Abstract
Curcumin has been extensively studied for its anti-inflammatory activities. However, its potential beneficial effects on various disease preventions and treatments are limited by its unstable structure. The β-diketone moiety renders curcumin to be rapidly metabolized by aldoketo reductase in liver. In the present study, a series of curcumin analogues with more stable chemical structures were synthesized and several compounds showed an enhanced ability to inhibit lipopolysaccharide (LPS)-induced TNF-α and IL-6 synthesis in macrophages.
Curcumin [1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, Fig. 1] is the major active constituent of turmeric, a yellow compound originally isolated from the plant Curcuma longa L. Tumeric has been widely used for centuries as a dietary spice and pigment.1 In addition to its unique flavor and color, tumeric has been extensively used in traditional medicine in China and India, particularly as an anti-inflammatory agent.2 During the last two decades, numerous studies have shown that curcumin has a variety of biological and pharmacological activities such as anti-carcinogen, immuno-modulation, anti-oxidant, anti-angiogenesis, and chemo-prevention.3–9
Fig. 1.
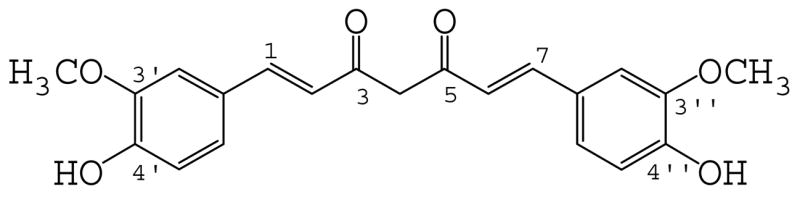
Chemical structure of curcumin.
Recent studies have demonstrated that inflammation is implicated in the pathogenesis of various diseases including cancer, atherosclerosis, diabetes, fatty liver, rheumatoid arthritis, and inflammatory bowel disease.10–13 Anti-inflammation is the major focus of current drug development.14 Cytokines are local mediators produced by lymphocytes and macrophages as well as by epithelial and mesenchymal cells.15 It has been demonstrated that cytokines are involved in a variety of biological processes and play a central role in the development of inflammation and immunity. TNF-α is a multifunctional cytokine produced primarily by activated monocytes/macrophages and plays a critical role in the initiation and continuation of inflammation and immunity.16 It is well-known that TNF-α is a key proinflammatory cytokine in the pathogenesis of various inflammatory diseases and cancer.13, 17 In addition to directly inducing inflammatory response, TNF-α is also able to induce other proinflammatory cytokines including IL-6, IL-1β, IL-8 and itself by activating NF-kB and MAP kinase signaling pathways.16,18 It has been shown that curcumin is able to inhibit the production of proinflammatory cytokines TNF-α and IL-6 in macrophages and various cancer cells.18,19 It also can inhibit the expression of other inflammatory mediators such as COX-2 and iNOS.20 However, preclinical and clinical studies have found that the potential beneficial effects of curcumin on various disease preventions and treatments are limited by its poor pharmacokinetic properties.3,21,22 It is believed that the presence of the active methylene group and β-diketone moiety contributes to the instability of curcumin under physiological conditions, poor absorption and fast metabolism.23 Recently, synthetic modifications of curcumin have been studied intensively in order to develop a molecule with enhanced properties and stability.23–26 In the present study, a series of curcumin analogues with more stable structures were synthesized and tested for anti-inflammatory activity in vitro. Several analogues showed an enhanced ability to inhibit lipopolysaccharide (LPS)-induced TNF-α and IL-6 in macrophages.
