Abstract
The paradigm of bacterial quorum sensing (QS), which mediates cell-density-dependent gene expression, usually has been studied in high-cell-density planktonic liquid cultures or in biofilms in which signal concentrations accumulate to sufficiently high levels to induce QS. Presumably under conditions with restricted diffusion of the signal molecule, smaller population sizes could achieve such a state of QS induction. The plant-pathogenic bacterium Pseudomonas syringae, in which QS controls traits involved in epiphytic fitness and virulence, occurs on leaf surfaces in aggregates of various sizes. Because leaves often harbor limited surface water, we investigated the size of aggregates that would permit QS in a nonsaturated environment. QS induction was visualized via dual fluorescence of P. syringae cells harboring a transcriptional fusion of mRFP1 with ahlI, which exhibits N-acyl homoserine lactone-dependent transcriptional activity, and a constitutive GFP marker to account for all P. syringae cells on a leaf. Confocal microscopy revealed that, on wet leaves, no QS induction was evident within 2 days after inoculation, but it increased rapidly with increasing aggregate sizes >40 and 22 cells per aggregate by 3 and 4 days, respectively. In contrast, QS induction was common in aggregates >33 cells by 2 days after inoculation on dry leaves and increased rapidly with increasing aggregate sizes >35 and 13 cells after 3 and 4 days, respectively. These observations demonstrate that small groups of cells experience QS conditions on dry leaves where signal diffusion is restricted. Quorum size of bacteria in non-water-saturated environments such as on leaves is small, and QS induction may be commonly operative.
Keywords: aggregate, diffusion, quorum sensing, epiphyte, bioreporter
Bacteria are able to sense cell population density via the production and perception of small diffusible signal molecules called autoinducers in a process termed “quorum sensing” (QS). The most studied process of QS involves the production of N-acyl homoserine lactones (AHL) in Gram-negative bacteria. At low cell densities, AHLs and an inactive form of the AHL receptor/transcription factor usually are produced at low levels. The local AHL concentration increases with population size as diffusional losses are balanced by a higher number of producing cells, resulting in AHL binding to the transcription factor. The activated transcription factor then initiates transcription of downstream genes, usually including the AHL synthase, resulting in a positive feedback loop and activating QS (1). Density-regulated genes vary among species and include those required for virulence, antibiotic production, pigmentation, nodulation, extracellular polysaccharide production, motility, and other processes (1–7). Such traits are thus expressed only when cell numbers increase in number to reach a so-called “quorum.”
The question “How many cells constitute a quorum?” usually has been addressed from the limited perspectives of cells either growing planktonically in shaken cultures or growing in biofilms in the presence of flowing liquids. The AHL signal concentration and cell density at which autoinductive QS regulation occur in broth cultures of several bacterial species has been described (1–7). AHL signals readily diffuse away from cells in these typical laboratory cultures, and it is required that they reach high population sizes to reach the threshold AHL concentration for QS to occur. Likewise, it has been shown that cells within biofilms apparently accumulate a high level of AHL and undergo QS induction sooner than cells at the periphery of biofilms in flow cells where the AHL can be dissipated by diffusional losses to the flowing liquid (8). In environments where the habitat is water-limited or water content fluctuates, the activation of QS would depend greatly on factors that control diffusional losses of AHLs. Redfield (9) states that “demonstrations of QS in the laboratory tells us nothing about the roles of autoinducers in the natural environment. We instead need to ask whether the regulation acts under natural conditions where QS is possible and whether sufficient autoinducer is produced under these conditions.” This concept, discussed by Redfield and others (1, 9, 10), has received little attention despite the extensive study of QS in many species. Our study addresses this issue in such an environment, the phyllosphere (11). Leaf surfaces are colonized by a variety of bacteria that are exposed to dramatic changes in environmental conditions such as water availability on which signal accumulation would be expected to depend.
To examine environmental dependency of quorum size, we have addressed the process of QS induction of the epiphytic bacterium Pseudomonas syringae pv. syringae (Pss), the causal agent of brown spot disease of bean and frost injury to plants (12). Pss can multiply to high population sizes on the surface of healthy leaves, and these large epiphytic populations precede disease (13). Pss forms aggregates of various sizes while growing on leaves, presumably in response to local differences in nutrient availability on the leaf. Although much less common than solitary cells or cells in small aggregates, large bacterial aggregates (>100 cells per aggregate) account for the majority of the cells on a leaf surface (14). Cells in large aggregates are more resistant to environmental stresses such as desiccation than solitary cells (15), suggestive of density-dependent behavior.
Pss mediates QS via its production of 3-oxo-hexanoyl-homoserine lactone (3OC6-HSL) by the synthase AhlI and the transcription factor AhlR (16, 17). QS positively regulates a variety of traits in Pss such as exopolysaccharide (EPS) production that contributes to its survival on leaf surfaces and negatively regulates swarming motility and thus host invasion and virulence (17–19). Because QS in Pss has been shown to increase in planktonic cell cultures at high cell concentrations, we cannot predict its QS-dependent behavior on leaves because it does not occur as dispersed cells in this habitat. The spatial aggregation of Pss cells in discrete cell assemblages of different sizes on leaves raises the question as to what a functional community of cells of this species is in such a natural habitat. That is, do cells in aggregates of different sizes achieve a QS-induced state that depends on the size of an individual cell aggregate, or does cross-talk caused by AHL diffusion enable proximal aggregates to achieve a quorum? Likewise, does the presence of water films, a common feature of leave surfaces, influence the number of cells that must be present before QS is achieved? Thus, although culture studies are informative, they do not enable the definition of a QS-induced state in its natural habitat where diffusion of the signal is restricted, as on a moist leaf surface. In this study, we report the quorum size of Pss on leaves and the effects of water availability the process of QS by using a whole-cell bioreporter responsive to AHL.
