Abstract
Despite the importance of metal ions in several catalytic functions, there has been, until recently, little molecular information available on the mechanisms whereby metal ions are actively taken up by mammalian cells. The classical concept for iron uptake into mammalian cells has been the endocytosis of transferrin-bound Fe3+ by the transferrin receptor. Studies with hypotransferrinaemic mice revealed that in the intestine mucosal transferrin is derived from the plasma and that its presence is not required in the intestinal lumen for dietary iron absorption. This suggests that, at least in the intestine, other non-receptor-mediated uptake systems exist. The molecular identification of metal ion transporters is of great importance, in particular since an increasing number of human diseases are thought to be related to disturbances in metal ion homeostasis, including metal ion overload and deficiency disorders (i.e. anaemia, haemochromatosis, Menkes disease, Wilson's disease), and neurodegenerative diseases (i.e. Alzheimer's, Friedreich's ataxia and Parkinson's diseases). Furthermore, susceptibilities to mycobacterial infections are caused by metal ion transporter defects. The pathological implications of disturbed metal ion homeostasis confirm the vital roles these metal ions play in the catalytic function of many enzymes, in gene regulation (zinc-finger proteins), and in free radical homeostasis. Recent insights have significantly advanced our knowledge of how metal ions are taken up or released by mammalian cells. The purpose of this review is to summarize these advances and to give an overview on the growing number of mammalian metal ion transporters.
Functional role of iron
Iron is required in all organisms for growth and crucial metabolic pathways. The redox potential of Fe2+/Fe3+ favours its use in a number of protein complexes, especially those involved in electron transfer. A number of proteins require iron for activity in the form of haeme or iron-sulfur clusters to transfer electrons. Iron complexes are not only necessary in the electron transport chain to supply cells with energy, but they are also affected by oxygen radicals (O2−·), and free Fe2+ is part of the Fenton reaction to generate reactive oxygen species (Henle & Linn, 1997). Therefore, the maintenance of iron homeostasis in the body as well as in the cells must be balanced, to supply enough iron for the metabolism, and to avoid excessive, toxic levels. Regulation of iron uptake also depends on the state of oxygenation. Studies of duodenal brush-border membranes in rat indicate that iron absorption is increased during chronic hypoxia (O'Riordan et al. 1997).
In the presence of oxygen, ferric iron (Fe3+) is the favoured species, but in the organism ferrous iron (Fe2+) is required. The uptake, and transport, of iron under physiological conditions requires special mechanisms, because Fe3+ has a very low solubility at neutral pH in oxygenated fluids (< 10−17 mol l−1 at pH 7.4; Harford, 1994). In daily diet two distinct forms of iron are present, namely non-haeme iron (Fe3+) and haeme iron. The rate-limiting step of iron uptake appears to be in the intestine, where high amounts of iron present in the diet have to be absorbed. In mammals, the best-studied uptake mechanism of iron is the process of transferrin receptor-mediated endocytosis (van Eijk & de Jong, 1992; Harford, 1994; Richardson & Ponka, 1997). However, there are two observations that indicate that this is not the pathway by which iron is taken up into the body. First, apo-transferrin is not available in the intestinal lumen, except from biliary excretion (Green et al. 1968; Iancu et al. 1995), which is insufficient to account for dietary iron absorption. Second, experiments with brush-border membrane vesicles suggested that other, non-receptor-mediated iron uptake systems exist in the intestine (Eastham et al. 1977; Teichmann & Stremmel, 1990). The acidic pH in the proximal intestine and/or the reduced pH of ∼6.0 in the unstirred layer close to the external surface of the intestinal brush-border membrane help to solubilize Fe2+, which is rendered in its reduced form by ascorbate, and a ferrireductase (Wien & Van Campen, 1991; Raja et al. 1992; Dorey et al. 1993; Inman et al. 1994; Jordan & Kaplan, 1994; Han et al. 1995; Umbreit et al. 1996).
Interestingly, the process of transferrin receptor-mediated endocytosis, thought to be the principal way of uptake of Fe3+ into non-intestinal cells, did not lead to an explanation of how iron can cross the endosomal membrane. Studies on the process of transferrin receptor-mediated endocytosis led to the observation that these endosomes need to be acidified. The low endosomal pH is necessary for release of iron from transferrin. Furthermore, the transfer from the endosomes into the cytosol requires the activity of an ferrireductase as well as an Fe2+ transporter, because iron exists primarily as Fe2+ in the cytosol (Dautry-Varsat, 1986; van Eijk & de Jong, 1992).
The recently cloned plant ferrireductase (Robinson et al. 1999) may shed light on eukaryotic iron acquisition. However, a mammalian homologue has not yet been identified. In yeast the combination of a ferrireductase/-oxidase reaction probably makes iron accessible for uptake from stable organic complexes (Askwith & Kaplan, 1998; Dancis, 1998).
Evidence for two types of iron transporters
The activity of more than one transporter for iron in the mammalian intestine was already promoted in 1977 by studies of Eastham and colleagues (Eastham et al. 1977) who found that vesicles from the brush-border or basolateral membrane show different kinetics of Fe2+ uptake. These findings were supported by those of Teichmann & Stremmel (1990) on Fe3+ uptake in intestinal vesicles and by studies of mice suffering from microcytic hypochromic anaemia. This anaemia is indistinguishable from iron deficiency anaemia but is unresponsive to increased dietary iron. In microcytic anaemia (mk) mice a transporter located in the brush-border membrane of microvillus cells was thought to be mutated, thereby blocking iron uptake into these cells. In sex-linked anaemia (sla) mice iron uptake into the microvillus cells is detectable, but the iron is not released into the serum (Anderson et al. 1998). These studies indicate two types of transporter proteins, located in the brush-border and basolateral membranes, respectively (Fig. 1).
Figure 1. Iron transport in intestinal (villus) cells.
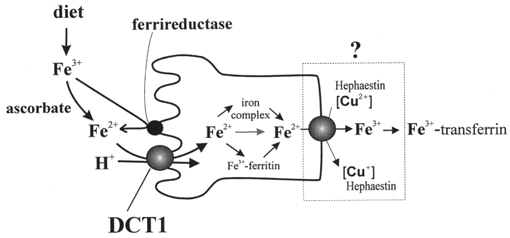
Dietary iron (Fe3+) in the lumen is taken up at the apical side by DCT1 after its reduction to Fe2+. In the cytosol, Fe2+ is bound to low molecular weight complexes, or proteins, or stored by binding to ferritin. At the basolateral side, Fe2+ is released from villus cells into the plasma by a transporter which requires a multicopper oxidase (the sla protein). The released Fe3+ is instantly transferred to transferrin.
In recent years, various iron transporters have been identified for lower organisms, including the Fe2+ transporters feoB from E. coli (Kammler et al. 1993), FET4 from yeast (Dix et al. 1994), the plant IRT1 (Eide et al. 1996), and the Fe3+ transporter FTR1 from yeast (Stearman et al. 1996). However, until recently the transporters which mediate direct uptake of iron and metal ions into mammalian cells remained elusive.
