Abstract
Progressive human immunodeficiency virus type 1 (HIV-1) infection is often associated with high plasma virus load (pVL) and impaired CD8+ T-cell function; in contrast, CD8+ T cells remain polyfunctional in long-term nonprogressors. However, it is still unclear whether CD8+ T-cell dysfunction is the cause or the consequence of high pVLs. Here, we conducted a longitudinal functional and phenotypic analysis of virus-specific CD8+ T cells in a cohort of patients with chronic HIV-1 infection. During the initiation and maintenance of successful antiretroviral therapy (ART), we assessed whether the level of pVL was associated with the degree of CD8+ T-cell dysfunction. Under viremic conditions, HIV-specific CD8+ T cells were dysfunctional with respect to cytokine secretion (gamma interferon, interleukin-2 [IL-2], and tumor necrosis factor alpha), and their phenotype suggested limited potential for proliferation. During ART, cytokine secretion by HIV-specific CD8+ T cells was gradually restored, IL-7Rα and CD28 expression increased dramatically, and PD-1 levels declined. Thus, prolonged ART-induced reduction of viral replication and, hence, presumably antigen exposure in vivo, allows a significant functional restoration of CD8+ T cells with the appearance of polyfunctional cells. These findings indicate that the level of pVL as a surrogate for antigen load has a dominant influence on the phenotypic and functional profile of virus-specific CD8+ T cells.
A large body of evidence indicates that virus-specific CD8+ T-cell responses play an important role in controlling human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) replication. During the acute phase of HIV/SIV infection, partial control of viral replication is associated with a marked expansion of virus-specific CD8+ T cells in infected individuals (2, 10, 14, 47). Furthermore, in vivo depletion of CD8+ T cells before or during SIV infection of rhesus macaques leads to a massive increase in viremia (40, 65). Moreover, long-term nonprogression in HIV type 1 (HIV-1) infection is associated with both vigorous CD8+ T-cell responses (45, 60) and with specific HLA class I alleles (4, 16, 17, 43, 52, 64). Persistent HIV/SIV replication is also associated with mutational escape at targeted CD8+ T-cell epitopes, suggesting an important selection pressure on the virus (2, 15, 31, 32, 44, 57, 62, 63). Ultimately, however, virus-specific CD8+ T cells are unable to control viral replication over prolonged periods of time in the vast majority of HIV-infected patients and SIV-infected macaques.
It has been demonstrated that HIV-specific CD8+ T cells from viremic patients are often dysfunctional; they usually express low levels of perforin (7, 8, 19, 25, 72, 73), have a poor ex vivo killing capacity (8, 20, 67, 72), and produce only a limited spectrum of cytokines (11, 13, 24). In addition, their proliferative capacity has been shown to be substantially reduced (51). The fact that both proliferation and perforin expression by HIV-specific CD8+ T cells are superior in long-term nonprogressors (LTNP) (51) may suggest a critical relationship between CD8+ T-cell functionality and plasma virus load (pVL). Furthermore, LTNP exhibit increased frequencies of polyfunctional HIV-specific T cells compared to progressors (3, 13, 33). These cells are able to perform multiple effector functions simultaneously, including the secretion of several cytokines and chemokines as well as degranulation, and are thought to be relevant for superior viral control in LTNP (3, 13, 29, 33, 49). However, it is not entirely clear whether improved T-cell functionality is the cause or the consequence of reduced pVL in LTNP.
In the present study, we assessed whether modulation of pVL by antiretroviral therapy (ART) induces important functional and phenotypic changes in CD8+ T cells from patients with chronic HIV-1 infection. We also addressed the time frame required for such changes to occur. Our longitudinal study indicates that pVL as a surrogate for antigen load has a profound influence on the functional capacities and phenotypic signatures of virus-specific CD8+ T cells. After prolonged ART, HIV-specific CD8+ T cells significantly increased their cytokine secretion capabilities, and substantial populations of polyfunctional cells emerged; furthermore, these CD8+ T cells developed phenotypic profiles characteristic of resting memory cells. These functional changes occurred slowly and required successful ART for a median duration of 30 months to develop. However, the significance of this functional improvement of CD8+ T cells is unclear since previous studies have firmly established that viral control is generally not increased after cessation of ART in chronically infected patients (27, 28, 36, 53, 54, 56).
MATERIALS AND METHODS
Study individuals.
Thirty-seven patients chronically infected with HIV-1 (subtype B) who were either ART naïve (n = 25) or had interrupted ART for >6 months (n = 12) were included in the study. Of the latter, the median duration without ART before study entry was 24 months (range, 8 to 66 months). Inclusion criteria were as follows: pVL of >4,000 RNA copies/ml and a CD4 count of >50 cells/μl. Three patients with minor inclusion criterion violations at baseline remained in the study (patient 16 with a pVL of 3,970 copies/ml and patients 12 and 14 with CD4 counts of 49 cells/μl). According to treatment history and baseline resistance testing, all patients had the potential for complete viral suppression (<50 copies/ml) within 6 months of ART initiation. Twenty-five patients, classified as the study group, initiated ART at week 0, while the remaining 12 patients, classified as the control group, remained untreated. Patient 29 stopped ART at week 5; therefore, later time points were not analyzed. The ART regimen usually consisted of two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) combined with a protease inhibitor (n = 15) or a non-NRTI (n = 8); two patients were treated with a triple-class combination (two NRTIs plus protease inhibitor and non-NRTI). Blood samples from the study group patients were taken at weeks 0, 2, 4, 12, and 24 as well as after a median of 2.5 years (shown as >24 weeks) following the onset of ART. In the control group, blood samples were taken at week 0, 12, and 24 as well as after a median of 1.6 years (shown as >24 weeks) from the beginning of the study. pVL was measured at every time point, and CD4 counts were measured at weeks 0, 12, and 24 and at 1.6 or 2.5 years. The clinical characteristics of all patients are summarized in Table 1. The study was approved by the local ethical committee, and written informed consent from all subjects was obtained according to the guidelines of the University Hospital, Zurich. HIV-seronegative donor samples were obtained from the Stiftung Zürcher Blutspendedienst, Swiss Red Cross.
TABLE 1.
