Abstract
In this study, we investigated the rate of colonization of skin of children with atopic dermatitis (AD) by methicillin-resistant Staphylococcus aureus (MRSA) and characterized the isolates. Active skin lesions in pediatric AD patients were cultured with Rodac Staph (Komed, Korea). S. aureus isolates were examined for drug susceptibilities, analyzed for the eta, etb, tst, and pvl genes, and typed using agr polymorphism, pulsed-field gel electrophoresis of SmaI-restricted chromosomal DNA, and staphylococcal cassette chromosome mec (SCCmec) typing. Eighty-seven (75.4%) of 115 patients had cultivable S. aureus isolates, 16 of which (18.3%) were MRSA. All MRSA isolates were susceptible to chloramphenicol, rifampin, cotrimoxazole, and ciprofloxacin. While methicillin-susceptible S. aureus (MSSA) isolates were composed of 23 isolates of singular types and nine clusters comprising 48 isolates, MRSA isolates were typed into three clones: eight isolates of pulsotype A-agr-1-SCCmec IV, five isolates of pulsotype B-agr-3-SCCmec IIb-etb positive, and three isolates of pulsotype C-agr-3-SCCmec IV. Three SCCmec IVA MRSA isolates were tst positive, but none were positive for the pvl or eta gene. Among 71 MSSA isolates, 7 isolates were tst positive, 6 of which were pulsotype F-agr-3, and 9 of 10 agr-4 isolates were eta positive. The average ages of patients carrying MSSA, SCCmec IVA MRSA, and SCCmec IIb MRSA were 7.7 ± 4.6, 3.1 ± 1.5, and 8.2 ± 3.1 years, respectively. Among the patients carrying MRSA, two patients had been treated with oral antimicrobials, and one had been admitted to the hospital 18 months previously. In conclusion, community-acquired MRSA isolates of a few clones colonized the skin of patients with AD without risk factors for the acquisition of hospital-acquired MRSA, which suggested that the skin of children with AD may represent a significant reservoir of MRSA colonization in the community.
Patients with atopic dermatitis (AD) tend to carry Staphylococcus aureus on their skin lesions (1), and superantigens and toxins of S. aureus allegedly exacerbate chronic inflammation of AD skin (4, 8, 9). As a result, antimicrobials have often been prescribed to control acute-phase AD (4). Eczematous lesions of AD patients are known to be a source of transmission of S. aureus (13, 15). Increasing incidences of community-acquired methicillin-resistant S. aureus (MRSA) (CA-MRSA) in skin and soft tissue infection raise concerns that AD skin would be a favorable reservoir for CA-MRSA.
A CA-MRSA outbreak was first described in United States in 1981 in association with intravenous drug users (40), but more recently, these strains have emerged as the pathogens most frequently found in patients with skin and soft tissue infections presenting to emergency departments in the United States (3, 23, 34). The most prevalent CA-MRSA clones in the United States have the USA300 pulsotype harboring staphylococcal cassette chromosome mec (SCCmec) IV and Panton-Valentine leukocidin (42, 43). The community-based epidemic of MRSA led us to think that that MRSA became as prevalent as penicillin-resistant S. aureus strains in the community, as suggested previously by Chambers (6). Although many Asian countries suffer from high rates of MRSA infection, there are few publications on the prevalence of CA-MRSA (7, 17). In South Korea, the overall MRSA rate in clinical isolates during the last decade has been reported to be approximately 70% regardless of the locations or sizes of hospitals (20, 29). Even though the origins of MRSA isolates are not clear, MRSA has been the major pathogen of skin infections and otitis media in South Korean outpatient clinics since the late 1990s (22, 28, 35). The epidemiology of CA-MRSA in South Korea requires urgent attention.
Therefore, in the present study, we evaluated the rate of colonization by MRSA in skin lesions of pediatric AD patients and characterized MRSA isolates obtained from those lesions.
MATERIALS AND METHODS
Patients and bacterial isolates.
