Abstract
The modulatory action of the α2δ subunit on various high-voltage-activated calcium channels has been demonstrated previously. However, very little is known about auxiliary subunit modulation of low-voltage-activated (LVA) calcium channels. We have examined the modulation of the α1G subunit corresponding to the neuronal T-type calcium channel by the ubiquitously expressed α2δ-1 and brain-specific α2δ-3 subunits.
The α1G subunit was expressed alone or in combination with either the α2δ-1 or α2δ-3 subunit in human embryonic kidney (HEK 293) cells and whole-cell barium currents were measured. The current density-voltage relationships for peak and sustained current, kinetics of current activation and inactivation, voltage dependence of current inactivation and time course of the recovery from inactivation were analysed for each type of expressed channel. No significant difference was found for any of the examined parameters.
These results suggest that the LVA α1G channel is not regulated by known auxiliary α2δ subunits.
Voltage-gated calcium channels have been purified and cloned from various tissues such as skeletal muscle, heart and brain. To date seven genes encoding α1 subunits of the high-voltage-activated (HVA) and two genes of the low-voltage-activated (LVA) calcium channels have been identified (reviewed by Hofmann et al. 1994; Strom et al. 1998; Perez-Reyes et al. 1998; Cribbs et al. 1998). HVA calcium channels form hetero-oligomeric complexes consisting of various combinations of an α1 protein with auxiliary β, α2δ and γ subunits. Modulation of HVA α1 expression and biophysical parameters related to channel gating by diverse regulatory subunits have been studied extensively (reviewed by Walker & De Waard, 1998). Whether the LVA calcium channels have the same subunit composition as the HVA channels remains unclear.
LVA T-type calcium channels (neuronal α1G and cardiac α1H) have only recently been cloned (Perez-Reyes et al. 1998; Cribbs et al. 1998; Klugbauer et al. 1998) and therefore analysis of regulation by auxiliary subunits was restricted previously to the manipulation of subunit expression in native cell lines. Lambert et al. (1997) and Leuranguer et al. (1998) employed antisense depletion of β subunits in nodus ganglion neurons or in the mammalian neuronal NG108-15 cell line, respectively, and showed that the β subunits had no effect on the T-type calcium channel. Wyatt et al. (1998) overexpressed α2δ and neuronal β2a subunits in undifferentiated NG108-15 cells and reported the modulation of activation and inactivation of LVA calcium channels by the α2δ, but not by the neuronal β2a, subunit.
Functional coexpression of the α2δ subunit with various combinations of α1 and β subunits has been reported to result in an increase in the current amplitude, an acceleration of current activation, a shift of the current-voltage (I-V) curve in a hyperpolarizing direction and an acceleration of the time course of current inactivation of HVA calcium channels (Singer et al. 1991; De Waard & Campbell, 1995; Gurnett et al. 1996; Bangalore et al. 1996; Felix et al. 1997; Qin et al. 1998; Shirokov et al. 1998). All authors cited analysed the modulation of HVA channels by the only previously known α2δ subunit, α2δ-1. In addition to the α2δ-1 subunit, two new subunits, α2δ-2 and α2δ-3, have been identified with 55 and 30 % homology to α2δ-1, respectively, and the neuronal α2δ-3 subunit has been shown to modulate both α1C and α1E subunits (Klugbauer et al. 1999).
In this study we took advantage of cloned α1G, encoding a neuronal T-type calcium channel, and two α2δ subunits: the ubiquitously expressed α2δ-1 and neuronal α2δ-3 subunits (Klugbauer et al. 1998, 1999). Upon functional expression in the human embryonic kidney (HEK 293) cell line, we show that the neuronal T-type calcium channel is not modulated by auxiliary α2δ subunits.
METHODS
Cloning of calcium channel subunits
The mouse α1G calcium channel subunit was identified by a PCR-based approach with primers derived from the genomic sequence C54D2.5 of the nematode Caenorhabditis elegans, which encodes a putative calcium channel. This PCR product was used to screen a mouse brain cDNA library, which led to the identification of a full-length cDNA (Klugbauer et al. 1998). The GenBank accession number for mouse α1G is AJ012569.
