Abstract
It is widely accepted that the heavily glycosylated glycoprotein gp120 on the surface of HIV-1 shields peptide epitopes from recognition by the immune system and may promote infection in vivo by interaction with dendritic cells and transport to tissue rich in CD4+ T cells such as lymph nodes. A conserved cluster of oligomannose glycans on gp120 has been identified as the epitope recognized by the broadly HIV-1-neutralizing monoclonal antibody 2G12. Oligomannose glycans are also the ligands for DC-SIGN, a C-type lectin found on the surface of dendritic cells. Multivalency is fundamental for carbohydrate–protein interactions, and mimicking of the high glycan density on the virus surface has become essential for designing carbohydrate-based HIV vaccines and antiviral agents. We report an efficient synthesis of oligomannose dendrons, which display multivalent oligomannoses in high density, and characterize their interaction with 2G12 and DC-SIGN by a glycan microarray binding assay. The solution and the surface binding analysis of 2G12 to a prototype oligomannose dendron clearly demonstrated the efficacy of dendrimeric display. We further showed that these glycodendrons inhibit the binding of gp120 to 2G12 and recombinant dimeric DC-SIGN with IC50 in the nanomolar range. A second-generation Man9 dendron was identified as a potential immunogen for HIV vaccine development and as a potential antiviral agent.
Keywords: glycodendron, high mannose, multivalency, HIV vaccine, antiviral agent
HIV infection is a massive global health problem with more than 33 million infected worldwide (1). An interesting feature of HIV is its densely glycosylated surface; the glycans account for ≈50% mass of the virus coat protein gp120 (2). This carbohydrate face of gp120 aids in immune evasion (3) and has been implicated in the enhancement of viral dissemination (4). Although the viral glycans are assembled by the host, their dense arrangements are relatively unique and the glycan shield has become an attractive potential target for the design of anti-HIV-1 agents including vaccine-induced antibodies. However, all efforts directed toward antibody-based vaccine development so far have failed. Recently, a broadly type-1 HIV neutralizing antibody, 2G12, was confirmed to recognize multiple high-mannose glycans on gp120, suggesting that these glycans may be used for the design of an HIV vaccine component to elicit “2G12-like” antibodies (5–7). A combination of crystallographic, biochemical, and modeling studies has shown that two Man9(GlcNAc)2 (Fig. 1), at positions 332 and 392, predominantly contribute to the gp120–2G12 interaction, while an oligomannose at position 339 may also contribute to the interaction (8).
Fig. 1.
Structures of Man9(GlcNAc)2 and synthetic Man4 and Man9.
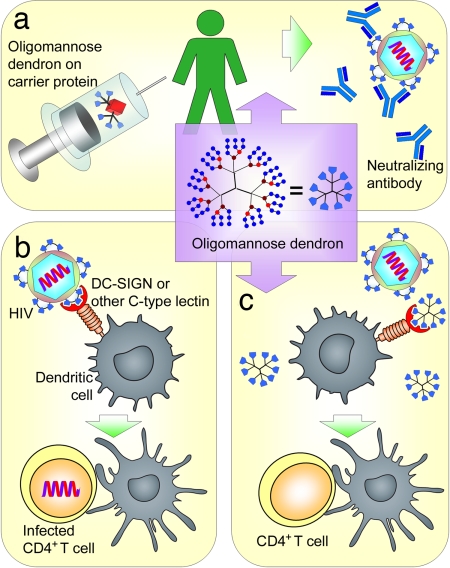
Another approach to anti-HIV activity is to block the interaction between HIV-1 and dendritic cells, which are associated with the enhanced infection of CD4+ T cells (Fig. 2b). It has been proposed that the mannose-binding C-type lectin DC-SIGN (dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin) on dendritic cells (9) interacts with the high-mannose glycans on HIV-1 and facilitates its dissemination (10), likely through the trans and cis mechanisms (11). This proposed mechanism is supported by studies that show that DC-SIGN binds the α1→3 and α1→6 mannotriose fragments (12, 13). Therefore, mimics of the multivalent N-linked high-mannose arrangement on gp120 have potential not only in HIV vaccine development (Fig. 2a), but also in the development of prophylactic antiviral agents that inhibit dendritic cell-mediated HIV-1 infection (Fig. 2c).