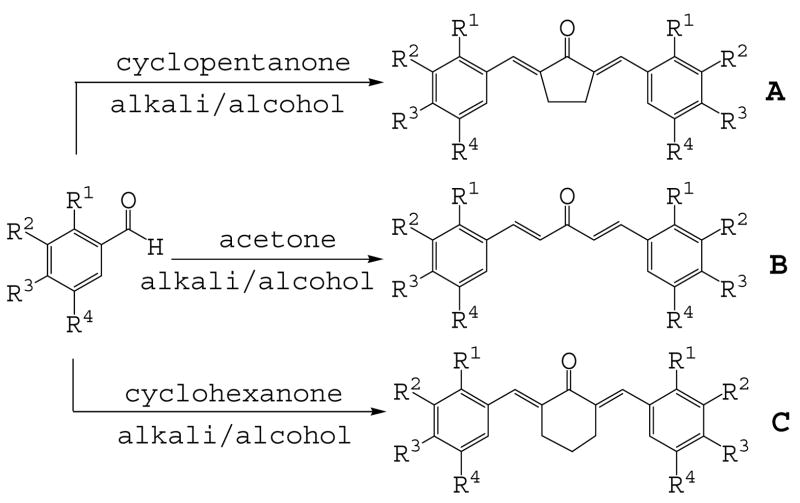
In order to identify the crucial structural motifs leading to anti-inflammatory activity and gain insight into future directions for designing new analogues with better activity, three series of mono-carbonyl curcumin analogues, 1,5-diaryl-1,4-pentadiene-3-ones (B), together with cyclopentanone (A) and cyclohexanone (C) analogues, were designed by deleting the methylene group and one carbonyl group. These compounds were also designed to examine the role of different substituents on the benzene ring and the influence of the structure of 5-C linker on inflammatory activities when the unstable methylene was absent. The structure and general synthesis of analogues designed are shown in Table 1, Scheme 1, and 2, respectively.
Table 1.
Structure of curcumin analogues
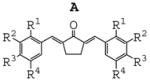 |
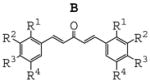 |
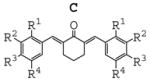 |
|||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 |

|
|
| 1 | H | H | OH | H | |
| 2 | H | OCH3 | OH | H | |
| 3 | Br | H | H | H | |
| 4 | F | CF3 | H | H |
|
| 5 | H | Br | H | H | |
| 6 | H | H | F | H | |
| 7 | H | H | OCH2CH3 | H | |
| 8 | H | OCH3 | OCH3 | OCH3 |
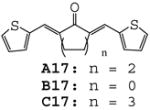
|
| 9 | H | H |
|
H | |
| 10 | H | H | N(CH3)2 | H | |
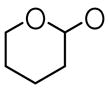
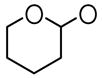
| 11 | H | OCH3 |
|
H | |
| 12 | H | H | OCH2CH=CH2 | H |
|
| 13 | H | OCH3 | OCH2CH=CH2 | H | |
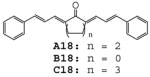
| 14 | H | H |
|
H | |
Scheme 1.
General synthesis of 1,5-diphenyl-1,4- pentadiene-3-ones and cyclic analogues
Scheme 2.
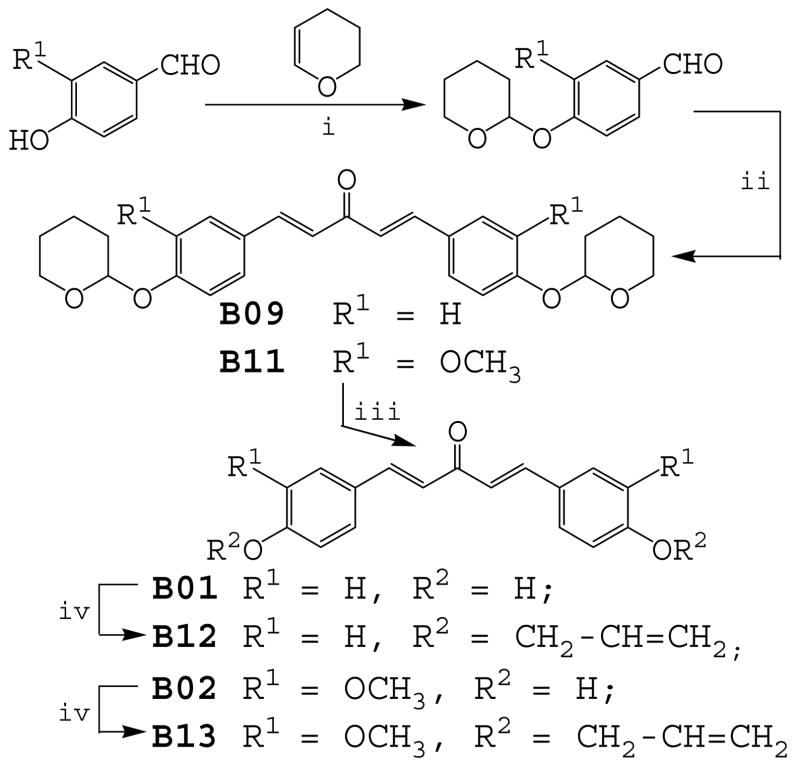
Synthesis of presented compounds. Reagents and conditions: (i) pyridine-PTSA, CH2Cl2, rt; (ii) CH3COCH3, NaOH/EtOH, rt; (iii) PTSA, MeOH, rt; (iv) CH2=CH-CH2Br, K2CO3/Acetone, reflux.