The novel α2δ-3 subunit was identified by an EST (expressed sequence tag) database search. An EST was found with the accession number AA190607, which had similarities with the α2δ subunit of a calcium channel (Klugbauer et al. 1999). A PCR was performed to obtain a probe for screening a mouse brain cDNA library. The GenBank accession number for α2δ-3 is AJ010949.
In each case the library clones were sequenced on both strands.
Transfection of HEK 293 cells
The full-length cDNAs of all subunits, i.e. α1C (Biel et al. 1990), α1G, α2δ-1 (Mikami et al. 1989) and α2δ-3 were cloned into the pcDNA 3 vector (Invitrogen). HEK 293 cells were transfected by lipofection with LipofectAMINE (Gibco BRL, Life Technologies) with the α1G subunit alone or with the α1G subunit together with one of the α2δ subunits (α2δ-1 or α2δ-3). When two subunits were coexpressed, the DNA mass ratio was 1 : 1. Expression of α2δ subunits was tested by parallel coexpression with the α1C channel using the same expression procedure and DNA concentration. For more details see Schuster et al. (1996).
Electrophysiological recordings
Ionic currents from transfected cells were recorded using the whole-cell configuration of the patch clamp method. Barium was used as the charge carrier. The extracellular solution contained (mM): N-methyl-D-glucamine, 125; BaCl2, 20; CsCl, 5; MgCl2, 1; Hepes, 10; and glucose, 5; pH 7.4 (HCl). The intracellular solution contained (mM): CsCl, 60; CaCl2, 1; EGTA, 11; MgCl2, 1; K2ATP, 5; Hepes, 10; and aspartic acid, 50; pH 7.4 (CsOH). Currents were recorded using an EPC-9 patch clamp amplifier and corresponding Pulse software from HEKA Electronics (Lambrecht, Germany). Patch pipettes were pulled from borosilicate glass. The pipette input resistance was typically between 1.8 and 2.2 MΩ. The capacity of individual cells ranged between 20 and 90 pF and series resistance (Rs) ranged between 3.0 and 5.0 MΩ. Capacity transients were compensated using the built-in procedure of the HEKA system.
The holding potential (Vh) in all experiments was -100 mV. The I-V relationship was measured by stepping to voltages between -80 and +60 mV for 40 ms at 0.2 Hz. Tail currents were analysed during 20 ms repolarizations to voltages between -90 and -40 mV following 5 ms depolarizations to -10 mV at 0.2 Hz. Steady-state inactivation was measured by a series of 5 s prepulses to voltages between -120 and -10 mV, followed by a 10 ms return to Vh and a 40 ms test pulse to -10 mV at 0.1 Hz. To analyse recovery from inactivation, the following pulse sequence was applied at 0.05 Hz: 40 ms test pulse to -10 mV, 5 ms return to Vh, 5 s long inactivating pulse to 0 mV, return to Vh with variable length (recovery period), second test pulse identical to the first one. The recovery period ranged from 5 to 5120 ms.
For the fitting of time courses of activation, inactivation and deactivation measured traces were not leak subtracted. Similarly, all traces shown except for α1C-derived currents in Fig. 1B are raw records. The amplitude of the holding current at -100 mV was typically less than 100 pA. For construction of the I-V relationships shown in Figs 1A and 2A and C, leak current was subtracted using the P/4 procedure.
Figure 1. Effect of the α2δ-3 subunit on the voltage dependence of α1C channel activation.
