Abstract
Resistance to Toxoplasma gondii depends on dendritic cells to recognize this pathogen and secrete IL-12, in turn promoting IFN-γ production from responding T cells. The adaptor protein, myeloid differentiation primary-response gene 88 (MyD88), is important for most Toll-like receptor (TLR) signaling, as well as IL-1R/IL-18R signals. There is considerable evidence that MyD88 is required for the innate sensing of T. gondii and IL-12 responses. Although Myd88−/− mice challenged with T. gondii have defective IL-12 and Th1 effector responses and succumb to disease, administration of IL-12 to Myd88−/− mice partially restores the Th1 response and yet fails to prolong survival. This finding suggested that MyD88 may mediate signals within T cells important for resistance to this pathogen. To evaluate the role of MyD88 in T cells under noncompetitive conditions, bone marrow chimeras were generated, in which the T cells lacked MyD88, but MyD88-dependent innate immune responses were intact. Upon challenge with T. gondii, these chimeric mice were more susceptible to disease, developing severe toxoplasmic encephalitis and succumbing within 30 days. Splenocytes and brain mononuclear cells isolated from infected chimeric mice produced less IFN-γ when cultured with a T. gondii-derived antigen. The increase in susceptibility observed was independent of signals via the IL-1R and IL-18R, suggesting a role for TLRs in MyD88-mediated T cell responses to T. gondii. These observations show that, in addition to a role for MyD88 in innate responses, T cell expression of MyD88 is necessary for prolonged resistance to a pathogen.
Keywords: adaptive immunity, intracellular parasite, protozoan parasite, IL-1 receptor family, Toll-like receptor
Myeloid differentiation primary-response gene 88 (MyD88) is an adaptor protein necessary for signal transduction of the IL-1R/IL-18R family and most Toll-like receptors (TLRs) (1, 2). Myd88−/− mice have defective proinflammatory cytokine responses, in particular production of the Th1 differentiation factor IL-12, and have increased susceptibility when challenged with a variety of bacterial and eukaryotic pathogens, including Listera monocytogenes (3, 4), Mycobacterium tuberculosis (5), Leishmania major (6), and Toxoplasma gondii (7). Consistent with its role in IL-12 responses, Myd88−/− mice repeatedly immunized with a T. gondii-derived antigen (8) are unable to establish a Th1 response, and thus a Th2 response predominates. For these reasons, it has been postulated that Myd88−/− mice have defects in innate pathogen recognition affecting IL-12 responses, which results in nonprotective adaptive immunity and increased susceptibility to intracellular pathogens (6).
During toxoplasmosis, the generation of Th1 effector responses has been shown to be strictly MyD88-dependent (7, 9). MyD88-dependent TLR11 participates in T. gondii resistance by recognition of a pathogen-derived profilin, but, unlike Myd88−/− mice, Tlr11−/− mice survive acute toxoplasmosis (9). Furthermore, TLR1, TLR2, TLR4, TLR6, TLR9, IL-1, and IL-18, all of which require MyD88 for signaling, are individually not required for control of this infection (7, 10–13). Thus, it remains unclear what pattern-recognition or cytokine receptors require MyD88 either singly or collectively for pathogen control.
Although much evidence implicates MyD88 as a key signaling component of innate responses, this molecule also is expressed in T cells. In Myd88−/− mice, a primary Th1 response to ovalbumin can be induced after regulatory T cell depletion. Nevertheless, primary and secondary immunization fail to establish T cell memory (14). Pasare and Medzhitov (14) propose that this lack of Th1 memory is due to defects in T cell differentiation or survival in the absence of MyD88-dependent signals. However, it is unclear whether deficient Th1 memory formation is due to the absence of an uncharacterized MyD88-dependent extrinsic signal from antigen-presenting cells or whether, instead, MyD88 provides T cell-intrinsic signals required for optimal function. Relevant to the latter possibility, we recently identified an MyD88-dependent PI3K-signaling pathway in CD4+ T cells that enhances survival and a T cell-dependent antibody response (15).
In this study, we demonstrate that T cell expression of MyD88 is necessary for resistance to T. gondii. Bone marrow chimeras were generated to specifically evaluate the role of MyD88 in T cells under noncompetitive conditions during an in vivo adaptive response. In reconstituted mice, T cells lacked MyD88, but MyD88-dependent innate responses were intact. Upon challenge with T. gondii, these chimeric mice were found to be more susceptible to infection, which was associated with severe toxoplasmic encephalitis and reduced antigen-specific IFN-γ production independent of IL-1R and IL-18R signaling. Our observations indicate a requirement for MyD88 in T cell-mediated resistance to a pathogen even in the setting of an intact innate response.
Results
A Model in Which T Cells Are MyD88-Deficient, but MyD88-Dependent Innate Responses Are Intact.