Functional cloning of rat DCT1
Using expression cloning with Xenopus laevis oocytes, our laboratory isolated the divalent cation/metal ion transporter (DCT1) from a duodenal cDNA library, prepared from mRNA from rats fed a low-iron diet (Gunshin et al. 1997). DCT1 was isolated by screening this library using a radiotracer assay of 55Fe2+ uptake in Xenopus oocytes. The isolated cDNA clone encodes a 561 amino acid polypeptide, which, when expressed in oocytes, increases the uptake of 55Fe2+ more than 200-fold compared with control (water injected) oocytes. The amino acid sequence predicts 12 membrane spanning domains, a glycosylated extracellular loop, a consensus transport motif (CTM) in the fourth intracellular loop, and a topology with N- and C-termini in the cytosol (see Fig. 3). The CTM is thought to interact with ATP coupling subunits, distinct from the nucleotide binding fold of ABC transporters. However, ATP depletion in oocytes did not affect DCT1-mediated iron uptake. DCT1 expression was found to be widespread in rat based on in situ hybridization and Northern blot analysis. Comparison of DCT1 expression in tissues from rats fed a normal diet or an iron-deficient diet for 3 weeks showed that iron deprivation triggers a strong increase of DCT1 mRNA levels in intestine and to some extent in all tissues examined. The finding of an iron-responsive element (IRE) in the 3′ untranslated region of the cloned DCT1 cDNA indicates regulation of DCT1 at the RNA stability level, analogous to the transferrin receptor mRNA. Recent studies in our laboratory on the function of the DCT1-IRE support this hypothesis and suggest that the iron-response element binding protein 1 (IRP1) binds to this IRE (M. A. Hediger, unpublished data).
Figure 3. Topology models of selected metal ion transporters.
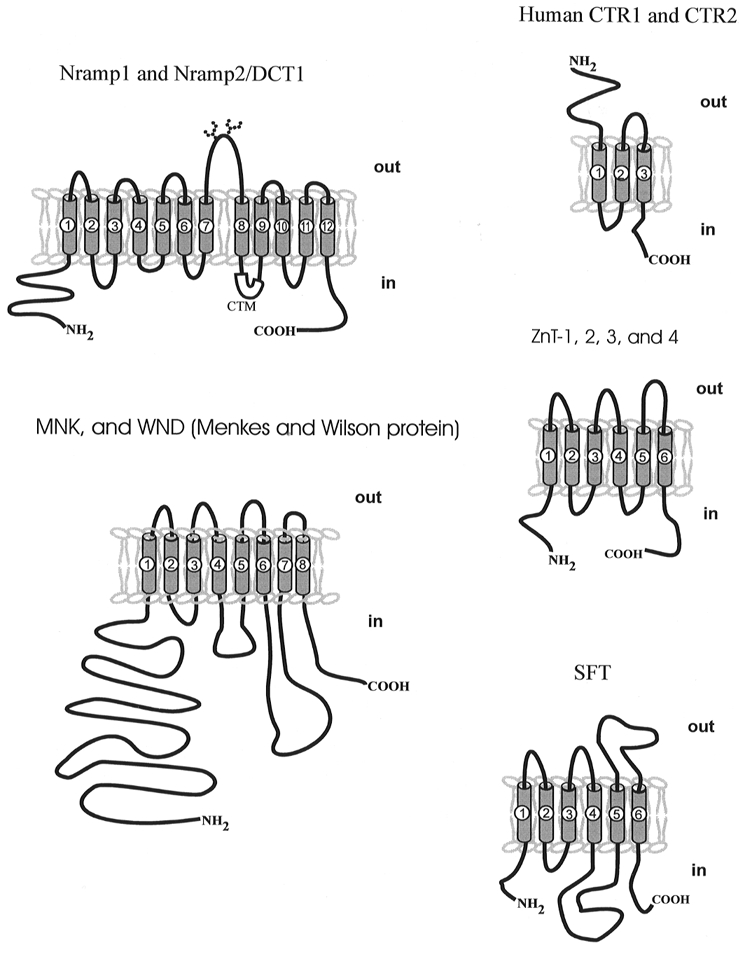
Predicted topology models of transporters mediating metal ion uptake (DCT1/Nramp2: Gunshin et al. 1997; CTRs: Zhou & Gitschier, 1997) and export (ZnTs: Palmiter & Findley, 1995; Palmiter et al. 1996a,b; Huang & Gitschier, 1997), WND/MNK (Tanzi et al. 1993; Mercer et al. 1993; Vulpe et al. 1993; Chelly et al. 1993; Petrukhin et al. 1994) and the iron transporter stimulator SFT (Gutierrez et al. 1997).
Electrophysiological studies of DCT1 expressed in oocytes revealed that the divalent cation transporter is electrogenic, with Fe2+ evoking currents of up to 1000 nA and an apparent Km for Fe2+ of ∼6 μM. These currents were both voltage- and pH-dependent, indicating that an acid pH is necessary for functional transport of iron. Furthermore, it was shown by these experiments that DCT1 transports not only Fe2+, but also Zn2+, Mn2+, Cu2+, Co2+, Cd2+ and Pb2+.
The cloning and characterization of DCT1 provided the first demonstration of an active, cellular uptake mechanism for divalent cations (including Fe2+) in mammalian cells. The functional properties of DCT1 show the need of an acidic environment, as found in the proximal duodenum, but also in the transferrin receptor-mediated endosomes. Localization studies by immunofluorescence and confocal microscopy show the presence of DCT1 primarily in recycling endosomes and also in the plasma membrane (Gruenheid et al. 1999). DCT1 turns out to be the rat homologue of the previously identified mouse and human Nramp2 and these proteins are homologous (∼70 % identical) to Nramp1, the natural resistance-associated macrophage protein (Vidal et al. 1993, 1995b). The biological functions of the Nramp proteins remained unknown, until DCT1 was identified. The name Nramp was given to these proteins because a defect in Nramp1 leads to lack of resistance to infections of macrophages (Vidal et al. 1995b; Gruenheid et al. 1997).
Positional cloning of Nramp2 (DCT1) in mouse and rat
Independently of our cloning of DCT1, Andrews and colleagues (Fleming et al. 1997) identified mouse Nramp2/DCT1 as an iron transporter, by using a genetic approach. That approach stems from their search for the gene for microcytic anaemia (mk) in mice. The severe microcytic hypochromic anaemia is indistinguishable from iron deficiency anaemia but is unresponsive to increased dietary iron. Iron injections, intended to circumvent the intestinal block, did not reverse the anaemia, suggesting a block of iron entry into red blood cell precursors. The locus for mk was found to be linked to an area of the mouse genome where the Nramp2/DCT1 gene was found earlier (Gruenheid et al. 1995; Vidal et al. 1995a). By using RT-PCR analysis for comparison of Nramp2/DCT1 cDNA in wild-type and mk mice, a single point-mutation, substituting arginine for glycine (G185R) within transmembrane domain 4 of mk-Nramp2/DCT1 was found (Fleming et al. 1997). Given the capacity of Nramp2/DCT1 to transport several other divalent heavy metal ions besides iron, the phenotype of the mk/mk mouse might be explained in terms of deficiencies of other metal ions such as Zn2+, Mn2+ and Cu2+ (Chua & Morgan, 1997).
Similar to mk mice, the Belgrade (b) rats have a severe hypochromic, microcytic anaemia that is associated with defects in erythroid iron utilization and intestinal iron uptake in the apical membrane of the enterocytes. Andrews and colleagues have reported that the b rats also have the G185R mutation in transmembrane domain 4 (Fleming et al. 1998). Expression of this mutant protein in eukaryotic cells did not result in stimulation of iron uptake (Su et al. 1998). It is likely that a subset of human patients with congenital anaemia also harbour mutations in DCT1. Indeed, isolated families with an apparently autosomal recessive inherited iron-deficiency anaemia, unresponsive to iron therapy, have been described (Hartman & Barker, 1996). The affected individuals have a phenotype reminiscent of that of the b rat and mk mouse.
