Abstract
Cortico-cortical inhibition and facilitation induced by paired transcranial magnetic stimulation (TMS) of the human motor cortex were investigated in the distal muscle opponens pollicis (OP) and the proximal muscle biceps brachii (BB) of normal subjects.
The test response evoked by TMS (125 % of motor threshold, MTh) in the relaxed OP and BB muscles was inhibited by a conditioning TMS (80 % of MTh) at short interstimulus intervals (ISIs; 2-5 ms) and facilitated at longer ISIs (10-25 ms). The test response was significantly less inhibited at short ISIs and more facilitated at long ISIs in the BB than OP.
The MTh at rest was significantly lower for the OP than for the BB, indicating a greater excitability of OP cortical area. However, the above pattern of inhibition and facilitation was preserved both when the stimulus intensity was adjusted to evoke test responses of matched size in the two muscles and within an ample range of conditioning stimulus intensities.
The use of a circular coil or a focal figure-of-eight coil produced no qualitative differences in the pattern of inhibition and facilitation in either muscle.
The significant difference in MTh between muscles was lost during voluntary activation. In both muscles, pre-innervation abolished the cortico-cortical facilitation and reduced the cortico-cortical inhibition. However, the latter remained larger in the OP than BB muscle.
We suggest that the different potency of intracortical inhibitory and facilitatory circuits directed towards distal and proximal arm muscles is related to their diverse prevalent functions.
Paired transcranial magnetic stimulation (TMS) of the human motor cortex, with a conditioning-test protocol, provides an indication of the excitability of the neuronal circuits underlying intracortical inhibition and facilitation, thus allowing investigation of some aspects of the functional organization of motor cortical output. Since the first description (Kujirai et al. 1993), this technique has been used to study the ipsilateral cortico-cortical interactions in various neurological disorders (Fong et al. 1993; Ridding et al. 1995a, b; Brown et al. 1996; Ziemann et al. 1996c; Yokota et al. 1996; Abbruzzese et al. 1997).
In normal subjects, the test motor potential (MEP) evoked in the intrinsic hand muscles is inhibited by a conditioning subthreshold stimulus at short interstimulus intervals (ISIs) of between 1 and 5 ms, possibly reflecting the activity of a subset of intracortical GABAergic interneurones (Ridding et al. 1995a; Ziemann et al. 1996a). Less is known of the mechanisms underlying the facilitation of the MEP that takes place in the same muscles with a conditioning stimulus at longer ISIs, between 10 and 25 ms, which is also likely to be at least in part cortical in origin (Ziemann et al. 1996b; Nakamura et al. 1997).
No data have been reported about the amplitude and time course of intracortical inhibition and facilitation for the proximal arm muscles. These muscles are known to have diverse functional roles and a different cortical representation, with a possible bilaterally distributed cortical motor outflow (Colebatch et al. 1990). Proximal arm muscles are relatively spared in upper motoneurone lesions (Colebatch & Gandevia, 1989) and the mechanisms of functional recovery after stroke appear quite different for arm and hand muscles (Turton et al. 1996). We recently found (Schieppati et al. 1996) a selective increase in MEP size in one of two distal muscles active in a ‘precision’ task (grip as compared with a ‘power’grasp), whereas an increase in MEP size was concurrently observed in two proximal muscles active in a different precision task (push as compared with ‘power’load). The differences in the distribution of these task-related effects across synergistic muscles active at proximal or distal joints might be the expression of a different ‘sculpturing’ effect of intracortical inhibitory or facilitatory mechanisms across different zones of the motor cortex.
In this paper, we investigated the possible differences in the intracortical facilitation and inhibition of the opponens pollicis (OP) and biceps brachii (BB) muscles of normal subjects to test whether the potency of cortico-cortical connections is the same in distal and proximal muscles.
METHODS
Subjects
Sixteen right-handed normal subjects, ten males and six females, aged 24-50 years, participated in the experiments. The use of magnetic brain stimulation had been approved by the local ethics committee and all subjects gave their written informed consent.
EMG recordings
Electromyographic activity (EMG) was recorded from the OP and BB muscles of the right side using pairs of Ag-AgCl disc electrodes placed 1 cm apart. The EMG signals were amplified (2000-5000 times), filtered (bandwidth, 50-2000 Hz; -3 dB), captured on a computer and converted by an analog-to-digital interface at a sampling rate of 2.5 kHz for further analysis. Each recorded epoch lasted 200 ms, of which 100 ms preceded the stimulus.
Paired magnetic stimulation
TMS was performed with two Magstim 200 stimulators connected to the same stimulating coil through a Bistim module. MEPs were evoked separately for each muscle in the same session. We used either a circular coil (outer diameter, 14 cm; maximum magnetic field, 2.5 T) positioned flat over the vertex with the current flowing in an anticlockwise direction when viewed from above in order to preferentially activate the left hemisphere, or a focal figure-of-eight coil oriented so that the induced electric current flowed in the brain in a posterior-to-anterior and lateral-to-medial direction and positioned at the best spot over the cortical representation areas for either the OP or the BB muscle.