AD patients were enrolled in our study at the times of their first visits to the pediatric allergy clinic of our hospital from June 2004 to April 2005. Eczematous skin lesions were imprinted with Rodac Staph (Komed, South Korea), and yellow colonies were selected after 48 h of incubation. Bacterial species identification and antimicrobial susceptibility testing were performed using the MicroScan PosCombo 1A system (Dade Behring, West Sacramento, CA). All isolates were stored in brain heart infusion broth containing 15% (vol/vol) glycerol. The first isolate obtained from each patient was investigated further. Patients' medical records were reviewed for basic demographics and clinical diagnoses, prior antimicrobial therapies, hospital admission histories, and places of residence.
Antimicrobial susceptibility.
The MicroScan PosCombo 1A (Dade-Behring) panel was used to determine bacterial susceptibility to penicillin, oxacillin, erythromycin, clindamycin, ciprofloxacin, ofloxacin, rifampin, gentamicin, cotrimoxazole, chloramphenicol, tetracycline, fusidic acid, quinupristin-dalfopristin, teicoplanin, and vancomycin. To determine inducible macrolide-lincosamide-streptogramin B (MLSB) resistance, the D-test (36) was performed on all S. aureus isolates that were clindamycin susceptible and erythromycin resistant.
DNA extraction.
MRSA isolates were subcultured on blood agar plates at 37°C overnight. Three to five isolated colonies were prepared for DNA extraction using the GeneElute bacterial genomic DNA kit (Sigma, St. Louis, MO). Lysostaphin and lysozyme were added for the lysis step at 10 units/ml and 45 mg/ml, respectively.
SCCmec typing and agr polymorphism.
PCR for agr polymorphism was performed using primers previously described by Gilot et al. (11). Type assignment of SCCmec elements from multiplex PCR was done as described previously by Oliveira and de Lencastre (38). For cases unresolved by these procedures, ccr typing and determining the location of IS1272 were undertaken as previously described (37).
PFGE.
Chromosomal DNA was digested with SmaI and electrophoresed using program 2 of the GenePath system (Bio-Rad Laboratories Inc., Hercules, CA) as previously described (21). The isolates showing six or fewer band differences by pulsed-field gel electrophoresis (PFGE) were counted to the same group of pulsotype. Cluster analysis of pulsotypes was done in the dendrogram type of the unweighted-pair group method using average linkages with the Dice coefficient using InfoQuest FP software, version 4.5 (Bio-Rad).
PCR for the eta, etb, tst, and pvl genes.
To detect the eta, etb, and tst genes, a multiplex PCR assay combining primers specific for eta, etb, and tst was performed (2). The pvl gene was detected with PCR using primers luk-PV-1 and luk-PV-2 (31).
RESULTS
Patients and bacterial isolates.
A total of 122 specimens were collected from 115 patients during the study. S. aureus was isolated from 92 (75.4%) specimens from 87 (75.7%) patients. Eighteen isolates from 16 (18.3%) patients were resistant to oxacillin by MicroScan. Forty-six (64.8%) of the 71 patients carrying methicillin-susceptible S. aureus (MSSA) were male, and their average age was 7.7 ± 4.6 years, The male-to-female ratio of 16 patients carrying MRSA was 7:9. While the average age of five patients carrying SCCmec IIb MRSA was 8.2 ± 3.1 years, that of 11 patients carrying SCCmec IVA MRSA was 3.1 ± 1.5 years. Two patients had been prescribed amoxicillin-clavulanate; one of them had also received mupirocin ointment, and the other patient had been admitted for pneumonia, which was treated with azithromycin 18 months prior to our study. All but two of our patients lived in metropolitan Seoul and its suburban area.
Antimicrobial susceptibility.
