates, are not pathogenic.
Sequence analysis of foamy virus isolates from the four subspecies of chimpanzee shows that the original HFV isolate is most closely related to SFV from Pan troglodytes schweinfurthii (474). Given that this subspecies is native to Kenya, there is a possibility that the original patient (4) may have caught the virus through contact with an infected chimpanzee, although laboratory contamination could also have been the source of this isolate. To indicate the simian origin of HFV, its current designation is SFVcpz(hu) or prototype foamy virus 1 (286). Other zoonoses of spumaviruses from primate species have included infections from African green monkey, bonobos, and, most recently, macaques (72). It is striking that the definitive studies on HFV that determined its true provenance depended on the use of PCR (436, 437, 474), which contrasts well with the difficulties associated with the use of this technique in studies of other human “rumor” viruses.
Primate Betaretroviruses
Primate betaretroviruses (previously designated type D retroviruses) are another group of viruses with the potential to infect humans (141, 157). These viruses naturally infect wild macaques in India and Indonesia and are also common infections in captive macaques. To date, seven serotypes, designated simian retrovirus 1 (SRV-1) to SRV-7, have been found in Asian macaques housed in primate centers in the United States or China and in feral monkeys in India (358). SRVs cause an immunosuppressive disease that resembles simian AIDS but which is distinct from that induced by SIV. The first SRV isolated, MPMV (SRV-3), was originally detected in 1970 in a macaque with breast cancer (92). Although this group of viruses is now not regarded as being oncogenic, the identification of MPMV in a mammary carcinoma helped to perpetuate the theory that a related virus might cause breast cancer in humans (see above) (523).
The most convincing case report of human SRV infection described MPMV infection in an AIDS patient with a B lymphoma (59, 146). Infectious virus was isolated from cultures of lymphoma tissue, and bone marrow DNA was positive by PCR with MPMV gag, pol, and env primers. Serum antibodies that were reactive with MPMV Gag and Env proteins were detected by immunoprecipitation and immunoblotting. This appears to be a genuine human MPMV infection since several lines of evidence were in agreement. However, whether the virus played any role in the pathogenesis of AIDS or the lymphoma could not be determined. Also, it was not known whether MPMV was acquired before, at the same time as, or after HIV infection, although it is possible that MPMV was acquired as an opportunistic infection after the development of AIDS.
While this appears to be a genuine human infection, other reports of SRV in humans have been more difficult to assess (204). Serological studies have either supported (224, 483) or disputed (88) human SRV infection. More recent large-scale studies in groups considered to be at risk for retroviral infection could not confirm SRV infection in humans, although a small percentage of patients with lymphoma scored as weak “indeterminate” positives (281). As with SFV and SIV, rare cases of genuine infection of primate handlers with SRVs have been described (282). In addition, SRVs grow efficiently in many human cell lines, and a number of reports that claimed the isolation of new human retrovirus turned out to be cases of laboratory contamination with SRV (19, 262, 496).
In the PCR age, there have been a few further attempts to detect SRV infections in humans. MPMV sequences were reported for individuals in Guinea (343). MPMV antibodies and sequences have also been detected in Russian children with Burkitt's lymphoma (225, 266). In that small study, purified lymphocytes from 6 of 8 children with lymphoma were positive by PCR for MPMV sequences, while 20 healthy donors were negative. Interestingly, five of the six positive children had at least one parent who also had evidence of MPMV infection.
In those reports of PCR detection, the sequences obtained were all almost identical to the original molecular clone of MPMV. Since the natural reservoir for MPMV is Asian macaques, it is reasonable to ask whether virus isolates from different hosts and on different continents would be so similar. Only one type D virus sequence from a primate not native to Asia has been reported, and this was obtained from captive Ethiopian baboons, which is most likely a result of cross-species transfer from macaques (183). This virus had 82 to 85% nucleotide sequence similarity to MPMV and SRV-2 in the Gag region. Thus, these instances of MPMV detection in humans need confirmation to be accepted as genuine.
HUMAN RNA “RUMOR” VIRUSES: PROVING CAUSATION
The past 25 years have seen extraordinary developments in the field of human retrovirology, with the emergence of HIV and AIDS being of overwhelming importance. In addition, the identification of HERVs and the recognition of zoonotic transfer as a mechanism of human infection have been important in shaping our view of the evolutionary relationships between these pathogens and ourselves. The ease with which cross-species transfer of retroviruses can occur has implications for plans for xenotransplantation of animal organs into humans (306). In light of these developments, the candidate human retroviruses discussed here all deserve our attention, whether genuine or not. The ease by which the PCR can be applied has led to an increase in the number of new putative retroviruses (and other viruses) associated with human disease. However, as we have described above, many of these data have not received acceptance among retrovirologists, and a number of controversial claims remain unresolved.
Why has it been so difficult to make progress in this field? One contributory factor has been that some of the claims for novel human retroviruses have relied on one or two high-profile papers, which have subsequently failed to be corroborated by others. The skepticism that exists in the virological community has perhaps deterred others from attempting to reproduce those preliminary reports experimentally. This is unfortunate, since it is important to validate any new virus quickly so that the importance of each claim can be assessed and erroneous reports can be corrected. For example, reports of HTLV sequences in MS (186, 408) were quickly scotched by negative findings from several groups (27, 114, 130, 237, 244, 366, 370, 412, 520). Similarly, large-scale serological and PCR analyses of HFV infections were crucial to the identification of this virus as a zoonotic agent from nonhuman primates (7, 436, 437).
Another problem with making sense of the disparate data on retroviruses and human disease is that few studies have attempted to link the various experimental observations in the same group of patients. For example, in psoriasis, a common chronic inflammatory disease of the skin, various observations that implicate retroviruses have been made. RVLPs have been observed in tissue biopsies, in derived cell lines, and in patient urine by EM (110, 230). A putative retroviral antigen designated pso-p27 was purified from these particles (231), and antibodies to pso-p27 were detected in patients (232). More recently, HERV-E RNA and Env proteins were found in psoriatic lesions (43, 336), and antibodies that were reactive with MLV Gag and Env in patient sera have been described (335). Interestingly, HERV-E expression is down-regulated by UV irradiation, which also clears psoriatic lesions (43, 214). While those reports provide intriguing evidence of retroviral activity in psoriasis, it is difficult to draw together the various strands, and none of the studies has related retrovirus expression to immunopathology. HERV-E is the latest target, but it remains to be seen whether this is linked to the previously observed RVLPs or to the serological reactivity.
Similar issues surround the putative role of retroviruses in thyroid diseases. HFV was linked to Graves' disease by serology, PCR, and Southern hybridization prior to the recognition that this virus is not a common human infection (268). In addition, sequences related to HIV-1 were detected in DNA from thyroid tissue and PBMCs of Graves' disease patients by Southern hybridization (100). Whether these data are due to cross-reaction with HERVs is unknown but unlikely given the absence of closely related elements in the human genome. Other studies reported that 87% of Graves' disease patients have antibodies to two proteins present in lysates of cells producing HIAP-I (235), but attempts to rescue a virus by cocultivation with H9 cells as was done for HIAP-I, HIAP-II, and HICRV (161, 162, 193) were unsuccessful (139). There are also reports on putative retroviruses in other thyroid disorders. For example, isolates of HFV were reported in patients with de Quervain thyroiditis (462, 525). As with psoriasis, there is nothing to link these various observations, and it is unclear whether they represent the detection of the same entity or how they might be related to disease.
A further difficulty in studying novel retroviral infections relates to the practicalities of obtaining an adequate number of tissue specimens. In order to demonstrate a strong and specific association between a disease and an etiologic agent, sufficient clinical specimens and appropriate controls are required for a robust statistical analysis. In some instances, this may not have been the case. Possibly, this has been due to genuine difficulties in obtaining sufficient specimens; for example, initial studies on a putative retrovirus in MS used cultured leptomeningeal cells from a single patient (387). Here, a rigorous examination of tissues from 30 to 40 patients would have been difficult due to the restricted availability of the appropriate material.
The clinical and pathogenetic heterogeneity of some of the proposed retrovirus-associated diseases such as SLE, RA, and MS can also present a problem in defining the role of infection because it is possible that a virus might be involved in a specific subtype of a particular disease but that such subtypes are not recognized by existing diagnostic criteria. For example, in MS, four distinct patterns of demyelinated plaques have been described (297). In comparison with established experimental models of MS, two of these patterns of demyelination resemble an autoimmune-mediated pathology directed against myelin, whereas the other two patterns resemble models where oligodendrocyte pathology is the primary lesion, with demyelination being a secondary event. Importantly, within an individual patient, all lesions show the same pattern, which is suggestive of a single mechanism. Therefore, distinct forms of MS in different patients may involve fundamentally different etiological factors (or combinations of factors) but may be difficult to distinguish clinically. An improved understanding of the different pathogenic mechanisms that play a role in complex diseases and how they relate to clinical markers would greatly assist the identification of the etiological factors involved.
Similarly, for some diseases, diagnostic criteria may change over time, and clinicians can differ in how they diagnose certain conditions. An example is BAC, which is currently classified as being a strictly noninvasive tumor (490). The classification of lung tumors has been revised several times over the last 30 years, and previous systems did not have such a restricted definition of BAC (490). Moreover, even under the previous classification, reports from European laboratories suggested that the frequency of BAC is around 1% of all lung cancers, while studies in the United States put the figure at around 25% (35, 341). Although genuine geographical differences exist for some human tumors, this does not appear to be the case in this instance, and the apparent variation in prevalence is more likely to be due to regional differences in how diagnostic criteria are applied (457). Clearly, such inconsistency in diagnosis has significance for virologists studying the potential involvement of a retrovirus in BAC.
Many of the diseases that are suspected to have a retroviral involvement are chronic conditions such as autoimmune inflammatory diseases, neurological disorders, and cancers that are thought to have a multifactorial etiology (Table 3). In addition to the postulated retroviral infection, other factors (genetic or environmental) are proposed to be required for the manifestation of symptoms. This may lead to difficulties in demonstrating a causal association, since the virus may be a common infection, and specificity may therefore be difficult to show without a good understanding of the other cofactors required. The specific association of the virus with the target cell of the disease can strengthen the evidence for a causative role but does not provide definitive proof. Together, these issues can confound studies on novel retroviruses since they create difficulties in determining the specificity of the disease association. When the effect of HERV expression is added, the challenge of assessing the role of the candidate virus can be appreciated. “Traditional” methodologies such as serology and RT assays can provide supporting evidence, but this is only circumstantial when these approaches are used alone. PCR or other molecular approaches are necessary to identify the agent and confirm that it as a human infection prior to analysis of disease association.
Assessing the Etiological Role
Guidelines for demonstrating causation in infectious disease were first set out by Henle and Koch in the 19th century (152) and are commonly cited as the definitive method of proving infectious disease etiology. However, while Koch's postulates have been an important concept in defining our understanding of acute infectious disease, it is now accepted that a broader framework is required for assessing the importance of a candidate pathogen in complex multifactorial disease (152). Such a framework needs to accommodate current knowledge of cell biology yet be flexible enough to allow for developing themes and new paradigms. A number of revisions to Koch's postulates have been suggested (133, 134, 152, 210, 220, 417, 510) and are exemplified by those proposed by Hill (210), who suggested nine criteria that may be used for evaluating the role of a putative causative factor (Table 7). These tests include determining the strength, specificity, and consistency of an association and evaluating whether such an association is biologically plausible and coherent. The association can also be tested experimentally or by drawing information from analogous situations, such as animal models. Although devised originally for environmental and occupational medicine, these tests can be applied to infectious diseases including chronic infections since they simply provide a structure for weighing the epidemiological, experimental, and clinical evidence for causation.
TABLE 7.
Hill's criteria for causation in diseasea
| Criterion | Association |
|---|---|
| Strength of association | What is the relative increased risk in disease after exposure to virus? |
| Consistency of association | How reproducible are the data with different methods and by different laboratories, etc.? |
| Specificity of association | Is the disease unique to exposure to virus? |
| Temporality | Does disease follow exposure? |
| Biological gradient | Does an increased dose of virus lead to more rapid onset or more severe symptoms? |
| Plausibility | Is a causal relationship biologically plausible? |
| Coherence | Is a relationship compatible with present knowledge of the disease? |
| Experiment | Does experimental manipulation or intervention affect disease outcome, e.g., therapy? |
| Analogy | Is there a comparable association with related virus or disease, e.g., animal models? |
The value of Hill's criteria can be illustrated by considering the data on the known human retroviruses. On this basis, there is an extremely strong case that HIV-1 causes AIDS in the absence of Koch's postulates being fulfilled. The strength and specificity of the association between virus and disease are high since HIV is found in all cases of AIDS (albeit by definition) and is not regularly associated with any other disease (12). This has been confirmed by a large number of independent studies from laboratories around the world. The knowledge that the major cellular targets of HIV-1 are CD4+ T lymphocytes and macrophages gives plausibility and coherence to the immunosuppressive pathology. Pharmaceutical intervention using highly active antiretroviral therapy to target HIV is clinically beneficial, providing experimental evidence. Finally, AIDS in macaques caused by SIV provides an analogous situation in animals. Thus, for HIV and AIDS, Hill's criteria are all met, and the case for causation is not doubted, except for a few diehard skeptics (465, 524).
HTLV-1 fits Hill's criteria less well since only around 1% of infected individuals develop ATL, and the incubation period between infection and onset of ATL may be as long as 50 years or more; thus, the strength of the association is low. Also, since HTLV-1 is present in at least two (and likely more) distinct diseases, the specificity of association of HTLV in ATL and HAM/TSP is weakened slightly. Nevertheless, the presence of the HTLV provirus clonally integrated into ATL cells and the uncovering of the central role of the viral Tax protein in activating cellular gene expression provide a plausible explanation for a role for HTLV-1 in tumorigenesis (23). Similar advances in understanding the immune response to HTLV-1 appear likely to provide an explanation of how virus infection results in the inflammatory pathogenesis of HAM/TSP (180, 506). Pharmaceutical intervention for HTLV with antiretroviral drugs has only recently been attempted, but early results indicate that it is clinically beneficial, at least in ATL (36, 481). Thus, the available evidence on HTLV-1 and ATL or HAM/TSP does not fit Hill's criteria as closely as does HIV-1 and AIDS, but taken together, a strong case exists for the involvement of the virus in these diseases, and indeed, such a role is not doubted.
These examples illustrate how Hill's criteria can provide a framework for testing an association of virus and disease, but they also show that we would not necessarily expect all of the criteria to be satisfied as long as the balance of evidence is in favor of a causal role (152). Therefore, when considering the evidence for putative new retroviruses in human disease, it is prudent to keep a broad mind and not dismiss out of hand those cases that do not fit an accepted dogma of how retroviral diseases should behave. Nevertheless, if we apply these criteria to some of the prominent candidate retroviruses described above, we can see that on this basis, there is much work to be done to demonstrate a causal role for any of these agents in human disease (Table 8). Most have an analogous disease in animals, but this has generally been the starting point for experimental studies. Aside from this, it is striking that insufficient independent reports have been published for any of these candidates.
TABLE 8.
Candidate retroviruses in human disease assessed by Hill's criteriaa
| Causal criterion | Association
|
|||||||
|---|---|---|---|---|---|---|---|---|
| HIV-1 and AIDS | HTLV-I and ATL | MMTV and breast cancer | HERV-W and MS | HERV-K18 and IDDM | HIAP and SS/ICL | HRV-5 and RA, SLE, lymphoma | XMRV | |
| Strength of association | Y | Y | N | NDb | NDb | ND | N | ND |
| Consistency of association | Y | Y | N | Yc | N | ND | N | ND |
| Specificity of association | Y | N | N | Nb | Nb | N | N | ND |
| Temporality | Y | Y | ND | Yb | Yb | ND | ND | ND |
| Biological gradient | Y | Y | ND | ND | ND | ND | ND | Y |
| Plausibility | Y | Y | N | ND | ND | ND | ND | ND |
| Coherence | Y | Y | N | ND | ND | ND | ND | Y |
| Experimentation | Y | Y | ND | ND | ND | ND | ND | ND |
| Analogy | Y | Y | Y | Y | Y | Y | Y | Y |
Y, yes; N, no; ND, not done/insufficient data.
Because endogenous.
For the Env protein.
Perhaps the strongest case exists for MMTV in breast cancer; however, despite studies that have been ongoing for over 50 years, more work is still needed to confirm or refute whether it has a role in human malignancy. The interest generated in recent years suggests that there are now sufficient laboratories involved to provide independent verification of research findings and resolve the issue. The preliminary report of MMTV integration in PBC patients (see above) is an exciting development and, if confirmed, will be an important milestone in the field. Similarly, the description of integration sites for XMRV in prostate cancer (125) validates this as a human infection. The finding that murine retroviruses can potentially infect humans raises the question of how much of the evidence on retroviral infection in humans is due to zoonoses such as these.
For the other candidates, few of Hill's criteria for causation have been met. The pathological role of the various human IAPs cannot be addressed until the coding genes are identified. The HRV-5 question appears to have been resolved by its identification as a rabbit ERV, and indeed, there was not a strong association with any specific disease despite the data being reproduced in different laboratories. MSRV and HERV-H in MS appear unlikely to act as replicating agents, although HERV-W expression in MS led to the discovery of the proinflammatory effects of its Env protein (13). The ubiquitous nature of HERV-W and HERV-K means that future studies on their contribution to disease need to focus on the role of individual proviral loci rather than these families as a whole. In this respect, the identification and characterization of insertionally polymorphic HERVs may be fruitful (345). The large body of literature on HERVs and disease has led to the identification of a number of specific molecules for future study, including HERV-K SAg, Rec and Np9, and syncytin-1. Other HERV proteins may yet prove to have pathological importance.
CONCLUDING REMARKS
As we have discussed above, the characterization of putative novel human retroviruses other than HIV and HTLV has led to a number of dead ends and produced little direct evidence of their involvement in disease. Advances in PCR and other molecular detection methods have greatly enhanced our ability to detect new infections but have not led to the identification of a confirmed retroviral pathogen. Nonetheless, there is still great interest in novel human retroviruses, as exemplified by recent papers on XMRV (125, 497). Given the level of skepticism in the field, new claims of retroviral infection require a substantial level of proof to convince virologists, and the experimental design must be sufficiently robust to withstand the most critical review. In particular, direct molecular evidence is much stronger than circumstantial data. The two reports on XMRV provide excellent examples of the way forward, although even here, independent confirmation of the presence of the virus in prostate cancer is still required.
Despite the experimental difficulties, the chronic diseases linked with retroviruses (Table 2) cause significant morbidity and mortality, so it is important to thoroughly evaluate any candidate pathogens even if there is a high chance of failure. The notion that the cause of any of these complex diseases is due to a single infectious etiological agent is most likely naïve, but the confirmation of a role for a virus (retroviral or other) would have far-reaching benefits for the diagnosis and treatment of what are frequently devastating conditions, and the search is therefore warranted. In the past 25 years, a high number of genuine novel human viruses have been discovered (250), and it appears highly likely that there are others still to be identified, some of which may be retroviruses. New genome-based molecular techniques are likely to increase the number of viruses discovered in human tissues and may circumvent the difficulties associated with identifying retroviruses by PCR. Ultimately, however, improved criteria for determining causation need to be defined.
Acknowledgments
We thank Mariam Andrawiss, Francois-Loic Cosset, Jean Dubuisson, Paul Kellam, Stuart Neil, and Peter Simmonds for critical reading of the manuscript and numerous colleagues for discussion of the issues presented here. We are grateful to Sophana Ung for help with preparing Fig. 1 and to Klaus Boller, Mark Boyd, Joseph DiRisi, David Fredricks, Sudhir Gupta, Sven Haahr, Norman Maclean, Andrew Mason, Ichiro Saito, Hervé Perron, Nurul Sarkar, and the copyright holders for permission to reuse previously published figures and tables.
We thank the Medical Research Council, the Arthritis Research Council, the Wellcome Trust, the ANRS, and the Scottish Environment and Rural Affairs Department for support.