Baseline characteristics of patients
| Treatment group | Patient no. | Gendera | Age (yrs) | pVL (RNA copies/ml) | CD4 count (cells/μl)b | ARTc | HLA genotype |
|---|---|---|---|---|---|---|---|
| Therapy | 01 | M | 43 | 48,500 | 185 | 3TC/TDF/EFV | A02/B40/B44 |
| 02 | M | 39 | 374,000 | 158 | AZT/3TC/EFV | A02/A29/B07/B44 | |
| 03 | M | 33 | 960,000 | 154 | AZT/3TC/EFV | A01/A02/B37/B15 | |
| 05 | F | 34 | 202,500 | 121 | AZT/3TC/ATVr | A11/A68/B35/B39 | |
| 06 | M | 32 | 90,000 | 147 | AZT/3TC/LPVr | A03/A11/B35 | |
| 09 | M | 30 | 403,000 | 91 | AZT/3TC/LPVr | A02/A24/B18/B53 | |
| 10 | M | 44 | 46,000 | 57 | AZT/3TC/LPVr | A30/A11/B15/B18 | |
| 12 | M | 39 | 2,450,000 | 49 | 3TC/TDF/EFV/LPVr | A01/A02/B51/B56 | |
| 13a | M | 36 | 17,800 | 189 | AZT/3TC/LPVr | A02/A68/B08/B51 | |
| 13b | M | 40 | 167,000 | 207 | 3TC/TDF/LPVr | A02/A24/B27/B15 | |
| 14 | M | 50 | 131,000 | 49 | 3TC/TDF/LPVr | A01/A11/B08/B51 | |
| 15 | M | 45 | 92,000 | 197 | DDI/TDF/EFV | A02/A03/B27/B57 | |
| 17 | M | 45 | 6,680 | 186 | DDI/TDF/EFV | A24/A11/40 | |
| 18 | M | 35 | 140,000 | 164 | TDF/EFV/LPVr | A02/A24/B35/B51 | |
| 20 | M | 41 | 12,700 | 275 | AZT/3TC/LPVr | A02/A11/B57/B51 | |
| 21 | M | 43 | 17,700 | 178 | ABC/TDF/ATVr | A01/A24/B49/B38 | |
| 22 | M | 27 | 23,400 | 288 | AZT/3TC/LPVr | A03/B35 | |
| 23 | F | 38 | 900,000 | 187 | 3TC/D4T/NFV | B47/B42 | |
| 24 | M | 37 | 211,000 | 334 | 3TC/TDF/EFV | A03/A11/B07 | |
| 25 | M | 44 | 42,600 | 219 | ABC/TDF/EFV | A03/B18/B35 | |
| 26 | M | 31 | 2,080,000 | 152 | 3TC/TDF/EFV/LPVr | A30/A29/B13/B44 | |
| 28 | M | 30 | 137,000 | 135 | AZT/3TC/EFV | A01/A03/B08/B38 | |
| 29 | F | 28 | 70,300 | 277 | AZT/3TC/LPVr | A01/A02/B08/B15 | |
| 31 | M | 41 | 11,200 | 289 | AZT/3TC/FPVr | A02/A03/B07/B15 | |
| 39 | M | 42 | 167000 | 548 | AZT/3TC/LPVr | NDd | |
| Median (range) for group (n = 25) | 39* (27-50) | 131,000 (6,680-2,450,000) | 185 (49-548) | ||||
| Control | 04 | M | 38 | 121,000 | 365 | A26/A11/B13/B44 | |
| 08 | F | 61 | 89,600 | 227 | A02/A32/B07/B40 | ||
| 16 | F | 36 | 3,970 | 185 | A30/A33/B35/B51 | ||
| 19 | M | 42 | 38,400 | 202 | A02/B13/B27 | ||
| 27 | M | 37 | 127,000 | 252 | A02/A03/B07/B44 | ||
| 30 | M | 27 | 147,000 | 342 | A02/B08/B27 | ||
| 32 | M | 38 | 60,200 | 353 | A01/A24/B47/B57 | ||
| 33 | M | 37 | 25,500 | 328 | A01/A03/B08/B15 | ||
| 34 | M | 59 | 23,100 | 330 | A01/A68/B44/B57 | ||
| 35 | M | 42 | 88,600 | 350 | A01/A03/B08/B35 | ||
| 36 | M | 28 | 20,800 | 364 | A24/A33/B44/B15 | ||
| 37 | M | 54 | 12,700 | 351 | A02/A32/B07 | ||
| Median (range) for group (n = 12) | 38 (27-61) | 49,300 (3,970-147,000) | 336 (185-365) |
M, male; F, female. The ratio of female to male patients was 3/22 in the therapy group and 2/10 in the control group.
CD4 count, absolute CD4 T cell count.
AZT, zidovudine; 3TC, lamivudine; ABC, abacavir; DDI, didanosine; D4T, stavudine; TDF, tenofovir disoproxil fumarate; EFV, efavirenz; ATV, atazanavir; LPV, lopinavir; FPV, Fos-amprenavir; r, ritonavir (booster dose).
ND, not determined.
HLA genotyping.
HLA genotyping (Table 1) was performed at a diagnostic laboratory by using sequence specific PCRs according to standard procedures. DNA for typing was extracted using the Protrans DNA isolation kit (Protrans, Germany).
Quantification of HIV-1 plasma virus load.
Plasma HIV RNA was quantified with an Amplicor HIV-1 Monitor test, version 1.5 (Roche Diagnostics, Rotkreuz, Switzerland), with a modification leading to a detection limit of 40 copies/ml (66).
Lymphocyte separation.
Fresh peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using Ficoll-Hypaque density gradient centrifugation and were cryopreserved for later analysis.
pMHC-I tetramers.
Tetrameric peptide-major histocompatibility complex class I (pMHC-I) complexes specific for HLA A2-, HLA B7-, or HLA B8-restricted epitopes derived from HIV were produced as previously described with minor modifications (5, 38). All tetramers were validated and titrated using PBMCs isolated from the HIV-1-infected patients in this study; in all cases, these reagents were conjugated to streptavidin-allophycocyanin and used as described previously (75).
Flow cytometric assessment of CD8+ T-cell function.
PBMCs from HIV-infected patients or healthy donors were thawed and cultured overnight in RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and glutamine (2 mM) (R-10) prior to stimulation. Antigens comprised an HIV-1 peptide pool containing overlapping 15-mer peptides provided by the NIH AIDS Research and Reference Reagent Program (Gag pool, 123 peptides) and HLA class I-restricted HIV or cytomegalovirus (CMV) optimal peptides purchased from NeoMPS, France (all at an individual peptide concentration of 2 μg/ml). These peptides were as follows: HLA-A2 restricted (HIV Gag p17 residues 77 to 85 [SLYNTVATL], HIV RT residues 464 to 472 [ILKEPVHGV] and CMV pp65 residues 495 to 503 [NLVPMVATV]) HLA-B8 restricted (HIV Gag p24 residues 127 to 135 [GEIYKRWII] and HIV Nef residues 90 to 97, [FLKEKGGL]), and HLA-B7 restricted (HIV Env gp41 residues 333 to 341 [IPRRIRQGL] and HIV Nef residues 75 to 83 [RPMTYKAAL]). Staphylococcus enterotoxin B ([SEB] 1 μg/ml; Sigma, Switzerland) was used as a positive control in all experiments. Anti-CD107a-fluorescein isothiocyanate (FITC) and the costimulatory antibodies anti-CD28 and anti-CD49d at 1 μg/ml each (all BD Biosciences, Switzerland) were added at the beginning of the 5-h stimulation period. Cultures established in the absence of peptide comprised the negative control in each case. All experiments were carried out in the presence of monensin A (2 μM; Sigma, Switzerland) to inhibit cytokine secretion. Cells were surface stained with anti-CD3-Pacific Blue and anti-CD8-peridinin chlorophyll protein (PerCP) and were then stained intracellularly with anti-gamma interferon [IFN-γ]-phycoerythrin (PE)-Cy7, anti-interleukin-2 [IL-2]-PE, and anti-tumor necrosis factor alpha [TNF-α]-allophycocyanin (all BD Biosciences, Switzerland). A minimum of 50,000 events in the small live cell scatter gate were collected. Cells were gated on CD3+ CD8+ T cells. Values of negative controls (incubation in absence of antigen) were usually below 0.1% cytokine-producing CD8+ T cells. Negative values were subtracted from test values, and values of >0.1% responding cells among CD8+ T cells were considered positive. Data were collected using an LSRII flow cytometer (BD Biosciences, Switzerland). Data files were analyzed using fluorescence-activated cell sorting (FACS) DIVA Software (BD Biosciences, Switzerland).
Intracellular perforin and GrB staining and CD8+ T-cell phenotyping.
PBMCs from HIV-infected patients or healthy donors were thawed, surface stained with anti-CD3-Pacific Blue, anti-CD8-PerCP, and anti-IL-7 receptor alpha (IL-7Rα)-PE, and then stained intracellularly with anti-perforin-FITC (all BD Biosciences, Switzerland). Gates for perforin stainings were set according to isotype control stainings. Alternatively, cells were surface stained with anti-CD3-Pacific Blue and anti-CD8-PerCP (both BD Biosciences, Switzerland) and then stained intracellularly with anti-granzyme B (GrB)-PE (Caltag Laboratories, Switzerland). For CD28 and PD-1 staining, cells were stained with anti-CD3-Pacific Blue, anti-CD8-PerCP, anti-PD-1-PE, and anti-CD28-FITC (all BD Biosciences, Switzerland). Where appropriate, experimental setups included extracellular pMHCI tetramer staining. Cells were kept on ice during the staining procedure to avoid degranulation. Data were collected using an LSRII flow cytometer (BD Biosciences, Switzerland) and analyzed using FACS DIVA Software (BD Biosciences, Switzerland).
Statistical analysis.
Statistical analyses were performed using SPSS for Windows, version 14.0.
RESULTS
Virologic and immunologic impact of ART in study cohort.
In this study, we investigated the longitudinal impact of declining HIV-1 pVL on the phenotype and functionality of CD8+ T cells in a cohort of 25 chronically HIV-1-infected patients after initiation of ART; we conducted similar analyses in a control group of 12 HIV-1-infected patients who remained untreated and in a group of 19 HIV-1 seronegative donors (Table 1).