Results
Development of a QS Biosensor for Use on Leaves.
A whole-cell QS bioreporter that harbors a gene encoding monomeric red fluorescent protein (mRFP1) (20) fused to the AHL-responsive promoter of ahlI (PahlI) to monitor the local accumulation of AHL indicative of QS was engineered. This AHL-producing biosensor (Pss strain B728a harboring plasmid pIRed) also exhibited bright green fluorescence whose intensity was independent of the amount of AHL produced by the strain or added exogenously because of the expression of the constitutive Ptrp:gfp marker gene to enable an accounting for all of the bioreporter cells on a leaf and to distinguish it from other resident bacterial epiphytes. Cells of Pss (pIRed) from a low-density culture (<107 cells per ml) in King's B (KB) broth without added AHL exhibited no red fluorescence in the absence of added 3OC6-HSL, indicating a lack of significant AHL accumulation and hence ahlI expression in the inoculum under these conditions. These cells exhibited weak red fluorescence within 5 h when 3OC6-HSL was added to a concentration of 100 nM, indicating a rapid response of PahlI to even low levels of AHL [supporting information (SI) Fig. 3]. Bright red fluorescence above background was observed when concentrations of 3OC6-HSL of 1 μM or more was added. After 16 h, all cells were more than two times more brightly red fluorescent than that seen within the 5-h treatments because of production of 3OC6-HSL by Pss itself and accumulation of mRFP1. Pss (pIRed) was as responsive to a given amount of added AHLs as Pss (pBQ9) (data not shown), a bioreporter harboring an PahlI:gfp reporter gene that was described in ref. 16.
QS on Wet Leaves.
Although Pss multiplied to large population sizes on moist leaves within 24 h after inoculation, QS-dependant expression of the fluorophore was not observed for 2 or more days after inoculation. Because Pss survival and growth on leaves is poor unless moisture is present soon after their deposition, plants were kept in a humid environment for the first 24 h after inoculation. Although a cell population size of Pss (pIRed) of ≈105 cells per leaf was present initially after plant inoculation, population sizes increased rapidly to ≈107 cells per leaf within 24 h of incubation in humid conditions where slight water condensation was visible on the leaf surface. Leaf population sizes increased insignificantly after 24 h under either wet or dry conditions. When sprayed onto bean plants, Pss (pIRed) grew normally compared with wild-type Pss. The number of cells of Pss (pIRed) in aggregates on leaves was easily quantified as a measure of green fluorescence, and the distribution of cells in aggregates was similar to that observed (14). Most of the cell aggregates observed (67%) consisted of 10 cells or fewer after 4 days' incubation under humid conditions, and aggregates as large as 2,475 cells were observed. Because of their large size, 70% of the total observed epiphytic cell population occurred in aggregates larger than 100 cells (SI Fig. 4A).
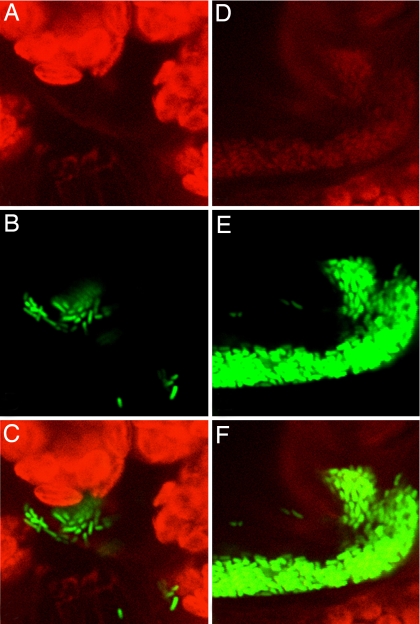
Cells of the Pss (pIRed) biosensor expressed sufficient red fluorescence to visualize them on plants. Inocula of cells grown only to low cell densities in culture exhibited no initial red fluorescence on leaves but could be readily detected because of their bright green fluorescence (Fig. 1 B and E). Likewise, most solitary cells and small cell aggregates visualized on leaves within a day of inoculation exhibited no red fluorescence (Fig. 1A). In contrast, cells of Pss (pIRed) in large cell aggregates that had formed 2 or more days after inoculation exhibited bright red fluorescence comparable to cells subjected to AHL concentrations ≥1 μM in vitro (Fig. 1D). Although the leaf had a low red autofluorescence, the use of confocal microscopy enabled clear differentiation of red fluorescence of bacterial cells to be differentiated from the background.
Fig. 1.