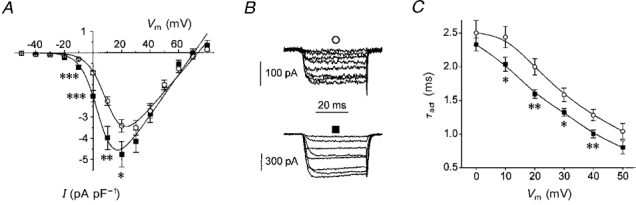
A, mean current density-voltage (I-V) relationships for 14 cells transfected with the α1C subunit only (○) and 14 cells cotransfected with α1C and α2δ-3 subunits (▪). The continuous lines are fits of mean data to the modified Boltzmann equation (Table 1). * P < 0.05, ** P < 0.01, *** P < 0.001, vs.α1C subunit only, Student's unpaired t test. B, examples of current records activated by voltage steps from the holding potential to voltages between -20 and +50 mV. ○, α1C channel, cell capacity 41 pF; ▪, α1C +α2δ-3 channel, cell capacity 79 pF. C, τact represents the time constant of a monoexponential fit to the ascending part of the inward current activated by voltage steps to the indicated membrane potentials (Vm). ○, α1C channel (n = 14 cells); ▪, α1C +α2δ-3 channel (n = 14 cells). * P < 0.05, ** P < 0.01, vs. α1C subunit only, Student's unpaired t test.
Figure 2. Voltage dependence of current activation.
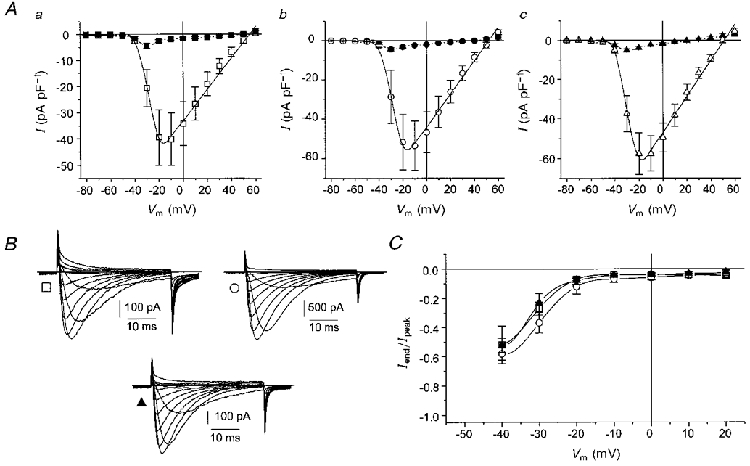
A, mean I-V relationships for 9 cells transfected with the α1G subunit only (□, ▪; a); 10 cells cotransfected with α1G and α2δ-1 subunits (○, •; b); and 11 cells cotransfected with α1G and α2δ-3 subunits (▵, ▴; c). Open symbols represent peak current amplitude, filled symbols represent sustained current amplitude measured at 39 ms. Continuous lines connecting open symbols are fits of mean data to the modified Boltzmann equation (Table 1). B, examples of current records activated by steps to voltages between -50 and +60 mV. □, α1G channel, cell capacity 25 pF; ○, α1G +α2δ-1 channel, cell capacity 58 pF; ▴, α1G +α2δ-3 channel, cell capacity 22 pF. C, I-V relationships for sustained current measured as described in A. Current amplitudes at 39 ms (Iend) were first normalized to the peak current amplitude (Ipeak) of each trace and then averaged. Symbols as in B.
Curve fittings were carried out using the Origin 5.0 software package (Microcal Inc., Northampton, MA, USA). The significance of observed differences was evaluated by Student's unpaired t test. A probability of 5 % or less was considered to be significant. All experimental values are given as means ±s.e.m.
RESULTS
Effect of α2δ-3 coexpression on voltage-dependent activation of the α1C calcium channel
As a control for the expression procedure, the experiments with the α1C calcium channel were performed in parallel (see also Klugbauer et al. 1999). From the results shown in Fig. 1 and Table 1 it can be seen that coexpression of the α2δ-3 subunit together with α1C was sufficient to shift significantly the voltage dependence of barium current (IBa) activation towards more negative membrane potentials (Fig. 1A) and to accelerate significantly the time course of current activation (Fig. 1C). Tail currents measured for α1C or α1C +α2δ-3 channels were too fast to be evaluated accurately and therefore were not compared.
Table 1.
