Abstract
Nonsteroidal anti-inflammatory drug (NSAID)-activated gene (NAG-1), a divergent member of the transforming growth factor β superfamily, was previously identified as a gene induced by several anti-tumorigenic compounds, including NSAIDs and peroxisome proliferator-activated receptor γ (PPARγ) ligands in humans. In this study, canine NAG-1 was characterised from a canine genomic database. Gene induction by some NSAIDs and PPARγ ligands was demonstrated in canine osteosarcoma cell lines. Phylogenetic analysis indicates that canine NAG-1 is more homologous with the corresponding mouse and rat genes than with human NAG-1. Expression of canine NAG-1 was increased by treatment with piroxicam and SC-560 (NSAIDs) and the PPARγ ligand rosiglitazone. This study demonstrates that canine NAG-1 is up-regulated by some anti-tumorigenic compounds in osteosarcoma cell lines and may provide an important target of chemotherapy in canine cancer.
Keywords: NAG-1, NSAID, PPARγ ligand, TGF-β, Canine osteosarcoma
1. Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used in the treatment of inflammatory disease. In addition, recent epidemiological studies in humans and experiments in spontaneous canine tumours and chemically-induced rodent tumours indicate that NSAIDs have anti-tumorigenic and chemotherapeutic effects in several different types of cancer (Reddy et al., 2000; Garcia Rodriguez and Huerta-Alvarez, 2001).
Treatment with the NSAID piroxicam had anti-tumorigenic activity against canine transitional cell carcinoma of the urinary bladder, accompanied by induction of tumour apoptosis and a reduction in angiogenic factor concentrations (Mohammed et al., 2003). A number of NSAIDs reduced cell proliferation rate, inhibited cell cycle progression and induced apoptosis in colorectal cancer cells ( Goldberg et al., 1996, Baek et al., 2001; 2002b). Administration of the NSAID sulindac dramatically reduced the number of tumours in the intestinal tract of Min mice, a strain containing a fully penetrant dominant mutation in the Apc gene that leads to the development of gastrointestinal adenomas (Boolbol et al., 1996). Thus, NSAIDs provide many benefits, including anti-tumorigenic effects.
The transforming growth factor β (TGF-β) superfamily constitutes a group of related cytokines that have activity regulating cell survival, proliferation, differentiation, apoptosis, extracellular matrix formation and immunosuppression (Kingsley, 1994). Although TGF-β is referred to as the “molecular Jekyll and Hyde of cancer,” TGF-β superfamily members act as potent growth suppressors via mechanisms including G1 cell cycle arrest, induction of apoptosis and expression of cell adhesion molecules (Bierie and Moses, 2006).
Recently, we identified NAG-1 (NSAID-activated gene), a member of the TGF-β superfamily, by polymerase chain reaction (PCR)-based subtractive hybridisation from an indomethacin-induced library in human colorectal cancer cells (Baek et al., 2001). Induction of NAG-1, subsequently, was observed following treatment with a number of other NSAIDs, including cyclooxygenase-1 (COX-1) specific, COX-2 specific and conventional NSAIDs (Baek et al., 2002b).
The protein encoded by NAG-1, which has been isolated by a variety of cloning strategies, is also known as macrophage inhibitory cytokine-1 (MIC-1) (Bootcov et al., 1997), placental transforming growth factor β (PTGFB) (Tan et al., 2000), prostate derived factor (PDF) (Paralkar et al., 1998), growth differentiation factor 15 (GDF15) (Hsiao et al., 2000) and placental bone morphogenetic protein (PLAB) (Hromas et al., 1997). The diversity of biological functions indicated by this nomenclature suggests that the biological role of the NAG-1 protein depends on cell context.