Nramp1 cloning and pathophysiology
Nramp1 (natural resistance-associated macrophage protein 1) was found by its impaired function in macrophages in response to infections with various species of mycobacteria and other intracellular parasites (Atkinson & Barton, 1998). The Nramp1 gene was identified by positional cloning, and found to be almost exclusively expressed in macrophages (Vidal et al. 1993, 1995b). The amino acid sequence is highly homologous to DCT1/Nramp2 (73 %), suggesting a similar metal ion transport function for Nramp1 in macrophages. Expression of Nramp1 in oocytes suggested a lower affinity for iron compared with DCT1 (Gunshin et al. 1997). However, expression levels were low in these experiments and further studies are required to elucidate the functional characteristics of Nramp1. In experiments in which Nramp1 was expressed in monkey COS-1 cells it was shown that iron uptake was not increased, but there was a reduced cellular iron content (Atkinson & Barton, 1998). Localization studies show that Nramp1 protein is present in the lysosomal compartment of macrophages, and in phagosomal membranes during phagocytosis (Gruenheid et al. 1997). Its localization does not overlap with that of DCT1/Nramp2, which is also expressed in these cells (Gruenheid et al. 1999). Given this localization, together with the observed metal ion transport, Nramp1 might play a role in resistance to infections by depleting the phagosome of Fe2+, Mn2+ or other essential divalent metal ions. Recently, it has been reported that tuberculosis infections in West Africans are linked to polymorphism in the Nramp1 gene (Bellamy et al. 1998), indicating that the successful development of treatment strategies for mycobacterial infections requires evaluation of these polymorphisms.
Additional iron transporters
Polarized epithelial cells have two different iron transporters, one in the apical and one in the basolateral membrane (Fig. 1). This is supported by findings in mice with sex-linked anaemia, sla mice. These mice suffer from microcytic anaemia similar to mk mice. mk animals have a disturbed uptake of iron in the intestine, whereas the export out of the intestine is normal. In contrast, the sla animals show the opposite disorder. Their uptake of iron into the villus cells appears normal, and is probably mediated by DCT1, but the release into the blood is compromised. Recent attempts to clone the basolateral transporter indicate that it is composed of at least two subunits, one necessary for Fe2+ transport, and the other one for oxidation of Fe2+ to Fe3+ (Anderson et al. 1998; McKie et al. 1998; Vulpe et al. 1999). The recently cloned hephaestin, deficient in sla mice, is a membrane-bound homologue of ceruloplasmin, and probably functions as multi-copper ferrioxidase. The protein contains only one transmembrane domain, indicating that hephaestin is not the basolateral iron transporter itself. Most probably it interacts with the transporter and facilitates release of iron into the blood by its ferrioxidase activity (Vulpe et al. 1999).
A stimulator of iron transport (SFT) has also been isolated (Gutierrez et al. 1997, 1998). This protein was reported to increase the uptake of iron into Xenopus oocytes, but it is not clear whether this is due to transport or (as indicated by the name) to stimulation of an endogenous transport protein, and whether Fe2+ or Fe3+ is the substrate in vivo (Yu & Wessling-Resnick, 1998a,b).
In addition to these iron transporters, frataxin, which is defective in the mitochondria of patients with Friedreich's ataxia has been isolated and shown to be an iron transport exit mechanism for mitochondria (Campuzano et al. 1996; Babcock et al. 1997; Kispal et al. 1997; Koutnikova et al. 1997; Wilson & Roof, 1997; Radisky et al. 1999). Its defect leads to iron accumulation in the myocardium of patients. Whether the human ABC7, a homologue of the yeast mitochondrial ABC-type iron transporter Atm1p, functions in addition to frataxin as an iron exporter in mitochondria has yet to be proven (Kispal et al. 1997; Csere et al. 1998).
Sensing body iron, iron transport and hereditary haemochromatosis
One of the most common genetic disorders in man, hereditary haemochromatosis (HH), is caused by the mutation of a MHC II protein, HFE. Recently it was reported that the HFE protein interacts with the transferrin receptor (TfR), and in the case of HH that this interaction is disrupted, giving rise to the long-lasting iron overload in the body (Parkkila et al. 1997; Feder et al. 1998; Gross et al. 1998; Zhou et al. 1998).
As alluded to above, DCT1 mRNA levels in the intestine are tightly controlled by serum iron levels. Intestinal crypt cells express HFE and TfR (Anderson et al. 1994; Wood & Han, 1998), whereas mature villus cells express DCT1, but not HFE (Anderson et al. 1994; Wood & Han, 1998). These findings led us to speculate that HFE and TfR together sense serum iron in crypt cells. We propose that HFE and TfR in crypt cells regulate the expression of the proteins involved in iron absorption in villus cells, including DCT1, via the IRE/IRP system (Fig. 2) (Waheed et al. 1999). In patients suffering from HH, this regulation may be disturbed in crypt cells, leading to a higher expression rate of proteins involved in iron absorption in mature villus and in increased intestinal iron absorption. This hypothesis is supported by studies of hypotransferrinaemic mice (Simpson et al. 1991). In these animals a hyperabsorption of iron is observed, in conjunction with a reduced level of transferrin expression (1-2 % of normal) (Buys et al. 1991). A recent report by Feder and coauthors (Roy et al. 1999) showed a direct effect of HFE on Tf-mediated, but not on non-Tf-mediated, iron uptake in a non-polarized cell line. In addition, Andrews and colleagues showed that, in heterozygous TfR knock-out mice, reduced levels of TfR expression give rise to microcytic, hypochromic anaemia, due to impaired iron uptake by maturating erythrocytes (Levy et al. 1999). The iron content in the liver and spleen of these mice was reduced, probably due to reduced intestinal iron absorption, and possibly as a consequence of disturbed iron sensing by TfR-HFE interaction in crypt cells. This concept is consistent with recent findings by Sly and colleagues addressing regulation of intestinal iron absorption in HFE knock-out mice. These mice were shown to have increased duodenal DCT1/Nramp2 levels, despite high serum iron concentration (Fleming et al. 1999).
Figure 2. Hypothetical model of iron sensing and uptake in the intestine and liver.
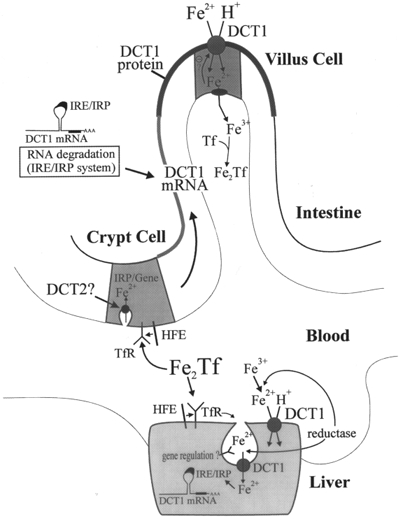
Two Fe3+ bound to Tf (Fe2TF) are taken up via the Tf-TfR cycle in the intestinal crypt cells as well as in other cells of the body such as hepatocytes. Intact HFE and TfR are required in crypt cells for iron absorption from the blood, thereby serving as a sensor of body iron. We hypothesize that the amount of iron absorbed in crypt cells determines the stability of DCT1 mRNA along the crypt-to-villus axis, and thereby the amount of DCT1 protein expressed in mature villus cells. A similar regulatory mechanism might exist for other proteins involved in intestinal iron absorption. In patients with atransferrinaemia or haemochromatosis this sensing is predicted to be disturbed, leading to increased iron uptake.
DCT1 as a metal ion transporter with broad selectivity
The finding that DCT1 mediates active cellular uptake of not only Fe2+, but also Zn2+, Mn2+, Cu2+, Co2+, Ni2+ and the toxic metal ions Pb2+ and Cd2+, was surprising. The existence of a common intestinal absorptive mechanism for a range of metals has important nutritional implications and emphasizes the potential interplay among these essential trace minerals at the level of their absorption. For example, dietary copper deficiency can cause microcytic anaemia, indistinguishable from iron-deficiency microcytic anaemia, suggesting that a defect in DCT1 may also lead to anaemia due to defective copper uptake. In addition, mk/mk mice also suffer from skin lesions reminiscent of defective zinc uptake. b rats are also reported to have widespread abnormalities in manganese metabolism, including impaired duodenal and reticulocyte uptake (Chua & Morgan, 1997; Savigni & Morgan, 1998). Thus, consistent with the substrate range which we determined for DCT1, the metabolism of a variety of metal ions including iron, manganese, cobalt (Savigni & Morgan, 1998) and zinc are deranged due to the DCT1 mutation in the mk and b alleles, underlining the importance of DCT1 in the metabolism of these metals.