The mRFP1-based biosensor for AHL expressed sufficient red fluorescence for visualization of Pss (pIRed) on plants by confocal fluorescent microscopy. (A–C) A small uninduced aggregate with a cell red pixel intensity of 0. (D–E) A large aggregate exhibiting QS-regulated mRFP1 production with a cell red pixel intensity of ≈42. A and D, red channel; B and E, green channel; C and F, composite. The width of each panel is ≈34 μm.
Only ≈0.31% of Pss (pIRed) cells exhibited red fluorescence indicative of QS induction after 1 day's incubation on wet leaves (Table 1). Although a slightly higher proportion of cells exhibited QS induction after incubation for 2 days on wet leaves (8.20%), a high percentage of cells exhibited QS induction only by the third and fourth day of incubation on wet leaves (86% and 90%, respectively).
Table 1.
Proportion of QS-induced cells in the Pss (pIRed) population
*Values compared within that day are significantly different (P < 0.005) determined by a test of proportions.
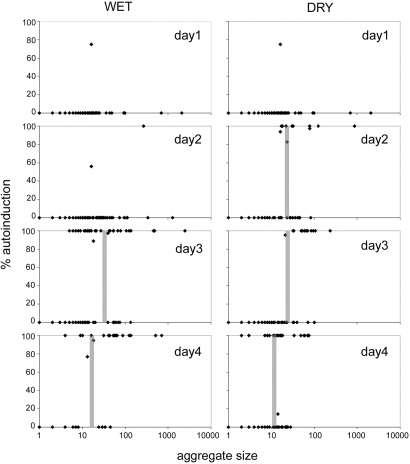
The frequency with which cells of Pss (pIRed) were QS-induced on moist leaves was strongly influenced by the size of the cell aggregate at a given incubation time. After only one day of incubation on moist leaves, even cell aggregates as large as 2,000 cells did not exhibit QS induction as measured by red fluorescence (Fig. 2). Only a single aggregate (269 cells) exhibited QS induction after 2 days of incubation. However, after 3 days of incubation, QS induction of cells was more evident, and the cell aggregate size at which QS induction was observed was progressively smaller. The “QS induction point” of the cells under a given condition was defined as the aggregate size at which the numbers of fluorescent and nonfluorescent aggregates were equal. Based on these criteria, the QS induction point for cells on wet plants 3 days postinoculation was ≈40 cells (Fig. 2), although at least a few QS-induced cell aggregates as small as 5 cells were observed. A similar distribution of aggregate sizes in which QS induction had occurred was seen after 4 days' incubation. The QS induction point was ≈22 cells, and a few cell aggregates as small as 4 cells were observed. No single cells were observed to be QS-induced. In nearly all cases, all cells within a cell aggregate on wet leaves exhibited the same QS status, being either all induced or not (Fig. 2).
Fig. 2.
QS induction observed in Pss (pIRed) inoculated on bean leaves occurs with increasing time and is affected by water availability. Each symbol represents a given cell aggregate observed by fluorescence microscopy on plants incubated only under wet conditions (Left) or under dry conditions after the first 24 h (Right). The vertical bars represent the QS induction point, that cell aggregate size at which the proportion of red fluorescent (QS-induced) cell aggregates and nonfluorescent aggregates are similar.
QS on Dry Leaves.
The viable population size of Pss (pIRed) remained high on leaves even after they were allowed to dry, and QS-induced cells were observed much sooner after inoculation and in smaller cell aggregates than on wet leaves. The distribution of aggregate sizes of Pss (pIRed) on leaves that were allowed to dry after 24 h of incubation in wet conditions followed the same pattern seen on leaves that remained wet for the entire incubation period. The majority (69%) of the cell aggregates observed were 10 cells or fewer in size. Although there were fewer cell aggregates >100 cells in size than on wet leaves, because of their relatively large size, ≈49% of the total population was found in such large cell aggregates on dry leaves (SI Fig. 3B). Approximately 65% of the total cell population occurred in cell aggregates of 50 cells or larger. Only ≈0.4% of all cells were QS-induced within 24 h of incubation under wet conditions (Table 1). When the surface of the plants was subsequently allowed to dry, a high proportion of all cells (69%) became QS-induced within 24 h of drying. The proportion of cells that were QS-induced remained high when incubated under such dry conditions for an additional 24 or 48 h (61% and 65%, respectively).
The QS induction of cells on dry leaves increased with both time and the size of the aggregate in which they occurred. No QS induction, even in cell aggregates as large as 2,000, cells was observed after 24 h under wet conditions. However, when leaves subsequently were dried, QS induction of cells was apparent within 24 h. The QS induction point for cells that had dried for 24 h was 33 cells, whereas some aggregates as small as 16 cells had become QS-induced (Fig. 2). After leaves had dried for 48 h, the QS induction point was 35 cells (Fig. 2), and some aggregates as small as 21 cells had become QS-induced. Further incubation of cells on dry leaves further decreased the size of aggregates that exhibited QS induction. After leaves had been dry for 72 h, the QS induction point was 13 cells, and aggregates as small as 2 cells exhibited QS induction. In no case, however, were single cells observed to be QS-induced. As on wet leaves, all cells in an aggregate usually had the same QS status suggestive of communication between the cells within that aggregate. Cells in very small aggregates nearby (as close as 5 μm) large aggregates usually did not share the same QS induction status.