In several types of cancer cells, the NAG-1 protein is thought to play a role as a pro-apoptotic/anti-tumorigenic gene. For example, NAG-1 is highly expressed in mature intestinal epithelial cells, but it is significantly reduced in human colorectal carcinoma samples and neoplastic intestinal polyps of Min mice (Kim et al., 2002). Furthermore, NAG-1 overexpression can reduce MDA-MB-468 and MCF-7 breast cancer cell viability by up to 80% (Li et al., 2000). Treatment of prostate cancer cells with purified NAG-1 protein induces apoptosis (Liu et al., 2003). It has also been shown that NAG-1 expression can be induced by p53 activation and that conditioned medium from cells overexpressing NAG-1 suppresses tumour cell growth (Tan et al., 2000). These data support the link between NAG-1, apoptosis and tumour suppressing activity.
NAG-1 is up-regulated by a number of anti-tumorigenic compounds in addition to NSAIDs, including peroxisome proliferator-activated receptor γ (PPARγ) ligands (Baek et al., 2003; 2004b; Yamaguchi et al., 2006a), phosphatidylinositol 3-kinase inhibitor (Yamaguchi et al., 2004) and chemopreventive dietary compounds, such as resveratrol (Baek et al., 2002a), indole-3-carbinol (Lee et al., 2005), conjugated linoleic acid (Lee et al., 2006) and epicatechin gallate (Baek et al., 2004a), as well as anti-inflammatory plant extracts (Yamaguchi et al., 2006b). Interestingly, induction of NAG-1 by these compounds occurs by multiple mechanisms at the levels of transcription and post-transcription. For instance, NSAIDs and resveratrol can transcriptionally activate NAG-1 through early growth response-1 and p53, respectively (Baek et al., 2002a; 2005).
NAG-1 has been well-characterised in humans, but it has not been studied previously in dogs. Since NSAIDs have been used for treatment of some canine cancers, the function of canine NAG-1 protein should also be explored in canine cancer cells. In this report, we characterise the canine NAG-1 sequence, demonstrate the distribution of NAG-1 expression in canine tissues and investigate canine NAG-1 expression in response to NSAIDs and PPARγ ligands.
2. Materials and methods
2.1. Cells and reagents
Canine osteosarcoma cell line CCL-183 was purchased from American Type Culture Collection and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, penicillin (100 U/mL), streptomycin (100 μg/mL), sodium pyruvate and L-glutamine (2 mM). NSAIDs used in this study were purchased as follows: [5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl) phenyl-2 (5H)-furanonel] (DFU) was purchased from Merck, sulindac sulphide and SC-560 were from Cayman Chemical Company and diclofenac and piroxicam were from Sigma. PPARγ ligands rosiglitazone and MCC-555 were purchased from Cayman Chemical Company. All compounds were dissolved in dimethyl sulphoxide (DMSO).
2.2. Preparation of RNA and semi-quantitative reverse transcriptase-PCR
Total RNA was extracted from CCL-183 cells using TRIzol reagent (Invitrogen). One microgram of total RNA was reverse-transcribed to produce single strand cDNAs using an iScript cDNA Synthesis Kit (Bio-Rad) according to the manufacturer’s protocol. On the basis of the predicted cDNA sequence of canine NAG-1 (GenBank XM_541938), primers were synthesised as follows: sense, 5′-gcacctgcagaccaagctga-3′; antisense, 5′-cgtccgggaacctacacgac-3′. REDTaq ReadyMix PCR Reaction Mixture (Sigma) was used for PCR. The thermocycling conditions for amplification of canine NAG-1, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and COX-2 genes were 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min.
Primer sequences used for canine GAPDH (GenBank NM_001003142) were: sense, 5′-gtgtccccacccccaatgta-3′; antisense, 5′-acccggttgctgtagccaaa-3. ′ For canine COX-2 (NM_001003354) the primer sequences are sense, 5′-aacacctgcagtttgctgtg-3′; antisense 5′-gcagctctgggtcaaacttc-3′. The final PCR products were electrophoresed on 1.4% agarose gels and photographed under ultraviolet light. Reverse transcriptase PCR (RT-PCR) for GAPDH was used as an equal loading control.