The similar affinity of transferrin for other metal ions (Zn2+, Mn2+, Cu2+ and Al3+) suggests that this mechanism mediates uptake of a variety of other metal ions. Thus, in non-intestinal tissues, the TfR-DCT1 endocytotic uptake pathway might allow uptake of not only iron but a range of other metal ions.
Uptake of manganese was previously thought to be related to iron uptake (Chua et al. 1996). At least three mechanisms were described based on studies of erythroid cells, one using manganese-transferrin, one involving a high-affinity Mn2+ transporter, and one involving a low-affinity Mn2+ transporter (Chua et al. 1996). It is likely that the previously reported high-affinity transporter for Mn2+ in erythroid cells involves TfR and DCT1, but this still needs verification (Savigni & Morgan, 1998).
Other metal ion uptake transporters
Thus far, several divalent cation transporters with an affinity for single metal ions have been identified in lower organisms. Most of these are thought to be mono-specific. They include the Fe2+ transporters feoB from E. coli (Kammler et al. 1993), FET4 from yeast (Dix et al. 1994), the plant IRT1 (Eide et al. 1996), and the Fe3+ transporter FTR1 from yeast (Stearman et al. 1996). The first Zn2+ transporters ZRT1 and 2 were isolated from yeast (Zhao & Eide, 1996a,b), and a Zn2+-translocating P-type ATPase in E. coli has been identified (Rensing et al. 1997), which was recently reported to function as a Pb2+-transporting P-type ATPase (Rensing et al. 1998). A manganese transporter belonging to the ABC transporter superfamily has been found in a cyanobacterial mutant strain (Sambongi et al. 1997). The Zn2+ transporters ZIP1 to 4 were identified in Arabidopsis (Grotz et al. 1998). SMF1 to 3 are yeast homologues of the mammalian Nramp proteins, and recent studies indicated that SMF1 is expressed in the yeast plasma membrane where it mediates Zn2+ and Mn2+ uptake (Supek et al. 1996). Studies in our laboratory indicate that SMF1 isoforms expressed in Xenopus oocytes mediate Fe2+ transport (X.-Z. Chen & M. A. Hediger, unpublished observations).
Recently, a human gene involved in high-affinity cellular uptake of copper (CTR-1) has been identified by complementation in yeast (Zhou & Gitschier, 1997). In yeast, three copper uptake proteins are described, CTR-1, CTR-2, and CTR-3. Whereas CTR-1 and CTR-3 are high-affinity copper transporters, CTR-2 is thought to be a low-affinity transporter (Askwith & Kaplan, 1998; Eide, 1998). Using CTR-1- and -3-deficient yeast, a mammalian cDNA library was screened for clones that restore copper uptake into these cells. Using this functional complementation approach, human CTR-1, which showed a low level of homology to yeast CTR-1, was isolated. The amino acid sequence of hCTR-1 predicts three transmembrane domains, analogous to the yeast CTR-1. The extracellular N-terminus has several histidine, serine and methionine residues, which are thought to be important for binding of copper ions in bacterial copper ATPases. A functional analysis of hCTR-1 has not yet been reported. The human CTR-1 sequence has been used for homology screening, giving rise to a putative second human copper transporter, hCTR-2. Its cDNA codes for a similar putative transporter, with pronounced differences in the N-terminus (Zhou & Gitschier, 1997). Clearly, more studies are required to establish the function, distribution and physiological relevance of the CTR transporters in human. The structure of these transporters is of particular interest since they only have three transmembrane domains.
There are a number of metal ion transporters identified in yeast, plant and bacteria, for which mammalian homologues have not yet been identified (Paulsen & Saier, 1997; Eng et al. 1998; Grotz et al. 1998). Thus, much work will be required to fully elucidate the molecular identity and properties of these metal ion transporters.
Metal ion export transporters
Several divalent cation export systems have been reported recently. These include the Zn2+ export systems ZnT-1 to ZnT-4 (Palmiter & Findley, 1995; Huang & Gitschier, 1997; Palmiter et al. 1996a,b; review articles: Huang, 1997; McMahon & Cousins, 1998), and two highly homologous P-type copper ATPase exporters, the Menkes (MNK) and Wilson's (WND) disease proteins (Mercer et al. 1993; Tanzi et al. 1993; Vulpe et al. 1993; Petrukhin et al. 1994). With respect to the ZnT family members, a naturally occurring gene defect of ZnT-4 in mouse has been reported, leading to the phenotype called ‘lethal milk’ (Huang & Gitschier, 1997). Targeted gene disruption of ZnT-3 did not lead to a specific phenotype. This was of great interest, since the protein is localized on synaptic vesicles of zinc-containing neurons in the hippocampus of wild-type animals, and is thereby associated with zinc transport into these vesicles (Wenzel et al. 1997; Cole et al. 1999).
The Menkes disease protein (MNK) is a P-type ATPase Cu2+ transporter. The MNK cDNA was the first mammalian heavy metal ion transporter cDNA to be cloned. In patients suffering from Menkes disease, the transport of Cu2+ is defective, leading to damage of certain tissues, neurodegeneration, and to death in early childhood. The cloning of the Menkes disease gene (MNK) was performed in part by positional cloning (Mercer et al. 1993; Chelly et al. 1993) and exon trapping (Vulpe et al. 1993). The cDNA (8.5 kb) predicts a protein of 1500 amino acids with eight transmembrane domains, six putative copper binding sites within a 600 amino acid N-terminal domain, an ATP-binding motif, a phosphatase domain, and several invariant amino acid residues in the proposed cation transduction channel (Vulpe & Packman, 1995; Lutsenko et al. 1997). Mutations in the MNK gene which cause Menkes disease give rise to accumulation of copper in cell cultures, and lead to a deficiency of enzymes that need copper for their activity. In patients, Cu2+ accumulates predominantly in the intestine and kidney, due to (re)absorption of Cu2+ in these organs. MNK is expressed in all tissues except the liver, where its function is probably mediated by the Wilson protein.
The Wilson's disease protein (WND) was thought to be related to a defect in the blood Cu2+ transport protein ceruloplasmin, since in this disease the amount of ceruloplasmin and its Cu2+ content are reduced. The clinical picture of Wilson's disease is hepatic cirrhosis and neuronal degradation in early childhood, due to the lack of Cu2+ release from the bile, and Cu2+ overload of hepatic cells. However, cloning of the WND gene showed that it encodes a Cu2+-ATPase consisting of 1465 amino acids, found to be expressed in liver and kidney (Tanzi et al. 1993). The sequence predicts eight transmembrane domains, six Cu2+ binding domains, and the characteristic P-type ATPase features. WND was cloned in part by positional cloning, and in part by its homology to MNK (54 % homology of the proteins). Experiments with hepatic cell lines revealed only low expression of WND on the plasma membrane. Most of its expression was found in the trans-Golgi network (Nagano et al. 1998), together with ceruloplasmin. This might indicate that Cu2+ is not released as a free ion into the blood, but is bound to ceruloplasmin, a copper transport protein thought to be secreted in the bile only after complete Cu2+ loading (Chowrimootoo et al. 1996). WND localized in the trans-Golgi network is thought to migrate close to the plasma membrane in a copper-dependent manner. Such a copper-dependent re-localization was also described for the MNK protein (Petris et al. 1996, 1998). Cu2+, but not Zn2+, Fe2+, Cd2+ or Co2+, was able to stimulate this migration of WND from the Golgi network to the plasma membrane (Hung et al. 1997). This specific effect of copper on the relocalization of WND is interesting, since zinc acetate is very efficient for the treatment of patients suffering from Wilson's disease, with minimal side effects (Brewer et al. 1994; Anderson et al. 1998). Other therapeutic strategies involve the use of Cu2+ chelating agents (d-penicillamine and trientine). However, these components show significant side effects. A detailed description of Cu2+-ATPases, their pathophysiological impacts, and their interaction with copper chaperons are presented elsewhere in excellent recent articles (Vulpe & Packman, 1995; DiDonato & Sarkar, 1997; Pufahl et al. 1997; Askwith & Kaplan, 1998).