Expression of Reporter Genes in Small Cell Aggregates on Leaves.
As ≈70% of the cell aggregates on both wet and dry leaves were 10 cells or fewer in size and thus may have been in microhabitats with limited nutritional resources needed to provide the metabolic ability to produce fluorescent reporter proteins, we determined whether such cells were capable of exhibiting a fluorescence phenotype if induced to do so. We determined the metabolic responsiveness of cells on leaves by visualizing green and red fluorescence of Pss (pVITIR, pBQ9RED), a reporter strain harboring an AHL-inducible PahlI:mRFP1 gene fusion and an iron-repressible Ppvd:gfp gene fusion. Inoculum of cells grown under high iron conditions were repressed for GFP expression, but when transferred to plants and incubated under both wet and dry as above, many single cells and small cell aggregates became brightly green fluorescent, which indicated that the cells experienced low iron conditions on the leaf, but more importantly, it demonstrated that the cells in small aggregates were capable of expressing reporter genes under these conditions on plants.
Discussion
This study was designed to determine not only the QS state of cells of different aggregate sizes in a natural habitat but also the extent to which factors such as the presence of liquid films would affect the process of QS induction, leading to AHL accumulation in cell aggregates on leaves. To investigate the process of QS by Pss on leaves it was necessary to construct a dual fluorescent reporter system including a constitutive GFP marker gene and an AHL-inducible mRFP1 reporter gene. The Pss (pIRed) biosensor was responsive to similarly low AHL concentrations as a gfp-based biosensor (17) and thus sufficiently sensitive to detect QS-induced cells in culture and on plants. The mRFP1-based biosensor allowed us to use the more visible GFP as a constitutive marker to account for all Pss cells growing on the leaf surface, which is inherently red fluorescent.
The temporal process of QS in Pss on leaves was strongly influenced both by the size of cell aggregates and by the availability of water on leaves. Similar to observations in earlier studies (14), the majority of cells were associated with large cell aggregates, even though the numbers of large aggregates are outnumbered by more solitary cells on leaves exposed to both wet and relatively dry conditions. Cell aggregates were slightly larger on plants exposed to prolonged wet conditions compared with plants wet for only 1 day, probably because of the greater availability of nutrients being leached from the plant. Both the total population size of Pss on leaves and the size of cell aggregates increased little after the first 24 h of incubation on leaves, presumably because of a substantial reduction of available nutrients over time (21). Although large cell aggregates had formed on leaves by 24-h incubation, little QS was apparent on wet leaves for 48 h, although QS increased only quite rapidly after this time (Fig. 2 and Table 1). Importantly, the cell aggregate size needed to achieve a quorum decreased over time under both wet and dry conditions. The minimum cell aggregate size at which QS was operative was always smaller on dry leaves than on wet leaves at a given incubation time. QS induction could be observed in many aggregates as small as 10 cells by the fourth day on dry leaves, whereas the by the fourth day of incubation on wet leaves, QS induction was seldom seen in such small cell aggregates. These results suggest that the AHL produced by Pss slowly accumulated to a threshold concentration needed to induce QS only many hours after cell aggregates had formed. Furthermore, the time needed for signal accumulation was lower under dry conditions, which presumably minimized the dissipation of the AHL away from the producing cells. It is unclear whether QS induction would ever occur in the many small aggregates on leaves under continuously wet conditions, because the diffusional loss of AHLs might exceed their rate of production. The finding that cell aggregates as small as two cells could eventually undergo QS induction on dry leaves is strong evidence that QS is a robust indicator of diffusional limitation as suggested by Redfield and others (1, 9, 10). This concept has been expanded further by Hense et al. (10) as an efficiency-sensing hypothesis. This model unifies the traditional population size concept of QS and that of diffusion sensing into a single process that measures cell density, mass-transfer properties, and spatial distribution to determine the efficiency by which AHL-producing cells express extracellular products and exhibit proper behavioral responses (10).
The nature of the leaf surface probably contributes to the constraint of AHLs near the site of their production in cell aggregates. The leaf surface is a hydrophobic habitat as the cuticle is composed of cutin, cutan, and primarily cuticular waxes: linear, long-chain aliphatic molecules. The 3OC6-HSL, produced by Pss, a lipophylic species with a Mr of 135, would have a relatively low mobility (10−3.8 k*) through the cuticle as estimated by Buchholz et al. (22). Others have noted that the mobility of polar compounds through the cuticle can increase up to 3-fold after absorbance of water into cuticular waxes when the relative humidity is increased from 0% to 100%, thereby increasing leaching of solutes through the cuticle (23). Thus, AHLs may be less likely to diffuse into the interior of plants through relatively dry compared with wet leaves. Because surface water availability both delayed the onset of QS in Pss and increased the size of cell aggregates that were needed for it to occur, it seems likely that AHL diffusion away from the producing cells is primarily limited by water availability. AHL diffusion would be expected both through the waxy cuticle into plant tissue and laterally across the leaf surface in water films. Given that AHLs are not appreciably volatile, the air–water interface of discontinuous water droplets on leaves is another diffusional barrier. Evaporation of AHL-containing droplets surrounding cell aggregates on leaves also would increase the concentrations of AHLs. Although studies have found that some plants can produce AHL mimics and inhibitors (24, 25), no such compounds capable of affecting the QS of Pss were observed from bean leaves (26). Because the bean leaf surface is acidic from proton excretion (27), the instability of AHLs in alkali pH would not affect signal accumulation.