2.3. Western blot analysis
Tissue samples were obtained from a research animal from another study that was euthanased in accordance with the institutional animal care and use committee protocol. Tissue lysates were prepared from samples stored at −70 °C by homogenisation in radioimmunoprecipitation assay (RIPA) buffer containing 1x phosphate-buffered saline, 1% Nonidet P-40, 0.5% sodium deoxycholate and 0.1% sodium dodecyl sulphate (SDS), supplemented with protease and phosphatase inhibitors (1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 mM phenylmethylsulphonyl fluoride, 0.1 mM Na3VO4 and 25 mM NaF). Samples were sonicated three times for 10 s, then centrifuged for 30 min at 12,000 g to remove debris and the supernatants were used for analysis.
Protein concentrations were determined by the bicinchronic acid (BCA) method using BCA protein assay reagent (Pierce) and bovine serum albumin as a standard. Samples were boiled in Laemmli sample buffer, electrophoresed on 16% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Osmonics). Blots were blocked with 5% skim milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T). The blots were incubated for 1 h at room temperature with a polyclonal anti-human NAG-1 antibody (1:1,000 dilution) (Baek et al., 2001), in 5% skim milk in TBS-T.
To examine whether the signal is specific to the canine NAG-1 protein band, the antibody was mixed with NAG-1 peptide (1 mg/mL) for 4 h at 4 °C and then followed by incubation with NAG-1 antibody as described. After washing, the blots were incubated with anti-rabbit horseradish peroxidase conjugated secondary antibody for 1 h and washed again. The signal was detected by the enhanced chemiluminescence system (Amersham).
2.4. Sequence analysis
The deduced amino acid sequence of the canine NAG-1 protein was generated by DIALIGN 2.2.1 (http://bibiserv.techfak.uni-bielefeld.de/dialign/). The sequence results were compared with those of other species within the National Center for Biomedical Information’s GenBank database and multiple alignments were performed by the AliBee program (GeneBee, http://www.genebee.msu.su/).
3. Results
3.1. Comparisons of predicted canine NAG-1 sequences with other species and molecular phylogenetic analysis
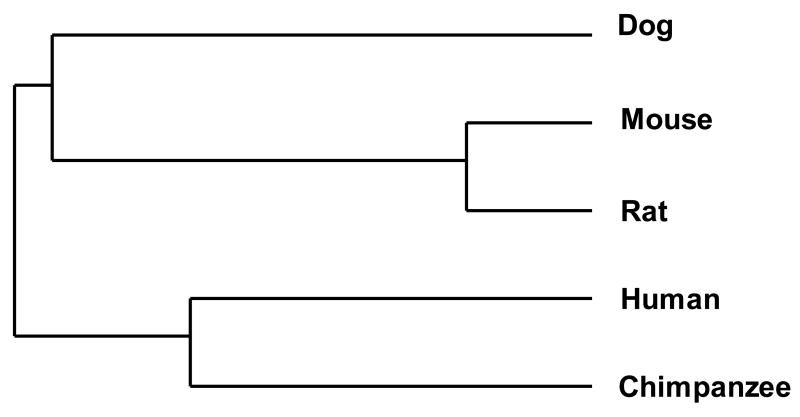
The deduced amino acid sequence of the canine NAG-1 protein was generated by DIALIGN 2.2.1 (Fig. 1). The full-length canine NAG-1 cDNA has 924 bp and 307 deduced amino acids. The deduced amino acid sequence of the NAG-1 protein from other species was obtained from the GenBank database and multiple alignments were generated by the AliBee program. This alignment shows nine conserved cysteine residues in the C-terminal end and an RXXR motif (Fig. 2a). As shown in Fig. 2b, phylogenetic analysis indicated the highest degree of homology between the canine NAG-1 protein and mouse and rat NAG-1.
Fig. 1.
Predicted nucleotide sequence (top) and deduced amino acid sequence (bottom) of canine NAG-1 (GenBank XM_541938).