Effect of coexpression of α2δ-1 and α2δ-3 subunits on parameters of the current density-voltage relationship of the α1G channel
| n | Vrev (mV) | V0.5 (mV) | k (mV) | |
|---|---|---|---|---|
| α1C | 14 | 73.6 ± 1.7 | 10.6 ± 1.3 | 6.2 ± 0.9 |
| α1C +α2δ-3 | 14 | 70.7 ± 1.8 | 4.9 ± 1.3** | 5.9 ± 1.0 |
| α1G | 9 | 54.2 ± 1.5 | −28.4 ± 0.6 | 4.0 ± 0.6 |
| α1G +α2δ-1 | 10 | 52.3 ± 1.1 | −28.1 ± 0.5 | 4.4 ± 0.4 |
| α1G +α2δ-3 | 11 | 51.1 ± 1.2 | −29.8 ± 0.5 | 4.1 ± 0.5 |
Peak I–V relationships shown in Figs 1A and 2A were fitted to a modified Boltzmann equation: I =Gmax (V - Vrev)/(1 + exp(-(V - V0.5)/k)), where Gmax is maximum conductance, Vrev is the reversal potential, V0.5 is the potential of half-maximal current activation and k is the slope factor. n, number of experiments (cells).
P < 0.01 vs.α1C subunit only, Student's unpaired t test.
Effect of α2δ-1 or α2δ-3 coexpression on biophysical parameters of I-V relationships of the α1G calcium channel
When expressed in HEK 293 cells, the α1G subunit generated inward IBa which activated at about -40 mV and reached a maximal peak amplitude at -10 mV (Fig. 2Aa). Individual current traces exhibited fast and strongly voltage-dependent inactivation which resulted in the crossing of successive current traces (Fig. 2B) and in a very small amplitude of current sustained after 39 ms of depolarization. As a result of the rapid increase in the speed of voltage-dependent inactivation during the first two suprathreshold depolarizations, the sustained current reached a maximal amplitude at -30 mV, i.e. 20 mV earlier than the peak current (Fig. 2Aa). These features are considered to be a signature pattern for the LVA T-type calcium channel and confirm that the subunit encodes a member of the neuronal LVA channel family. Upon coexpression of the α2δ-1 or α2δ-3 subunit, none of the above mentioned characteristics of expressed channels changed (Fig. 2Ab, Ac and B). The mean expressed current densities were -40.1 ± 9.9 pA pF−1 for the α1G channel, -53.6 ± 12.4 pA pF−1 for the α1G +α2δ-1 channel and -57.6 ± 10.4 pA pF−1 for the α1G +α2δ-3 channel. These values are not significantly different. Parameters characterizing the voltage dependence of current activation, i.e. reversal potential (Vrev), potential of half-maximal current activation (V0.5) and the slope factor k were not significantly affected by coexpression of either α2δ subunit (Table 1). To facilitate comparison of the voltage dependence of current maintained after 39 ms of depolarization, the amplitudes of maintained current were normalized to the peak amplitude of individual current traces. I-V relationships for all channel types virtually overlapped (Fig. 2C).
The characteristics of IBa carried through channels formed from the α1G subunit alone or from α1G in combination with either of the α2δ subunits were comparable to those reported by Perez-Reyes et al. (1998) upon expression of the α1G channel in Xenopus oocytes. The shift by +10 mV along the voltage axis is most probably caused by the higher concentration of charge carrier (20 vs. 10 mM Ba2+) used in our experiments. The steeper slope of voltage dependence of current activation in our study may be due to a voltage drop on non-compensated Rs. This usually ranged between 2.5 and 10 mV (the highest value of Rs was 5 MΩ and the IBa amplitude typically ranged between 0.5 and 2 nA). However, because these values were unaltered upon coexpression of α2δ subunits, the comparison of voltage-dependent parameters was not affected.