Protocol I (9 subjects)
Paired stimuli were applied in relaxed subjects according to the procedure originally described by Kujirai et al. (1993); the second stimulus, referred to as the test stimulus, was delivered at an intensity equal to 125 % of motor threshold (MTh) at rest, while the first conditioning stimulus was subthreshold (80 %) and was timed to precede the test stimulus at ISIs of between 2 and 25 ms for each muscle. For either muscle, MTh was defined as the lowest stimulus intensity evoking an MEP with an amplitude of at least 50 μV in the relaxed muscles in 50 % of eight trials. To determine MTh, the stimulus intensity was changed in steps of 1 % of the maximum stimulator output.
Protocol II (7 subjects)
The test stimulus intensity was adjusted in order to evoke motor responses of a matched size (approximately 0.5-1.0 mV peak-to-peak amplitude) in the two relaxed muscles, while the conditioning stimulus was still 80 % of the rest MTh for each muscle. Only ISIs of 3 ms (inhibition) and 12 ms (facilitation) were tested.
Protocol III (6 subjects)
The effect of varying the intensity of the conditioning stimulus was investigated using the focal coil. For either the OP or the BB relaxed muscles, the intensity of the conditioning stimulus was progressively reduced in 5 % steps of the stimulator output starting from the rest threshold values for each muscle. The test stimulus intensity was adjusted in order to evoke motor responses of a matched size (approximately 0.4-0.8 mV peak-to-peak amplitude) in the two muscles. Only ISIs of 3 ms (inhibition) and 12 ms (facilitation) were tested.
Protocol IV (5 subjects)
To investigate the influence of pre-innervation, the subjects were requested to maintain a voluntary tonic contraction of the target muscles (approximately 5-10 % of maximum EMG activity, with the help of an audiovisual feedback) by opposing the thumb to the index finger (for the OP) or sustaining a load secured at the wrist with the elbow joint flexed at about 30 deg (for the BB). It was checked that the EMG activity in the prestimulus time period was kept constant and similar across the different trials. The circular coil was used and the test stimulus intensity was adjusted in order to evoke motor responses of matched size (approximately 1.0 mV peak-to-peak amplitude) in the two muscles; the conditioning stimulus intensity was 80 % of the active MTh. Only ISIs of 3 and 12 ms were tested.
For all the experimental protocols, at least eight non-conditioned and eight conditioned trials were collected in each subject for each ISI. A random order of presentation of the different ISIs was used. Conditioned and non-conditioned stimuli were randomly intermixed and given every 10 s. Throughout the experiment we checked that the threshold intensity did not change and that the conditioning stimulus alone did not evoke any muscle potential. Muscle relaxation was checked by audiovisual EMG monitoring. The resting trials in which background EMG activity was present in the prestimulus time period were rejected off-line.
Data analysis
Measurements were made on individual responses. The mean size (area of the rectified EMG signal) of the conditioned responses, at each ISI, was then calculated and expressed as a percentage of the mean size of the non-conditioned responses. In protocol I, for statistical evaluation of the differences between the two muscles, the ISIs were divided into two groups (ISIs of 2-5 and 10-25 ms) since opposite effects on the test response could be expected for such ISIs (see Ridding et al. 1995a). A two-way analysis of variance (ANOVA) was used in order to evaluate the effect of muscle (OP/BB), different ISIs, and the interaction between muscle and ISI (muscle*ISI). In protocol III, a two-way analysis of covariance (ANCOVA) was used in order to evaluate the effect of muscle (OP/BB) and intensity of the conditioning stimulus. In protocols II and IV, the differences between OP and BB MEPs conditioned at ISIs of 3 and 12 ms were evaluated using Student's two-tailed paired t test. P < 0.05 was considered significant.
Input-output curves
The input-output curves of MEPs from the OP and BB muscles were constructed for four subjects both at rest and during voluntary contraction (approximately 5-10 % of the maximum) as described above. A circular coil was used and the stimulus intensity was progressively increased in 5-10 % steps up to 100 % stimulator output. At least four stimuli were delivered every 10 s for each step. Student's two-tailed paired t test was used to compare intensity threshold values at rest and during voluntary contraction.
RESULTS
Cortical stimulation at rest
The time course of the changes induced in the test MEPs (125 % of MTh at rest) of OP and BB muscles by the conditioning subthreshold (80 % of MTh) stimuli was roughly the same in the two muscles of nine subjects (Fig. 1). However, in the BB the amount of inhibition at ISIs of 2-5 ms was smaller (ANOVA, n = 9, F = 4.03, P < 0.05; no muscle*ISI significant interaction) and the amount of facilitation at ISIs of 10-25 ms was much larger (F = 22.98, P < 0.0001; no muscle*ISI significant interaction) than in the OP muscle. Examples of intracortical inhibition (ISI, 3 ms) and facilitation (ISI, 10 ms) of OP and BB MEPs in one representative subject are shown in Fig. 2. In the above experiments, we routinely used a large round coil to enable the use of 125 % threshold intensity to evoke the test MEPs in both muscles of all the subjects. This invariably corresponded to a higher stimulator output for the BB muscle, owing to its higher rest MTh than that for OP. However, the time course and relative magnitude of the cortico-cortical interactions in the BB muscle were remarkably similar when in three subjects (having low MTh for the BB at rest) both a circular and a focal figure-of-eight coil was used (Fig. 3A). In addition, in two subjects the time course of cortico-cortical interactions in BB and OP was investigated using the focal coil (Fig. 3B). The BB MEP still remained less inhibited (pooled data, ANOVA, F = 19.8, P < 0.0001) and more facilitated (pooled data, ANOVA, F = 31.2, P < 0.0001) than the OP MEP.