Fig. 2.
Alignment of NAG-1 amino acid sequence from the dog, human, mouse, rat, and chimpanzee (GenBank: human, AAH08962; mouse, NP_035949; rat, CAA09891; chimpanzee, XP_524157). (a) Amino acid sequence alignment of the C-terminal region begins with the first conserved cysteine residue. Conserved cysteine residues are marked with an asterisk (*). Bold letter indicates RXXR motifs. (b) Phylogenetic tree based on NAG-1 amino acid sequence homology among the dog, human, mouse, rat, and chimpanzee.
3.2. Tissue distribution of canine NAG-1
To investigate the tissue distribution of the canine NAG-1 protein, Western blot analysis was performed using canine kidney, skeletal muscle, jejunum, lung, spleen, heart and liver using a polyclonal anti-human NAG-1 antibody. Strong bands were observed in kidney, lung and liver (Fig. 3a). High-level expression of NAG-1 protein in liver is also seen in the mouse (Hsiao et al., 2000a).
Fig. 3.
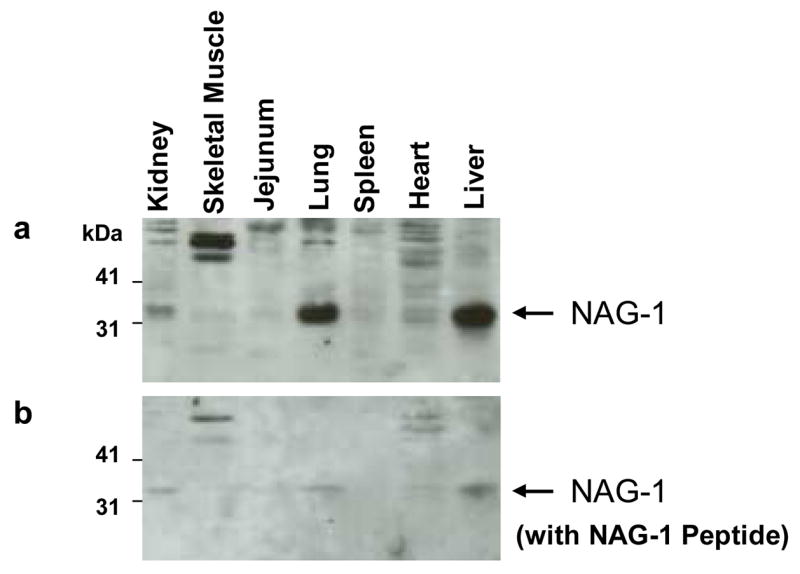
Expression profiles of NAG-1 protein in canine tissues demonstrated by Western blot analysis using 30 μg of cell lysate from each tissue separated by SDS-PAGE. (a) Polyclonal anti-human NAG-1 antibody. Canine NAG-1 appeared as a band ~ 35 kDa. (b) Result from incubating NAG-1 antibody with human NAG-1 peptide.
We also determined whether human NAG-1 antibody (Baek et al., 2001) specifically recognises canine NAG-1 protein. As shown in Fig. 3b, incubation of NAG-1 peptide with the antibody markedly attenuated the detection of specific bands (~35 kDa), demonstrating that anti-human NAG-1 antibody recognises canine NAG-1 protein. Interestingly, the intensity of an unexpected band (~60 kDa) seen in skeletal muscle was also diminished. This band probably represents pro-NAG-1 hemidimer (Bauskin et al., 2000).
3.3. Effects of NSAIDs and PPARγ ligands on NAG-1 expression in CCL-183
NAG-1 induction by NSAIDs is well established in human colorectal cancer cell lines, as well as osteosarcoma cell lines (Baek et al., 2002a). Based on this information, we sought to analyse NAG-1 induction in canine cancer cells. Canine osteosarcoma CCL-183 cells were treated with DMSO or NSAIDs (DFU, diclofenac, piroxicam, SC-560 and sulindac sulphide) to determine if these compounds induce NAG-1 in canine osteosarcoma cells. Plasmid containing canine NAG-1 cDNA was used as a positive RT-PCR control. As shown in Fig. 4a, NAG-1 induction was evident in piroxicam and SC-560 treated cells.