Effect of α2δ-1 or α2δ-3 coexpression on the time and voltage dependence of activation, inactivation and deactivation of the current through the α1G calcium channel
To compare the time courses of current activation we fitted the current trace between the intercept with the zero current line and the small plateau around the current peak with a single exponential. The time constants for current activation (τact) decreased with increasing membrane potential over the entire tested interval of voltages (Fig. 3A). At each tested membrane potential, values of τact were not significantly different between α1G, α1G +α2δ-1 and α1G +α2δ-3 channels.
Figure 3. Time and voltage dependence of current activation, inactivation and deactivation.
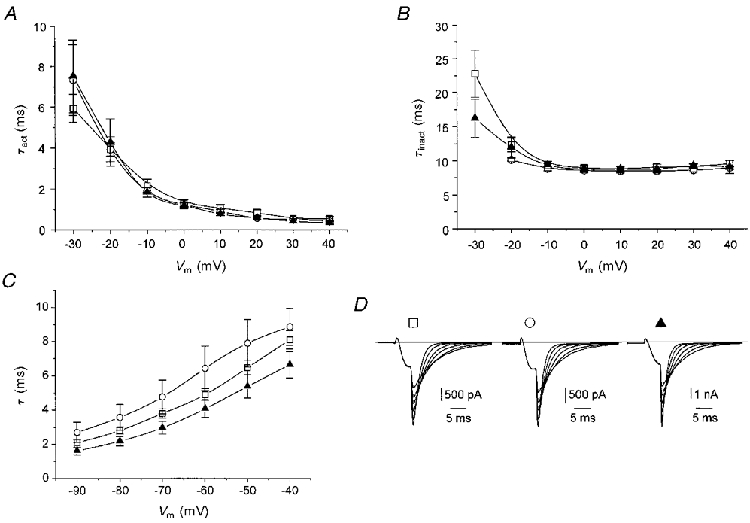
A, τact represents the time constant of a monoexponential fit to the ascending part of the current activated by voltage steps to the indicated membrane potentials. □, α1G channel (n = 9 cells); ○, α1G +α2δ-1 channel (n = 10 cells); ▴, α1G +α2δ-3 channel (n = 11 cells). B, τinact represents the time constant of a monoexponential fit to the descending part of the current activated by voltage steps to the indicated membrane potentials. □, α1G channel (n = 9 cells); ○, α1G +α2δ-1 channel (n = 10 cells); ▴, α1G +α2δ-3 channel (n = 11 cells). C, time constants of current decay evaluated by monoexponential fits of tail currents measured after repolarization to membrane voltages indicated. □, α1G channel (n = 7 cells); ○, α1G +α2δ-1 channel (n = 4 cells); ▴, α1G +α2δ-3 channel (n = 8 cells). D, examples of tail current records. □, α1G channel, cell capacity 33 pF; ○, α1G +α2δ-1 channel, cell capacity 36 pF; ▴, α1G +α2δ-3 channel, cell capacity 37 pF.
The time courses of current inactivation during the depolarizing step could be fitted with a single exponential. The time constants for current inactivation (τinact) decreased rapidly during the first two suprathreshold pulses and afterwards remained constant (Fig. 3B). Similar to the activation time constants, τinact was not significantly different between α1G, α1G +α2δ-1 and α1G +α2δ-3 channels.
Another distinctive characteristic of T-type calcium channel gating is slow kinetics of deactivation, which can be measured as the time constant of tail current decay. We compared the time constants of tail current decay of α1G, α1G +α2δ-1 and α1G +α2δ-3 channels at repolarizing potentials ranging from -90 to -40 mV (Fig. 3C and D). No significant difference amongst the three combinations was found.