The acute susceptibility of Myd88−/− mice to T. gondii is associated with defective innate pathogen recognition and diminished IL-12 responses (7, 9). One prediction of this model is that exogenous IL-12 would improve the survival of Myd88−/− mice challenged with T. gondii by enhancing IFN-γ production from responding T cells, enabling a protective Th1 response. Nonetheless, a regimen of IL-12 previously shown to be adequate to control T. gondii infection in Il12−/− mice (16) was not protective in Myd88−/− mice [supporting information (SI) Fig. 6]. Despite exogenous IL-12 enhancing a Th1 response in Myd88−/− mice, and reciprocally suppressing a Th2 response, these effects were nonprotective during T. gondii infection. These data strongly suggest that the abnormal response of Myd88−/− mice to T. gondii is not merely due to deficient induction of IL-12.
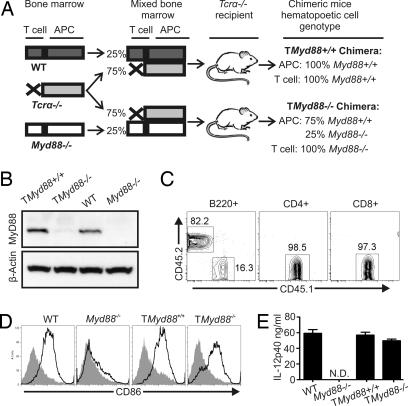
In light of our previous studies showing altered responses of MyD88-deficient T cells (15), we considered whether the remarkable susceptibility of Myd88−/− mice to T. gondii might reflect a T cell-intrinsic requirement for MyD88 for control of this pathogen. To specifically evaluate the role of MyD88 in T cells under noncompetitive conditions, bone marrow chimeras were generated whose T cells lacked MyD88, but that had intact MyD88-dependent innate immune responses (TMyd88−/− chimeric mice). As illustrated in Fig. 1A, marrow cells from mice deficient in the α/β T cell receptor (Tcrα−/−) and Myd88−/− mice were mixed in a 3:1 ratio, respectively, and used to reconstitute irradiated Tcrα−/− recipients. Tcrα−/− mice were used as recipients to ensure that the entire α/β T cell compartment was derived from the bone marrow graft. Thus, the T cell compartment in reconstituted TMyd88−/− chimeric mice is solely derived from Myd88−/− precursors, whereas the non-T cell hematopoietic compartment is predominantly Myd88+/+ (at least 75%). To control for conditioning manipulations, sham chimeric mice were generated in an analogous manner by using a marrow mixture from Tcrα−/− mice and Myd88+/+ littermates (TMyd88+/+ control mice).
Fig. 1.
A mouse model in which T cells are MyD88-deficient, but MyD88-dependent innate responses are intact. (A) Scheme representing the hematopoietic reconstitution strategy. (B) Twelve weeks after reconstitution, T cells were isolated from mice (>90% purity), and an immunoblot was performed to assess MyD88 expression. (C) Chimeric mice were generated as illustrated in A, except that the Myd88−/− mice were CD45.1+, whereas the other marrow donors and the recipients were CD45.2+. After 12 weeks, flow cytometry was performed on PBMCs; data represent two individually reconstituted cohorts. (D) Splenocytes were cultured for 24 h in the presence or absence of CpG DNA and then analyzed by flow cytometry for B cell expression of CD86. Open histogram, CpG DNA stimulated; shaded histogram, unstimulated. These data are representative of three experiments. (E) Mice from each cohort were injected with STAg, and serum was obtained after 3 h for ELISA; these data are representative of three experiments; N.D., none detected. For all experiments, unless otherwise indicated, data represent at least three experiments.
Twelve weeks after transplant, T cells were purified from TMyd88−/− chimeric mice, and the absence of MyD88 protein was confirmed by immunoblot (Fig. 1B). In addition, TMyd88−/− chimeric mice reconstituted with congenic bone marrow precursors similarly showed all T cells to be derived from CD45.1xMyd88−/− cells, whereas B cells demonstrated CD45 isoform chimerism in the predicted proportion (Fig. 1C). Flow cytometry of the reconstituted hematopoietic cells found a similar distribution of CD4+ and CD8+ T cells and B cells in the spleen and lymph nodes of TMyd88−/− chimeric mice compared with wild-type mice (data not shown).
To evaluate innate responses in TMyd88−/− chimeric mice, we first used CpG DNA, which activates APCs via TLR9 in an MyD88-dependent manner. In contrast to B cells from Myd88−/− mice, the majority of B cells from TMyd88−/− chimeric mice were CpG-responsive as measured by CD86 up-regulation (Fig. 1D). The innate response in TMyd88−/− chimeric mice was next evaluated by challenge with a T. gondii-derived antigen extract (STAg). The response to STAg is MyD88-dependent and results in the production of IL-12 (9). As expected, Myd88−/− mice were unresponsive to i.p. injection of STAg, but TMyd88−/− chimeric mice responded robustly as measured by serum IL-12p40 (Fig. 1E). As an additional test of the innate immune compartment, we injected mice with LPS, which induces TLR4/MyD88-dependent production of proinflammatory cytokines. Unlike Myd88−/− mice, TMyd88−/− chimeric mice given a lethal dose of LPS succumbed within 72 h, as did MyD88-sufficient controls (SI Fig. 7).