The apparent constraint of AHL movement on leaves contrasts with observations made in the rhizosphere. Studies of the “calling distance” between an AHL source strain and a biosensor strain suggested that a single AHL source cell could produce enough AHL to be perceived by adjacent cells on the root (28). AHLs could be sensed as far as 78 μm from the nearest producing microbe in the rhizosphere (28) and at concentrations exceeding 40 nM (29) although most AHL was detected only within a few micrometers of the producing strain. Because a point source of AHL applied to roots could diffuse over entire the root surface (possibly conducted by a mucoid matrix on the root) and activate QS biosensor cells (29), it remains unclear whether the sources of AHLs on colonized roots were only from the nearest neighboring bacterial cell on the root and not from nearby larger cell aggregates or multiple single-cell AHL sources. In contrast, the heterogeneity of the available nutrients and leaf topology contribute to a clustering of cells on the leaf surface (14, 21), making the probability of interaggregate signal perception low, especially so in dry diffusion-limited conditions. Because we seldom observed solitary cells within 5 μm of larger cell aggregates to have the same QS status of the aggregate, especially under dry conditions, it appears that AHL-mediated cross-talk occurs only under very limited distances on leaves and the interaggregate signaling caused by spatial clustering as modeled by Hense et al. (10) is uncommon on dry leaves.
Because QS controls a variety of traits in Pss that contribute to epiphytic fitness and virulence, factors that influence QS induction on leaves will contribute greatly to its behavior. EPS production in Pss is positively regulated by QS (19). This increased EPS production enables greater survival of epiphytes under desiccating conditions on leaves (5, 19, 30, 31). Because QS was induced much more rapidly and in smaller cell aggregates on dry leaves than wet leaves, we would expect that such decreases in water availability would lead to increased AHL concentrations and thus QS-induced EPS production. Motility also is an important behavior of Pss on leaves. QS suppresses swarming in Pss B728a and QS-deficient strains invade leaves more readily than wild-type strains, thereby causing a higher incidence of disease (19). Nonmotile mutants of Pss are less able to survive desiccation stresses on leaves, apparently because they cannot access protected sites in or on the leaf surface (32). Repression of swarming motility associated with AHL accumulation upon drying of the leaf would allow Pss to conserve resources invested in flagella or surfactants when movement is not possible. Presumably it would be disadvantageous for Pss to move away from sites where abundant resources existed, and large cell aggregates that formed there would undergo QS and thus suppress motility. The QS behaviors of Pss would thus tend to cause its populations to be spatially aggregated because sites favorable for growth and survival on leaves are uncommon. High-velocity raindrops are required for increases in epiphytic populations of Pss under field conditions (33) and facilitate their emigration to neighboring leaves (34). Because the binding of AHLs to QS regulators in bacteria can be reversible (35), washing of leaves by rain should rapidly decrease the local concentration of AHLs around epiphytic bacteria and disperse the aggregated cells that are enclosed an EPS matrix, which would enable cell motility and enhance exploration of the leaf surface for nutrients and subsequent invasion of the leaf.
Although the phyllosphere is a unique environment in many respects, the environmental conditions studied here that affect QS in Pss are applicable to bacterial species residing in other spatially structured, non-water-saturated habitats. Bacteria in nonhydrated environments such as soils also might be expected to exhibit cell-density-dependent behaviors governed by QS at small local spatial densities because they are found in small water-filled pores that usually are discontinuous. The same also may be true of oral biofilms that experience periodic liquid flow and signaling is limited to separated cell clusters (36, 37). Animal pathogens that use QS-dependent gene expression might become QS-induced after being engulfed by macrophages. The rapid environmental change from being suspended in large volumes of plasma to transitioning into a small phagosome would cause signal(s) to rapidly accumulate, as may be the case when Staphylococcus epidermis up-regulates virulence, resistance, and stress survival-associated genes when QS-induced (38). Similarly, captured bacteria in the food vacuole of an amoeba would be in a high spatial density and thus may enter a QS state, thereby inducing traits for escape or survival from the would-be assailant.
Our studies support the suggestions of other studies that AHL levels are highest under conditions in which the diffusion of this signal molecule is constrained. Most previous studies of the process of QS in culture or in natural systems have relied on estimations of AHL concentrations from extractions of signal molecule from whole communities and extrapolation to indicate the possibility of QS, or by use of secondary signal biosensors. For example, because many as 108 cells of the bacterial symbiont Vibrio fisherii can be found within the light organs of an adult bobtail squid. This community accumulates 3OC6-HSL to concentrations exceeding 100 nm, >40 times the concentration needed to induce QS induction and thus bioluminescence in liquid culture (39). The squid light organ epithelial cells (35) and lung epithelial tissue (40) apparently do not provide a complete barrier to diffusion of 3OC6-HSL and yet signal may still accumulate in organs. AHL levels also have been found in biofilms of Pseudomonas aeruginosa at concentrations of 1 mM, 1,000 times greater than those necessary to induce QS in shaken cultures (41). The highest levels of expression of lasI, indicative of AHL accumulation, were only seen within or near the substratum of a biofilm and not at the periphery where AHLs might be expected to be lost to the surrounding liquid milieu by diffusion (8). Although Pss accumulates a maximal level of ≈400 nM 3OC6-HSL in broth culture (17), artificially produced aggregates of Pss in small agar discs have been found to accumulate more than nine times this amount of AHL (G.D. and R. Shepherd, unpublished results). Such results all suggest that the process of QS induction is enhanced when there is less diffusion of AHL signal away from the producing cells.