Fig. 2.
Two strategies to target HIV-1 by oligomannose dendrons. (a) These glycodendrons can be conjugated to carrier proteins and serve as vaccines. (b) HIV-1 can bind dendritic cell-surface DC-SIGN or other mannose-binding proteins to enhance CD4+ T cell infection. (c) Oligomannose dendrons can inhibit the binding of HIV-1 to dendritic cell-surface DC-SIGN or other mannose-binding proteins to prevent dendritic cell-enhanced CD4+ T cell infection.
Previous work in our laboratories has demonstrated that oligomannoses corresponding to the D3 and/or D1 arm of Man9(GlcNAc)2 can mimic the complete glycan in disrupting the gp120–2G12 interaction (14, 15). However, monomeric oligosaccharides bind to 2G12 weakly (Fig. 3a). Likewise, among various monomeric oligomannoses, the highly branched Man9(GlcNAc)2 is more potent in binding DC-SIGN (12); however, the affinity is low (13). Because the importance of multivalency in carbohydrate–protein interactions is well established (16, 17), our design of HIV vaccines or anti-HIV carbohydrates is based on multivalent presentation of Man9(GlcNAc)2 and related glycans (18–20).
Fig. 3.
Monomeric and multivalent oligomannose binding to 2G12 complex. (a) Monomeric Man4 in solution. (b) Pseudo-multivalent Man4 on surface. (c) Multivalent Man9 on dendrimeric scaffold. (d) Man9 dendron on surface. The figure is not drawn to scale.
We have demonstrated that the interaction of Man4 to 2G12 is greatly improved when Man4 is immobilized onto a glass slide (Fig. 3b) (21). However, this surface-generated pseudo-multivalency is not suitable for vaccine purposes. To achieve the multivalent display of oligomannose on a carrier protein, we turned our attention to branched scaffolds (Fig. 3c). Enhancement of carbohydrate–protein interactions by multivalency (22–25) has been reported using glycoclusters (26, 27), glycodendrimers/glycodendrons (28, 29), and glycopolymers (30). Among these architectural categories, glycodendrons were chosen for our study, because (i) a relatively large number of carbohydrates can be displayed with a high density, (ii) their valency and size can be easily adjusted, and (iii) they can be selectively functionalized for conjugation to other biomolecules. Over the past several years, many groups have found that glycodendrimers/glycodendrons exhibit strikingly enhanced affinity against target proteins (23, 31, 32). More recently, different synthetic approaches and applications of these macromolecules have been explored (33–36).
Results and Discussion
Syntheses of Oligomannose Dendrons.
We chose an AB3 type dendrimeric skeleton (37) because of its high loading number that can be achieved in a few generations. A versatile ligation reaction was also needed to conjugate sterically demanding oligomannose to the dendrons. We exploited the copper(I) catalyzed alkyne-azide 1,3-dipolar cycloaddition reaction (CuAAC) for this conjugation (38).
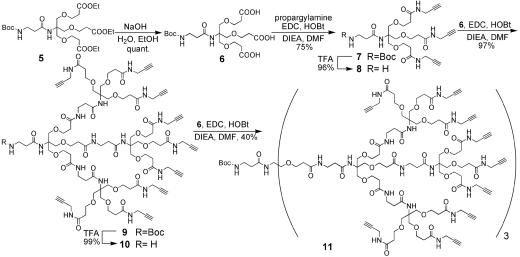
To facilitate homogeneity of the dendrimeric scaffold, we designed the synthesis by means of a convergent approach (39); terminal alkyne groups were installed on building block 6 (Scheme 1), providing 7 as the first-generation tris-alkyne. Installing the alkyne at an early stage not only avoided incomplete alkynyl installation for later generation dendrons, but also saved a final deprotection step at the end of the synthesis. Tris-alkyne 7 was deprotected in trifluoroacetic acid solution to give 8, which was condensed to give a second generation alkynyl dendron 9. Using similar deprotection and condensation conditions, the third-generation alkynyl dendron 11 was synthesized.