Taken together, these data show TMyd88−/− chimeric mice and their reconstituted hematopoietic APCs have intact innate responses to pathogen-associated molecules. In this chimeric model, under noncompetitive conditions, T cell responses are not limited by detectable deficits in innate recognition, thus providing an opportunity to specifically evaluate the role of MyD88 in T cells during an adaptive response.
T Cell Expression of MyD88 Is Required for Resistance to T. gondii.
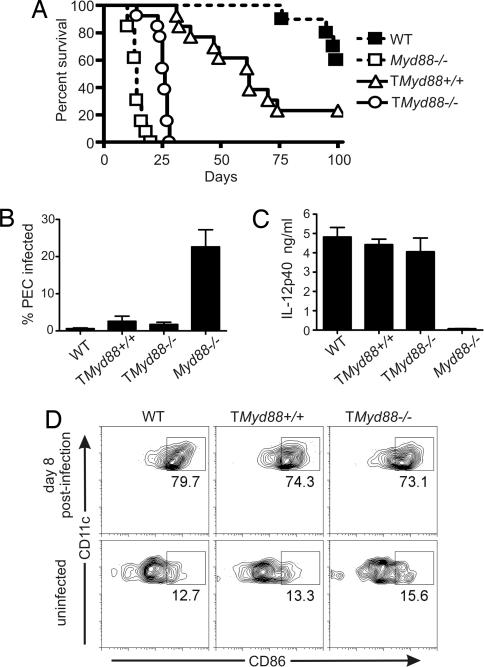
Using TMyd88−/− chimeric mice, we next determined whether T cell expression of MyD88 is necessary for resistance to T. gondii. Strikingly, all TMyd88−/− chimeric mice succumbed to infection by day 30 and had significantly increased mortality compared with TMyd88+/+ control mice (P < 0.0001) (Fig. 2A). TMyd88+/+ control mice survived >30 days after infection, with a median survival of 62 days. However, as observed previously (17), they exhibited somewhat reduced resistance compared with wild-type mice (Fig. 2A).
Fig. 2.
T cell expression of MyD88 is required for resistance to the pathogen T. gondii independent of the innate response. (A) Mice were inoculated i.p. with T. gondii and monitored for survival. Data are pooled from two experiments (n = 13 each cohort). (B) PECs isolated from mice on day 7 after infection was used to prepare cytospin smears. Data are the mean of three pooled experiments (n = 6). (C) Sera obtained from mice on day 5 of infection were evaluated by ELISA. Data are representative of three experiments. (D) Cells were isolated from draining lymph nodes and spleen on day 8 of infection and analyzed by flow cytometry. Data are representative of two experiments (n = 6 total for each cohort).
Examination of peritoneal exudative cells (PECs) in TMyd88−/− chimeric mice on day 7 of infection found only rare parasite-infected cells similar to MyD88-sufficient mice (Fig. 2B), indicating acute control of the pathogen at the inoculation site. This finding was in contrast to PECs from unmanipulated Myd88−/− mice, which had much larger numbers of parasitized cells. Furthermore, measurement of IL-12p40 in sera 5 days after infection revealed a comparable proinflammatory response in TMyd88−/− chimeric mice and MyD88-sufficient controls (Fig. 2C). This finding was in contrast to sera from unmanipulated Myd88−/− mice, which had minimal IL-12p40 responses after infection. Flow-cytometry analysis of lymphoid tissue (mesenteric, para-aortic, and spleen) from TMyd88−/− chimeric mice and MyD88-sufficient controls revealed a similar percentage of mature dendritic cells as measured by CD86 expression (Fig. 2D). These data indicate that, despite their increased susceptibility to disease, TMyd88−/− chimeric mice mount an innate response to T. gondii comparable to MyD88-sufficient controls.
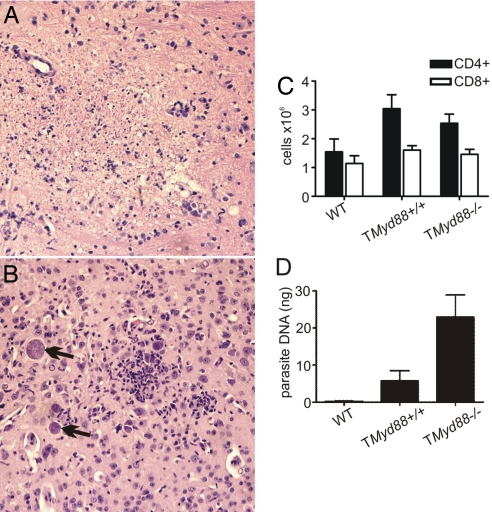
In infected mice, toxoplasmic encephalitis is the major cause of morbidity and mortality (18). Concordant with their increased susceptibility, the brains of TMyd88−/− chimeric mice killed near median survival (days 25–27 after infection) demonstrated parenchymal regions of necrosis (Fig. 3A) associated with free tachyzoites (seen at higher power) (data not shown). In comparison, TMyd88+/+ control mice killed near median survival (days 60–62 after infection) demonstrated more focal cellular infiltrates associated with bradyzoite tissue cysts (Fig. 3B). Analysis of brain-derived mononuclear cells (BMNCs) on day 23 of infection revealed similar T cell numbers within the brains of TMyd88−/− chimeric mice and controls (Fig. 3C). This finding indicated that the increased susceptibility of TMyd88−/− chimeric mice to toxoplasmic encephalitis was not due to quantitative differences in the T cell infiltrate. Furthermore, consistent with the histopathological findings, TMyd88−/− chimeric mice brains had an increased parasite burden compared with controls (Fig. 3D). Collectively, these data indicate that TMyd88−/− chimeric mice have increased susceptibility to disease and an increased parasite burden. Given their intact innate immune responses, these findings suggest an impaired adaptive response during T. gondii infection.
