Abstract
We performed patch-clamp recordings on acutely isolated dendritic segments and cell somata of rat neocortical pyramidal neurons to determine and compare the relative density of G protein-activated K+ (GIRK) currents in the two cellular compartments.
Hyperpolarizing voltage ramps and elevation of extracellular K+ concentration to 40 mM served to identify inwardly rectifying K+ currents. Near-saturating concentrations of adenosine (100 μm), baclofen (20 μm) and serotonin (20 μm) all produced robust GIRK currents in cell somata as well as in dendritic segments that were completely abolished by Ba2+ (200 μm). In addition to agonist-activated GIRK currents, both somata and dendrites displayed a constitutive Ba2+-sensitive inward rectification.
In order to compare the relative strengths of GIRK current responses in the two compartments, GIRK conductance was normalized to surface area. In contrast to intrinsic, G protein-independent inward rectification, which was comparable in size in the two compartments, all three agonists evoked significantly larger GIRK conductances in dendrites than in somata.
Our data suggest that several neurotransmitters might employ GIRK currents as a tool to directly modulate the electrical properties of dendrites. In concert with voltage-dependent K+ currents and the hyperpolarization-activated cation current (Ih) of the dendrite, GIRK currents should dampen dendritic excitability and thus influence various aspects of dendritic signal integration.
Several neuromodulators including adenosine (via A1 receptors), serotonin (via 5-HT1A receptors) and GABA (via GABAB receptors) target G protein-activated inwardly rectifying K+ (GIRK) currents to influence the excitability of CNS neurons (Nicoll et al. 1990; Yamada et al. 1998). GIRK current activation occurs in a direct, membrane-delimited fashion, with the Gβγ-subunit serving as the major gating particle (Dascal, 1997). It has long been disputed whether GIRK currents serve as effectors of G protein-coupled receptors on both the presynaptic and the postsynaptic side, leading to inhibition of transmitter release and hyperpolarization of the postsynaptic neuron, respectively. This issue was resolved only recently by combining transgenic mice technology with electrophysiology: recordings from brain slices of normal and transgenic mice lacking a subunit essential for functional GIRK channels demonstrated that GIRK currents of pyramidal cells are confined to the postsynaptic side, and play no role in the inhibition of transmitter release (Lüscher et al. 1997). One important, but still missing piece of information to further elucidate the function of GIRK currents in the postsynaptic neuron is their relative density along the somatodendritic axis. Recordings from different types of CNS neurons in brain slices and after acute isolation have provided ample evidence that various G protein-coupled receptors are capable of evoking GIRK currents in cell somata (e.g. Gähwiler & Brown, 1985; Andrade et al. 1986; Alzheimer & ten Bruggencate, 1991; Misgeld et al. 1995; Sodickson & Bean 1998). In contrast, evidence for dendritic GIRK currents is scarce and indirect, based mainly on intracellular recordings from pyramidal neurons in vitro, where local application of baclofen to apical dendrites was found to induce hyperpolarization of the (somatically recorded) membrane potential (Newberry & Nicoll, 1985; Luhmann & Prince, 1991). Given the functional significance attributed to dendritic ion channels, information on the density of ion currents in somatic vs. dendritic compartments is essential for understanding their specific role in neuronal computation. Within this context, the purpose of the present study was to determine whether dendrites of pyramidal neurons exhibit appreciable GIRK currents, and, if so, to relate dendritic to somatic GIRK current density. In order to obtain GIRK current measurements from the different cellular compartments of neocortical pyramidal neurons under favourable voltage- and space-clamp conditions, we took advantage of the fact that established cell dissociation protocols yield not only acutely isolated cell somata, but also isolated dendritic segments which are also amenable to patch-clamp recording, as first published by Kavalali et al. (1997). Using adenosine, serotonin, and the GABAB receptor agonist baclofen to evoke GIRK current responses, we report here that dendrites of neocortical pyramidal neurons display significantly higher GIRK current densities than the respective cell somata.