All MRSA isolates were susceptible to ciprofloxacin, ofloxacin, rifampin, cotrimoxazole, chloramphenicol, quinupristin-dalfopristin, teicoplanin, and vancomycin. The susceptibilities to erythromycin, clindamycin, gentamicin, tetracycline, and fusidic acid were 17.6%, 58.8%, 41.2%, 94.1%, and 88.2%, respectively. All MSSA isolates were susceptible to rifampin, cotrimoxazole, quinupristin-dalfopristin, teicoplanin, and vancomycin. Their susceptibilities against chloramphenicol, ofloxacin, and ciprofloxacin were 95.7%, 94.1%, and 94.1%, respectively. They were more susceptible to erythromycin (59.4%), clindamycin (95.9%), and gentamicin (99.7%) but were less susceptible to fusidic acid (55.1%) than MRSA. All 28 isolates, including 7 MRSA isolates that were resistant to erythromycin and susceptible to clindamycin, were D-test positive, except for a single MSSA isolate.
SCCmec typing, agr polymorphism, and PFGE.
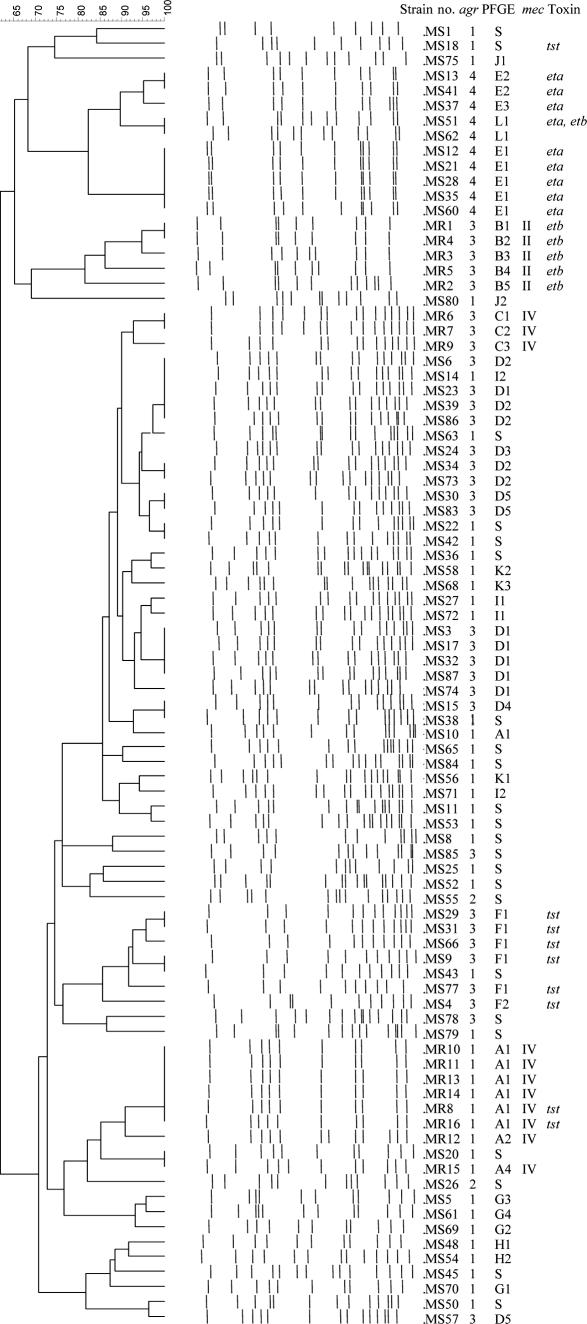
The 16 MRSA isolates were all mecA positive, dcs positive, and kdp negative. Of those isolates, five isolates were mecI positive and were positive for pUB110, except one. The other 11 isolates were mecI negative, IS1272 positive, and pUB110 positive. All MRSA isolates were positive for ccrA2, indicating the possible combinations of ccr type and mec type of 2A and 2B. Therefore, the former five isolates were SCCmec type II, kdp-negative variant IIb (14), and the latter 11 isolates were SCCmec type IV, pUB110-positive variant IVA (25, 35). Of 11 SCCmec IVA isolates, 8 had pulsotype A-agr-1, while 3 had pulsotype C-agr-3. Five SCCmec IIb isolates were all of pulsotype B-agr-3 (Fig. 1).
FIG. 1.
Cluster analysis of pulsotype, agr polymorphism, SCCmec typing, and toxin gene profiles of 87 S. aureus isolates according to agr polymorphism.