The median pVL before onset of therapy in the study group was 131,000 copies/ml (range, 6,680 to 2,450,000 copies/ml), which declined significantly during ART (median, 40 copies/ml at week 24) and remained below 50 copies/ml in treated patients throughout the study. In the control group, pVL increased marginally during the observation period (median at week 0, 49,300 copies/ml; median at week 24, 62,000 copies/ml). After the initiation of ART, CD4+ T-cell counts increased from a median of 185 cells/μl at week 0 to 315 cells/μl at week 24 and to 508 cells/μl at the late time point in the study group. In the control group, CD4+ T-cell counts decreased from 336 cells/μl at week 0 to 275 cells/μl at week 24 and reached a nadir of 208 cells/μl at the later time points (data not shown). The percentages of CD8+ T cells in the study and control groups were stable (approximately 44% of lymphocytes) over the period of analysis (data not shown).
We measured three different phenotypic markers (IL-7Rα, PD-1, and CD28) which have been previously associated with CD8+ T-cell function. In addition, we analyzed six separate CD8+ T-cell functions to assess degranulation capacity (CD107a), cytokine secretion (IFN-γ, IL-2, and TNF-α) and granule content (perforin and GrB).
Increase in IL-7Rα expression with prolonged time on ART.
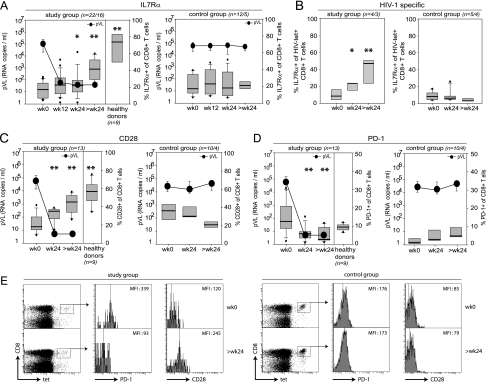
To analyze the impact of declining pVL on the differentiation stage of CD8+ T cells, we first measured the expression of IL-7Rα on CD8+ T cells from study and control group patients (Fig. 1A and B). Recent studies indicate that effector T cells with high expression levels of IL-7Rα preferentially survive and differentiate into long-lived memory cells (9, 37, 41, 59). While the majority of antigen-experienced CD8+ T cells specific for cleared pathogens express IL-7Rα, CD8+ T-cell populations specific for persistent viruses like Epstein-Barr virus, CMV, and HIV contain only low levels of IL-7Rα-expressing cells, thereby indicating the predominance of cells with an effector and not a resting memory phenotype. This most likely reflects continuous or repetitive exposure to cognate antigen (21, 59, 74). In comparison to healthy donors, the frequency of IL-7Rα expression was massively reduced in viremic HIV-infected patients (Fig. 1A), potentially reflecting chronic immune activation; approximately 80% of all CD8+ T cells were IL-7Rα−. Importantly, after onset of successful ART and subsequent decline in pVL, the frequency of IL-7Rα-expressing CD8+ T cells increased significantly in the study group, while no changes were observed in the viremic control group (Fig. 1A). This indicates a slow change from an effector toward a memory phenotype. However, even after long-term ART, the levels of IL-7Rα-expressing CD8+ T cells were somewhat diminished in treated HIV-patients compared to healthy donors (patients at >24 weeks, 41.5%; healthy donors, 73.9%) (Fig. 1A). Consistent with these findings in the total CD8+ T-cell population, we found that the frequencies of IL-7Rα-expressing HIV-specific CD8+ T cells also increased substantially in patients undergoing ART whereas no changes occurred in viremic controls (Fig. 1B).
FIG. 1.
Phenotypic CD8+ T cell analysis. (A) Longitudinal analysis of IL-7Rα expression on CD8+ T cells from patients in the study group (n = number of analyzed patients at week 0 to 24/number of patients analyzed at >24 weeks), healthy donors and control group patients (n = number of analyzed patients at week 0 to 24/number analyzed at >24 weeks). PBMC were gated on CD3+ CD8+ T cells. Box plots represent the 25th and 75th percentiles, black lines depict the median, whiskers indicate the 90th and 10th percentile, and dots represent outliers. Black circles represent the median pVL (RNA copies/ml). *, P < 0.05; **, P < 0.01 (unpaired Students t test; refers to values at week 0). (B) IL-7Rα expression on HIV-specific CD8+ T cells. (C) CD28 expression on CD8+ T cells. Cells were gated in CD3+ CD8+ T cells. (D) PD-1 expression on CD8+ T cells. (E) Representative PD-1 and CD28 expression profiles on HIV-specific CD8+ T cells at week 0 and at >24 weeks (study group: patient 02, HLA-B7 restricted, gp41 residues 333 to 341 [IPRRIRQGL]-specific CD8+ T-cell response; control group: patient 33, HLA-B8 restricted, Nef residues 90 to 97 [FLKEKGGL]-specific CD8+ T-cell response). MFI, mean fluorescence intensity. wk, week.
Increase in CD28 expression with prolonged time on ART.
We next analyzed the expression of the T-cell costimulatory molecule CD28, which is down-regulated on antigen-experienced, terminally differentiated CD8+ T cells with restricted proliferative potential (7, 69). Similarly to IL-7Rα, the frequency of CD28+ CD8+ T cells was significantly reduced in viremic HIV-1-infected patients compared to healthy donors (Fig. 1C). The declining pVL after initiation of ART led to a robust and significant increase in the frequency of CD28-expressing CD8+ T cells in the study group approaching the levels observed in healthy controls, while CD28+ CD8+ T cells in the viremic control group further declined (Fig. 1C). Furthermore, CD28 expression also increased on HIV-specific CD8+ T cells in study group patients but not in untreated controls (Fig. 1E). These results strengthen our previous finding that prolonged suppression of pVL by ART causes a phenotypic shift in CD8+ T cells from an effector to a memory type.
Decrease in PD-1 expression with declining pVL.
It has recently been shown in untreated HIV-infected patients that increased PD-1 expression on CD8+ T cells correlates not only with pVL and functional exhaustion of CD8+ T cells but also with disease progression (23, 61, 71, 77). Therefore, we longitudinally investigated the frequency of PD-1-expressing CD8+ T cells in our patients. A significant decline in PD-1 expression was observed within 24 weeks of successful ART both in total and HIV-specific CD8+ T cells (Fig. 1D and E). In contrast, PD-1 expression on CD8+ T cells from the viremic control group tended to increase over the study period (Fig. 1D). Thus, PD-1 expression on CD8+ T cells is critically influenced by the level of pVL.
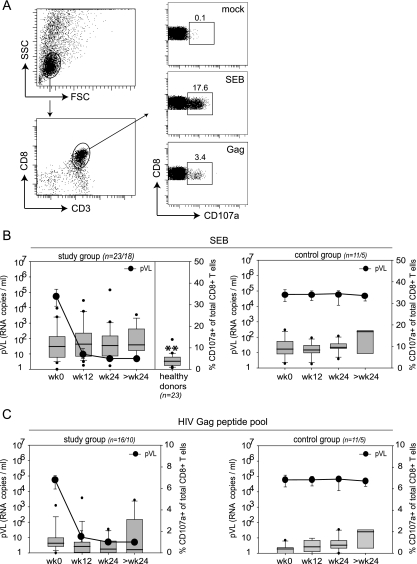
Elevated levels of degranulating CD8+ T cells in HIV-1-infected patients.
We next assessed the functional changes in CD8+ T cells from HIV-infected patients initiating ART and compared them to untreated infected controls and healthy donors. First, we analyzed the degranulation capacity of CD8+ T cells from HIV-1-infected individuals by measuring the appearance of the lysosomal-associated membrane protein 1 (CD107a) on the surface of CD8+ T cells following SEB and HIV-1 Gag peptide pool stimulation (12) (Fig. 2). Although CD107a staining is a functional readout for CD8+ T cells, previous studies have also demonstrated that this assay can be used to determine the frequency of antigen-specific CD8+ T cells in HIV-infected individuals with a comparable accuracy to MHC-I tetrameric complexes (12, 78). Compared to a group of healthy donors, the frequency of CD8+ T cells that degranulated upon polyclonal SEB stimulation was significantly increased in viremic HIV-1-infected patients, and these frequencies remained increased despite declining pVL in treated patients (Fig. 2B). No significant changes occurred in the untreated control group.