METHODS
Wistar rats (2-3 weeks old) were anaesthetized with ketamine- xylazine solution (RBI, 1 ml kg−1i.p.) and decapitated. Coronal slices, 400 μm thick, were prepared from the sensorimotor cortex and processed as described previously (Alzheimer, 1994). Briefly, slices were incubated for 30 min in warmed (30°C) Ringer solution which was allowed to equilibrate with room temperature (21-24°C) for another 30 min. Small pieces of slice tissue (1-2 mm2) were then incubated for 90 min at 29°C in standard bath solution (for composition, see below) containing 19 U ml−1 papain. After washing, tissue pieces were maintained in standard bath solution at room temperature. Before each recording session, individual tissue pieces were mechanically dissociated using Pasteur pipettes of increasingly smaller bore diameter. After dissociation, the cell suspension was immediately transferred to the recording chamber which was mounted on the stage of an inverted microscope equipped with Hoffman modulation optics.
Whole-cell recordings were performed on visually identified segments of presumed dendritic origin, and, for comparison, on cell somata of pyramidal neurons. Current responses were evoked and recorded using an Axopatch 200 amplifier in conjunction with a TL-1 interface and pCLAMP 6.0 software (all from Axon Instruments). Current signals were sampled at 4-5 kHz and filtered at 1 kHz (-3 dB). All recordings were made at room temperature. Standard bath solution was composed of (mM): NaCl, 150; KCl, 3; CaCl2, 2; MgCl2, 2; Hepes, 10; and d-glucose, 10 (pH 7.4). After whole-cell access was established, GIRK currents were investigated in an extracellular solution containing (mM): NaCl, 110; KCl, 40; MgCl2, 3; EGTA, 5; Hepes, 6; D-glucose, 10; and tetrodotoxin (TTX), 0.001 (pH 7.4). Patch pipettes were filled with (mM): potassium gluconate, 135; Hepes, 5; MgCl2, 3; EGTA, 5; Na2-ATP, 2; and Na-GTP, 2 (pH 7.25). When filled with pipette solution, electrode resistances in the bath and in the whole-cell configuration (before series resistance compensation, 70-75%) were 4-8 and 10-15 MΩ, respectively, for somatic recordings, and 15-18 and 25-35 MΩ, respectively, for dendritic recordings. From this, we calculated a maximum steady-state voltage error of 1.6 mV for dendritic GIRK current recordings and 2.7 mV for somatic GIRK current recordings. Voltage readings were corrected for liquid junction potential. Since we wished to express GIRK currents in terms of normalized conductances for later comparison among different neuronal compartments, we estimated membrane areas with the use of the whole-cell compensation circuit of the Axopatch 200 amplifier assuming a specific membrane capacitance of 1 μF cm−2. In order to ensure the validity of this procedure in recordings from small dendritic segments, the electronically obtained value was cross-checked by geometric calculations using photographs such as the ones depicted in Fig. 1A. A plot of electronically vs. morphometrically estimated dendritic surface area yielded a linear correlation very close to 1 (Fig. 1B) corroborating the validity of both approaches. Rapid substance application to isolated dendrites was achieved by means of a remotely controlled, solenoid-operated Y-tube system. Adenosine, serotonin and baclofen were all purchased from Sigma.
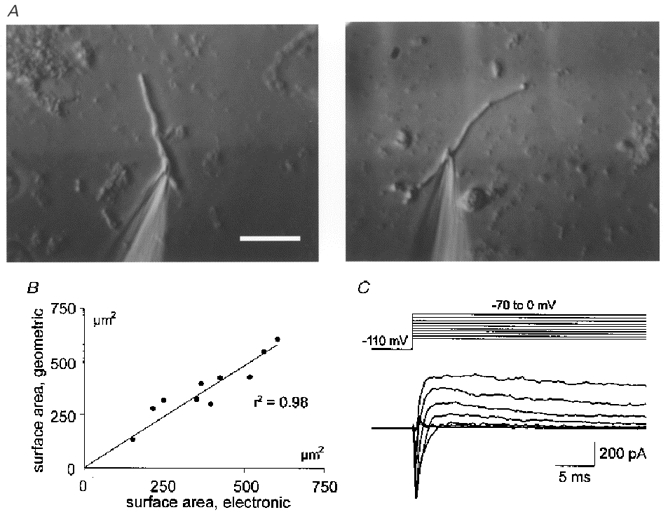
Figure 1. Morphological and electrical properties of acutely isolated dendritic segments.