Effect of α2δ-1 or α2δ-3 coexpression on steady-state inactivation and recovery from inactivation of the current through the α1G calcium channel
The dependence of channel availability on the resting membrane potential was tested using a 5 s conditioning prepulse (Fig. 4A). Considering that the time constants of IBa inactivation were approximately 10 ms at most membrane potentials (Fig. 3B) we assumed that this length of prepulse would be sufficient to obtain steady-state inactivation. At each membrane potential the proportion of non-inactivated channels was virtually identical for all three channels and the V0.5 (potential of half-maximal inactivation) values calculated from fits to the Boltzmann equation were very similar. Nevertheless, close inspection of Fig. 4A revealed slight differences in the slopes of the steady-state inactivation curves. The slope values were significantly different between α1G and α1G +α2δ-1 channels (P < 0.05). This deviation in slope values was the only significant difference found in all analyses performed. However, considering the sharp steepness of all three curves and the fact that the relative number of available channels do not differ at any prepulse voltage this difference has no functional implications for the channel gating.
Figure 4. Voltage dependence of current availability and time course of recovery from voltage-dependent inactivation.
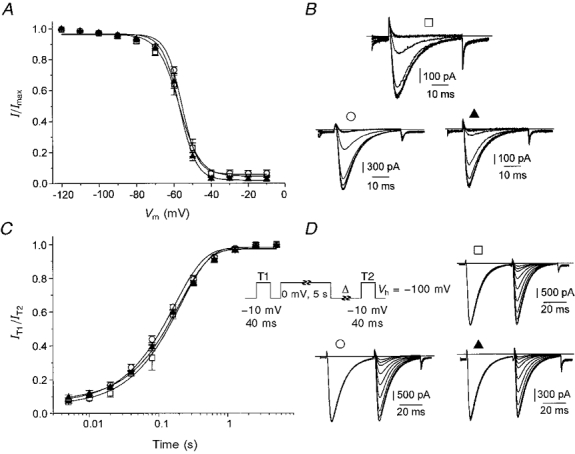
A, steady-state inactivation curves for α1G (□, n = 8 cells), α1G +α2δ-1 (○, n = 9 cells) and α1G +α2δ-3 (▴, n = 10 cells) channels. Continuous lines represent the fits of experimental points to the Boltzmann equation. Resulting V0.5 (potential of half-maximal current inactivation) and k values were -57.3 ± 0.6 mV and 5.6 ± 0.5 mV for the α1G channel, -55.9 ± 0.6 mV and 4.2 ± 0.4 mV for the α1G +α2δ-1 channel and -57.2 ± 0.5 mV and 4.6 ± 0.9 mV for the α1G +α2δ-3 channel. B, examples of currents measured during the steady-state inactivation protocol. □, α1G channel, cell capacity 25 pF; ○, α1G +α2δ-1 channel, cell capacity 92 pF; ▴, α1G +α2δ-3 channel, cell capacity 22 pF. C, proportion of current available during the second test pulse T2 plotted against recovery time (Δ). The voltage protocol is shown in the inset; Vh, holding potential. Continuous lines represent monoexponential fits to the experimental points. Individual time constants of recovery were 202 ± 17 ms for the α1G channel (□, n = 5 cells), 171 ± 15 ms for the α1G +α2δ-1 channel (○, n = 4 cells) and 206 ± 18 ms for the α1G +α2δ-3 channel (▴, n = 7 cells). D, examples of currents measured during both test pulses T1 and T2. The interval between the two test pulses was omitted. □, α1G channel, cell capacity 38 pF; ○, α1G +α2δ-1 channel, cell capacity 37 pF; ▴, α1G +α2δ-3 channel, cell capacity 23 pF.
The recovery from voltage-dependent inactivation of the α1G channel was monoexponential and was complete in less than 2 s (Fig. 4C). Coexpression of either α2δ-1 or α2δ-3 did not significantly affect the recovery time constant.
DISCUSSION
This study was undertaken to investigate whether the neuronal α1G calcium channel is modulated by two different auxiliary α2δ subunits. Our results show that the α1G channel is not regulated by either the α2δ-1 or α2δ-3 subunit.