Fig. 4.
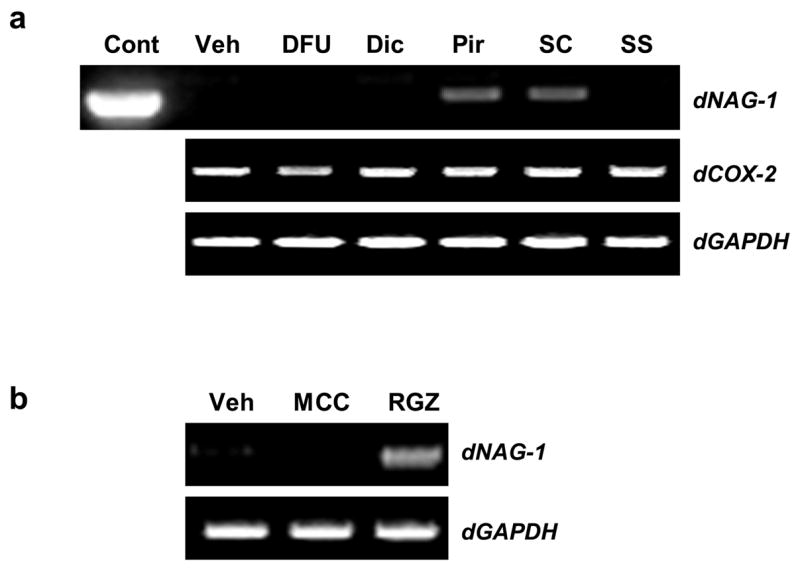
Effects of NSAID and PPARγ ligands on NAG-1 expression in canine osteosarcoma cancer cells determined by RT-PCR. (a) CCL-183 cells were treated for 24 h with DMSO vehicle (Veh) or NSAIDs: DFU: 5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl) phenyl-2 (5H)-furanonel, 100 μM; Dic: diclofenac, 100 μM; Pir: piroxicam, 100 μM; SC: SC-560, 50 μM; SS: sulindac sulphide, 30 μM. Plasmid containing canine NAG-1 cDNA was used as a positive control (Cont). (b) CCL-183 cells were treated for 24 h with vehicle (Veh) or PPARγ ligands: MCC: MCC-555, 5 μM; RGZ: rosiglitazone, 5 μM. RT-PCR was performed using total RNA isolated from cells. Equal loading was demonstrated by GAPDH RT-PCR.
COX-2 expression was also analysed because its expression has been known to be repressed by some NSAIDs (Xu et al., 1999). Treatment with DFU reduced COX-2 expression when compared to the vehicle, but other NSAIDs did not affect COX-2 expression.
PPARγ ligands have been characterised as having anti-tumorigenic effects in numerous types of human cancer showing induction of apoptosis and NAG-1 expression (Yamaguchi et al., 2006a). To see if the same phenomenon occurred in canine osteosarcoma, CCL-183 cells were treated with MCC-555 and rosiglitazone, both of which induce NAG-1 expression in human colorectal cancer cells. As shown in Fig. 4b, treatment with rosiglitazone induced NAG-1 expression, but treatment with MCC-555 did not.
4. Discussion
In the last decade, studies with microarray and PCR-based subtractive hybridisation have identified a number of genes that are targeted by anti-tumorigenic compounds. NAG-1 was identified from an indomethacin-induced gene library in our laboratory (Baek and Eling, 2006). Human NAG-1 encodes a protein with homology to members of the TGF-β superfamily. Intra- and inter-molecular bonds formed by six to nine conserved cysteine residues create a structure characteristic of the TGF-β superfamily.