A, typical examples of dissociated dendritic segments with patch pipettes attached, photographed from the stage of an inverted microscope equipped with Hoffman modulation optics. Scale bar, 20 μm. B, for eleven dendritic segments of various sizes, surface area was determined both electronically and morphometrically. As indicated by the plot of the respective data, the two independent methods yielded almost identical values. C, inward and subsequent outward current responses in a voltage-clamped dendritic segment, evoked by successive depolarizing test pulses at physiological ion gradients. Due to the high series resistance of the recording electrode, activation of inward (Na+) currents was not properly controlled.
Data are presented as means ±s.e.m. Statistical analysis (one-way ANOVA with Bonferroni post-test comparison) was done with the use of Graphpad prism 2.0.
RESULTS
Representative examples of dendritic segments used for patch-clamp recordings are illustrated in Fig. 1A. Based on their cylindrical tube-like shape, their diameter (2-3 μm) which was distinctly larger than that of axons, and their close morphological resemblance to apical dendrites which occasionally remained attached to pyramidal cell somata after dissociation, these processes were identified as segments of dendritic origin, most probably representing parts of the shaft of the main apical dendrite. Typically, dendritic segments were 40-80 μm long and displayed no branching. Their mean surface area was 381.4 ± 42.9 μm2 determined electronically, and 372.3 ± 39.6 μm2 determined geometrically for the same dendritic segments (n = 11, Fig. 1B). Dendritic segments retained their active properties after isolation as demonstrated by the voltage-clamp protocol of Fig. 1C successive depolarizing voltage steps evoked typical sequences of fast inward (presumed Na+) currents followed by slow outward (presumed K+) currents.
GIRK currents were investigated using 700 ms voltage ramps from -20 to -130 mV in an extracellular solution designed to enhance current flow through GIRK channels and to abolish current flow through voltage-dependent Na+ and Ca2+ channels (see Methods). Initial experiments using isotonic K+ solutions were abandoned, since this condition often impaired recordings from dendritic segments, whereas elevation of extracellular K+ to 40 mM resulted in stable recordings. Adenosine (100 μm), baclofen (20 μm) and serotonin (20 μm) were applied at near-saturating concentrations (cf. Sodickson & Bean, 1998). As illustrated in Fig. 2, all three compounds produced a considerable increase in hyperpolarizing inward rectification of dendritic segments. In order to confirm that the agonist-induced inwardly rectifying current was indeed mediated by GIRK channels, we added Ba2+ in the presence of the agonist at a low concentration (200 μm), at which it is thought to specifically block inwardly rectifying K+ channels, and observed a complete reversal of the drug action (Fig. 2).
Figure 2. Activation of GIRK currents by serotonin, adenosine and baclofen.