Lack of modulation of α1G channels by auxiliary subunits would be an exception amongst the calcium channels. All HVA calcium channels identified so far, i.e. α1A, α1B, α1C, α1D and α1S and the α1E channel (which has some properties similar to those of the LVA channel) have been shown to be modulated by auxiliary subunits (reviewed by Walker & De Waard, 1998). When the above mentioned α1 subunits are expressed alone, either in Xenopus oocytes or in mammalian cell lines, their gating-related properties differ from the properties of their native analogues. The time dependence of channel inactivation is particularly slow for HVA α1 channels when compared with that of their native counterparts. This deviation is relieved by coexpression of auxiliary subunits (for review see Walker & De Waard, 1998).
In contrast, the current conducted by expressed α1G subunits is very similar to that of neuronal T-type calcium channels (reviewed by Huguenard, 1996; Randall & Tsien, 1997; Lambert et al. 1997; Leuranguer et al. 1998; Wyatt et al. 1998). It is therefore possible that T-type calcium channels are not associated with regulatory subunits. Indeed, the amino acid sequence of the α1G subunit supports the notion that this channel is not modulated by β subunits (Perez-Reyes et al. 1998; Cribbs et al. 1998; Klugbauer et al. 1998) since the connector between the I and II repeats lacks the sequence identified as a binding site for the β subunit. This suggestion is supported by the work of Lambert et al. (1997) and Leuranguer et al. (1998) who have shown that the neuronal T-type calcium channel is not affected by antisense depletion of known β subunits.
Regulation of the kinetics of channel activation and inactivation has been attributed to the transmembrane δ segment of the α2δ subunit (Felix et al. 1997). The corresponding interaction region on the α1 subunit has not yet been identified and therefore no suggestions can be derived from the α1G sequence. Nevertheless, coexpression of the α2δ-1 subunit with the α1G subunit performed in this work did not alter any gating-related biophysical parameter of the α1G channel.
In addition to the first known α2δ subunit, α2δ-1, two new subunits, α2δ-2 and α2δ-3, have been identified, which show 55 and 30 % homology with α2δ-1, respectively (Klugbauer et al. 1998). Because the α2δ-3 subunit is expressed specifically in brain, it was considered possible that it may have a specific function in the regulation of α1G and/or other neuronal calcium channels. The α2δ-3 subunit was shown to be partially specific to the neuronal α1E channel compared with the ubiquitously expressed α1C channel (Klugbauer et al. 1999). As we have shown here, α2δ-3 also failed to influence the α1G channel.
In contrast to our results, Wyatt et al. (1998) recently reported the effects of the α2δ subunit on voltage-dependent activation and the sustained component of T-type calcium channels in the mammalian NG108-15 cell line. Even if the current measured by Wyatt et al. (1998) was predominantly a T-type calcium current, immunostaining of undifferentiated NG108-15 cells confirmed the presence of α1A, α1B, α1C, α1D and α1E proteins. Staining for α1C was very weak with little membrane association (Wyatt et al. 1998). The α2δ subunit is known to facilitate membrane trafficking of the α1C subunit (Shistik et al. 1995). It is therefore possible that the non-inactivated current component which Wyatt et al. (1998) observed upon coexpression of the α2δ subunit during depolarizing pulses positive to -30 mV reflects the upregulation of previously unmeasurable L-type calcium channels.
To summarize, the results of this study suggest that the expressed neuronal α1G channel is not regulated by α2δ-1 or α2δ-3 subunits. Together with the work of Lambert et al. (1997) and Leuranguer et al. (1998), we may conclude that the biophysical properties of neuronal T-type calcium channels are not regulated by presently known auxiliary subunits of the HVA channel. We cannot exclude the possibility that α2δ subunits do associate with the α1G subunit without affecting current characteristics under control conditions, but that they may regulate its pharmacological properties. Also, the possibility of regulation of T-type calcium channels by a not yet identified class of subunits remains open.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft and Fond der Chemie.