Structure of human metal ion transporters
The diversity of the predicted structures of metal ion transporters from different transporter families is striking. Table 1 summarizes these differences and Fig. 3 shows topology models of selected transporters. Except for the DCT1/NRAMP2 family, metal transporters do not comply with the 12 transmembrane domain (TMs) dogma of transport proteins. The MNK and WND proteins have eight putative TMs consistent with the structure of other ATP-driven transporters. Interestingly, the ZnTs and the SFT have only six TMs and the CTRs only three TMs. The low number of TMs in the CTRs gives rise to the question of whether these transporters function as homo- or heteromultimers, since a single protein would probably not be able to form a pore complex sufficient for the translocation of the metal ion.
Table 1.
Mammalian metal ion transporters
| Name | Metal transported | Function | TM | Tissue distribution | Disease | Reference(s) |
|---|---|---|---|---|---|---|
| DCT1/ Nramp2 | Fe2+, Zn2+, Mn2+ Cu2+, Co2+, Cd2+, Pb2+ | Uptake/ endosomal exit | 12 | Widespread(intestine, kidney liver, neurons, etc.) | HaemochromatosisA, microcytic anaemiaG | Gruenheid et al. 1995; Vidal et al. 1995a; Gunshin et al. 1997 |
| Nramp1 | Fe2+, Mn2+, other? | Phagosomal/ lysosomal exit | 12 | Macrophages | Infectious susceptibilityG | Vidal et al. 1995b; Gunshin et al. 1997 |
| SFT | Fe2+/Fe3+ | Uptake | 6 | Ubiquitous | — | Gutierrez et al. 1997, 1998 |
| Frataxin/FRDA | Fe2+/Fe3+ | Mitochondrial export | n.d. | Neuronal | Friedreich's ataxiaG | Babcock et al. 1997; Koutnikova et al. 1997; Wilson & Roof, 1997; Radisky et al. 1999 |
| hCTR1 | Cu2+ | Uptake (yeast) | 3 | Ubiquitous | — | Zhou & Gitschier, 1997 |
| hCTR2 | Cu2+ | n.d. | 3 | Ubiquitous | — | Zhou & Gitschier, 1997 |
| ZnT-1 | Zn2+ | Basolateral exit | 6 | Ubiquitous(intestine, kidney) | — | Palmiter & Findley, 1995 |
| ZnT-2 | Zn2+ | Vacuolar exit | 6 | n.d. | — | Palmiter et al. 1996a |
| ZnT-3 | Zn2+ | Synaptic vesicles | 6 | Brain, neurons | — | Palmiter et al. 1996b |
| ZnT-4 | Zn2+ | Export/lactation | 6 | Mammary gland | Lethal milkG | Huang & Gitschier, 1997 |
| MNK | Cu2+ | Basolateral exit(intestine) | 8 | Ubiquitous, except liver | Menkes diseaseG | Mercer et al. 1993; Vulpe et al. 1993; Chelly et al. 1993 |
| WND | Cu2+ | Exit, biliary excretion | 8 | Liver, kidney | Wilson's diseaseG | Tanzi et al. 1993; Petrukhin et al. 1994 |
TM, transmembrane domain; A, acquired; G, genetic; FRDA, Friedreich's ataxia; n.d., not determined.
What are the pharmacological opportunities in the metal ion transporter field?
Treatment of disturbances in metal ion homeostasis often involves supplementation of the missing metal ion, or metal ion chelation. In the case of Cu2+ overload in Wilson's disease, treatment with zinc is very effective in preventing symptoms (Anderson et al. 1998; Brewer et al. 1994). In hereditary haemochromatosis, weekly phlebotomy is the usual treatment in early diagnosis (Barton et al. 1998). Blocking of DCT1 in the intestine by suitable pharmaceutical substances might offer an alternative treatment, but the impact on the uptake of other metal ions needs to be considered. An important consideration for dietary metal supplementation is that iron supplementation will down-regulate DCT1. Furthermore, competition between iron and other divalent metal ions for a single absorptive site can be expected when using multiple trace element supplementation. Nramp1 dysfunction leads to failure to protect against pathogen resistance (Bellamy et al. 1998; Skamene et al. 1998). In addition, an implication of Nramp1 in rheumatoid arthritis has been discussed (Govoni & Gros, 1998). Whether Nramp1 can be used as a therapeutic target in certain diseases needs to be evaluated.
At glutamatergic synapses, glutamate is co-released with zinc which is thought to interact with NMDA receptors, possibly modulating synaptic transmission (Huang, 1997). Although a gene knock-out in mice of ZnT-3, which transports Zn2+ into synaptic vesicles, was shown to give no specific phenotype (Cole et al. 1999), modulation of Zn2+ transport might lead to subtle changes of the transmission process. Thus, the zinc transporter in neurons may be a potential target for modulation of glutamatergic transmission. Whether DCT1 is involved in re-uptake of released Zn2+ remains to be determined.
Metal ion transporters might also contribute to inappropriate accumulation of metal ions in affected neurons in the substantia nigra in patients with Parkinson's disease (Jellinger et al. 1993; Gerlach et al. 1994; Hirsch, 1994; Hirsch & Faucheux, 1998). The dysfunction of metal ion transport may be involved in other neurodegenerative diseases (Gerlach et al. 1994; Multhaup et al. 1997; Atwood et al. 1998) opening a variety of pharmacological opportunities in the metal ion transporter field.