Most of the compounds were synthesized by coupling the appropriate aromatic aldehyde with cyclohexanone, cyclopentanone or acetone in an alkaline solution, respectively. Details of the reaction routes, yields, melting points, NMR and ESI-MS analysis are described in online supplemental data. All reactions were carried out with a ratio of 2:1 of substituted arylaldehydes to ketones. Incorporation of two aryl-rings was confirmed using 1H NMR analysis by detecting the absence of methyl (B analogues) or methylene (A and C analogues) next to the carbonyl group.
For the synthesis of analogues 1 and 2 in alkaline conditions, the hydroxyls of 4-hydroxybenzaldehyde and 3-methoxy-4-hydroxybenzaldehyde were protected with an easily removable group, 3,4-dihydro-α-pyran. Scheme 2 illustrates a representative example of the preparation of the tetrahydropyran-2-yl-protected derivative and its reaction with acetone to obtain compounds 9 and 11. Analogues 1 and 2 were prepared by deprotection with a catalytic amount of p-tolunesulfonic acid, and compounds 12 and 13 were obtained by further etherification with allyl bromide. The anti-inflammatory activities of curcumin and its analogues were measured as the ability of these compounds to inhibit LPS-induced TNF-α and IL-6 expression in mouse J774A.1 macrophages using Enzyme-linked immunosorbent assays (ELISA).27 Briefly, cells were pre-treated with 10 μM of curcumin, each analogue or vehicle control for 2 h, then treated with LPS (0.5 μg/ml) for 24 h. At the end of treatment, the culture media were collected and centrifuged at 14,000 rpm for 5 min. TNF-α and IL-6 levels in the media were determined by ELISA using mouse TNF-α and mouse IL-6 ELISA MaxTM Set Deluxe Kits (Biolegend, USA). The total protein concentrations of the viable cell pellets were determined using Bio-Rad protein assay reagents. Total amounts of the TNF-α and IL-6 in the media were normalized to the total protein amount in the viable cell pellets.