REFERENCES
- 1.Acha-Orbea, H., D. Finke, A. Attinger, S. Schmid, N. Wehrli, S. Vacheron, I. Xenarios, L. Scarpellino, K. M. Toellner, I. C. MacLennan, and S. A. Luther. 1999. Interplays between mouse mammary tumor virus and the cellular and humoral immune response. Immunol. Rev. 168287-303. [DOI] [PubMed] [Google Scholar]
- 2.Acha-Orbea, H., and H. R. MacDonald. 1995. Superantigens of mouse mammary tumor virus. Annu. Rev. Immunol. 13459-486. [DOI] [PubMed] [Google Scholar]
- 3.Achong, B. G., and M. A. Epstein. 1978. Preliminary seroepidemiological studies on the human syncytial virus. J. Gen. Virol. 40175-181. [DOI] [PubMed] [Google Scholar]
- 4.Achong, B. G., P. W. Mansell, M. A. Epstein, and P. Clifford. 1971. An unusual virus in cultures from a human nasopharyngeal carcinoma. J. Natl. Cancer Inst. 46299-307. [PubMed] [Google Scholar]
- 5.Achong, B. G., P. A. Trumper, and B. C. Giovanella. 1976. C-type virus particles in human tumours transplanted into nude mice. Br. J. Cancer 34203-206.183801 [Google Scholar]
- 6.Aguzzi, A., K. Bothe, E. F. Wagner, A. Rethwilm, and I. Horak. 1992. Human foamy virus: an underestimated neuropathogen? Brain Pathol. 261-69. [PubMed] [Google Scholar]
- 7.Ali, M., G. P. Taylor, R. J. Pitman, D. Parker, A. Rethwilm, R. Cheingsong-Popov, J. N. Weber, P. D. Bieniasz, J. Bradley, and M. O. McClure. 1996. No evidence of antibody to human foamy virus in widespread human populations. AIDS Res. Hum. Retrovir. 121473-1483. [DOI] [PubMed] [Google Scholar]
- 8.Allander, T., K. Andreasson, S. Gupta, A. Bjerkner, G. Bogdanovic, M. A. Persson, T. Dalianis, T. Ramqvist, and B. Andersson. 2007. Identification of a third human polyomavirus. J. Virol. 814130-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allander, T., M. T. Tammi, M. Eriksson, A. Bjerkner, A. Tiveljung-Lindell, and B. Andersson. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. USA 10212891-12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambinder, R. F. 2000. Gammaherpesviruses and “hit-and-run” oncogenesis. Am. J. Pathol. 1561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews, W. D., P. W. Tuke, A. Al-Chalabi, P. Gaudin, S. Ijaz, M. J. Parton, and J. A. Garson. 2000. Detection of reverse transcriptase activity in the serum of patients with motor neurone disease. J. Med. Virol. 61527-532. [DOI] [PubMed] [Google Scholar]
- 12.Anonymous. 2000. The Durban declaration. Nature 40615-16. [DOI] [PubMed] [Google Scholar]
- 13.Antony, J. M., G. van Marle, W. Opii, D. A. Butterfield, F. Mallet, V. W. Yong, J. L. Wallace, R. M. Deacon, K. Warren, and C. Power. 2004. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat. Neurosci. 71088-1095. [DOI] [PubMed] [Google Scholar]
- 14.Araujo, A., and W. W. Hall. 2004. Human T-lymphotropic virus type II and neurological disease. Ann. Neurol. 5610-19. [DOI] [PubMed] [Google Scholar]
- 15.Arbach, H., V. Viglasky, F. Lefeu, J. M. Guinebretière, V. Ramirez, N. Bride, N. Boualaga, T. Bauchet, J. P. Peyrat, M. C. Mathieu, S. Mourah, M. P. Podgorniak, J. M. Seignerin, K. Takada, and I. Joab. 2006. Epstein-Barr virus (EBV) genome and expression in breast cancer tissue: effect of EBV infection of breast cancer cells on resistance to paclitaxel (Taxol). J. Virol. 80845-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arimori, S., K. Ichimura, M. Tokunaga, and K. Morita. 1990. Retrovirus-like particles in human thymomas. Tokai J. Exp. Clin. Med. 15219-225. [PubMed] [Google Scholar]
- 17.Armbruester, V., M. Sauter, E. Krautkraemer, E. Meese, A. Kleiman, B. Best, K. Roemer, and N. Mueller-Lantzsch. 2002. A novel gene from the human endogenous retrovirus K expressed in transformed cells. Clin. Cancer Res. 81800-1807. [PubMed] [Google Scholar]
- 18.Armbruester, V., M. Sauter, K. Roemer, B. Best, S. Hahn, A. Nty, A. Schmid, S. Philipp, A. Mueller, and N. Mueller-Lantzsch. 2004. Np9 protein of human endogenous retrovirus K interacts with ligand of Numb protein X. J. Virol. 7810310-10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asikainen, K., M. Vesanen, T. Kuittinen, and A. Vaheri. 1993. Identification of human type D retrovirus as a contaminant in a neuroblastoma cell line. Arch. Virol. 129357-361. [DOI] [PubMed] [Google Scholar]
- 20.Axel, R., S. C. Gulati, and S. Spiegelman. 1972. Particles containing RNA-instructed DNA polymerase and virus-related RNA in human breast cancers. Proc. Natl. Acad. Sci. USA 693133-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Axel, R., J. Schlom, and S. Spiegelman. 1972. Presence in human breast cancer of RNA homologous to mouse mammary tumour virus RNA. Nature 23532-36. [DOI] [PubMed] [Google Scholar]
- 22.Azar, G. A., and J. Thibodeau. 2002. Human endogenous retrovirus IDDMK1,222 and mouse mammary tumor virus superantigens differ in their ability to stimulate murine T cell hybridomas. Immunol. Lett. 8187-91. [DOI] [PubMed] [Google Scholar]
- 23.Azran, I., Y. Schavinsky-Khrapunsky, and M. Aboud. 2004. Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badenhoop, K., H. Donner, J. Neumann, J. Herwig, R. Kurth, K. H. Usadel, and R. R. Tönjes. 1999. IDDM patients neither show humoral reactivities against endogenous retroviral envelope protein nor do they differ in retroviral mRNA expression from healthy relatives or normal individuals. Diabetes 48215-218. [DOI] [PubMed] [Google Scholar]
- 25.Balda, B. R., R. Hehlmann, J. R. Cho, and S. Spiegelman. 1975. Oncornavirus-like particles in human skin cancers. Proc. Natl. Acad. Sci. USA 723697-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ball, J. K., H. Diggelmann, G. A. Dekaban, G. F. Grossi, R. Semmler, P. A. Waight, and R. F. Fletcher. 1988. Alterations in the U3 region of the long terminal repeat of an infectious thymotropic type B retrovirus. J. Virol. 622985-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bangham, C. R., S. Nightingale, J. K. Cruickshank, and S. Daenke. 1989. PCR analysis of DNA from multiple sclerosis patients for the presence of HTLV-I. Science 246821-824. [PubMed] [Google Scholar]
- 28.Banki, K., J. Maceda, E. Hurley, E. Ablonczy, D. H. Mattson, L. Szegedy, C. Hung, and A. Perl. 1992. Human T-cell lymphotropic virus (HTLV)-related endogenous sequence, HRES-1, encodes a 28-kDa protein: a possible autoantigen for HTLV-I gag-reactive autoantibodies. Proc. Natl. Acad. Sci. USA 891939-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bannert, N., and R. Kurth. 2006. The evolutionary dynamics of human endogenous retroviral families. Annu. Rev. Genomics Hum. Genet. 7149-173. [DOI] [PubMed] [Google Scholar]
- 30.Bannert, N., and R. Kurth. 2004. Retroelements and the human genome: new perspectives on an old relation. Proc. Natl. Acad. Sci. USA 101(Suppl. 2)14572-14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbacid, M., D. Bolognesi, and S. A. Aaronson. 1980. Humans have antibodies capable of recognizing oncoviral glycoproteins: demonstration that these antibodies are formed in response to cellular modification of glycoproteins rather than as consequence of exposure to virus. Proc. Natl. Acad. Sci. USA 771617-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barmak, K., E. Harhaj, C. Grant, T. Alefantis, and B. Wigdahl. 2003. Human T cell leukemia virus type I-induced disease: pathways to cancer and neurodegeneration. Virology 3081-12. [DOI] [PubMed] [Google Scholar]
- 33.Barré-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vézinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220868-871. [DOI] [PubMed] [Google Scholar]
- 34.Bar-Sinai, A., N. Bassa, M. Fischette, M. M. Gottesman, D. C. Love, J. A. Hanover, and J. Hochman. 2005. Mouse mammary tumor virus Env-derived peptide associates with nucleolar targets in lymphoma, mammary carcinoma, and human breast cancer. Cancer Res. 657223-7230. [DOI] [PubMed] [Google Scholar]
- 35.Barsky, S. H., R. Cameron, K. E. Osann, D. Tomita, and E. C. Holmes. 1994. Rising incidence of bronchioloalveolar lung carcinoma and its unique clinicopathologic features. Cancer 731163-1170. [DOI] [PubMed] [Google Scholar]
- 36.Bazarbachi, A., D. Ghez, Y. Lepelletier, R. Nasr, H. de Thé, M. E. El-Sabban, and O. Hermine. 2004. New therapeutic approaches for adult T-cell leukaemia. Lancet Oncol. 5664-672. [DOI] [PubMed] [Google Scholar]
- 37.Beatty, B., S. Mai, and J. Squire. 2002. FISH: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 38.Belshaw, R., A. L. Dawson, J. Woolven-Allen, J. Redding, A. Burt, and M. Tristem. 2005. Genomewide screening reveals high levels of insertional polymorphism in the human endogenous retrovirus family HERV-K(HML2): implications for present-day activity. J. Virol. 7912507-12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belshaw, R., V. Pereira, A. Katzourakis, G. Talbot, J. Pačes, A. Burt, and M. Tristem. 2004. Long-term reinfection of the human genome by endogenous retroviruses. Proc. Natl. Acad. Sci. USA 1014894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benoist, C., and D. Mathis. 2001. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat. Immunol. 2797-801. [DOI] [PubMed] [Google Scholar]
- 41.Berkhout, B., M. Jebbink, and J. Zsíros. 1999. Identification of an active reverse transcriptase enzyme encoded by a human endogenous HERV-K retrovirus. J. Virol. 732365-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernhard, W. 1958. Electron microscopy of tumor cells and tumor viruses; a review. Cancer Res. 18491-509. [PubMed] [Google Scholar]
- 43.Bessis, D., J. P. Molès, N. Basset-Séguin, A. Tesniere, C. Arpin, and J. J. Guilhou. 2004. Differential expression of a human endogenous retrovirus E transmembrane envelope glycoprotein in normal, psoriatic and atopic dermatitis human skin. Br. J. Dermatol. 151737-745. [DOI] [PubMed] [Google Scholar]
- 44.Best, S., P. Le Tissier, G. Towers, and J. P. Stoye. 1996. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382826-829. [DOI] [PubMed] [Google Scholar]
- 45.Bhadra, S., M. M. Lozano, S. M. Payne, and J. P. Dudley. 2006. Endogenous MMTV proviruses induce susceptibility to both viral and bacterial pathogens. PLoS Pathog. 2e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bieda, K., A. Hoffmann, and K. Boller. 2001. Phenotypic heterogeneity of human endogenous retrovirus particles produced by teratocarcinoma cell lines. J. Gen. Virol. 82591-596. [DOI] [PubMed] [Google Scholar]
- 47.Bieniasz, P. D., A. Rethwilm, R. Pitman, M. D. Daniel, I. Chrystie, and M. O. McClure. 1995. A comparative study of higher primate foamy viruses, including a new virus from a gorilla. Virology 207217-228. [DOI] [PubMed] [Google Scholar]
- 48.Billich, C., C. Sauder, R. Frank, S. Herzog, K. Bechter, K. Takahashi, H. Peters, P. Staeheli, and M. Schwemmle. 2002. High-avidity human serum antibodies recognizing linear epitopes of Borna disease virus proteins. Biol. Psych. 51979-987. [DOI] [PubMed] [Google Scholar]
- 49.Bindra, A., S. Muradrasoli, R. Kisekka, H. Nordgren, F. Warnberg, and J. Blomberg. 2007. Search for DNA of exogenous mouse mammary tumor virus-related virus in human breast cancer samples. J. Gen. Virol. 881806-1809. [DOI] [PubMed] [Google Scholar]
- 50.Birkmayer, G. D., B. R. Balda, and F. Miller. 1974. Oncorna-viral information in human melanoma. Eur. J. Cancer 10419-424. [DOI] [PubMed] [Google Scholar]
- 51.Bishop, J. M., N. Quintrell, E. Medeiros, and H. E. Varmus. 1974. Of birds and mice and men: comments on the use of animal models and molecular hybridization in the search for human tumor viruses. Cancer 341421-1426. [DOI] [PubMed] [Google Scholar]
- 52.Bjerregaard, B., S. Holck, I. J. Christensen, and L. I. Larsson. 2006. Syncytin is involved in breast cancer-endothelial cell fusions. Cell. Mol. Life Sci. 631906-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blaise, S., N. de Parseval, L. Benit, and T. Heidmann. 2003. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. USA 10013013-13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blaise, S., N. de Parseval, and T. Heidmann. 2005. Functional characterization of two newly identified human endogenous retrovirus coding envelope genes. Retrovirology 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blond, J. L., F. Besème, L. Duret, O. Bouton, F. Bedin, H. Perron, B. Mandrand, and F. Mallet. 1999. Molecular characterization and placental expression of HERV-W, a new human endogenous retrovirus family. J. Virol. 731175-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blond, J. L., D. Lavillette, V. Cheynet, O. Bouton, G. Oriol, S. Chapel-Fernandes, B. Mandrand, F. Mallet, and F. L. Cosset. 2000. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 743321-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boeke, J. D., and J. P. Stoye. 1997. Retrotransposons, endogenous retroviruses, and the evolution of retroelements, p. 343-435. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- 58.Boese, A., M. Sauter, U. Galli, B. Best, H. Herbst, J. Mayer, E. Kremmer, K. Roemer, and N. Mueller-Lantzsch. 2000. Human endogenous retrovirus protein cORF supports cell transformation and associates with the promyelocytic leukemia zinc finger protein. Oncogene 194328-4336. [DOI] [PubMed] [Google Scholar]
- 59.Bohannon, R. C., L. A. Donehower, and R. J. Ford. 1991. Isolation of a type D retrovirus from B-cell lymphomas of a patient with AIDS. J. Virol. 655663-5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boller, K., O. Janssen, H. Schuldes, R. R. Tönjes, and R. Kurth. 1997. Characterization of the antibody response specific for the human endogenous retrovirus HTDV/HERV-K. J. Virol. 714581-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boller, K., H. König, M. Sauter, N. Mueller-Lantzsch, R. Löwer, J. Löwer, and R. Kurth. 1993. Evidence that HERV-K is the endogenous retrovirus sequence that codes for the human teratocarcinoma-derived retrovirus HTDV. Virology 196349-353. [DOI] [PubMed] [Google Scholar]
- 62.Boneva, R. S., A. J. Grindon, S. L. Orton, W. M. Switzer, V. Shanmugam, A. I. Hussain, V. B. Bhullar, M. E. Chamberland, W. Heneine, T. M. Folks, and L. E. Chapman. 2002. Simian foamy virus infection in a blood donor. Transfusion 42886-891. [DOI] [PubMed] [Google Scholar]
- 63.Bossi, P., S. Perrot, N. Dupin, A. G. Marcelin, D. Griffiths, J. M. Huraux, C. J. Menkes, and V. Calvez. 1999. Human retrovirus-5 and Sjogren's syndrome. Clin. Microbiol. Infect. 5105-106. [DOI] [PubMed] [Google Scholar]
- 64.Bothe, K., A. Aguzzi, H. Lassmann, A. Rethwilm, and I. Horak. 1991. Progressive encephalopathy and myopathy in transgenic mice expressing human foamy virus genes. Science 253555-557. [DOI] [PubMed] [Google Scholar]
- 65.Bower, M., C. Palmieri, and T. Dhillon. 2006. AIDS-related malignancies: changing epidemiology and the impact of highly active antiretroviral therapy. Curr. Opin. Infect. Dis. 1914-19. [DOI] [PubMed] [Google Scholar]
- 66.Boyd, M. T., N. Maclean, and D. G. Oscier. 1989. Detection of retrovirus in patients with myeloproliferative disease. Lancet i814-817. [DOI] [PubMed] [Google Scholar]
- 67.Brand, A., D. J. Griffiths, C. Hervé, E. Mallon, and P. J. Venables. 1999. Human retrovirus-5 in rheumatic disease. J. Autoimmun. 13149-154. [DOI] [PubMed] [Google Scholar]
- 68.Bringuier, A.-F., and M.-L. Labat. 1995. Retroviral expression in a patient who has paraarticular ossification. Clin. Orthop. Relat. Res. 313239-252. [PubMed] [Google Scholar]
- 69.Brodsky, I., B. Foley, and D. Gillespie. 1993. Expression of human endogenous retrovirus (HERV-K) in chronic myeloid leukemia. Leuk. Lymphoma 11(Suppl. 1)119-123. [DOI] [PubMed] [Google Scholar]
- 70.Bromham, L., F. Clark, and J. J. McKee. 2001. Discovery of a novel murine type C retrovirus by data mining. J. Virol. 753053-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bronson, D. L., E. E. Fraley, J. Fogh, and S. S. Kalter. 1979. Induction of retrovirus particles in human testicular tumor (Tera-1) cell cultures: an electron microscopic study. J. Natl. Cancer Inst. 63337-339. [PubMed] [Google Scholar]
- 72.Brooks, J. I., E. W. Rud, R. G. Pilon, J. M. Smith, W. M. Switzer, and P. A. Sandstrom. 2002. Cross-species retroviral transmission from macaques to human beings. Lancet 360387-388. [DOI] [PubMed] [Google Scholar]
- 73.Broussard, S. R., A. G. Comuzzie, K. L. Leighton, M. M. Leland, E. M. Whitehead, and J. S. Allan. 1997. Characterization of new simian foamy viruses from African nonhuman primates. Virology 237349-359. [DOI] [PubMed] [Google Scholar]
- 74.Brown, P., M. C. Moreau-Dubois, and D. C. Gajdusek. 1982. Persistent asymptomatic infection of the laboratory mouse by simian foamy virus type 6: a new model of retrovirus latency. Arch. Virol. 71229-234. [DOI] [PubMed] [Google Scholar]
- 75.Brown, P., G. Nemo, and D. C. Gajdusek. 1978. Human foamy virus: further characterization, seroepidemiology, and relationship to chimpanzee foamy viruses. J. Infect. Dis. 137421-427. [DOI] [PubMed] [Google Scholar]
- 76.Brudek, T., T. Christensen, H. J. Hansen, J. Bobecka, and A. Møller-Larsen. 2004. Simultaneous presence of endogenous retrovirus and herpes virus antigens has profound effect on cell-mediated immune responses: implications for multiple sclerosis. AIDS Res. Hum. Retrovir. 20415-423. [DOI] [PubMed] [Google Scholar]
- 77.Brudek, T., P. Lühdorf, T. Christensen, H. J. Hansen, and A. Møller-Larsen. 2007. Activation of endogenous retrovirus reverse transcriptase in multiple sclerosis patient lymphocytes by inactivated HSV-1, HHV-6 and VZV. J. Neuroimmunol. 187147-155. [DOI] [PubMed] [Google Scholar]
- 78.Buehring, G. C., S. M. Philpott, and K. Y. Choi. 2003. Humans have antibodies reactive with bovine leukemia virus. AIDS Res. Hum. Retrovir. 191105-1113. [DOI] [PubMed] [Google Scholar]
- 79.Burmeister, T., A. D. Ebert, W. Pritze, C. Loddenkemper, S. Schwartz, and E. Thiel. 2004. Insertional polymorphisms of endogenous HERV-K113 and HERV-K115 retroviruses in breast cancer patients and age-matched controls. AIDS Res. Hum. Retrovir. 201223-1229. [DOI] [PubMed] [Google Scholar]
- 80.Burmeister, T., S. Schwartz, and E. Thiel. 2001. A PCR primer system for detecting oncoretroviruses based on conserved DNA sequence motifs of animal retroviruses and its application to human leukaemias and lymphomas. J. Gen. Virol. 822205-2213. [DOI] [PubMed] [Google Scholar]
- 81.Büscher, K., S. Hahn, M. Hofmann, U. Trefzer, M. Ozel, W. Sterry, J. Löwer, R. Löwer, R. Kurth, and J. Denner. 2006. Expression of the human endogenous retrovirus-K transmembrane envelope, Rec and Np9 proteins in melanomas and melanoma cell lines. Melanoma Res. 16223-234. [DOI] [PubMed] [Google Scholar]
- 82.Büscher, K., U. Trefzer, M. Hofmann, W. Sterry, R. Kurth, and J. Denner. 2005. Expression of human endogenous retrovirus K in melanomas and melanoma cell lines. Cancer Res. 654172-4180. [DOI] [PubMed] [Google Scholar]
- 83.Calattini, S., S. A. Chevalier, R. Duprez, S. Bassot, A. Froment, R. Mahieux, and A. Gessain. 2005. Discovery of a new human T-cell lymphotropic virus (HTLV-3) in Central Africa. Retrovirology 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Callahan, R., and G. H. Smith. 2000. MMTV-induced mammary tumorigenesis: gene discovery, progression to malignancy and cellular pathways. Oncogene 19992-1001. [DOI] [PubMed] [Google Scholar]