References
- Anderson GJ, Murphy TL, Cowley L, Evans BA, Halliday JW, McLaren GD. Mapping the gene for sex-linked anemia - An inherited defect of intestinal iron absorption in the mouse. Genomics. 1998;48:34–39. doi: 10.1006/geno.1997.5138. [DOI] [PubMed] [Google Scholar]
- Anderson GJ, Powell LW, Halliday JW. The endocytosis of transferrin by rat intestinal epithelial cells. Gastroenterology. 1994;106:414–422. doi: 10.1016/0016-5085(94)90600-9. [DOI] [PubMed] [Google Scholar]
- Anderson LA, Hakojarvi SL, Boudreaux SK. Zinc acetate treatment in Wilson's disease. Annals of Pharmacotherapy. 1998;32:78–87. doi: 10.1345/aph.17075. [DOI] [PubMed] [Google Scholar]
- Askwith CC, Kaplan J. Iron and copper transport in yeast and its relevance to human disease. Trends in Biochemical Sciences. 1998;23:135–138. doi: 10.1016/s0968-0004(98)01192-x. [DOI] [PubMed] [Google Scholar]
- Atkinson PP, Barton CH. Ectopic expression of NRAMP1 in COS-1 cells modulates iron accumulation. FEBS Letters. 1998;425:239–242. doi: 10.1016/s0014-5793(98)00236-1. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NM, Romano DM, Hartshorn MA, Tanzi RE, Bush AI. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. Journal of Biological Chemistry. 1998;273:12817–12826. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- Babcock M, De Silva D, Oaks R, Davis-Kaplan S, Jiralerspong S, Montermini L, Pandolfo M, Kaplan J. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science. 1997;276:1709–1712. doi: 10.1126/science.276.5319.1709. [DOI] [PubMed] [Google Scholar]
- Barton JC, McDonnell SM, Adams PC, Brissot P, Powell LW, Edwards CQ, Cook JD, Kowdley KV. Management of hemochromatosis. Hemochromatosis Management Working Group. Annals of Internal Medicine. 1998;129:932–939. doi: 10.7326/0003-4819-129-11_part_2-199812011-00003. [DOI] [PubMed] [Google Scholar]
- Bellamy R, Ruwende C, Corrah T, McAdam KP, Whittle HC, Hill AV. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. New England Journal of Medicine. 1998;338:640–644. doi: 10.1056/NEJM199803053381002. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Dick RD, Yuzbasiyan-Gurkan V, Johnson V, Wang Y. Treatment of Wilson's disease with zinc. XIII: Therapy with zinc in presymptomatic patients from the time of diagnosis. Journal of Laboratory and Clinical Medicine. 1994;123:849–858. [PubMed] [Google Scholar]
- Buys SS, Martin CB, Eldridge M, Kushner JP, Kaplan J. Iron absorption in hypotransferrinemic mice. Blood. 1991;78:3288–3290. [PubMed] [Google Scholar]
- Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- Chelly J, Tumer Z, Tonnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, Horn N, Monaco AP. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nature Genetics. 1993;3:14–19. doi: 10.1038/ng0193-14. [DOI] [PubMed] [Google Scholar]
- Chowrimootoo GF, Ahmed HA, Seymour CA. New insights into the pathogenesis of copper toxicosis in Wilson's disease: evidence for copper incorporation and defective canalicular transport of caeruloplasmin. Biochemical Journal. 1996;315:851–855. doi: 10.1042/bj3150851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua AC, Morgan EH. Manganese metabolism is impaired in the Belgrade laboratory rat. Journal of Comparative Physiology B. 1997;167:361–369. doi: 10.1007/s003600050085. [DOI] [PubMed] [Google Scholar]
- Chua AC, Stonell LM, Savigni DL, Morgan EH. Mechanisms of manganese transport in rabbit erythroid cells. The Journal of Physiology. 1996;493:99–112. doi: 10.1113/jphysiol.1996.sp021367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proceedings of the National Academy of Sciences of the USA. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csere P, Lill R, Kispal G. Identification of a human mitochondrial ABC transporter, the functional orthologue of yeast Atm1p. FEBS Letters. 1998;441:266–270. doi: 10.1016/s0014-5793(98)01560-9. [DOI] [PubMed] [Google Scholar]
- Dancis A. Genetic analysis of iron uptake in the yeast Saccharomyces cerevisiae. Journal of Pediatrics. 1998;132:S24–S29. doi: 10.1016/s0022-3476(98)70524-4. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A. Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor. Biochimie. 1986;68:375–381. doi: 10.1016/s0300-9084(86)80004-9. [DOI] [PubMed] [Google Scholar]
- DiDonato M, Sarkar B. Copper transport and its alterations in Menkes and Wilson diseases. Biochimica et Biophysica Acta. 1997;1360:3–16. doi: 10.1016/s0925-4439(96)00064-6. [DOI] [PubMed] [Google Scholar]
- Dix DR, Bridgham JT, Broderius MA, Byersdorfer CA, Eide DJ. The FET4 gene encodes the low affinity Fe(II) transport protein of Saccharomyces cerevisiae. Journal of Biological Chemistry. 1994;269:26092–26099. [PubMed] [Google Scholar]
- Dorey C, Cooper C, Dickson DP, Gibson JF, Simpson RJ, Peters TJ. Iron speciation at physiological pH in media containing ascorbate and oxygen. British Journal of Nutrition. 1993;70:157–169. doi: 10.1079/bjn19930113. [DOI] [PubMed] [Google Scholar]
- Eastham EJ, Bell JI, Douglas AP. Iron-transport characteristics of vesicles of brush-border and basolateral plasma membrane from the rat enterocyte. Biochemical Journal. 1977;164:289–294. doi: 10.1042/bj1640289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide DJ. The molecular biology of metal ion transport in Saccharomyces cerevisiae. Annual Review of Nutrition. 1998;18:441–469. doi: 10.1146/annurev.nutr.18.1.441. [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proceedings of the National Academy of Sciences of the USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng BH, Guerinot ML, Eide D, Saier MHJ. Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. Journal of Membrane Biology. 1998;166:1–7. doi: 10.1007/s002329900442. [DOI] [PubMed] [Google Scholar]
- Feder JN, Penny DM, Irrinki A, Lee VK, Lebron JA, Watson N, Tsuchihashi Z, Sigal E, Bjorkman PJ, Schatzman RC. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proceedings of the National Academy of Sciences of the USA. 1998;95:1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic belgrade (b) rat: evidence of a role for nramp2 in endosomal iron transport. Proceedings of the National Academy of Sciences of the USA. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MD, Trenor CC, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nature Genetics. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- Fleming RE, Migas MC, Zhou X, Jiang J, Britton RS, Brunt EM, Tomatsu S, Waheed A, Bacon BR, Sly WS. Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: Increased duodenal expression of the iron transporter DMT1. Proceedings of the National Academy of Sciences of the USA. 1999;96:3143–3148. doi: 10.1073/pnas.96.6.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach M, Ben-Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases. Journal of Neurochemistry. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- Govoni G, Gros P. Macrophage NRAMP1 and its role in resistance to microbial infections. Inflammatory Research. 1998;47:277–284. doi: 10.1007/s000110050330. [DOI] [PubMed] [Google Scholar]
- Green R, Charlton R, Seftel H, Bothwell T, Mayet F, Adams B, Finch C, Layrisse M. Body iron excretion in man: a collaborative study. American Journal of Medicine. 1968;45:336–353. doi: 10.1016/0002-9343(68)90069-7. [DOI] [PubMed] [Google Scholar]
- Gross CN, Irrinki A, Feder JN, Enns CA. Co-trafficking of HFE, a nonclassical major histocompatibility complex class I protein, with the transferrin receptor implies a role in intracellular iron regulation. Journal of Biological Chemistry. 1998;273:22068–22074. doi: 10.1074/jbc.273.34.22068. [DOI] [PubMed] [Google Scholar]
- Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proceedings of the National Academy of Sciences of the USA. 1998;95:7220–7224. doi: 10.1073/pnas.95.12.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S, Canonne-Hergaux F, Gauthier S, Hackam DJ, Grinstein S, Gros P. The iron transport protein NRAMP2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. Journal of Experimental Medicine. 1999;189:831–841. doi: 10.1084/jem.189.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S, Cellier M, Vidal S, Gros P. Identification and characterization of a second mouse Nramp gene. Genomics. 1995;25:514–525. doi: 10.1016/0888-7543(95)80053-o. [DOI] [PubMed] [Google Scholar]