Among the 71 MSSA isolates, 35 were of the agr-1 type, 24 were of the agr-3 type, 10 were of the agr-4 type, and only 2 were of the agr-2 type (Fig. 1). In PFGE analyses, 48 MSSA isolates were distributed into nine clusters: pulsotype D for 17 isolates with agr-3, pulsotype E for 8 isolates with agr-4, pulsotype F for 6 isolates with agr-3, pulsotype G for 4 isolates with agr-1, and 5 other pulsotypes composed of two to three isolates per each group; however, the other 23 MSSA isolates were the solitary type (Fig. 1).
Toxin gene profiles.
All S. aureus isolates were negative for the pvl gene. Among the 16 MRSA isolates, 2 were tst positive and 5 were etb positive. Two of the tst-positive isolates were pulsotype A-agr-1-SCCmec IVA, while the five etb-positive isolates were all of pulsotype C-agr-3-SCCmec IIb (Fig. 1). Among the 71 MSSA isolates, tst was positive in six pulsotype F-agr-3 isolates and two pulsotype A-agr-1 isolates. eta was positive in all eight pulsotype E-agr-4 isolates and one pulsotype L-agr-4 isolate, which was the only etb-positive isolate (Fig. 1).
DISCUSSION
Consistent with previous studies (12, 16), S. aureus colonization was found in 75.7% of pediatric AD lesions, with MRSA accounting for 18.4% of S. aureus isolates in skin lesions of pediatric AD patients. This is the first report on the carriage rates of MRSA in skin lesions of pediatric AD patients. The carriage rate found by us is much higher than the recently reported rates of colonization by MRSA in healthy Asian schoolchildren. These rates were 5.1% in South Korea (30), 4.3% in Japan (14), and 1.9% in Taiwan (19). Considering a predilection of S. aureus for damaged skin and the frequent exposure of AD patients to antimicrobials, the high rate of colonization by MRSA noted in our study may not be surprising. Recently, there was a case report of a child with severe AD who presented with CA-MRSA skin abscesses (41). A high rate of colonization by MRSA can be worrisome for AD patients because it predisposes them to having invasive cutaneous infections. In addition, the average age of patients from whom SCCmec IV isolates were obtained was significantly younger than that of patients from whom SCCmec II isolates were cultured. These findings suggest that two discrete CA-MRSA clones were spread in different time periods. The high colonization rate and clonality of MRSA seen in this study indicate that AD patients can be a potential source of CA-MRSA transmission.
All the MRSA isolates were community acquired, and only two patients had risk factors for hospital-acquired MRSA (HA-MRSA), such as previous hospitalization and prior antibiotic therapy (24). SCCmec IVA was predominant among the MRSA isolates in our study. In addition, all such isolates were susceptible to cotrimoxazole and ciprofloxacin, which is unusual among MRSA strains isolated in South Korean hospitals (20, 29). Although the outbreak of staphylococcal scalded skin syndrome by MRSA that occurred in the Kyungnam province involved patients with no risk factors for HA-MRSA, and all isolates were clonal by PFGE, the MRSA isolates showed characteristics of typical HA-MRSA isolates, such as multidrug resistance and SCCmec type II (32). Therefore, the MRSA isolate was assumed to be a hospital-derived clone introduced into the community. However, an SCCmec IV clone has been found in community settings such as in neonates born at primary obstetrics clinics (26), in a surveillance of healthy schoolchildren (30), and in cases of bovine mastitis (27). As was the case in this study, such SCCmec IV clones were pUB110 positive and of type IVA and did not show multidrug resistance (5, 26). Even though there has been a lack of data on the prevalence of CA-MRSA infections, those reports suggest the emergence of CA-MRSA in South Korea.