- 85.Cameron, K. R., S. M. Birchall, and M. A. Moses. 1978. Isolation of foamy virus from patient with dialysis encephalopathy. Lancet ii796. [DOI] [PubMed] [Google Scholar]
- 86.Caporale, M., C. Cousens, P. Centorame, C. Pinoni, M. De las Heras, and M. Palmarini. 2006. Expression of the jaagsiekte sheep retrovirus envelope glycoprotein is sufficient to induce lung tumors in sheep. J. Virol. 808030-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cech, T. R., T. M. Nakamura, and J. Lingner. 1997. Telomerase is a true reverse transcriptase. A review. Biochemistry (Moscow) 621202-1205. [PubMed] [Google Scholar]
- 88.Charman, H. P., R. Rahman, M. H. White, N. Kim, and R. V. Gilden. 1977. Radioimmunoassay for the major structural protein of Mason-Pfizer monkey virus: attempts to detect the presence of antigen or antibody in humans. Int. J. Cancer 19498-504. [DOI] [PubMed] [Google Scholar]
- 89.Chen, J. M., P. D. Stenson, D. N. Cooper, and C. Ferec. 2005. A systematic analysis of LINE-1 endonuclease-dependent retrotranspositional events causing human genetic disease. Hum. Genet. 117411-427. [DOI] [PubMed] [Google Scholar]
- 90.Choi, Y., J. W. Kappler, and P. Marrack. 1991. A superantigen encoded in the open reading frame of the 3′ long terminal repeat of mouse mammary tumour virus. Nature 350203-207. [DOI] [PubMed] [Google Scholar]
- 91.Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244359-362. [DOI] [PubMed] [Google Scholar]
- 92.Chopra, H. C., and M. M. Mason. 1970. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 302081-2086. [PubMed] [Google Scholar]
- 93.Christensen, T. 2005. Association of human endogenous retroviruses with multiple sclerosis and possible interactions with herpes viruses. Rev. Med. Virol. 15179-211. [DOI] [PubMed] [Google Scholar]
- 94.Christensen, T., P. D. Sørensen, H. Riemann, H. J. Hansen, and A. Møller-Larsen. 1998. Expression of sequence variants of endogenous retrovirus RGH in particle form in multiple sclerosis. Lancet 3521033. [DOI] [PubMed] [Google Scholar]
- 95.Christensen, T., P. D. Sørensen, H. Riemann, H. J. Hansen, M. Munch, S. Haahr, and A. Møller-Larsen. 2000. Molecular characterization of HERV-H variants associated with multiple sclerosis. Acta Neurol. Scand. 101229-238. [DOI] [PubMed] [Google Scholar]
- 96.Christensen, T., A. W. Jensen, M. Munch, S. Haahr, P. D. Sørensen, H. Riemann, H. J. Hansen, and A. Møller-Larsen. 1997. Characterization of retroviruses from patients with multiple sclerosis. Acta Neurol. Scand. Suppl. 16949-58. [DOI] [PubMed] [Google Scholar]
- 97.Christensen, T., L. Pedersen, P. D. Sørensen, and A. Møller-Larsen. 2002. A transmissible human endogenous retrovirus. AIDS Res. Hum. Retrovir. 18861-866. [DOI] [PubMed] [Google Scholar]
- 98.Christensen, T., P. D. Sørensen, H. J. Hansen, and A. Møller-Larsen. 2003. Antibodies against a human endogenous retrovirus and the preponderance of env splice variants in multiple sclerosis patients. Mult. Scler. 96-15. [DOI] [PubMed] [Google Scholar]
- 99.Christensen, T., R. R. Tönjes, J. zur Megede, K. Boller, and A. Møller-Larsen. 1999. Reverse transcriptase activity and particle production in B lymphoblastoid cell lines established from lymphocytes of patients with multiple sclerosis. AIDS Res. Hum. Retrovir. 15285-291. [DOI] [PubMed] [Google Scholar]
- 100.Ciampolillo, A., V. Marini, R. Mirakian, M. Buscema, T. Schulz, R. Pujol-Borrell, and G. F. Bottazzo. 1989. Retrovirus-like sequences in Graves' disease: implications for human autoimmunity. Lancet i1096-1100. [DOI] [PubMed] [Google Scholar]
- 101.Clavel, F., D. Guétard, F. Brun-Vézinet, S. Chamaret, M. A. Rey, M. O. Santos-Ferreira, A. G. Laurent, C. Dauguet, C. Katlama, C. Rouzioux, D. Klatzmann, J. L. Champalimaud, and L. Montagnier. 1986. Isolation of a new human retrovirus from West African patients with AIDS. Science 233343-346. [DOI] [PubMed] [Google Scholar]
- 102.Coffin, J. M., S. H. Hughes, and H. E. Varmus (ed.). 1997. Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- 103.Colmegna, I., and R. F. Garry. 2006. Role of endogenous retroviruses in autoimmune diseases. Infect. Dis. Clin. N. Am. 20913-929. [DOI] [PubMed] [Google Scholar]
- 104.Conrad, B., E. Weidmann, G. Trucco, W. A. Rudert, R. Behboo, C. Ricordi, H. Rodriquez-Rilo, D. Finegold, and M. Trucco. 1994. Evidence for superantigen involvement in insulin-dependent diabetes mellitus aetiology. Nature 371351-355. [DOI] [PubMed] [Google Scholar]
- 105.Conrad, B., R. N. Weissmahr, J. Boni, R. Arcari, J. Schupbach, and B. Mach. 1997. A human endogenous retroviral superantigen as candidate autoimmune gene in type I diabetes. Cell 90303-313. [DOI] [PubMed] [Google Scholar]
- 106.Cordonnier, A., J. F. Casella, and T. Heidmann. 1995. Isolation of novel human endogenous retrovirus-like elements with foamy virus-related pol sequence. J. Virol. 695890-5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Costas, J. 2002. Characterization of the intragenomic spread of the human endogenous retrovirus family HERV-W. Mol. Biol. Evol. 19526-533. [DOI] [PubMed] [Google Scholar]
- 108.Crawford, D. H., B. G. Achong, N. M. Teich, S. Finerty, J. L. Thompson, M. A. Epstein, and B. C. Giovanella. 1979. Identification of murine endogenous xenotropic retrovirus in cultured multicellular tumour spheroids from nude-mouse-passaged nasopharyngeal carcinoma. Int. J. Cancer 231-7. [DOI] [PubMed] [Google Scholar]
- 109.Cunha, G. R., S. W. Hayward, Y. Z. Wang, and W. A. Ricke. 2003. Role of the stromal microenvironment in carcinogenesis of the prostate. Int. J. Cancer 1071-10. [DOI] [PubMed] [Google Scholar]
- 110.Dalen, A. B., L. Hellgren, O. J. Iversen, and J. Vincent. 1983. A virus-like particle associated with psoriasis. Acta Pathol. Microbiol. Immunol. Scand. 91221-229. [DOI] [PubMed] [Google Scholar]
- 111.Dalton, A. J. 1972. Further analysis of the detailed structure of type B and C particles. J. Natl. Cancer Inst. 481095-1099. [PubMed] [Google Scholar]
- 112.Deas, J. E., J. J. Thompson, C. D. Fermin, L. L. Liu, D. Martin, R. F. Garry, and W. R. Gallaher. 1999. Viral induction, transmission and apoptosis among cells infected by a human intracisternal A-type retrovirus. Virus Res. 6119-27. [DOI] [PubMed] [Google Scholar]
- 113.Deb-Rinker, P., T. A. Klempan, R. L. O'Reilly, E. F. Torrey, and S. M. Singh. 1999. Molecular characterization of a MSRV-like sequence identified by RDA from monozygotic twin pairs discordant for schizophrenia. Genomics 61133-144. [DOI] [PubMed] [Google Scholar]
- 114.Dekaban, G. A., and G. P. Rice. 1990. Retroviruses and multiple sclerosis. II. Failure of gene amplification techniques to detect viral sequences unique to the disease. Neurology 401254-1258. [DOI] [PubMed] [Google Scholar]
- 115.De las Heras, M., S. H. Barsky, P. Hasleton, M. Wagner, E. Larson, J. Egan, A. Ortín, J. A. Gimenez-Mas, M. Palmarini, and J. M. Sharp. 2000. Evidence for a protein related immunologically to the jaagsiekte sheep retrovirus in some human lung tumours. Eur. Respir. J. 16330-332. [DOI] [PubMed] [Google Scholar]
- 116.De las Heras, M., P. Murcia, A. Ortín, J. Azúa, L. Borderias, R. Álvarez, J. A. Jiménez-Más, A. Marchetti, and M. Palmarini. 2007. Jaagsiekte sheep retrovirus is not detected in human lung adenocarcinomas expressing antigens related to the Gag polyprotein of betaretroviruses. Cancer Lett. 25822-30. [DOI] [PubMed] [Google Scholar]
- 117.Denne, M., M. Sauter, V. Armbruester, J. D. Licht, K. Roemer, and N. Mueller-Lantzsch. 2007. Physical and functional interactions of human endogenous retrovirus proteins Np9 and Rec with the promyelocytic leukemia zinc finger protein. J. Virol. 815607-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Parseval, N., J. Casella, L. Gressin, and T. Heidmann. 2001. Characterization of the three HERV-H proviruses with an open envelope reading frame encompassing the immunosuppressive domain and evolutionary history in primates. Virology 279558-569. [DOI] [PubMed] [Google Scholar]
- 119.Dewannieux, M., S. Blaise, and T. Heidmann. 2005. Identification of a functional envelope protein from the HERV-K family of human endogenous retroviruses. J. Virol. 7915573-15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dewannieux, M., F. Harper, A. Richaud, C. Letzelter, D. Ribet, G. Pierron, and T. Heidmann. 2006. Identification of an infectious progenitor for the multiple-copy HERV-K human endogenous retroelements. Genome Res. 161548-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dhellin, O., J. Maestre, and T. Heidmann. 1997. Functional differences between the human LINE retrotransposon and retroviral reverse transcriptases for in vivo mRNA reverse transcription. EMBO J. 166590-6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.di Giovine, F. S., S. Bailly, J. Bootman, N. Almond, and G. W. Duff. 1994. Absence of lentiviral and human T cell leukemia viral sequences in patients with rheumatoid arthritis. Arthritis Rheum. 37349-358. [DOI] [PubMed] [Google Scholar]
- 123.Dolei, A., C. Serra, G. Mameli, M. Pugliatti, G. Sechi, M. C. Cirotto, G. Rosati, and S. Sotgiu. 2002. Multiple sclerosis-associated retrovirus (MSRV) in Sardinian MS patients. Neurology 58471-473. [DOI] [PubMed] [Google Scholar]
- 124.Donehower, L. A., R. C. Bohannon, R. J. Ford, and R. A. Gibbs. 1990. The use of primers from highly conserved pol regions to identify uncharacterized retroviruses by the polymerase chain reaction. J. Virol. Methods 2833-46. [DOI] [PubMed] [Google Scholar]
- 125.Dong, B., S. Kim, S. Hong, J. Das Gupta, K. Malathi, E. A. Klein, D. Ganem, J. L. Derisi, S. A. Chow, and R. H. Silverman. 2007. An infectious retrovirus susceptible to an IFN antiviral pathway from human prostate tumors. Proc. Natl. Acad. Sci. USA 1041655-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dourmashkin, R. R., D. Bucher, and J. S. Oxford. 1993. Small virus-like particles bud from the cell membranes of normal as well as HIV-infected human lymphoid cells. J. Med. Virol. 39229-232. [DOI] [PubMed] [Google Scholar]
- 127.Douvas, A., Y. Takehana, G. Ehresmann, T. Chernyovskiy, and E. S. Daar. 1996. Neutralization of HIV type 1 infectivity by serum antibodies from a subset of autoimmune patients with mixed connective tissue disease. AIDS Res. Hum. Retrovir. 121509-1517. [DOI] [PubMed] [Google Scholar]
- 128.Dube, S., S. Bachman, T. Spicer, J. Love, D. Choi, E. Esteban, J. F. Ferrer, and B. J. Poiesz. 1997. Degenerate and specific PCR assays for the detection of bovine leukaemia virus and primate T cell leukaemia/lymphoma virus pol DNA and RNA: phylogenetic comparisons of amplified sequences from cattle and primates from around the world. J. Gen. Virol. 781389-1398. [DOI] [PubMed] [Google Scholar]
- 129.Dunn, C. A., P. Medstrand, and D. L. Mager. 2003. An endogenous retroviral long terminal repeat is the dominant promoter for human β1,3-galactosyltransferase 5 in the colon. Proc. Natl. Acad. Sci. USA 10012841-12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ehrlich, G. D., J. B. Glaser, V. Bryz-Gornia, J. Maese, T. A. Waldmann, B. J. Poiesz, S. J. Greenberg, et al. 1991. Multiple sclerosis, retroviruses, and PCR. Neurology 41335-343. [DOI] [PubMed] [Google Scholar]
- 131.Ellermann, V., and O. Bang. 1908. Experimentelle Leukämie bei Hühnern. Zentralbl. Bakteriol. Parasite. Infekt. 46595-609. [Google Scholar]
- 132.Etkind, P., J. Du, A. Khan, J. Pillitteri, and P. H. Wiernik. 2000. Mouse mammary tumor virus-like ENV gene sequences in human breast tumors and in a lymphoma of a breast cancer patient. Clin. Cancer Res. 61273-1278. [PubMed] [Google Scholar]
- 133.Evans, A. 1976. Causation and disease: the Henle-Koch postulates revisited. Yale J. Biol. Med. 49175-195. [PMC free article] [PubMed] [Google Scholar]
- 134.Evans, A. S., and N. E. Mueller. 1990. Viruses and cancer. Causal associations. Ann. Epidemiol. 171-92. [DOI] [PubMed] [Google Scholar]
- 135.Faedo, M., C. E. Ford, R. Mehta, K. Blazek, and W. D. Rawlinson. 2004. Mouse mammary tumor-like virus is associated with p53 nuclear accumulation and progesterone receptor positivity but not estrogen positivity in human female breast cancer. Clin. Cancer Res. 104417-4419. [DOI] [PubMed] [Google Scholar]
- 136.Feldman, S. P., J. Schlom, and S. Spiegelman. 1973. Further evidence for oncornaviruses in human milk: the production of cores. Proc. Natl. Acad. Sci. USA 701976-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Feller, W. F., and H. C. Chopra. 1968. A small virus-like particle observed in human breast cancer by means of electron microscopy. J. Natl. Cancer Inst. 401359-1373. [PubMed] [Google Scholar]
- 138.Feller, W. F., and H. C. Chopra. 1971. Virus-like particles in human milk. Cancer 281425-1430. [DOI] [PubMed] [Google Scholar]
- 139.Fierabracci, A., C. P. Upton, N. Hajibagheri, and G. F. Bottazzo. 2001. Lack of detection of retroviral particles (HIAP-1) in the H9 T cell line co-cultured with thyrocytes of Graves' disease. J. Autoimmun. 16457-462. [DOI] [PubMed] [Google Scholar]
- 140.Fina, F., S. Romain, L. Ouafik, J. Palmari, F. Ben Ayed, S. Benharkat, P. Bonnier, F. Spyratos, J. A. Foekens, C. Rose, M. Buisson, H. Gerard, M. O. Reymond, J. M. Seigneurin, and P. M. Martin. 2001. Frequency and genome load of Epstein-Barr virus in 509 breast cancers from different geographical areas. Br. J. Cancer 84783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fine, D., and G. Schochetman. 1978. Type D primate retroviruses: a review. Cancer Res. 383123-3139. [PubMed] [Google Scholar]
- 142.Firouzi, R., A. Rolland, M. Michel, E. Jouvin-Marche, J. J. Hauw, C. Malcus-Vocanson, F. Lazarini, L. Gebuhrer, J. M. Seigneurin, J. L. Touraine, K. Sanhadji, P. N. Marche, and H. Perron. 2003. Multiple sclerosis-associated retrovirus particles cause T lymphocyte-dependent death with brain hemorrhage in humanized SCID mice model. J. Neurovirol. 979-93. [DOI] [PubMed] [Google Scholar]
- 143.Flescher, E., M. J. Dauphinee, D. Fossum, J. Ledbetter, and N. Talal. 1992. Signal transduction in Sjögren's syndrome T cells. Abnormalities associated with a newly described human A-type retrovirus. Arthritis Rheum. 351068-1074. [DOI] [PubMed] [Google Scholar]
- 144.Ford, C. E., M. Faedo, and W. D. Rawlinson. 2004. Mouse mammary tumor virus-like RNA transcripts and DNA are found in affected cells of human breast cancer. Clin. Cancer Res. 107284-7289. [DOI] [PubMed] [Google Scholar]
- 145.Ford, C. E., D. Tran, Y. Deng, V. T. Ta, W. D. Rawlinson, and J. S. Lawson. 2003. Mouse mammary tumor virus-like gene sequences in breast tumors of Australian and Vietnamese women. Clin. Cancer Res. 91118-1120. [PubMed] [Google Scholar]
- 146.Ford, R. J., L. A. Donehower, and R. C. Bohannon. 1992. Studies on a type D retrovirus isolated from an AIDS patient lymphoma. AIDS Res. Hum. Retrovir. 8742-751. [PubMed] [Google Scholar]
- 147.Forsman, A., D. Ushameckis, A. Bindra, Z. Yun, and J. Blomberg. 2003. Uptake of amplifiable fragments of retrotransposon DNA from the human alimentary tract. Mol. Genet. Genomics 270362-368. [DOI] [PubMed] [Google Scholar]
- 148.Forsman, A., D. Uzameckis, L. Ronnblom, E. Baecklund, A. Aleskog, A. Bindra, R. Pipkorn, S. Lejniece, S. Kozireva, M. Murovska, and J. Blomberg. 2003. Single-tube nested quantitative PCR: a rational and sensitive technique for detection of retroviral DNA. Application to RERV-H/HRV-5 and confirmation of its rabbit origin. J. Virol. Methods 1111-11. [DOI] [PubMed] [Google Scholar]
- 149.Fox, R. I., C. A. Robinson, J. G. Curd, F. Kozin, and F. V. Howell. 1986. Sjögren's syndrome. Proposed criteria for classification. Arthritis Rheum. 29577-585. [DOI] [PubMed] [Google Scholar]
- 150.Frank, O., M. Giehl, C. Zheng, R. Hehlmann, C. Leib-Mosch, and W. Seifarth. 2005. Human endogenous retrovirus expression profiles in samples from brains of patients with schizophrenia and bipolar disorders. J. Virol. 7910890-10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Frank, O., C. Verbeke, N. Schwarz, J. Mayer, A. Fabarius, R. Hehlmann, C. Leib-Mosch, and W. Seifarth. 12 December 2007. Variable transcriptional activity of endogenous retroviruses in human breast cancer. J. Virol. doi: 10.1128/JVI.02115-07. [DOI] [PMC free article] [PubMed]
- 152.Fredricks, D. N., and D. A. Relman. 1996. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin. Microbiol. Rev. 918-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Frendo, J. L., D. Olivier, V. Cheynet, J. L. Blond, O. Bouton, M. Vidaud, M. Rabreau, D. Evain-Brion, and F. Mallet. 2003. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol. Cell. Biol. 233566-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fujinami, R. S., M. G. von Herrath, U. Christen, and J. L. Whitton. 2006. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin. Microbiol. Rev. 1980-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Furmanski, P., C. Longley, D. Fouchey, R. Rich, and M. A. Rich. 1974. Normal human mammary cells in culture: evidence for oncornavirus-like particles. J. Natl. Cancer Inst. 52975-977. [DOI] [PubMed] [Google Scholar]
- 156.Galli, U. M., M. Sauter, B. Lecher, S. Maurer, H. Herbst, K. Roemer, and N. Mueller-Lantzsch. 2005. Human endogenous retrovirus rec interferes with germ cell development in mice and may cause carcinoma in situ, the predecessor lesion of germ cell tumors. Oncogene 243223-3228. [DOI] [PubMed] [Google Scholar]
- 157.Garder, M., M. Endres, and P. Barry. 1994. The simian retroviruses: SIV and SRV, p. 133-276. In J. A. Levy (ed.), The Retroviridae, vol. 3. Plenum Press, New York, NY. [Google Scholar]
- 158.Gardner, M. B., J. N. Ihle, R. J. Pillarisetty, N. Talal, E. L. Dubois, and J. A. Levy. 1977. Type C virus expression and host response in diet-cured NZB/W mice. Nature 268341-344. [DOI] [PubMed] [Google Scholar]
- 159.Garrison, K. E., R. B. Jones, D. A. Meiklejohn, N. Anwar, L. C. Ndhlovu, J. M. Chapman, A. L. Erickson, A. Agrawal, G. Spotts, F. M. Hecht, S. Rakoff-Nahoum, J. Lenz, M. A. Ostrowski, and D. F. Nixon. 2007. T cell responses to human endogenous retroviruses in HIV-1 infection. PLoS Pathog. 3e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Garry, R. F. 1990. Extensive antigenic mimicry by retrovirus capsid proteins. AIDS Res. Hum. Retrovir. 61361-1362. [DOI] [PubMed] [Google Scholar]
- 161.Garry, R. F., C. D. Fermin, D. J. Hart, S. S. Alexander, L. A. Donehower, and H. Luo-Zhang. 1990. Detection of a human intracisternal A-type retroviral particle antigenically related to HIV. Science 2501127-1129. [DOI] [PubMed] [Google Scholar]
- 162.Garry, R. F., C. D. Fermin, P. F. Kohler, M. L. Markert, and H. Luo. 1996. Antibodies against retroviral proteins and nuclear antigens in a subset of idiopathic CD4+ T lymphocytopenia patients. AIDS Res. Hum. Retrovir. 12931-940. [DOI] [PubMed] [Google Scholar]
- 163.Garry, R. F., A. M. Krieg, W. P. Cheevers, R. C. Montelaro, H. Golding, C. D. Fermin, and W. R. Gallaher. 1995. Retroviruses and their roles in chronic inflammatory diseases and autoimmunity, p. 491-603. In J. A. Levy (ed.), The Retroviridae, vol. 4. Plenum Press, New York, NY. [Google Scholar]