Materials and Methods
Bacterial Strains and Culture Conditions.
P. syringae pv. syringae B728a (42) (hereafter called Pss) was maintained on KB medium (43) and grown at 28°C. Escherichia coli DH5a and TOP10 (Invitrogen) strains used for routine cloning were maintained on Luria media and cultured at 37°C. Concentrations of the appropriate antibiotics used were 50 μg/ml kanamycin; 100 μg/ml rifampicin; and 20 μg/ml spectinomycin.
Construction of AHL Reporter.
The stable plasmid pIRed harboring both a mRFP1 reporter gene fusion and a gfp marker gene was constructed through the following steps. The mRFP1 protein coding sequence was amplified from pRSETB (20) with the primers 5′-AAGGATCATATGGCCTCCTCCGAGGACGTCATC-3′ and 5′-GTTAACTTAGGCGCCGGTGGAGTG-3′. The 0.8-kb fragment was cloned into pCR-Blunt II-TOPO (Invitrogen) and the sequence of mRFP1 determined by sequencing. The mRFP1 gene then was cloned into pBQ9 (17) as a NdeI–HpaI fragment 3′ of the ahlI promoter (PahlI), replacing most of the gfp gene present, creating pBQ9RED. The PahlI:mRFP1 gene fusion was reamplified with the primers 5′-CAGCTCGAGCTGATCCTGGTGCGTGTGGGCATCGGC-3′ and 5′-GCTCTCGAGTTAGGCGCCGGTGGAGTG-3′ and then digested with XhoI. The resulting 1-kb PahlI :mRFP1 XhoI fragment was ligated into the SalI site of pKLN42 containing the Ptrp:gfp. Gene orientation was determined via sequencing and plasmid pIRed, wherein the PahlI :mRFP1 and Ptrp:gfp gene fusion were divergent, was chosen. Plasmid pIRed was introduced into Pss by electroporation as in other studies (17). A second reporter strain was constructed by electroporation of the pBQ9RED plasmid into Pss B728a (pVITIR), which contains an iron starvation inducible Ppvd:gfp gene fusion (44).
QS Induction in Vitro.
Pss harboring pIRed or pBQ9 were grown to a low cell density of ≈107 cells per ml in KB broth, a cell concentration at which QS induction has yet to occur. 3OC6-HSL dissolved in ethyl acetate was placed in glass culture tubes, air-dried, and resuspended with KB broth to appropriate concentrations. Tubes then were inoculated with Pss cells to a concentration of 106 cells per ml. After 5 and 16 h of growth, samples were mounted on ProbeOn Plus (Fisher Scientific) microscope slides with Aqua-Polymount (Polysciences, Inc.). Cells were analyzed at ×1,000 magnification on a Zeiss 510 UV/Vis Meta laser scanning confocal microscope. GFP fluorescence was visualized by excitation using a single-line 488-nm laser and emissions filtered with a 505- to 550-nm bandpass. The mRFP1 was excited with a single-line 543 laser, and emissions were collected with filters set to a 560- to 615-nm bandpass.
Microscopy of Cells on Leaves.
QS-uninduced cells of Pss (pIRed) or Pss (pVITIR, pBQ9RED) were grown to a low cell density (107 cells per ml), harvested by centrifugation, and resuspended in 1 mM potassium phosphate buffer to a final concentration of 106 cells per ml. QS-induced cells of Pss (pVITIR, pBQ9RED) were grown to high cell density on 10 μM FeCl3 KB agar, harvested by centrifugation, and resuspended in 1 mM potassium phosphate buffer to a final concentration of 106 cells per ml. The appropriate suspension (≈1 ml/plant) then was sprayed onto leaves of 14-day-old bean plants (Phaseolus vulgaris cv. Bush Blue Lake 274). Plants then were placed in a humid chamber at room temperature (≈28°C) and periodically exposed to a fine mist of water to maintain high relative humidity (≈100% R.H.) wherein a fine film of water condensation was apparent on the leaf surface. Artificial light was maintained for 16-h periods within the 24-h cycle. After 24 h, plants either were taken out of the chamber and allowed to dry at ambient R.H. (≈60% R.H.) or remained in the humid chamber.