Fig. 3.
TMyd88−/− chimeric mice have increased susceptibility to toxoplasmic encephalitis. (A) Brain histopathology of TMyD88−/− chimeric mice is shown near median survival days 25–27 after infection. Parenchymal regions of necrotic encephalitis are evident in the center of the pictogram. (B) Brain histopathology of TMyD88+/+ control mice is shown near median survival days 60–62 after infection. Focus of cellular infiltrate is in the center of the pictogram; arrows indicate bradyzoite tissue cysts. The data are representative of mice in two experiments (n = 6 total). (Magnification: ×100.) (C) BMNCs were prepared from mice on day 23 of infection, counted, and analyzed by flow cytometry to enumerate T cells. Data are representative of three experiments. (D) Quantitative real-time PCR of parasite DNA isolated from the brains of mice on day 23 of infection. Pooled data are from three experiments, with at least two mice in each group per experiment.
TMyd88−/− Chimeric Mice Have Fewer IFN-γ-Producing Responder T Cells.
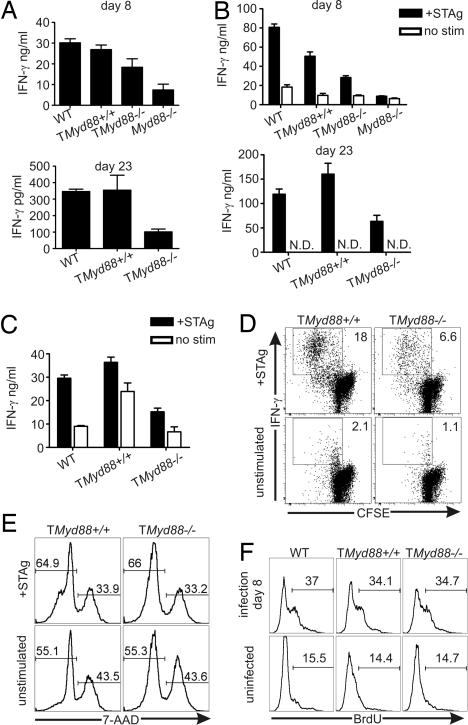
The production of IFN-γ by effector T cells is indispensable for acquired resistance to toxoplasmosis (19, 20). Consistent with reduced survival, sera obtained from TMyd88−/− chimeric mice on days 8 and 23 of infection had less IFN-γ than MyD88-sufficient controls (Fig. 4A). To further evaluate the antigen-specific T cell response in this model, splenocytes were isolated from mice on days 8 and 23 of infection and cultured with STAg. Cell cultures from TMyd88−/− chimeric mice produced less IFN-γ compared with those from MyD88-sufficient controls (Fig. 4B). Because TMyd88−/− chimeric mice infected with T. gondii developed advanced brain pathology (Fig. 3A), we examined the T cell response within this organ. Similar to antigen-specific responses of spleen-derived cells, cultures of BMNCs from TMyd88−/− chimeric mice produced less IFN-γ in the presence of STAg (Fig. 4C).
Fig. 4.
TMyd88−/− chimeric mice have less IFN-γ-producing responder T cells. (A) Sera obtained from mice on day 8 (Upper) and day 23 (Lower) of T. gondii infection were analyzed by ELISA. (B) Splenocytes were isolated from mice on day 8 (Upper) and day 23 (Lower) of infection and cultured in the presence or absence of STAg. After 72 h, supernatants were analyzed by ELISA. (C) BMNCs prepared from mice on day 23 of infection were cultured in the presence or absence of STAg, and after 72 h cell-free supernatants were assessed by ELISA. (D) Splenocytes were isolated from mice on day 23 of infection, cultured in the presence or absence of STAg for 48 h, and then processed for intracellular IFN-γ. (E) Splenocytes were isolated from mice on day 23 after infection, cultured in the presence or absence of STAg for 72 h, and then analyzed by flow cytometry. Gate is on CD4+ T cells. (F) Mice were pulsed with BrdU on days 5 and 6 of T. gondii infection, and splenocytes were analyzed by flow cytometry on day 8. Gate is on CD4+ T cells. Data represent two experiments (n = 6 mice total). N.D., none detected. For all experiments, unless otherwise indicated, data are representative of three experiments.