FIG. 2.
Longitudinal assessment of the degranulation capacity of CD8+ T cells. (A) Representative FACS plots and gating strategy (patient 38). PBMC were gated on small lymphocytes and subsequently on CD3+ CD8+ T cells. The right graphs show CD107a staining of gated cells after mock, SEB, or Gag peptide pool stimulation. Numbers indicate the percentage of CD107+ CD3+ CD8+ T cells. (B and C) Box plots show the percentages of degranulating CD8+ T cells; boxes indicate the 25th and 75th percentiles, black lines depict the median, whiskers indicate the 90th and 10th percentile, and dots represent outliers. Black circles represent the median pVL (RNA copies/ml). The graphs in panel B show the degranulation capacity of CD8+ T cells from study and control groups upon SEB stimulation compared to healthy donors (n = number of analyzed patients at week 0 to 24/number analyzed at >24 weeks). **, P < 0.01 (unpaired students t test; refers to values at week 0). The graphs in panel C show results of stimulation with a pool of overlapping peptides covering the complete HIV-1 Gag protein. wk, week.
The frequency of degranulating CD8+ T cells following stimulation with the HIV-1 Gag peptide pool tended to decrease slightly during ART-induced viral suppression, which is in agreement with previous studies (18, 22, 42, 55). In untreated controls, the frequencies of HIV-specific degranulating CD8+ T cells increased slightly over time (Fig. 2C).
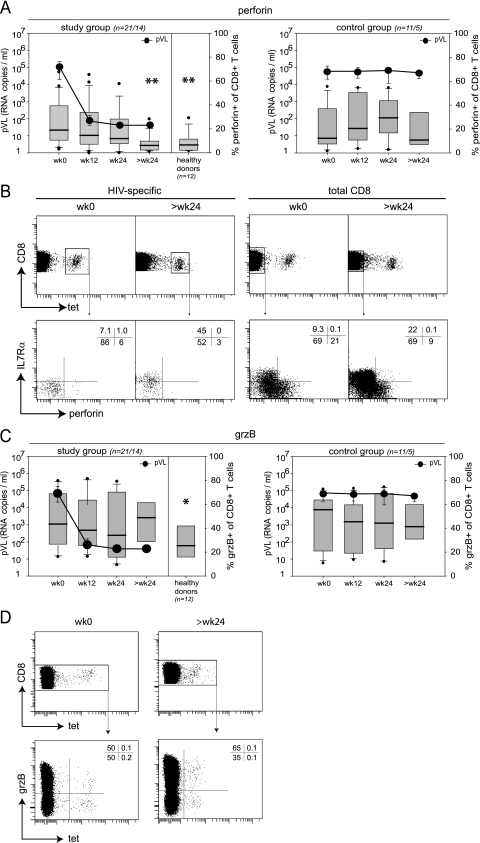
Decreasing frequencies of perforin but not GrB-containing CD8+ T cells with declining pVL.
Degranulation capacity on its own is not sufficient to indicate potent cytotoxic CD8+ T-cell effector function because this also depends critically on the contents of lytic granules (reviewed in reference 48). Therefore, we longitudinally analyzed the frequency of GrB and perforin expression in CD8+ T cells from HIV-1-infected patients as a function of decreasing pVL in comparison to untreated patients and healthy donors (Fig. 3).
FIG. 3.
Longitudinal analysis of CD8+ T-cell granule content. (A and C) Comparison of perforin and GrB expression in total CD8+ T cells from study group patients, control group patients, and healthy donors (n = number of analyzed patients at week 0 to 24/number analyzed at >24 weeks). PBMC were gated on CD3+ CD8+ T cells. Box plots represent the 25th and 75th percentiles, black lines depict the median, whiskers indicate the 90th and 10th percentiles, and dots represent outliers. Black circles represent the median pVL (RNA copies/ml). *, P < 0.05; **, P < 0.01 (unpaired students t test; refers to values at week 0). (A) Perforin expression. (C) GrB expression. (B and D) Representative FACS plots for assessment of perforin and GrB expression in HIV-specific and total CD8+ T cells. Plots in upper rows are gated on CD3+ CD8+ T cells. Numbers indicate the percentages of cells in the respective quadrants. (B) Representative intracellular perforin and extracellular IL-7Rα staining of HIV-specific or total CD8+ T cells (patient 20, HLA-A2 restricted, Gag p17 residues 77 to 85 [SLYNTVATL]-specific CD8+ T-cell response). (D) Representative intracellular GrB staining of HIV-specific or total CD8+ T cells (patient 29, HLA-A2 restricted, RT residues 464 to 472 [ILKEPVHGV]-specific CD8+ T-cell response). wk, week; grzB, granzyme B.
Before onset of ART, perforin was expressed by a median of 19% of total CD8+ T cells from viremic patients (Fig. 3A), while GrB was detected in a median of 42% of total CD8+ T cells (Fig. 3C). Compared to healthy donors, frequencies of perforin-positive CD8+ T cells and to a lesser extent of GrB-positive CD8+ T cells from viremic patients were elevated; only 6.6% and 20.3% of CD8+ T cells from healthy donors expressed perforin or GrB, respectively (Fig. 3A and C). This indicates an expansion of CD8+ T cells with effector function in the peripheral blood of HIV-1-infected patients. However, with prolonged ART, the frequency of HIV-specific and total CD8+ T cells expressing perforin declined significantly in the study group, thus indicating a contraction of the CD8+ T-cell population with immediate cytolytic effector function with ART (Fig. 3A and B). In the control group patients with persistent viremia, no significant changes in perforin expression were observed over a similar period of time (Fig. 3A). In contrast to the declining levels of perforin expression with ART, we did not observe any significant changes in GrB expression in total or HIV-specific CD8+ T cells over time in either group (Fig. 3C and D).
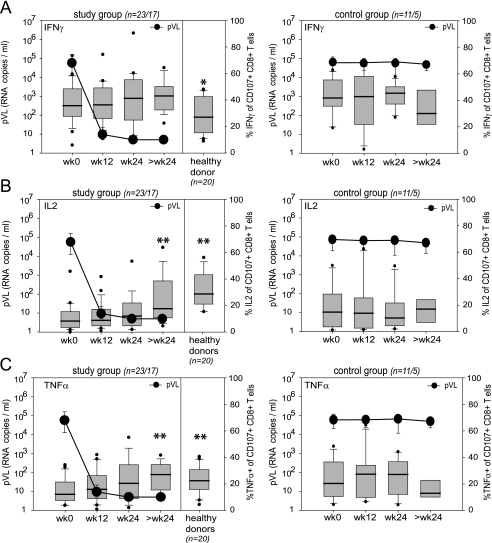
Dynamics of cytokine secretion by CD8+ T cells after onset of ART.
Next, we compared the cytokine secretion capacity of degranulating CD8+ T cells (Fig. 4) under conditions of declining and suppressed pVL in study group patients, in viremic control group patients, and in healthy control individuals. We initially focused our analysis on the cytokine secretion capacity of degranulating cells as we have shown previously that degranulation is a robust effector function to assess antigen-reactive CD8+ T cells under conditions of persistent exposure to viral antigen (1). After SEB stimulation, a median of 35.8% of CD107a+ CD8+ T cells secreted IFN-γ in HIV-infected patients at week 0, followed by TNF-α (median, 12.1%) and finally IL-2 (median, 8.3%). In healthy donors, however, these frequencies were of equal magnitude (IL-2, 28.7%; IFN-γ, 27.1%; and TNF-α, 22.4%) (Fig. 4). After initiation of ART, a significant recovery of cytokine (IL-2 and TNF-α) secretion capacity within degranulating CD8+ T cells was observed, while IFN-γ secretion capacity remained constant. In sharp contrast, no changes in IL-2 or TNF-α secretion capacities were observed in the control group where the pVL remained constant (Fig. 4). Thus, we conclude that the increase in CD8+ T-cell cytokine secretion capacity on ART reflects an overall improvement of CD8+ T-cell functionality (Fig. 4), which is closely linked to the decline of HIV replication and thus pVL as a surrogate for antigen load.