Figure 1. Intracortical inhibition and facilitation of distal and proximal arm muscles induced by paired transcranial magnetic cortical stimulation.
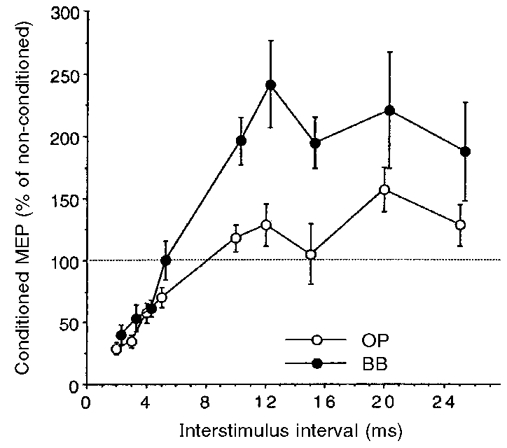
Time course of paired-pulse inhibition (at ISIs of 2-5 ms) and facilitation (at ISIs of 10-25 ms) in the opponens pollicis (OP; ○) and biceps brachii (BB; •) muscles. A circular coil was used and stimulus intensity was set at 80 % of the rest motor threshold (MTh) for the conditioning stimulus and at 125 % of the MTh for the test stimulus for both muscles. Each point corresponds to the mean size (±s.e.m.) of the conditioned motor evoked potential (MEP) in 9 subjects. Changes induced by the conditioning subthreshold stimuli were significantly different in the two muscles. In the BB, inhibition at ISIs of 2-5 ms was less marked and facilitation at ISIs of 10-25 ms was more marked than in the OP. Note that the filled circles are slightly shifted to the right to avoid superimposition of error bars.
Figure 2. Examples of intracortical inhibition (ISI, 3 ms) and facilitation (ISI, 10 ms) in OP and BB muscles.
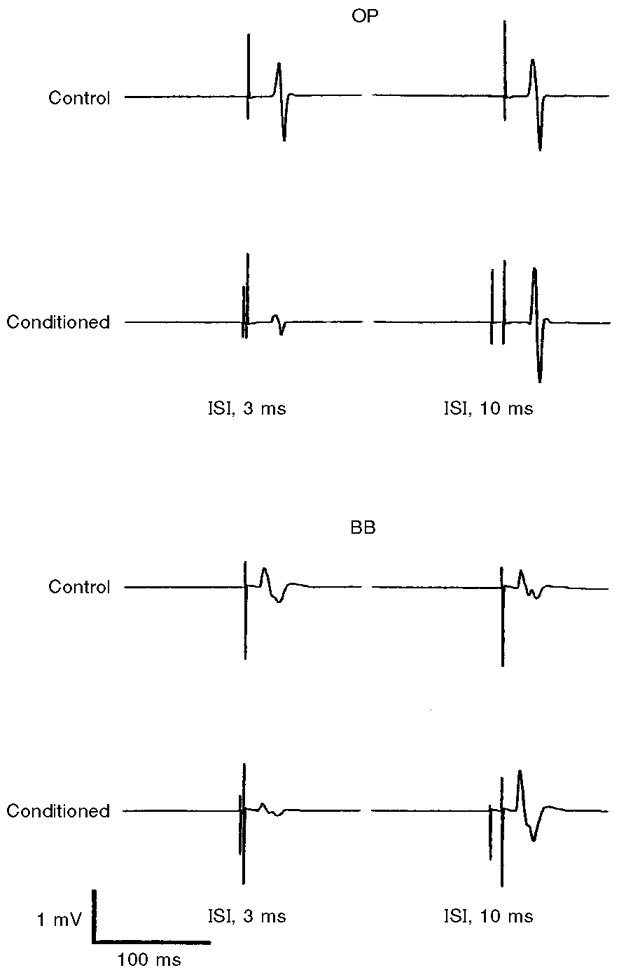
Each trace is the average of 5 recordings from the OP and BB muscles in a single representative subject. The test MEP of the OP muscle was largely inhibited at an ISI of 3 ms and slightly facilitated at an ISI of 10 ms. Less inhibition and more facilitation was observed for the corresponding ISIs in the BB muscle. Stimulus intensities and coil as in Fig. 1.
Figure 3. Comparison of intracortical inhibition and facilitation induced by circular and figure-of-eight coils.