Scheme 1.
Syntheses of first-, second-, and third-generation alkynyl dendrimeric scaffolds. EDC, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; DIEA, diisopropylethylamine; DMF, dimethylformamide.
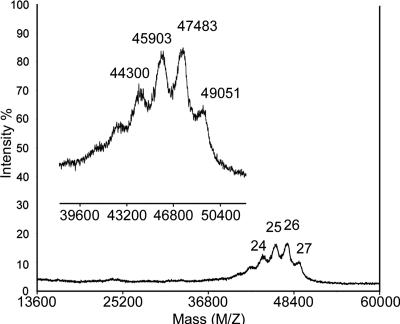
Next, we coupled azido-Man4 or Man9 (Fig. 1) to different generations of alkynyl dendrons (Scheme 2) via CuAAC (40). The reaction proceeded rapidly (<0.5 h) as monitored by matrix-assisted laser desorption/ionization–time-of-flight (MALDI-TOF) mass spectrometry, which also measured the conjugation copy number. We found that with a longer reaction time and extra CuI complex, the reaction did not reach completion in the most sterically congested cases (15, 17, and 18) [Fig. 4 and supporting information (SI) Figs. 8 and 9]. However, for compound 4, even for the third generation, a near-maximum occupancy was achieved. In comparison, using conventional amide coupling conjugation, only 8 of 32 sites were occupied by a hexasaccharide on a PAMAM dendrimer (36). The average numbers of attached oligomannose per dendron are summarized in Table 1.
Scheme 2.
Conjugation of oligomannose to alkynyl scaffolds by CuAAC reaction.
Fig. 4.
Representative MALDI-TOF mass spectrum for glycodendron 18.
Table 1.
Average copy number of oligomannose dendrons, determined by MALDI-TOF mass spectrometry
| Compound | High mannose type | Dendron generation | Expected copy number | Average copy number | Yield, % |
|---|---|---|---|---|---|
| 3 | Man4-N3 | 1 | 1 | — | |
| 13 | Man4 | 1 | 3 | 3 | 44 |
| 14 | Man4 | 2 | 9 | 9 | 77 |
| 15 | Man4 | 3 | 27 | 25 | 97 |
| 4 | Man9-N3 | 1 | 1 | — | |
| 16 | Man9 | 1 | 3 | 3 | 73 |
| 17 | Man9 | 2 | 9 | 9 | 98 |
| 18 | Man9 | 3 | 27 | 25 | 98 |
Interaction of Oligomannose Dendrons with 2G12.
Binding of glycodendrons to 2G12 was compared with the corresponding monomers 3 and 4. In previous studies, Man4 1 was immobilized (Fig. 3b) onto an N-hydroxysuccinimide (NHS) activated glass slide (normal NHS density) to determine the surface dissociation constant (KD,surf) of various proteins (21). In a modified assay, fluorescently labeled antibody and 2G12 were coincubated with oligomannosyl dendrons 13–18 (Fig. 3c) and competed for immobilized Man4 on the same slide (Fig. 5).
Fig. 5.
Measurement of oligomannose dendrons–2G12 complex interaction by glycan array competition assay. (a) Design of glycan array-based competition assay (not drawn to scale). (b) Representative microarray slide. 16 (bottom row) and 17 (top row) are used as inhibitors; concentrations from left to right are 50 μM, 5 μM, 500 nM, 50 nM, 5 nM, 500 pM, 50 pM, and 0. (Scale bar: 1 mm.) (c) Inhibition curves of 16 and 17 for the determination of solution dissociation constant.