- Gruenheid S, Pinner E, Desjardins M, Gros P. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. Journal of Experimental Medicine. 1997;185:717–730. doi: 10.1084/jem.185.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, MacKenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Yu J, Wessling-Resnick M. Characterization and chromosomal mapping of the human gene for SFT, a stimulator of Fe transport. Biochemical and Biophysical Research Communications. 1998;253:739–742. doi: 10.1006/bbrc.1998.9836. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Yu JM, Rivera S, Wessling-Resnick M. Functional expression cloning and characterization of SFT, a stimulator of Fe transport. Journal of Cellular Biology. 1997;139:895–905. doi: 10.1083/jcb.139.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han O, Failla ML, Hill AD, Morris ER, Smith JCJ. Reduction of Fe(III) is required for uptake of nonheme iron by Caco-2 cells. Journal of Nutrition. 1995;125:1291–1299. doi: 10.1093/jn/125.5.1291. [DOI] [PubMed] [Google Scholar]
- Harford JB. Cellular iron homeostasis: a paradigm for mechanisms of posttranscriptional control of gene expression. Progress in Liver Disease. 1994;12:47–62. [PubMed] [Google Scholar]
- Hartman KR, Barker JA. Microcytic anemia with iron malabsorption: an inherited disorder of iron metabolism. American Journal of Hematology. 1996;51:269–275. doi: 10.1002/(SICI)1096-8652(199604)51:4<269::AID-AJH4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Henle ES, Linn S. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. Journal of Biological Chemistry. 1997;272:19095–19098. doi: 10.1074/jbc.272.31.19095. [DOI] [PubMed] [Google Scholar]
- Hirsch EC. Biochemistry of Parkinson's disease with special reference to the dopaminergic systems. Molecular Neurobiology. 1994;9:135–142. doi: 10.1007/BF02816113. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Faucheux BA. Iron metabolism and Parkinson's disease. Movement Disorders. 1998;13:39–45. [PubMed] [Google Scholar]
- Huang EP. Metal ions and synaptic transmission: think zinc. Proceedings of the National Academy of Sciences of the USA. 1997;94:13386–13387. doi: 10.1073/pnas.94.25.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nature Genetics. 1997;17:292–297. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
- Hung IH, Suzuki M, Yamaguchi Y, Yuan DS, Klausner RD, Gitlin JD. Biochemical characterization of the Wilson disease protein and functional expression in the yeast Saccharomyces cerevisiae. Journal of Biological Chemistry. 1997;272:21461–21466. doi: 10.1074/jbc.272.34.21461. [DOI] [PubMed] [Google Scholar]
- Iancu TC, Shiloh H, Raja KB, Simpson RJ, Peters TJ, Perl DP, Hsu A, Good PF. The hypotransferrinaemic mouse: ultrastructural and laser microprobe analysis observations. Journal of Pathology. 1995;177:83–94. doi: 10.1002/path.1711770113. [DOI] [PubMed] [Google Scholar]
- Inman RS, Coughlan MM, Wessling-Resnick M. Extracellular ferrireductase activity of K562 cells is coupled to transferrin-independent iron transport. Biochemistry. 1994;33:11850–11857. doi: 10.1021/bi00205a022. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Kienzl E, Rumpelmaier G, Paulus W, Riederer P, Stachelberger H, Youdim MB, Ben-Shachar D. Iron and ferritin in substantia nigra in Parkinson's disease. Advances in Neurology. 1993;60:267–272. [PubMed] [Google Scholar]
- Jordan I, Kaplan J. The mammalian transferrin-independent iron transport system may involve a surface ferrireductase activity. Biochemical Journal. 1994;302:875–879. doi: 10.1042/bj3020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammler M, Schon C, Hantke K. Characterization of the ferrous iron uptake system of Escherichia coli. Journal of Bacteriology. 1993;175:6212–6219. doi: 10.1128/jb.175.19.6212-6219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispal G, Csere P, Guiard B, Lill R. The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Letters. 1997;418:346–350. doi: 10.1016/s0014-5793(97)01414-2. [DOI] [PubMed] [Google Scholar]
- Koutnikova H, Campuzano V, Foury F, Dolle P, Cazzalini O, Koenig M. Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nature Genetics. 1997;16:345–351. doi: 10.1038/ng0897-345. [DOI] [PubMed] [Google Scholar]
- Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nature Genetics. 1999;21:396–399. doi: 10.1038/7727. [DOI] [PubMed] [Google Scholar]
- Lutsenko S, Petrukhin K, Cooper MJ, Gilliam CT, Kaplan JH. N-terminal domains of human copper-transporting adenosine triphosphatases (the Wilson's and Menkes disease proteins) bind copper selectively in vivo and in vitro with stoichiometry of one copper per metal-binding repeat. Journal of Biological Chemistry. 1997;272:18939–18944. doi: 10.1074/jbc.272.30.18939. [DOI] [PubMed] [Google Scholar]
- McKie AT, Wehr K, Simpson RJ, Peters TJ, Hentze MW, Farzaneh F. Molecular cloning and characterisation of a novel duodenal-specific gene implicated in iron absorption. Biochemical Society Transactions. 1998;26:S264. doi: 10.1042/bst026s264. [DOI] [PubMed] [Google Scholar]
- McMahon RJ, Cousins RJ. Mammalian zinc transporters. Journal of Nutrition. 1998;128:667–670. doi: 10.1093/jn/128.4.667. [DOI] [PubMed] [Google Scholar]
- Mercer JF, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, Lockhart P, Grimes A, Bhave M, Siemieniak D. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nature Genetics. 1993;3:20–25. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- Multhaup G, Ruppert T, Schlicksupp A, Hesse L, Beher D, Masters CL, Beyreuther K. Reactive oxygen species and Alzheimer's disease. Biochemical Pharmacology. 1997;54:533–539. doi: 10.1016/s0006-2952(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Nagano K, Nakamura K, Urakami KI, Umeyama K, Uchiyama H, Koiwai K, Hattori S, Yamamoto T, Matsuda I, Endo F. Intracellular distribution of the Wilson's disease gene product (ATPase7B) after in vitro and in vivo exogenous expression in hepatocytes from the LEC rat, an animal model of Wilson's disease. Hepatology. 1998;27:799–807. doi: 10.1002/hep.510270323. [DOI] [PubMed] [Google Scholar]
- O'Riordan DK, Debnam ES, Sharp PA, Simpson RJ, Taylor EM, Srai SS. Mechanisms involved in increased iron uptake across rat duodenal brush-border membrane during hypoxia. The Journal of Physiology. 1997;500:379–384. doi: 10.1113/jphysiol.1997.sp022028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Cole TB, Findley SD. ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO Journal. 1996a;15:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proceedings of the National Academy of Sciences of the USA. 1996b;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Findley SD. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO Journal. 1995;14:639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkila S, Waheed A, Britton RS, Bacon BR, Zhou XY, Tomatsu S, Fleming RE, Sly WS. Association of the transferrin receptor in human placenta with HFE, the protein defective in hereditary hemochromatosis. Proceedings of the National Academy of Sciences of the USA. 1997;94:13198–13202. doi: 10.1073/pnas.94.24.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen IT, Saier MH., Jr A novel family of ubiquitous heavy metal ion transport proteins. Journal of Membrane Biology. 1997;156:99–103. doi: 10.1007/s002329900192. [DOI] [PubMed] [Google Scholar]
- Petris MJ, Camakaris J, Greenough M, Lafontaine S, Mercer JB. A C-terminal di-leucine is required for localization of the Menkes protein in the trans-Golgi network. Human Molecular Genetics. 1998;7:2063–2071. doi: 10.1093/hmg/7.13.2063. [DOI] [PubMed] [Google Scholar]
- Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO Journal. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- Petrukhin K, Lutsenko S, Chernov I, Ross BM, Kaplan JH, Gilliam TC. Characterization of the Wilson disease gene encoding a P-type copper transporting ATPase: genomic organization, alternative splicing, and structure/function predictions. Human Molecular Genetics. 1994;3:1647–1656. doi: 10.1093/hmg/3.9.1647. [DOI] [PubMed] [Google Scholar]
- Pufahl RA, Singer CP, Peariso KL, Lin SJ, Schmidt PJ, Fahrni CJ, Culotta VC, Penner-Hahn JE, O'Halloran TV. Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science. 1997;278:853–856. doi: 10.1126/science.278.5339.853. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Babcock MC, Kaplan J. The yeast frataxin homologue mediates mitochondrial iron efflux. Evidence for a mitochondrial iron cycle. Journal of Biological Chemistry. 1999;274:4497–4499. doi: 10.1074/jbc.274.8.4497. [DOI] [PubMed] [Google Scholar]
- Raja KB, Simpson RJ, Peters TJ. Investigation of a role for reduction in ferric iron uptake by mouse duodenum. Biochimica et Biophysica Acta. 1992;1135:141–146. doi: 10.1016/0167-4889(92)90129-y. [DOI] [PubMed] [Google Scholar]
- Rensing C, Mitra B, Rosen BP. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proceedings of the National Academy of Sciences of the USA. 1997;94:14326–14331. doi: 10.1073/pnas.94.26.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing C, Sun Y, Mitra B, Rosen BP. Pb(II)-translocating P-type ATPases. Journal of Biological Chemistry. 1998;273:32614–32617. doi: 10.1074/jbc.273.49.32614. [DOI] [PubMed] [Google Scholar]
- Richardson DR, Ponka P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochimica et Biophysica Acta. 1997;1331:1–40. doi: 10.1016/s0304-4157(96)00014-7. [DOI] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Roy CN, Penny DM, Feder JN, Enns CA. The hereditary hemochromatosis protein, HFE, specifically regulates transferrin-mediated iron uptake in HeLa cells. Journal of Biological Chemistry. 1999;274:9022–9028. doi: 10.1074/jbc.274.13.9022. [DOI] [PubMed] [Google Scholar]
- Sambongi Y, Wakabayashi T, Yoshimizu T, Omote H, Oka T, Futai M. Caenorhabditis elegans cDNA for a Menkes/Wilson disease gene homologue and its function in a yeast CCC2 gene deletion mutant. Journal of Biochemistry. 1997;121:1169–1175. doi: 10.1093/oxfordjournals.jbchem.a021711. [DOI] [PubMed] [Google Scholar]
- Savigni DL, Morgan EH. Transport mechanisms for iron and other transition metals in rat and rabbit erythroid cells. The Journal of Physiology. 1998;508:837–850. doi: 10.1111/j.1469-7793.1998.837bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ, Lombard M, Raja KB, Thatcher R, Peters TJ. Iron absorption by hypotransferrinaemic mice. British Journal of Haematology. 1991;78:565–570. doi: 10.1111/j.1365-2141.1991.tb04490.x. [DOI] [PubMed] [Google Scholar]
- Skamene E, Schurr E, Gros P. Infection genomics: Nramp1 as a major determinant of natural resistance to intracellular infections. Annual Review of Medicine. 1998;49:275–287. doi: 10.1146/annurev.med.49.1.275. [DOI] [PubMed] [Google Scholar]
- Stearman R, Yuan DS, Yamaguchi-Iwai Y, Klausner RsD, Dancis A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science. 1996;271:1552–1557. doi: 10.1126/science.271.5255.1552. [DOI] [PubMed] [Google Scholar]
- Su MA, Trenor CC, Fleming JC, Fleming MD, Andrews NC. The G185R mutation disrupts function of the iron transporter Nramp2. Blood. 1998;92:2157–2163. [PubMed] [Google Scholar]
- Supek F, Supekova L, Nelson H, Nelson N. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proceedings of the National Academy of Sciences of the USA. 1996;93:5105–5110. doi: 10.1073/pnas.93.10.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nature Genetics. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- Teichmann R, Stremmel W. Iron uptake by human upper small intestine microvillous membrane vesicles. Indication for a facilitated transport mechanism mediated by a membrane iron-binding protein. Journal of Clinical Investigation. 1990;86:2145–2153. doi: 10.1172/JCI114953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbreit JN, Conrad ME, Moore EG, Desai MP, Turrens J. Paraferritin: a protein complex with ferrireductase activity is associated with iron absorption in rats. Biochemistry. 1996;35:6460–6469. doi: 10.1021/bi951927s. [DOI] [PubMed] [Google Scholar]
- van Eijk HG, De Jong G. The physiology of iron, transferrin, and ferritin. Biological Trace Element Research. 1992;35:13–24. doi: 10.1007/BF02786234. [DOI] [PubMed] [Google Scholar]
- Vidal S, Belouchi AM, Cellier M, Beatty B, Gros P. Cloning and characterization of a second human NRAMP gene on chromosome 12q13. Mammalian Genome. 1995a;6:224–230. doi: 10.1007/BF00352405. [DOI] [PubMed] [Google Scholar]
- Vidal S, Gros P, Skamene E. Natural resistance to infection with intracellular parasites: molecular genetics identifies Nramp1 as the Bcg/Ity/Lsh locus. Journal of Leukocyte Biology. 1995b;58:382–390. doi: 10.1002/jlb.58.4.382. [DOI] [PubMed] [Google Scholar]
- Vidal SM, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nature Genetics. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nature Genetics. 1999;21:195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- Vulpe CD, Packman S. Cellular copper transport. Annual Review of Nutrition. 1995;15:293–322. doi: 10.1146/annurev.nu.15.070195.001453. [DOI] [PubMed] [Google Scholar]
- Waheed A, Parkkila S, Saarnio J, Fleming RE, Zhou XY, Tomatsu S, Britton RS, Bacon BR, Sly WS. Association of HFE protein with transferrin receptor in crypt enterocytes of human duodenum. Proceedings of the National Academy of Sciences of the USA. 1999;96:1579–1584. doi: 10.1073/pnas.96.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter R. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proceedings of the National Academy of Sciences of the USA. 1997;94:12676–12681. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wien EM, van Campen DR. Ferric iron absorption in rats: relationship to iron status, endogenous sulfhydryl and other redox components in the intestinal lumen. Journal of Nutrition. 1991;121:825–831. doi: 10.1093/jn/121.6.825. [DOI] [PubMed] [Google Scholar]
- Wilson RB, Roof DM. Respiratory deficiency due to loss of mitochondrial DNA in yeast lacking the frataxin homologue. Nature Genetics. 1997;16:352–357. doi: 10.1038/ng0897-352. [DOI] [PubMed] [Google Scholar]
- Wood RJ, Han O. Recently identified molecular aspects of intestinal iron absorption. Journal of Nutrition. 1998;128:1841–1844. doi: 10.1093/jn/128.11.1841. [DOI] [PubMed] [Google Scholar]
- Yu JM, Wessling-Resnick M. Influence of copper depletion on iron uptake mediated by SFT, a stimulator of Fe transport. Journal of Biological Chemistry. 1998a;273:6909–6915. doi: 10.1074/jbc.273.12.6909. [DOI] [PubMed] [Google Scholar]
- Yu J, Wessling-Resnick M. Structural and functional analysis of SFT, a stimulator of Fe transport. Journal of Biological Chemistry. 1998b;273:21380–21385. doi: 10.1074/jbc.273.33.21380. [DOI] [PubMed] [Google Scholar]
- Zhao H, Eide D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proceedings of the National Academy of Sciences of the USA. 1996a;93:2454–2458. doi: 10.1073/pnas.93.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Eide D. The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. Journal of Biological Chemistry. 1996b;271:23203–23210. doi: 10.1074/jbc.271.38.23203. [DOI] [PubMed] [Google Scholar]
- Zhou B, Gitschier J. hCTR1 - A human gene for copper uptake identified by complementation in yeast. Proceedings of the National Academy of Sciences of the USA. 1997;94:7481–7486. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XY, Tomatsu S, Fleming RE, Parkkila S, Waheed A, Jiang J, Fei Y, Brunt EM, Ruddy DA, Prass CE, Schatzman RC, O'Neill R, Britton RS, Bacon BR, Sly WS. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proceedings of the National Academy of Sciences of the USA. 1998;95:2492–2497. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]