- 164.Garson, J. A., P. W. Tuke, P. Giraud, G. Paranhos-Baccala, and H. Perron. 1998. Detection of virion-associated MSRV-RNA in serum of patients with multiple sclerosis. Lancet 35133. [DOI] [PubMed] [Google Scholar]
- 165.Gaudin, P., S. Ijaz, P. W. Tuke, F. Marcel, A. Paraz, J. M. Seigneurin, B. Mandrand, H. Perron, and J. A. Garson. 2000. Infrequency of detection of particle-associated MSRV/HERV-W RNA in the synovial fluid of patients with rheumatoid arthritis. Rheumatology 39950-954. [DOI] [PubMed] [Google Scholar]
- 166.Gaudin, P., F. Moutet, P. W. Tuke, and J. A. Garson. 1999. Absence of human retrovirus 5 in French patients with rheumatoid arthritis: comment on the article by Griffiths et al. Arthritis Rheum. 422492-2494. [DOI] [PubMed] [Google Scholar]
- 167.Gaynor, A. M., M. D. Nissen, D. M. Whiley, I. M. Mackay, S. B. Lambert, G. Wu, D. C. Brennan, G. A. Storch, T. P. Sloots, and D. Wang. 2007. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 3e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gazdar, A. F., J. S. Butel, and M. Carbone. 2002. SV40 and human tumours: myth, association or causality? Nat. Rev. Cancer 2957-964. [DOI] [PubMed] [Google Scholar]
- 169.Gelman, I. H., J. Zhang, E. Hailman, H. Hanafusa, and S. S. Morse. 1992. Identification and evaluation of new primer sets for the detection of lentivirus proviral DNA. AIDS Res. Hum. Retrovir. 81981-1989. [DOI] [PubMed] [Google Scholar]
- 170.Gerwin, B. I., P. S. Ebert, H. C. Chopra, S. G. Smith, J. P. Kvedar, S. Albert, and M. J. Brennan. 1973. DNA polymerase activities of human milk. Science 180198-201. [DOI] [PubMed] [Google Scholar]
- 171.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de Thé. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii407-410. [DOI] [PubMed] [Google Scholar]
- 172.Gifford, R., and M. Tristem. 2003. The evolution, distribution and diversity of endogenous retroviruses. Virus Genes 26291-315. [DOI] [PubMed] [Google Scholar]
- 173.Giovannoni, G., and G. Ebers. 2007. Multiple sclerosis: the environment and causation. Curr. Opin. Neurol. 20261-268. [DOI] [PubMed] [Google Scholar]
- 174.Goedert, J. J., M. E. Sauter, L. P. Jacobson, R. L. Vessella, M. W. Hilgartner, S. F. Leitman, M. C. Fraser, and N. G. Mueller-Lantzsch. 1999. High prevalence of antibodies against HERV-K10 in patients with testicular cancer but not with AIDS. Cancer Epidemiol. Biomarkers Prev. 8293-296. [PubMed] [Google Scholar]
- 175.Goff, S. P. 2007. Host factors exploited by retroviruses. Nat. Rev. Microbiol. 5253-263. [DOI] [PubMed] [Google Scholar]
- 176.Goff, S. P. 2000. Retroviridae: the retroviruses and their replication, p. 1871-1939. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 177.Goff, S. P. 2004. Retrovirus restriction factors. Mol. Cell 16849-859. [DOI] [PubMed] [Google Scholar]
- 178.Golding, H., F. A. Robey, F. T. Gates III, W. Linder, P. R. Beining, T. Hoffman, and B. Golding. 1988. Identification of homologous regions in human immunodeficiency virus I gp41 and human MHC class II β 1 domain. I. Monoclonal antibodies against the gp41-derived peptide and patients' sera react with native HLA class II antigens, suggesting a role for autoimmunity in the pathogenesis of acquired immune deficiency syndrome. J. Exp. Med. 167914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Golding, H., G. M. Shearer, K. Hillman, P. Lucas, J. Manischewitz, R. A. Zajac, M. Clerici, R. E. Gress, R. N. Boswell, and B. Golding. 1989. Common epitope in human immunodeficiency virus (HIV) I-GP41 and HLA class II elicits immunosuppressive autoantibodies capable of contributing to immune dysfunction in HIV I-infected individuals. J. Clin. Investig. 831430-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Goon, P. K., E. Hanon, T. Igakura, Y. Tanaka, J. N. Weber, G. P. Taylor, and C. R. Bangham. 2002. High frequencies of Th1-type CD4(+) T cells specific to HTLV-1 Env and Tax proteins in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Blood 993335-3341. [DOI] [PubMed] [Google Scholar]
- 181.Gourley, M., and F. W. Miller. 2007. Mechanisms of disease: environmental factors in the pathogenesis of rheumatic disease. Nat. Clin. Pract. Rheumatol. 3172-180. [DOI] [PubMed] [Google Scholar]
- 182.Granieri, E., I. Casetta, M. R. Tola, and P. Ferrante. 2001. Multiple sclerosis: infectious hypothesis. Neurol. Sci. 22179-185. [DOI] [PubMed] [Google Scholar]
- 183.Grant, R. F., S. K. Windsor, C. J. Malinak, C. R. Bartz, A. Sabo, R. E. Benveniste, and C. C. Tsai. 1995. Characterization of infectious type D retrovirus from baboons. Virology 207292-296. [DOI] [PubMed] [Google Scholar]
- 184.Grassmann, R., M. Aboud, and K. T. Jeang. 2005. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene 245976-5985. [DOI] [PubMed] [Google Scholar]
- 185.Green, J. E., S. H. Hinrichs, J. Vogel, and G. Jay. 1989. Exocrinopathy resembling Sjögren's syndrome in HTLV-1 tax transgenic mice. Nature 34172-74. [DOI] [PubMed] [Google Scholar]
- 186.Greenberg, S. J., G. D. Ehrlich, M. A. Abbott, B. J. Hurwitz, T. A. Waldmann, and B. J. Poiesz. 1989. Detection of sequences homologous to human retroviral DNA in multiple sclerosis by gene amplification. Proc. Natl. Acad. Sci. USA 862878-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Griffiths, D., and P. Venables. 1999. Author's reply. Arthritis Rheum. 422493-2494. [Google Scholar]
- 188.Griffiths, D. J., S. P. Cooke, C. Hervé, S. P. Rigby, E. Mallon, A. Hajeer, M. Lock, V. Emery, P. Taylor, P. Pantelidis, C. B. Bunker, R. du Bois, R. A. Weiss, and P. J. Venables. 1999. Detection of human retrovirus 5 in patients with arthritis and systemic lupus erythematosus. Arthritis Rheum. 42448-454. [DOI] [PubMed] [Google Scholar]
- 189.Griffiths, D. J., P. J. Venables, R. A. Weiss, and M. T. Boyd. 1997. A novel exogenous retrovirus sequence identified in humans. J. Virol. 712866-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Griffiths, D. J., C. Voisset, P. J. Venables, and R. A. Weiss. 2002. Novel endogenous retrovirus in rabbits previously reported as human retrovirus 5. J. Virol. 767094-7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Grigoriadis, N., and G. M. Hadjigeorgiou. 2006. Virus-mediated autoimmunity in multiple sclerosis. J. Autoimmune Dis. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Grossberg, S. E., V. M. Kushnaryov, L. W. Cashdollar, K. P. Raisch, G. Miller, and H. Y. Sun. 1997. A human B-lymphoblastoid cell line constitutively producing Epstein-Barr herpesvirus and JHK retrovirus. Res. Virol. 148191-206. [DOI] [PubMed] [Google Scholar]
- 193.Gupta, S., C. E. Ribak, S. Gollapudi, C. H. Kim, and S. Z. Salahuddin. 1992. Detection of a human intracisternal retroviral particle associated with CD4+ T-cell deficiency. Proc. Natl. Acad. Sci. USA 897831-7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Haahr, S., M. Sommerlund, A. Møller-Larsen, R. Nielsen, and H. J. Hansen. 1991. Just another dubious virus in cells from a patient with multiple sclerosis? Lancet 337863-864. [DOI] [PubMed] [Google Scholar]
- 195.Hackett, J., Jr., P. Swanson, D. Leahy, E. L. Anderson, S. Sato, R. P. Roos, R. Decker, and S. G. Devare. 1996. Search for retrovirus in patients with multiple sclerosis. Ann. Neurol. 40805-809. [DOI] [PubMed] [Google Scholar]
- 196.Hart, D. J., H. Luo, and R. F. Garry. 1996. Biochemical characterization of the reverse transcriptase of a human intracisternal A-type particle (HIAP). AIDS Res. Hum. Retrovir. 121367-1372. [DOI] [PubMed] [Google Scholar]
- 197.Harwood, C. A., P. J. Spink, T. Surentheran, I. M. Leigh, E. M. de Villiers, J. M. McGregor, C. M. Proby, and J. Breuer. 1999. Degenerate and nested PCR: a highly sensitive and specific method for detection of human papillomavirus infection in cutaneous warts. J. Clin. Microbiol. 373545-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hasuike, S., K. Miura, O. Miyoshi, T. Miyamoto, N. Niikawa, Y. Jinno, and M. Ishikawa. 1999. Isolation and localization of an IDDMK1,2-22-related human endogenous retroviral gene, and identification of a CA repeat marker at its locus. J. Hum. Genet. 44343-347. [DOI] [PubMed] [Google Scholar]
- 199.Hauser, S. L., C. Aubert, J. S. Burks, C. Kerr, O. Lyon-Caen, G. de Thé, and M. Brahic. 1986. Analysis of human T-lymphotrophic virus sequences in multiple sclerosis tissue. Nature 322176-177. [DOI] [PubMed] [Google Scholar]
- 200.Haverkos, H. W. 2004. Viruses, chemicals and co-carcinogenesis. Oncogene 236492-6499. [DOI] [PubMed] [Google Scholar]
- 201.Haynes, B. F., J. Fleming, E. W. St. Clair, H. Katinger, G. Stiegler, R. Kunert, J. Robinson, R. M. Scearce, K. Plonk, H. F. Staats, T. L. Ortel, H. X. Liao, and S. M. Alam. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 3081906-1908. [DOI] [PubMed] [Google Scholar]
- 202.Held, W., G. A. Waanders, A. N. Shakhov, L. Scarpellino, H. Acha-Orbea, and H. R. MacDonald. 1993. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell 74529-540. [DOI] [PubMed] [Google Scholar]
- 203.Hendrick, J. C., C. Francois, C. M. Calberg-Bacq, C. Colin, P. Franchimont, L. Gosselin, S. Kozma, and P. M. Osterrieth. 1978. Radioimmunoassay for protein p28 of murine mammary tumor virus in organs and serum of mice and search for related antigens in human sera and breast cancer extracts. Cancer Res. 381826-1831. [PubMed] [Google Scholar]
- 204.Heneine, W., N. W. Lerche, T. Woods, T. Spira, J. M. Liff, W. Eley, J. L. Yee, J. E. Kaplan, and R. F. Khabbaz. 1993. The search for human infection with simian type D retroviruses. J. Acquir. Immune Defic. Syndr. 61062-1066. [PubMed] [Google Scholar]
- 205.Heneine, W., W. M. Switzer, P. Sandstrom, J. Brown, S. Vedapuri, C. A. Schable, A. S. Khan, N. W. Lerche, M. Schweizer, D. Neumann-Haefelin, L. E. Chapman, and T. M. Folks. 1998. Identification of a human population infected with simian foamy viruses. Nat. Med. 4403-407. [DOI] [PubMed] [Google Scholar]
- 206.Herndier, B. G., B. T. Shiramizu, N. E. Jewett, K. D. Aldape, G. R. Reyes, and M. S. McGrath. 1992. Acquired immunodeficiency syndrome-associated T-cell lymphoma: evidence for human immunodeficiency virus type 1-associated T-cell transformation. Blood 791768-1774. [PubMed] [Google Scholar]
- 207.Herniou, E., J. Martin, K. Miller, J. Cook, M. Wilkinson, and M. Tristem. 1998. Retroviral diversity and distribution in vertebrates. J. Virol. 725955-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hervé, C. A., E. B. Lugli, A. Brand, D. J. Griffiths, and P. J. Venables. 2002. Autoantibodies to human endogenous retrovirus-K are frequently detected in health and disease and react with multiple epitopes. Clin. Exp. Immunol. 12875-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hiatt, K. M., and W. E. Highsmith. 2002. Lack of DNA evidence for jaagsiekte sheep retrovirus in human bronchioloalveolar carcinoma. Hum. Pathol. 33680. [DOI] [PubMed] [Google Scholar]
- 210.Hill, A. B. 1965. The environment and disease:association and causation. Proc. R. Soc. Med. 58295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hirschl, S., O. Schanab, H. Seppele, A. Waltenberger, J. Humer, K. Wolff, H. Pehamberger, and T. Muster. 2007. Sequence variability of retroviral particles derived from human melanoma cells melanoma-associated retrovirus. Virus Res. 123211-215. [DOI] [PubMed] [Google Scholar]
- 212.Hishikawa, T., H. Ogasawara, H. Kaneko, T. Shirasawa, Y. Matsuura, I. Sekigawa, Y. Takasaki, H. Hashimoto, S. Hirose, S. Handa, R. Nagasawa, and N. Maruyama. 1997. Detection of antibodies to a recombinant gag protein derived from human endogenous retrovirus clone 4-1 in autoimmune diseases. Viral Immunol. 10137-147. [DOI] [PubMed] [Google Scholar]
- 213.Ho, D. D., Y. Cao, T. Zhu, C. Farthing, N. Wang, G. Gu, R. T. Schooley, and E. S. Daar. 1993. Idiopathic CD4+ T-lymphocytopenia—immunodeficiency without evidence of HIV infection. N. Engl. J. Med. 328380-385. [DOI] [PubMed] [Google Scholar]
- 214.Hohenadl, C., H. Germaier, M. Walchner, M. Hagenhofer, M. Herrmann, M. Stürzl, P. Kind, R. Hehlmann, V. Erfle, and C. Leib-Mösch. 1999. Transcriptional activation of endogenous retroviral sequences in human epidermal keratinocytes by UVB irradiation. J. Investig. Dermatol. 113587-594. [DOI] [PubMed] [Google Scholar]
- 215.Holder, W. D., Jr., and S. A. Wells, Jr. 1983. Antibody reacting with the murine mammary tumor virus in the serum of patients with breast carcinoma: a possible serological detection method for breast carcinoma. Cancer Res. 43239-244. [PubMed] [Google Scholar]
- 216.Holtzman, M. J., J. W. Tyner, E. Y. Kim, M. S. Lo, A. C. Patel, L. P. Shornick, E. Agapov, and Y. Zhang. 2005. Acute and chronic airway responses to viral infection: implications for asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2132-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hoover, E. A., R. G. Olsen, W. D. Hardy, Jr., J. P. Schaller, and L. E. Mathes. 1976. Feline leukemia virus infection: age-related variation in response of cats to experimental infection. J. Natl. Cancer Inst. 57365-369. [DOI] [PubMed] [Google Scholar]
- 218.Horwitz, M. S., M. T. Boyce-Jacino, and A. J. Faras. 1992. Novel human endogenous sequences related to human immunodeficiency virus type 1. J. Virol. 662170-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hsiao, F. C., M. Lin, A. Tai, G. Chen, and B. T. Huber. 2006. Epstein-Barr virus transactivates the HERV-K18 superantigen by docking to the human complement receptor 2 (CD21) on primary B cells. J. Immunol. 1772056-2060. [DOI] [PubMed] [Google Scholar]
- 220.Huebner, R. J. 1957. Criteria for etiologic association of prevalent viruses with prevalent diseases; the virologist's dilemma. Ann. N. Y. Acad. Sci. 67430-438. [DOI] [PubMed] [Google Scholar]
- 221.Hughes, J. F., and J. M. Coffin. 2004. Human endogenous retrovirus K solo-LTR formation and insertional polymorphisms: implications for human and viral evolution. Proc. Natl. Acad. Sci. USA 1011668-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hughes, S. H. 1982. Sequence of the long terminal repeat and adjacent segments of the endogenous avian virus Rous-associated virus 0. J. Virol. 43191-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Humer, J., A. Waltenberger, A. Grassauer, M. Kurz, J. Valencak, R. Rapberger, S. Hahn, R. Löwer, K. Wolff, M. Bergmann, T. Muster, B. Mayer, and H. Pehamberger. 2006. Identification of a melanoma marker derived from melanoma-associated endogenous retroviruses. Cancer Res. 661658-1663. [DOI] [PubMed] [Google Scholar]
- 224.Hunsmann, G., R. M. Flügel, and R. Walder. 1990. Retroviral antibodies in Indians. Nature 345120. [DOI] [PubMed] [Google Scholar]
- 225.Ilyin, K. V., J. G. Kzhyshkowska, L. R. Imamova, T. D. Mashkova, A. S. Ostashkin, A. V. Kiselev, G. A. Gordina, V. I. Kurmashow, and A. V. Itkes. 2001. Type D retrovirus specific sequences in lymphocytes of the children with Burkitt-type lymphoma and their parents. Immunol. Lett. 7851-54. [DOI] [PubMed] [Google Scholar]
- 226.Indik, S., W. H. Günzburg, P. Kulich, B. Salmons, and F. Rouault. 2007. Rapid spread of mouse mammary tumor virus in cultured human breast cells. Retrovirology 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Indik, S., W. H. Günzburg, B. Salmons, and F. Rouault. 2005. Mouse mammary tumor virus infects human cells. Cancer Res. 656651-6659. [DOI] [PubMed] [Google Scholar]
- 228.Indik, S., W. H. Günzburg, B. Salmons, and F. Rouault. 2005. A novel, mouse mammary tumor virus encoded protein with Rev-like properties. Virology 3371-6. [DOI] [PubMed] [Google Scholar]
- 229.Itescu, S., and R. Winchester. 1992. Diffuse infiltrative lymphocytosis syndrome: a disorder occurring in human immunodeficiency virus-1 infection that may present as a sicca syndrome. Rheum. Dis. Clin. N. Am. 18683-697. [PubMed] [Google Scholar]
- 230.Iversen, O. J. 1990. The expression of retrovirus-like particles in psoriasis. J. Investig. Dermatol. 9541S-43S. [DOI] [PubMed] [Google Scholar]
- 231.Iversen, O. J., and A. B. Dalen. 1985. The major internal protein, p27, of a retrovirus-like particle is expressed in blood lymphocytes from psoriatic patients. Arch. Virol. 85197-207. [DOI] [PubMed] [Google Scholar]
- 232.Iversen, O. J., and E. Rødahl. 1985. The major internal protein, p27, of a retrovirus-like particle participates in immune complex formation in psoriasis. Arch. Virol. 8637-45. [DOI] [PubMed] [Google Scholar]
- 233.Iwakura, Y., M. Tosu, E. Yoshida, M. Takiguchi, K. Sato, I. Kitajima, K. Nishioka, K. Yamamoto, T. Takeda, M. Hatanaka, H. Yamamoto, and T. Sekiguchi. 1991. Induction of inflammatory arthropathy resembling rheumatoid arthritis in mice transgenic for HTLV-I. Science 2531026-1028. [DOI] [PubMed] [Google Scholar]
- 234.Jaeckel, E., S. Heringlake, D. Berger, G. Brabant, G. Hunsmann, and M. P. Manns. 1999. No evidence for association between IDDMK1,222, a novel isolated retrovirus, and IDDM. Diabetes 48209-214. [DOI] [PubMed] [Google Scholar]
- 235.Jaspan, J. B., H. Luo, B. Ahmed, S. Tenenbaum, T. Voss, D. M. Sander, K. Bollinger, T. Baquet, and R. F. Garry. 1995. Evidence for a retroviral trigger in Graves' disease. Autoimmunity 20135-142. [DOI] [PubMed] [Google Scholar]
- 236.Jern, P., G. O. Sperber, and J. Blomberg. 2005. Use of endogenous retroviral sequences (ERVs) and structural markers for retroviral phylogenetic inference and taxonomy. Retrovirology 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jocher, R., A. Rethwilm, L. Kappos, and V. ter Meulen. 1990. Search for retroviral sequences in peripheral blood mononuclear cells and brain tissue of multiple sclerosis patients. J. Neurol. 237352-355. [DOI] [PubMed] [Google Scholar]
- 238.Johnson, R., and J. B. Kaneene. 1992. Bovine leukemia virus and enzootic bovine leukosis. Vet. Bull. 62287-312. [Google Scholar]
- 239.Johnston, J. B., C. Silva, J. Holden, K. G. Warren, A. W. Clark, and C. Power. 2001. Monocyte activation and differentiation augment human endogenous retrovirus expression: implications for inflammatory brain diseases. Ann. Neurol. 50434-442. [DOI] [PubMed] [Google Scholar]
- 240.Jones, M. S., A. Kapoor, V. V. Lukashov, P. Simmonds, F. Hecht, and E. Delwart. 2005. New DNA viruses identified in patients with acute viral infection syndrome. J. Virol. 798230-8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kalter, S. S., R. J. Helmke, R. L. Heberling, M. Panigel, A. K. Fowler, J. E. Strickland, and A. Hellman. 1973. C-type particles in normal human placentas. J. Natl. Cancer Inst. 501081-1084. [DOI] [PubMed] [Google Scholar]
- 242.Kalyanaraman, V. S., M. G. Sarngadharan, M. Robert-Guroff, I. Miyoshi, D. Golde, and R. C. Gallo. 1982. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science 218571-573. [DOI] [PubMed] [Google Scholar]
- 243.Kamradt, T., and N. A. Mitchison. 2001. Tolerance and autoimmunity. N. Engl. J. Med. 344655-664. [DOI] [PubMed] [Google Scholar]
- 244.Kaneko, K., S. Sato, T. Miyatake, and S. Tsuji. 1991. Absence of highly homologous sequence to HTLV-I in Japanese multiple sclerosis. Neurology 4131-34. [DOI] [PubMed] [Google Scholar]
- 245.Kaplan, H. S. 1980. Prospects for the etiologic involvement of RNA tumor viruses in human cancer, p. 485-514. In J. R. Stephenson (ed.), Molecular biology of RNA tumor viruses. Academic Press, New York, NY.