The IC50 values of these glycodendrons are shown in Table 2. A strong increase in avidity to 2G12 was noted compared with monomeric oligomannose for the first (13 and 16) and second generations (14 and 17), whereas the third-generation glycodendron (15 and 18) remained at the same level as the second-generation glycodendron. To assess the validity of the new glycan microarray assay, we performed the standard gp120/2G12 competition ELISA, in which the analytes compete with surface-bound gp120 for uncomplexed 2G12 (15). IC50 in both assays improved rapidly with increasing generation for both Man4 and Man9 dendrons. However, this glycan microarray cannot discriminate the efficacy of second- and third-generation Man4 dendrons, which showed a 40-fold IC50 difference in a gp120/2G12 ELISA. We believe the incompatibility was due to the relatively low density of Man4 on the microarray, which was not accurately mimicking the high-density glycan presentation on the surface of gp120. To correct this, high-NHS-density slides were used for Man4 immobilization. The resulting high-density Man4 slide exhibited a matched trend in both assays (Table 2), suggesting that higher surface Man4 density better mimics the surface of gp120. The solution dissociation constants (KD,sol) for each glycodendron, as determined with high-density Man4 slides, are reported in Table 2.
Table 2.
Oligomannose dendrons as inhibitors of 2G12 binding to multivalent glycan displays
| Compound | Glycan array assay, normal denisty, IC50, μM |
Glycan array assay, high-density |
2G12/gp120 ELISA IC50, μM |
||||
|---|---|---|---|---|---|---|---|
| IC50, μM |
KD,sol, μM | ||||||
| Per dendron | Per oligomannose | Per dendron | Per oligomannose | Per dendron | Per oligomannose | ||
| 3 | 42 | 42 | 2,100 | 2,100 | 350 ± 98 | 1,100 | 1,100 |
| 13 | 0.29 | 0.87 | 100 | 300 | 17 ± 5.6 | 160 | 480 |
| 14 | 0.0046 | 0.041 | 1.2 | 11 | 0.21 ± 0.088 | 10* | 90* |
| 15 | 0.0041 | 0.10 | 0.022 | 0.56 | 0.0039 ± 0.0013 | 0.24 | 6.0 |
| 4 | 18 | 18 | 1,000 | 1,000 | 180 ± 99 | 530 | 530 |
| 16 | 0.095 | 0.29 | 3.5 | 11 | 0.61 ± 0.19 | 36 | 107 |
| 17 | 0.0030 | 0.027 | 0.020 | 0.18 | 0.0034 ± 0.00049 | 0.54 | 4.8 |
| 18 | 0.0031 | 0.078 | 0.018 | 0.46 | 0.0031 ± 0.00041 | 0.29 | 7.3 |
* Extrapolated from the concentration of 40% inhibition.
In both ELISA and high-density glycan array assays, the improvement of IC50 for the dendrons relative to monomeric oligosaccharide is much greater than an additive effect, as shown in Table 2. All glycodendrons showed superior IC50 per oligomannose unit relative to monomeric ligands, showing a synergistic multivalent effect.
ELISA results using dendrons 15, 17, and 18 showed improved inhibition compared with published Man9(GlcNAc)2 clusters (27). This may be partly due to the dendrons' higher valency and/or better presentation to the multiple-antibody glycan-binding sites on 2G12. It also has been demonstrated that a high-mannose dimer can be synthesized in a distance-fixed manner to give superior 2G12 affinity (18), but the internal flexibility, which should be minimized for an optimal synthetic vaccine (42), cannot be controlled in this case. Alternatively, the use of a rigid high-mannose cluster constrained the flexibility (27), but this control may decay rapidly when a longer linker is used to make a larger cluster. The use of glycodendrons may be a solution to this dilemma because the oligosaccharide valency increases as the structure grows larger, and therefore the internal flexibility is controlled to a certain level even within a large structure.