MRSA isolates showed two agr types, agr-1 and agr-3, and MSSA isolates also were mainly of types agr-1 and agr-3. The prevalent CA-MRSA strain circulating in France, Switzerland, and Australia has agr-3 and the USA300 clone, which is an epidemic clone in United States, and in Europe, it has agr-1 (43). There has not been a reported case of agr-2 CA-MRSA. Because agr-2, which seems to have benefits in surviving in the hospital setting (33), is the type frequently found in cases of HA-MRSA in South Korea (46), the absence of agr-2 in MRSA isolates reported in this study was consistent with the community origin of the isolates reported here. Compared to the MSSA isolates composed of heterogeneous pulsotypes, all the MRSA isolates were clustered into a few clones by PFGE analysis. The MRSA isolates of each clusters also shared common types in SCCmec, agr polymorphism, and toxin profile: pulsotype A-agr-1-SCCmec IVA, pulsotype B-agr-3-SCCmec IVA, and pulsotype C-agr-3-SCCmec IIb-etb positive. Healthy schoolchildren in the Kyungnam province were also found to carry both SCCmec II and SCCmec IV MRSA clones (30). It thus appears that both SCCmec IV and SCCmec II clones of CA-MRSA have emerged in South Korea. CA-MRSA isolates in Taiwan and Japan did not always harbor SCCmec IV (7, 14, 39). SCCmec II is also predominant among CA-MRSA isolates in Japan (45), SCCmec III occurred frequently, and a novel SCCmec type (type V) was found among CA-MRSA isolates in Taiwan (7). MRSA isolates were all negative for pvl, and etb was exclusively correlated with the pulsotype B-SCCmec IIb clone in this study. As in this study, SCCmec IIb, first described in Japanese CA-MRSA isolates, also carries etb (45). There was no pvl gene found in CA-MRSA isolates from South Korea or Japan (14, 26, 30, 32, 39), while the pvl gene was present in those from Taiwan (44). Combined with the findings that eta was confined to agr-4 MSSA and tst was found in MRSA or MSSA isolates of the agr-1 or agr-3 type, these toxin genes indicate the evolution and spread of certain S. aureus strains. In Asian countries, CA-MRSA clones seem to have an origin distinct from those of CA-MRSA epidemic clones in Australia, the United States, and Europe (7, 14, 17, 18, 39). Well-organized prospective surveillance is thus required to understand the epidemiology of CA-MRSA in South Korea.
Consistent with the previous reports of CA-MRSA, the MRSA isolates were susceptible to antimicrobials of many different classes, as were MSSA isolates; however, MLSB resistance was common in erythromycin-resistant, clindamycin-susceptible isolates. Clindamycin is a treatment option for CA-MRSA infections in the United States because the isolates were usually susceptible to clindamycin and MLSB induction test negative (10). In South Korea, clindamycin should not be used for clindamycin-susceptible CA-MRSA infections without MLSB induction testing. Fortunately, skin and soft tissue infection can be treated without antimicrobial therapy if the area of infection is drained properly (34). However, as is the case with otitis media, CA-MRSA infection of tissues other than skin and soft tissue offers a challenge to antimicrobial therapy in South Korea (28).
In conclusion, AD patients showed high rates of MRSA colonization, and such patients may represent a significant reservoir of CA-MRSA. The major MRSA clone demonstrated known characteristics of CA-MRSA, including SCCmec type IV and a lack of multidrug resistance. MRSA isolates showed clonality by agr typing, PFGE, SCCmec typing, and toxin assays, suggesting a clonal spread of CA-MRSA.
Acknowledgments
This work was supported by the Asan Institute for Life Science (grant 2004-0660).
We thank Teruyo Ito at Juntendo University for valuable advice on SCCmec typing.
Footnotes
Published ahead of print on 3 January 2008.