At various times after inoculation, 1-cm square sections of the primary leaves were sampled and mounted on glass slides with a Whatman paper base with Aqua-Polymount (Polysciences, Inc.). Bacterial cells were analyzed at ×1,000 magnification on a Zeiss 510 UV/Vis Meta laser scanning confocal microscope. The leaf surface was scanned for green fluorescent cells indicating the presence of the gfp marker gene. GFP fluorescence was visualized by excitation using a single-line 488-nm laser, and emission was collected with filters set to a 505- to 550-nm bandpass. The AHL-induced mRFP1 was excited with a single-line 543 laser, and emissions were collected with filters set to a 560- to 615-nm bandpass to block plant autofluorescence, specifically chloroplast fluorescence at wavelengths >615 nm. Multiple z-section scans acquired at 1 μM increments were taken of large cell aggregates. The size of large three-dimensional aggregates of GFP-expressing cells was determined by the estimation of the area of an aggregate within a single z section divided by the area of a single cell, and then results of multiple z sections were added to estimate the total population size of the entire aggregate.
Statistical Analysis.
A test of proportions was performed by using R 2.5.0 (Free Software Foundation, Inc.) to determine the significance (P < 0.005) of the proportions of QS-induced cells on plants exposed to different humidity treatments. Descriptive statistical analysis were performed by using Statistica (Statsoft Inc.).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Steve Ruzin and Denise Schichnes at the College of Natural Resources Biological Imaging Center for support with microscopy. We also acknowledge Tracy Powell for helpful comments on the manuscript. This research was funded in part by United Stated Department of Agriculture National Research Initiative Grant 2004-35319-14145 and an Environmental Protection Agency STAR fellowship awarded to G.D.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711723105/DC1.
References
- 1.Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 2.Eberl L. N-acyl homoserinelactone-mediated gene regulation in gram-negative bacteria. Syst Appl Microbiol. 1999;22:493–506. doi: 10.1016/S0723-2020(99)80001-0. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez JE, Marketon MM. Quorum sensing in nitrogen-fixing rhizobia. Microbiol Mol Biol Rev. 2003;67:574–592. doi: 10.1128/MMBR.67.4.574-592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 5.von Bodman SB, Bauer WD, Coplin DL. Quorum sensing in plant-pathogenic bacteria. Annu Rev Phytopathol. 2003;41:455–482. doi: 10.1146/annurev.phyto.41.052002.095652. [DOI] [PubMed] [Google Scholar]
- 6.Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 7.Whitehead NA, Barnard AML, Slater H, Simpson NJL, Salmond GPC. Quorum-sensing in gram-negative bacteria. FEMS Microbiol Rev. 2001;25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 8.De Kievit TR, Gillis R, Marx S, Brown C, Iglewski BH. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: Their role and expression patterns. Appl Environ Microbiol. 2001;67:1865–1873. doi: 10.1128/AEM.67.4.1865-1873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redfield RJ. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 2002;10:365–370. doi: 10.1016/s0966-842x(02)02400-9. [DOI] [PubMed] [Google Scholar]
- 10.Hense BA, et al. Opinion: Does efficiency sensing unify diffusion and quorum sensing? Nat Rev Microbiol. 2007;5:230–239. doi: 10.1038/nrmicro1600. [DOI] [PubMed] [Google Scholar]
- 11.Ruinen J. The phyllosphere. 1. An ecologically neglected milieu. Plant Soil. 1961;15:81–109. [Google Scholar]
- 12.Hirano SS, Upper CD. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiol Mol Biol Rev. 2000;64:624–653. doi: 10.1128/mmbr.64.3.624-653.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouse DI, Nordheim EV, Hirano SS, Upper CD. A model relating the probability of foliar disease incidence to the population frequencies of bacterial plant-pathogens. Phytopathology. 1985;75:505–509. [Google Scholar]
- 14.Monier JM, Lindow SE. Frequency, size, and localization of bacterial aggregates on bean leaf surfaces. Appl Environ Microbiol. 2004;70:346–355. doi: 10.1128/AEM.70.1.346-355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monier JM, Lindow SE. Differential survival of solitary and aggregated bacterial cells promotes aggregate formation on leaf surfaces. Proc Natl Acad Sci USA. 2003;100:15977–15982. doi: 10.1073/pnas.2436560100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumenyo CK, Mukherjee A, Chun W, Chatterjee AK. Genetic and physiological evidence for the production of N-acyl homoserine lactones by Pseudomonas syringae pv. syringae and other fluorescent plant pathogenic Pseudomonas species. Eur J Plant Pathol. 1998;104:569–582. [Google Scholar]
- 17.Quinones B, Pujol CJ, Lindow SE. Regulation of AHL production and its contribution to epiphytic fitness in Pseudomonas syringae. Mol Plant Microbe Interact. 2004;17:521–531. doi: 10.1094/MPMI.2004.17.5.521. [DOI] [PubMed] [Google Scholar]
- 18.Kitten T, Kinscherf TG, McEvoy JL, Willis DK. A newly identified regulator is required for virulence and toxin production in Pseudomonas syringae. Mol Microbiol. 1998;28:917–929. doi: 10.1046/j.1365-2958.1998.00842.x. [DOI] [PubMed] [Google Scholar]