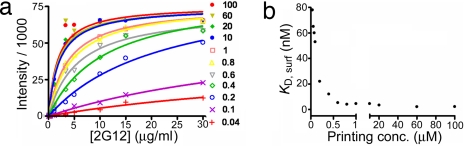
Based on the binding data in Table 2, the second-generation Man9 dendron 17 is a promising candidate for vaccine development. It was chosen because it has a similar IC50 to the third-generation dendrons (15 and 18) in disrupting the gp120–2G12 interaction but has a smaller size to facilitate synthesis and carrier protein conjugation. To further test its multivalent efficacy, 17 was immobilized onto a normal-NHS-density slide (Fig. 3d) at varying printing concentrations. The measured KD,surf of 17 (3.5 nM; SI Materials and Methods and SI Table 4) was significantly stronger than the KD,surf of 2 (830 nM) on the same slide (21), indicating that the density of Man9 on the dendron is higher than on the glass slide. The finding that the saturated KD,surf of 17 is comparable to the KD,sol for 17 (3.4 nM; Fig. 6 and Table 2) also suggested that the enhanced 2G12 complex avidity came from dendrimeric display of Man9 rather than the pseudo-multivalency arising from the close proximity of the surface-immobilized molecules. Moreover, the KD,surf of 17 remains strong in the case of lower printing concentration, which is contrary to the results observed in Man9 monomer, in which a a high critical printing concentration was observed. We reasoned that part of this phenomenon may arise from the high density of Man9 on glycodendron 17, so that it does not require dense surface immobilization to achieve tight binding to 2G12. Overall, the glycodendron 17 appears to be an effective mimic of the gp120 surface, and it is suitable for conjugation to a carrier protein as a vaccine candidate. The critical printing concentration of 17 for 2G12 complex binding was found to be 400 nM (Fig. 6, SI Materials and Methods, and SI Table 4), compared with 40 μM for Man4 on the same surface. The detection limit of this glycodendron slide for the 2G12 complex was 0.05 μg/ml (Man4 slide: 3 μg/ml), which is low enough to be suitable for diagnostic use.
Fig. 6.
Properties of glycodendron 17-coated slide. (a) Representative binding curves of fluorescent 2G12 complex with glycodendron 17 arrayed on glass slide at different printing concentration, ranging from 40 nM to 100 μM. (b) Calculated KD,surf plot against 17 printing concentrations.
Interaction of Oligomannose Dendron with DC-SIGN.
The success in enhancing oligomannose–2G12 complex binding by dendrimeric scaffolds encouraged us to test their affinity to DC-SIGN. Because the optimal size and the oligomannose density for 2G12 complex seem to be achieved at the second-generation Man9 dendron 17, we tested this construct for DC-SIGN binding in a similar glycan microarray assay. Competitive binding was performed on the same high-density Man4 slide, with the Fc-DC-SIGN fusion protein detected with Cy3-labeled anti-human IgG antibody. The Man9 dendron 17 showed good competition against surface-bound Man4 for Fc-DC-SIGN, whereas the Man4 dendron 14 was weaker (Table 3). These results are consistent with a previous report showing that the monomeric branched high mannose binds better than linear glycans (12).
Table 3.
Oligomannose dendrons as inhibitors of Fc-DC-SIGN binding to multivalent glycans
| Compound | Glycan array assay IC50, μM | gp120/DC-SIGN-Fc ELISA IC50, μM |
|---|---|---|
| 14 | 0.16 | 0.020 |
| 17 | 0.026 | 0.008 |
| d-Mannose | — | 8,500 |
As the next step, we determined whether these oligomannose dendrons can interfere with the binding between gp120 and DC-SIGN, which is likely a key step for dendritic cell-mediated CD4+ T cell HIV infection. We performed gp120/Fc-DC-SIGN ELISA, in a similar setting as gp120/2G12 ELISA, to evaluate the inhibition activity of glycodendrons 14 and 17. Indeed, these second-generation glycodendrons demonstrated excellent inhibition activity in the nanomolar range, in contrast to the millimolar range from the reference mannose (Table 3). In these experiments, no inhibition was observed for the unglycosylated alkynyl dendron 9 (up to 0.1 mM), showing that the multivalent oligomannose is responsible for DC-SIGN binding.
The next question we asked was whether these glycodendrons bind DC-SIGN presented on cell surface. We synthesized fluorescence-labeled dendrons 14 and 17 by connecting fluorescein to the amine group via an oligo(ethylene glycol) linker and used flow cytometry to monitor their interaction to cell-surface DC-SIGN. As shown in Fig. 7, both glycodendrons stain DC-SIGN-expressing Jurkat cells (expression level are shown in SI Fig. 12) with a stronger fluorescent intensity compared with the negative control Jurkat cells. The results indicate that the oligomannose dendrons interact with DC-SIGN on cell surface. In the same setting, we found that immature monocyte-derived dendritic cells (MDDC) were intensely labeled by these fluorescent dendrons (Fig. 7). Because DC-SIGN is not the only mannose-binding lectin on MDDC, it is possible that the multivalent high-mannose glycans also bind to other mannose-binding proteins, which may also contribute to viral transmission.