FIG. 4.
Longitudinal assessment of the cytokine secretion capacity of degranulating CD8+ T cells. Cytokine (IFN-γ, IL-2, and TNF-α) secretion capacity of degranulating (CD107a+) CD8+ T cells upon SEB stimulation from study group patients compared to patients from the control group and healthy donors (n = number of analyzed patients at week 0 to 24/number analyzed at >24 weeks). Box plots represent the 25th and 75th percentiles, black lines depict the median, whiskers indicate the 90th and 10th percentiles, and dots represent outliers. Black circles represent the median pVL (RNA copies/ml). *, P < 0.05; **, P < 0.01 (unpaired students t test; refers to values at week 0). Data are shown for IFN-γ (A), IL-2 (B), and TNF-α (C). wk, week.
We confirmed that these changes in cytokine production capacity are not confined to degranulating CD8+ T cells but also hold for total CD8+ T cells. CD8+ T cells from viremic study group patients predominantly secreted IFN-γ (median, 5.8%) before initiation of ART (week 0), followed by TNF-α (median, 2.2%) and finally IL-2 (median, 1.4%), which is concordant with previous findings (13). In contrast, healthy donors exhibited similar frequencies of CD8+ T cells that were able to secrete IL-2 (median, 4.2%), TNF-α (median, 4.1%), and IFN-γ (median, 3.3%). Upon initiation of ART, the frequencies of IL-2- and TNF-α-secreting CD8+ T cells increased continuously, leading to a statistically significant increase for the latest time point of analysis compared to baseline (data not shown). In contrast, the frequencies of IL-2- and TNF-α-secreting CD8+ T cells remained low in the untreated control group. The frequencies of IFN-γ-secreting CD8+ T cells also showed a tendency to increase with declining pVL, although this did not reach statistical significance (not shown).
Selective impairment of cytokine secretion in HIV-specific CD8+ T cells.
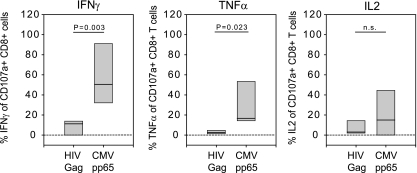
We next analyzed whether impaired cytokine production by CD8+ T cells is independent of antigen specificity in viremic patients or whether it is preferentially found in HIV-specific CD8+ T cells. To achieve this, we compared the cytokine expression capacity of degranulating (CD107a+) CD8+ T cells from viremic patients (week 0) upon stimulation with HIV-1 Gag pool peptides or with a CMV pp65-derived peptide (Fig. 5). Before initiation of ART, the proportion of degranulating CD8+ T cells capable of producing IFN-γ or TNF-α upon HIV-1-specific stimulation was significantly reduced in comparison to CMV pp65 peptide stimulation, suggesting a predominant functional defect in HIV-specific CD8+ T cells (Fig. 5). Both HIV- and CMV-specific cells demonstrated very low frequencies of IL-2 production, consistent with previous studies (1, 71, 80).
FIG. 5.
Comparison of the cytokine secretion capacity of HIV- and CMV-specific CD8+ T cells at week 0. Cytokine secretion capacity of degranulating (CD107a+) CD8+ T cells from viremic patients (week 0, n = 8) exhibiting an HLA-A2-restricted CMV, pp65 residues 495 to 503 (NLVPMVATV)-specific CD8+ T-cell response. PBMCs were stimulated with the CMV pp65 peptide or with the Gag pool peptides. Box plots represent the 25th and 75th percentiles; black lines depict the median. P, unpaired students t test.
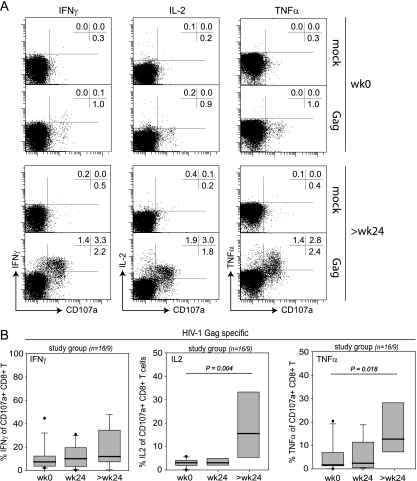
We then analyzed whether the cytokine production defect in HIV-specific CD8+ T cells was restored by ART-induced suppression of HIV replication. Indeed, upon stimulation with the HIV-1 Gag peptide pool, we observed an increase in IFN-γ, IL-2, and TNF-α secretion capacity in patients with long-term suppression of HIV-1 replication (median, 114 weeks). These increases were profound and statistically significant for IL-2 and TNF-α at late time points (Fig. 6A and B). Thus, our findings demonstrate that HIV-specific CD8+ T cells are particularly impaired with regard to cytokine secretion in viremic patients. Importantly, this functional defect is regulated by the level of persistent antigen exposure and can be restored in vivo by long-term reduction of pVL as a surrogate for antigen load.
FIG. 6.
Longitudinal changes in cytokine secretion capacity of HIV Gag-specific CD8+ T cells. (A) Representative FACS plots for assessment of degranulation, IFN-γ, IL-2, and TNF-α production upon mock or Gag peptide pool stimulation (patient 02). Plots are gated on CD3+ CD8+ T cells. The upper graphs show staining results from week 0, and the lower graphs show staining results at >24 weeks. Numbers indicate the percentages of cells in the respective quadrants. (B) Longitudinal analysis of cytokine production of degranulating CD8+ T cells upon stimulation with the HIV-1 Gag peptide pool (n = number of analyzed patients at weeks 0 to 24/number analyzed at >24 weeks). Box plots represent the 25th and 75th percentiles, black lines depict the median, whiskers indicate the 90th and 10th percentiles, and dots represent outliers. P, unpaired students t test. wk, week.
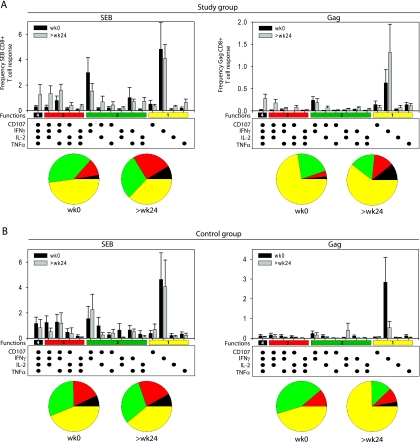
Emergence of polyfunctional CD8+ T cells after prolonged ART.
Next, we addressed whether the overall improvement of cytokine production during ART was evident on a single-cell level. We therefore assessed the simultaneous ability of CD8+ T cells to execute four different effector functions: degranulation, IFN-γ, TNF-α, and IL-2 secretion. As shown in Fig. 7, prolonged suppression of pVL by ART allows for the development of polyfunctional CD8+ T cells as assessed after SEB or HIV-specific stimulation (Fig. 7A). In particular, prolonged ART was associated with the appearance of CD8+ T cells exhibiting three or four simultaneous functions. In contrast, no gain in polyfunctionality was observed in the viremic control group either after SEB or after HIV Gag stimulation (Fig. 7B).
FIG. 7.
Polyfunctionality of CD8+ T cells. Simultaneous production of the cytokines IFN-γ, TNF-α, and IL-2 as well as degranulation was assessed at week 0 and at >24 weeks in the study (A; n = 24) and control (B; n = 9) group patients after SEB (left panels) or after HIV Gag peptide pool (right panels) stimulation. CD8+ T-cell responses were classified in 15 categories according to the diagram shown underneath the bar graphs. Black bars indicate the percentages of CD8+ T cells in a given category at week 0, gray bars show values at >24 weeks. In the pie charts, the total percentage of responses with a given number of functionalities is color coded as follows: yellow, 1 functionality; green, 2 functionalities; red, 3 functionalities; and black, 4 functionalities. wk, week.