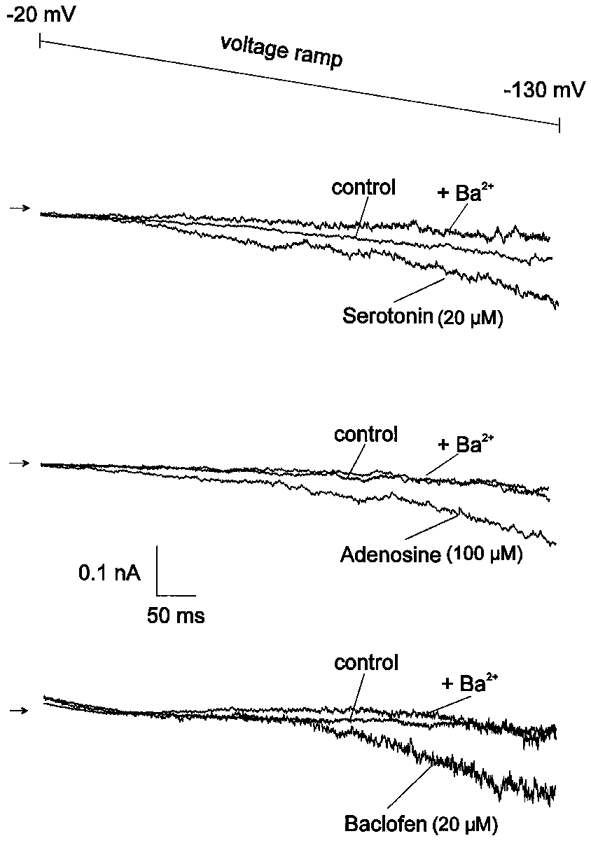
In Ca2+-free solution containing K+ (40 mM) and TTX (1 μm), all three agonists produced a substantial enhancement of Ba2+-sensitive inward rectification which was monitored by 700 ms voltage ramps from -20 to -130 mV. The sequence of recordings was as follows: control solution, agonist solution, agonist solution with Ba2+. Arrows indicate zero current level. The recordings were obtained from three different dendritic segments.
For the dendritic segments (n = 6) and somata (n = 18) exposed to baclofen, 67% of each proved sensitive. Adenosine evoked GIRK current responses in 60% of all dendritic segments (n = 15) and 63% of all somata (n = 8) tested. Serotonin-induced GIRK current responses were observed in 50% of all dendritic segments (n = 6) and 57% of all somata (n = 14). Compared with the data of Sodickson & Bean (1998), where baclofen, serotonin and 2-chloroadenosine induced GIRK currents in 100, 93 and 98%, respectively, of all dissociated hippocampal pyramidal cell somata examined, the fraction of non-responders in our preparation is substantially higher. Assuming that all three agonists converge onto the same effector system (Andrade et al. 1986; Sodickson & Bean 1998), this most probably reflects less robust receptor expression rather than lack of GIRK channels, since virtually no dissociated pyramidal cell somata of rat neocortex were found to be insensitive to all three agonists applied subsequently in the same recording session (T. Sickmann & C. Alzheimer, unpublished observations).
In order to compare the density of agonist-evoked GIRK currents between somata and dendrites, we subtracted control current traces from traces recorded in the presence of each agonist to obtain the current-voltage (I-V) relationship of the GIRK current. Then we calculated the maximum slope conductance (between -110 and -130 mV) and normalized the value to the membrane area. The histogram of Fig. 3 summarizes the normalized GIRK conductances evoked by the three agonists in each cellular compartment. Comparison of the columns indicated that, for all compounds, GIRK current densities were significantly higher in dendritic segments than in somata (P < 0.05).
Figure 3. Normalized GIRK conductances in dendrites and somata of pyramidal neurons.
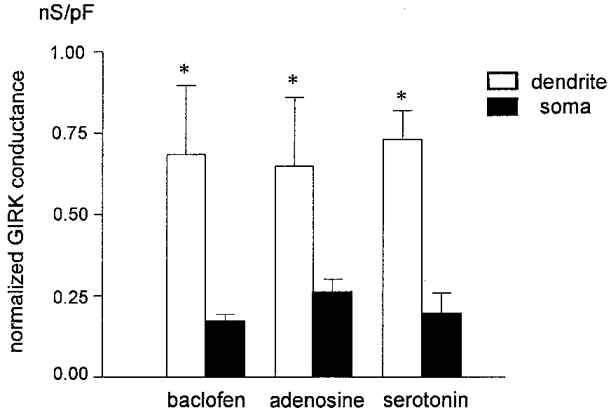
To allow a comparison between different cellular compartments, GIRK conductances were normalized to membrane area. The histogram summarizes data from dendrites (n = 4) and somata (n = 12) exposed to baclofen (20 μm), dendrites (n = 9) and somata (n = 5) exposed to adenosine (100 μm), and dendrites (n = 3) and somata (n = 8) exposed to serotonin (20 μm). The histogram only includes data from positively responding somata and dendrites. Whereas baclofen, adenosine and serotonin produced remarkably similar responses within a given neuronal compartment, the response to each agonist differed significantly between dendrites and somata (*P < 0.05).