REFERENCES
- 1.Akiyama, H., O. Yamasaki, J. Tada, and J. Arata. 2000. Adherence characteristics and susceptibility to antimicrobial agents of Staphylococcus aureus strains isolated from skin infections and atopic dermatitis. J. Dermatol. Sci. 23155-160. [DOI] [PubMed] [Google Scholar]
- 2.Becker, K., R. Roth, and G. Peters. 1998. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J. Clin. Microbiol. 362548-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharya, D., H. Carleton, C. J. Tsai, E. J. Baron, and F. Perdreau-Remington. 2007. Differences in clinical and molecular characteristics of skin and soft tissue methicillin-resistant Staphylococcus aureus isolates between two hospitals in Northern California. J. Clin. Microbiol. 451798-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardona, I. D., S. H. Cho, and D. Y. Leung. 2006. Role of bacterial superantigens in atopic dermatitis: implications for future therapeutic strategies. Am. J. Clin. Dermatol. 7273-279. [DOI] [PubMed] [Google Scholar]
- 5.Cha, H. Y., D. C. Moon, C. H. Choi, J. Y. Oh, Y. S. Jeong, Y. C. Lee, S. Y. Seol, D. T. Cho, H. H. Chang, S. W. Kim, and J. C. Lee. 2005. Prevalence of the ST239 clone of methicillin-resistant Staphylococcus aureus and differences in antimicrobial susceptibilities of ST239 and ST5 clones identified in a Korean hospital. J. Clin. Microbiol. 433610-3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers, H. F. 2001. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C. J., and Y. C. Huang. 2005. Community-acquired methicillin-resistant Staphylococcus aureus in Taiwan. J. Microbiol. Immunol. Infect. 38376-382. [PubMed] [Google Scholar]
- 8.Diep, B. A., H. A. Carleton, R. F. Chang, G. F. Sensabaugh, and F. Perdreau-Remington. 2006. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 1931495-1503. [DOI] [PubMed] [Google Scholar]
- 9.Durand, G., M. Bes, H. Meugnier, M. C. Enright, F. Forey, N. Liassine, A. Wenger, K. Kikuchi, G. Lina, F. Vandenesch, and J. Etienne. 2006. Detection of new methicillin-resistant Staphylococcus aureus clones containing the toxic shock syndrome toxin 1 gene responsible for hospital- and community-acquired infections in France. J. Clin. Microbiol. 44847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank, A. L., J. F. Marcinak, P. D. Mangat, J. T. Tjhio, S. Kelkar, P. C. Schreckenberger, and J. P. Quinn. 2002. Clindamycin treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatr. Infect. Dis. J. 21530-534. [DOI] [PubMed] [Google Scholar]
- 11.Gilot, P., G. Lina, T. Cochard, and B. Poutrel. 2002. Analysis of the genetic variability of genes encoding the RNA III-activating components agr and TRAP in a population of Staphylococcus aureus strains isolated from cows with mastitis. J. Clin. Microbiol. 404060-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong, J. Q., L. Lin, T. Lin, F. Hao, F. Q. Zeng, Z. G. Bi, D. Yi, and B. Zhao. 2006. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br. J. Dermatol. 155680-687. [DOI] [PubMed] [Google Scholar]
- 13.Hare, R., and E. M. Cooke. 1961. Self-contamination of patients with staphylococcal infections. Br. Med. J. 2333-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hisata, K., K. Kuwahara-Arai, M. Yamanoto, T. Ito, Y. Nakatomi, L. Cui, T. Baba, M. Terasawa, C. Sotozono, S. Kinoshita, Y. Yamashiro, and K. Hiramatsu. 2005. Dissemination of methicillin-resistant staphylococci among healthy Japanese children. J. Clin. Microbiol. 433364-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeger, P. H., and P. Elsner. 1988. Staphylococcal scalded skin syndrome: transmission of exfoliatin-producing Staphylococcus aureus by an asymptomatic carrier. Pediatr. Infect. Dis. J. 7340-342. [PubMed] [Google Scholar]
- 16.Hoeger, P. H., W. Lenz, A. Boutonnier, and J. M. Fournier. 1992. Staphylococcal skin colonization in children with atopic dermatitis: prevalence, persistence, and transmission of toxigenic and nontoxigenic strains. J. Infect. Dis. 1651064-1068. [DOI] [PubMed] [Google Scholar]