To confirm these observations, splenocytes isolated from mice on day 23 of infection were CFSE-labeled and cultured with STAg. Antigen-specific CD4+ T cell proliferation and effector response were evaluated by CFSE dilution and intracellular IFN-γ staining, respectively. As shown in Fig. 4D, TMyd88−/− chimeric mice had less IFN-γ+ responding CD4+ T cells compared with controls. We considered the possibility that Myd88−/− T cells primed in TMyd88−/− chimeric mice during the immune response to T. gondii may have an increased propensity for cell death in culture. Nonetheless, as measured by flow cytometry using exclusion of the vital stain 7-amino-actinomycin D (7-AAD), the survival of Myd88−/− CD4+ T cells from infected TMyd88−/− chimeric mice was comparable to controls (Fig. 4E). Thus, diminished IFN-γ in these cultures and the observation of reduced responder frequency are unlikely to be caused by increased T cell death ex vivo. Thus, in both lymphoid and brain tissue, TMyd88−/− chimeric mice infected with T. gondii generate fewer antigen-specific IFN-γ-producing T cells.
To evaluate whether reduced IFN-γ production in infected TMyd88−/− chimeric mice was associated with poor T cell responses in vivo, animals were pulsed with BrdU on days 5 and 6 of infection and killed on day 8. These experiments revealed comparable levels of T cell proliferation in TMyd88−/− chimeric mice and controls (Fig. 4F). In addition, there were similar numbers of T cells in spleens on day 7 of infection (TMyd88−/−: CD4+ 9.5 ± 1.6 × 106, CD8+ 5.6 ± 1.3 × 106; TMyd88+/+: CD4+ 8.8 ± 1.5 × 106, CD8+ 5.3 ± 0.9 × 106; data pooled from three experiments of a least three mice each). These data suggest that MyD88 deficiency limited to the T cell compartment alters the relative levels of IFN-γ produced in response to T. gondii without affecting the expansion or survival of responding T cells, in turn affecting the ability to maintain prolonged resistance.
Deficient IL-1R/IL-18R Signaling in T Cells Is Not the Cause of Increased Susceptibility of TMyd88−/− Chimeric Mice.
MyD88 is required for signaling via multiple T cell-expressed TLRs (15, 21–30), which may play a role in the immune response to T. gondii. MyD88 also is used for downstream signaling via IL-1R and IL-18 R, raising the possibility that TMyd88−/− chimeric mice have increased susceptibility due to defects in these pathways. However, other groups have reported that IL-18-deficient mice (11) and mice lacking caspase-1, which is responsible for converting precursors of IL-1β and IL-18 into an active form, have unimpaired resistance to T. gondii (12). To evaluate whether our results reflected impairment in a pathway initiated by IL-18-independent engagement of the IL-18R (31) or IL-1-independent engagement of the IL-1R, we challenged IL-18R-deficient and IL-1R-deficient mice with T. gondii. Consistent with previous reports, neither of these transgenic strains had increased susceptibility to T. gondii (data not shown). Thus, in TMyd88−/− chimeric mice, the absence of signals mediated by IL-1R or IL-18R within T cells is unlikely to be the cause of reduced resistance.
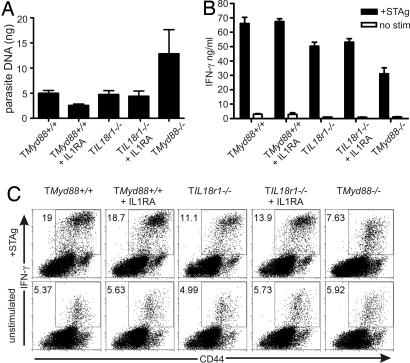
Considering the potential for functional redundancy within the IL-1R/IL-18R receptor family, it remained possible that the increased susceptibility in TMyd88−/− chimeric mice was due to disruption of both IL-1R and IL-18R signals, perhaps superimposed on the experimental conditions of bone marrow reconstitution. Unfortunately, mice with a targeted mutation in both the IL-1R and IL-18R genes are unavailable, and the close proximity of these genes within the IL-1R/IL-18R cluster on chromosome 1 make crossing the Il1r1−/− and IL18r1−/− strains impractical. Therefore, in a manner analogous to that used in previous experiments (Fig. 1A), chimeric mice were generated by using a marrow mixture from Tcrα−/− mice and Il18r1−/− mice (TIl18r1−/− chimeric mice). TIl18r1−/− chimeric mice and controls were infected with T. gondii and were administered recombinant IL-1 receptor antagonist (IL-1Ra) daily. The dose of IL-1Ra given (500 mg/kg) was sufficient to rescue 80% of wild-type mice from a highly lethal challenge with 40 mg/kg LPS (SI Fig. 8). Because prolonged administration of IL-1Ra was impractical, we used parasite burden and IFN-γ production as surrogate measures in place of survival for the response to T. gondii, and we examined these parameters on day 23, the time just preceding mortality in TMyd88−/− chimeric mice. On day 23 of infection, the brains of TIl18r1−/− chimeric mice given IL-1Ra had markedly less parasite DNA compared with TMyd88−/− chimeric mice, with levels comparable to wild-type reconstitution controls (Fig. 5A). Splenocytes isolated from mice on day 23 of infection and cultured with STAg revealed that IFN-γ production was only in part dependent on IL-18R and was independent of IL-1R (Fig. 5 B and C). These data further support that the T cell-intrinsic role of MyD88 during the response to T. gondii is independent of IL-1R and IL-18R signaling.