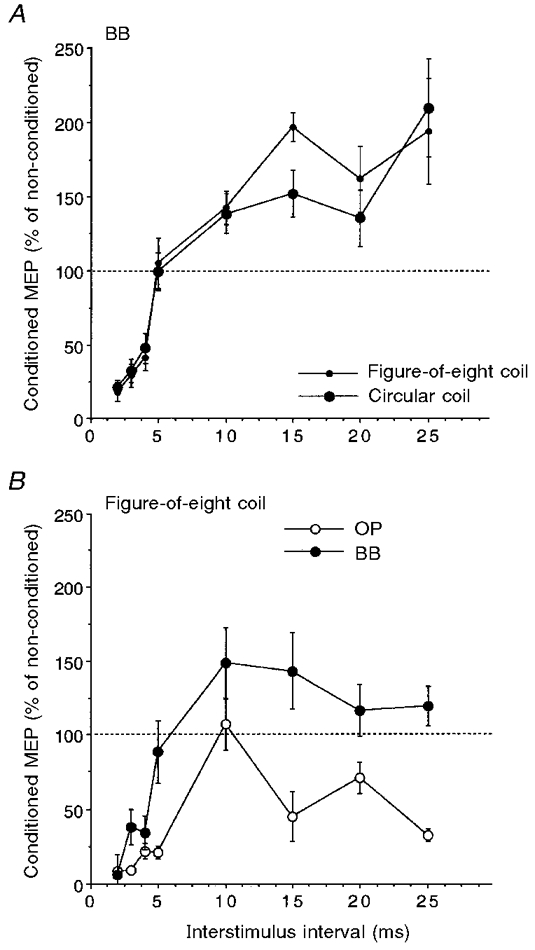
A, time course of inhibition (at ISIs of 2-5 ms) and facilitation (at ISIs of 10-25 ms) induced in the BB muscle using a circular (large filled circles) and a focal figure-of-eight (small filled circles) coil. Stimulus intensities as in Fig. 1. Each point corresponds to the mean size (±s.e.m.) of the conditioned MEP in 3 subjects. Changes induced by the conditioning subthreshold stimuli were remarkably similar with either coil. B, time course of inhibition and facilitation in the OP (○) and BB (•) muscles. A focal figure-of-eight coil was used and stimulus intensity was set at 80 % of the rest MTh for the conditioning stimulus and at 125 % of the MTh for the test stimulus for both muscles. Each point corresponds to the mean size (±s.e.m.) of the conditioned MEP in 2 subjects. In BB, inhibition at ISIs of 2-5 ms was less marked and facilitation at ISIs of 10-25 ms was more marked than in the OP.
The differences observed between proximal and distal muscles in the percentage changes of the conditioned MEP amplitude might be partly related to the absolute MEP size in the two muscles. In fact, the size of test MEPs was significantly (two-tailed paired t test, n = 9, P < 0.05) smaller in the BB muscle (1.35 ± 0.83 mV ms; mean ±s.d.) than in the OP muscle (3.60 ± 2.44 mV ms). This reflects the different input-output curves of the two muscles at rest, illustrated for four subjects in Fig. 4A; the growth of MEP size at progressively increasing stimulus intensities was earlier, larger and steeper in the OP than in the BB. In addition, the stimulus intensity corresponding to the MTh at rest was slightly but significantly (two-tailed paired t test, n = 9, P < 0.02) lower for the OP muscle (48.5 ± 4.3 % of the maximal stimulator output; mean ±s.d.) than for the BB muscle (54.4 ± 8.7 %). By plotting the percentage mean inhibition (at ISIs of 2-5 ms) or facilitation (at ISIs of 10-25 ms) of the conditioned MEPs observed in each subject against the absolute mean non-conditioned MEP size (Fig. 5) it can be seen that, although most BB values lay on the left part of the curve, an indication of less inhibition and more facilitation in the BB was present. This would indeed point to a real smaller cortico-cortical inhibitory effect for the BB than for the OP muscle, irrespective of any effect connected to the size of the test MEPs.
Figure 4. Input-output curves of OP and BB muscles upon transcranial magnetic cortical stimulation with a circular coil.
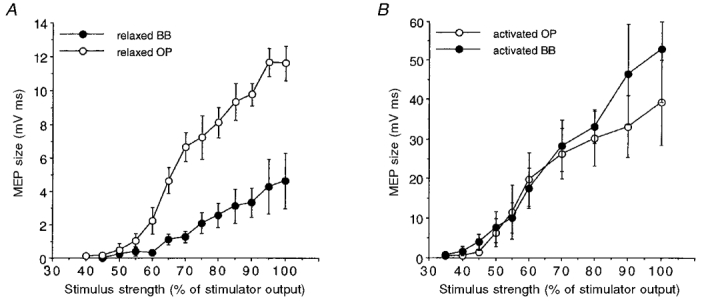
The graphs show the relationships between stimulus intensity and MEP size of the OP (○) and BB (•) muscles at rest (A) and during voluntary activation (5-10 % of maximum EMG activity; B). Each point corresponds to the mean size (±s.e.m.) of the MEP in 4 subjects. A larger and steeper increase in MEP size of the OP was observed at rest, while during voluntary activation the curves were similar for both the OP and BB. Note the different range of the two ordinates.
Figure 5. Relationship between the degree of intracortical inhibition and facilitation and absolute MEP size in OP and BB muscles.
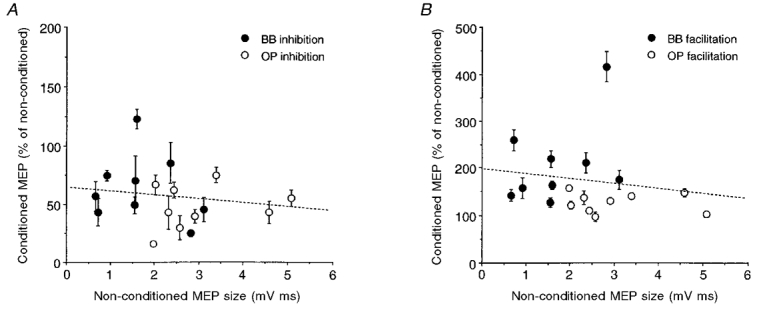
The percentage mean inhibition (±s.e.m.) at ISIs of 2-5 ms (A) and facilitation at ISIs of 10-25 ms (B) of each subject (n = 9) are plotted against the respective absolute mean size of the test MEPs. In spite of test MEPs being of smaller size in the BB (•) than in the OP (○), conditioning stimulation seemed to induce quantitatively different inhibitory and facilitatory effects in the two muscles. The slopes of the dashed lines (best fitting the pooled data) are not significantly different from zero. Stimulus intensities and coil as in Fig. 1.