- 17.Hsu, L. Y., T. H. Koh, K. Singh, M. L. Kang, A. Kurup, and B. H. Tan. 2005. Dissemination of multisusceptible methicillin-resistant Staphylococcus aureus in Singapore. J. Clin. Microbiol. 432923-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, H., N. M. Flynn, J. H. King, C. Monchaud, M. Morita, and S. H. Cohen. 2006. Comparisons of community-associated methicillin-resistant Staphylococcus aureus (MRSA) and hospital-associated MSRA infections in Sacramento, California. J. Clin. Microbiol. 442423-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, Y. C., L. H. Su, C. J. Chen, and T. Y. Lin. 2005. Nasal carriage of methicillin-resistant Staphylococcus aureus in school children without identifiable risk factors in northern Taiwan. Pediatr. Infect. Dis. J. 24276-278. [DOI] [PubMed] [Google Scholar]
- 20.Kim, J. S., H. S. Kim, W. Song, H. C. Cho, K. M. Lee, and E. C. Kim. 2004. Antimicrobial resistance profiles of Staphylococcus aureus isolated in 13 Korean hospitals. Kor. J. Lab. Med. 24223-229. [Google Scholar]
- 21.Kim, M. N., S. H. Hwang, Y. J. Pyo, H. M. Mun, and C. H. Pai. 2002. Clonal spread of Staphylococcus aureus heterogeneously resistant to vancomycin in a university hospital in Korea. J. Clin. Microbiol. 401376-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, Y. J. 2001. A study of prevalence and antibiotic susceptibilities of Staphylococcus aureus in the bacterial skin infection of dermatology outpatients. Kor. J. Dermatol. 39866-871. [Google Scholar]
- 23.King, M. D., B. J. Humphrey, Y. F. Wang, E. V. Kourbatova, S. M. Ray, and H. M. Blumberg. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144309-317. [DOI] [PubMed] [Google Scholar]
- 24.Klevens, R. M., M. A. Morrison, S. K. Fridkin, A. Reingold, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, G. Fosheim, L. K. McDougal, and F. C. Tenover. 2006. Community-associated methicillin-resistant Staphylococcus aureus and healthcare risk factors. Emerg. Infect. Dis. 121991-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko, K. S., Y. S. Kim, J. H. Song, J. S. Yeom, H. Lee, S. I. Jung, D. R. Jeong, S. W. Kim, H. H. Chang, H. K. Ki, C. Moon, W. S. Oh, K. R. Peck, and N. Y. Lee. 2005. Genotypic diversity of methicillin-resistant Staphylococcus aureus isolates in Korean hospitals. Antimicrob. Agents Chemother. 493583-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko, K. S., S. Park, K. R. Peck, E. J. Shin, W. S. Oh, N. Y. Lee, and J. H. Song. 2006. Molecular characterization of methicillin-resistant Staphylococcus aureus spread by neonates transferred from primary obstetrics clinics to a tertiary care hospital in Korea. Infect. Control Hosp. Epidemiol. 27593-597. [DOI] [PubMed] [Google Scholar]
- 27.Kwon, N. H., K. T. Park, J. S. Moon, W. K. Jung, S. H. Kim, J. M. Kim, S. K. Hong, H. C. Koo, Y. S. Joo, and Y. H. Park. 2005. Staphylococcal cassette chromosome mec (SCCmec) characterization and molecular analysis for methicillin-resistant Staphylococcus aureus and novel SCCmec subtype IVg isolated from bovine milk in Korea. J. Antimicrob. Chemother. 56624-632. [DOI] [PubMed] [Google Scholar]
- 28.Lee, J. K., S. H. Kim, A. S. Na, C. G. Kim, and Y. B. Cho. 2003. Treatment of methicillin-resistant Staphylococcus aureus (MRSA) otorrhea in pediatric patients. Kor. J. Otolaryngol. Head Neck Surg. 4616-20. [Google Scholar]
- 29.Lee, K., Y. A. Kim, Y. J. Park, H. S. Lee, M. Y. Kim, E. C. Kim, D. Yong, and Y. Chong. 2004. Increasing prevalence of vancomycin-resistant enterococci, and cefoxitin-, imipenem- and fluoroquinolone-resistant gram-negative bacilli: a KONSAR study in 2002. Yonsei Med. J. 45598-608. [DOI] [PubMed] [Google Scholar]
- 30.Lee, Y. S., S. H. Ma, J. C. Lee, J. O. Cha, J. I. Yoo, E. K. Shin, and G. T. Chung. 2006. The prevalence of nasal colonization with methicillin-resistant Staphylococcus aureus among children in South Korea, abstr. C2-1135, p. 122. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother.