Fig. 5.
Chimeric mice with simultaneous disruption of both IL-1R- and IL-18R-mediated signals are less susceptible to T. gondii than TMyd88−/− chimeric mice. (A) Chimeric mice and controls were infected with T. gondii, and the indicated groups were given 500 mg/kg IL-1Ra daily. Mice were killed on day 23 of infection, and quantitative real-time PCR was performed on DNA isolated from brains to assess parasite burden. Data are from one experiment with seven to eight mice per group. (B) Splenocytes were isolated from mice on day 23 of infection and cultured in the presence or absence of STAg. After 72 h, supernatants were analyzed by ELISA. (C) Splenocytes were isolated from mice on day 23 of infection, cultured in the presence or absence of STAg for 48 h, and then processed for intracellular IFN-γ. Gate is on CD4+ T cells.
Discussion
The superfamily of transmembrane receptors defined by the intracellular Toll/IL-1 receptor (TIR) domain plays a major role in the early immune response. This superfamily can be divided into two groups based on the extracellular domain of the receptors: those having an Ig-like domain, like the IL-1R/IL-18R family; and those having a leucine-rich repeat motif, such as TLRs (32). The IL-1R/IL-18R family includes the IL-1 receptor type I (IL-1RI), IL-1R accessory protein, the decoy receptor IL-1R type II, the IL-18 receptors (IL-18Rα and IL-18Rβ), the putative regulatory receptor T1/ST2, and other poorly characterized receptors, including IL-1R-related protein 2 (IL-1Rrp2). The IL-1R/IL-18R family and all TLRs, except TLR3, signal through a pathway that uses the adaptor protein, MyD88. MyD88 is one of five TIR domain-containing adaptors; as such, it links these receptors to other downstream signaling molecules, like the IL-1R-associated kinase family (2). However, MyD88 also interacts with proteins that lack TIR domains, such as PI3K (15, 33), IFN-γ receptor 1 (34), IFN regulatory factor (IRF) 1 (35), IRF5 (36), and IRF7 (37).
Because activated CD4+ T cells contain a MyD88-dependent signaling pathway that affects T cell function and survival (15), we considered whether MyD88 directly mediates signals in T cells important for resistance to T. gondii, a pathogen to which resistance is highly dependent on MyD88 and cell-mediated immunity (7, 9, 10). To evaluate the role of MyD88 in T cells under noncompetitive conditions, we generated bone marrow chimeras in which T cells lacked MyD88 but MyD88-dependent innate immune responses were shown to be intact. Upon challenge with T. gondii, these chimeric mice were more susceptible to disease, establishing that T cell expression of MyD88 is necessary for prolonged resistance to this pathogen.
Necropsy of TMyd88−/− chimeric mice moribund with T. gondii infection revealed brain histopathology consistent with poor parasite control. This finding appeared similar to that described in infected mice after T cell depletion or treatment with monoclonal antibody to IFN-γ (38, 39). Consistent with these findings, we found that TMyd88−/− chimeric mice had less IFN-γ-producing CD4+ T cells during the immune response to T. gondii. Given that IL-1 is a ubiquitously expressed proinflammatory cytokine that costimulates T cells (40) and IL-18 is a major inducer of IFN-γ production, we considered the possibility that the simultaneous disruption of both of these pathways in TMyd88−/− chimeric mice was the cause of increase susceptibility and decreased IFN-γ production. By generating TIl18r1−/− chimeric mice and treating with high doses of IL-1Ra during infection, we evaluated this possibility under the experimental conditions of bone marrow reconstitution. It was not surprising that chimeras with T cells lacking IL-18Rα produced less antigen-specific IFN-γ ex vivo; however, infected TIl18r1−/− chimeric mice treated with IL-1Ra demonstrated a greater degree of resistance to infection (as assessed by parasite burden) than TMyd88−/− chimeric mice. Thus, our findings support the role of a MyD88-dependent signaling pathway in T cells that affects function, separate from IL-1R/IL-18R-dependent effects. Although the receptors T1/ST2 and IL-1Rrp2 are thought to be MyD88-dependent, these receptors do not bind IL-1 or IL-18 (32, 41), and their role in T. gondii infection is uncharacterized.