DISCUSSION
In the present study, we investigated longitudinal changes in the functionality and phenotype of CD8+ T cells in a cohort of chronically HIV-1-infected patients on commencement of ART. Our results clearly demonstrate that the quality of HIV-specific CD8+ T cells changes fundamentally during successful ART. Cells acquire a phenotype of resting memory cells (CD28 and CD127high) and exhibit reduced expression of markers associated with dysfunction (i.e., PD-1). More importantly, CD8+ T cells increase their functional repertoire particularly with respect to TNF-α and IL-2 production; furthermore, a sizeable proportion of specific cells become polyfunctional. This indicates that pVL as a surrogate for antigen load has a profound and direct influence on T-cell functionality. The overall improvement of CD8+ T-cell function is not due to the presence of increased frequencies of naïve CD8+ T cells after prolonged ART as naïve CD8+ T cells are poor cytokine producers in 5-h stimulation assays (data not shown), and functional improvement was observed for both SEB-reactive and HIV-specific CD8+ T cells.
It has been reported previously that HIV-specific CD8+ T cells from viremic patients are impaired in cytokine production (11, 13, 24, 46, 58, 68), and it was proposed that this might be a consequence of persistent antigen exposure, which induces a state of functional exhaustion. Thus, only a fraction of HIV-specific CD8+ T cells detectable by tetramer staining produced IFN-γ upon stimulation (30, 46, 58, 67), and this fraction increased with long-term ART (58). Here, we support and extend these findings by showing that HIV-specific CD8+ T cells from viremic patients are significantly impaired with regard to not only IFN-γ but also IL-2 and TNF-α production whereas degranulation capacity (CD107a) is largely conserved.
Recently, we have reported a similar dichotomy in CD8+ T-cell effector functions in chronic murine lymphocytic choriomeningitis virus infection, where prolonged in vivo antigen exposure led to severely impaired cytokine production while degranulation and cytolytic activity were maintained at a cellular level (1, 68). Furthermore, a detailed cross-sectional analysis of various CD8+ T-cell functions in HIV-infected individuals showed that degranulation was a relatively robust effector function in most cases (13). In the present longitudinal study we demonstrate that this selective dysfunction is dependent on the level of antigen, since ART-induced suppression of HIV replication led to in vivo restoration of IL-2, IFN-γ, and TNF-α secretion capacity in degranulating HIV-specific and bulk CD8+ T cells. Whether this overall restoration of cytokine secretion capacity in CD8+ T cells is caused by a functional improvement on a single-cell level or whether it is due to preferential survival and expansion of cytokine-producing cells is at present unclear.
The overall functional restoration of CD8+ T cells seems to be a slow process, which became clearly apparent only after more than 2 years of complete viral suppression. Therefore, previous studies analyzing several CD8 T-cell functions simultaneously may have missed such an effect of ART since the longitudinal follow-up of these patients may have been too short (13). In addition, addressing these questions in a cross-sectional design may be less appropriate because very large patient groups would be needed to compensate for the substantial individual variation of these parameters.
The most crucial question raised by our data concerns the significance of the observed changes in CD8+ T-cell functionality for the control of HIV replication. We have not formally tested this possibility by interruption of ART in the present study since current treatment guidelines do not favor treatment interruptions. However, it seems highly unlikely that HIV control would be enhanced in these patients after cessation of ART despite the presence of polyfunctional cells. Several large studies in comparable patient populations with chronic HIV infection have firmly established that HIV control is not improved after interruption of prolonged ART; the viral set point in these studies was usually very similar before the onset and after the cessation of ART (27, 28, 36, 54, 56). Therefore, the question remains whether polyfunctionality of T cells is indeed a predictive correlate of protective immunity in HIV infection or whether it mainly reflects reduced in vivo antigen exposure, a milieu which seems to be a prerequisite for the development of such cells both in humans and in mice (1, 13, 34, 49, 76). Our data clearly support the latter, and previous data on polyfunctional T cells in LTNP could be interpreted similarly (13). Nevertheless, for progress to be made toward a T-cell-based vaccine, it is highly desirable that a solid correlate of protective immunity can be identified. Although a recent HIV vaccination trial unfortunately had to be halted prematurely due to lack of efficacy, careful immunological analyses in these individuals may help to clarify whether the functional profile of T cells constitutes such a correlate of protection or not (6).
Comparable to HIV-specific CD8+ T cells, the functionality and the phenotype of HIV-specific CD4+ T cells are also influenced by the level of antigen exposure in vivo. In LTNP and patients on therapy, the phenotype and cytokine production capacity of HIV-specific CD4+ T cells was comparable to CD4+ T-cell responses directed against non-HIV antigens; in contrast, HIV-specific CD4+ T cells from untreated viremic patients showed reduced proliferation and IL-2 production capacity and exhibited an effector phenotype (26, 35, 39, 50, 70, 79).
The exhaustion of cytokine secretion capacity in CD8+ T cells is inversely correlated with the frequency of PD-1-expressing CD8+ T cells, indicating that PD-1 expression is a marker for an exhausted CD8+ T-cell population. It has been reported previously that PD-1 expression levels on HIV-specific CD8+ T cells are correlated with pVL and that blockade of the PD-1/PD-1L pathway leads to restoration of CD8+ T-cell function in vitro (23, 61, 71, 77). However, because we observed that the frequency of PD-1-positive CD8+ T cells and dysfunctionality are both regulated by the level of viremia, it seems more likely that increased PD-1 expression and cellular dysfunction are rather the result than the cause of high viremia.
Taking these results together, we show here that a high level of HIV viremia causes a selective functional impairment of HIV-specific CD8+ T cells with regard to cytokine secretion but not with respect to degranulation capacity and GrB expression (68). The fact that prolonged treatment with ART allowed the restoration of cytokine secretion capacity by CD8+ T cells suggests that the level of antigen exposure in vivo is the main cause of CD8+ T-cell dysfunction. Furthermore, persistent exposure to antigen led to the differentiation of CD8+ T cells with an effector phenotype, and a sustained decrease in pVL as a surrogate for antigen load induced a transition toward a memory phenotype with a polyfunctional profile. Whether these polyfunctional memory-type CD8+ T cells are able to mediate any superior protection is unlikely based on our disappointing experiences with treatment interruption trials in chronic HIV infection. However, it is conceivable that the polyfunctional T cells that arise spontaneously in untreated HIV infection in LTNP are different from the polyfunctional T cells that develop under prolonged ART; this is a testable hypothesis that merits further investigation.
Acknowledgments
This work was supported by the Roche Research Fund for Biology, the Swiss National Science Foundation, the Vontobel Foundation, and by an unrestricted educational grant from Abbott AG (Switzerland). D.A.P. is a Medical Research Council (United Kingdom) Senior Clinical Fellow.
We thank our patients for their commitment to the study, Rainer Weber for general support, Christina Grube for excellent patient care, Friederike Burgener for excellent laboratory assistance, and Markus Weber, Barbara Rüsi, and Eduardo Meyer for HLA genotyping.
The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH: HIV-1 Con B Gag peptides—complete set.
Footnotes
Published ahead of print on 16 January 2008.