- 246.Karlsson, H., S. Bachmann, J. Schroder, J. McArthur, E. F. Torrey, and R. H. Yolken. 2001. Retroviral RNA identified in the cerebrospinal fluids and brains of individuals with schizophrenia. Proc. Natl. Acad. Sci. USA 984634-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Karpas, A., U. Kämpf, A. Sidèn, M. Koch, and S. Poser. 1986. Lack of evidence for involvement of known human retroviruses in multiple sclerosis. Nature 322177-178. [DOI] [PubMed] [Google Scholar]
- 248.Katzourakis, A., M. Tristem, O. G. Pybus, and R. J. Gifford. 2007. Discovery and analysis of the first endogenous lentivirus. Proc. Natl. Acad. Sci. USA 1046261-6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kaye, B. R. 1989. Rheumatologic manifestations of infection with human immunodeficiency virus (HIV). Ann. Intern. Med. 111158-167. [DOI] [PubMed] [Google Scholar]
- 250.Kellam, P. 1998. Molecular identification of novel viruses. Trends Microbiol. 6160-165. [DOI] [PubMed] [Google Scholar]
- 251.Kempf, W., M. E. Kadin, A. M. Dvorak, C. C. Lord, G. Burg, N. L. Letvin, and I. J. Koralnik. 2003. Endogenous retroviral elements, but not exogenous retroviruses, are detected in CD30-positive lymphoproliferative disorders of the skin. Carcinogenesis 24301-306. [DOI] [PubMed] [Google Scholar]
- 252.Keydar, I., T. Ohno, R. Nayak, R. Sweet, F. Simoni, F. Weiss, S. Karby, R. Mesa-Tejada, and S. Spiegelman. 1984. Properties of retrovirus-like particles produced by a human breast carcinoma cell line: immunological relationship with mouse mammary tumor virus proteins. Proc. Natl. Acad. Sci. USA 814188-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Keydar, I., G. Selzer, S. Chaitchik, M. Hareuveni, S. Karby, and A. Hizi. 1982. A viral antigen as a marker for the prognosis of human breast cancer. Eur. J. Cancer Clin. Oncol. 181321-1328. [DOI] [PubMed] [Google Scholar]
- 254.Khabbaz, R. F., W. Heneine, J. R. George, B. Parekh, T. Rowe, T. Woods, W. M. Switzer, H. M. McClure, M. Murphey-Corb, and T. M. Folks. 1994. Infection of a laboratory worker with simian immunodeficiency virus. N. Engl. J. Med. 330172-177. [DOI] [PubMed] [Google Scholar]
- 255.Khabbaz, R. F., T. Rowe, M. Murphey-Corb, W. M. Heneine, C. A. Schable, J. R. George, C. P. Pau, B. S. Parekh, M. D. Lairmore, J. W. Curran, J. E. Kaplan, G. Schochetman, and T. M. Folks. 1992. Simian immunodeficiency virus needlestick accident in a laboratory worker. Lancet 340271-273. [DOI] [PubMed] [Google Scholar]
- 256.Kim, A., H. S. Jun, L. Wong, D. Stephure, D. Pacaud, R. A. Trussell, and J. W. Yoon. 1999. Human endogenous retrovirus with a high genomic sequence homology with IDDMK1,222 is not specific for type I (insulin-dependent) diabetic patients but ubiquitous. Diabetologia 42413-418. [DOI] [PubMed] [Google Scholar]
- 257.Kleiman, A., N. Senyuta, A. Tryakin, M. Sauter, A. Karseladze, S. Tjulandin, V. Gurtsevitch, and N. Mueller-Lantzsch. 2004. HERV-K(HML-2) GAG/ENV antibodies as indicator for therapy effect in patients with germ cell tumors. Int. J. Cancer 110459-461. [DOI] [PubMed] [Google Scholar]
- 258.Komurian-Pradel, F., G. Paranhos-Baccala, F. Bedin, A. Ounanian-Paraz, M. Sodoyer, C. Ott, A. Rajoharison, E. Garcia, F. Mallet, B. Mandrand, and H. Perron. 1999. Molecular cloning and characterization of MSRV-related sequences associated with retrovirus-like particles. Virology 2601-9. [DOI] [PubMed] [Google Scholar]
- 259.Koprowski, H., E. C. DeFreitas, M. E. Harper, M. Sandberg-Wollheim, W. A. Sheremata, M. Robert-Guroff, C. W. Saxinger, M. B. Feinberg, F. Wong-Staal, and R. C. Gallo. 1985. Multiple sclerosis and human T-cell lymphotropic retroviruses. Nature 318154-160. [DOI] [PubMed] [Google Scholar]
- 260.Kozireva, S., S. Lejniece, J. Blomberg, and M. Murovska. 2001. Human retrovirus type 5 sequences in non-Hodgkin's lymphoma of T cell origin. AIDS Res. Hum. Retrovir. 17953-956. [DOI] [PubMed] [Google Scholar]
- 261.Kozireva, S., G. Nemceva, I. Danilane, O. Pavlova, J. Blomberg, and M. Murovska. 2001. Prevalence of blood-borne viral infections (cytomegalovirus, human herpesvirus-6, human herpesvirus-7, human herpesvirus-8, human T-cell lymphotropic virus-I/II, human retrovirus-5) among blood donors in Latvia. Ann. Hematol. 80669-673. [DOI] [PubMed] [Google Scholar]
- 262.Krause, H., V. Wunderlich, and W. Uckert. 1989. Molecular cloning of a type D retrovirus from human cells (PMFV) and its homology to simian acquired immunodeficiency type D retroviruses. Virology 173214-222. [DOI] [PubMed] [Google Scholar]
- 263.Krieg, A. M., and A. D. Steinberg. 1990. Retroviruses and autoimmunity. J. Autoimmun. 3137-166. [DOI] [PubMed] [Google Scholar]
- 264.Kuff, E. L., and K. K. Lueders. 1988. The intracisternal A-particle gene family: structure and functional aspects. Adv. Cancer Res. 51183-276. [DOI] [PubMed] [Google Scholar]
- 265.Kurtzke, J. F. 1993. Epidemiologic evidence for multiple sclerosis as an infection. Clin. Microbiol. Rev. 6382-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kzhyshkowska, J. G., A. V. Kiselev, G. A. Gordina, V. I. Kurmashow, N. M. Portjanko, A. S. Ostashkin, and K. V. Ilyin. 1996. Markers of type D retroviruses in children with Burkitt's-type lymphoma. Immunol. Lett. 53101-104. [DOI] [PubMed] [Google Scholar]
- 267.Labat, M. L. 1996. Retroviruses and bone diseases. Clin. Orthop. Relat. Res. 1996287-309. [DOI] [PubMed] [Google Scholar]
- 268.Lagaye, S., P. Vexiau, V. Morozov, V. Guénebaut-Claudet, J. Tobaly-Tapiero, M. Canivet, G. Cathelineau, J. Périès, and R. Emanoil-Ravier. 1992. Human spumaretrovirus-related sequences in the DNA of leukocytes from patients with Graves disease. Proc. Natl. Acad. Sci. USA 8910070-10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lal, R. B., D. L. Rudolph, K. P. Griffis, K. Kitamura, M. Honda, J. E. Coligan, and T. M. Folks. 1991. Characterization of immunodominant epitopes of gag and pol gene-encoded proteins of human T-cell lymphotropic virus type I. J. Virol. 651870-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lan, M. S., A. Mason, R. Coutant, Q. Y. Chen, A. Vargas, J. Rao, R. Gomez, S. Chalew, R. Garry, and N. K. Maclaren. 1998. HERV-K10s and immune-mediated (type 1) diabetes. Cell 9514-16. [DOI] [PubMed] [Google Scholar]
- 271.Landry, J. R., A. Rouhi, P. Medstrand, and D. L. Mager. 2002. The Opitz syndrome gene Mid1 is transcribed from a human endogenous retroviral promoter. Mol. Biol. Evol. 191934-1942. [DOI] [PubMed] [Google Scholar]
- 272.Lapatschek, M., S. Dürr, R. Löwer, C. Magin, H. Wagner, and T. Miethke. 2000. Functional analysis of the env open reading frame in human endogenous retrovirus IDDMK1,222 encoding superantigen activity. J. Virol. 746386-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.La Placa, M., T. Bianchi, F. Vitone, L. Muratori, C. Varotti, D. Gibellini, and M. C. Re. 2004. Serum antibody reactivity to human intracisternal A-type particle retrovirus proteins in systemic sclerosis patients. Acta Derm. Venereol. 84177-180. [DOI] [PubMed] [Google Scholar]
- 274.La Placa, M., F. Vitone, T. Bianchi, C. Vincenzi, D. Gibellini, M. C. Re, and A. Tosti. 2004. Serum antibodies against human intracisternal A-type particle (HIAP) endogenous retrovirus in alopecia areata patients: a hallmark of autoimmune disease? J. Investig. Dermatol. 123407-409. [DOI] [PubMed] [Google Scholar]
- 275.Lavie, L., M. Kitova, E. Maldener, E. Meese, and J. Mayer. 2005. CpG methylation directly regulates transcriptional activity of the human endogenous retrovirus family HERV-K(HML-2). J. Virol. 79876-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lee, W. J., H. J. Kwun, H. S. Kim, and K. L. Jang. 2003. Activation of the human endogenous retrovirus W long terminal repeat by herpes simplex virus type 1 immediate early protein 1. Mol. Cells 1575-80. [PubMed] [Google Scholar]
- 277.Lee, Y. N., and P. D. Bieniasz. 2007. Reconstitution of an infectious human endogenous retrovirus. PLoS Pathog. 3e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lefebvre, S., B. Hubert, F. Tekaia, M. Brahic, and J. F. Bureau. 1995. Isolation from human brain of six previously unreported cDNAs related to the reverse transcriptase of human endogenous retroviruses. AIDS Res. Hum. Retrovir. 11231-237. [DOI] [PubMed] [Google Scholar]
- 279.Leiter, E. H., J. W. Fewell, and E. L. Kuff. 1986. Glucose induces intracisternal type A retroviral gene transcription and translation in pancreatic beta cells. J. Exp. Med. 16387-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Leiter, E. H., and E. L. Kuff. 1984. Intracisternal type A particles in murine pancreatic B cells. Immunocytochemical demonstration of increased antigen (p73) in genetically diabetic mice. Am. J. Pathol. 11446-55. [PMC free article] [PubMed] [Google Scholar]
- 281.Lerche, N. W., W. Heneine, J. E. Kaplan, T. Spira, J. L. Yee, and R. F. Khabbaz. 1995. An expanded search for human infection with simian type D retrovirus. AIDS Res. Hum. Retrovir. 11527-529. [DOI] [PubMed] [Google Scholar]
- 282.Lerche, N. W., W. M. Switzer, J. L. Yee, V. Shanmugam, A. N. Rosenthal, L. E. Chapman, T. M. Folks, and W. Heneine. 2001. Evidence of infection with simian type D retrovirus in persons occupationally exposed to nonhuman primates. J. Virol. 751783-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Levin, M. C., S. M. Lee, F. Kalume, Y. Morcos, F. C. Dohan, Jr., K. A. Hasty, J. C. Callaway, J. Zunt, D. Desiderio, and J. M. Stuart. 2002. Autoimmunity due to molecular mimicry as a cause of neurological disease. Nat. Med. 8509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Li, M. D., T. D. Lemke, D. L. Bronson, and A. J. Faras. 1996. Synthesis and analysis of a 640-bp pol region of novel human endogenous retroviral sequences and their evolutionary relationships. Virology 2171-10. [DOI] [PubMed] [Google Scholar]
- 285.Li, W. H., Z. Gu, H. Wang, and A. Nekrutenko. 2001. Evolutionary analyses of the human genome. Nature 409847-849. [DOI] [PubMed] [Google Scholar]
- 286.Linial, M. L., H. Fan, B. Hahn, R. Löwer, J. Neil, S. Quackenbush, A. Rethwilm, P. Sonigo, J. Stoye, and M. Tristem. 2005. Retroviridae, p. 421-440. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA.
- 287.Liu, B., Y. Wang, S. M. Melana, I. Pelisson, V. Najfeld, J. F. Holland, and B. G. Pogo. 2001. Identification of a proviral structure in human breast cancer. Cancer Res. 611754-1759. [PubMed] [Google Scholar]
- 288.Liu, S. L., and A. D. Miller. 2007. Oncogenic transformation by the jaagsiekte sheep retrovirus envelope protein. Oncogene 26789-801. [DOI] [PubMed] [Google Scholar]
- 289.Llewelyn, M., and J. Cohen. 2002. Superantigens: microbial agents that corrupt immunity. Lancet Infect. Dis. 2156-162. [DOI] [PubMed] [Google Scholar]
- 290.Loh, P. C., F. Matsuura, and C. Mizumoto. 1980. Seroepidemiology of human syncytial virus: antibody prevalence in the Pacific. Intervirology 1387-90. [DOI] [PubMed] [Google Scholar]
- 291.Lolli, F., S. Fredrikson, S. Kam-Hansen, and H. Link. 1987. Increased reactivity to HTLV-I in inflammatory nervous system diseases. Ann. Neurol. 2267-71. [DOI] [PubMed] [Google Scholar]
- 292.Loveday, C., and R. S. Tedder. 1993. Enzyme-linked immunosorbent assays for the measurement of human immunodeficiency virus, type 1 reverse transcriptase antigen and antibodies. J. Virol. Methods 41181-192. [DOI] [PubMed] [Google Scholar]
- 293.Löwer, R. 1999. The pathogenic potential of endogenous retroviruses: facts and fantasies. Trends Microbiol. 7350-356. [DOI] [PubMed] [Google Scholar]
- 294.Löwer, R., K. Boller, B. Hasenmaier, C. Korbmacher, N. Muller-Lantzsch, J. Löwer, and R. Kurth. 1993. Identification of human endogenous retroviruses with complex mRNA expression and particle formation. Proc. Natl. Acad. Sci. USA 904480-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Löwer, R., J. Löwer, and R. Kurth. 1996. The viruses in all of us: characteristics and biological significance of human endogenous retrovirus sequences. Proc. Natl. Acad. Sci. USA 935177-5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Löwer, R., R. R. Tönjes, K. Boller, J. Denner, B. Kaiser, R. C. Phelps, J. Löwer, R. Kurth, K. Badenhoop, H. Donner, K. H. Usadel, T. Miethke, M. Lapatschek, and H. Wagner. 1998. Development of insulin-dependent diabetes mellitus does not depend on specific expression of the human endogenous retrovirus HERV-K. Cell 9511-14. [DOI] [PubMed] [Google Scholar]
- 297.Lucchinetti, C., W. Bruck, J. Parisi, B. Scheithauer, M. Rodriguez, and H. Lassmann. 2000. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 47707-717. [DOI] [PubMed] [Google Scholar]
- 298.Lugert, R., H. König, R. Kurth, and R. R. Tönjes. 1996. Specific suppression of false-positive signals in the product-enhanced reverse transcriptase assay. BioTechniques 20210-217. [PubMed] [Google Scholar]
- 299.Luppi, P., M. M. Zanone, H. Hyoty, W. A. Rudert, C. Haluszczak, A. M. Alexander, S. Bertera, D. Becker, and M. Trucco. 2000. Restricted TCR Vβ gene expression and enterovirus infection in type I diabetes: a pilot study. Diabetologia 431484-1497. [DOI] [PubMed] [Google Scholar]
- 300.Macfarlane, C., and P. Simmonds. 2004. Allelic variation of HERV-K(HML-2) endogenous retroviral elements in human populations. J. Mol. Evol. 59642-656. [DOI] [PubMed] [Google Scholar]
- 301.Mack, D. H., and J. J. Sninsky. 1988. A sensitive method for the identification of uncharacterized viruses related to known virus groups: hepadnavirus model system. Proc. Natl. Acad. Sci. USA 856977-6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.MacKie, R. M. 1998. Incidence, risk factors and prevention of melanoma. Eur. J. Cancer 34(Suppl. 3)S3-S6. [DOI] [PubMed] [Google Scholar]
- 303.Madeley, C. 1995. Traditional techniques of viral diagnosis, p. 1-32. In U. Desselberger (ed.), Medical virology: a practical approach. IRL Press, Oxford, United Kingdom.