NAG-1 is a dimeric secreted protein consisting of a long propeptide separated from the mature protein by furin-like proconvertase at a conserved RXXR sequence (Bauskin et al., 2000). Based on GenBank database comparison, the predicted canine NAG-1 amino acid sequence has nine cysteine residues and an RXXR sequence conserved with human, mouse, rat and chimpanzee NAG-1, suggesting that canine NAG-1 protein has a biological activity (Fig. 2).
It has been known that the human NAG-1 transcript is highly expressed in placenta and at lower levels in the colon, kidney and prostate (Paralkar et al., 1998a). Canine NAG-1 is highly expressed in the liver and lung, whereas human NAG-1 is not expressed in liver (Fig. 3). On the other hand, mouse NAG-1 is highly expressed in the liver and expressed at moderate levels in the kidney (Hsiao et al., 2000a), indicating a different distribution of basal NAG-1 expression among species. However, phylogenetic tree analysis (Fig. 2b) indicates that canine NAG-1 is more closely related to mouse NAG-1 than the corresponding human molecule in tissue distribution.
NSAIDs represent a promising approach to cancer therapy (Ulrich et al., 2006) because they inhibit COX-1 and/or COX-2, which are responsible for the formation of prostaglandins from arachidonic acid. Overexpression of COX-2 is often observed in many types of cancer cells and results in an increase of cell survival (Lin et al., 2001) and metastasis (Tsujii et al., 1997) and a reduction of apoptosis (Nzeako et al., 2002). COX inhibition by NSAIDs at the protein level is likely to be closely related to their anti-tumorigenic effects.
However, NSAIDs can also act through COX-independent mechanisms (Grosch et al., 2006). Induction of canine NAG-1 was observed only in the presence of piroxicam or SC-560. On the other hand, the NSAIDs diclofenac and sulindac sulphide did not induce NAG-1. Furthermore, NAG-1 induction was not observed in the presence of the COX-2 specific inhibitor DFU (Fig. 4). These data suggest that COX inhibition does not necessarily increase NAG-1 expression and a COX-independent pathway may be involved in NAG-1 induction by NSAID.
Several studies demonstrated that the PPARγ ligand rosiglitazone inhibits cell growth (Brockman et al., 1998). Rosiglitazone also markedly induced NAG-1 expression. Since canine NAG-1 is induced by both NSAID and PPARγ ligand treatment, it can be inferred that NAG-1 induction occurs via several mechanistic pathways.
5. Conclusion
The canine NAG-1 gene is more homologous with mouse and rat NAG-1, than with the corresponding human gene. Some NSAIDs and rosiglitazone up-regulate canine NAG-1, consistent with the notion that the NAG-1 protein may play an important role as a mediator for chemotherapeutic agents in canine cancer. Further studies will be required to elucidate the pathways for NAG-1 induction by NSAID and PPARγ ligands in dogs.