REFERENCES
- 1.Agnellini, P., P. Wolint, M. Rehr, J. Cahenzli, U. Karrer, and A. Oxenius. 2007. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc. Natl. Acad. Sci. USA 1044565-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407386-390. [DOI] [PubMed] [Google Scholar]
- 3.Almeida, J. R., D. A. Price, L. Papagno, Z. A. Arkoub, D. Sauce, E. Bornstein, T. E. Asher, A. Samri, A. Schnuriger, I. Theodorou, D. Costagliola, C. Rouzioux, H. Agut, A. G. Marcelin, D. Douek, B. Autran, and V. Appay. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2042473-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altfeld, M., M. M. Addo, E. S. Rosenberg, F. M. Hecht, P. K. Lee, M. Vogel, X. G. Yu, R. Draenert, M. N. Johnston, D. Strick, T. M. Allen, M. E. Feeney, J. O. Kahn, R. P. Sekaly, J. A. Levy, J. K. Rockstroh, P. J. Goulder, and B. D. Walker. 2003. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS 172581-2591. [DOI] [PubMed] [Google Scholar]
- 5.Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 27494-96. [Erratum, 280:1821, 1998.] [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. 2007. HIV vaccine failure prompts Merck to halt trial. Nature 449390. [DOI] [PubMed] [Google Scholar]
- 7.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8379-385. [DOI] [PubMed] [Google Scholar]
- 8.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 19263-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann, M. F., P. Wolint, K. Schwarz, P. Jager, and A. Oxenius. 2005. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J. Immunol. 1754686-4696. [DOI] [PubMed] [Google Scholar]
- 10.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415335-339. [DOI] [PubMed] [Google Scholar]
- 11.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 7511983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 28165-78. [DOI] [PubMed] [Google Scholar]
- 13.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 1074781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 686103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3205-211. [DOI] [PubMed] [Google Scholar]
- 16.Carrington, M., G. W. Nelson, M. P. Martin, T. Kissner, D. Vlahov, J. J. Goedert, R. Kaslow, S. Buchbinder, K. Hoots, and S. J. O'Brien. 1999. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 2831748-1752. [DOI] [PubMed] [Google Scholar]
- 17.Carrington, M., and S. J. O'Brien. 2003. The influence of HLA genotype on AIDS. Annu. Rev. Med. 54535-551. [DOI] [PubMed] [Google Scholar]
- 18.Casazza, J. P., M. R. Betts, L. J. Picker, and R. A. Koup. 2001. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J. Virol. 756508-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410106-111. [DOI] [PubMed] [Google Scholar]
- 20.Chen, G., P. Shankar, C. Lange, H. Valdez, P. R. Skolnik, L. Wu, N. Manjunath, and J. Lieberman. 2001. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood 98156-164. [DOI] [PubMed] [Google Scholar]
- 21.Colle, J. H., J. L. Moreau, A. Fontanet, O. Lambotte, M. Joussemet, J. F. Delfraissy, and J. Theze. 2006. CD127 expression and regulation are altered in the memory CD8 T cells of HIV-infected patients—reversal by highly active antiretroviral therapy (HAART). Clin. Exp. Immunol. 143398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalod, M., M. Dupuis, J. C. Deschemin, D. Sicard, D. Salmon, J. F. Delfraissy, A. Venet, M. Sinet, and J. G. Guillet. 1999. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8+ responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J. Virol. 737108-7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443350-354. [DOI] [PubMed] [Google Scholar]
- 24.Edwards, B. H., A. Bansal, S. Sabbaj, J. Bakari, M. J. Mulligan, and P. A. Goepfert. 2002. Magnitude of functional CD8+ T-cell responses to the Gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 762298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellefsen, K., A. Harari, P. Champagne, P. A. Bart, R. P. Sekaly, and G. Pantaleo. 2002. Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections. Eur. J. Immunol. 323756-3764. [DOI] [PubMed] [Google Scholar]
- 26.Emu, B., E. Sinclair, D. Favre, W. J. Moretto, P. Hsue, R. Hoh, J. N. Martin, D. F. Nixon, J. M. McCune, and S. G. Deeks. 2005. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J. Virol. 7914169-14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fagard, C., M. Le Braz, H. Gunthard, H. H. Hirsch, M. Egger, P. Vernazza, E. Bernasconi, A. Telenti, C. Ebnother, A. Oxenius, T. Perneger, L. Perrin, and B. Hirschel. 2003. A controlled trial of granulocyte macrophage-colony stimulating factor during interruption of HAART. AIDS 171487-1492. [DOI] [PubMed] [Google Scholar]
- 28.Fagard, C., A. Oxenius, H. Gunthard, F. Garcia, M. Le Braz, G. Mestre, M. Battegay, H. Furrer, P. Vernazza, E. Bernasconi, A. Telenti, R. Weber, D. Leduc, S. Yerly, D. Price, S. J. Dawson, T. Klimkait, T. V. Perneger, A. McLean, B. Clotet, J. M. Gatell, L. Perrin, M. Plana, R. Phillips, and B. Hirschel. 2003. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch. Intern. Med. 1631220-1226. [DOI] [PubMed] [Google Scholar]
- 29.Genesca, M., T. Rourke, J. Li, K. Bost, B. Chohan, M. B. McChesney, and C. J. Miller. 2007. Live attenuated lentivirus infection elicits polyfunctional simian immunodeficiency virus Gag-specific CD8+ T cells with reduced apoptotic susceptibility in rhesus macaques that control virus replication after challenge with pathogenic SIVmac239. J. Immunol. 1794732-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 7410249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goulder, P. J., and D. I. Watkins. 2004. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4630-640. [DOI] [PubMed] [Google Scholar]
- 32.Goulder, P. J. R., R. E. Phillips, B. Colbert, S. McAdam, G. Ogg, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T lymphocyte response associated with progression to AIDS. Nat. Med. 3212-217. [DOI] [PubMed] [Google Scholar]
- 33.Harari, A., C. Cellerai, F. B. Enders, J. Kostler, L. Codarri, G. Tapia, O. Boyman, E. Castro, S. Gaudieri, I. James, M. John, R. Wagner, S. Mallal, and G. Pantaleo. 2007. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc. Natl. Acad. Sci. USA 10416233-16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harari, A., V. Dutoit, C. Cellerai, P. A. Bart, R. A. Du Pasquier, and G. Pantaleo. 2006. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev. 211236-254. [DOI] [PubMed] [Google Scholar]
- 35.Harari, A., S. Petitpierre, F. Vallelian, and G. Pantaleo. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103966-972. [DOI] [PubMed] [Google Scholar]
- 36.Hatano, H., S. Vogel, C. Yoder, J. A. Metcalf, R. Dewar, R. T. Davey, Jr., and M. A. Polis. 2000. Pre-HAART HIV burden approximates post-HAART viral levels following interruption of therapy in patients with sustained viral suppression. AIDS 141357-1363. [DOI] [PubMed] [Google Scholar]
- 37.Huster, K. M., V. Busch, M. Schiemann, K. Linkemann, K. M. Kerksiek, H. Wagner, and D. H. Busch. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 1015610-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hutchinson, S. L., L. Wooldridge, S. Tafuro, B. Laugel, M. Glick, J. M. Boulter, B. K. Jakobsen, D. A. Price, and A. K. Sewell. 2003. The CD8 T cell coreceptor exhibits disproportionate biological activity at extremely low binding affinities. J. Biol. Chem. 27824285-24293. [DOI] [PubMed] [Google Scholar]
- 39.Iyasere, C., J. C. Tilton, A. J. Johnson, S. Younes, B. Yassine-Diab, R. P. Sekaly, W. W. Kwok, S. A. Migueles, A. C. Laborico, W. L. Shupert, C. W. Hallahan, R. T. Davey, Jr., M. Dybul, S. Vogel, J. Metcalf, and M. Connors. 2003. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 7710900-10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 41191-1198. [DOI] [PubMed] [Google Scholar]
- 42.Kalams, S. A., P. J. Goulder, A. K. Shea, N. G. Jones, A. K. Trocha, G. S. Ogg, and B. D. Walker. 1999. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J. Virol. 736721-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaslow, R. A., M. Carrington, R. Apple, L. Park, A. Munoz, A. J. Saah, J. J. Goedert, C. Winkler, S. J. O'Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2405-411. [DOI] [PubMed] [Google Scholar]