In contrast to agonist-evoked GIRK currents, constitutive K+ channel-mediated inward rectification did not differ between dendrites and somata. Evidence for constitutive, apparently G protein-independent inward rectification was first suggested by the observation that Ba2+ often not only reversed the action of the agonist, but also further reduced inward rectification beyond control level (Fig. 2). We investigated this intrinsic effect of Ba2+ by subtracting current traces recorded in the presence of Ba2+ from control traces. Normalization of the respective conductances revealed that dendrites (0.15 ± 0.06 nS pF−1, n = 16) and somata (0.14 ± 0.02 nS pF−1, n = 14) display equal densities of constitutive inward rectification.
DISCUSSION
Our data provide direct evidence that several types of G protein-coupled receptor are functionally linked to GIRK channels in dendrites of rat neocortical pyramidal cells. The functional importance of dendritic GIRK currents is underlined by the observation that their conductance per unit membrane area significantly exceeded that of GIRK currents recorded at the soma. At the molecular level, GIRK channels are heterotetramers of the Kir3 (GIRK) subfamily of inwardly rectifying K+ channels (Dascal, 1997; Yamada et al. 1998). In the rat neocortex, Kir3.1, 3.2 and 3.3 subunits are abundantly expressed, whereas, apart from in layer VI, the fourth cloned subunit, Kir3.4, is not detectable in appreciable amounts (Karschin et al. 1996; Murer et al. 1997). The possibility that dendrites exhibit GIRK currents was raised by immunocytochemical studies on the subcellular distribution of GIRK channel subunits in various brain regions (Liao et al. 1996; Ponce et al. 1996; Drake et al. 1997; Murer et al. 1997). In neocortical pyramidal neurons, Kir3.1 immunoreactivity is prominent in apical dendrites, but rather faint in somata, whereas Kir3.2 antibodies produce equally strong signals in the two compartments (Liao et al. 1996; Ponce et al. 1996). Since Kir3.1 immunoreactivity in the dendrite is predominantly targeted to perisynaptic regions known to express G protein-linked receptors at high density (Drake et al. 1997), a functional coupling between GIRK channels containing the Kir3.1 subunit and these receptors appeared likely. Our data now lend electrophysiological support to this notion, suggesting that the dendritic GIRK currents observed here were mediated by G protein-gated tetrameric K+ channels, most probably containing Kir3.1 and Kir3.2 subunits. In the absence of immunocytochemical data on the subcellular location of the Kir3.3 protein, the contribution of this subunit to dendritic GIRK channel assembly remains to be determined.
In addition to subunits of the Kir3 subfamily, in situ hybridization demonstrated that neocortical neurons also express transcripts of all three subunits of the Kir2 (IRK) subfamily (Kir2.1-2.3, Karschin et al. 1996). Ion channels arising from an assembly of Kir2.x subunits have been termed ‘classical’ inwardly rectifying K+ channels, which, like GIRK channels, are sensitive to Ba2+, but are not activated by transmitters (Isomoto et al. 1997). Instead, they seem to be constitutively active and are thought to contribute predominantly to resting K+ conductance in many tissues (Isomoto et al. 1997). Thus Kir2 channels are a prime candidate to account for the basal, apparently G protein-independent Ba2+-sensitive inward rectification that we observed in both somata and dendrites of neocortical pyramidal cells. Although we cannot exclude the possibility that GIRK channels display some degree of spontaneous background activity in the absence of G protein activation, the fact that GIRK conductance, but not constitutive inward rectification, differed between soma and dendrite would argue against them being mediated by the same Kir channel subfamily.