Fig. 7.
Oligomannose dendrons bind cell-surface receptors. Flow cytometry histograms showing fluorescein-labeled glycodendrons 14 (green in a and c) and 17 (blue in b and d) binds DC-SIGN-expressing Jurkat cells (a and b) or MDDCs (c and d). Mock-transfected Jurkat cells stained with the same conditions are shown in red in a and b. The fluorescent levels of mock-transfection control are the same as unstained cells. Unstained MDDCs serve as the negative control (red) for glycodendron-stained MDDCs in c and d.
Sexual transmission is a major route for HIV infection, in which the dendritic cells enhance the infection of CD4+ T cells. Therefore, inhibiting the gp120–DC-SIGN interaction, which is likely the key step of HIV–dendritic cells binding, has become a strategy for preventing infection (41). Our glycodendrons inhibit the DC-SIGN–gp120 interaction, demonstrating their potential as antiviral agent for preventing sexual transmission of HIV-1. Furthermore, as well defined structures, our glycodendrons may be useful for investigating the “macro” structure requirement of ligands for DC-SIGN or other receptors. Future studies may focus on understanding the role of these lectins in the immune system and how they may be exploited by pathogens.
In conclusion, we have developed a convenient strategy for the efficient syntheses of oligomannose dendrons, in which the high-density oligomannose mimics the glycans on the surface of HIV-1 and the monomeric glycan immobilized on glass slides. The binding properties of these glycodendrons were characterized by glycan microarray assay. The inhibition of glycodendrons on gp120 interacting with 2G12 and DC-SIGN demonstrated that these glycodendrons, especially the second-generation Man9 dendron, have the potential for use in the development of both carbohydrate vaccine candidates and antiviral agents. HIV uses its glycan shield to evade the immune response, but the unusual high glycan density and the existence of conserved oligomannosides, evidenced by the discovery of the broadly neutralizing antibody 2G12, suggest that targeting of these carbohydrates may be a promising approach (41–43). From this point of view, multivalent display of carbohydrates that have higher binding affinity/avidity may be a practical solution for inducing 2G12-like antibodies and blocking mannose-binding-protein-mediated viral infection.
Materials and Methods
Details for the synthesis of the compounds, microarray experiments, protein expression, and cell-based experiments can be found in SI Materials and Methods.
IC50 Determined by the Microarray Competitor Assay for 2G12 Complex.
Serial diluted competitors (1.5 μl) were mixed with 1.5 μl of 50 μg/ml (based on 2G12, for high-density Man4 slide: 15 μg/ml) 2G12-Cy3-labeled goat anti-human IgG complex (44). The 3-μl mixtures in PBS-BT buffer (1% BSA and 0.05% Tween 20 in PBS) were applied directly to each subarray. After incubation in a humidified chamber for 1 h, the slide was rinsed sequentially with PBS, PBS-T buffer (0.05% Tween 20 in PBS), and distilled water, and then centrifuged at 200 × g for 5 min to ensure a complete dryness. The array was then imaged at 5-Å resolution with an A595 laser on an ArrayWorx microarray reader (Applied Precision) to measure the fluorescence. ArrayVision 8.0 was used for the fluorescence analysis and extraction of data (Applied Precision). Binding curves are shown in SI Figs. 10 and 11.
Competition ELISA.