- 44.Kelleher, A. D., C. Long, E. C. Holmes, R. L. Allen, J. Wilson, C. Conlon, C. Workman, S. Shaunak, K. Olson, P. Goulder, C. Brander, G. Ogg, J. S. Sullivan, W. Dyer, I. Jones, A. J. McMichael, S. Rowland-Jones, and R. E. Phillips. 2001. Clustered mutations in HIV-1 Gag are consistently required for escape from HLA-B27-restricted cytotoxic T lymphocyte responses. J. Exp. Med. 193375-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein, M. R., C. A. van Baalen, A. M. Holwerda, S. R. Kerkhof Garde, R. J. Bende, I. P. Keet, J. K. Eeftinck-Schattenkerk, A. D. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 1811365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kostense, S., K. Vandenberghe, J. Joling, D. Van Baarle, N. Nanlohy, E. Manting, and F. Miedema. 2002. Persistent numbers of tetramer+ CD8+ T cells, but loss of interferon-γ+ HIV-specific T cells during progression to AIDS. Blood 992505-2511. [DOI] [PubMed] [Google Scholar]
- 47.Koup, R. A., J. T. Safrit, Y. Coa, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lieberman, J. 2003. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat. Rev. Immunol. 3361-370. [DOI] [PubMed] [Google Scholar]
- 49.Makedonas, G., and M. R. Betts. 2006. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin. Immunopathol. 28209-219. [DOI] [PubMed] [Google Scholar]
- 50.McNeil, A. C., W. L. Shupert, C. A. Iyasere, C. W. Hallahan, J. A. Mican, R. T. Davey, Jr., and M. Connors. 2001. High-level HIV-1 viremia suppresses viral antigen-specific CD4+ T cell proliferation. Proc. Natl. Acad. Sci. USA 9813878-13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 31061-1068. [DOI] [PubMed] [Google Scholar]
- 52.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long-term nonprogressors. Proc. Natl. Acad. Sci. USA 972709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oxenius, A., and B. Hirschel. 2003. Structured treatment interruptions in HIV infection: benefit or disappointment? Expert Rev. Anti Infect. Ther. 1129-139. [DOI] [PubMed] [Google Scholar]
- 54.Oxenius, A., A. R. McLean, M. Fischer, D. A. Price, S. J. Dawson, R. Hafner, C. Schneider, H. Joller, B. Hirschel, R. E. Phillips, R. Weber, and H. F. Gunthard. 2002. Human immunodeficiency virus-specific CD8+ T-cell responses do not predict viral growth and clearance rates during structured intermittent antiretroviral therapy. J. Virol. 7610169-10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oxenius, A., D. A. Price, P. J. Easterbrook, C. A. O'Callaghan, A. D. Kelleher, J. A. Whelan, G. Sontag, A. K. Sewell, and R. E. Phillips. 2000. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA 973382-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oxenius, A., D. A. Price, H. F. Gunthard, S. J. Dawson, C. Fagard, L. Perrin, M. Fischer, R. Weber, M. Plana, F. Garcia, B. Hirschel, A. McLean, and R. E. Phillips. 2002. Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc. Natl. Acad. Sci. USA 9913747-13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oxenius, A., D. A. Price, A. Trkola, C. Edwards, E. Gostick, H. T. Zhang, P. J. Easterbrook, T. Tun, A. Johnson, A. Waters, E. C. Holmes, and R. E. Phillips. 2004. Loss of viral control in early HIV-1 infection is temporally associated with sequential escape from CD8+ T cell responses and decrease in HIV-1-specific CD4+ and CD8+ T cell frequencies. J. Infect. Dis. 190713-721. [DOI] [PubMed] [Google Scholar]
- 58.Oxenius, A., A. K. Sewell, S. J. Dawson, H. F. Gunthard, M. Fischer, G. M. Gillespie, S. L. Rowland-Jones, C. Fagard, B. Hirschel, R. E. Phillips, and D. A. Price. 2002. Functional discrepancies in HIV-specific CD8+ T-lymphocyte populations are related to plasma virus load. J. Clin. Immunol. 22363-374. [DOI] [PubMed] [Google Scholar]
- 59.Paiardini, M., B. Cervasi, H. Albrecht, A. Muthukumar, R. Dunham, S. Gordon, H. Radziewicz, G. Piedimonte, M. Magnani, M. Montroni, S. M. Kaech, A. Weintrob, J. D. Altman, D. L. Sodora, M. B. Feinberg, and G. Silvestri. 2005. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 1742900-2909. [DOI] [PubMed] [Google Scholar]
- 60.Pantaleo, G., J. F. Demarest, T. Schacker, M. Vaccarezza, O. J. Cohen, M. Daucher, C. Graziosi, S. S. Schnittman, T. C. Quinn, G. M. Shaw, L. Perrin, G. Tambussi, A. Lazzarin, R. P. Sekaly, H. Soudeyns, L. Corey, and A. S. Fauci. 1997. The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia. Proc. Natl. Acad. Sci. USA 94254-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2032281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phillips, R. E., S. Rowland-Jones, D. F. Nixon, F. M. Gotch, J. P. Edwards, A. O. Ogunlesi, J. G. Elvin, J. A. Rothbard, C. R. M. Bangham, C. R. Rizza, and A. J. McMichael. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354453-459. [DOI] [PubMed] [Google Scholar]
- 63.Price, D., P. J. R. Goulder, P. Klenerman, A. Sewell, M. Troop, P. Easterbrook, C. R. M. Bangham, and R. E. Phillips. 1997. Positive selection of cytotoxic T cell escape variants during primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 941890-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scherer, A., J. Frater, A. Oxenius, J. Agudelo, D. A. Price, H. F. Gunthard, M. Barnardo, L. Perrin, B. Hirschel, R. E. Phillips, and A. R. McLean. 2004. Quantifiable cytotoxic T lymphocyte responses and HLA-related risk of progression to AIDS. Proc. Natl. Acad. Sci. USA 10112266-12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 66.Schockmel, G. A., S. Yerly, and L. Perrin. 1997. Detection of low HIV-1 RNA levels in plasma. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 14179-183. [DOI] [PubMed] [Google Scholar]
- 67.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8+ T cells in chronic human immunodeficiency virus infection. Blood 963094-3101. [PubMed] [Google Scholar]
- 68.Snyder-Cappione, J. E., A. A. Divekar, G. M. Maupin, X. Jin, L. M. Demeter, and T. R. Mosmann. 2006. HIV-specific cytotoxic cell frequencies measured directly ex vivo by the Lysispot assay can be higher or lower than the frequencies of IFN-gamma-secreting cells: anti-HIV cytotoxicity is not generally impaired relative to other chronic virus responses. J. Immunol. 1762662-2668. [DOI] [PubMed] [Google Scholar]
- 69.Takata, H., and M. Takiguchi. 2006. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J. Immunol. 1774330-4340. [DOI] [PubMed] [Google Scholar]
- 70.Tilton, J. C., M. R. Luskin, A. J. Johnson, M. Manion, C. W. Hallahan, J. A. Metcalf, M. McLaughlin, R. T. Davey, Jr., and M. Connors. 2007. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. J. Virol. 812713-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 121198-1202. [DOI] [PubMed] [Google Scholar]
- 72.Trimble, L. A., and J. Lieberman. 1998. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downmodulate CD3 zeta, the signaling chain of the T-cell receptor complex. Blood 91585-594. [PubMed] [Google Scholar]
- 73.Tussey, L. G., U. S. Nair, M. Bachinsky, B. H. Edwards, J. Bakari, K. Grimm, J. Joyce, R. Vessey, R. Steigbigel, M. N. Robertson, J. W. Shiver, and P. A. Goepfert. 2003. Antigen burden is major determinant of human immunodeficiency virus-specific CD8+ T cell maturation state: potential implications for therapeutic immunization. J. Infect. Dis. 187364-374. [DOI] [PubMed] [Google Scholar]
- 74.van Leeuwen, E. M., G. J. de Bree, E. B. Remmerswaal, S. L. Yong, K. Tesselaar, I. J. ten Berge, and R. A. van Lier. 2005. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood 1062091-2098. [DOI] [PubMed] [Google Scholar]
- 75.Whelan, J. A., P. R. Dunbar, D. A. Price, M. A. Purbhoo, F. Lechner, G. S. Ogg, G. Griffiths, R. E. Phillips, V. Cerundolo, and A. K. Sewell. 1999. Specificity of CTL interactions with peptide-MHC class I tetrameric complexes is temperature dependent. J. Immunol. 1634342-4348. [PubMed] [Google Scholar]
- 76.Wherry, E. J., D. L. Barber, S. M. Kaech, J. N. Blattman, and R. Ahmed. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA 10116004-16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wherry, E. J., C. L. Day, R. Draenert, J. D. Miller, P. Kiepiela, T. Woodberry, C. Brander, M. Addo, P. Klenerman, R. Ahmed, and B. D. Walker. 2006. HIV-specific CD8 T cells express low levels of IL-7Rα: implications for HIV-specific T cell memory. Virology 353366-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolint, P., M. R. Betts, R. A. Koup, and A. Oxenius. 2004. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J. Exp. Med. 199925-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Younes, S. A., B. Yassine-Diab, A. R. Dumont, M. R. Boulassel, Z. Grossman, J. P. Routy, and R. P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 1981909-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zimmerli, S. C., A. Harari, C. Cellerai, F. Vallelian, P. A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA 1027239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]