Acknowledgments
We thank Ms. Min Hwang for her technical assistance. We thank Ms. Misty R. Bailey for her critical reading of manuscript. This work was supported by grants from the National Institutes of Health (K22 ES011657) and Center of Excellence at The University of Tennessee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baek SJ, Eling TE. Changes in gene expression contribute to cancer prevention by COX inhibitors. Progress in Lipid Research. 2006;45:1–16. doi: 10.1016/j.plipres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Kim JS, Moore SM, Lee SH, Martinez J, Eling TE. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Molecular Pharmacology. 2005;67:356–364. doi: 10.1124/mol.104.005108. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Kim JS, Jackson FR, Eling TE, McEntee MF, Lee SH. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis. 2004a;25:2425–2432. doi: 10.1093/carcin/bgh255. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Kim JS, Nixon JB, DiAugustine RP, Eling TE. Expression of NAG-1, a transforming growth factor-β superfamily member, by troglitazone requires the early growth response gene EGR-1. Journal of Biological Chemistry. 2004b;279:6883–6892. doi: 10.1074/jbc.M305295200. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Wilson LC, Hsi LC, Eling TE. Troglitazone, a peroxisome proliferator-activated receptor γ (PPARγ) ligand, selectively induces the early growth response-1 gene independently of PPARγ. A novel mechanism for its anti-tumorigenic activity. Journal of Biological Chemistry. 2003;278:5845–5853. doi: 10.1074/jbc.M208394200. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002a;23:425–434. doi: 10.1093/carcin/23.3.425. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Wilson LC, Lee CH, Eling TE. Dual function of nonsteroidal anti-inflammatory drugs (NSAIDs): inhibition of cyclooxygenase and induction of NSAID-activated gene. Journal of Pharmacology and Experimental Therapeutics. 2002b;301:1126–1131. doi: 10.1124/jpet.301.3.1126. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-β superfamily member that has proapoptotic and antitumorigenic activities. Molecular Pharmacology. 2001;59:901–908. [PubMed] [Google Scholar]
- Bauskin AR, Zhang HP, Fairlie WD, He XY, Russell PK, Moore AG, Brown DA, Stanley KK, Breit SN. The propeptide of macrophage inhibitory cytokine (MIC-1), a TGF-β superfamily member, acts as a quality control determinant for correctly folded MIC-1. EMBO Journal. 2000;19:2212–2220. doi: 10.1093/emboj/19.10.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Moses HL. TGFB: the molecular Jekyll and Hyde of cancer. Nature Reviews Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, Abreu-Goris M, Newmark HL, Lipkin ML, DeCosse JJ, Bertagnolli MM. Cyclooxygenase-2 overexpresssion and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Research. 1996;56:2556–2560. [PubMed] [Google Scholar]
- Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-β superfamily. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman JA, Gupta RA, Dubois RN. Activation of PPARγ leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology. 1998;115:1049–1055. doi: 10.1016/s0016-5085(98)70072-1. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12:88–93. doi: 10.1097/00001648-200101000-00015. [DOI] [PubMed] [Google Scholar]
- Goldberg Y, Nassif II, Pittas A, Tsai LL, Dynlacht BD, Rigas B, Shiff SJ. The anti-proliferative effect of sulindac and sulindac sulfide on HT-29 colon cancer cells: alterations in tumor suppressor and cell cycle- regulatory proteins. Oncogene. 1996;12:893–901. [PubMed] [Google Scholar]
- Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. Journal of the National Cancer Institute. 2006;98:736–747. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- Hromas R, Hufford M, Sutton J, Xu D, Li Y, Lu L. PLAB, a novel placental bone morphogenetic protein. Biochimica et Biophysica Acta. 1997;1354:40–44. doi: 10.1016/s0167-4781(97)00122-x. [DOI] [PubMed] [Google Scholar]
- Hsiao EC, Koniaris LG, Zimmers-Koniaris T, Sebald SM, Huynh TV, Lee SJ. Characterization of growth-differentiation factor 15, a transforming growth factor β superfamily member induced following liver injury. Molecular and Cellular Biology. 2000;20:3742–3751. doi: 10.1128/mcb.20.10.3742-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Baek SJ, Flake GP, Loftin CD, Calvo BF, Eling TE. Expression and regulation of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) in human and mouse tissue. Gastroenterology. 2002;122:1388–1398. doi: 10.1053/gast.2002.32972. [DOI] [PubMed] [Google Scholar]