This conclusion was supported by comparison of intracortical inhibition and facilitation in the two muscles using test MEPs of a similar size. When the stimulus intensity was adjusted to evoke test MEPs of a matched size in the two muscles of seven subjects at rest, the same subthreshold (80 %) conditioning stimulus still induced - in BB with respect to OP - less inhibition at an ISI of 3 ms (OP conditioned MEP: 15.4 ± 7.9 % (mean ±s.d.) of non-conditioned MEP; BB conditioned MEP: 39.6 ± 19.5 %; two-tailed paired t test, n = 7, P < 0.05) and more facilitation at an ISI of 12 ms (OP conditioned MEP: 126.1 ± 43.2 %; BB conditioned MEP: 213.8 ± 86.5 %; two-tailed paired t test, n = 7, P < 0.01). Absolute MEP sizes (mean values) observed in these subjects are shown in Fig. 6A.
Figure 6. Comparison of intracortical inhibition and facilitation of size-matched MEPs of OP and BB muscles.
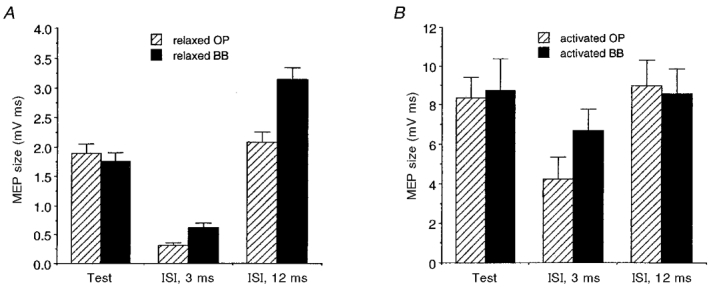
The mean size (±s.e.m.) of test and conditioned MEPs (at ISIs of 3 and 12 ms) of the OP ( ) and BB (▪) muscles is plotted both for rest and active conditions. At rest (A; n = 7), significantly less inhibition and more facilitation was observed in the BB than in the OP muscle. In both muscles, voluntary activation (B; 5-10 % of maximum EMG activity; n = 5) clearly reduced the inhibition at an ISI of 3 ms (which remained significant only in the OP muscle) and abolished the facilitation at an ISI of 12 ms. A circular coil was used with a stimulus intensity evoking test MEPs of a matched size in the two muscles, while the conditioning stimulus was always 80 % of the MTh at rest (A) or during activation (B).
) and BB (▪) muscles is plotted both for rest and active conditions. At rest (A; n = 7), significantly less inhibition and more facilitation was observed in the BB than in the OP muscle. In both muscles, voluntary activation (B; 5-10 % of maximum EMG activity; n = 5) clearly reduced the inhibition at an ISI of 3 ms (which remained significant only in the OP muscle) and abolished the facilitation at an ISI of 12 ms. A circular coil was used with a stimulus intensity evoking test MEPs of a matched size in the two muscles, while the conditioning stimulus was always 80 % of the MTh at rest (A) or during activation (B).
The different MTh in the relaxed condition between OP and BB muscles and its effect on the absolute strength of the conditioning stimulus might account for some of the observed results. To examine this possibility we investigated the effect of systematically changing the conditioning stimulus intensity. In six subjects, intracortical inhibition (ISI, 3 ms) and facilitation (ISI, 12 ms) were compared in the two muscles by progressively decreasing the intensity of the conditioning stimulus in 5 % steps of the stimulator output starting from the relaxed MTh value. In each subject the maximum amount of intracortical inhibition was greater in the OP than in the BB size-matched MEPs (mean ±s.d. MTh: OP, 54.8 ± 5.5 % of the stimulator output; BB, 59.6 ± 5.8 %), irrespective of the absolute values of the conditioning stimulus intensity. Intracortical inhibition (ISI, 3 ms) was significantly greater in the OP than in the BB muscle (ANCOVA, n = 6; muscle: F = 4.3, P < 0.05; intensity: F = 6.11, P < 0.05) (Fig. 7A). On the other hand, the intracortical facilitation (ISI, 12 ms) was significantly greater in the BB than in the OP muscle (ANCOVA, n = 6; muscle: F = 5.81, P < 0.005; intensity: F = 22.1, P < 0.01) (Fig. 7B).
Figure 7. Effect of varying the conditioning stimulus intensity on intracortical inhibition and facilitation of size-matched MEPs of OP and BB muscles.