- 19.Quinones B, Dulla G, Lindow SE. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol Plant Microbe Interact. 2005;18:682–693. doi: 10.1094/MPMI-18-0682. [DOI] [PubMed] [Google Scholar]
- 20.Campbell RE, et al. A monomeric red fluorescent protein. Proc Natl Acad Sci USA. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leveau JHJ, Lindow SE. Appetite of an epiphyte: Quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc Natl Acad Sci USA. 2001;98:3446–3453. doi: 10.1073/pnas.061629598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchholz A, Baur P, Schonherr J. Differences among plane species in cuticular permeabilities and solute mobilities are not caused by differential size selectivities. Planta. 1998;206:322–328. [Google Scholar]
- 23.Schreiber L. Polar paths of diffusion across plant cuticles: New evidence for an old hypothesis. Ann Bot (London) 2005;95:1069–1073. doi: 10.1093/aob/mci122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teplitski M, Robinson JB, Bauer WD. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol Plant Microbe Interact. 2000;13:637–648. doi: 10.1094/MPMI.2000.13.6.637. [DOI] [PubMed] [Google Scholar]
- 25.Gao M, Teplitski M, Robinson JB, Bauer WD. Production of substances by Medicago truncatula that affect bacterial quorum sensing. Mol Plant Microbe Interact. 2003;16:827–834. doi: 10.1094/MPMI.2003.16.9.827. [DOI] [PubMed] [Google Scholar]
- 26.Karamanoli K, Lindow SE. Disruption of N-acyl homoserine lactone-mediated cell signaling and iron acquisition in epiphytic bacteria by leaf surface compounds. Appl Environ Microbiol. 2006;72:7678–7686. doi: 10.1128/AEM.01260-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Volkenburgh E, Cleland RE. Proton excretion and cell expansion in bean leaves. Planta. 1980;148:273–278. doi: 10.1007/BF00380038. [DOI] [PubMed] [Google Scholar]
- 28.Gantner S, et al. In situ quantitation of the spatial scale of calling distances and population density-independent N-acylhomoserine lactone-mediated communication by rhizobacteria colonized on plant roots. FEMS Microbiol Ecol. 2006;56:188–194. doi: 10.1111/j.1574-6941.2005.00037.x. [DOI] [PubMed] [Google Scholar]
- 29.Steidle A, et al. Visualization of N-acylhomoserine lactone-mediated cell–cell communication between bacteria colonizing the tomato rhizosphere. Appl Environ Microbiol. 2001;67:5761–5770. doi: 10.1128/AEM.67.12.5761-5770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindow SE, Brandl MT. Microbiology of the phyllosphere. Appl Environ Microbiol. 2003;69:1875–1883. doi: 10.1128/AEM.69.4.1875-1883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loh J, Pierson EA, Pierson LS, III, Stacey G, Chatterjee A. Quorum sensing in plant-associated bacteria. Curr Opin Plant Biol. 2002;5:285–290. doi: 10.1016/s1369-5266(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 32.Haefele DM, Lindow SE. Flagellar motility confers epiphytic fitness advantages upon Pseudomonas syringae. Appl Environ Microbiol. 1987;53:2528–2533. doi: 10.1128/aem.53.10.2528-2533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirano SS, Baker LS, Upper CD. Raindrop momentum triggers growth of leaf-associated populations of Pseudomonas syringae on field-grown snap bean plants. Appl Environ Microbiol. 1996;62:2560–2566. doi: 10.1128/aem.62.7.2560-2566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butterworth J, Mccartney HA. The dispersal of bacteria from leaf surfaces by water splash. J Appl Bacteriol. 1991;71:484–496. [Google Scholar]
- 35.Kaplan HB, Greenberg EP. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985;163:1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egland PG, Palmer RJ, Kolenbrander PE. Interspecies communication in Streptococcus gordonii-Veillonella atypica biofilms: Signaling in flow conditions requires juxtaposition. Proc Natl Acad Sci USA. 2004;101:16917–16922. doi: 10.1073/pnas.0407457101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolenbrander PE, et al. Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao YF, et al. Characterization of the Staphylococcus epidermidis accessory-gene regulator response: Quorum-sensing regulation of resistance to human innate host defense. J Infect Dis. 2006;193:941–948. doi: 10.1086/500246. [DOI] [PubMed] [Google Scholar]
- 39.Boettcher KJ, Ruby EG. Detection and quantification of Vibrio fischeri autoinducer from symbiotic squid light organs. J Bacteriol. 1995;177:1053–1058. doi: 10.1128/jb.177.4.1053-1058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H, et al. Detection of N-acylhomoserine lactones in lung tissues of mice infected with Pseudomonas aeruginosa. Microbiology. 2000;146:2481–2493. doi: 10.1099/00221287-146-10-2481. [DOI] [PubMed] [Google Scholar]
- 41.Charlton TS, et al. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: Application to a model bacterial biofilm. Environ Microbiol. 2000;2:530–541. doi: 10.1046/j.1462-2920.2000.00136.x. [DOI] [PubMed] [Google Scholar]
- 42.Loper JE, Lindow SE. Lack of evidence for in situ fluorescent pigment production by Pseudomonas-syringae Pv syringae on bean leaf surfaces. Phytopathology. 1987;77:1449–1454. [Google Scholar]
- 43.King EO, Ward MK, Rainey DE. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:310–317. [PubMed] [Google Scholar]
- 44.Joyner DC, Lindow SE. Heterogeneity of iron bioavailability on plants assessed with a whole-cell GFP-based bacterial biosensor. Microbiology. 2000;146:2435–2445. doi: 10.1099/00221287-146-10-2435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.