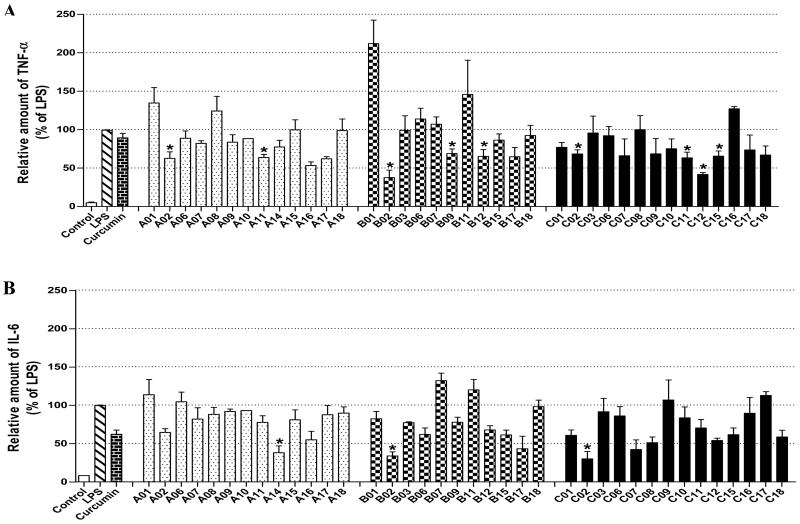
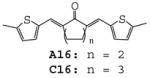
The results indicate that curcumin and its analogues inhibited LPS-induced TNF-α and IL-6 expression to various degrees. The cyclohexanone-derived C compounds were more effective than acetone-derived A and cyclopentanone-derived B compounds. Among these compounds, A02, A11, B02, B09, B12, C02, C11, C12 and C15 are more potent than curcumin in inhibiting LPS-induced TNF-α expression (Fig. 2A). Compounds B02, C02 and A14 showed better inhibitory effect than curcumin on LPS-induced IL-6 expression (Fig. 2B). A14, a novel compound with a long chain substituent group of 3-(dimethylamino) propoxy, showed a similar inhibitory effect on LPS-induced TNF-α expression as curcumin, but has more potent inhibitory effect on LPS-induced IL-6 expression than curcumin. However, the similar dimethylamino-substituted compounds (A10, B10 and C10) showed similar or less inhibitory effects on LPS-induced TNF-α and IL-6 expression as curcumin, indicating that nitrogenous substitution by itself does not enhance the anti-inflammatory activity. On the other hand, B12 and C12 with a long chain allyloxyl substituent group showed stronger inhibitory effect on LPS-induced TNF-α, indicating that the length and flexibility of the substituent groups may be favorable to the anti-inflammatory activity. Among eight heterocyclo-substituted compounds 15~17, A16, A17 and C15 exhibited moderate activity on inhibiting LPS-induced TNF-α secretion and B17 displayed a strong inhibition on IL-6 expression (43.1%). Among curcumin-like compounds, A02, B02 and C02 showed the best inhibition activities (Fig 2), while A01, B01 and C01 has less or opposite activates, suggesting that the presence of a 3-methyoxy group is critical to the activity.
Fig. 2.
Inhibition of LPS-induced TNF-α and IL-6 by curcumin and its analogues in J774A.1 macrophages. Cells were pretreated with curcumin or its analogues (10 μM) for 2 h, then treated with LPS (0.5 μg/ml) for 24 h. TNF-α (A) and IL-6 (B) levels in the culture media were measured by ELISA. The results were expressed as percent of LPS control. Each bar represents mean ± S.E. of five independent experiments. Statistical significance relative to LPS was indicated, *p < 0.05.
In summary, three series of mono-carbonyl analogues of curcumin were synthesized. Their structures were identified by NMR analysis and anti-inflammatory activities were examined by measuring the inhibitory effect on LPS-induced TNF-α and IL-6 expression in macrophages using ELISA. Although the synthesis of several analogues has been reported previously, the inhibitory activates on LPS-induced TNF-a and IL-6 expression have not been explored. In the present study, several novel analogues with better inhibitory effect than curcumin were identified. The results suggest that the properties and position of the substituent and the space of the linking chain determine the anti-inflammatory activities. However, the underlying mechanisms by which curcumin and its derivatives inhibited LPS-induced TNF-α and IL-6 expression remain unknown and are the focus of our current research. These new compounds would be useful for development of new anti-inflammatory drugs to treat various inflammatory diseases.