- 304.Maeda, N., M. Palmarini, C. Murgia, and H. Fan. 2001. Direct transformation of rodent fibroblasts by jaagsiekte sheep retrovirus DNA. Proc. Natl. Acad. Sci. USA 984449-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Magin, C., R. Löwer, and J. Löwer. 1999. cORF and RcRE, the Rev/Rex and RRE/RxRE homologues of the human endogenous retrovirus family HTDV/HERV-K. J. Virol. 739496-9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Magre, S., Y. Takeuchi, and B. Bartosch. 2003. Xenotransplantation and pig endogenous retroviruses. Rev. Med. Virol. 13311-329. [DOI] [PubMed] [Google Scholar]
- 307.Malassiné, A., S. Blaise, K. Handschuh, H. Lalucque, A. Dupressoir, D. Evain-Brion, and T. Heidmann. 2007. Expression of the fusogenic HERV-FRD Env glycoprotein (syncytin 2) in human placenta is restricted to villous cytotrophoblastic cells. Placenta 28185-191. [DOI] [PubMed] [Google Scholar]
- 308.Malassiné, A., K. Handschuh, V. Tsatsaris, P. Gerbaud, V. Cheynet, G. Oriol, F. Mallet, and D. Evain-Brion. 2005. Expression of HERV-W Env glycoprotein (syncytin) in the extravillous trophoblast of first trimester human placenta. Placenta 26556-562. [DOI] [PubMed] [Google Scholar]
- 309.Mallet, F., O. Bouton, S. Prudhomme, V. Cheynet, G. Oriol, B. Bonnaud, G. Lucotte, L. Duret, and B. Mandrand. 2004. The endogenous retroviral locus ERVWE1 is a bona fide gene involved in hominoid placental physiology. Proc. Natl. Acad. Sci. USA 1011731-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Mameli, G., V. Astone, G. Arru, S. Marconi, L. Lovato, C. Serra, S. Sotgiu, B. Bonetti, and A. Dolei. 2007. Brains and peripheral blood mononuclear cells of multiple sclerosis (MS) patients hyperexpress MS-associated retrovirus/HERV-W endogenous retrovirus, but not human herpesvirus 6. J. Gen. Virol. 88264-274. [DOI] [PubMed] [Google Scholar]
- 311.Mameli, G., V. Astone, K. Khalili, C. Serra, B. E. Sawaya, and A. Dolei. 2007. Regulation of the syncytin-1 promoter in human astrocytes by multiple sclerosis-related cytokines. Virology 362120-130. [DOI] [PubMed] [Google Scholar]
- 312.Mangeney, M., N. de Parseval, G. Thomas, and T. Heidmann. 2001. The full-length envelope of an HERV-H human endogenous retrovirus has immunosuppressive properties. J. Gen. Virol. 822515-2518. [DOI] [PubMed] [Google Scholar]
- 313.Mant, C., and J. Cason. 2004. A human murine mammary tumour virus-like agent is an unconvincing aetiological agent for human breast cancer. Rev. Med. Virol. 14169-177. [DOI] [PubMed] [Google Scholar]
- 314.Mant, C., C. Gillett, C. D'Arrigo, and J. Cason. 2004. Human murine mammary tumour virus-like agents are genetically distinct from endogenous retroviruses and are not detectable in breast cancer cell lines or biopsies. Virology 318393-404. [DOI] [PubMed] [Google Scholar]
- 315.Marrack, P., G. M. Winslow, Y. Choi, M. Scherer, A. Pullen, J. White, and J. W. Kappler. 1993. The bacterial and mouse mammary tumor virus superantigens; two different families of proteins with the same functions. Immunol. Rev. 13179-92. [DOI] [PubMed] [Google Scholar]
- 316.Martin, J., E. Herniou, J. Cook, R. W. O'Neill, and M. Tristem. 1997. Human endogenous retrovirus type I-related viruses have an apparently widespread distribution within vertebrates. J. Virol. 71437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Mason, A. L., G. H. Farr, L. Xu, S. G. Hubscher, and J. M. Neuberger. 2004. Pilot studies of single and combination antiretroviral therapy in patients with primary biliary cirrhosis. Am. J. Gastroenterol. 992348-2355. [DOI] [PubMed] [Google Scholar]
- 318.Mason, A. L., L. Xu, L. Guo, S. Munoz, J. B. Jaspan, M. Bryer-Ash, Y. Cao, D. M. Sander, Y. Shoenfeld, A. Ahmed, J. Van de Water, M. E. Gershwin, and R. F. Garry. 1998. Detection of retroviral antibodies in primary biliary cirrhosis and other idiopathic biliary disorders. Lancet 3511620-1624. [DOI] [PubMed] [Google Scholar]
- 319.Mathias, S. L., A. F. Scott, H. H. Kazazian, Jr., J. D. Boeke, and A. Gabriel. 1991. Reverse transcriptase encoded by a human transposable element. Science 2541808-1810. [DOI] [PubMed] [Google Scholar]
- 320.Maul, G. G., S. A. Jimenez, E. Riggs, and D. Ziemnicka-Kotula. 1989. Determination of an epitope of the diffuse systemic sclerosis marker antigen DNA topoisomerase I: sequence similarity with retroviral p30gag protein suggests a possible cause for autoimmunity in systemic sclerosis. Proc. Natl. Acad. Sci. USA 868492-8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Mayer, J., M. Sauter, A. Racz, D. Scherer, N. Mueller-Lantzsch, and E. Meese. 1999. An almost-intact human endogenous retrovirus K on human chromosome 7. Nat. Genet. 21257-258. [DOI] [PubMed] [Google Scholar]
- 322.McAllister, R. M., M. Nicolson, M. B. Gardner, R. W. Rongey, S. Rasheed, P. S. Sarma, R. J. Huebner, M. Hatanaka, S. Oroszlan, R. V. Gilden, A. Kabigting, and L. Vernon. 1972. C-type virus released from cultured human rhabdomyosarcoma cells. Nat. New Biol. 2353-6. [DOI] [PubMed] [Google Scholar]
- 323.McClure, M. A. 2000. The complexities of genome analysis, the retroid agent perspective. Bioinformatics 1679-95. [DOI] [PubMed] [Google Scholar]
- 324.Medstrand, P., and J. Blomberg. 1993. Characterization of novel reverse transcriptase encoding human endogenous retroviral sequences similar to type A and type B retroviruses: differential transcription in normal human tissues. J. Virol. 676778-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Medstrand, P., J. R. Landry, and D. L. Mager. 2001. Long terminal repeats are used as alternative promoters for the endothelin B receptor and apolipoprotein C-I genes in humans. J. Biol. Chem. 2761896-1903. [DOI] [PubMed] [Google Scholar]
- 326.Medstrand, P., M. Lindeskog, and J. Blomberg. 1992. Expression of human endogenous retroviral sequences in peripheral blood mononuclear cells of healthy individuals. J. Gen. Virol. 732463-2466. [DOI] [PubMed] [Google Scholar]
- 327.Meiering, C. D., and M. L. Linial. 2001. Historical perspective of foamy virus epidemiology and infection. Clin. Microbiol. Rev. 14165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Melana, S. M., I. Nepomnaschy, M. Sakalian, A. Abbott, J. Hasa, J. F. Holland, and B. G. Pogo. 2007. Characterization of viral particles isolated from primary cultures of human breast cancer cells. Cancer Res. 678960-8965. [DOI] [PubMed] [Google Scholar]
- 329.Menard, A., R. Amouri, M. Michel, F. Marcel, A. Brouillet, J. Belliveau, C. Geny, L. Deforges, C. Malcus-Vocanson, M. Armstrong, O. Lyon-Caen, B. Mandrand, T. Dobransky, F. Rieger, and H. Perron. 1997. Gliotoxicity, reverse transcriptase activity and retroviral RNA in monocyte/macrophage culture supernatants from patients with multiple sclerosis. FEBS Lett. 413477-485. [DOI] [PubMed] [Google Scholar]
- 330.Mendez, E. A., K. B. DeSalvo, Y. Cao, R. F. Garry, and L. R. Espinoza. 2001. Familial erosive arthritis associated with seroreactivity to human intracisternal retroviral particle type I (HIAP-I). Rheumatology 40227-228. [DOI] [PubMed] [Google Scholar]
- 331.Mertz, J. A., M. S. Simper, M. M. Lozano, S. M. Payne, and J. P. Dudley. 2005. Mouse mammary tumor virus encodes a self-regulatory RNA export protein and is a complex retrovirus. J. Virol. 7914737-14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Mesa-Tejada, R., I. Keydar, M. Ramanarayanan, T. Ohno, C. Fenoglio, and S. Spiegelman. 1978. Detection in human breast carcinomas of an antigen immunologically related to a group-specific antigen of mouse mammary tumor virus. Proc. Natl. Acad. Sci. USA 751529-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mi, S., X. Lee, X. Li, G. M. Veldman, H. Finnerty, L. Racie, E. LaVallie, X. Y. Tang, P. Edouard, S. Howes, J. C. Keith, Jr., and J. M. McCoy. 2000. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403785-789. [DOI] [PubMed] [Google Scholar]
- 334.Miné, M., J. M. Chen, M. Brivet, I. Desguerre, D. Marchant, P. de Lonlay, A. Bernard, C. Férec, M. Abitbol, D. Ricquier, and C. Marsac. 2007. A large genomic deletion in the PDHX gene caused by the retrotranspositional insertion of a full-length LINE-1 element. Hum. Mutat. 28137-142. [DOI] [PubMed] [Google Scholar]
- 335.Molès, J. P., J. C. Hadi, and J. J. Guilhou. 2003. High prevalence of an IgG response against murine leukemia virus (MLV) in patients with psoriasis. Virus Res. 9497-101. [DOI] [PubMed] [Google Scholar]
- 336.Molès, J. P., A. Tesniere, and J. J. Guilhou. 2005. A new endogenous retroviral sequence is expressed in skin of patients with psoriasis. Br. J. Dermatol. 15383-89. [DOI] [PubMed] [Google Scholar]
- 337.Møller-Larsen, A., and T. Christensen. 1998. Isolation of a retrovirus from multiple sclerosis patients in self-generated iodixanol gradients. J. Virol. Methods 73151-161. [DOI] [PubMed] [Google Scholar]
- 338.Moore, D. H., J. Charney, B. Kramarsky, E. Y. Lasfargues, N. H. Sarkar, M. J. Brennan, J. H. Burrows, S. M. Sirsat, J. C. Paymaster, and A. B. Vaidya. 1971. Search for a human breast cancer virus. Nature 229611-614. [DOI] [PubMed] [Google Scholar]
- 339.Morgan, D., and I. Brodsky. 2004. Human endogenous retrovirus (HERV-K) particles in megakaryocytes cultured from essential thrombocythemia peripheral blood stem cells. Exp. Hematol. 32520-525. [DOI] [PubMed] [Google Scholar]
- 340.Morgan, R. A., and R. C. Huang. 1984. Correlation of undermethylation of intracisternal A-particle genes with expression in murine plasmacytomas but not in NIH/3T3 embryo fibroblasts. Cancer Res. 445234-5241. [PubMed] [Google Scholar]
- 341.Mornex, J. F., F. Thivolet, M. De las Heras, and C. Leroux. 2003. Pathology of human bronchioloalveolar carcinoma and its relationship to the ovine disease. Curr. Top. Microbiol. Immunol. 275225-248. [DOI] [PubMed] [Google Scholar]
- 342.Morozov, V. A., S. Lagaye, J. Löwer, and R. Löwer. 2004. Detection and characterization of betaretroviral sequences, related to sheep jaagsiekte virus, in Africans from Nigeria and Cameroon. Virology 327162-168. [DOI] [PubMed] [Google Scholar]
- 343.Morozov, V. A., S. Lagaye, L. Lyakh, and J. ter Meulen. 1996. Type D retrovirus markers in healthy Africans from Guinea. Res. Virol. 147341-351. [DOI] [PubMed] [Google Scholar]
- 344.Morse, B., P. G. Rotherg, V. J. South, J. M. Spandorfer, and S. M. Astrin. 1988. Insertional mutagenesis of the myc locus by a LINE-1 sequence in a human breast carcinoma. Nature 33387-90. [DOI] [PubMed] [Google Scholar]
- 345.Moyes, D., D. J. Griffiths, and P. J. Venables. 2007. Insertional polymorphisms: a new lease of life for endogenous retroviruses in human disease. Trends Genet. 23326-333. [DOI] [PubMed] [Google Scholar]
- 346.Moyes, D. L., A. Martin, S. Sawcer, N. Temperton, J. Worthington, D. J. Griffiths, and P. J. Venables. 2005. The distribution of the endogenous retroviruses HERV-K113 and HERV-K115 in health and disease. Genomics 86337-341. [DOI] [PubMed] [Google Scholar]
- 347.Muller, H. K., G. Ball, M. A. Epstein, B. G. Achong, G. Lenoir, and A. Levin. 1980. The prevalence of naturally occurring antibodies to human syncytial virus in East African populations. J. Gen. Virol. 47399-406. [DOI] [PubMed] [Google Scholar]
- 348.Munch, M., A. Møller-Larsen, T. Christensen, N. Morling, H. J. Hansen, and S. Haahr. 1995. B-lymphoblastoid cell lines from multiple sclerosis patients and a healthy control producing a putative new human retrovirus and Epstein-Barr virus. Mult. Scler. 178-81. [DOI] [PubMed] [Google Scholar]
- 349.Mura, M., P. Murcia, M. Caporale, T. E. Spencer, K. Nagashima, A. Rein, and M. Palmarini. 2004. Late viral interference induced by transdominant Gag of an endogenous retrovirus. Proc. Natl. Acad. Sci. USA 10111117-11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Murovska, M., S. Lejniece, S. Kozireva, M. Koulikovska, H. Yin, and J. Blomberg. 2000. Human retrovirus 5 sequences in peripheral blood cells of patients with B-cell non-Hodgkin's lymphoma. Int. J. Cancer 85762-770. [DOI] [PubMed] [Google Scholar]
- 351.Murphy, V. J., L. C. Harrison, W. A. Rudert, P. Luppi, M. Trucco, A. Fierabracci, P. A. Biro, and G. F. Bottazzo. 1998. Retroviral superantigens and type 1 diabetes mellitus. Cell 959-11. [DOI] [PubMed] [Google Scholar]
- 352.Murray, R. S., B. Brown, D. Brian, and G. F. Cabirac. 1992. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann. Neurol. 31525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Mustafa, F., S. Bhadra, D. Johnston, M. Lozano, and J. P. Dudley. 2003. The type B leukemogenic virus truncated superantigen is dispensable for T-cell lymphomagenesis. J. Virol. 773866-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Muster, T., A. Waltenberger, A. Grassauer, S. Hirschl, P. Caucig, I. Romirer, D. Födinger, H. Seppele, O. Schanab, C. Magin-Lachmann, R. Löwer, B. Jansen, H. Pehamberger, and K. Wolff. 2003. An endogenous retrovirus derived from human melanoma cells. Cancer Res. 638735-8741. [PubMed] [Google Scholar]
- 355.Myers, G., and H. Lu. 1996. HIV and molecular mimicry, p. 78-87. In B. Korber, C. Brander, B. Haynes, R. Koup, J. P. Moore, B. D. Walker, P. M. D'Sousa, and G. Myers (ed.), HIV molecular immunology database 1996, vol. IV. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, NM. [Google Scholar]
- 356.Nakagawa, K., V. Brusic, G. McColl, and L. C. Harrison. 1997. Direct evidence for the expression of multiple endogenous retroviruses in the synovial compartment in rheumatoid arthritis. Arthritis Rheum. 40627-638. [DOI] [PubMed] [Google Scholar]
- 357.Nakamura, H., A. Kawakami, M. Tominaga, A. Hida, S. Yamasaki, K. Migita, Y. Kawabe, T. Nakamura, and K. Eguchi. 2000. Relationship between Sjögren's syndrome and human T-lymphotropic virus type I infection: follow-up study of 83 patients. J. Lab. Clin. Med. 135139-144. [DOI] [PubMed] [Google Scholar]
- 358.Nandi, J. S., S. Van Dooren, A. K. Chhangani, and S. M. Mohnot. 2006. New simian beta retroviruses from rhesus monkeys (Macaca mulatta) and langurs (Semnopithecus entellus) from Rajasthan, India. Virus Genes 33107-116. [DOI] [PubMed] [Google Scholar]
- 359.Nellåker, C., Y. Yao, L. Jones-Brando, F. Mallet, R. H. Yolken, and H. Karlsson. 2006. Transactivation of elements in the human endogenous retrovirus W family by viral infection. Retrovirology 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Nelson, W. G., A. M. De Marzo, and W. B. Isaacs. 2003. Prostate cancer. N. Engl. J. Med. 349366-381. [DOI] [PubMed] [Google Scholar]
- 361.Nemo, G. J., P. W. Brown, C. J. Gibbs, Jr., and D. C. Gajdusek. 1978. Antigenic relationship of human foamy virus to the simian foamy viruses. Infect. Immun. 2069-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Neuberger, J. 1997. Primary biliary cirrhosis. Lancet 350875-879. [DOI] [PubMed] [Google Scholar]
- 363.Neumann-Haefelin, D., U. Fleps, R. Renne, and M. Schweizer. 1993. Foamy viruses. Intervirology 35196-207. [DOI] [PubMed] [Google Scholar]
- 364.Nevels, M., B. Tauber, T. Spruss, H. Wolf, and T. Dobner. 2001. “Hit-and-run” transformation by adenovirus oncogenes. J. Virol. 753089-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Nevins, J. R. 2001. Cell transformation by viruses, p. 245-283. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 366.Nishimura, M., A. Adachi, M. Maeda, I. Akiguchi, A. Ishimoto, and J. Kimura. 1990. Human T lymphotrophic virus type I may not be associated with multiple sclerosis in Japan. J. Immunol. 1441684-1688. [PubMed] [Google Scholar]
- 367.Nishioka, K., T. Sumida, and T. Hasunuma. 1996. Human T lymphotropic virus type I in arthropathy and autoimmune disorders. Arthritis Rheum. 391410-1418. [DOI] [PubMed] [Google Scholar]
- 368.Nowak, J., D. Januszkiewicz, M. Pernak, I. Liwen, M. Zawada, J. Rembowska, K. Nowicka, K. Lewandowski, H. Hertmanowska, and M. Wender. 2003. Multiple sclerosis-associated virus-related pol sequences found both in multiple sclerosis and healthy donors are more frequently expressed in multiple sclerosis patients. J. Neurovirol. 9112-117. [DOI] [PubMed] [Google Scholar]
- 369.Ohta, M., K. Ohta, F. Mori, H. Nishitani, and T. Saida. 1986. Sera from patients with multiple sclerosis react with human cell T lymphotropic virus-I Gag proteins but not Env proteins—Western blotting analysis. J. Immunol. 1373440-3443. [PubMed] [Google Scholar]
- 370.Oksenberg, J. R., R. Mantegazza, K. Sakai, C. C. Bernard, and L. Steinman. 1990. HTLV-I sequences are not detected in peripheral blood genomic DNA or in brain cDNA of multiple sclerosis patients. Ann. Neurol. 28574-577. [DOI] [PubMed] [Google Scholar]
- 371.Oluwole, S. O., Y. Yao, S. Conradi, K. Kristensson, and H. Karlsson. 2007. Elevated levels of transcripts encoding a human retroviral envelope protein (syncytin) in muscles from patients with motor neuron disease. Amyotroph. Lateral Scler. 867-72. [DOI] [PubMed] [Google Scholar]
- 372.Ono, M. 1986. Molecular cloning and long terminal repeat sequences of human endogenous retrovirus genes related to types A and B retrovirus genes. J. Virol. 58937-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Ono, M., T. Yasunaga, T. Miyata, and H. Ushikubo. 1986. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J. Virol. 60589-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ortín, A., E. Minguijon, P. Dewar, M. Garcia, L. M. Ferrer, M. Palmarini, L. Gonzalez, J. M. Sharp, and M. De las Heras. 1998. Lack of a specific immune response against a recombinant capsid protein of jaagsiekte sheep retrovirus in sheep and goats naturally affected by enzootic nasal tumour or sheep pulmonary adenomatosis. Vet. Immunol. Immunopathol. 61229-237. [DOI] [PubMed] [Google Scholar]
- 375.Otowa, T., M. Tochigi, M. Rogers, T. Umekage, N. Kato, and T. Sasaki. 2006. Insertional polymorphism of endogenous retrovirus HERV-K115 in schizophrenia. Neurosci. Lett. 408226-229. [DOI] [PubMed] [Google Scholar]
- 376.Pal, B. K., S. Mohan, R. Nimo, and M. B. Gardner. 1983. Wild mouse retrovirus-induced neurogenic paralysis in laboratory mice. I. Virus replication and expression in central nervous system. Arch. Virol. 77239-247. [DOI] [PubMed] [Google Scholar]
- 377.Palmarini, M., and H. Fan. 2001. Retrovirus-induced ovine pulmonary adenocarcinoma, an animal model for lung cancer. J. Natl. Cancer Inst. 931603-1614. [DOI] [PubMed] [Google Scholar]
- 378.Patarca, R., and W. A. Haseltine. 1985. A major retroviral core protein related to EPA and TIMP. Nature 318390. [DOI] [PubMed] [Google Scholar]
- 379.Patience, C., G. R. Simpson, A. A. Colletta, H. M. Welch, R. A. Weiss, and M. T. Boyd. 1996. Human endogenous retrovirus expression and reverse transcriptase activity in the T47D mammary carcinoma cell line. J. Virol. 702654-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Patience, C., Y. Takeuchi, and R. A. Weiss. 1997. Infection of human cells by an endogenous retrovirus of pigs. Nat. Med. 3282-286. [DOI] [PubMed] [Google Scholar]
- 381.Paul, T. A., S. L. Quackenbush, C. Sutton, R. N. Casey, P. R. Bowser, and J. W. Casey. 2006. Identification and characterization of an exogenous retrovirus from Atlantic salmon swim bladder sarcomas. J. Virol. 802941-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Pavlíček, A., J. Pačes, D. Elleder, and J. Hejnar. 2002. Processed pseudogenes of human endogenous retroviruses generated by LINEs: their integration, stability, and distribution. Genome Res. 12391-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Perl, A., J. D. Rosenblatt, I. S. Chen, J. P. DiVincenzo, R. Bever, B. J. Poiesz, and G. N. Abraham. 1989. Detection and cloning of new HTLV-related endogenous sequences in man. Nucleic Acids Res. 176841-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Perron, H., R. Firouzi, P. Tuke, J. A. Garson, M. Michel, F. Beseme, F. Bedin, F. Mallet, E. Marcel, J. M. Seigneurin, B. Mandrand, et al. 1997. Cell cultures and associated retroviruses in multiple sclerosis. Acta Neurol. Scand. Suppl. 16922-31. [DOI] [PubMed] [Google Scholar]