References
- Bangalore R, Mehrke G, Gingrich K, Hofmann F, Kass RS. Influence of L-type Ca channel α2/δ-subunit on ionic and gating current in transiently transfected HEK 293 cells. American Journal of Physiology. 1996;270:H1521–1528. doi: 10.1152/ajpheart.1996.270.5.H1521. [DOI] [PubMed] [Google Scholar]
- Biel M, Ruth P, Bosse E, Hullin R, Stühmer W, Flockerzi V, Hofmann F. Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung. FEBS Letters. 1990;269:409–412. doi: 10.1016/0014-5793(90)81205-3. [DOI] [PubMed] [Google Scholar]
- Cribbs LL, Lee J-H, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E. Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family. Circulation Research. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- De Waard M, Campbell KP. Subunit regulation of the neuronal α1A Ca2+ channel expressed in Xenopus oocytes. The Journal of Physiology. 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel α2δ subunit. Journal of Neuroscience. 1997;17:6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. 10.1016/S0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- Hofmann F, Biel M, Flockerzi V. Molecular basis for Ca2+ channel diversity. Annual Review of Neuroscience. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annual Review of Physiology. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Lacinová L, Hofmann F. Identification of novel subunits of the voltage gated calcium channel family. Naunyn-Schmiedeberg's Archives of Pharmacology. 1998;358(suppl. 1):R687. [Google Scholar]
- Klugbauer N, Lacinová L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel α2δ subunit. Journal of Neuroscience. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert RC, Maulet Y, Mouton J, Beatie R, Volsen S, De Waard M, Feltz A. T-type Ca2+ current properties are not modified by Ca2+ channel β subunit depletion in nodus ganglion neurons. Journal of Neuroscience. 1997;17:6621–6628. doi: 10.1523/JNEUROSCI.17-17-06621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuranguer V, Bourinet E, Lory P, Nargeot J. Antisense depletion of β-subunits fails to affect T-type calcium channels properties in a neuroblastoma cell line. Neuropharmacology. 1998;37:701–708. doi: 10.1016/s0028-3908(98)00060-4. 10.1016/S0028-3908(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–233. doi: 10.1038/340230a0. 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee J-H. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–899. doi: 10.1038/36110. 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- Qin N, Olcese R, Stefani E, Birnbaumer L. Modulation of human neuronal α1E-type calcium channel by α2δ-subunit. American Journal of Physiology. 1998;274:C1324–1331. doi: 10.1152/ajpcell.1998.274.5.C1324. [DOI] [PubMed] [Google Scholar]
- Randall AD, Tsien RW. Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology. 1997;36:879–893. doi: 10.1016/s0028-3908(97)00086-5. 10.1016/S0028-3908(97)00086-5. [DOI] [PubMed] [Google Scholar]
- Schuster A, Lacinová L, Klugbauer N, Ito H, Birnbaumer L, Hofmann F. The IVS6 segment of the L-type calcium channel is critical for the action of dihydropyridines and phenylalkylamines. EMBO Journal. 1996;15:2365–2370. [PMC free article] [PubMed] [Google Scholar]
- Shirokov R, Ferreira G, Yi J, Ríos E. Inactivation of gating currents of L-type calcium channels. Specific role of the α2δ subunit. Journal of General Physiology. 1998;111:807–823. doi: 10.1085/jgp.111.6.807. 10.1085/jgp.111.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shistik E, Ivanina T, Puri T, Hosey M, Dascal N. Ca2+ current enhancement by α2/δ and β subunits in Xenopus oocytes: contribution of changes in channel gating and α1 protein level. The Journal of Physiology. 1995;489:55–62. doi: 10.1113/jphysiol.1995.sp021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Ruther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nature Genetics. 1998;19:260–263. doi: 10.1038/940. 10.1038/940. [DOI] [PubMed] [Google Scholar]
- Walker D, De Waard M. Subunit interaction sites in voltage-dependent Ca2+ channels: role in channel function. Trends in Neurosciences. 1998;21:148–154. doi: 10.1016/s0166-2236(97)01200-9. 10.1016/S0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Page KM, Berrow NS, Brice NL, Dolphin AC. The effect of overexpression of auxiliary Ca2+ channel subunits on native Ca2+ channel currents in undifferentiated mammalian NG108–15 cells. The Journal of Physiology. 1998;510:347–360. doi: 10.1111/j.1469-7793.1998.347bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