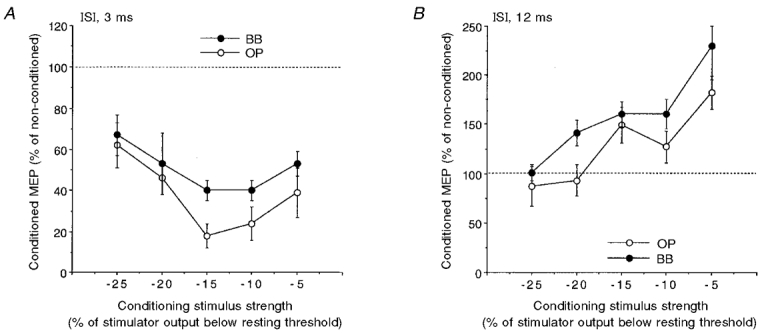
Intracortical inhibition (ISI, 3 ms; A) and facilitation (ISI, 12 ms; B) in the relaxed OP (○) and BB (•) muscles. A figure-of-eight coil with a stimulus intensity evoking test MEPs of a matched size in the two muscles was used. The conditioning stimulus intensity was progressively reduced in 5 % steps of the stimulator output starting from the rest threshold value. Each point corresponds to the mean value (±s.e.m.) in 6 subjects. The maximum amount of intracortical inhibition (A) was greater in the OP than in the BB, irrespective of the absolute conditioning stimulus intensity; conversely, the maximum amount of intracortical facilitation (B) was greater in the BB than in the OP muscle.
Cortical stimulation during voluntary contraction
The effect of voluntary tonic contraction (approximately 5-10 % of maximum) on intracortical inhibition and facilitation of size-matched test MEPs in the OP and BB muscles was investigated in five subjects. In both muscles, voluntary pre-innervation of the target muscles markedly reduced the inhibition at an ISI of 3 ms (OP conditioned MEP: 48.4 ± 18.0 % (mean ±s.d.); BB conditioned MEP: 78.8 ± 13.0 %) and almost abolished the facilitation at an ISI of 12 ms (OP conditioned MEP: 105.0 ± 8.0 %; BB conditioned MEP: 103.0 ± 13.0 %). However, the BB test MEP obtained during voluntary contraction was still significantly less inhibited at an ISI of 3 ms than the OP test MEP (two-tailed paired t test, n = 5, P < 0.05; Fig. 6B).
In this regard, it should be noted that the input-output curves of the OP and BB muscles were similar during voluntary activation (Fig. 4B), the MEP size increase being larger in the BB muscle at high stimulation intensity (Fig. 4B). The MTh during activation was not significantly different in the two muscles (OP: 43.7 ± 6.3 % (mean ±s.d.) of the maximal stimulator output; BB: 39.1 ± 5.5 %; two-tailed paired t test, n = 8, P > 0.1). Actually, with respect to the rest condition, the MTh was slightly lower for the BB than for the OP muscle.
DISCUSSION
The present results show that ipsilateral intracortical inhibition and facilitation operate in a similar way in the proximal arm muscles (BB) and distal hand muscles (OP). The time course of the effects is analogous in both muscles, but quantitative differences are present, whereby inhibition appears to be smaller and facilitation larger for the proximal than distal muscles. Corticomotoneuronal (CM) projections to the distal intrinsic hand muscles, therefore, seem to be more prone to short-latency inhibitory influences of cortical origin than those to the proximal arm muscles. Conversely, the latter appear to be more susceptible to longer-latency facilitatory influences of presumably cortical origin.
It has been suggested (Ziemann et al. 1996b) that intracortical inhibition and facilitation are two separate phenomena and that their effects within the motor cortex may be explained by the convergence of two independent inputs onto a common neurone, possibly the pyramidal cell itself. If this is the case, the different balance between inhibitory and facilitatory influences observed in distal and proximal muscles would suggest that the inhibitory connections operating for the CM cells directed to the proximal arm muscles are less potent and that a larger amount of facilitatory influence is present.
Superimposable results obtained with the circular and figure-of-eight coil indicate that the extent of the motor cortical area invaded by the stimulus current is not accountable for the differential effects in the two muscles. However, the direction of the current induced with different orientations of the focal coil is critical as far as the amount of cortico-cortical inhibition is concerned (Werhahn et al. 1994; Hanajima et al. 1998). We consistently used a posterior-to-anterior and lateral-to-medial orientation of the focal coil for stimulating the cortical areas of the two muscles; the similarity of the results obtained using either coil would suggest that the I3 wave was similarly affected in each muscle in both conditions.
As tested by separate experiments, the observed differences in the amount of intracortical inhibition and facilitation cannot be explained by the absolute differences in the size of the test MEPs in the two muscles. As expected, the size of the motor responses evoked by TMS in the relaxed OP and BB muscles increased with increasing stimulus intensity, without reaching a plateau level within the range of the stimulator ouput (Devanne et al. 1997). The input-output relationships were similar in the two muscles at low stimulus intensities and in this range it was possible to obtain MEPs of the same size in both muscles. All the same, conditioning TMS induced less inhibition and more facilitation in the BB muscle.
On the other hand, the differences in the amplitudes and time courses of conditioned OP and BB MEPs cannot be explained by the higher MTh in BB than in OP relaxed muscles, so that 80 % of the relaxed threshold was not the same relative to the active threshold between the two muscles. By using various intensities of the conditioning stimuli we still observed a significantly greater inhibition in the OP muscle within an ample range of the conditioning stimulus intensities adopted.
Judging from the thresholds for relaxed and active muscles, -15 % intensity of the conditioning stimulus must mean around the threshold for active muscle in the OP and even suprathreshold in the BB. If so, it might be argued that the observed effects are not entirely cortical and that a spinal contribution should also be considered. A control experiment was, therefore, made in two subjects to test, with the focal coil, the influence of a conditioning stimulus equal to 90 % of active MTh on the relaxed muscles. In this case, the effects must be produced at a cortical level. Figure 8 (upper panel) shows that the intracortical inhibition still remained much greater (pooled data, two-tailed unpaired t test, P < 0.0001) in the OP than in the BB relaxed muscles.