- 385.Perron, H., J. A. Garson, F. Bedin, F. Beseme, G. Paranhos-Baccala, F. Komurian-Pradel, F. Mallet, P. W. Tuke, C. Voisset, J. L. Blond, B. Lalande, J. M. Seigneurin, B. Mandrand, et al. 1997. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. Proc. Natl. Acad. Sci. USA 947583-7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Perron, H., C. Geny, O. Genoulaz, J. Pellat, J. Perret, and J. M. Seigneurin. 1991. Antibody to reverse transcriptase of human retroviruses in multiple sclerosis. Acta Neurol. Scand. 84507-513. [DOI] [PubMed] [Google Scholar]
- 387.Perron, H., C. Geny, A. Laurent, C. Mouriquand, J. Pellat, J. Perret, and J. M. Seigneurin. 1989. Leptomeningeal cell line from multiple sclerosis with reverse transcriptase activity and viral particles. Res. Virol. 140551-561. [DOI] [PubMed] [Google Scholar]
- 388.Perron, H., B. Gratacap, B. Lalande, O. Genoulaz, A. Laurent, C. Geny, M. Mallaret, P. Innocenti, E. Schuller, P. Stoebner, and J. M. Seigneurin. 1992. In vitro transmission and antigenicity of a retrovirus isolated from a multiple sclerosis patient. Res. Virol. 143337-350. [DOI] [PubMed] [Google Scholar]
- 389.Perron, H., E. Jouvin-Marche, M. Michel, A. Ounanian-Paraz, S. Camelo, A. Dumon, C. Jolivet-Reynaud, F. Marcel, Y. Souillet, E. Borel, L. Gebuhrer, L. Santoro, S. Marcel, J. M. Seigneurin, P. N. Marche, and M. Lafon. 2001. Multiple sclerosis retrovirus particles and recombinant envelope trigger an abnormal immune response in vitro, by inducing polyclonal Vβ16 T-lymphocyte activation. Virology 287321-332. [DOI] [PubMed] [Google Scholar]
- 390.Perron, H., B. Lalande, B. Gratacap, A. Laurent, O. Genoulaz, C. Geny, M. Mallaret, E. Schuller, P. Stoebner, and J. M. Seigneurin. 1991. Isolation of retrovirus from patients with multiple sclerosis. Lancet 337862-863. [DOI] [PubMed] [Google Scholar]
- 391.Perron, H., F. Lazarini, K. Ruprecht, C. Pechoux-Longin, D. Seilhean, V. Sazdovitch, A. Creange, N. Battail-Poirot, G. Sibai, L. Santoro, M. Jolivet, J. L. Darlix, P. Rieckmann, T. Arzberger, J. J. Hauw, and H. Lassmann. 2005. Human endogenous retrovirus (HERV)-W ENV and GAG proteins: physiological expression in human brain and pathophysiological modulation in multiple sclerosis lesions. J. Neurovirol. 1123-33. [DOI] [PubMed] [Google Scholar]
- 392.Perron, H., and J. M. Seigneurin. 1999. Human retroviral sequences associated with extracellular particles in autoimmune diseases: epiphenomenon or possible role in aetiopathogenesis? Microbes Infect. 1309-322. [DOI] [PubMed] [Google Scholar]
- 393.Perron, H., M. Suh, B. Lalande, B. Gratacap, A. Laurent, P. Stoebner, and J. M. Seigneurin. 1993. Herpes simplex virus ICP0 and ICP4 immediate early proteins strongly enhance expression of a retrovirus harboured by a leptomeningeal cell line from a patient with multiple sclerosis. J. Gen. Virol. 7465-72. [DOI] [PubMed] [Google Scholar]
- 394.Perzova, R. N., T. P. Loughran, S. Dube, J. Ferrer, E. Esteban, and B. J. Poiesz. 2000. Lack of BLV and PTLV DNA sequences in the majority of patients with large granular lymphocyte leukaemia. Br. J. Haematol. 10964-70. [DOI] [PubMed] [Google Scholar]
- 395.Pichon, J. P., B. Bonnaud, P. Cleuziat, and F. Mallet. 2006. Multiplex degenerate PCR coupled with an oligo sorbent array for human endogenous retrovirus expression profiling. Nucleic Acids Res. 34e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Pille, E. R., V. M. Mzokova, A. P. Andreeva, and O. G. Andzhaparidze. 1977. Possible role of measles, rubella and mumps viruses in multiple sclerosis. Acta Virol. 21139-145. [PubMed] [Google Scholar]
- 397.Piper, K. E., A. D. Hanssen, D. G. Lewallen, E. L. Matteson, D. R. Osmon, M. C. Duffy, R. A. Hagan, J. M. Steckelberg, and R. Patel. 2006. Lack of detection of human retrovirus-5 proviral DNA in synovial tissue and blood specimens from individuals with rheumatoid arthritis or osteoarthritis. Arthritis Rheum. 55123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Pogo, B. G., S. M. Melana, J. F. Holland, J. F. Mandeli, S. Pilotti, P. Casalini, and S. Menard. 1999. Sequences homologous to the mouse mammary tumor virus env gene in human breast carcinoma correlate with overexpression of laminin receptor. Clin. Cancer Res. 52108-2111. [PubMed] [Google Scholar]
- 399.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 777415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Portis, J. L. 2002. Perspectives on the role of endogenous human retroviruses in autoimmune diseases. Virology 2961-5. [DOI] [PubMed] [Google Scholar]
- 401.Price, E. J., and P. J. Venables. 1995. The etiopathogenesis of Sjögren's syndrome. Semin. Arthritis Rheum. 25117-133. [DOI] [PubMed] [Google Scholar]
- 402.Pyra, H., J. Böni, and J. Schüpbach. 1994. Ultrasensitive retrovirus detection by a reverse transcriptase assay based on product enhancement. Proc. Natl. Acad. Sci. USA 911544-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Query, C. C., and J. D. Keene. 1987. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell 51211-220. [DOI] [PubMed] [Google Scholar]
- 404.Rakoff-Nahoum, S., P. J. Kuebler, J. J. Heymann, M. E. Sheehy, G. M. Ortiz, G. S. Ogg, J. D. Barbour, J. Lenz, A. D. Steinfeld, and D. F. Nixon. 2006. Detection of T lymphocytes specific for human endogenous retrovirus K (HERV-K) in patients with seminoma. AIDS Res. Hum. Retrovir. 2252-56. [DOI] [PubMed] [Google Scholar]
- 405.Rakowicz-Szulczynska, E. M., D. G. McIntosh, and M. L. Smith. 1999. Giant syncytia and virus-like particles in ovarian carcinoma cells isolated from ascites fluid. Clin. Diagn. Lab. Immunol. 6115-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Ranki, A., E. Johansson, and K. Krohn. 1988. Interpretation of antibodies reacting solely with human retroviral core proteins. N. Engl. J. Med. 318448-449. [DOI] [PubMed] [Google Scholar]
- 407.Ranki, A., P. Kurki, S. Riepponen, and E. Stephansson. 1992. Antibodies to retroviral proteins in autoimmune connective tissue disease. Relation to clinical manifestations and ribonucleoprotein autoantibodies. Arthritis Rheum. 351483-1491. [DOI] [PubMed] [Google Scholar]
- 408.Reddy, E. P., M. Sandberg-Wollheim, R. V. Mettus, P. E. Ray, E. DeFreitas, and H. Koprowski. 1989. Amplification and molecular cloning of HTLV-I sequences from DNA of multiple sclerosis patients. Science 243529-533. [DOI] [PubMed] [Google Scholar]
- 409.Relman, D. A., T. M. Schmidt, R. P. MacDermott, and S. Falkow. 1992. Identification of the uncultured bacillus of Whipple's disease. N. Engl. J. Med. 327293-301. [DOI] [PubMed] [Google Scholar]
- 410.Reus, K., J. Mayer, M. Sauter, D. Scherer, N. Muller-Lantzsch, and E. Meese. 2001. Genomic organization of the human endogenous retrovirus HERV-K(HML-2.HOM) (ERVK6) on chromosome 7. Genomics 72314-320. [DOI] [PubMed] [Google Scholar]
- 411.Reuss, F. U. 1992. Expression of intracisternal A-particle-related retroviral element-encoded envelope proteins detected in cell lines. J. Virol. 661915-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Richardson, J. H., K. W. Wucherpfennig, N. Endo, P. Rudge, A. G. Dalgleish, and D. A. Hafler. 1989. PCR analysis of DNA from multiple sclerosis patients for the presence of HTLV-I. Science 246821-823. [PubMed] [Google Scholar]
- 413.Richman, L. K., R. J. Montali, R. L. Garber, M. A. Kennedy, J. Lehnhardt, T. Hildebrandt, D. Schmitt, D. Hardy, D. J. Alcendor, and G. S. Hayward. 1999. Novel endotheliotropic herpesviruses fatal for Asian and African elephants. Science 2831171-1176. [DOI] [PubMed] [Google Scholar]
- 414.Rigby, S. P., D. J. Griffiths, R. F. Jarrett, R. A. Weiss, and P. J. Venables. 1998. A new human retrovirus: a role in lymphoma? Am. J. Med. 10499-100. [DOI] [PubMed] [Google Scholar]
- 415.Rigby, S. P., D. J. Griffiths, R. A. Weiss, and P. J. Venables. 1997. Human retrovirus-5 proviral DNA is rarely detected in salivary gland biopsy tissues from patients with Sjögren's syndrome. Arthritis Rheum. 402016-2021. [DOI] [PubMed] [Google Scholar]
- 416.Ristevski, S., D. F. Purcell, J. Marshall, D. Campagna, S. Nouri, S. P. Fenton, D. A. McPhee, and G. Kannourakis. 1999. Novel endogenous type D retroviral particles expressed at high levels in a SCID mouse thymic lymphoma. J. Virol. 734662-4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Rivers, T. 1937. Viruses and Koch's postulates. J. Bacteriol. 331-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Rodriguez, M. P., R. Alvarez, D. G. del Barco, V. Falcon, M. C. de la Rosa, and J. de la Fuente. 1998. Characterization of a virus isolated from the cerebrospinal fluid of patients with epidemic neuropathy. Ann. Trop. Med. Parasitol. 9297-105. [PubMed] [Google Scholar]
- 419.Rolland, A., E. Jouvin-Marche, M. Saresella, P. Ferrante, R. Cavaretta, A. Créange, P. Marche, and H. Perron. 2005. Correlation between disease severity and in vitro cytokine production mediated by MSRV (multiple sclerosis associated retroviral element) envelope protein in patients with multiple sclerosis. J. Neuroimmunol. 160195-203. [DOI] [PubMed] [Google Scholar]
- 420.Rolland, A., E. Jouvin-Marche, C. Viret, M. Faure, H. Perron, and P. N. Marche. 2006. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J. Immunol. 1767636-7644. [DOI] [PubMed] [Google Scholar]
- 421.Rose, T. M., E. R. Schultz, J. G. Henikoff, S. Pietrokovski, C. M. McCallum, and S. Henikoff. 1998. Consensus-degenerate hybrid oligonucleotide primers for amplification of distantly related sequences. Nucleic Acids Res. 261628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Ross, S. R., J. J. Schofield, C. J. Farr, and M. Bucan. 2002. Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus. Proc. Natl. Acad. Sci. USA 9912386-12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Roucoux, D. F., and E. L. Murphy. 2004. The epidemiology and disease outcomes of human T-lymphotropic virus type II. AIDS Rev. 6144-154. [PubMed] [Google Scholar]
- 424.Rous, P. 1911. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J. Exp. Med. 13397-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Roy-Burman, P., R. W. Rongey, B. E. Henderson, and M. B. Gardner. 1973. Attempts to detect RNA tumour virus in human milk. Nat. New Biol. 244146. [DOI] [PubMed] [Google Scholar]
- 426.Ruprecht, K., K. Obojes, V. Wengel, F. Gronen, K. S. Kim, H. Perron, J. Schneider-Schaulies, and P. Rieckmann. 2006. Regulation of human endogenous retrovirus W protein expression by herpes simplex virus type 1: implications for multiple sclerosis. J. Neurovirol. 1265-71. [DOI] [PubMed] [Google Scholar]
- 427.Sander, D., S. Soble, B. Choi, H. Jaspan, W. Gallaher, C. Fermin, J. Jaspan, Y. Cao, P. Kohler, H. Lou, and R. Garry. 1996. HIAP-I: a serological and molecular characterization of a novel retrovirus associated with autoimmune disease. FASEB J. 101700. [Google Scholar]
- 428.Sanger, F., G. M. Air, B. G. Barrell, N. L. Brown, A. R. Coulson, C. A. Fiddes, C. A. Hutchison, P. M. Slocombe, and M. Smith. 1977. Nucleotide sequence of bacteriophage phi X174 DNA. Nature 265687-695. [DOI] [PubMed] [Google Scholar]
- 429.Sarkar, N. H., and D. H. Moore. 1972. On the possibility of a human breast cancer virus. Nature 236103-106. [DOI] [PubMed] [Google Scholar]
- 430.Sauter, M., K. Roemer, B. Best, M. Afting, S. Schommer, G. Seitz, M. Hartmann, and N. Mueller-Lantzsch. 1996. Specificity of antibodies directed against Env protein of human endogenous retroviruses in patients with germ cell tumors. Cancer Res. 564362-4365. [PubMed] [Google Scholar]
- 431.Schiavetti, F., J. Thonnard, D. Colau, T. Boon, and P. G. Coulie. 2002. A human endogenous retroviral sequence encoding an antigen recognized on melanoma by cytolytic T lymphocytes. Cancer Res. 625510-5516. [PubMed] [Google Scholar]
- 432.Schlom, J., and S. Spiegelman. 1971. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science 174840-843. [DOI] [PubMed] [Google Scholar]
- 433.Schmitz, C., D. Marchant, S. J. Neil, K. Aubin, S. Reuter, M. T. Dittmar, and Á. McKnight. 2004. Lv2, a novel postentry restriction, is mediated by both capsid and envelope. J. Virol. 782006-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Schubbert, R., D. Renz, B. Schmitz, and W. Doerfler. 1997. Foreign (M13) DNA ingested by mice reaches peripheral leukocytes, spleen, and liver via the intestinal wall mucosa and can be covalently linked to mouse DNA. Proc. Natl. Acad. Sci. USA 94961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Schulte, A. M., C. Malerczyk, R. Cabal-Manzano, J. J. Gajarsa, H. J. List, A. T. Riegel, and A. Wellstein. 2000. Influence of the human endogenous retrovirus-like element HERV-E.PTN on the expression of growth factor pleiotrophin: a critical role of a retroviral Sp1-binding site. Oncogene 193988-3998. [DOI] [PubMed] [Google Scholar]
- 436.Schweizer, M., and D. Neumann-Haefelin. 1995. Phylogenetic analysis of primate foamy viruses by comparison of pol sequences. Virology 207577-582. [DOI] [PubMed] [Google Scholar]
- 437.Schweizer, M., R. Turek, H. Hahn, A. Schliephake, K. O. Netzer, G. Eder, M. Reinhardt, A. Rethwilm, and D. Neumann-Haefelin. 1995. Markers of foamy virus infections in monkeys, apes, and accidentally infected humans: appropriate testing fails to confirm suspected foamy virus prevalence in humans. AIDS Res. Hum. Retrovir. 11161-170. [DOI] [PubMed] [Google Scholar]
- 438.Seifarth, W., O. Frank, U. Zeilfelder, B. Spiess, A. D. Greenwood, R. Hehlmann, and C. Leib-Mösch. 2005. Comprehensive analysis of human endogenous retrovirus transcriptional activity in human tissues with a retrovirus-specific microarray. J. Virol. 79341-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Seifarth, W., U. Krause, C. Hohenadl, C. Baust, R. Hehlmann, and C. Leib-Mösch. 2000. Rapid identification of all known retroviral reverse transcriptase sequences with a novel versatile detection assay. AIDS Res. Hum. Retrovir. 16721-729. [DOI] [PubMed] [Google Scholar]
- 440.Seifarth, W., H. Skladny, F. Krieg-Schneider, A. Reichert, R. Hehlmann, and C. Leib-Mösch. 1995. Retrovirus-like particles released from the human breast cancer cell line T47-D display type B- and C-related endogenous retroviral sequences. J. Virol. 696408-6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Selmi, C., S. R. Ross, A. A. Ansari, P. Invernizzi, M. Podda, R. L. Coppel, and M. E. Gershwin. 2004. Lack of immunological or molecular evidence for a role of mouse mammary tumor retrovirus in primary biliary cirrhosis. Gastroenterology 127493-501. [DOI] [PubMed] [Google Scholar]
- 442.Shah, K. V. 2007. SV40 and human cancer: a review of recent data. Int. J. Cancer 120215-223. [DOI] [PubMed] [Google Scholar]
- 443.Sharp, P. M., E. Bailes, R. R. Chaudhuri, C. M. Rodenburg, M. O. Santiago, and B. H. Hahn. 2001. The origins of acquired immune deficiency syndrome viruses: where and when? Philos. Trans. R. Soc. Lond. B Biol. Sci. 356867-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Shattles, W. G., S. M. Brookes, P. J. Venables, D. A. Clark, and R. N. Maini. 1992. Expression of antigen reactive with a monoclonal antibody to HTLV-1 P19 in salivary glands in Sjögren's syndrome. Clin. Exp. Immunol. 8946-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Shen, Y., H. Zhu, and T. Shenk. 1997. Human cytomagalovirus IE1 and IE2 proteins are mutagenic and mediate “hit-and-run” oncogenic transformation in cooperation with the adenovirus E1A proteins. Proc. Natl. Acad. Sci. USA 943341-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Shen-Ong, G. L., and M. D. Cole. 1984. Amplification of a specific set of intracisternal A-particle genes in a mouse plasmacytoma. J. Virol. 49171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Shih, A., R. Misra, and M. G. Rush. 1989. Detection of multiple, novel reverse transcriptase coding sequences in human nucleic acids: relation to primate retroviruses. J. Virol. 6364-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Shiramizu, B., B. G. Herndier, and M. S. McGrath. 1994. Identification of a common clonal human immunodeficiency virus integration site in human immunodeficiency virus-associated lymphomas. Cancer Res. 542069-2072. [PubMed] [Google Scholar]
- 449.Sigurdsson, B., and P. A. Palsson. 1958. Visna of sheep; a slow, demyelinating infection. Br. J. Exp. Pathol. 39519-528. [PMC free article] [PubMed] [Google Scholar]
- 450.Silver, J., T. Maudru, K. Fujita, and R. Repaske. 1993. An RT-PCR assay for the enzyme activity of reverse transcriptase capable of detecting single virions. Nucleic Acids Res. 213593-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Simmons, A. 2001. Herpesvirus and multiple sclerosis. Herpes 860-63. [PubMed] [Google Scholar]
- 452.Simpson, G. R., C. Patience, R. Löwer, R. R. Tönjes, H. D. Moore, R. A. Weiss, and M. T. Boyd. 1996. Endogenous D-type (HERV-K) related sequences are packaged into retroviral particles in the placenta and possess open reading frames for reverse transcriptase. Virology 222451-456. [DOI] [PubMed] [Google Scholar]
- 453.Slattery, J. P., G. Franchini, and A. Gessain. 1999. Genomic evolution, patterns of global dissemination, and interspecies transmission of human and simian T-cell leukemia/lymphotropic viruses. Genome Res. 9525-540. [PubMed] [Google Scholar]
- 454.Smith, D. K., J. J. Neal, S. D. Holmberg, et al. 1993. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. An investigation of cases in the United States. N. Engl. J. Med. 328373-379. [DOI] [PubMed] [Google Scholar]
- 455.Snyder, H. W., Jr., and E. Fleissner. 1980. Specificity of human antibodies to oncovirus glycoproteins: recognition of antigen by natural antibodies directed against carbohydrate structures. Proc. Natl. Acad. Sci. USA 771622-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Sommerlund, M., G. Pallesen, A. Møller-Larsen, H. J. Hansen, and S. Haahr. 1993. Retrovirus-like particles in an Epstein-Barr virus-producing cell line derived from a patient with chronic progressive myelopathy. Acta Neurol. Scand. 8771-76. [DOI] [PubMed] [Google Scholar]
- 457.Sørensen, J. B., F. R. Hirsch, A. Gazdar, and J. E. Olsen. 1993. Interobserver variability in histopathologic subtyping and grading of pulmonary adenocarcinoma. Cancer 712971-2976. [DOI] [PubMed] [Google Scholar]
- 458.Sotgiu, S., G. Arru, G. Mameli, C. Serra, M. Pugliatti, G. Rosati, and A. Dolei. 2006. Multiple sclerosis-associated retrovirus in early multiple sclerosis: a six-year follow-up of a Sardinian cohort. Mult. Scler. 12698-703. [DOI] [PubMed] [Google Scholar]
- 459.Spiegelman, S., R. Axel, and J. Schlom. 1972. Virus-related RNA in human and mouse mammary tumors. J. Natl. Cancer Inst. 481205-1211. [PubMed] [Google Scholar]
- 460.Spiegelman, S., I. Keydar, R. Mesa-Tejada, T. Ohno, M. Ramanarayanan, R. Nayak, J. Bausch, and C. Fenoglio. 1980. Possible diagnostic implications of a mammary tumor virus related protein in human breast cancer. Cancer 46879-892. [DOI] [PubMed] [Google Scholar]
- 461.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 812123-2135. [DOI] [PubMed] [Google Scholar]
- 462.Stancek, D., M. Stanceková-Gressnerová, M. Janotka, P. Hnilica, and D. Oravec. 1975. Isolation and some serological and epidemiological data on the viruses recovered from patients with subacute thyroiditis de Quervain. Med. Microbiol. Immunol. (Berlin) 161133-144. [DOI] [PubMed] [Google Scholar]
- 463.Stauffer, Y., S. Marguerat, F. Meylan, C. Ucla, N. Sutkowski, B. Huber, T. Pelet, and B. Conrad. 2001. Interferon-α-induced endogenous superantigen. A model linking environment and autoimmunity. Immunity 15591-601. [DOI] [PubMed] [Google Scholar]
- 464.Stauffer, Y., G. Theiler, P. Sperisen, Y. Lebedev, and C. V. Jongeneel. 2004. Digital expression profiles of human endogenous retroviral families in normal and cancerous tissues. Cancer Immun. 42. [PubMed] [Google Scholar]