Why did all three agonists induce a stronger GIRK current response in dendrites than in somata? The preferential dendritic location of the Kir3.1 subunit, which is an essential component of many neuronal GIRK channels (Yamada et al. 1998), would be consistent with the notion that a higher density of functional GIRK channels is present in dendrites than in somata. However, without additional immunohistochemical or biochemical data, we cannot exclude the possibility that an increase in receptor density along the somatodendritic axis, or a more efficient receptor-effector coupling in the dendrite might primarily account for the prominent GIRK current response in the dendrite.
Whereas intrinsically active, presumed Kir2 channels should play a role mainly in determining the resting membrane potential of the dendrite, dendritic GIRK current should primarily serve as an instrument by which several neurotransmitters can directly modulate the electrical properties of dendrites. In the last few years, Na+, K+, Ca2+ and cation currents (Ih) have been identified in dendrites of CNS neurons (Huguenard et al. 1989; Stuart & Sakmann, 1994; Andreasen & Lambert 1995; Magee & Johnston, 1995; Hoffman et al. 1997; Kavalali et al. 1997; Magee 1998; for review see Johnston et al. 1996). Given the functional significance of dendritically located ion currents (Yuste & Tank, 1996), it is not surprising that their density in somatic and dendritic compartments appears to be delicately regulated. Some currents display approximately equal densities (Na+ currents: Stuart & Sakmann, 1994; Magee & Johnston, 1995) or decrease in density along the somatodendritic axis of pyramidal neurons (high-threshold Ca2+ currents: Magee & Johnston, 1995), whereas other currents such as the transient (A-type) K+ current (Hoffman et al. 1997), low-threshold Ca2+ currents (Magee & Johnston, 1995) and, as shown here, GIRK currents, are stronger in dendrites than in somata, suggesting that they are particularly important for the electroresponsiveness of dendrites. Active conductances in the dendrites have been implicated in a number of functions, including backpropagation of action potentials (Stuart et al. 1997), amplification or attenuation of excitatory synaptic input (Lipowsky et al. 1996; Hoffman et al. 1997), and coupling of electrical events to biochemical events at the subcellular level (Mulkey & Malenka, 1992). Due to dendritic membrane hyperpolarization and shunting, GIRK channel activation would influence the threshold and shape of dendritic action potentials, counteract amplification of excitatory synaptic input in the subthreshold voltage range, reduce the availability of NMDA channels by favouring their voltage-sensitive Mg2+ block, and influence biochemical processes initiated by Ca2+ influx through voltage-dependent channels. Since GIRK channels appear to be present in dendritic shafts as well as in spines, where they are immediately adjacent to asymmetric (excitatory) synapses (Drake et al. 1997), GIRK currents would not only affect electrical events occurring along the shaft, but also modulate properties of individual excitatory synapses associated with synaptic plasticity.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 391 and Al 294/7) and the Friedrich-Baur-Stiftung. C. A. is a Heisenberg-Fellow of the Deutsche Forschungsgemeinschaft.