Figure 8. Comparison of intracortical inhibition and facilitation induced by a subthreshold conditioning stimulus in relaxed and activated OP and BB muscles.
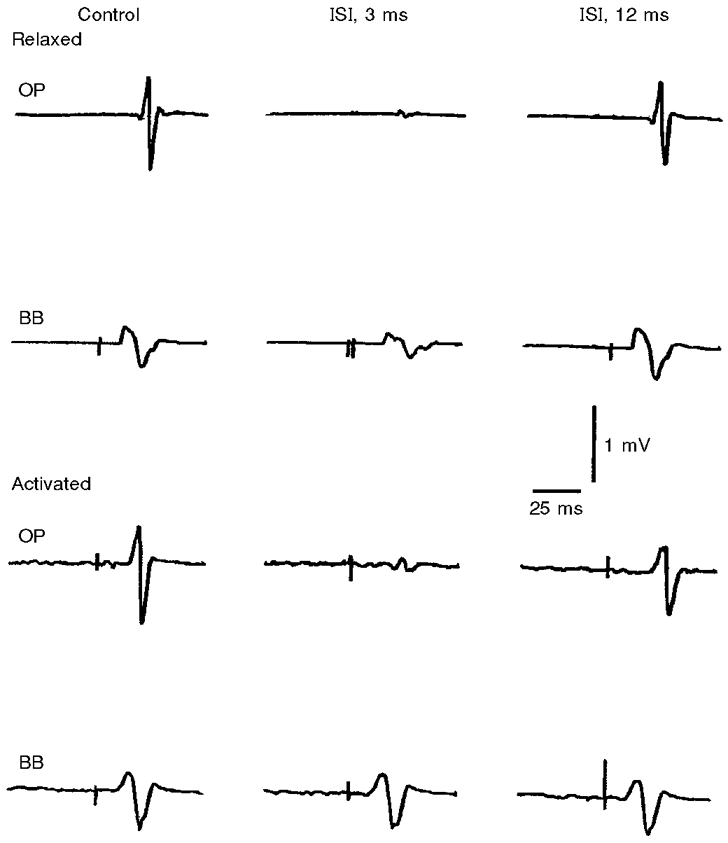
Each trace is the average of 5 recordings from the OP and BB muscles in a single subject. The intensity of the conditioning stimulus was set at 90 % of the active MTh for each muscle. Intracortical inhibition (ISI, 3 ms) was more pronounced in the OP than in the BB muscle under both relaxed (upper panel) and activated (lower panel) conditions. With this conditioning stimulus intensity no consistent intracortical facilitation (ISI, 12 ms) was observed in either muscle under both conditions.
Differences in the balance between intracortical inhibition and facilitation between the distal and proximal muscles were also found when the cortical excitability was investigated during a slight tonic contraction of the target muscles. Others have shown that the threshold differences between OP and BB muscles decrease during mild voluntary activation (Mazzocchio et al. 1994) and that the input- output relationships assume a non-linear sigmoidal shape for both muscles, possibly influenced by the characteristics of motoneuronal recruitment and synchronization (Devanne et al. 1997). Contraction of the target muscles reduced the intracortical inhibition and almost abolished the intracortical facilitation in both the OP and BB muscles. This was confirmed in our study also using the focal coil with a conditioning stimulus equal to 90 % of the active MTh (Fig. 8, lower panel). These findings had already been reported for the intrinsic hand muscles (Ridding et al. 1995c) and attributed to a reduced excitability of intracortical circuits in the cortical areas projecting to the target muscle. While a tuning down of the inhibitory influences might be important for the recruitment of CM cells in the intended motor activity, such an explanation cannot account for the ‘switch off’ of the facilitatory influences. One might postulate that the motor drive induces excitatory effects that are more effective on the CM cells than on inhibitory interneurones, thus accounting for the reduced intracortical inhibition; however, the same motor drive might lead to a sort of functional ‘occlusion’ of facilitatory interneurones because of saturated excitability of CM cells. Whatever mechanisms are responsible for the qualitatively similar changes in the two muscles with intracortical inhibition and facilitation during voluntary contraction, such changes are quantitatively different. In fact, intracortical inhibition was reduced in both muscles, remaining significantly effective only in the OP muscle, while intracortical facilitation was apparently abolished in both muscles during pre-innervation.
The findings observed both at rest and during voluntary activation of the target muscles point to a different potency of inhibitory and facilitatory circuits directed towards the OP and those directed towards the BB muscle. This different organization of intracortical motor control mechanisms is probably related to the prevalent function of the distal and proximal muscles in man. Intrinsic hand muscles are mainly involved in finely controlled motor tasks, where a sharp, sudden modulation of the produced force can often be required. A strong inhibitory control, therefore, might be necessary in the case of distal muscles. In addition, it could help in achieving a selective contraction of a single muscle (Turton et al. 1993; Schieppati et al. 1996). In contrast, proximal arm muscles are frequently engaged, often together with synergistic muscles, in tonic postural motor tasks, where force modulation can be less substantial and more progressive, with the result that the balance between inhibitory and facilitatory control may be shifted towards the latter.