The importance of MyD88 for TLR signaling within cells of the innate immune system and B cells is well established. The triggering of TLRs on antigen-presenting cells allows for infectious non-self-discrimination and initiates events important to adaptive immunity, such as costimulatory molecule expression and enhanced antigen presentation (42). In addition to TLR expression in innate immune cells, several reports demonstrate TLR expression in murine and human T cells (21–25, 27, 29, 30, 43, 44), and TLR agonists directly costimulate T cell activation and modulate effector function (15, 21–30). Whether TLR signaling within T cells affects the in vivo adaptive response to infection has not been evaluated. Our results provide in vivo evidence in support of a T cell-intrinsic MyD88-dependent pathway that directly modulates effector function. At present, it cannot be determined from our experiments whether this requirement for MyD88 in T cells is to enable TLR signaling or another known function of the molecule, such as MyD88-dependent posttranscriptional regulation (34), or perform a function that has not yet been described. Nonetheless, our data indicate a role for MyD88 in T cell-mediated resistance to a pathogen independent of the well established role for MyD88 in linking innate and adaptive immunity by APC-mediated pathogen recognition. Together these findings imply that the increased susceptibility of Myd88−/− mice upon challenge with certain pathogens may in part be due to abnormal cell-mediated immunity caused by the lack of MyD88-dependent signaling in T cells, a possibility that has largely been underappreciated.
Materials and Methods
Mice.
Myd88−/− mice were provided by S. Akira (Osaka University, Osaka, Japan), and other strains were purchased from The Jackson Laboratory. All mice were on the B6 background. Animals were housed within the University of Pennsylvania University Laboratory Animal Resources facility and treated according to approved protocols.
Experimental Infection.
An i.p. injection of 20 cysts of T. gondii ME49 strain, isolated from brains of infected CBA/CaJ mice, was used for infection. In some experiments, infected Myd88−/− mice were given 250 ng recombinant mouse IL-12 (R&D Systems) or PBS i.p. 3 h postinfection and then daily (16). PECs were obtained from day 7-infected mice and used to prepare cytospins, which were stained for histological analysis. The mean percentage of cells infected with T. gondii was determined by counting 500 cells per smear. For measurement of parasite burden, the 35-fold repetitive T. gondii B1 gene was amplified by real-time PCR as described (45).
Generation of Mixed Bone Marrow Chimeric Mice.
Marrow from femur and tibia was obtained by flushing with a needle. Erythrocytes were lysed from the marrow suspension, and T-cells were depleted (anti-CD90 MicroBeads; Miltenyi Biotec). Prepared marrow cells from Tcrα−/− and Myd88−/− mice were combined in a 3:1 ratio, respectively. This mixture was used to generate TMyd88−/− chimeric mice. Marrow cells from Tcrα−/− mice and Myd88+/+ littermates were combined in the same way to generate TMyd88+/+ control mice. Recipient mice were irradiated with 1,000 rads, followed 8–18 h later by i.v. injection of 2–3 × 106 marrow cells. In all experiments, mice were used after 12 weeks, at which time the hematopoietic compartment was determined reconstituted by flow cytometry.
Immunoblotting.
T cells were isolated (Pan-T cell isolation kit; Miltenyi Biotec) to >90% purity. Cell lysates were loaded at 2 × 106 cell equivalents per well and resolved on an SDS/10% PAGE gel. Proteins were probed with rabbit anti-mouse MyD88 (FL-296; Santa Cruz Biotechnologies) at a 1:500 dilution.
Analysis of Immune Responses.
STAg was prepared as described (46). Then, 20 μg was given to mice i.p., and serum was obtained after 3 h for IL-12p40 ELISA. In other studies, 1 mg LPS (Escherichia coli 055:B5; Sigma–Aldrich) was given to mice i.p., and 72-h survival was determined. To assess splenocyte responses, cells were cultured alone or with the addition of 1 μM CpG DNA. After 24 h, the cells were analyzed by FACS for B cell expression of CD86. To assess ex vivo responses, erythrocyte-depleted splenocytes were cultured for 72 h in the presence or absence of 25 μg/ml STAg. Cell-free supernatants were collected for cytokine measurement by ELISA after 72 h incubation. The 7-AAD staining solution (BD PharMingen) was used according to the manufacturer's instructions. For intracellular cytokine staining, splenocytes were cultured with or without 25 μg/ml STAg for 48 h; washed; resuspended in media containing 2 μM monensin (BD PharMingen), 5 ng/ml phorbol 12-myristate 13-acetate, and 500 ng/ml ionomycin; and cultured for an additional 5 h. Cells were then washed, stained with surface antibodies, and fixed and permeabilized by using the Cytofix/Cytoperm kit (BD PharMingen). BMNCs were prepared as described (47).
Statistical Analysis.
The Mantel–Haenzel log-rank test was used to determine the statistical significance of survival data. P < 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS.
This work was supported by National Institutes of Health Grants R01-AI-062789 (to L.A.T. and C.A.H.) and K08-AI-070153 (to D.F.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706663105/DC1.