References
- Alzheimer C. A novel voltage-dependent cation current in rat neocortical neurones. The Journal of Physiology. 1994;479:199–205. doi: 10.1113/jphysiol.1994.sp020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer C, ten Bruggencate G. Postsynaptic inhibition by adenosine in hippocampal CA3 neurons: Co2+-sensitive activation of an inwardly rectifying K+ conductance. Pflügers Archiv. 1991;419:288–295. doi: 10.1007/BF00371109. [DOI] [PubMed] [Google Scholar]
- Andrade R, Malenka RC, Nicoll RA. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Andreasen M, Lambert JDC. Regenerative properties of pyramidal cell dendrites in area CA1 of the rat hippocampus. The Journal of Physiology. 1995;483:421–441. doi: 10.1113/jphysiol.1995.sp020595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. Signalling via the G protein-activated K+ channels. Cellular Signalling. 1997;9:551–573. doi: 10.1016/s0898-6568(97)00095-8. [DOI] [PubMed] [Google Scholar]
- Drake CT, Bausch SB, Milner TA, Chavkin C. GIRK1 immunoreactivity is present predominantly in dendrites, dendritic spines, and somata in the CA1 region of the hippocampus. Proceedings of the National Academy of Sciences of the USA. 1997;94:1007–1012. doi: 10.1073/pnas.94.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler BH, Brown DA. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proceedings of the National Academy of Sciences of the USA. 1985;82:1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Hamill OP, Prince DA. Sodium channels in dendrites of rat cortical pyramidal neurons. Proceedings of the National Academy of Sciences of the USA. 1989;86:2473–2477. doi: 10.1073/pnas.86.7.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and function. Japanese The Journal of Physiology. 1997;47:11–39. doi: 10.2170/jjphysiol.47.11. [DOI] [PubMed] [Google Scholar]
- Johnston D, Magee JC, Colbert CM, Cristie BR. Active properties of neuronal dendrites. Annual Review of Neuroscience. 1996;19:165–186. doi: 10.1146/annurev.ne.19.030196.001121. [DOI] [PubMed] [Google Scholar]
- Karschin C, Dissmann E, Stühmer W, Karschin A. IRK (1–3) and GIRK (1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. Journal of Neuroscience. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Zhuo M, Bito H, Tsien RW. Dendritic Ca2+ channels characterized by recordings from isolated hippocampal dendritic segments. Neuron. 1997;18:651–663. doi: 10.1016/s0896-6273(00)80305-0. [DOI] [PubMed] [Google Scholar]
- Liao YJ, Jan YN, Jan LY. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. Journal of Neuroscience. 1996;16:7137–7150. doi: 10.1523/JNEUROSCI.16-22-07137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky R, Gillessen T, Alzheimer C. Dendritic Na+ channels amplify EPSPs in hippocampal CA1 pyramidal cells. Journal of Neurophysiology. 1996;76:2181–2191. doi: 10.1152/jn.1996.76.4.2181. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. Journal of Neurophysiology. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. Journal of Neuroscience. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Johnston D. Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. The Journal of Physiology. 1995;487:67–90. doi: 10.1113/jphysiol.1995.sp020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Progress in Neurobiology. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Murer G, Adelbrecht C, Lauritzen I, Lesage F, Lazdunski M, Agid Y, Raisman-Vozari R. An immunocytochemical study on the distribution of the two G-protein-gated inward rectifier potassium channels (GIRK2 and GIRK4) in the adult rat brain. Neuroscience. 1997;80:345–357. doi: 10.1016/s0306-4522(97)00001-8. [DOI] [PubMed] [Google Scholar]
- Newberry NR, Nicoll RA. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. The Journal of Physiology. 1985;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiological Reviews. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Ponce A, Bueno E, Kentros C, Vega-Saenz de Miera E, Chow A, Hillman D, Chen S, Zhu L, Wu MB, Wu X, Rudy B, Thornhill WB. G-protein-gated inward rectifier K+ channel proteins (GIRK1) are present in the soma and dendrites as well as in nerve terminals of specific neurons in the brain. Journal of Neuroscience. 1996;16:1990–2001. doi: 10.1523/JNEUROSCI.16-06-01990.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. Neurotransmitter activation of inwardly rectifying potassium current in dissociated hippocampal CA3 neurons: interactions among multiple receptors. Journal of Neuroscience. 1998;18:8153–8162. doi: 10.1523/JNEUROSCI.18-20-08153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Häusser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends in Neurosciences. 1997;20:125–131. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacological Reviews. 1998;50:723–757. [PubMed] [Google Scholar]
- Yuste R, Tank DW. Dendritic integration in mammalian neurons, a century after Cajal. Neuron. 1996;16:701–716. doi: 10.1016/s0896-6273(00)80091-4. [DOI] [PubMed] [Google Scholar]