The observed differences in the extent of intracortical inhibition and facilitation are in keeping with previous reports pointing to a different functional organization of the CM system for distal and proximal arm muscles. The susceptibility of CM cells to excitation is determined by several factors, including cell size and the relative magnitudes of facilitatory and inhibitory inputs. Several studies indicate that a complex overlapping of output zones exists in the primary motor cortex, with single CM cells being connected with the activity of several muscles (for a review, see Turton et al. 1993). In monkeys, direct monosynaptic CM projections are more extensive for the distal than for the proximal muscles (see Porter & Lemon, 1993). A stronger CM influence on motoneurones innervating the distal muscles has also been demonstrated in man (Palmer & Ashby, 1992). Nevertheless, it has been shown (Colebatch et al. 1990) that spinal motoneurones innervating the proximal arm muscles receive an additional medium-latency facilitation, besides the monosynaptic cortical projection. This observation has been related to activity in small diameter corticospinal fibres (possibly of bilateral origin) or to an indirect polysynaptic pathway. Indeed, in the monkey, proximal muscle activity is related mostly to slow pyramidal tract neurones (Matsunami & Hamada, 1980). It might be postulated, therefore, that the contribution of smaller size CM cells is greater with respect to the proximal arm muscles than distal hand muscles.
In fact, it has been shown that the more rapidly conducting pyramidal fibres are more susceptible to stroke (Branston et al. 1988), hence, the existence of a larger number of small neurones in the cortical representation areas of proximal muscles may partly explain why the latter are relatively spared in stroke (Colebatch & Gandevia, 1989) and why their functional recovery is poorly correlated with the behaviour of MEPs (Turton et al. 1996).
References
- Abbruzzese G, Buccolieri A, Marchese R, Trompetto C, Mandich P, Schieppati M. Intracortical inhibition and facilitation are abnormal in Huntington's disease: a paired magnetic stimulation study. Neuroscience Letters. 1997;228:87–90. doi: 10.1016/s0304-3940(97)00363-7. 10.1016/S0304-3940(97)00363-7. [DOI] [PubMed] [Google Scholar]
- Branston NM, Bentivoglio P, Momma F, Symon L. Changes in pyramidal tract conduction with experimental brain-stem ischaemia in the monkey. Electroencephalography and Clinical Neurophysiology. 1988;69:469–475. doi: 10.1016/0013-4694(88)90069-7. [DOI] [PubMed] [Google Scholar]
- Brown P, Ridding MC, Werhahn KJ, Rothwell JC, Marsden CD. Abnormalities of the balance between inhibition and excitation in the motor cortex of patients with cortical myoclonus. Brain. 1996;119:309–317. doi: 10.1093/brain/119.1.309. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112:749–763. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Rothwell JC, Day BL, Thompson PD, Marsden CD. Cortical outflow to proximal arm muscles in man. Brain. 1990;113:1843–1856. doi: 10.1093/brain/113.6.1843. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Experimental Brain Research. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Fong JKY, Werhahn KJ, Rothwell JC, Shorvon SD, Thompson PD, Day BL, Marsden CD. Motor cortex excitability in focal and generalized epilepsy. The Journal of Physiology. 1993;459:468P. [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. The Journal of Physiology. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. The Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami K, Hamada I. Antidromic latency of the monkey pyramidal tract neuron related to ipsilateral hand movements. Neuroscience Letters. 1980;16:245–249. doi: 10.1016/0304-3940(80)90005-1. 10.1016/0304-3940(80)90005-1. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R, Rothwell JC, Day BL, Thompson PD. Effect of tonic voluntary activity on the excitability of human motor cortex. The Journal of Physiology. 1994;474:261–267. doi: 10.1113/jphysiol.1994.sp020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. The Journal of Physiology. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. The Journal of Physiology. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal Function and Voluntary Movement. Oxford: Clarendon Press; 1993. pp. 186–190. [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes of motor cortical circuitry in patients with Parkinson's disease. Annals of Neurology. 1995a;37:181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. Journal of Neurology, Neurosurgery and Psychiatry. 1995b;59:493–498. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. The Journal of Physiology. 1995c;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati M, Trompetto C, Abbruzzese G. Selective facilitation of responses to cortical stimulation of proximal and distal arm muscles by precision tasks in man. The Journal of Physiology. 1996;491:551–562. doi: 10.1113/jphysiol.1996.sp021239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton A, Fraser C, Flament D, Werner W, Bennett KMB, Lemon R. Organisation of cortico-motoneuronal projections from the primary motor cortex: evidence for task-related function in monkey and in man. In: Thilmann AF, Burke DJ, Rymer WZ, editors. Spasticity: Mechanisms and Management. Berlin: Springer-Verlag; 1993. pp. 8–23. [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalography and Clinical Neurophysiology. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. 10.1016/0924-980X(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JKY, Meyer B-U, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseus muscle. Electroencephalography and Clinical Neurophysiology. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Yokota T, Yoshino A, Inaba A, Saito Y. Double cortical stimulation in amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery and Psychiatry. 1996;61:596–600. doi: 10.1136/jnnp.61.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Annals of Neurology. 1996a;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. The Journal of Physiology. 1996b;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Winter M, Reimers K, Reimers CD, Paulus W. Hyperexcitability of motor cortex in amyotrophic lateral sclerosis: evidence from paired magnetic stimulation. Electroencephalography and Clinical Neurophysiology. 1996c;99:346. 10.1016/0013-4694(96)88453-7. [Google Scholar]