- 31.Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 291128-1132. [DOI] [PubMed] [Google Scholar]
- 32.Ma, S. H., E. J. Kim, J. C. Lee, and Y. S. Lee. 2005. Clinical features and microbial characteristics of community-acquired MRSA staphylococcal scalded skin syndrome. Kor. J. Infect. Dis. Suppl. 2S232. [Google Scholar]
- 33.Moise-Broder, P. A., G. Sakoulas, G. M. Eliopoulos, J. J. Schentag, A. Forrest, and R. C. Moellering, Jr. 2004. Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin. Infect. Dis. 381700-1705. [DOI] [PubMed] [Google Scholar]
- 34.Moran, G. J., A. Krishnadasan, R. J. Gorwitz, G. E. Fosheim, L. K. McDougal, R. B. Carey, and D. A. Talan. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355666-674. [DOI] [PubMed] [Google Scholar]
- 35.Nam, E. C., M. N. Kim, and K. S. Lee. 1999. Surgical results of MRSA-isolated chronic otitis media. Kor. J. Otolaryngol. 421238-1243. [Google Scholar]
- 36.National Committee for Clinical Laboratory Standards. 2004. Performance standards for antimicrobial susceptibility testing. Fourteenth informational supplement (NCCLS document M100-S14). National Committee for Clinical Laboratory Standards, Wayne, PA.
- 37.Okuma, K., K. Iwakawa, J. D. Turnidge, W. B. Grubb, J. M. Bell, F. G. O'Brien, G. W. Coombs, J. W. Pearman, F. C. Tenover, M. Kapi, C. Tiensasitorn, T. Ito, and K. Hiramatsu. 2002. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 404289-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliveira, D. C., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 462155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piao, C., T. Karasawa, K. Totsuka, T. Uchiyama, and K. Kikuchi. 2005. Prospective surveillance of community-onset and healthcare-associated methicillin-resistant Staphylococcus aureus isolated from a university-affiliated hospital in Japan. Microbiol. Immunol. 49959-970. [DOI] [PubMed] [Google Scholar]
- 40.Saravolatz, L. D., N. Markowitz, L. Arking, D. Pohlod, and E. Fisher. 1982. Methicillin-resistant Staphylococcus aureus. Epidemiologic observations during a community-acquired outbreak. Ann. Intern. Med. 9611-16. [DOI] [PubMed] [Google Scholar]
- 41.Suh, L. M., P. J. Honig, and A. C. Yan. 2006. Methicillin-resistant Staphylococcus aureus skin abscesses in a pediatric patient with atopic dermatitis: a case report. Cutis 78113-116. [PubMed] [Google Scholar]
- 42.Tenover, F. C., L. K. McDougal, R. V. Goering, G. Killgore, S. J. Projan, J. B. Patel, and P. M. Dunman. 2006. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J. Clin. Microbiol. 44108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, C. C., W. T. Lo, M. L. Chu, and L. K. Siu. 2004. Epidemiological typing of community-acquired methicillin-resistant Staphylococcus aureus isolates from children in Taiwan. Clin. Infect. Dis. 39481-487. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi, T., Y. Yokota, J. Terajima, T. Hayashi, M. Aepfelbacher, M. Ohara, H. Komatsuzawa, H. Watanabe, and M. Sugai. 2002. Clonal association of Staphylococcus aureus causing bullous impetigo and the emergence of new methicillin-resistant clonal groups in Kansai district in Japan. J. Infect. Dis. 1851511-1516. [DOI] [PubMed] [Google Scholar]
- 46.Yoon, H. J., J. Y. Choi, K. Lee, D. Yong, J. M. Kim, and Y. G. Song. 2007. Accessory gene regulator group polymorphisms in methicillin-resistant Staphylococcus aureus: an association with clinical significance. Yonsei Med. J. 30176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]