Supplementary Material
Acknowledgments
This work was supported by The Program of New Century Excellent Talents in Universities (2006), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (2007) and NIH grants (AI-68432 and AT-04148) and A.D. Williams Award (to H. Zhou), USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Govindarajan VS. Crit Rev Food Sci Nutr. 1980;12(3):199. doi: 10.1080/10408398009527278. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Adv Exp Med Biol. 2007;595:1. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 3.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Life Sci. 2006;78:2081. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Menon VP, Sudheer AR. Adv Exp Med Biol. 2007;595:105. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 5.Arbiser JL, Klauber N, Rohan R, van LR, Huang MT, Fisher C, Flynn E, Byers HR. Mol Med. 1998;4:376. [PMC free article] [PubMed] [Google Scholar]
- 6.Kowluru RA, Kanwar M. Nutr Metab (Lond) 2007;4:8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sidhu GS, Singh AK, Thaloor D, Banaudha KK, Patnaik GK, Srimal RC, Maheshwari RK. Wound Repair Regen. 1998;6:167. doi: 10.1046/j.1524-475x.1998.60211.x. [DOI] [PubMed] [Google Scholar]
- 8.Gautam SC, Gao X, Dulchavsky S. Adv Exp Med Biol. 2007;595:321. doi: 10.1007/978-0-387-46401-5_14. [DOI] [PubMed] [Google Scholar]
- 9.Thangapazham RL, Sharma A, Maheshwari RK. AAPS J. 2006;8:E443. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams MD, Nadler JL. Curr Diab Rep. 2007;7:242. doi: 10.1007/s11892-007-0038-y. [DOI] [PubMed] [Google Scholar]
- 11.Shoelson SE, Herrero L, Naaz A. Gastroenterology. 2007;132:2169. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 12.Paoletti R, Bolego C, Poli A, Cignarella A. Vasc Health Risk Manag. 2006;2:145. doi: 10.2147/vhrm.2006.2.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnamoorthy S, Honn KV. Cancer Metastasis Rev. 2006;25:481. doi: 10.1007/s10555-006-9016-0. [DOI] [PubMed] [Google Scholar]
- 14.Rainsford KD. Subcell Biochem. 2007;42:3. doi: 10.1007/1-4020-5688-5_1. [DOI] [PubMed] [Google Scholar]
- 15.Papadakis KA, Targan SR. Inflamm Bowel Dis. 2000;6:303. doi: 10.1002/ibd.3780060408. [DOI] [PubMed] [Google Scholar]
- 16.Newton RC, Decicco CP. J Med Chem. 1999;42:2295. doi: 10.1021/jm980541n. [DOI] [PubMed] [Google Scholar]
- 17.Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. J Lipid Res. 2007;48:751. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Cho JW, Lee KS, Kim CW. Int J Mol Med. 2007;19:469. [PubMed] [Google Scholar]
- 19.Chan MM. Pharmacol. 1995;49:1551. doi: 10.1016/0006-2952(95)00171-u. [DOI] [PubMed] [Google Scholar]
- 20.Rao CV. Adv Exp Med Biol. 2007;595:213. doi: 10.1007/978-0-387-46401-5_9. [DOI] [PubMed] [Google Scholar]
- 21.Sharma RA, Steward WP, Gescher A. J Adv Exp Med Biol. 2007;595:453. doi: 10.1007/978-0-387-46401-5_20. [DOI] [PubMed] [Google Scholar]
- 22.Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ, Berry DP. Br J Cancer. 2004;90:1011. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sardjiman SS, Reksohadiprodjo MS, Hakim L, van der Goot H, Timmerman H. European Journal of Medicinal Chemistry. 1997;32:625. [Google Scholar]
- 24.Handler N, Jaeger W, Puschacher H, Leisser K, Erker T. Chem Pharm Bull (Tokyo) 2007;55:64. doi: 10.1248/cpb.55.64. [DOI] [PubMed] [Google Scholar]
- 25.Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo Y, Takahashi A, Takahashi S, Kato S, Suzuki T, Ishioka C, Iwabuchi Y, Shibata H. Mol Cancer Ther. 2006;5:2563. doi: 10.1158/1535-7163.MCT-06-0174. [DOI] [PubMed] [Google Scholar]
- 26.Weber WM, Hunsaker LA, Roybal CN, Bobrovnikova-Marjon EV, Abcouwer SF, Royer RE, Deck LM, Vander Jagt DL. Bioorg Med Chem. 2006;14:2450. doi: 10.1016/j.bmc.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 27.Zhou H, Jarujaron S, Gurley EC, Chen L, Ding H, Studer E, Pandak J, Hu W, Zou T, Wang JY, Hylemon PB. Atherosclerosis. 2007;195:e134. doi: 10.1016/j.atherosclerosis.2007.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.