- 465.Stewart, G. T. 2000. The Durban Declaration is not accepted by all. Nature 407286. [DOI] [PubMed] [Google Scholar]
- 466.Stewart, T. H., R. D. Sage, A. F. Stewart, and D. W. Cameron. 2000. Breast cancer incidence highest in the range of one species of house mouse, Mus domesticus. Br. J. Cancer 82446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Stoye, J. P. 1999. The pathogenic potential of endogenous retroviruses: a sceptical view. Trends Microbiol. 7430. [DOI] [PubMed] [Google Scholar]
- 468.Stoye, J. P., and J. M. Coffin. 1987. The four classes of endogenous murine leukemia virus: structural relationships and potential for recombination. J. Virol. 612659-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Stransky, G., J. Vernon, W. K. Aicher, L. W. Moreland, R. E. Gay, and S. Gay. 1993. Virus-like particles in synovial fluids from patients with rheumatoid arthritis. Br. J. Rheumatol. 321044-1048. [DOI] [PubMed] [Google Scholar]
- 470.Sun, R., E. Grogan, D. Shedd, A. F. Bykovsky, V. M. Kushnaryov, S. E. Grossberg, and G. Miller. 1995. Transmissible retrovirus in Epstein-Barr virus-producer B95-8 cells. Virology 209374-383. [DOI] [PubMed] [Google Scholar]
- 471.Sutkowski, N., G. Chen, G. Calderon, and B. T. Huber. 2004. Epstein-Barr virus latent membrane protein LMP-2A is sufficient for transactivation of the human endogenous retrovirus HERV-K18 superantigen. J. Virol. 787852-7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Sutkowski, N., B. Conrad, D. A. Thorley-Lawson, and B. T. Huber. 2001. Epstein-Barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. Immunity 15579-589. [DOI] [PubMed] [Google Scholar]
- 473.Sutkowski, N., T. Palkama, C. Ciurli, R. P. Sekaly, D. A. Thorley-Lawson, and B. T. Huber. 1996. An Epstein-Barr virus-associated superantigen. J. Exp. Med. 184971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Switzer, W. M., V. Bhullar, V. Shanmugam, M. E. Cong, B. Parekh, N. W. Lerche, J. L. Yee, J. J. Ely, R. Boneva, L. E. Chapman, T. M. Folks, and W. Heneine. 2004. Frequent simian foamy virus infection in persons occupationally exposed to nonhuman primates. J. Virol. 782780-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Szabo, S., A. M. Haislip, and R. F. Garry. 2005. Of mice, cats, and men: is human breast cancer a zoonosis? Microsc. Res. Tech. 68197-208. [DOI] [PubMed] [Google Scholar]
- 476.Tai, A. K., M. Lin, F. Chang, G. Chen, F. Hsiao, N. Sutkowski, and B. T. Huber. 2006. Murine Vβ3+ and Vβ7+ T cell subsets are specific targets for the HERV-K18 Env superantigen. J. Immunol. 1773178-3184. [DOI] [PubMed] [Google Scholar]
- 477.Talal, N., M. J. Dauphinee, H. Dang, S. S. Alexander, D. J. Hart, and R. F. Garry. 1990. Detection of serum antibodies to retroviral proteins in patients with primary Sjögren's syndrome (autoimmune exocrinopathy). Arthritis Rheum. 33774-781. [DOI] [PubMed] [Google Scholar]
- 478.Talal, N., R. F. Garry, P. H. Schur, S. Alexander, M. J. Dauphinee, I. H. Livas, A. Ballester, M. Takei, and H. Dang. 1990. A conserved idiotype and antibodies to retroviral proteins in systemic lupus erythematosus. J. Clin. Investig. 851866-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Tamura, N., A. Iwase, K. Suzuki, N. Maruyama, and S. Kira. 1997. Alveolar macrophages produce the Env protein of a human endogenous retrovirus, HERV-E 4-1, in a subgroup of interstitial lung diseases. Am. J. Respir. Cell Mol. Biol. 16429-437. [DOI] [PubMed] [Google Scholar]
- 480.Tamura, N., I. Sekigawa, H. Hashimoto, N. Yamamoto, and S. Kira. 1997. Syncytial cell formation in vivo by type C retroviral particles in the systemic lupus erythematosus (SLE) lung. Clin. Exp. Immunol. 107474-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Taylor, G. P., P. Goon, Y. Furukawa, H. Green, A. Barfield, A. Mosley, H. Nose, A. Babiker, P. Rudge, K. Usuku, M. Osame, C. R. Bangham, and J. N. Weber. 2006. Zidovudine plus lamivudine in human T-lymphotropic virus type-I-associated myelopathy: a randomised trial. Retrovirology 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Theodorou, V., M. Boer, B. Weigelt, J. Jonkers, M. van der Valk, and J. Hilkens. 2004. Fgf10 is an oncogene activated by MMTV insertional mutagenesis in mouse mammary tumors and overexpressed in a subset of human breast carcinomas. Oncogene 236047-6055. [DOI] [PubMed] [Google Scholar]
- 483.Thiry, L., S. Sprecher-Goldberger, M. Bossens, J. Cogniaux-Le Clerc, and P. Vereerstraeten. 1978. Neutralization of Mason-Pfizer virus by sera from patients treated for renal disease. J. Gen. Virol. 41587-597. [DOI] [PubMed] [Google Scholar]
- 484.Tisch, R., and H. McDevitt. 1996. Insulin-dependent diabetes mellitus. Cell 85291-297. [DOI] [PubMed] [Google Scholar]
- 485.Titus-Ernstoff, L., K. M. Egan, P. A. Newcomb, J. A. Baron, M. Stampfer, E. R. Greenberg, B. F. Cole, J. Ding, W. Willett, and D. Trichopoulos. 1998. Exposure to breast milk in infancy and adult breast cancer risk. J. Natl. Cancer Inst. 90921-924. [DOI] [PubMed] [Google Scholar]
- 486.Todaro, G. J., P. Arnstein, W. P. Parks, E. H. Lennette, and R. J. Huebner. 1973. A type-C virus in human rhabdomyosarcoma cells after inoculation into NIH Swiss mice treated with antithymocyte serum. Proc. Natl. Acad. Sci. USA 70859-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Todd, J. A. 1999. From genome to aetiology in a multifactorial disease, type 1 diabetes. Bioessays 21164-174. [DOI] [PubMed] [Google Scholar]
- 488.Tönjes, R. R., F. Czauderna, and R. Kurth. 1999. Genome-wide screening, cloning, chromosomal assignment, and expression of full-length human endogenous retrovirus type K. J. Virol. 739187-9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Tönjes, R. R., R. Löwer, K. Boller, J. Denner, B. Hasenmaier, H. Kirsch, H. König, C. Korbmacher, C. Limbach, R. Lugert, R. C. Phelps, J. Scherer, K. Thelen, J. Löwer, and R. Kurth. 1996. HERV-K: the biologically most active human endogenous retrovirus family. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13(Suppl. 1):S261-S267. [DOI] [PubMed] [Google Scholar]
- 490.Travis, W. D., K. Garg, W. A. Franklin, I. I. Wistuba, B. Sabloff, M. Noguchi, R. Kakinuma, M. Zakowski, M. Ginsberg, R. Padera, F. Jacobson, B. E. Johnson, F. Hirsch, E. Brambilla, D. B. Flieder, K. R. Geisinger, F. Thunnissen, K. Kerr, D. Yankelevitz, T. J. Franks, J. R. Galvin, D. W. Henderson, A. G. Nicholson, P. S. Hasleton, V. Roggli, M. S. Tsao, F. Cappuzzo, and M. Vazquez. 2006. Bronchioloalveolar carcinoma and lung adenocarcinoma: the clinical importance and research relevance of the 2004 World Health Organization pathologic criteria. J. Thorac. Oncol. 1S13-S19. [PubMed] [Google Scholar]
- 491.Tristem, M. 1996. Amplification of divergent retroelements by PCR. BioTechniques 20608-612. [DOI] [PubMed] [Google Scholar]
- 492.Tristem, M. 2000. Identification and characterization of novel human endogenous retrovirus families by phylogenetic screening of the human genome mapping project database. J. Virol. 743715-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Tuke, P. W., H. Perron, F. Bedin, F. Beseme, and J. A. Garson. 1997. Development of a pan-retrovirus detection system for multiple sclerosis studies. Acta Neurol. Scand. Suppl. 16916-21. [DOI] [PubMed] [Google Scholar]
- 494.Turner, G., M. Barbulescu, M. Su, M. I. Jensen-Seaman, K. K. Kidd, and J. Lenz. 2001. Insertional polymorphisms of full-length endogenous retroviruses in humans. Curr. Biol. 111531-1535. [DOI] [PubMed] [Google Scholar]
- 495.Tustin, R. C. 1969. Ovine jaagsiekte. J. S. Afr. Vet. Med. Assoc. 403-23. [PubMed] [Google Scholar]
- 496.Uckert, W., V. Wunderlich, E. Bender, G. Sydow, and D. Bierwolf. 1980. The protein pattern of PMF virus, a type-D retrovirus from malignant permanent human cell lines. Arch. Virol. 64155-166. [DOI] [PubMed] [Google Scholar]
- 497.Urisman, A., R. J. Molinaro, N. Fischer, S. J. Plummer, G. Casey, E. A. Klein, K. Malathi, C. Magi-Galluzzi, R. R. Tubbs, D. Ganem, R. H. Silverman, and J. L. DeRisi. 2006. Identification of a novel gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2e25. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 498.Urnovitz, H. B., and W. H. Murphy. 1996. Human endogenous retroviruses: nature, occurrence, and clinical implications in human disease. Clin. Microbiol. Rev. 972-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Vallée, H., and H. Carré. 1904. Nature infectieuse de l'anemie du cheval. C. R. Acad. Sci. 139331-333. [Google Scholar]
- 500.van der Hoek, L., K. Pyrc, M. F. Jebbink, W. Vermeulen-Oost, R. J. Berkhout, K. C. Wolthers, P. M. Wertheim-van Dillen, J. Kaandorp, J. Spaargaren, and B. Berkhout. 2004. Identification of a new human coronavirus. Nat. Med. 10368-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Varmus, H. 1988. Retroviruses. Science 2401427-1435. [DOI] [PubMed] [Google Scholar]
- 502.Venables, P. J., S. M. Brookes, D. Griffiths, R. A. Weiss, and M. T. Boyd. 1995. Abundance of an endogenous retroviral envelope protein in placental trophoblasts suggests a biological function. Virology 211589-592. [DOI] [PubMed] [Google Scholar]
- 503.Verma, I. M. 1977. The reverse transcriptase. Biochim. Biophys. Acta 4731-38. [DOI] [PubMed] [Google Scholar]
- 504.Vernon, M. L., J. M. McMahon, and J. J. Hackett. 1974. Additional evidence of type-C particles in human placentas. J. Natl. Cancer Inst. 52987-989. [DOI] [PubMed] [Google Scholar]
- 505.Villesen, P., L. Aagaard, C. Wiuf, and F. S. Pedersen. 2004. Identification of endogenous retroviral reading frames in the human genome. Retrovirology 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Vine, A. M., A. G. Heaps, L. Kaftantzi, A. Mosley, B. Asquith, A. Witkover, G. Thompson, M. Saito, P. K. Goon, L. Carr, F. Martinez-Murillo, G. P. Taylor, and C. R. Bangham. 2004. The role of CTLs in persistent viral infection: cytolytic gene expression in CD8+ lymphocytes distinguishes between individuals with a high or low proviral load of human T cell lymphotropic virus type 1. J. Immunol. 1735121-5129. [DOI] [PubMed] [Google Scholar]
- 507.Voisset, C., O. Bouton, F. Bedin, L. Duret, B. Mandrand, F. Mallet, and G. Paranhos-Baccala. 2000. Chromosomal distribution and coding capacity of the human endogenous retrovirus HERV-W family. AIDS Res. Hum. Retrovir. 16731-740. [DOI] [PubMed] [Google Scholar]
- 508.Voisset, C., R. E. Myers, A. Carne, P. Kellam, and D. J. Griffiths. 2003. Rabbit endogenous retrovirus-H encodes a functional protease. J. Gen. Virol. 84215-225. [DOI] [PubMed] [Google Scholar]
- 509.Voisset, C., R. R. Tönjes, P. Breyton, B. Mandrand, and G. Paranhos-Baccalà. 2001. Specific detection of RT activity in culture supernantants of retrovirus-producing cells, using synthetic DNA as competitor in polymerase enhanced reverse transcriptase assay. J. Virol. Methods 94187-193. [DOI] [PubMed] [Google Scholar]
- 510.Vonka, V. 2000. Causality in medicine: the case of tumours and viruses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 3551831-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Wang, D., L. Coscoy, M. Zylberberg, P. C. Avila, H. A. Boushey, D. Ganem, and J. L. DeRisi. 2002. Microarray-based detection and genotyping of viral pathogens. Proc. Natl. Acad. Sci. USA 9915687-15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Wang, D., A. Urisman, Y. T. Liu, M. Springer, T. G. Ksiazek, D. D. Erdman, E. R. Mardis, M. Hickenbotham, V. Magrini, J. Eldred, J. P. Latreille, R. K. Wilson, D. Ganem, and J. L. DeRisi. 2003. Viral discovery and sequence recovery using DNA microarrays. PLoS Biol. 1E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Wang, Y., V. Go, J. F. Holland, S. M. Melana, and B. G. Pogo. 1998. Expression of mouse mammary tumor virus-like env gene sequences in human breast cancer. Clin. Cancer Res. 42565-2568. [PubMed] [Google Scholar]
- 514.Wang, Y., J. F. Holland, I. J. Bleiweiss, S. Melana, X. Liu, I. Pelisson, A. Cantarella, K. Stellrecht, S. Mani, and B. G. Pogo. 1995. Detection of mammary tumor virus env gene-like sequences in human breast cancer. Cancer Res. 555173-5179. [PubMed] [Google Scholar]
- 515.Wang, Y., J. D. Jiang, D. Xu, Y. Li, C. Qu, J. F. Holland, and B. G. Pogo. 2004. A mouse mammary tumor virus-like long terminal repeat superantigen in human breast cancer. Cancer Res. 644105-4111. [DOI] [PubMed] [Google Scholar]
- 516.Wang-Johanning, F., A. R. Frost, B. Jian, L. Epp, D. W. Lu, and G. L. Johanning. 2003. Quantitation of HERV-K env gene expression and splicing in human breast cancer. Oncogene 221528-1535. [DOI] [PubMed] [Google Scholar]
- 517.Wang-Johanning, F., A. R. Frost, G. L. Johanning, M. B. Khazaeli, A. F. LoBuglio, D. R. Shaw, and T. V. Strong. 2001. Expression of human endogenous retrovirus K envelope transcripts in human breast cancer. Clin. Cancer Res. 71553-1560. [PubMed] [Google Scholar]
- 518.Wang-Johanning, F., J. Liu, K. Rycaj, M. Huang, K. Tsai, D. G. Rosen, D. T. Chen, D. W. Lu, K. F. Barnhart, and G. L. Johanning. 2007. Expression of multiple human endogenous retrovirus surface envelope proteins in ovarian cancer. Int. J. Cancer 12081-90. [DOI] [PubMed] [Google Scholar]
- 519.Weber, G., J. Shendure, D. M. Tanenbaum, G. M. Church, and M. Meyerson. 2002. Identification of foreign gene sequences by transcript filtering against the human genome. Nat. Genet. 30141-142. [DOI] [PubMed] [Google Scholar]
- 520.Weinshenker, B. G., G. Dekaban, and G. P. Rice. 1990. Retroviruses and multiple sclerosis. I. Analysis of seroreactivity by Western blot and radioimmune assay. Neurology 401251-1253. [DOI] [PubMed] [Google Scholar]
- 521.Weis, S., I. C. Llenos, S. Sabunciyan, J. R. Dulay, L. Isler, R. Yolken, and H. Perron. 2007. Reduced expression of human endogenous retrovirus (HERV)-W GAG protein in the cingulate gyrus and hippocampus in schizophrenia, bipolar disorder, and depression. J. Neural Transm. 114645-655. [DOI] [PubMed] [Google Scholar]
- 522.Weiss, R., N. Teich, H. Varmus, and J. Coffin (ed.). 1984. RNA tumor viruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 523.Weiss, R. A. 1984. The search for human RNA tumor viruses, p. 1205-1281. In R. Weiss, N. Teich, H. Varmus, and J. Coffin (ed.), RNA tumor viruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 524.Weiss, R. A., and H. W. Jaffe. 1990. Duesberg, HIV and AIDS. Nature 345659-660. [DOI] [PubMed] [Google Scholar]
- 525.Werner, J., and H. Gelderblom. 1979. Isolation of foamy virus from patients with de Quervain thyroiditis. Lancet ii258-259. [DOI] [PubMed] [Google Scholar]
- 526.White, J., A. Herman, A. M. Pullen, R. Kubo, J. W. Kappler, and P. Marrack. 1989. The Vβ-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell 5627-35. [DOI] [PubMed] [Google Scholar]
- 527.White, R. A., S. G. McNulty, N. N. Nsumu, L. A. Boydston, B. P. Brewer, and K. Shimizu. 2005. Positional cloning of the Ttc7 gene required for normal iron homeostasis and mutated in hea and fsn anemia mice. Genomics 85330-337. [DOI] [PubMed] [Google Scholar]
- 528.Wilkerson, M. J., W. C. Davis, T. V. Baszler, and W. P. Cheevers. 1995. Immunopathology of chronic lentivirus-induced arthritis. Am. J. Pathol. 1461433-1443. [PMC free article] [PubMed] [Google Scholar]
- 529.Wilkinson, D. A., D. L. Mager, and J.-A. C. Leong. 1994. Endogenous human retroviruses, p. 465-535. In J. A. Levy (ed.), The Retroviridae, vol. 3. Plenum Press, New York, NY. [Google Scholar]
- 530.Witkin, S. S., N. H. Sarkar, D. W. Kinne, R. A. Good, and N. K. Day. 1980. Antibodies reactive with the mouse mammary tumor virus in sera of breast cancer patients. Int. J. Cancer 25721-725. [DOI] [PubMed] [Google Scholar]
- 531.Witt, A., B. Hartmann, E. Marton, R. Zeillinger, M. Schreiber, and E. Kubista. 2003. The mouse mammary tumor virus-like env gene sequence is not detectable in breast cancer tissue of Austrian patients. Oncol. Rep. 101025-1029. [PubMed] [Google Scholar]
- 532.Wolfe, N. D., W. Heneine, J. K. Carr, A. D. Garcia, V. Shanmugam, U. Tamoufe, J. N. Torimiro, A. T. Prosser, M. Lebreton, E. Mpoudi-Ngole, F. E. McCutchan, D. L. Birx, T. M. Folks, D. S. Burke, and W. M. Switzer. 2005. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc. Natl. Acad. Sci. USA 1027994-7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Wolfe, N. D., W. M. Switzer, J. K. Carr, V. B. Bhullar, V. Shanmugam, U. Tamoufe, A. T. Prosser, J. N. Torimiro, A. Wright, E. Mpoudi-Ngole, F. E. McCutchan, D. L. Birx, T. M. Folks, D. S. Burke, and W. Heneine. 2004. Naturally acquired simian retrovirus infections in central African hunters. Lancet 363932-937. [DOI] [PubMed] [Google Scholar]
- 534.Woodland, D. L. 2002. Immunity and retroviral superantigens in humans. Trends Immunol. 2357-58. [DOI] [PubMed] [Google Scholar]
- 535.Wootton, S. K., C. L. Halbert, and A. D. Miller. 2005. Sheep retrovirus structural protein induces lung tumours. Nature 434904-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Wu, J., T. Zhou, J. He, and J. D. Mountz. 1993. Autoimmune disease in mice due to integration of an endogenous retrovirus in an apoptosis gene. J. Exp. Med. 178461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Xu, L., M. Sakalian, Z. Shen, G. Loss, J. Neuberger, and A. Mason. 2004. Cloning the human betaretrovirus proviral genome from patients with primary biliary cirrhosis. Hepatology 39151-156. [DOI] [PubMed] [Google Scholar]
- 538.Xu, L., Z. Shen, L. Guo, B. Fodera, A. Keogh, R. Joplin, B. O'Donnell, J. Aitken, W. Carman, J. Neuberger, and A. Mason. 2003. Does a betaretrovirus infection trigger primary biliary cirrhosis? Proc. Natl. Acad. Sci. USA 1008454-8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Xu, Y., N. Stange-Thomann, G. Weber, R. Bo, S. Dodge, R. G. David, K. Foley, J. Beheshti, N. L. Harris, B. Birren, E. S. Lander, and M. Meyerson. 2003. Pathogen discovery from human tissue by sequence-based computational subtraction. Genomics 81329-335. [DOI] [PubMed] [Google Scholar]
- 540.Yamano, S., J. N. Renard, F. Mizuno, Y. Narita, Y. Uchida, H. Higashiyama, H. Sakurai, and I. Saito. 1997. Retrovirus in salivary glands from patients with Sjögren's syndrome. J. Clin. Pathol. 50223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Yang, J., H. P. Bogerd, S. Peng, H. Wiegand, R. Truant, and B. R. Cullen. 1999. An ancient family of human endogenous retroviruses encodes a functional homolog of the HIV-1 Rev protein. Proc. Natl. Acad. Sci. USA 9613404-13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Yeh, J., M. Ahmed, S. A. Mayyasi, and A. A. Alessi. 1975. Detection of an antigen related to Mason-Pfizer virus in malignant human breast tumors. Science 190583-584. [DOI] [PubMed] [Google Scholar]
- 543.Yi, J. M., H. M. Kim, and H. S. Kim. 2004. Expression of the human endogenous retrovirus HERV-W family in various human tissues and cancer cells. J. Gen. Virol. 851203-1210. [DOI] [PubMed] [Google Scholar]
- 544.Yousem, S. A., S. D. Finkelstein, P. A. Swalsky, A. Bakker, and N. P. Ohori. 2001. Absence of jaagsiekte sheep retrovirus DNA and RNA in bronchioloalveolar and conventional human pulmonary adenocarcinoma by PCR and RT-PCR analysis. Hum. Pathol. 321039-1042. [DOI] [PubMed] [Google Scholar]
- 545.Zammarchi, F., M. Pistello, A. Piersigilli, R. Murr, C. Di Cristofano, A. G. Naccarato, and G. Bevilacqua. 2006. MMTV-like sequences in human breast cancer: a fluorescent PCR/laser microdissection approach. J. Pathol. 209436-444. [DOI] [PubMed] [Google Scholar]
- 546.Zangen, R., S. Harden, D. Cohen, P. Parrella, and D. Sidransky. 2002. Mouse mammary tumor-like env gene as a molecular marker for breast cancer? Int. J. Cancer 102304-307. [DOI] [PubMed] [Google Scholar]
- 547.zur Hausen, H. 2001. Proliferation-inducing viruses in nonpermissive systems as possible causes of human cancers. Lancet 357381-384. [DOI] [PubMed] [Google Scholar]