Microtiter plate wells (flat-bottom; Costar type 3690 from Corning) were coated with 50 ng per well gp120JR-FL overnight at 4°C in PBS. All subsequent steps were performed at room temperature. The wells were then washed four times with PBS/0.05% (vol/vol) Tween 20 (Sigma) before blocking for 1 h with 3% (mass/vol) BSA. IgG 2G12, diluted to 0.5 μg/ml (25 ng per well) with 1% (mass/vol) BSA/0.02% (vol/vol) Tween 20/PBS (PBS-BT), was then added for 2 h to the antigen-coated wells in the presence of serially diluted oligomannoses or glycodendrons. Unbound Ab was removed by washing four times as described above. Bound 2G12 was detected with an alkaline phosphatase-conjugated goat anti-human IgG F(ab′)2 Ab (Pierce) diluted 1:1,000 in PBS-BT. After 1 h, the wells were washed four times, and bound Ab was visualized with p-nitrophenyl phosphate substrate (Sigma) and monitored at 405 nm.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Prof. William Greenberg for suggestions to this manuscript. S.-K.W. thanks Drs. Hing-Ken Lee and Cheng-Yuan Huang for useful discussion. This work was supported by The Skaggs Institute for Chemical Biology.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712326105/DC1.
References
- 1.Cohen J. New estimates scale back scope of HIV/AIDS epidemic. Science. 2007;318:1360–1361. doi: 10.1126/science.318.5855.1360. [DOI] [PubMed] [Google Scholar]
- 2.Barin F, et al. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science. 1985;228:1094–1096. doi: 10.1126/science.2986291. [DOI] [PubMed] [Google Scholar]
- 3.Reitter JN, Means RE, Desrosiers RC. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 4.Lue J, et al. Addition of a single gp120 glycan confers increased binding to dendritic cell-specific ICAM-3-grabbing nonintegrin and neutralization escape to HIV type 1. J Virol. 2002;76:10299–10306. doi: 10.1128/JVI.76.20.10299-10306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchacher A, et al. Generation of human monoclonal antibodies against HIV-1 proteins: Electrofusion and Epstein–Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 6.Trkola A, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of HIV type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scanlan CN, et al. The broadly neutralizing anti-HIV type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calarese DA, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances transinfection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 10.van Kooyk Y, Geijtenbeek TB. DC-SIGN: Escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: Infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinberg H, Mitchell DA, Drickamer K, Weis WI. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294:2163–2166. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 14.Lee HK, et al. Reactivity-based one-pot synthesis of oligomannoses: Defining antigens recognized by 2G12, a broadly neutralizing anti-HIV-1 antibody. Angew Chem Int Ed. 2004;43:1000–1003. doi: 10.1002/anie.200353105. [DOI] [PubMed] [Google Scholar]
- 15.Calarese DA, et al. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci USA. 2005;102:13372–13377. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Lee YC, Lee RT. Carbohydrate–protein interactions: Basis of glycobiology. Acc Chem Res. 1995;28:321–327. [Google Scholar]
- 18.Dudkin VY, et al. Toward fully synthetic carbohydrate-based HIV antigen design: on the critical role of bivalency. J Am Chem Soc. 2004;126:9560–9562. doi: 10.1021/ja047720g. [DOI] [PubMed] [Google Scholar]
- 19.Ni J, Song H, Wang Y, Stamatos NM, Wang LX. Toward a carbohydrate-based HIV-1 vaccine: Synthesis and immunological studies of oligomannose-containing glycoconjugates. Bioconjugate Chem. 2006;17:493–500. doi: 10.1021/bc0502816. [DOI] [PubMed] [Google Scholar]
- 20.Krauss IJ, et al. Fully synthetic carbohydrate HIV antigens designed on the logic of the 2G12 antibody. J Am Chem Soc. 2007;129:11042–11044. doi: 10.1021/ja074804r. [DOI] [PubMed] [Google Scholar]
- 21.Liang PH, Wang SK, Wong CH. Quantitative analysis of carbohydrate–protein interactions using glycan microarrays: Determination of surface and solution dissociation constants. J Am Chem Soc. 2007;129:11177–11184. doi: 10.1021/ja072931h. [DOI] [PubMed] [Google Scholar]