References
- 1.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 3.Seki E, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 4.Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: No role for either in macrophage listericidal activity. J Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 5.Fremond CM, et al. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790–1799. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muraille E, et al. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J Immunol. 2003;170:4237–4241. doi: 10.4049/jimmunol.170.8.4237. [DOI] [PubMed] [Google Scholar]
- 7.Scanga CA, et al. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 8.Jankovic D, et al. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(-/-) setting. Immunity. 2002;16:429–439. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 9.Yarovinsky F, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 10.Debierre-Grockiego F, et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 11.Vossenkamper A, et al. Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. Eur J Immunol. 2004;34:3197–3207. doi: 10.1002/eji.200424993. [DOI] [PubMed] [Google Scholar]
- 12.Hitziger N, Dellacasa I, Albiger B, Barragan A. Dissemination of Toxoplasma gondii to immunoprivileged organs and role of Toll/interleukin-1 receptor signalling for host resistance assessed by in vivo bioluminescence imaging. Cell Microbiol. 2005;7:837–848. doi: 10.1111/j.1462-5822.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 13.Minns LA, et al. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J Immunol. 2006;176:7589–7597. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- 14.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Gelman AE, et al. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4(+) T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25:783–793. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yap G, Pesin M, Sher A. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol. 2000;165:628–631. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- 17.Yap GS, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med. 1999;189:1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichmann G, et al. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect Immun. 2000;68:1312–1318. doi: 10.1128/iai.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharton-Kersten TM, et al. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 20.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: The major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caron G, et al. Direct stimulation of human T cells via TLR5 and TLR7/8: Flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005;175:1551–1557. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 23.Cottalorda A, et al. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur J Immunol. 2006;36:1684–1693. doi: 10.1002/eji.200636181. [DOI] [PubMed] [Google Scholar]
- 24.Crellin NK, et al. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J Immunol. 2005;175:8051–8059. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- 25.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imanishi T, et al. Cutting edge: TLR2 directly triggers Th1 effector functions. J Immunol. 2007;178:6715–6719. doi: 10.4049/jimmunol.178.11.6715. [DOI] [PubMed] [Google Scholar]
- 27.Komai-Koma M, et al. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaRosa DF, et al. CpG DNA inhibits CD4+CD25+ Treg suppression through direct MyD88-dependent costimulation of effector CD4+ T cells. Immunol Lett. 2007;108:183–188. doi: 10.1016/j.imlet.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng G, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 30.Sutmuller RP, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutcher I, et al. Interleukin 18-independent engagement of interleukin 18 receptor-alpha is required for autoimmune inflammation. Nat Immunol. 2006;7:946–953. doi: 10.1038/ni1377. [DOI] [PubMed] [Google Scholar]
- 32.Boraschi D, Tagliabue A. The interleukin-1 receptor family. Vitam Horm. 2006;74:229–254. doi: 10.1016/S0083-6729(06)74009-2. [DOI] [PubMed] [Google Scholar]
- 33.Ojaniemi M, et al. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 34.Sun D, Ding A. MyD88-mediated stabilization of interferon-gamma-induced cytokine and chemokine mRNA. Nat Immunol. 2006;7:375–381. doi: 10.1038/ni1308. [DOI] [PubMed] [Google Scholar]
- 35.Negishi H, et al. Evidence for licensing of IFN-gamma-induced IFN regulatory factor 1 transcription factor by MyD88 in Toll-like receptor-dependent gene induction program. Proc Natl Acad Sci USA. 2006;103:15136–15141. doi: 10.1073/pnas.0607181103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaoka A, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 37.Honda K, et al. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc Natl Acad Sci USA. 2004;101:15416–15421. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gazzinelli RT, et al. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 39.Suzuki Y, Conley FK, Remington JS. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;143:2045–2050. [PubMed] [Google Scholar]
- 40.Joseph SB, Miner KT, Croft M. Augmentation of naive, Th1 and Th2 effector CD4 responses by IL-6, IL-1 and TNF. Eur J Immunol. 1998;28:277–289. doi: 10.1002/(SICI)1521-4141(199801)28:01<277::AID-IMMU277>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Towne JE, et al. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J Biol Chem. 2004;279:13677–13688. doi: 10.1074/jbc.M400117200. [DOI] [PubMed] [Google Scholar]
- 42.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 43.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 44.Mansson A, Adner M, Cardell LO. Toll-like receptors in cellular subsets of human tonsil T cells: Altered expression during recurrent tonsillitis. Respir Res. 2006;7:36–45. doi: 10.1186/1465-9921-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 46.Villegas EN, Lieberman LA, Carding SR, Hunter CA. Susceptibility of interleukin-2-deficient mice to Toxoplasma gondii is associated with a defect in the production of gamma interferon. Infect Immun. 2002;70:4757–4761. doi: 10.1128/IAI.70.9.4757-4761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol. 2005;165:63–74. doi: 10.1016/j.jneuroim.2005.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.