- Kingsley DM. The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes and Development. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim JS, Yamaguchi K, Eling TE, Baek SJ. Indole-3-carbinol and 3,3′-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochemical and Biophysical Research Communications. 2005;328:63–69. doi: 10.1016/j.bbrc.2004.12.138. [DOI] [PubMed] [Google Scholar]
- Lee SH, Yamaguchi K, Kim JS, Eling TE, Safe S, Park Y, Baek SJ. Conjugated linoleic acid stimulates an anti-tumorigenic protein NAG-1 in an isomer specific manner. Carcinogenesis. 2006;27:972–981. doi: 10.1093/carcin/bgi268. [DOI] [PubMed] [Google Scholar]
- Li PX, Wong J, Ayed A, Ngo D, Brade AM, Arrowsmith C, Austin RC, Klamut HJ. Placental transforming growth factor-B is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression. Journal of Biological Chemistry. 2000;275:20127–20135. doi: 10.1074/jbc.M909580199. [DOI] [PubMed] [Google Scholar]
- Lin MT, Lee RC, Yang PC, Ho FM, Kuo ML. Cyclooxygenase-2 inducing Mcl-1-dependent survival mechanism in human lung adenocarcinoma CL1.0 cells: involvement of phosphatidylinositol 3-kinase/Akt pathway. Journal of Biological Chemistry. 2001;276:48997–49002. doi: 10.1074/jbc.M107829200. [DOI] [PubMed] [Google Scholar]
- Liu T, Bauskin AR, Zaunders J, Brown DA, Pankhurst S, Russell PJ, Breit SN. Macrophage inhibitory cytokine 1 reduces cell adhesion and induces apoptosis in prostate cancer cells. Cancer Research. 2003;63:5034–5040. [PubMed] [Google Scholar]
- Mohammed SI, Craig BA, Mutsaers AJ, Glickman NW, Snyder PW, deGortari AE, Schlittler DL, Coffman KT, Bonney PL, Knapp DW. Effects of the cyclooxygenase inhibitor, piroxicam, in combination with chemotherapy on tumor response, apoptosis, and angiogenesis in a canine model of human invasive urinary bladder cancer. Molecular Cancer Therapeutics. 2003;2:183–188. [PubMed] [Google Scholar]
- Nzeako UC, Guicciardi ME, Yoon JH, Bronk SF, Gores GJ. COX-2 inhibits Fas-mediated apoptosis in cholangiocarcinoma cells. Hepatology. 2002;35:552–559. doi: 10.1053/jhep.2002.31774. [DOI] [PubMed] [Google Scholar]
- Paralkar VM, Vail AL, Grasser WA, Brown TA, Xu H, Vukicevic S, Ke HZ, Qi H, Owen TA, Thompson DD. Cloning and characterization of a novel member of the transforming growth factor-β/bone morphogenetic protein family. Journal of Biological Chemistry. 1998;273:13760–13767. doi: 10.1074/jbc.273.22.13760. [DOI] [PubMed] [Google Scholar]
- Reddy BS, Hirose Y, Lubet R, Steele V, Kellof GJ, Paulson S, Seibert K, Rao CV. Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Research. 2000;60:293–297. [PubMed] [Google Scholar]
- Tan M, Wang Y, Guan K, Sun Y. PTGF-β, a type β transforming growth factor (TGF-β) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-β signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:109–114. doi: 10.1073/pnas.97.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils, and pharmacogenetics. Nature Reviews Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- Xu XM, Sansores-Garcia L, Chen XM, Matijevic-Aleksic N, Du M, Wu KK. Suppression of inducible cyclooxygenase 2 gene transcription by aspirin and sodium salicylate. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5292–5297. doi: 10.1073/pnas.96.9.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Lee SH, Eling TE, Baek SJ. A novel peroxisome proliferator-activated receptor γ ligand, MCC-555, induces apoptosis via posttranscriptional regulation of NAG-1 in colorectal cancer cells. Molecular Cancer Therapeutics. 2006a;5:1352–1361. doi: 10.1158/1535-7163.MCT-05-0528. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Liggett JL, Kim NC, Baek SJ. Anti-proliferative effect of horehound leaf and wild cherry bark extracts on human colorectal cancer cells. Oncology Reports. 2006b;15:275–281. [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Lee SH, Eling TE, Baek SJ. Identification of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) as a novel downstream target of phosphatidylinositol 3-kinase/AKT/GSK-3β pathway. Journal of Biological Chemistry. 2004;279:49617–49623. doi: 10.1074/jbc.M408796200. [DOI] [PubMed] [Google Scholar]