- 22.Kiessling LL, Gestwicki JE, Strong LE. Synthetic multivalent ligands as probes of signal transduction. Angew Chem Int Ed. 2006;45:2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem Rev. 2002;102:555–578. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- 24.Lindhorst TK. Artificial multivalent sugar ligands to understand and manipulate carbohydrate–protein interactions. Top Curr Chem. 2002;218:201–235. [Google Scholar]
- 25.Kitov PI, et al. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature. 2000;403:669–672. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- 26.Lee YC. Synthesis of some cluster glycosides suitable for attachment to proteins or solid matrices. Carbohydr Res. 1978;67:509–514. [Google Scholar]
- 27.Wang LX, Ni J, Singh S, Li H. Binding of high-mannose-type oligosaccharides and synthetic oligomannose clusters to human antibody 2G12: Implications for HIV-1 vaccine design. Chem Biol. 2004;11:127–134. doi: 10.1016/j.chembiol.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Roy R. A decade of glycodendrimer chemistry. Trends Glycosci Glycotechnol. 2003;15:291–310. [Google Scholar]
- 29.Turnbull WB, Stoddart JF. Design and synthesis of glycodendrimers. J Biotechnol. 2002;90:231–255. doi: 10.1016/s1389-0352(01)00062-9. [DOI] [PubMed] [Google Scholar]
- 30.Gestwicki JE, Kiessling LL. Inter-receptor communication through arrays of bacterial chemoreceptors. Nature. 2002;415:81–84. doi: 10.1038/415081a. [DOI] [PubMed] [Google Scholar]
- 31.Roy R, Baek MG, Rittenhouse-Olson K. Synthesis of N,N′-bis(acrylamido)acetic acid-based T-antigen glycodendrimers and their mouse monoclonal IgG antibody binding properties. J Am Chem Soc. 2001;123:1809–1816. doi: 10.1021/ja002596w. [DOI] [PubMed] [Google Scholar]
- 32.Krist P, et al. Fluorescent labeled thiourea-bridged glycodendrons. ChemBioChem. 2004;5:445–452. doi: 10.1002/cbic.200300669. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Megia E, Correa J, Rodriguez-Meizoso I, Riguera R. A click approach to unprotected glycodendrimers. Macromolecules. 2006;39:2113–2120. [Google Scholar]
- 34.Suda Y, et al. Immobilization and clustering of structurally defined oligosaccharides for sugar chips: An improved method for surface plasmon resonance analysis of protein-carbohydrate interactions. Bioconjugate Chem. 2006;17:1125–1135. doi: 10.1021/bc0600620. [DOI] [PubMed] [Google Scholar]
- 35.Pukin AV, et al. Strong inhibition of cholera toxin by multivalent GM1 derivatives. ChemBioChem. 2007;8:1500–1503. doi: 10.1002/cbic.200700266. [DOI] [PubMed] [Google Scholar]
- 36.de Paz JL, Noti C, Böhm F, Werner S, Seeberger PH. Potentiation of fibroblast growth factor activity by synthetic heparin oligosaccharide glycodendrimers. Chem Biol. 2007;14:879–887. doi: 10.1016/j.chembiol.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Chung HH, et al. Dendritic oligoguanidines as intracellular translocators. Biopolymers. 2004;76:83–96. doi: 10.1002/bip.10597. [DOI] [PubMed] [Google Scholar]
- 38.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Crespo L, et al. Peptide and amide bond-containing dendrimers. Chem Rev. 2005;105:1663–1681. doi: 10.1021/cr030449l. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Z, Fahrni CJ. A fluorogenic probe for the copper(I)-catalyzed azide-alkyne ligation reaction: Modulation of the fluorescence emission via 3(n,π*)-1(π,π*) inversion. J Am Chem Soc. 2004;126:8862–8863. doi: 10.1021/ja049684r. [DOI] [PubMed] [Google Scholar]
- 41.Balzarini J. Targeting the glycans of glycoproteins: A novel paradigm for antiviral therapy. Nat Rev Microbiol. 2007;5:583–597. doi: 10.1038/nrmicro1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scanlan CN, Offer J, Zitzmann N, Dwek RA. Exploiting the defensive sugars of HIV-1 for drug and vaccine design. Nature. 2007;446:1038–1045. doi: 10.1038/nature05818. [DOI] [PubMed] [Google Scholar]
- 43.Botos I, Wlodawer A. Proteins that bind high-mannose sugars of the HIV envelope. Prog Biophys Mol Biol. 2005;88:233–282. doi: 10.1016/j.pbiomolbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Blixt O, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.